Abstract
Lipopolysaccharide (LPS) administered i.p. increases significantly the activation of c-Fos in neurons of the nucleus of the solitary tract (NTS), which in turn activates hypothalamus-pituitary-adrenal axis. The vagus nerve appears to play a role in conveying cytokines signals to the central nervous system (CNS), since -in rodent models of sepsis- bilateral vagotomy abolishes increases in plasmatic glucocorticoid levels, but does not suppress c-Fos NTS activation. Considering that NTS also receives sensory inputs from carotid body chemoreceptors, we evaluated c-Fos activation and plasmatic cortisol levels 90 min after i.p. administration of 15 mg/kg LPS. Experiments were performed in male Sprague–Dawley rats, in control conditions and after bilateral carotid neurotomy (BCN). LPS administration significantly increases the number of c-Fos positive NTS neurons and plasmatic cortisol levels in animals with intact carotid/sinus nerves. When LPS was injected after BCN, the number of c-Fos positive NTS neurons, and plasmatic cortisol levels were not significantly modified. Our data suggest that carotid body chemoreceptors might mediate CNS activation during sepsis.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
26.1 Introduction
Inflammation induced by lipopolysaccharide (LPS) enhances neural and endocrine response through an increase in release of glucocorticoids and catecholamines, which in turn regulates pro-inflammatory cytokines (Steinman 2004). The effects of LPS may result from either direct actions of pro-inflammatory cytokines on central nervous system (CNS) structures, or mediated by activation of autonomic afferent pathways (Dantzer et al. 2000).
The role of peripheral sensory nerves in immunomodulation is not yet fully understood. Neuroimmune chemosensory transduction would begin at vagal paraganglia glomus cells, innervated by vagal afferent neurons (Berthoud et al. 1995), the somata of which are located in the nodose ganglion, and their central projection ending primarily within the dorsal vagal complex of the medulla oblongata. Thus, immunosensory inputs could: (1) initiate local cardiorespiratory reflexes, (2) convey information about the inflammatory process, and (3) activate specific signs of diseases. However, neither tumor necrosis factor (TNF)-α nor interleukin (IL)-1β has significant effects on the frequency of action potentials recorded from vagal nerve paraganglia (Mac Grory et al. 2010). In addition, in rodents exposed to LPS, bilateral subdiaphragmatic vagotomy hinders some sickness symptoms (Bluthe et al. 1994), but failed to prevent the activation –assessed by the induction of c-Fos protein (Hoffman et al. 1993)– of the nucleus tractus solitarii (NTS) (Wan et al. 1994; Hermann et al. 2001). Thus, NTS activation and the anti-inflammatory response induced by the administration of LPS could be mediated by the carotid body (CB) chemoreceptors (Fernandez et al. 2008, 2011; Zapata et al. 2011).
The carotid body is the largest paraganglion in the body (Mascorro and Yates 1980), and like other paraganglia, it has specialized glomus cells innervated by the carotid/sinus nerve, which forms its first synapse CNS at the NTS. In consequence, inflammation-derived sensory inputs originated from carotid body chemoreceptors can be differentially processed in the medulla. It is noteworthy that central (ascending or descending) projections from the NTS provide a neuronal substrate for interaction between immunosensory signals, hypothalamic-pituitary-adrenal axis, and autonomous nervous system as an immunomodulatory mechanism (Pavlov et al. 2003).
26.2 Materials and Methods
Male Sprague–Dawley rats (100–120 g), anesthetized with Na-pentobarbitone (60 mg/kg intraperitoneally, i.p.) and spontaneously breathing, were placed in supine position on a regulated heating pad. Body temperature was maintained about 37°C using a rectal thermistor probe. Right saphenous vein was cannulated for blood sampling. At the end of experiments, animals were euthanized by an overdose of anesthetic.
A ventral incision in the neck was performed to identify and section both carotid/sinus nerves (BCN, n = 12) close to the carotid body. Thereafter, either saline (Control group) or 15 mg/kg LPS (from Escherichia coli Serotype 0127:B8. Sigma-Aldrich Corp, USA) was administered i.p. After 90-min, blood samples were collected in heparin-containing tubes, centrifuged at 1,000 g for 15-min and plasma were stored at −80°C until use. Then, animals were fixed by formaldehyde trans-cardiac perfusion. Same procedure was used for Control animals (Co, n = 14) where both carotid/sinus nerves remained intact.
The brainstems were removed and post-fixed for 24 h before being transferred into sucrose solution. NTS sections were obtained on cryostat and processed for c-Fos protein activation in response to LPS. Briefly, slices were rinsed, blocked, and incubated with primary antibodies against rat c-Fos (Rabbit anti-c-Fos (Ab-5), Calbiochem, Merck, Germany). Then, washed sections were incubated with biotinylated secondary antibodies (Goat ant-Rabbit IgG, Jackson ImmunoResearch Laboratories, Inc., USA) and immunohistochemical detection was revealed with avidin-biotin-peroxidase complex and peroxidase substrate (Vectastain Elite ABC Kit, Vector Laboratories, Inc., USA). Finally, sections were mounted on glass slides and analyzed under light microscopy. c-Fos-labeled nuclei were counted manually (Hermann et al. 2001). Results are presented as number of c-Fos positive cells/mm2. Dorsal medulla areas were identified according to Paxinos and Watson atlas (Paxinos and Watson 2007).
Cortisol plasma levels were measured by ELISA with cortisol EIA Kit, according to manufacturer’s instructions (Cayman Chemical Company, USA). Briefly, 50 μL of plasma samples were added to 96-well plate. Non-specific binding (NSB), maximum binding (B0), and an eight-point standard curve were also included. Then, cortisol tracer and antiserum were added and the plate was incubated overnight at 4°C. Finally, Ellman’s reagent was added and the reaction was developed for 90-min. Plate was read at 405 nm. Cortisol concentrations were obtained by subtracting the NSB absorbance from B0 absorbance (corrected B0). Then, B/B0 (sample or standard bound/maximum bound) were calculated subtracting NBS absorbance from the absorbance of the samples or standard curve, and divided by the corrected B0. Data from standard curve were transformed using Logit, were Logit (B/B0) = ln [(B/B0) / (1 – B/B0)], and plotted as Logit (B/B0) vs. cortisol concentration.
Data are expressed as Means ± Standard Deviations of the Mean (SD). Statistical analyses were performed using GraphPad Prism© for Windows (GraphPad Software, CA, USA). Statistical differences were considered as significant when P < 0.05. All protocols were approved by The Commission of Bioethics of the Universidad Andres Bello. Na-pentobarbitone was kindly provided by Dr. Patricio Zapata.
26.3 Results
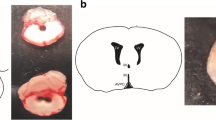
All medullary brainstem sections containing the NTS and the area postrema (AP) were analyzed for the presence of c-Fos stained nuclei. Saline-treated control rats (Co, n = 6), with both carotid/sinus nerves intact, showed discrete number of c-Fos labeled neurons in the NTS (Fig. 26.1a). Systemic administration of LPS induced a significant increase in NTS c-Fos labeling (n = 6) (Fig. 26.1b). Since the number of c-Fos positive cells after the i.p. administration of both saline and LPS was symmetrical even after bilateral carotid neurotomy (BCN) (P > 0.05. Paired t test), c-Fos count data were pooled (left NTS + right NTS). The graphic representation of the total number of activated neurons (per square mm) is displayed in Fig. 26.2, showing fivefold increases in c-Fos expression in response to systemic endotoxin challenge.
Photomicrographs of coronal histological sections of the NTS at the level of the area postrema (AP). c-Fos activation in response to i.p. challenge of either saline (a, c) or 15 mg/kg LPS (b, d). The latter is characterized by the dark staining cells in b. Systemic LPS evoked a substantial, bilateral, and symmetrical increase in c-Fos activation in the NTS excised from control rats, which was suppressed by bilateral carotid neurotomy (BCN). CC, central canal; R, right; L, left; DMNV, dorsal motor nucleus of vagus. Scale bar, 400 μm (10x original magnification). Inset in Co-LPS, 40x original magnification
Cell counts of c-Fos-activated neurons in the NTS from control (Co) and bilaterally carotid neurotomized (BCN) rats treated i.p. with either saline (open bars) or 15 mg/kg LPS (closed bars). Pooled data showed that LPS increased the number of c-Fos positive neurons in control animals, while in BCN animals, no increase was observed. Values, Means ± SD (n = 6), are expressed per mm2. *P < 0.05, Kruskal-Wallis ANOVA followed by Dunn’s multiple comparisons test
BCN decreased the number c-Fos positive cells in saline-treated rats (n = 6) (Fig. 26.1c) and suppressed LPS-induced c-Fos increase in the NTS (Fig. 26.1d) (n = 6), when BCN was performed prior to endotoxin challenge. Conversely, the number of c-Fos positive nuclei in the area postrema was slightly increased in LPS-treated Co and BCN animals, but did not reach statistical significance (P > 0.05, Kruskal-Wallis test, not shown).
Plasmatic cortisol levels measured 90-min after endotoxin challenge in both saline- (n = 7) and LPS-treated (n = 7) control rats showed a threefold increase in LPS-treated rats, but no changes were observed in saline-treated rats (Fig. 26.3).
Plasmatic cortisol response to i.p. administration of saline (open bars) or 15 mg/kg LPS (closed bars) in control (Co) and bilaterally carotid neurotomized (BCN) rats. LPS-induced plasma cortisol increase was suppressed by prior bilateral carotid chemodenervation. Values, Means ± SD (Co, n = 7; BCN, n = 6). *P < 0.05, Kruskal-Wallis ANOVA followed by Dunn’s multiple comparisons test
When saline (n = 6) was administered after BCN, no significant changes in plasmatic cortisol were observed. Noteworthy BCN (n = 6) suppressed the cortisol increases induced by LPS. Moreover, plasmatic levels were even lower than those measured in saline-treated control group (Fig. 26.3).
26.4 Discussion
LPS-induced systemic inflammation is known to evoke NTS activation but bilateral carotid chemodenervation suppresses both NTS activation and plasma cortisol increase in septic rats. Under our experimental conditions, NTS demonstrated a significant increase in c-Fos nuclear protein labeling after systemic challenge of endotoxin in rats with both carotid nerves intact. Vagus nerve appears to be important in conveying inflammation-derived signals to the central nervous system (CNS), but bilateral cervical vagotomy performed in septic rats had a subtle effect on the NTS activation (Hermann et al. 2001). On the contrary, in our experiments we found that bilateral carotid neurotomy (BCN) performed prior to LPS administration suppresses c-Fos activation in the NTS, indicating that carotid body chemoreceptors exert a key effect on the responsiveness of the NTS to LPS and/or immune cells-derived afferent inputs to CNS. It must be mentioned that in this case both vagus nerves remained intact in all animals.
It is also known that chronic hypoxia induces local increase of pro-inflammatory cytokines in the CB, regardless the model studied; intermittent, hypobaric or hypoxic hypoxia (Del Río et al. 2011; Liu et al. 2009; Lam et al. 2008; respectively). We reported that systemic or topical -upon the CB- application of LPS diminished the ventilatory chemoreflex evoked by acute hypoxia, but we did not find any histological evidence of inflammation evoked by that challenge (Fernandez et al. 2008). Considering that apoptosis was not observed, the aforementioned reduction in the chemoreflex may be mediated by release of inhibitory transmitter (De Laurentis et al. 2002). Taken together, above arguments suggest that only a tonical stimulation of the CB induces a local inflammatory response, clearly not enough to produce a systemic inflammatory response syndrome. A possible synergic interaction between a hypoxia stimulated CB and LPS has not been studied yet.
Although nodose neurons may still detect LPS-related signals and convey the information to the CNS through their synaptic inputs to the NTS (Hosoi et al. 2005), we recently described that in septic rats, LPS acts directly upon carotid body chemoreceptors and their sensory ganglion, inducing the local production of TNF-α (Fernandez et al. 2011), which in fact activates NTS neurons (Emch et al. 2000; Hermann and Rogers 2009). Furthermore, in the present work the number of c-Fos positive nuclei in the NTS obtained from BCN rats treated with saline was significantly lower than saline-treated control animals, confirming the tonic discharge from the carotid body chemoreceptors upon NTS.
Carotid body chemoreceptors seem to be an afferent pathway for endocrine response to endotoxemia. In fact, BCN suppresses the cortisol increases induced by LPS. Thus, acting through the NTS, central projections from the carotid body provide a neuronal substrate for interaction between immunosensory signals and hypothalamic-pituitary-adrenal axis, as an immunomodulatory mechanism activated during sepsis.
References
Berthoud HR, Kressel M, Neuhuber WL (1995) Vagal afferent innervation of rat abdominal paraganglia as revealed by anterograde DiI-tracing and confocal microscopy. Acta Anat (Basel) 152:127–132
Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R (1994) Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III 317:499–503
Dantzer R, Konsman JP, Bluthe RM, Kelley KW (2000) Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci 85:60–65
De Laurentis A, Pisera D, Caruso C, Candolfi M, Mohn C, Rettori V, Seilicovich A (2002) Lipopolysaccharide- and tumor necrosis factor–induced changes in prolactin secretion and dopaminergic activity in the hypothalamic-pituitary axis. Neuroimmunomodulation 10:30–39
Del Río R, Moya EA, Iturriaga R (2011) Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Res 1395:74–85
Emch GS, Hermann GE, Rogers RC (2000) TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol 279:G582–G586
Fernandez R, Gonzalez S, Rey S, Cortes PP, Maisey KR, Reyes EP, Larrain C, Zapata P (2008) Lipopolysaccharide-induced carotid body inflammation in cats: functional manifestations, histopathology and involvement of tumour necrosis factor-alpha. Exp Physiol 93:892–907
Fernandez R, Nardocci G, Simon F, Martin A, Becerra A, Rodriguez-Tirado C, Maisey KR, Cuna-Castillo C, Cortes PP (2011) Lipopolysaccharide signaling in the carotid chemoreceptor pathway of rats with sepsis syndrome. Respir Physiol Neurobiol 175:336–348
Hermann GE, Rogers RC (2009) TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: implications for autonomic control. Brain Res 1273:72–82
Hermann GE, Emch GS, Tovar CA, Rogers RC (2001) c-Fos generation in the dorsal vagal complex after systemic endotoxin is not dependent on the vagus nerve. Am J Physiol Regul Integr Comp Physiol 280:R289–R299
Hoffman GE, Smith MS, Verbalis JG (1993) c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14:173–213
Hosoi T, Okuma Y, Matsuda T, Nomura Y (2005) Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Auton Neurosci 120:104–107
Lam SY, Tipoe GL, Liong EC, Fung ML (2008) Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem Cell Biol 130:549–559
Liu X, He L, Stensaas L, Dinger B, Fidone S (2009) Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am J Physiol Lung Cell Mol Physiol 296:L158–L166
Mac Grory B, O’Connor ET, O’Halloran KD, Jones JF (2010) The effect of pro-inflammatory cytokines on the discharge rate of vagal nerve paraganglia in the rat. Respir Physiol Neurobiol 171:122–127
Mascorro JA, Yates RD (1980) Paraneurons and paraganglia: histological and ultrastructural comparisons between intraganglionic paraneurons and extra-adrenal paraganglion cells. Adv Biochem Psychopharmacol 25:201–213
Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9:125–134
Paxinos G, Watson CR (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, Burlington, p 456
Steinman L (2004) Elaborate interactions between the immune and nervous systems. Nat Immunol 5:575–581
Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM (1994) Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull 34:7–14
Zapata P, Larrain C, Reyes P, Fernandez R (2011) Immunosensory signalling by carotid body chemoreceptors. Respir Physiol Neurobiol 178:370–374
Acknowledgments
Special thanks are due to Mrs. Carolina Larraín for proofreading the manuscript. This work was supported by grant DI-40-11/R (to RF), from the Division for Research of the Universidad Andres Bello (UNAB).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Reyes, EP., Abarzúa, S., Martin, A., Rodríguez, J., Cortés, P.P., Fernández, R. (2012). LPS-Induced c-Fos Activation in NTS Neurons and Plasmatic Cortisol Increases in Septic Rats Are Suppressed by Bilateral Carotid Chemodenervation. In: Nurse, C., Gonzalez, C., Peers, C., Prabhakar, N. (eds) Arterial Chemoreception. Advances in Experimental Medicine and Biology, vol 758. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4584-1_26
Download citation
DOI: https://doi.org/10.1007/978-94-007-4584-1_26
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4583-4
Online ISBN: 978-94-007-4584-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)