Abstract
High flow nasal insufflations (NI) can improve gas exchange and alleviate dyspnea in patients with acute respiratory failure. In the present study we investigated the effects of high flow nasal insufflations in COPD patients with chronic hypercapnic respiratory failure (HRF). Seventeen patients with severe COPD and HRF were recruited. We delivered a mixture of 20 L/min room air and 2 L/min O2 through a nasal cannula either into both nostrils (NI) or into one nostril (Partial NI). Respiratory pattern and PaCO2 responses under NI were compared with low flow oxygen of 2 L/min. High flow nasal insufflations led to a systematic reduction in respiratory rate from 19.8 ± 4.2 at baseline to 18.0 ± 4.7 during NI (p < 0.008) and 18.1 ± 5.2 breaths/min during Partial NI (P < 0.03). The mean group inspiratory duty cycle (TI/TT) and mean group PaCO2 remained constant between all experimental conditions. Individual responses to NI were heterogeneous: six patients demonstrated marked reductions in respiratory rate (>20% fall from baseline), another group (n = 6) demonstrated no change in respiratory rate but marked reductions in arterial carbon dioxide of more than 8 mmHg. In conclusion, high flow (20 L/min) nasal insufflations of warm and humidified air during wakefulness for 45 min reduced respiratory rate without deterioration of hypercapnia. Our data indicate that high flow NI improved efficiency of breathing and may be used as an adjunct to low flow oxygen for preventing hypercapnic respiratory failure in severely ill COPD patients.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Chronic obstructive pulmonary disease (COPD) has a large impact on health worldwide. It is the fourth leading cause of both chronic morbidity and mortality in Western societies (Halbert et al. 2006; Mathers and Loncar 2006; Jemal et al. 2005; Fang et al. 2011). Disturbances in oxygenation and ventilation are commonly observed in patients with COPD and are recognized causes of increased mortality in COPD (Anthonisen 1983). Thus, a major target for treatment is the improvement in both oxygenation and ventilation, both of which have been shown to reduce mortality, particularly in patients with severe COPD (Report of the Medical Research Council 1981; Nocturnal Oxygen Therapy Trial Group 1980).
Low flow oxygen at a rate of 2–5 L/min is the mainstream treatment option for reducing mortality in hypoxemic patients with severe COPD. Oxygen administration for at least 15–18 h/day leads to almost 50% decrease in 5 year mortality compared with untreated controls. Although long-term low flow oxygen (LTOT) clearly benefits patients’ outcome, many patients do not adhere to this treatment. The primary reasons for low adherence are complaints of drying out of nasal mucosa and frequent nose bleeds, lack of alleviating dyspnea, and high cost of oxygen treatment. Non-invasive ventilation (NIV) via a nasal/facial mask has been proposed to offer an alternative or adjunct to LTOT, but adherence rates are even lower than those for LTOT (Clini et al. 2002; Wijkstra et al. 2003; McEvoy et al. 2009). Thus, low flow oxygen and NIV are hampered by either significant side effects or reduced adherence leaving a majority of patients with severe COPD insufficiently treated.
Recently, insufflation of high nasal airflow of warm and humidified room air has been introduced to stabilize breathing pattern in adults and children with obstructive sleep apnea (Schneider et al. 2000; McGinley et al. 2009). Moreover, several case reports indicate that this treatment can improve gas exchange and alleviate dyspnea (Roca et al. 2010). The effects of high flow nasal insufflation of room air (NI at 20 L/min) on gas exchange and breathing pattern in patients with severe COPD remain unclear. Therefore, in the present study we examined the effects of nasal insufflation on arterial blood gases, respiratory rate and inspiratory duty cycle in COPD patients who had chronic hypercapnic respiratory failure and required 1–2 L/min oxygen for maintaining normal levels of oxyhemoglobin levels. We hypothesized that nasal insufflation of room air would stabilize breathing pattern and gas exchange.
4.2 Methods
4.2.1 Subjects
This study was approved by the Ethics Committee of the University Witten-Herdecke. This study is registered under clinical trials.gov NCT01090544. Each participant was provided with the study information and enrolled in the study upon written consent. Baseline demographics and respiratory function are displayed in Table 4.1. All participants were recruited from the Pneumological Ward of the HELIOS Klinik Hagen-Ambrock and were currently receiving treatment for an acute exacerbation of COPD. As a usual part of clinical treatment arterial blood gas analysis was performed within the 2 days prior to their planned discharge. Subjects were asked to participate if they had mild to moderate hypoxia on supplemental oxygen (PaO2 < 80 mmHg) and hypercapnic respiratory failure (pCO2 > 50 mmHg, pH > 7.35). Exclusion criteria included: respiratory acidosis (pH < 7.30), unstable cardiovascular conditions, decompensated renal insufficiency, acute pneumonia, disturbed metabolic status and uncontrolled diabetes mellitus.
4.2.2 Procedures
Arterial blood gas exchange was measured by obtaining ∼5 μL of arterial blood at the end of each experiment from an earlobe. Blood gas analysis (Radiometer Ltd., Germany) were performed within 1–5 min after drawing each arterial sample. The PaO2 and PaCO2 values obtained at the end of each condition were recorded for statistical analysis.
Breathing responses were measured continuously by monitoring airflow with a nasal cannula and respiratory effort with inductive plethysmography. In addition, heart rate and oxyhemoglobin saturation (SaO2) were monitored by pulse oximetry and transcutaneous CO2 through a probe attached to the earlobe (SenTec® V-sign™ SenTec AG, Switzerland) to determine steady-state and to ensure safety of patients throughout the entire experiment. All physiologic signals were amplified and recorded continuously and digitized and stored for off-line analysis (Alice®4 Diagnostic Sleep System, Respironics-Philips, Hamburg, Germany).
A constant flow rate of up to 20 L/min was delivered at the nose. A heater and humidifier (TNI®20s oxy, TNI medical, Freiburg, Germany) were used to blend the clinically supplied compressed air and oxygen and to keep it at 30–33°C and 80%, respectively. A customized nasal cannula was used to deliver a combination of oxygen and high-flow heated humidified room air.
4.2.3 Experimental Protocol
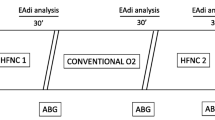
The experiment was performed prior to midday and carried out with the patients sitting comfortably, semi-recumbent in a bed, receiving low flow oxygen at a rate of 2 L/min. Three experimental conditions were achieved by administering combination of NI and oxygen for 45 min periods in a random order as described in brief below. There was a 15-min baseline period between each condition.
-
Baseline Condition: Standard low-flow oxygen at a rate of 2 L/min through a standard oxygen nasal cannula.
-
High-flow NI Condition: A combination of low-flow oxygen (as in the Baseline Condition) plus room air high-flow NI at 20 L/min both delivered through a custom made nasal cannula.
-
Partial NI: The same combination of low-flow oxygen plus room air high-flow NI at 20 L/min, delivered through only one nostril to achieve a lower PaO2 than the High-flow NI condition.
4.2.4 Data Analysis
Variables measured were: respiratory rate (ƒ) and inspiratory duty cycle (TI/TT), where TI = inspiratory time and TT = respiratory cycle length. The ƒ was calculated each minute and an average value was determined from the 45-min period for an individual in every condition. In each individual, an average TI/TT was taken from 15 breaths of the last minute of each condition.
Parameters were expressed as the mean for each patient in each experimental condition. Group data were reported as means ± SD. To examine responses in breathing patterns to High-flow NI, two-way ANOVA were performed with the patient number treated as a random factor and the NI flow rate treated as a fixed factor. When the ANOVA revealed a significant differences (p < 0.05), post hoc analysis was performed with paired t-tests to determine which levels of NI differed from Baseline. A p-value of less than 0.05 was considered significant.
4.3 Results
A total of 17 subjects were included in the study. Compared with Baseline, High-flow NI and Partial NI were associated with stable physiologic and clinical conditions. No subject demonstrated dyspnea or an increase in heart rate of greater than 10 bpm. There was no severe hypoxemia as defined by a prolonged (>5 min) drop in SaO2 below 85%. There was no group mean difference in the transcutaneous CO2 between any conditions (Baseline: 57.3 ± 10.1; High-flow NI 55.1 ± 8.7; Partial NI: 54.3 ± 10.2 mmHg; ANOVA; p = 0.23).
4.3.1 Effect of Nasal Insufflation on Blood Gas Levels
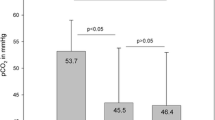
During the experimental conditions, NI decreased inspired FiO2 because of the high flow rate of room air entrained with a constant level of supplemental oxygen that was unchanged from baseline. The group mean PaO2 fell from the Baseline 62.3 ± 8.8 mmHg to 52.5 ± 8.1 mmHg (p < 0.01) during High-flow NI, and to 55.3 ± 10.2 mmHg during Partial NI (p < 0.03) (Fig. 4.1). There was no change in PaCO2 between all experimental conditions (Baseline: 63.7 ± 9.2 mmHg vs. High-flow: 60.6.4 ± 8.3 mmHg and Partial NI: 59.8 ± 7.9 mmHg; ANOVA; p = 0.17).
4.3.2 Breathing Pattern von Nasal Insufflation
For the group, the respiratory rate fell from 19.8 ± 4.2 at baseline to 18.0 ± 4.7 breaths/min during High-flow NI (p < 0.01) (Fig. 4.2). There was no difference in the respiratory rate between High-flow NI and Partial NI (18.1 ± 5.2 breaths/min, p = 0.98). The inspiratory duty cycle (TI/TT) remained constant throughout all experimental conditions (0.39 ± 0.06 vs. 0.40 ± 0.07 and 0.37 ± 0.05; baseline vs. high-flow NI and Partial NI, respectively).
4.3.3 Individual Respiratory Rate and Arterial Blood Gas Responses
There was no association between changes in respiratory rate and either PaO2 or PaCO2. Figure 4.3 shows individual responses in PaO2 (Fig. 4.3a), PaCO2 (Fig. 4.3b) and respiratory rate (Fig. 4.3c) between baseline and high flow NI. In approximately 1/3rd of patients, PaO2 remained above 55 mmHg during High-flow NI (n = 6). Similarly, approximately 1/3rd of the patients lowered their PaCO2 more than 4 mmHg during High-flow NI (n = 6), while there were 3 individuals during High-flow NI whose PaCO2 worsened more than 4 mmHg. More than 75% of patients lowered the respiratory rate by >2 breaths/min, while the remaining four individuals had no change in their respiratory rate (≤1 breath/min).
4.4 Discussion
The major finding of our study is that high flow nasal insufflation (NI) of 20 L/min over a 45-min period during wakefulness led to a systematic reduction in respiratory rate without worsening hypercapnia and dyspnea. This reduction in respiratory rate was present even after lowering PaO2 with partial NI. Second, in approximately 1/3rd of patients, the reduction in respiratory rate was associated with a significant improvement in hypercapnia (>4 mmHg). Third, respiratory rate responses were independent of blood gas changes. Taken together, high flow (20 L/min) nasal insufflation of warm and humidified appears to improve the efficiency of breathing and may be used as an adjunct to low flow oxygen for alleviating dyspnea and improving arterial CO2 levels in some COPD patients with severe hypoxic, hypercapnic respiratory failure.
Nasal insufflation of warm air has been used to stabilize breathing in patients with mild upper airway obstruction during sleep in adults and children. The mechanisms for alleviating upper airway obstruction appear to be through a mild (∼2 cmH2O) increase in end-expiratory pressure (PEEP) (McGinley et al. 2007; Groves and Tobin 2007; Parke et al. 2009). It is possible that the improvement in respiratory efficiency was due to an increase in end-expiratory pressure. PEEP is known to improve ventilation perfusion mismatch which helps to improve gas exchange. In the current study we show that despite lowering partial oxygen pressure, dyspnea did not develop and hypercapnia improved in 1/3rd of patients. Thus, it is possible that NI has improved ventilation perfusion mismatch, thereby improving gas exchange for carbon dioxide
A reduction in respiratory rate with both partial and high flow NI was approximately 10% compared with baseline low flow oxygen. Our data do not indicate that this reduction was associated with an increase in tidal volume, since respiratory effort and inspiratory duty cycle remained constant. Further support for this is given by a clinical observation that none of the patients developed dyspnea or an increase in heart rate that may have indicated augmented cardiovascular stress. Thus, it is likely that the reduction in respiratory rate with nasal insufflation was associated with a reduction in minute ventilation. Because we did not quantify airflow, the mechanisms of the reductions in minute ventilation remain unclear.
While the respiratory rate decreased in the majority of patients (13 of 17), there was a heterogeneous response to nasal insufflation. Some patients demonstrated marked reductions in respiratory rate (>20% from baseline) and others demonstrated no change in respiratory rate, but had marked reductions in arterial carbon dioxide of more than 8 mmHg. Thus, some patients respond to high-flow NI by lowering the respiratory rate, and presumably minute ventilation, while others appear to maintain ventilation, but decrease carbon dioxide levels. The mechanisms for this heterogeneous response are unclear. It is possible that NI has either improved alveolar ventilation through an increase in PEEP as mentioned above. Alternatively, NI may have either reduced anatomical dead space or carbon dioxide production. Regardless of the mechanisms, NI appears to improve efficiency of breathing and our data suggest may be used as an adjunct to oxygen therapy to prevent/treat hypercapnic respiratory failure in some patients.
4.5 Limitations
Our current study was designed as a pilot trial for examining the safety of NI in COPD patients with severe hypoxic, hypercapnic respiratory failure. As such our study has several limitations. First, patients did not accept a nasal or full face mask for capturing tidal volume. Similarly, we could not use a calibrated inductive plethysmography signal due to the patients’ illness and the inability to prevent body movements or coughs. The lack of measuring tidal volume limits our ability to explore mechanisms of our findings. Second, our patient population consisted of patients with severe COPD and hypercapnic respiratory failure who required supplemental oxygen. The lack of a baseline period without oxygen or NI may mask some of the physiologic effects of NI. We could not, however, remove supplemental oxygen at baseline to assess the breathing pattern and gas exchange on room air in these patients without producing significant hypoxia, dyspnea, or anxiety. Third, we did not adjust the O2 supply to maintain a constant fraction of inspired O2 (FiO2) during all trials. The blend of 2 L/min oxygen with 20 L/min NI may have diluted the FiO2 close to room-air, which resulted in lower arterial oxygen pressures during the NI trials. Nevertheless, approximately 1/3rd of the patients maintained PaO2 above 55 mmHg during NI, indicating that some patients may maintain oxygenation with NI alone.
4.6 Implications
NI might assist in the treatment of COPD, specifically the prevention of hypoxemia and hypercapnia, both of which are independent risk factors for mortality and morbidity. Our study demonstrates that a proportion of hypoxic COPD patients may be able to maintain normoxia with high flow NI. It is conceivable that NI may be utilized as an adjunct to improve oxygenation in hypoxic COPD patients. Second, NI led to a reduction in respiratory rate of ∼10%, and in some patients this reduction was associated with a reduction in hypercapnia. This finding suggests a reduction in the work of breathing, whose increase was associated with the development of muscle fatigue leading to respiratory failure. Thus, NI may serve as a treatment option to reduce work of breathing and to counteract the development of respiratory failure in some COPD patients. Third, sleep is associated with worsening of hypoxia and hypercapnia in the majority of COPD patients. High flow NI was easily tolerated in our patient population making nocturnal use of NI easily applicable. If the NI reduces the respiratory rate without worsening hypercapnia during sleep, high flow therapy may be a promising therapy option for treating sleep disordered breathing in severely ill COPD patients as well.
References
Anthonisen, N. R. (1983). Long-term oxygen therapy. Annals of Internal Medicine, 99, 519–527.
Clini, E., Sturani, C., Rossi, A., Viaggi, S., Corrado, A., Donner, C. F., & Ambrosino, N. (2002). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. European Respiratory Journal, 20, 529–538.
Fang, X., Wang, X., & Bai, C. (2011). COPD in China: The burden and importance of proper management. Chest, 139, 920–929.
Groves, N., & Tobin, A. (2007). High flow nasal oxygen generates positive airway pressure in adult volunteers. Australian Critical Care, 20, 126–131.
Halbert, R. J., Natoli, J. L., Gano, A., Badamgarav, E., Buist, A. S., & Mannino, D. M. (2006). Global burden of COPD: Systematic review and meta-analysis. European Respiratory Journal, 28, 523–532.
Jemal, A., Ward, E., Hao, Y., & Thun, M. (2005). Trends in the leading causes of death in the United States, 1970–2002. Journal of the American Medical Association, 294, 1255–1259.
Mathers, C. D., & Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine, 3, e442.
McEvoy, R. D., Pierce, R. J., Hillman, D., Esterman, A., Ellis, E. E., Catcheside, P. G., O’Donoghue, F. J., Barnes, D. J., & Grunstein, R. R. (2009). Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax, 64, 561–566.
McGinley, B. M., Patil, S. P., Kirkness, J. P., Smith, P. L., Schwartz, A. R., & Schneider, H. (2007). A nasal cannula can be used to treat obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 15(176), 194–200.
McGinley, B., Halbower, A., Schwartz, A. R., Smith, P. L., Patil, S. P., & Schneider, H. (2009). Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics, 124, 179–188.
Nocturnal Oxygen Therapy Trial Group. (1980). Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Annals of Internal Medicine, 93, 391–398.
Parke, R., McGuinness, S., & Eccleston, M. (2009). Nasal high-flow therapy delivers low level positive airway pressure. British Journal of Anaesthesia, 103, 886–890.
Report of the Medical Research Council Working Party. (1981). Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet, 1, 681–686.
Roca, O., Riera, J., Torres, F., & Masclans, J. R. (2010). High-flow oxygen therapy in acute respiratory failure. Respiratory Care, 55, 408–413.
Schneider, H., O’Hearn, D. J., Leblanc, K., Smith, P. L., O’Donnell, C. P., Eisele, D. W., Peter, J. H., & Schwartz, A. R. (2000). High-flow transtracheal insufflation treats obstructive sleep apnea. A pilot study. American Journal of Respiratory and Critical Care Medicine, 161, 1869–1876.
Wijkstra, P. J., Lacasse, Y., Guyatt, G. H., Casanova, C., Gay, P. C., Meecham Jones, J., & Goldstein, R. S. (2003). A meta-analysis of nocturnal noninvasive positive pressure ventilation in patients with stable COPD. Chest, 124, 337–343.
Acknowledgments
Supported by P50 HL084945, HL105546, and TNI medical Freiburg, Germany.
Conflicts of Interest: The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Nilius, G., Franke, KJ., Domanski, U., Rühle, KH., Kirkness, J.P., Schneider, H. (2013). Effects of Nasal Insufflation on Arterial Gas Exchange and Breathing Pattern in Patients with Chronic Obstructive Pulmonary Disease and Hypercapnic Respiratory Failure. In: Pokorski, M. (eds) Respiratory Regulation - Clinical Advances. Advances in Experimental Medicine and Biology, vol 755. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4546-9_4
Download citation
DOI: https://doi.org/10.1007/978-94-007-4546-9_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4545-2
Online ISBN: 978-94-007-4546-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)