Abstract
Present study is completed on soils developed from different sources and types of materials (fluvial, lacustrine, marine, sandy and fluvio-sandy, and calcareous deposits). These soils belong to orders entisols and aridisols. The diverse geological nature of the deposits on which these soils are developed is reflected in the wide variation of soil characteristics (morphologies, clay, cation-exchange capacity, carbonate equivalents, and oxides of Fe, Al, Si, and Mn). All soils are alkaline in reaction, ECe (salinity) ranges between 0.66 and 8.0 dS m−1, clay (6.2–57.5%), cation-exchange capacity (3.0–79.1 cmolc kg−1), organic matter (0.29–2.68%), and calcium carbonate equivalents (0.07–55.62%). Total Fe, Al, and Mn concentrations differed greatly between soils, and the majority of the Fe and Mn occurred in crystalline form. Aluminum oxides are amorphous especially in clayey soils. Total free Si was similar to or exceeded to those of amorphous Si in all soils except calcareous ones. The soils developed from different materials are classified, such as fluvial (Vertic Torrifluvents, Typic Torrifluvents, Typic Fluvaquents, and Typic Ustifluvents), lacustrine (Typic Xerofluvents and Typic Fluvaquents), marine (Typic Xeropsamments and Typic Psammiaquents), sandy (Typic Quartzipsamments and Typic Torripsamments), and calcareous (Typic Haplocalcids).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
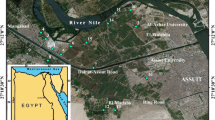
The Nile Delta and the Nile River Valley of Egypt is one of the oldest agricultural areas in the world. These areas are under continuous cultivation for at least 5,000 years. Egypt is situated at the northeast extremity of Africa; it lies between the latitudes 31° N and 22° N and between the longitudes 25° E and 34° E. Its gross area is 1,001,450 km2, of which about 3.6% is suitable for agricultural production. Physiographically, Egypt is divided into three main regions, the delta, the Nile Valley, and the deserts (Amer and Abo-Zeid 1989).
Soil properties and classifications are influenced by the type of materials from which the soils are developed and the conditions of depositional environments. Pedologists intend to establish relationships between the deposition environments and soils morphological, physical, chemical, and mineralogical characteristics as well as soil classifications (Perttjhon 1984).
The soil parent materials could be from sources such as rocks and mineral mixtures, sediments from floods, glacial deposits, and aeolian deposits (Raymond and Roy 1995). Fluvial deposits include all sediments deposited by the running waters. These deposits are generally poorly sorted and reveal evidence of rapid but interrupted deposition. The alluvial soils are developed through the deposition of alluvium brought by streams to the land (Worcoster 1969). The lacustrine deposits are settled out of stagnant lakes, whereas the marine deposits originate from stream action and deposited in the sea (FAO-SF 1964; Selley 1982).
It is well established that the type and source of the soil materials and the depositional environments control the soil properties and classifications due to particle-size distribution and the mineralogical composition. Present study was completed with the objective to investigate the influence of different sources of soil materials on soil properties and classifications and based on the basis of these results to discuss management issues of these soils.
2 Materials and Methods
2.1 Field Description and Sample Collection
Eighteen soil profiles from cultivated entisols and aridisols representing different geological deposits of Egypt (fluvial, lacustrine, marine, sandy and fluvio-sandy, and calcareous) were selected for the present study. Fluvial soils were selected from the Nile Delta, lacustrine soils (Burullus lake, north of the Nile Delta), sandy marine soils (north of Nile Delta by the Mediterranean sea), and calcareous soils (north of the western desert of Egypt). Soil profiles were dug, various genetic horizons and layers described using standard FAO procedures (FAO 1970), as well as using American system of soil classification (Soil Survey Staff 2006). The horizons were described for soil color (moist, dry), soil texture, structure, and consistence (wet, moist, dry), and effervescence test was made to check calcium carbonates. Soil samples were collected from representative depths of the soil profiles.
2.2 Laboratory Procedures
The soil samples were processed prior to their analyses in the laboratory. The samples were air-dried and ground to pass through a 2-mm sieve to obtain fine earth fraction. The soil samples were analyzed for routine physical and chemical characteristics using the procedures referred by Sparks et al. (1996). EC of the soil saturation extract (ECe) was measured by standard EC meter. Soluble cations (Na, K, Ca, Mg) and anions (CO3, HCO3, Cl, SO4) were measured using standard titration method and atomic absorption spectrophotometer (AAS) as appropriate. Soil reaction (pH) was measured in a 1:2.5 soil-deionized water suspension (Thomas 1996). Calcium carbonate equivalents were determined by calcimeter. Organic matter content was determined by the Walkley-Black method (Walkley 1947). Cation-exchange capacity (CEC) was measured by saturating the soil exchange complex with 1N ammonium acetate solution at pH 7 (Sumner and Miller 1996); the ammonium was replaced and measured to represent CEC. Extractable cations (Na, K, Ca, Mg) were determined in 1N ammonium acetate extract; the difference between the extractable and soluble cations represented as exchangeable cations. Particle-size distribution analysis was accomplished by standard pipette method (Gee and Bauder 1986) following by wet sieving to quantify five sand subfractions (very coarse, coarse, medium, fine, and very fine). Soil texture was determined using USDA textural triangle (Soil Survey Division Staff 1993). Total Fe, Al, and Mn were extracted by using mixture of concentrated nitric acid, concentrated hydrochloric acid, and 30% hydrogen peroxide according to USEPA method (USEPA 1995). Total free iron oxides (FIO) were extracted with 3 M sodium citrate, 1 M sodium bicarbonate, and 1 g sodium dithionite in a water bath at 85 °C (Mehra and Jackson 1960); Al, Mn, Si, and Ca were also measured in the same extract. Amorphous Fe oxides as Fe (Feox) were extracted with 0.175 M ammonium oxalate + 0.1 M oxalic acid adjusted to pH 3.0. Calcareous soils were pretreated with 1N sodium acetate (pH 5.5) to remove carbonates according to Loeppert and Inskeep (1996). The P, Al, Mn, Si, and Ca were measured in this extract. The Fe, Al, Mn, Si, and Ca were measured by atomic absorption spectrometry (Varian, SpectrAA-400 Plus, Australia).
3 Results and Discussion
In this section, soils developed from different sources of materials are described.
3.1 Fluvial Soils
3.1.1 Field Description, Soil Classification, Physical, and Chemical Characteristics
Table 23.1 illustrates general aspects and soil classification of the fluvial soil profiles. Generally, the soil color is very dark grayish brown (moist) and dark grayish brown (dry); massive structure and soil consistency is sticky and plastic (wet), firm (moist), and hard (dry). These properties are due to the type of sediments with varying clay contents, cemented materials, and type of clay minerals. Based on the genetic horizons development and the sequence in the profiles, the fluvial profiles are classified as Typic Torrifluvents, Typic Fluvaquents, Typic Ustifluvents, and Vertic Torrifluvents (Table 23.1 ).
Silty clay (heavy) is the common soil texture in various soil horizons of all soil profiles (Table 23.2 ), except at few depths where other textures were found (clay loam, silty clay loam). The ranges of primary soil particles are clay (17.0–57.5%), silt (14.1–53.8%), and sand (<1–68.9%). The sand was irregularly distributed with depth, the dominant being in the fine and very fine fractions (250–50 μm).
Data in Table 23.2 showed that fluvial soils (P1, P2, P4, and P5) are either nonsaline (ECe < 2 dS m−1), or they are (P3 and P6) slightly saline (ECe 4–8 dS m−1). There is slight but an insignificant in-depth difference of salt distribution within the profile. The highest ECe was recorded at the subsurface (P6); this could be attributed to the mobility of salts downward with descending irrigation water or from below through rising water table. Relatively water soluble Na+ is dominant over the Ca2+, Mg2+, and K+. General trend for cations (Na > Ca + Mg > K) and anions (Cl > SO4 > HCO3 > CO3) was observed. In P1, different trend exists for cations (Ca > Mg > Na > K) and anions (Cl > HCO3 > SO4 > CO3). Increasing and decreasing trend of other properties is shown in Table 23.2 . The soil reaction (pHs) in general is in the moderately alkaline range (7.9–8.4).
The organic matter is low (0.88–1.26%). The relatively high value at the surface is due to the residual effect of cultivation on these soils and the addition of organic manure and plant residues to improve soils physical and fertility aspects. All profiles indicate different trends in distribution of total CaCO3 with depth. However, most of them present less than 3%, other between 3 and 5%. From such low quantities, it is difficult to confirm the calcification process. Higher quantities at surface (Sparks 1995) can be due to secondary carbonates.
The CEC values are due to higher clay contents in the profiles. The CEC shows irregular trend of distribution in the profiles; this could be due to the type of sediments, clay minerals, and organic matter and perhaps due to fine clay illuviation; however, this has not been confirmed in the present study. The values of the exchangeable Na and CEC were used to determine soil sodicity, which shows soils to be in general highly sodic with ESPs ranging between 27.6 and 60.6.
3.2 Lacustrine Soils
3.2.1 Field Description, Soil Classification, and Physical and Chemical Characteristics
General aspects and soil classification of four lacustrine profiles are presented in Table 23.3 . Field description showed very dark grayish brown color in the moist state of most layers in all profiles, while in dry state, the dominant color was dark grayish brown. The structure in all layers of four tested profiles was massive and massive blocky structure except for the surface layer in P1 and P2, where it was coarse to medium blocky structure. The soil consistence was sticky and plastic in surface layers and very sticky, very plastic in the deepest ones in the wet state, firm in moist state, and hard in dry state.
These features are mainly due to the nature of sediment, the increase of clay content, and cemented materials as well as the type of clay minerals. The classifications of all lacustrine profiles were Typic Fluvaquents (P2 and P4) and Typic Xerfluvents (P1 and P3).
Silty clay is the common soil texture, followed by silty clay loam and in one depth (P4), it is clay loam (Table 23.4 ). Table 23.4 illustrates heavy texture at surface and subsurface layers and also in the deepest layers, while the texture was relatively light in the middle layers in profile nos. 2, 3, and 4. Such variations in soil texture are due to multi-depositional cycles. The clay (21.4–56.3%) and sands (0.98–55.05%) were distributed variably. The fine and very fine sand (250–50 μm) was the dominant fractions. The differences in soil texture, CaCO3 content, and CEC values are closely related to the matter of sedimentation mode and the nature of parent materials.
The ECe is relatively higher at deeper layers due to leaching. The difference is due to type of irrigation water used, clay content, and drainage conditions. Salt distributions throughout the profiles were irregular and differ in profiles. The soil reaction (pHs) ranges between 7.93 and 9.13 (moderately to strongly alkaline reaction), and ECe is generally less than 4 dS m−1 (Table 23.4 ). Water soluble sodium was dominant among cations, other cations trend Mg2+ > Ca2+ > K+, and among anions, Cl− was dominant over others trending SO4 > HCO3. Lacustrine soils exhibited more soluble Mg2+ and K+ relative to other soils. Similar trends are reported earlier (Ali 1985).
The organic matter content is low. The relatively higher but insignificant values at surface are due to cultivation and addition of organic manure and plant residues. Surface and subsurface layers (P2, P3, and P4) have high CaCO3 (17.3%), while the deepest layers of P4 have the lowest (0.07%). The high values of CaCO3 are due to the presence of broken and complete shells.
The CEC values vary greatly and have irregular trend due to sedimentation regime, clay, and organic matter contents. The soils (P1 and P2) are sodic where ESP > 15; other profiles are non-sodic (ESP < 15). The sodic soils are to be amended with gypsum based on gypsum requirement to improve properties of these profiles.
3.3 Marine Soils
3.3.1 Field Description, Soil Classification, and Physical and Chemical Characteristics
Table 23.5 presents soil properties and classification. The color is variable dominantly light yellowish brown (dry) and light olive brown (moist). The dominance of light color, structureless, nonsticky, nonplastic and friable properties, and in depth difference in sand, silt, and clay are due to the nature of sandy sediments. The soil classification is Typic Xerpsamments (P1) and Typic Psammiaquents (P1 and P3). Sand is the texture in all profiles at all depths, very fine and fine sands together dominate in sand subfractions, other fraction ranges in their mean values as 2.0–4.6 (silt) and 4.23–5.5 (clay).
Table 23.6 presents physical and chemical characteristics, revealing generally organic matter and CaCO3 (<1%); CEC ranges between 3.1 (P3) and 10 cmol kg−1 (P1), and ESP is lowest 6.3 in P3 and the highest 62.9 in P1. The ECe is very low (<0.73 dS m−1) in P1, <3.2 dS m−1 in P2, and <7 dS m−1 in P3. This shows that P1 and P2 are sodic and P3 is saline at subsurface (below 30 cm). Such a development is due to the use of irrigation water and high drainage conditions in sandy texture that leached the salts.
Soluble Na and chlorides dominate among other cations and anions, respectively. The pH is in the range of moderately to strongly alkaline (P1 and P2) and very strongly alkaline (P3). The pH is above the optimum range (pH 6.6–7.3) where most of the plant nutrients become available to plants. It is not clear why the pH in P1 and P2 is lower than P3, the former are sodic profiles, which usually give pHs > 9.00 (Richards 1954).
3.4 Sandy and Fluvio-Sandy Soils
3.4.1 Field Description, Soil Classification, and Physical and Chemical Characteristics
Variable color, structure, and consistence observed during profile description (Table 23.7 ). Table 23.8 shows sandy (P1) and variable texture (clay loam, loam, and sandy loam) in P2. Sand being dominant over silt and clay in both profiles. Profile 2 represents the zone between fluvial and sandy deposits, and there is large difference in texture due to multi-depositional regime. Similar findings were reported by El-Shahawi (1994). The difference in color and other characteristics is due to type of sediment and the management of these soils. The CaCO3 is low (less than 3%), organic matter (<1%), soil reaction (pHs) is very strongly alkaline in all layers of both profiles, except upper layer of P2 (strongly alkaline), and hence will restrict nutrient availability to plants. The CEC is relatively lower in P1 than P2, this is due to high clay content and perhaps type of clay minerals in P2. Soluble Na and chlorides dominate among other cations and anions, respectively. All layers (P1 and P2) are nonsaline and non-sodic (ECe < 4 dS m−1 and ESP < 15), except upper surface in P2 which is saline. Profiles are classified as Typic Quartzipsamments (P1) and Typic Torripsamments (P2). In a previous study, Shaheen (1999) has reported similar results.
3.5 Calcareous Soils
3.5.1 Field Description, Soil Classification, and Physical and Chemical Characteristics
Variable color, texture, and consistence observed during profile description (Table 23.9 ). Table 23.10 shows silty loam being the dominant texture (P1 and P2) and variety of textures in P3. Compared to all other profiles investigated, these soils are heavier in soil texture, and silt being dominant. The trend of primary particles is silt > clay > sand. This can be attributed to the type of parent material developed under marine and lagoonal conditions high in carbonate contents (Moustafa 1987).
The difference in color and other characteristics is due to type of sediment and the management of these soils. All profiles are highly calcareous, and the CaCO3 is relatively higher in P1 compared to other two profiles. The high-CaCO3 contents correspond to high silt content (El-Gamal 1992). The organic matter in general is <1%, and at surface of all profiles, it is present up to 1.61%. Soil reaction (pH) is strongly alkaline in all layers (P1 and P2) and very strongly alkaline (P3), and hence will restrict nutrient availability to plants. The mean CEC is higher (11.5–17.8 cmolc kg-1) due to high clay content. The entire profiles (P1, P2, P3) are nonsaline and non-sodic (ECe < 4 dS m−1 and ESP < 15). All profiles are classified as Typic Haplocalcids. The trend of soluble cations (Na > Ca > K) and soluble anions is same (Cl > SO4 > HCO3) as for other profiles.
4 Sesquioxides in the Tested Profiles
Table 23.11 reveals sequioxides of profiles, showing the dominance of total Fe and Al when expressed on g kg−1 basis. Total Fe being the lowest in marine soils (6.1 g kg−1), and the highest in lacustrine soils (58.4 g kg−1). Total aluminum follows same trend as for total Fe (lowest 5.9 g kg−1 in marine and highest 64.9 g kg−1 in lacustrine soils). The general trend of citrate-bicarbonate-dithionite extractable Fe, Mn, Al, Si, and Ca is found as Ca dominates over the others, Mn being the lowest, whereas Fe and Al codominate over other ammonium oxalate extractable contents (Mn, Si, Ca). The variations of sequioxides with respect to profiles developed from different sediments can be seen in Table 23.11 .
5 Conclusions and Recommendations
The classification of profiles and their basic properties are investigated. The profiles vary considerably in physical and chemical properties depending on the geological nature of the deposits (fluvial, lacustrine, marine, calcareous, sandy, and fluvio-sandy). The fluvial, lacustrine, marine, and sandy and fluvio-sandy soils are classified as entisols, whereas calcareous soils as aridisols. The soils exhibited quite different physical and chemical properties. The pH is above optimum range of nutrient availability; therefore, the soil needs careful management to improve nutrient efficiency in soil. The sodic soils need to be amended by gypsum application to reduce sodicity. The diverse geological nature of these deposits is reflected in the wide variation of clay content, carbonates content, and in the form of Fe, Al, Si, and Mn oxides.
References
Ali RA (1985) Mineralogical and chemical composition of some Egyptian soils in relation to potassium status. PhD thesis, Tanta University
Amer MH, Abo-Zeid M (1989) History of land drainage of Egypt. In: Amer MH, Ridder NA (eds) Land drainage in Egypt. DRI, Cairo
El-Gamal AA (1992) Different approaches for evaluation of soil development and uniformity at the area between Nubaria and El hager canal. MSc thesis, Tanta University
El-Shahawi MI (1994) Pedochemical studies on some Egyptian soils. PhD thesis, Tanta University
FAO (1970) Guidelines to soil description. FAO Bull, Rome
FAO-SF (1964) High dam soil survey. UAR, United Nations Development Program, FAO, Rome
Gee GW, Bauder JW (1986) Particle size analysis. In: Klute A (ed) Methods of soil analysis: physical and mineralogy methods, part 1, 2nd edn. ASA/SSSA, Madison, pp 383–412
Loeppert RH, Inskeep WP (1996) Iron. In: Sparks DL (ed) Methods of soil analysis: chemical methods, part 3. ASA/SSSA, Madison, pp 639–664
Mehra OP, Jackson ML (1960) Iron oxides removal from soils and clays by dithionate-citrate system buffered with sodium bicarbonate. Clays Clay Miner 7:317–327
Moustafa AM (1987) Pedological studies on new formations in some calcareous soils of the western desert, Egypt. MSc thesis, Alexandria University
Perttjhon EJ (1984) Sedimentary rocks, 3rd edn. CBC Publishers and Distributors, Delhi
Raymond WA, Roy LD (1995) Soils in our environment. Prentice Hall, Englewood Cliffs
Richards L (ed) (1954) Diagnosis and improvement of saline and alkali soils, vol 60, USDA handbook. US Salinity Lab Staff, Washington, DC
Selley RC (1982) An introduction to sedimentology, 2nd edn. Academic, London
Shaheen SM (1999) Some pedological and geochemical studies of some Egyptian soils. MSc thesis, Tanta University
Soil Survey Division Staff (1993) Soil survey manual. Handbook No 18. USDA, Washington, DC
Soil Survey Staff (2006) Key of soil taxonomy, 10th edn. USDA-NRCS, Govt Printing Office, Washington, DC
Sparks DL (1995) Environmental soil chemistry. Academic, New York
Sparks DL, Page AL, Helmke PA, Loppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Summner ME (1996) Methods of soil analysis: chemical methods, part 3. ASA/SSSA, Madison
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Sparks DL, Page AL, Helmke PA, Loppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Summner ME (eds) Methods of soil analysis: chemical methods, part 3. ASA/SSSA, Madison, pp 1201–1230
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL, Page AL, Helmke PA, Loppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Summner ME (eds) Methods of soil analysis: chemical methods, part 3. ASA/SSSA, Madison, pp 475–490
United States Environmental Protection Agency (USEPA) (1995) Test methods for evaluating solid wastes. USEPA SW 846. US Govt Printing Office, Washington, DC
Walkley A (1947) A critical examination of a rapid method for determining soil organic matter. Soil Sci 63:654–661
Worcoster GP (1969) A text book of geomorphology, 2nd edn. Affiliated East–West Pvt Ltd, New Delhi
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Shaheen, S.M., Abo-Waly, M.E., Ali, R.A. (2013). Classification, Characterization, and Management of Some Agricultural Soils in the North of Egypt. In: Shahid, S., Taha, F., Abdelfattah, M. (eds) Developments in Soil Classification, Land Use Planning and Policy Implications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5332-7_23
Download citation
DOI: https://doi.org/10.1007/978-94-007-5332-7_23
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5331-0
Online ISBN: 978-94-007-5332-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)