Abstract
In 2005, Q fever was diagnosed on two dairy goat farms and 2 years later it emerged in the human population in the south of the Netherlands. From 2007 to 2010, more than 4,000 human cases were notified with an annual seasonal peak. The outbreaks in humans were mainly restricted to the south of the country in an area with intensive dairy goat farming. In the most affected areas, up to 15% of the population may have been infected. The epidemic resulted in a serious burden of disease, with a hospitalisation rate of 20% of notified cases and is expected to result in more cases of chronic Q fever among risk groups in the coming years. The most important risk factor for human Q fever is living close (<5 km) to an infected dairy goat farm. Occupational exposure plays a much smaller role. In 2009 several veterinary control measures were implemented including mandatory vaccination of dairy goats and dairy sheep, improved hygiene measures, and culling of pregnant animals on infected farms. The introduction of these drastic veterinary measures has probably ended the Q fever outbreak, for which the Netherlands was ill-prepared.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Since its first description in abattoir workers in Australia in 1935, Q fever has been considered primarily an occupational disease for abattoir workers, sheep shearers, farmers, and veterinarians. Occasional outbreaks among the general population have been described in different countries but these were mostly confined to small areas and were of short duration. The 2007–2010 epidemic of Q fever in the Netherlands with more than 4,000 notified human cases was unique. We describe the different aspects of this exceptionally large epidemic, primarily from the human health perspective, and provide details of ongoing research that will add considerably to the global knowledge base of Q fever. Topics covered are the surveillance of acute Q fever before and during the epidemic; the challenges in laboratory diagnostics; the long-term effects of Q fever; prevention of severe disease by vaccination; risks for pregnant women; and the drastic veterinary measures on dairy goat and dairy sheep farms that were implemented from 2009 and that have probably played a major role in stopping the epidemic.
17.2 Surveillance of Acute Q Fever and Diagnostic Criteria
17.2.1 Q Fever as a Rarity Before 2007
The diagnosis of Q fever was very rare in the Netherlands before 1977, despite increasing numbers of reported cases from other countries. Extensive studies were carried out between 1951 and 1956 on cattle (n = 524) and on patients with atypical pneumonia (n = 6,000). These studies tested serum samples using complement fixation test (CFT) and used animal (guinea pig) cultures. None of them revealed a positive result (Wolff and Kouwenaar 1954). Then in 1956, just as these studies were being phased out, the first three human cases of Q fever were diagnosed in the Netherlands (Westra et al. 1958; Dekking and Zanen 1958). One patient worked at a slaughterhouse, one was thought to have been infected in Switzerland, and a third had spent time living near sheep. When 28 of these sheep were serologically analysed, one tested positive.
17.2.2 An Increase in Reported Cases
In 1976 Q fever was added to the list of notifiable diseases in the Netherlands. This was quickly followed by a rise in the number of reported cases – an average of 2–3 a year between 1977 and 1980, and an average of 20 a year up until 2007. Thirty-three Q fever cases diagnosed between 1979 and 1983 have been described in more detail (Richardus et al. 1984a). An in-house developed immunofluorescence assay (IFA) was used and IgM phase II ≥1:16 was considered reactive. Apart from the usual clinical presentation, epidemiological analysis to identify possible sources showed that 67% of these patients had acquired Q fever in the Netherlands, while the rest were probably infected in a variety of other European countries. However, a reanalysis of the data shows that the serologic profiles described in the patients diagnosed with acute Q fever were quite heterogeneous, with mismatches of CFT and IFA results. Therefore, the group of patients described was a heterogeneous group with acute, past resolved and chronic infections.
17.2.3 Early Seroprevalence Studies
Within the context of increasing number of cases, extensive serologic studies were conducted in the Netherlands in the period between 1982 and 1985 (Richardus et al. 1984b, 1985, 1987), using CFT and IFA with Coxiella burnetii antigen phase II from Virion (Virion Ltd., Zurich, Switzerland). For IFA, IgG antibodies were tested to the phase II antigen, with a cut-off of ≥1:16. The study tested a selection of serum samples from groups of people considered to be at high risk of infection. This approach showed very high seropositive rates among veterinarians working with large domesticated animals (84%) and with small domesticated animals (77%), as well as taxidermists (70%) and wool spinners (58%). However, a range of high seropositivity rates (14–73%) was also found in the control groups. The authors suggest that these high rates were achieved because the IFA used in their study was more sensitive than the CFT used in previous studies. No control experiments with CFT were performed in this study, and the IFA results were not confirmed with additional titrations.
Unfortunately, the original data are not available for statistical reanalysis. If transmission rates in the 1970s and 1980s were high, then serologic evidence of this should be evident in older age groups in recent serological surveys. We speculate that the lack of specificity in the in-house IFA, combined with a low cut-off may have influenced the seroprevalence rates of this study.
17.2.4 The Need for Clearer Analysis Before the 2007 Epidemic
When the Dutch Q fever epidemic began in 2007, some speculated that the disease may have been previously overlooked because of under-diagnosis and misclassification, and that increased awareness had created a pseudo-epidemic through the misclassification of acute infections (Van Knapen, personal communication). Indeed, a considerable amount of misclassification is possible if diagnosis relies on detection of IgM phase II, which can persist for months or even years. Clearly, the pre-epidemic situation needed analysis. In addition a change in laws and regulations regarding infectious diseases in the Netherlands also started to have an impact.
17.2.5 Changing Laws and Regulations
The analysis of national data regarding disease incidence relies on notifications. Regulations that control notification are important as regulatory changes can influence epidemiological curves. When Q fever was added to the list of notifiable diseases in 1976, clinicians were legally required to notify public health authorities of Q fever patients, and municipalities (advised by municipal health services) were obliged to enforce legal actions to curb epidemics when necessary. The use of the Q fever notification system was analysed in 2002 (van Gageldonk-Lafeber et al. 2003). Aggregated data from between 1988 and 2002, retrieved from laboratories, hospital admissions and discharge records, were compared to the national data set of Q fever notifications. This analysis showed that only 50% of diagnosed cases were reported by clinicians because laboratories were not required to provide notification at that time. In 2008, a new law was introduced in the Netherlands to comply with international health regulations. According to this law, laboratories were also obliged to provide notification. This approach was expected to improve notification records – as each case would be notified by both the laboratory and the consulting clinician.
17.2.6 Possible Q Fever Clusters Before 2007 Detected Retrospectively
Clinical Q fever in animals was diagnosed in the Netherlands in 2005 in two dairy goat herds with high abortion rates (Wouda and Dercksen 2007). Van den Wijngaard et al. (2011) speculated that unrecognised outbreaks might have preceded the first recognised outbreak in 2007. With this in mind, they used space-time scan statistics and syndromic surveillance to search for hidden Q fever clusters before and during 2007. Hospitalisation data for lower respiratory, hepatitis and endocarditis infections occurring between 2005 and 2008 were aggregated by week, age group and postal codes. Alternative causes of outbreaks were excluded by reviewing all mandatory notified diseases with similar clinical presentation in the same period. Surveillance data on influenza-like illness were also included to assess whether clusters of hospital admissions for lower respiratory tract infections could be due to influenza. From 2005 to 2008, a total of 20 lower respiratory tract infection clusters and two hepatitis clusters were detected. Scan statistics for space-time clusters detected one specific cluster – the Q fever epidemic in 2007. However, ten other clusters were also detected that could be due to other causes, including a major confirmed Legionella outbreak. Three clusters which occurred earlier than the recorded outbreak – two in 2005 and one 2006 – could be due to Q fever because there was a Q fever-affected farm nearby and there was no alternative explanation for the cluster. In 2007, a number of clusters of lower respiratory tract infection and one hepatitis cluster were also found, and could be attributed to the actual Q fever epidemic. Three clusters in 2007 could not be attributed to Q fever, because they could not be linked to Q fever abortion waves on farms.
In conclusion, Q fever may have spread unnoticed among humans before 2007, and routine cluster scanning may facilitate earlier detection of comparable epidemics in the future. There is indeed circumstantial evidence of limited clustered spreading of C. burnetii among humans before 2007, but this analysis also confirms that the major outbreak started in 2007. However, once the Q fever epidemic was established, it may have resulted in an increased number of diagnoses, influencing epidemic curves (van der Hoek et al. 2010a).
17.2.7 Recent Serosurveillance: The PIENTER Study
In 2006, a population-based seroprevalence study was carried out by the National Institute for Public Health and the Environment to evaluate the Dutch National Immunisation Programme. This programme (PIENTER) has been described in detail by van der Klis et al. (2009). It was a national survey in which participants were asked to donate blood and complete a questionnaire on demographics, health perception and activities related to infectious diseases. Data and sample collection was finalised in June 2007, after which the stored serum samples were used to screen for the presence of C. burnetii antibodies (Schimmer et al. 2011).
Given the screening considerations described above, this study used a combined test strategy to measure seroprevalence, using an enzyme-linked immunosorbent assay (ELISA) IgG phase II (Serion Immundiagnostica, Würzburg, Germany) on the study group of 5,654 samples, followed by a confirmation of positives by IFA (Focus Diagnostics, Cypress, California, USA). IFA was also used to estimate the ELISA’s false negative rates on 504 randomly chosen ELISA negative samples.
Of the 5,654 samples tested, 85 were positive with the ELISA IgG phase II. Of these, 47 had borderline levels and 15 were negative in an IFA IgG phase II screened with 1:32. This resulted in a seroprevalence of 1.5% using ELISA to screen and IFA to confirm. In the 504 ELISA negative samples tested in IFA, six (1.2%) had titres ranging from 1:32 to 1:128. Using IFA as the ‘agreed standard’, the adjusted seroprevalence estimate was 2.4%. These results underscore the problems encountered while comparing different seroprevalence studies.
The results from this study yielded a low seroprevalence in the Netherlands before 2007, but the low numbers still represent a considerable amount of exposure. Seropositivity in males was higher than in females and increased with age. No regional differences were observed, even when sheep, goat and cattle densities were examined. However, higher seroprevalence was associated with increasing age, being born abroad (specifically in Turkey), keeping ruminants and having occupational contact with animals. In conclusion, this study supports the concept of the massive localised introduction of C. burnetii in the human population from 2007 onwards (Schimmer et al. 2011).
Clearly, Q fever has been circulating at a low level in the Netherlands since the 1950s. There may have been a temporary increase in exposure during the 1980s, but data from the older serosurveys and notifications may lack accuracy. Recent studies have confirmed a massive exposure of Q fever in the Netherlands from 2007 onwards.
17.3 The Dutch Epidemic from 2007 to 2010
17.3.1 Concerns Rise in 2007
Between March and June 2007, six cases of acute Q fever were notified by regional microbiology laboratories to public health authorities in the province of North Brabant in the south of the Netherlands. These patients were admitted with atypical pneumonia to a number of hospitals in the province. Concerned, the regional Municipal Health Service (MHS) analysed the cases in detail, but could not link them epidemiologically. Then a general practitioner from a nearby village reported an excess of patients with pneumonia at his practice. Initially, these patients were mistakenly thought to have Mycoplasma pneumoniae infection due to serologic cross-reactions, but they were eventually confirmed as having acute Q fever in July 2007 (Roest et al. 2011; van Steenbergen et al. 2007).
Eventually, a total of 168 human cases were notified in North Brabant in 2007. Dairy goats were identified as the source of the human Q fever cases in North Brabant – the Animal Health Service confirmed a considerable number of Q fever-induced abortions at several farms in the region. The unusually hot and dry weather in the spring of 2007 may have caused airborne transmission of contaminated dust particles. The outbreak seemed to have been concentrated around a single village, but a specific point source could not be identified. A case-control study was performed in the village (Karagiannis et al. 2009) and contact with manure, hay and straw were shown to be risk factors. It was shown that people living in the eastern part of the village close to ruminant farms (one of which had a recent history of abortion problems) were at higher risk than people living in other parts of the village. Contact with animals and the consumption of raw milk products were not significant risk factors in the multivariable analysis. In general acute Q fever seemed not to be related to the working environment but there were reports of incidental cases that occurred after visits to dairy goat farms with abortion problems.
17.3.2 Source of Epidemiological Data
The epidemiological data comes from the national registry of notifiable infectious diseases. Attending physicians and heads of microbiology laboratories have a legal obligation to notify the diagnosis of human Q fever to the MHS, which enters the cases into an anonymous national electronic database (‘Osiris’) monitored by the Centre for Infectious Disease Control. Since the beginning of 2007, notification criteria for acute Q fever in Osiris have been a combination of clinical presentation matching Q fever, with either a four-fold IgG titre rise or a positive IgM phase II antibody test measured by IFA, ELISA, or CFT. During the course of the outbreaks, certain adaptations were made to the notification criteria. In July 2008, a clinical presentation matching Q fever was further defined as fever, or pneumonia, or hepatitis. In February 2010, an additional laboratory criterion was the detection by polymerase chain reaction (PCR) of C. burnetii DNA in serum or respiratory material. However, diagnoses based on PCR were already accepted before that time. Given the above criteria, misclassification was possible when isolated IgM was used as a sole measure, since IgM can be a false positive, or persist for months after a past resolved infection. Moreover, clinical symptoms may be aspecific.
Another important source of epidemiological data was a questionnaire routinely dispatched to notified acute Q fever cases by the MHS, which included questions about environmental risk factors and clinical characteristics. This questionnaire was received from 74% of notified cases with onset of illness in 2007 and from 93% of notified cases with onset of illness in 2008.
17.3.3 –2010 Overview: Not an Isolated Incident
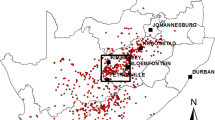
As the number of notifications increased from May 2008, it became evident that the 2007 outbreak was not an isolated incident. A total of 3,489 Q fever patients who experienced onset of disease between 2007 and 2009 were notified. Of these, 194 cases had a date of onset in 2007, 982 in 2008, and 2,313 in 2009. The epidemic curve (Fig. 17.1) shows a seasonal pattern, with most cases occurring in spring and early summer. The highest incidences were seen in the south of the country, mainly in the province of North Brabant; the affected area expanded to the north and the south during the epidemic (Fig. 17.2). Patient characteristics from 2007 to 2009 were presented by Schneeberger et al. (2010a). The median age of the confirmed notified patients was 50 years, and over 60% of them were male.
The additional MHS questionnaires showed that only a small proportion of patients lived on a farm or worked in the agriculture or meat processing sectors. However, notified patients frequently reported that they had been in contact with a diverse number of animals and animal products.
In 2007, the percentage of hospitalized patients (50%) was largely influenced by active case findings in a retrospective survey among hospitalised cases (van der Hoek et al. 2010b). In 2008 and 2009 it was 20%, still much higher than the 2–5% hospitalisation rate reported in the literature (Raoult et al. 2000). Fever was the most frequently reported symptom (92%), followed by fatigue (78%) and headache (69%). Pneumonia was diagnosed in 62% of patients, while endocarditis (3%) and hepatitis (<1%) were relatively rare. Underlying diseases were frequently reported. Almost 49% of patients smoked, which is relatively high compared to percentages in the general population (30% for males and 24% for females, according to Statistics Netherlands).
17.3.4 Diagnostic Delay and Influence of Influenza A(H1N1)
All notified patients in 2007 for whom additional laboratory data was available were diagnosed either by IFA or CFT. In 2008, 3% of cases were diagnosed by PCR. The most popular method in 2008 was still IFA (75%), although CFT, ELISA, PCR and other methods were also used. In 2009, 79% of notified patients were diagnosed serologically and 20% by PCR. IFA was used in more than half of the cases in 2009, CFT was used in more than a quarter of the cases and ELISA was used in 14% of cases.
The median diagnostic delay (the delay between the date of onset of illness and the date of C. burnetti diagnosis) decreased from 82 days in 2007, to 28 days in 2008, to 20 days in 2009 (van der Hoek et al. 2010a). The diagnostic delay was due to lack of awareness by medical staff and the delay in making a definitive diagnosis, as routine diagnostics mainly relied on seroconversion in convalescent serum. Increased awareness and improved routine laboratory services, such as the introduction of IFA, ELISA, and in 2009 PCR, have reduced this diagnostic delay.
Under conditions of high incidence, the positive predictive values of tests are very high. In the autumn of 2009, pandemic influenza A(H1N1) 2009 with more or less similar symptoms interfered with the analysis of the Q fever epidemic. In this third year of the Q fever outbreak, a high background prevalence of antibodies to C. burnetii, specifically positive IgM titres, were common in most of the affected areas. This made it much more difficult to determine the exact start of an acute Q fever episode, thus making notifications less reliable. In fact, many patients diagnosed in the laboratory in 2010 had probably experienced clinical signs of acute Q fever much earlier. Under these circumstances, the persistence of IgM makes it difficult to measure the actual decline of the incidence of the disease.
17.3.5 A Link with Goats and Sheep
In May 2008, an outbreak of Q fever occurred in a psychiatric care institution in Nijmegen near the 2007 outbreak area (Koene et al. 2011). At least 28 in-patients, staff, and visitors had laboratory confirmed Q fever illness and several patients in the institution developed atypical pneumonia. It was discovered that these patients had been in close contact with lambs as part of their therapy sessions. Then a large number of goats unexpectedly aborted their offspring on a farm close by, and Q fever was confirmed in the farmer and his wife living there (Roest et al. 2011). An urban cluster identified in 2008 was found to be related to a goat farm with high abortion rates in the area. Patients lived downwind of the goat farm and a house located <2 km from the farm was associated with a higher risk of Q fever infection, compared to a house located at ≥5 km (Schimmer et al. 2010). In 2009, 59% of notified human cases lived within a 5 km zone of a bulk tank milk-positive dairy goat or sheep farm, and 12% (roughly one million people) of the Dutch population lived within such zones (Roest et al. 2011; van der Hoek et al. 2010b). The available evidence in the Netherlands points to dairy goat farms with Q fever-induced abortion problems as the main source of the human outbreaks with a smaller role for dairy sheep and non-dairy sheep.
17.3.6 Transmission from Animals to Humans
Infection of humans is caused by inhalation of contaminated aerosols that can spread over some distance. Especially when infected pregnant small ruminants abort, billions of C. burnetii end up in the environment while fewer than 10 organisms are sufficient to seed an infection (Benenson and Tigertt 1956). The organism’s ability to persist in the environment may result in a continued risk for infection weeks to months after the birthing event.
The size of the community outbreak in the Netherlands suggests that transmission predominantly takes place through wide-scale environmental contamination or multiple point-source contamination sites. There is strong epidemiological evidence that most human cases are caused by abortion waves on dairy goat farms. People living close to such farms are at risk. Infected farms that have no abortion waves can still be infectious when there is close contact with animals. The transmission route is the same, through inhalation of contaminated aerosols, but the dose is much lower, hence closer contact is required for infection. Based on detailed information from notified patients, occupational exposure can explain only a small proportion of the acute Q fever cases in the Netherlands.
Despite the evidence pointing towards dairy goat farms with Q fever-induced abortion problems, there were a number of such farms without any human cases in the surrounding population. In the 5 km areas around 27 farms with clinical abortion problems, environmental data sets were collected. This showed clear differences between areas with and without transmission to humans in vegetation density and in average groundwater conditions (van der Hoek et al. 2011a). Areas without transmission had higher vegetation densities, based on remotely sensed satellite imagery, and relatively shallow groundwater conditions suggesting that vegetation and soil moisture are relevant factors in the transmission of C. burnetii from infected small ruminant farms to humans.
Alternative routes of transmission are unlikely to have played an important role. Q fever is a zoonotic disease with no convincing evidence for human-to-human transmission. Information from notified acute Q fever patients makes it very unlikely that consumption of unpasteurised dairy products has played an important role. In 2008 the manure streams from dairy goat farms were investigated in some detail. Manure was often transported to other parts of the country to be used for example in flower bulb cultivation but in the recipient areas no Q fever cases were reported (unpublished data). It was therefore concluded that manure did not play an important role. More than 2000 ticks have been collected from sheep and the environment but no C. burnetii was detected. Dairy goats in the Netherlands are kept indoors in deep litter stables and are not affected by ticks.
17.4 Veterinary Control Measures
17.4.1 The Veterinary Situation at the Beginning of the Outbreak
The world’s largest Q fever epidemic recorded to date occurred in an area densely populated with people and domesticated animals, suggesting that animal farming in such areas poses a risk for zoonotic diseases such as Q fever in humans. Initially, the evidence to link the outbreak to goat farming was largely circumstantial, in the absence of DNA fingerprinting techniques for C. burnetii that could have matched bacteria from human and animal samples. While there was no sound evidence base for control measures, the subsequent rapid expansion in the scale of epidemic was unforeseen. National and regional public health authorities were largely unprepared for an outbreak of this magnitude, and international literature on smaller outbreaks provided insufficient guidance on several key issues – such as appropriate control measures, the possible effects of the epidemic on pregnant women, the most adequate therapy for acute Q fever, the identification and classification of chronic Q fever, and the use of the Australian human vaccine for Q fever. The most affected province, North Brabant, has a surface area of 5,100 km2 and currently houses 2.4 million people and 6.4 million livestock (80,000 sheep, 135,000 goats, 660,000 cows and 5.5 million pigs) (Statistics Netherlands 2011), with a goat density that increased five-fold between 1990 and 2007. In retrospect, abortion waves due to C. burnetii infection among the goat population were reported from 2005 onwards, although they were not recognized as such at that time.
17.4.2 Veterinary Measures in Response to the 2008 Outbreak
The widespread pattern of the outbreak in 2008 was alarming and pointed to several clusters with multiple sources. In June 2008, the government announced the mandatory notification of Q fever on dairy goat and sheep farms with >5% abortions due to C. burnetii infection, and introduced appropriate hygiene measures.Footnote 1 During a period of 90 days following the detection of Q fever at a farm, a manure removal ban and visiting restrictions were implemented.1 However, no restrictions on the transport of animals from infected farms were imposed, and other possible veterinary measures to contain the outbreak, such as a breeding ban, were not included.
Then, in October 2008, the Dutch government authorised the voluntary vaccination of animals on large dairy goat and sheep and recreational farms using the non-registered Coxevac® vaccine (Ceva Santé Animale, France). From November 2008, goat and sheep at smaller farms were also vaccinated. However because of limited vaccine availability – just 80,000 doses – vaccinations could only be provided within a 45 km radius of the outbreak source. In February 2009, a nationwide hygiene protocol became mandatory for all dairy goat and sheep farms, whether infected or not.1 The eradication of vermin became compulsory, the cleaning of stables during lambing season and for 30 days afterwards was forbidden, and manure had to be stored and covered for at least 90 days before use.
17.4.3 Veterinary Measures in Response to the 2009 Outbreak
Despite these measures, the outbreak was still far from contained. Over 2,000 new human acute Q fever cases were notified from late March 2009 onwards, in a larger area than in 2008. In response, in April 2009, the government extended the vaccination campaign to include a compulsory vaccination programme.1 Farms with a public function and dairy goat and sheep farms with more than 50 animals in the epidemic centre had to vaccinate their animals before 2010. All Q fever infected farms outside of the area were also obliged to vaccinate their animals. Vaccination of animals on farms in the rest of the Netherlands was still on a voluntary basis. In July 2009, the pasteurization of manure for a minimum of 1 h at 70°C was permitted instead of a decomposition period of 90 days.1 Restrictions on incoming and outgoing animal transport on C. burnetii infected farms were imposed from October 2009 onwards.1
17.4.4 Bulk Tank Milk Monitoring
Also from October 2009 onwards, the government set out a new strategy to identify infected farms that did not have an abortion rate above 5%. Farms with more than 50 dairy goats or sheep were obliged to participate in Q fever bulk tank milk monitoring.1 Bulk milk tanks were sampled once every 2 months (and later on once every 2 weeks) and tested for the presence of C. burnetii DNA using a real-time PCR by the Animal Health Service. To separate infected farms from non-infected farms, a cycle threshold (Ct) of 36 as detected by a-real-time PCR test targeting IS1111 was used. This threshold, which was set arbitrarily, is close to the detection limit of real-time PCR tests, implying that the outcome of the test in the lower range (from Ct 34 to 36) is determined stochastically. Positive samples were forwarded for confirmatory testing to the Central Veterinary Institute, and confirmed farms were declared infected. Infected farms were identified on the basis of a positive PCR outcome only, as information on background values of C. burnetii DNA load in goat bulk tank milk samples was unavailable. This approach may well have resulted in farms being declared infected when they posed no threat to human health.
17.4.5 Drastic Veterinary Measures
In December 2009, Zembla, a current affairs television programme co-produced by the Dutch Broadcasting Association and a Dutch public newscaster, raised critical concerns about the role of the Dutch government in containing the Q fever outbreak. The Dutch government responded to the increasing concerns by administrators, professionals, and the public by making the location of the 55 C. burnetii-infected farms public, announcing a breeding ban on infected farms, and increasing the frequency of tank milk monitoring from bi-monthly to bi-weekly.1 The decision was taken to implement the most radical measure possible, the pre-emptive culling of all pregnant goats on infected farms.1 Male goats on infected farms were also culled, as they could supposedly transmit the disease via semen.
A total of 50,355 goats and sheep were culled from 21 December 2009 to June 2010 on 89 bulk tank milk positive farms. Of 517 culling-workers, involved, 17.5% seroconverted for antibodies to C. burnetii despite use of personal protective equipment (Whelan et al. 2011). Seroprevalence of C. burnetii in workers before the culling activity was 13%, which is similar to findings among blood donors residing in the high-incidence area in the Netherlands in 2009 and in similar high-risk occupational groups internationally (Anderson et al. 2011). Symptomatic infection was recorded in 31% of the seroconverters. A strong dose-response relationship was shown between risk of seroconversion and number of hours worked on the farms and working inside the stable (in close proximity to the animals). In other settings internationally, a risk-gradient has also been shown with close direct and indirect animal contact over time (Porten et al. 2006; Casolin 1999). Given the high risk of infection during culling activities, additional preventive measures should be taken. The Health Council of the Netherlands (2010) has already issued first advice on risk groups suitable for human vaccination against Q fever. However this advice does not extend to culling workers.
17.4.6 Effect of Veterinary Measures in 2010
Exponential spread of Q fever did not occur during the spring of 2010, as feared. Still, almost 400 new Q fever patients were diagnosed during that year. By June 2010, all dairy goats and sheep had been vaccinated twice with Coxevac, and on July 15th the breeding ban for non-infected farms was lifted. The reason for the approximately five-fold decrease in the number of human infections between 2009 and 2010 is mainly attributed to the culling of pregnant goats and sheep, and the vaccination programme. Other factors could have contributed such as hygiene measures, climate, and increasing immunity among the general population.
17.4.7 Identification of the Source of the Outbreak
Recent genotyping studies point to a multi-strain bacterium in both livestock and humans (Huijsmans et al. 2011) as the cause of the Dutch Q fever outbreak and not simply one highly virulent C. burnetii strain. Several conditions may have favoured the introduction and rapid spread of C. burnetii among livestock in the Netherlands since 2005. In general, goats are kept in large herds in ‘deep litter stables’ – stables on concrete floors with pits. Straw is regularly added to these deep litter stables, which allows for relatively unhygienic conditions as potentially infected excreta such as urine, faeces and birth products are not regularly removed. Furthermore, the straw is often bought from countries such as France and Germany, which might be a source of C. burnetii. Relatively high quantities of C. burnetii DNA were measured in samples of stocked straw that had not yet been used in deep litter stables (unpublished observation of M.H.A. Hermans and P.C. Wever). Newborn goats are often fed raw cow colostrum. Colostrum is the highly nutritious milk produced by mammals just before giving birth, and cow colostrum may contain large quantities of C. burnetii DNA (unpublished observations of M.H.A. Hermans and P.C. Wever). Therefore, the role of straw and colostrum are interesting for further investigation as potential sources of multi-strain C. burnetii infection in goats. When the pits in deep litter stables are full – which happens two or three times a year – everything (including manure and birth products) is removed and spread over the fields or transported elsewhere. Further-more, the open air stables allow wind to blow through and take C. burnetii-infected dust into the environment. April 2007, May and June of 2008, and April of 2009 were unusually dry by Dutch standards – this type of weather condition combined with wind has been documented to play an important role in other Q fever outbreaks (Hawker et al. 1998).
17.5 Laboratory Issues
17.5.1 The Optimization of Acute Q Fever Diagnostics in the Netherlands During an Outbreak
Prior to recognition of the Q fever outbreak in 2007, Q fever diagnostics were performed by a limited number of Dutch microbiology laboratories. The National Institute for Public Health and the Environment functioned as a reference laboratory using the IFA as a reference method, while other regional microbiology laboratories used the CFT. Following recognition of the scale of the outbreak in mid 2007, a number of microbiology laboratories in the epidemic area began to offer Q fever diagnostics. The laboratories chose to use either IFA or CFT, depending in part on the type of serologic assays already in use by them. Seroconversion can be detected earlier by IFA compared to CFT (between 10 and 15 days after infection, versus 2–3 weeks, respectively). In addition, IFA allows the separate analysis of IgM and IgG antibodies against C. burnetii phase II and phase I antigens (IgM-II, IgG-II, IgM-I and IgG-I antibodies). Analysis of these four antibodies makes it possible to identify various stages in the acute Q fever infection. The presence of solitary IgM-II antibodies – the first antibody to appear in the serologic response – indicates early acute infection, whereas the presence of IgG-II, IgM-I and IgG-I antibodies reflects a later stage of acute infection. CFT, in contrast, has been reported to be less prone to false positive results than IFA, especially with detection of IgM-II antibodies. Both methods are labour-intensive, non-automated and subject to inter- and intraobserver variation (Maurin and Raoult 1999; Wegdam-Blans et al. 2010).
An important drawback to the serological diagnosis of acute Q fever is the lag phase in antibody response of up to 3 weeks after the onset of clinical symptoms. In 2008, the ongoing outbreak resulted in the development of real-time PCR assays targeting the multicopy IS1111 insertion element by several microbiology laboratories. These assays were used for the detection of C. burnetii DNA in serum, respiratory samples, urine specimens, tissues and amniotic fluids. Subsequently, an interlaboratory evaluation of different DNA extraction and real time PCR methods for the detection of C. burnetii DNA in serum was conducted. Overall, a low degree of variation was observed in the sensitivity of the evaluated real time PCR assays, although assays amplifying short DNA fragments yielded better results than those producing a large DNA fragment (Tilburg et al. 2010). Next, performance of one of the PCR was evaluated retrospectively on serum samples of acute Q fever patients at various stages of the serological response. C. burnetii DNA was detected in serum from 98% of seronegative acute Q fever patients and in 90% of patients with solitary IgM-II antibodies. Ultimately, the PCR became negative as the serological response to C. burnetii further developed, with subsequent appearance of IgG-II, IgM-I and IgG-I antibodies (Schneeberger et al. 2010a, b).
17.5.2 Increasing Diagnostic Demands
To cope with the surge in diagnostic demands, which occurred during 2009 (e.g. over 18,000 requests for Q fever diagnostics were received by one single laboratory), several microbiology laboratories in the epidemic area began using algorithms to provide accurate, fast, cost-effective and standardized acute Q fever diagnostics. One such algorithm used an ELISA for IgM-II antibodies, performed on an automated processing system as an initial screening step. In the case of a positive or dubious ELISA result, IFA was performed as a confirmation step. PCR was performed after a negative ELISA result and when the serum sample was either acquired ≤14 days after onset of disease or referred by a hospital physician (noted for their lack in providing a date of onset of disease). When acute Q fever diagnostics on the first serum sample were non-conclusive, a second serum sample was requested after 14 days. Overall, this diagnostic approach led to a significant reduction in the number of labour-intensive, non-automated IFA tests performed, with an increased diagnostic yield on first serum samples due to the introduction of PCR techniques. Likewise, an algorithm was introduced using the ELISA for IgM-II antibodies as a screening step followed by CFT as a confirmation step (Wegdam-Blans et al. 2009).
17.5.3 A Consensus on Q Fever Diagnosis
In 2010, the National Institute for Public Health and the Environment and the Dutch Society for Medical Microbiology formed a working group to develop a consensus on the microbiological diagnosis of acute Q fever. In September 2010, a consensus document was published recommending the use of algorithms in which PCR, ELISA for IgM-II antibodies and either IFA or CFT were incorporated (Wegdam-Blans et al. 2010). The diagnosis ‘confirmed acute Q fever’ is established by a single positive PCR result with an appropriate clinical presentation or an IgG-II seroconversion or a four-fold or higher increase in IgG-II titre detected by IFA or CFT (requiring multiple serum samples). The diagnosis of ‘possible acute Q fever’ is made by a positive IgM-II result (in the presence or absence of IgG-II, IgM-I, IgG-I antibodies) in a single serum sample with an appropriate clinical presentation and should be confirmed by either an IgG-II seroconversion or a four-fold or higher increase in IgG-II titre in a follow-up serum sample. The introduction of the term ‘possible acute Q fever’ was the result of an increasing number of patients with past resolved Q fever and persisting antibody titres against phase II antigens in particular, as well as reported false-positive IgM-II results from IFA and ELISA.
17.5.4 Cross-reactions in Serologic Tests for Coxiella burnetii
While screening methods should be very sensitive and can be less specific, several screening methods for infectious diseases have shown cross-reactions with other infections. For Q fever, most cross-reactions described in the literature are those with other agents, which cause pulmonary infections such as Legionella pneumophila (Dwyer et al. 1988; Finidori et al. 1992).
In the Netherlands, patients with a pulmonary infection are usually screened for several pathogens. This approach recognises cross-reactions with other agents that cause pulmonary infections. The Q fever epidemic in the Netherlands was first thought to be caused by Mycoplasma pneumoniae, because CFT results of several patients showed low titres against this organism. Therefore, we investigated cross-reactions between sera from patients with high Mycoplasma titres and the ELISA screening assay for Q fever. No cross-reactions were found.
Cross-reactions with other pathogens are less likely to be recognised. We investigated cross-reactivity in sera taken from patients with recent Epstein-Barr virus (EBV) and Cytomegalovirus (CMV) infections (IgM positive with low avidity). In a Coxiella screening ELISA (IgM phase II), 16/72 EBV IgM positive sera reacted while 7/33 CMV IgM positive sera reacted. In both EBV IgM- and CMV IgM-positive patients, the results in the Q fever ELISA were generally low positive. When performing an IFA phase I and II assay on the ELISA positive samples, all EBV IgM positive patients became negative. However, 40% of CMV IgM positive patients also had positive IFA tests and we concluded that these patients probably had two infections going on at around the same time.
When screening patients with a proven seroconversion for C. burnetii, we found very few cross-reactions in tests for EBV IgM (2%) but somewhat more in tests for CMV IgM (8%). Unfortunately, we were unable to follow these patients to see if these were true double infections or not. We concluded that recent EBV and CMV infections can cause cross-reactive antibodies against C. burnetii in ELISA tests, but not in IFA tests. It is possible that a recent Q fever infection can also cause cross-reactions in the test for CMV IgM. Therefore, screening with ELISA, followed by confirmation with IFA, is a good way to exclude false positive tests for C. burnetii due to recent EBV and CMV infections.
17.5.5 Seroprevalence Surveys
Strategies for diagnosing acute and chronic Q fever in individual patients differ from population-based seroprevalence surveys. Acute Q fever is diagnosed mainly by the detection of antibodies of IgM and IgG subclasses against phase II of C. burnetii. The diagnosis of chronic Q fever relies on high titres of phase I IgG antibodies; the sole presence of phase II IgG antibodies against C. burnetii indicates a previous infection (Tissot Dupont et al. 1994; Waag et al. 1995). IgG-II antibody levels remain constantly high for almost a year and then slowly decrease, remaining detectable for years after first detection (Dupuis et al. 1985). Therefore, the study of seroprevalence relies on the detection of IgG-antibodies against phase II of C. burnetii in serum samples– antibodies can be detected by CFT, IFA or ELISA. What we need to establish is: which test works best?
17.5.6 The IFA/ELISA Debate: The Need for a Standard
Studies in the late 1980s and early 1990s reported that the ELISA was a more sensitive and specific method than either the IFA or CFT (Cowley et al. 1992; Peter et al. 1988). Field et al. (2002) evaluated the performance of an ELISA IgG kit (Panbio) against an in-house IFA. The two tests had moderate (53%) agreement; the ELISA had a sensitivity of 71% and a specificity of 96%. A number of different in-house IFAs were evaluated against ELISA, using different methods and cut-offs (D’Harcourt et al. 1996; Setiyono et al. 2005) and the results are illustrative of the difficulties in comparing studies of the serodiagnosis and seroprevalence of Q fever. Nevertheless, IFA has been proclaimed the gold standard reference method in the literature (Fournier et al. 1998), although this method is laborious when compared to the ELISA (which is easier to automate and more suitable for testing large sample numbers). Commercial ELISA and IFA tests are both in current use for the diagnosis of acute and chronic Q fever and in seroprevalence studies globally (Anderson et al. 2009; Gilsdorf et al. 2008; McCaughey et al. 2008). The uncertainty regarding a valid standardised test, coupled with insufficient knowledge about the specific fate of antibodies against C. burnetii makes the seroepidemiology of Q fever a difficult undertaking.
Serum samples from a case-control study conducted in 2007 in the Netherlands to investigate the source and routes of transmission in this outbreak were used to evaluate the performance of one commercially available ELISA (Serion Immundiagnostica, Würzburg, Germany) and one IFA (Focus Diagnostics, Cypress, California, USA). Four hundred and eighty-seven human sera were evaluated in terms of sensitivity, specificity and kappa value. The sensitivity and specificity of ELISA for the detection of IgG phase II antibodies were 59% and 97%, respectively (Blaauw et al. 2011). Seroprevalence varied depending on the method used; it was 12.7% when tested with IFA and 6.2% when tested with the ELISA. When measuring IgM antibodies to phase II Coxiella antigen, the two tests were comparable, with a kappa value of 0.89. Sensitivity was 82% and specificity 100%. These results support the concept that the ELISA performs reasonably well when diagnosing acute Q fever. However, in past infections, as defined by the sole presence of IgG antibodies, low positive samples have been missed by ELISA. More longitudinal studies using different test systems are needed to measure the levels of antibodies to C. burnetii in human serum. A single standard must be agreed on and defined in order to be able to easily compare results from various serosurveys.
17.6 Q Fever and Pregnancy During the 2007–2010 Q Fever Outbreaks in the Netherlands
17.6.1 The International Literature
When it became apparent that Q fever had become a major problem in the Netherlands from 2007 onwards, discussion arose about the health threat to pregnant women (Schimmer et al. 2009; van der Hoek et al. 2010b). An estimated 90% of acute Q fever infections in pregnancy present without clinical signs, which is much higher than among non-pregnant persons. International literature suggests that untreated acute Q fever infection during pregnancy may result in adverse pregnancy outcomes in up to 81% of cases (Tissot Dupont et al. 2007; Carcopino et al. 2007; Langley et al. 2003). These outcomes include abortion or intra-uterine foetal death and premature delivery or low birth weight. Furthermore, the risk of developing chronic Q fever infection is reported to be higher in pregnant women (Maurin and Raoult 1999; Carcopino et al. 2009). The only way to detect subclinical Q fever is through screening of serum for antibodies to C. burnetii.
17.6.2 The First Year of the Epidemic
In 2007 the Q fever outbreak was confined to a relatively small area. In July 2007, the Outbreak Management Team of the Netherlands decided to offer all pregnant women living in that area a screening test for Q fever. This decision was based on the fact that a policy based on signs and or symptoms was not possible (Parker et al. 2006) as asymptomatic infections could carry the same risk for adverse pregnancy outcome and chronic infection as symptomatic cases. Testing of all pregnant women in outbreak situations was also common policy in other countries. In France, the recommendation was to treat pregnant women testing positive and for those testing negative, to repeat testing on a monthly basis until delivery (Tissot Dupont et al. 2007). Public health practitioners in the south of the Netherlands tried to identify all women who were pregnant or who had recently delivered in the affected area. They were contacted by letter and offered the test. Out of 29 women identified through midwives and obstetricians working in the area, 19 responded, were interviewed and underwent serological testing with IFA (Focus Diagnostics) in which a titre of 1:64 was considered positive. None of these women experienced or had experienced signs or symptoms of Q fever. Two women however had serological evidence of a recent infection and one of an older infection (Meekelenkamp et al. 2009). The two women with serological evidence of recent infection were treated with cotrimoxazole for the duration of the pregnancy, as recommended in the literature (Carcopino et al. 2007). Both delivered under strict hygiene measures and both pregnancies and deliveries were without complications. Birth products tested by PCR all were negative. In none of the neonates there was serological or PCR evidence of vertical transmission of Q fever.
17.6.3 –2010
In the following years, the epidemic spread to a much larger geographic area with a total population of almost two million people. This raised the question whether screening of pregnant women for acute Q fever infection was necessary or feasible. The adverse effects from untreated Q fever infections were compared with the possible side effects of long-term antibiotic treatment during pregnancy. In July 2008 an international meeting organized by the Centre for Infectious Disease Control and the Health Council of the Netherlands met to discuss the feasibility of screening of all pregnant women for recent Q fever infection.
In all reported studies, there are a limited number of pregnant women with Q fever for whom pregnancy outcomes (adverse or otherwise) have been reported (<100 women for all studies combined). Most reports concern retrospectively collected data, which don’t allow quantification of the risk for an adverse outcome of an infection during pregnancy. Based on this information and the fact that the epidemic had spread to a larger geographical area, the recommendation was that screening of all pregnant women for recent Q fever was not recommended (Health Council of the Netherlands 2008). Several retrospective and prospective studies were set up in response to the urgent need for better quantification of the risk of adverse pregnancy outcome among pregnant women with acute Q fever in early pregnancy.
17.6.4 A Population-Based Retrospective Follow-up Study
In a retrospective study, the presence of antibodies against C. burnetii during pregnancy was determined by testing sera that had routinely been collected in the Prenatal Screening for Infectious Diseases and Erythrocyte Immunization (PSIE) programme (http://www.rivm.nl/pns_en/). This screening programme for hepatitis B, syphilis, and HIV is offered to all pregnant women in the Netherlands at around the 12th week of pregnancy. Samples were available in a high percentage of pregnancies as they are often stored for a period of 1 year after initial analysis. Sera were analysed using an IFA for detection of IgG and IgM antibodies. A recent infection was defined as the presence of anti-phase II IgM and anti-phase II IgG antibodies with a titre of ≥1:64. A possible infection was defined as a solitary IgM II ≥1:64 and a past infection as the presence of anti-phase I and II IgG antibodies without IgM being present. Information on pregnancy outcome was obtained from the Netherlands Perinatal Registry (PRN) – a database that represents the joint efforts of the professional organizations of midwives, gynaecologists, obstetrically-trained general practitioners and paediatricians in the Netherlands. The PRN contains perinatal data from 16 weeks of gestation onwards for 96% of all births in the Netherlands. It was estimated that 60% of pregnant women were included in the study during the study period, based on the registered number of births. In this study, almost 4.5% of women had a recent or past infection. The presence of antibodies against C. burnetii was not significantly associated with an adverse pregnancy outcome as measured by: preterm delivery (gestational age below 37 weeks), low birth weight <2,500 g, birth weight for gestational age <10th percentile, foetal or neonatal mortality, congenital malformation and 5-min Apgar score <7 (van der Hoek et al. 2011b).
17.6.5 A Prospective Screen and Treat Study
In 2010 a clustered randomized controlled trial among pregnant women within the area of high transmission was started in the Netherlands (Munster et al. 2010a). The study participants were recruited by the midwives in these high risk areas. The midwife centres were randomized to recruit pregnant women from the control group or the intervention group. When taking part in the intervention group, blood samples were taken and tested immediately for Q fever. Patients were referred to a hospital for further pregnancy monitoring and long-term bacteriostatic treatment, if found positive for acute or chronic Q fever. In the control arm, blood samples were stored and analyzed for Q fever only after delivery. If tested positive for Q fever after pregnancy, antibiotics were started if needed as part of regular health care. The objective of the study was to measure differences in obstetric or maternal complications in Q fever positive women between screened and control group. Because the outbreak of Q fever in the Netherlands was successfully managed, relatively few pregnant women included in this study experienced a recent infection with C. burnetti. By September 2010, 815 samples had been examined, showing an overall seroprevalence of 15%, but with only 4% having a serologic profile suggesting recent infection (Munster et al. 2010b). The final results of this study are not yet available.
17.6.6 No Evidence of Adverse Effects on Pregnancy Outcome in the Netherlands
Data from the literature on the effects of Q fever infection in pregnant women are limited. Currently the best available evidence with regard to adverse pregnancy outcomes comes from a large case series and from several case reports documenting one to two cases (Carcopino et al. 2007; Denman and Woods 2009; Jover-Diaz et al. 2001; Stein and Raoult 1998; Rey et al. 2000). Case reports and case series have methodological limitations and selective publication of severe outcomes cannot be ruled out. In contrast, in the Dutch outbreak the presence of antibodies against C. burnetii in early pregnancy was not associated with adverse pregnancy outcome. This might be explained by a possible difference in pathogenicity of different bacterial strains or because we were not able to include early miscarriages in the study. We have to conclude that in the Dutch outbreak of Q fever, no evidence was found for adverse effects on pregnancy outcome among pregnant women with an asymptomatic Q fever infection in early pregnancy. Based on this, there is insufficient basis for recommending large-scale screening of pregnant women in high incidence areas.
17.7 Long-Term Effects of Acute Q Fever
17.7.1 From Acute to Chronic Illness
According to the literature, 60% of infected Q fever patients are asymptomatic, while 20% of patients develop mild symptoms (CSL 2009). The remaining 20% present with more severe symptoms including high fever, severe headache, night sweating, nausea, diarrhoea, pneumonia, hepatitis, pericarditis, myocarditis, neurological symptoms and weight loss (Mertens et al. 2007). The acute illness spontaneously resolves after 2–6 weeks (Marrie 1990). However, the organism or its partly degraded remains can persist in bone marrow, which can cause future episodes. Chronic illness after acute Q fever can express itself in different forms (Karakousis et al. 2006; Wildman et al. 2002). Classic Q fever endocarditis may take 10–15 years to develop and presents with cardiac vegetations that contain viable Coxiella bacteria. Recrudescent granulomatous infections can also occur. Patients with these two forms present with elevated levels of antibodies and persistent presence of viable C. burnetii. Another long-term effect of Q fever is QFS (post-Q fever fatigue syndrome). Contrary to the first two forms, QFS may present while there are no viable Coxiella and antibody levels are low or negligible. This is confusing for clinicians and patients alike.
17.7.2 Laboratory Diagnosis of Chronic Q Fever
Acute Q fever may develop into chronic Q fever in 2% of patients, a potentially lethal disease with endocarditis as the main presentation (ECDC 2010). Patients with previous cardiac valve pathology, aneurysms or vascular grafts, the immunocompromised and women who are infected during pregnancy are at risk of chronic Q fever (Maurin and Raoult 1999). An IFA IgG phase I antibody titre ≥1:800 is considered highly predictive for chronic Q fever (Tissot Dupont et al. 1994, 2007; Landais et al. 2007). The final diagnosis of chronic Q fever is made when a suspect serologic profile is combined with a positive PCR (Fenollar et al. 2004). However, considerable uncertainties exist about the value of serology to identify chronic cases, and the value of a positive PCR is not completely clear. At the regional laboratory of Jeroen Bosch Hospital (‘s-Hertogenbosch, the Netherlands), located at the epicentre of the Dutch outbreak, we evaluated the serologic profiles of 686 patients diagnosed with acute Q fever in 2007 and 2008 at 3, 6 and 12 months after diagnosis (van der Hoek et al. 2011c). Our results differ from data provided by others, as high IgG phase I antibody titres at a 3-month follow-up were not predictive for chronic Q fever and IgG phase I antibody titres greater than IgG phase II antibody titres were rarely seen. An IgG phase I ≥1:1,024 at 6 months seemed to have the highest sensitivity for detecting chronic Q fever, but the probability that cases with this profile actually had chronic Q fever is low. Chronic Q fever cases show a persistently high (≥1:1,024) or increasing IgG phase I antibody titre, combined with a persistently high (≥1:4,096) IgG phase II antibody titre. A serologic cut-off at ≥1:1,024 (or at the previously proposed ≥1:800) provides adequate sensitivity and positive predictive value. The study confirmed that IgG phase I is a good screening test, in our case with a cut-off of ≥1:1,024, at a follow-up of between 6 and 12 months after the acute Q fever episode. A more stringent follow-up scheme is required for patients with clinical risk factors. Based on the experience gained since 2007, the serologic follow-up strategy is now one analysis at 9 months after an episode of acute Q fever. For patients with specific risk factors, the follow-up strategy at 3, 6 and 12 months is maintained, with serology combined with PCR. The diagnosis of chronic Q fever and the decisions about treatment were made by a multidisciplinary team of medical specialists, based on serologic profile, PCR results, the presence of clinical risk factors, clinical presentation, and other patient characteristics. Of the 686 acute Q fever cases that were followed up, 1.6% converted to a classic chronic case with microbiological evidence (van der Hoek et al. 2011c). In the epidemic in the Netherlands, we found that the antibody titre of IgG phase I ≥1:1,024 is not useful for immunocompromised patients and every follow-up serum sample must be tested by PCR independently of the serological profile. In endocarditis patients, we concluded that the PCR in a minority of patients is negative despite having vegetations on echocardiography. Almost every vascular patient has a chronic serological profile and a positive PCR.
A Dutch consensus on chronic Q fever was recently formulated (Wegdam-Blans et al. 2011). A distinction is made between ‘proven’, ‘probable’, and ‘possible’ chronic Q fever. Proven chronic Q fever requires (1) a positive PCR in tissue or blood in the absence of an acute Q fever infection; or (2) an IFA phase I IgG titre ≥1:1,024 and evidence of endocarditis; or (3) an IFA phase I IgG titre ≥1:1,024 and evidence of vascular infection by radiologic imaging.
17.7.3 Fatigue in Q Fever Patients
Following acute Q fever, up to 60% of patients may experience post-infection fatigue symptoms. These symptoms can persist for 6–12 months, after which they spontaneously resolve (Ayres et al. 1998). Post-infection fatigue also occurs after other infectious diseases such as Lyme disease (Marques 2008). In 10–15% of Q fever patients, fatigue can last from 5 to 10 years (Marmion et al. 1996) and is then often referred to as QFS, with a symptom presentation similar to chronic fatigue syndrome (CFS). Some studies state that cytokine deregulation and immuno-modulation due to the persistence of C. burnetii may be responsible for prolonged fatigue, but others contradict this (Penttila et al. 1998). An impaired or deregulated immune response or the long-term persistence of the bacteria or its antigens and the immune response may also play a role.
17.7.4 A Typical Q Fever Patient
‘Jan Verkerk’ is a 48-year-old self-employed male. He ran a small, family-owned bicycle shop and did most of the work himself. He was also an active sportsman, running 15 km three times a week and cycling daily. He had no known underlying physical or psychological diseases. In May 2007 he developed Q fever and visited his general practitioner (GP) for the first time in years, presenting with high fever and pneumonia. These acute symptoms disappeared during ensuing weeks, but 1 year later and despite his best efforts, he still hadn’t resumed running at his normal level. He was feeling constantly tired and was struggling to manage his business. He did not sleep well due to night sweating, he was unable to concentrate, and he suffered muscle and joint pains. He visited his GP many times, but several blood tests revealed nothing. He felt misunderstood by his GP, who seemed unable to help him, and he worried that if he did not recover he might not be able to manage his shop any longer. After speaking with other patients who told a similar story, he called the Department of Infectious Diseases of the Municipal Health Service to find out if this was a normal experience, whether others had similar problems, what further investigations could be done, and how he could be treated for his persisting symptoms.
17.7.5 The Health Status of Q Fever Patients After Long-Term Follow-up
In response to the many signals and questions about persisting symptoms, particularly fatigue in Q fever patients from the 2007 cohort, the collaborative multidisciplinary study Q-Quest I was started in 2008 (Morroy et al. 2011). A validated questionnaire, the Nijmegen Clinical Screening Instrument (NCSI), was used to obtain a detailed assessment of the health status in Q fever patients 12–26 months after the onset of their illness. This study is the largest and longest follow-up study of Dutch Q fever patients from the 2007 and 2008 outbreaks. In 2009, we asked 870 Q fever patients from the 2007 and 2008 outbreaks to complete the questionnaire based on an empirical definition of health status (Vercoulen et al. 2008), covering physiological functioning, symptoms, functional impairment and quality of life (QoL) as the main domains. These domains were subdivided into eight sub-domains: subjective symptoms, dyspnoea emotions, fatigue, behavioural impairment, subjective impairment, general QoL, health related quality of life, and satisfaction with relations (Peters et al. 2009). We compared the NCSI scores of these Q fever patients with normal data from healthy individuals and patients with severe chronic obstructive pulmonary disease (COPD).
Our findings demonstrate that in comparison to healthy individuals, Q fever patients – especially those that were hospitalized – present 12–26 months after the onset of illness with more severe clinically relevant subjective symptoms, functional impairment and impaired quality of life. The long-term health status of two-thirds of Q fever patients was severely affected for at least one sub-domain. Year of illness onset, level of education and smoking behaviour had no significant influence on sub-domain mean scores. Published data on the health status and its sub-domains of Q fever patients are scarce. Hatchette et al. (2003) reported that 52% of Q fever patients were symptomatic and had an impaired QoL 27 months after infection, using the 36-Item Short Form Health Survey (SF-36), with significantly lower scores, compared to non-infected controls in the domains of physical pain, function and role, emotional role and social function.
In Q-Quest I, the sub-domains ‘general QoL’ and ‘fatigue’ were severely and clinically impaired, compared to the reference group. More than half, 59% of patients had abnormal (mild to severe) fatigue, similar to other publications, which indicate that 60% of patients reported protracted fatigue (CSL 2009) and up to 69% fatigue (Ayres et al. 1998) 5 years after infection. A small study on Dutch patients that measured a 1-year follow-up and also used the NCSI reported a higher rate of 53% of patients with severe fatigue (Limonard et al. 2010), whereas the Q-Quest I study reported 44%.
Health status can be impaired after pneumonia regardless of the causative organism. Dutch pneumonia patients had significantly affected SF-36 scores 18 months after pneumonia on the subscales ‘physical function’ and ‘general health status’ (El Moussaoui et al. 2006). Survivors of a Legionnaire’s Disease outbreak in the Netherlands 17 months after infection reported severely impaired SF-36 domains: ‘physical role function’, ‘general health’ and ‘vitality’ (Lettinga et al. 2002). Up to 75% of patients reported fatigue. In Q-Quest I hospitalization in the acute phase was significantly related to long-term behavioural impairment (OR 2.8, 95% CI 1.5–5.1), poor health-related quality of life (OR 2.3, 95% CI 1.5–4.0), and subjective symptoms (OR 1.9, 95% CI 1.1–3.6) (Morroy et al. 2011). Severity of initial illness generally has a negative influence on long-term QoL (Lowry and Pakenham 2008; Testa and Simonson 1996). Similarly, the severity of the acute Q fever symptoms predicts long-term symptoms (Hickie et al. 2006). Hospitalization can be seen as an indicator of the severity of the initial infection. We conclude that Q fever patients with severe acute illness are more likely to experience long-term impaired QoL. Lung or heart disease, depression and arthritis also significantly affected the long-term health status of Q fever patients. Other authors state that underlying heart (de Leon et al. 2009; Juenger et al. 2002) or lung disease (Daudey et al. 2010), arthritis (Garip et al. 2010), depression (Beard et al. 2010) and diabetes (Glasgow et al. 1997) all have a negative effect on the health status in different sub-domains. In Q-Quest I this effect was also found for all underlying conditions, except for diabetes. It was not possible to compare data with existing studies as most of these studies focus on specific diseases (such as COPD) and grades of severity.
17.7.6 The Q Fever Patient Society
In 2007 and 2008 the Q fever outbreaks in the province of North Brabant did not receive much media attention. In 2009, the outbreaks expanded to a larger area outside Brabant and patient numbers rose to over 2,000. The number of patients presenting with long-term effects grew, the precautionary veterinary measures were stepped up and media attention increased as a result. This fed the public interest. Then, at the height of the Q fever epidemic in 2009, most of the media attention switched to the influenza pandemic. Some general practitioners and other medical doctors and public health officials felt that the concurrent Q fever outbreak received insufficient attention. At the same time, GPs and patients increasingly reported long-term complaints. In November 2009, with help and financial support from the Province of North Brabant, a Q fever Patient’s Society was founded. This society offers patients a platform to meet and express concerns and needs such as on treatment options.
Several hospitals now run Q fever out-patient departments for follow-up of Q fever patients but care in these centres is not standardized. In June 2010, the Patient’s Society requested the Minister of Health to focus attention on patients with long-term complaints after acute Q fever infection. The National Institute for Public Health and the Environment was asked to draft guidelines on the treatment of long-term complaints after acute Q fever, and the product of a multidisciplinary working group is expected in 2012.
17.7.7 An Opportunity for More Research and Understanding
Many questions on the late effects of Q fever remain unanswered, such as the effectiveness of treatment of QFS (Rimes and Chalder 2005) with cognitive behavioural treatment and graded exercise therapy. The outbreaks in the Netherlands offer a unique opportunity for prospective research (the Q-Quest II study) on the long-term health outcomes in Q fever patients. With more than 4,000 acute Q fever cases reported up to November 2010 and symptoms that can last for 10 years or more, a considerable burden of disease in coming years is expected for patients and the affected communities. GPs and other medical doctors should be aware that Q fever patients may present with long-term symptoms, especially if they have been hospitalized or have co-morbidity (heart or lung disease, or depression). Ongoing research on the treatment and recovery of Q fever patients should offer a better understanding of the delayed and long-term effects of this zoonosis. There is a particular need for randomised clinical trials to test the effectiveness of treatment options.
17.8 Q Fever Vaccination in the Netherlands
17.8.1 Vaccination Decisions During the Q Fever Epidemic
The annual Q fever epidemics that began in 2007 prompted Dutch policy makers to consider introducing a human vaccination programme to protect people at risk for severe outcomes of the disease. However, early live attenuated and sub-unit vaccines were abandoned because of low efficacy and safety concerns, leaving just one human Q fever vaccine. This whole-cell vaccine was developed and registered in Australia and is licensed under the name Q-vax. It is not registered in the Netherlands or in any other European country. There were logistical and legal constraints to introducing a non-registered vaccine that required extensive testing of subjects before vaccination. Human vaccination can play no role in controlling the epidemic but the increasing number of reports of long-term effects in patients with chronic Q fever eventually caused both professionals and decision makers to reconsider the introduction of the vaccine in the Netherlands. At the same time, hospitals and public health services were confronted with an increasing number of worried Q fever patients, both acute and chronic, some of whom travelled to Australia at their own expense to be vaccinated. In 2010, the Government asked the Health Council of the Netherlands to advise on the possible use of the vaccine.
17.8.2 The Q-Vax Vaccine
Q-vax consists of formaline inactivated C. burnetii and was developed by CSL limited (CLS Biotherapies). It has been licensed in Australia to protect at-risk slaughterhouse employees and veterinary professionals (Ackland et al. 1994). In this respect, the vaccine was quite successful and is still in use (Gilroy et al. 2001; Marmion et al. 1984).
Analysis of the vaccine’s efficacy in selected groups of professionals with a potentially high attack rate shows a protection rate of 97% (Gefenaite et al. 2011). Vaccinating subjects without a measurable immune response to C. burnetii is safe, but does commonly result in mild local reactions (33–48%) or mild systemic reactions (9%) such as headache (Marmion et al. 1990). Between 2002 and 2006, a large campaign in Australia saw the vaccination of 50,000 patients, resulting in eight serious adverse events requiring hospital admission and one life-threatening event. No deaths have ever been recorded after vaccination (Gidding et al. 2009). It is noteworthy that this data comes from a specific group of young and predominantly healthy males, the vaccine is only given to subjects over 15 years of age, and it is not administered to pregnant women.
Data is not available on the effectiveness of the vaccine in persons other than healthy workers. Furthermore, the vaccine can only be given to those not previously in contact with C. burnetii, as vaccinating subjects that have already mounted an immunological response may lead to serious adverse reactions such as sterile abscesses and systemic symptoms of inflammation. To prevent this, serology and skin testing must be performed to identify those who have previously had contact with C. burnetii. Although these tests are not complicated per se, they can be difficult to organise and require specific skills such as administering and interpreting of the skin test. To further complicate matters, laboratory tests are not standardised, and different serologic tests systems and cut-off values are used.
17.8.3 Target Groups for Vaccination
Patients affected with Q fever come mainly from specific areas in the south of the Netherlands. However, considerable differences occur within the affected area, and people living near affected farms may be especially affected. Nevertheless, localised mass vaccination has never been considered.
Preliminary data indicate that high numbers of professionals have been infected with C. burnetii – studies performed among goat and sheep farmers and veterinarians showed seroprevalence figures of up to 80%. Since the majority of these risk groups had already been exposed with a limited burden of disease, it was decided not to vaccinate them. However, those just starting out in a high-risk career, such as veterinarians, could be considered as candidates for vaccination.
The Q fever vaccine could be of use for population groups with underlying disease that make them at risk for long-term effects. Although these long-term effects are quite rare, they can be very serious and include endocarditis and the infection of large blood vessels (Landais et al. 2007; Botelho-Nevers et al. 2007). The treatment of chronic Q fever requires long-term (>1.5 years) antibiotic treatment and sometimes cardiovascular surgical interventions.
Patients with pathologic heart valves or blood vessels are particularly at risk. However, most of the studies in this area have been performed retrospectively and suffer from considerable selection bias. This means that the true contribution and magnitude of the risk associated with pre-existent factors is not known. It is also unclear whether minor valve or vessel pathology could develop into serious pathology during chronic Q fever. Furthermore, little is known about the incubation period of serious long-term effects of chronic Q fever.
Once the decision has been made to vaccinate patients at risk, these uncertainties matter and must be considered. For example, the screening of all acute Q fever patients for heart defects with echocardiography (as advised in the international literature) was not feasible during the large-scale Dutch outbreak (Limonard et al. 2010; Botelho-Nevers et al. 2007). Similar screening options for aneurysms in a given population may also not be feasible.
For these reasons, defining and selecting patient groups for vaccination is not a simple matter. In 2010, the Health Council of the Netherlands (2010) identified the following groups as eligible for vaccination:
-
Patients who have had endocarditis in the past
-
Patients with artificial heart valves
-
Patients with significant congenital heart anomalies, including those that required repair with grafts
-
Patients with structural defects of the aortic or mitral valve
-
Patients with known aneurysm of the aorta
-
Patients with vascular grafts
-
Patients with severe peripheral vascular disease (such as Buerger’s disease)
17.8.4 Deciding to Vaccinate
Even though the use of the vaccine in certain groups has been advocated, this vaccine is not licensed in the Netherlands and its administration will not be part of a nationally steered programme. However, it is considered part of health care under the responsibility of the treating physician. Together with the patient, the physician must weigh the potential benefits and disadvantages of vaccine administration. These decisions need to be made with full awareness of the medical and the epidemiological risks involved.
Vaccine administration can only be carried out after a professional skin test reading and serology result analysis. This requires a standardised process with similar cut-off titres and specificity tests, as well as centralised vaccination to realise standardised quality of care. Therefore, even though the vaccine has not been added to the national vaccine programme, its introduction in 2011 was coordinated by the National Institute of Public Health and the Environment, in collaboration with a commercial partner, regional public health departments and local physicians. In the vaccination campaign, early 2011, 1,354 people were vaccinated, all from the defined high risk groups.
17.9 Conclusion
Between 2007 and 2009, the Netherlands experienced an unprecedented series of seasonal outbreaks of Q fever. Dairy goats are clearly implicated in these outbreaks. In 2010 there were a much lower number of notified acute Q fever cases than in 2009, probably due to the drastic veterinary interventions such as culling of pregnant goats on infected farms, vaccination, and hygiene measures. But the risk of Q fever outbreaks and possibly other zoonotic diseases remains high because of the cohabitation of 2.4 million inhabitants with 6.4 million animals in the province of North Brabant. A great deal of knowledge has been generated in the past few years but many questions remain. Ongoing research, including 20 PhD projects, is expected to significantly advance the knowledge base. Attention is now shifting from acute Q fever to the problem of long-term effects of Q fever, the extent of which is not yet known and which poses important challenges for diagnosis and treatment.
Notes
- 1.
Information from official documents of the Ministry of Agriculture, Nature and Food Quality. Available at http://overheid-op.sdu.nl/cgi/login
References
Ackland JR, Worswick DA, Marmion BP (1994) Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Aust 160:704–708
Anderson AD, Kruszon-Moran D, Loftis AD et al (2009) Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg 81:691–694
Anderson AD, Baker TR, Littrell AC et al (2011) Seroepidemiologic survey for Coxiella burnetii among hospitalized US troops deployed to Iraq. Zoonoses Public Health 58:276–283
Ayres JG, Flint N, Smith EG et al (1998) Post-infection fatigue syndrome following Q fever. QJM 91:105–123
Beard C, Weisberg RB, Keller MB (2010) Health-related quality of life across the anxiety disorders: findings from a sample of primary care patients. J Anxiety Disord 24:559–564
Benenson AS, Tigertt WD (1956) Studies on Q fever in man. Trans Assoc Am Physicians 69:98–104
Blaauw GJ, Notermans DW, Schimmer B et al (2011) The application of an enzyme-linked immunosorbent assay or an immunofluorescent assay test leads to different estimates of seroprevalence of Coxiella burnetii in the population. Epidemiol Infect. doi:10.1017/S0950268811000021
Botelho-Nevers E, Fournier PE, Richet H et al (2007) Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis 26:635–640
Carcopino X, Raoult D, Bretelle F et al (2007) Managing Q fever during pregnancy: the benefits of long-term cotrimoxazole therapy. Clin Infect Dis 45:548–555
Carcopino X, Raoult D, Bretelle F et al (2009) Q fever during pregnancy: a cause of poor fetal and maternal outcome. Ann N Y Acad Sci 1166:79–89
Casolin A (1999) Q fever in New South Wales Department of Agriculture workers. J Occup Environ Med 41:273–278
Cowley R, Fernandez F, Freemantle W et al (1992) Enzyme immunoassay for Q fever: comparison with complement fixation and immunofluorescence tests and dot immunoblotting. J Clin Microbiol 30:2451–2455
CSL (2009) A guide to Q-fever and Q-fever vaccination. CSL Biotherapies, Parkville, ABN 66120398067
D’Harcourt SC, Soto AB, Burgos VC et al (1996) Comparison of immunofluorescence with enzyme immunoassay for detection of Q fever. Eur J Clin Microbiol Infect Dis 15:749–752
Daudey L, Peters JB, Molema J et al (2010) Health status in COPD cannot be measured by the St George’s Respiratory Questionnaire alone: an evaluation of the underlying concepts of this questionnaire. Respir Res 11:98
de Leon CF, Grady KL, Eaton C et al (2009) Quality of life in a diverse population of patients with heart failure: baseline findings from the heart failure adherence and retention trial (HART). J Cardiopulm Rehabil Prev 29:171–178
Dekking F, Zanen HC (1958) Q-fever in the Netherlands. Ned Tijdschr Geneeskd 102:65–68
Denman J, Woods M (2009) Acute Q fever in pregnancy: report and literature review. Intern Med J 39:479–481
Dupuis G, Peter O, Peacock M et al (1985) Immunoglobulin responses in acute Q fever. J Clin Microbiol 22:484–487
Dwyer DE, Gibbons VL, Brady LM et al (1988) Serological reaction to Legionella pneumophila group 4 in a patient with Q fever. J Infect Dis 158:499–500
ECDC (2010) Risk assessment on Q fever. European Centre for Disease Prevention and Control, Stockholm. doi:10.2900/28860
El Moussaoui R, Opmeer BC, de Borgie CA et al (2006) Long-term symptom recovery and health-related quality of life in patients with mild-to-moderate-severe community-acquired pneumonia. Chest 130:1165–1172
Fenollar F, Fournier PE, Raoult D (2004) Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol 42:4919–4924
Field PR, Santiago A, Chan SW et al (2002) Evaluation of a novel commercial enzyme-linked immunosorbent assay detecting Coxiella burnetii-specific immunoglobulin G for Q fever prevaccination screening and diagnosis. J Clin Microbiol 40:3526–3529
Finidori JP, Raoult D, Bornstein N et al (1992) Study of cross-reaction between Coxiella burnetii and Legionella pneumophila using indirect immunofluorescence assay and immunoblotting. Acta Virol 36:459–465
Fournier PE, Marrie TJ, Raoult D (1998) Diagnosis of Q fever. J Clin Microbiol 36:1823–1834
Garip Y, Eser F, Bodur H (2010) Health-related quality of life in rheumatoid arthritis: comparison of RAQoL with other scales in terms of disease activity, severity of pain, and functional status. Rheumatol Int. doi:10.1007/s00296-009-1353-1
Gefenaite G, Munster JM, van Houdt R et al (2011) Effectiveness of the Q fever vaccine: a meta-analysis. Vaccine 29:395–398
Gidding HF, Wallace C, Lawrence GL et al (2009) Australia’s national Q fever vaccination program. Vaccine 27:2037–2041
Gilroy N, Formica N, Beers M et al (2001) Abattoir-associated Q fever: a Q fever outbreak during a Q fever vaccination program. Aust N Z J Public Health 25:362–367
Gilsdorf A, Kroh C, Grimm S et al (2008) Large Q fever outbreak due to sheep farming near residential areas, Germany, 2005. Epidemiol Infect 136:1084–1087
Glasgow RE, Ruggiero L, Eakin EG et al (1997) Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care 20:562–567
Hatchette TF, Hayes M, Merry H et al (2003) The effect of C. burnetii infection on the quality of life of patients following an outbreak of Q fever. Epidemiol Infect 130:491–495
Hawker JI, Ayres JG, Blair I et al (1998) A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health 1:180–187
Health Council of the Netherlands (2008) Advisory letter: meeting on Q fever in the Netherlands. The Hague: Health Council of the Netherlands, publication no. 2008/28. [in Dutch, summary in English] Accessible at: http://www.gezondheidsraad.nl/sites/default/files/201008_r.pdf
Health Council of the Netherlands (2010) Human vaccination against Q fever. The Hague: Health Council of the Netherlands, publication no. 2010/08E. Accessible at: http://www.gezondheidsraad.nl/sites/default/files/201008E.pdf
Hickie I, Davenport T, Wakefield D et al (2006) Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333:575
Huijsmans CJ, Schellekens JJ, Wever PC et al (2011) Single-nucleotide-polymorphism genotyping of Coxiella burnetii during a Q fever outbreak in The Netherlands. Appl Environ Microbiol 77:2051–2057
Jover-Diaz F, Robert-Gates J, Andreu-Gimenez L et al (2001) Q fever during pregnancy: an emerging cause of prematurity and abortion. Infect Dis Obstet Gynecol 9:47–49
Juenger J, Schellberg D, Kraemer S et al (2002) Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 87:235–241
Karagiannis I, Schimmer B, van Lier A et al (2009) Investigation of a Q fever outbreak in a rural area of The Netherlands. Epidemiol Infect 137:1283–1294
Karakousis PC, Trucksis M, Dumler JS (2006) Chronic Q fever in the United States. J Clin Microbiol 44:2283–2287
Koene RP, Schimmer B, Rensen H et al (2011) A Q fever outbreak in a psychiatric care institution in The Netherlands. Epidemiol Infect 139:13–18
Landais C, Fenollar F, Thuny F et al (2007) From acute Q fever to endocarditis: serological follow-up strategy. Clin Infect Dis 44:1337–1340
Langley JM, Marrie TJ, Leblanc JC et al (2003) Coxiella burnetii seropositivity in parturient women is associated with adverse pregnancy outcomes. Am J Obstet Gynecol 189:228–232
Lettinga KD, Verbon A, Nieuwkerk PT et al (2002) Health-related quality of life and posttraumatic stress disorder among survivors of an outbreak of Legionnaires disease. Clin Infect Dis 35:11–17
Limonard GJ, Peters JB, Nabuurs-Franssen MH et al (2010) Detailed analysis of health status of Q fever patients 1 year after the first Dutch outbreak: a case-control study. QJM 103:953–958
Lowry TJ, Pakenham KI (2008) Health-related quality of life in chronic fatigue syndrome: predictors of physical functioning and psychological distress. Psychol Health Med 13:222–238
Marmion BP, Ormsbee RA, Kyrkou M et al (1984) Vaccine prophylaxis of abattoir-associated Q fever. Lancet ii:1411–1414
Marmion BP, Ormsbee RA, Kyrkou M et al (1990) Vaccine prophylaxis of abattoir-associated Q fever: eight years’ experience in Australian abattoirs. Epidemiol Infect 104:275–287
Marmion BP, Shannon M, Maddocks I et al (1996) Protracted debility and fatigue after acute Q fever. Lancet 347:977–978
Marques A (2008) Chronic Lyme disease: a review. Infect Dis Clin North Am 22:341–348
Marrie TJ (1990) Acute Q fever. In: Marrie TJ (ed) Q fever. The disease, vol 1. CRC Press, Boca Raton
Maurin M, Raoult D (1999) Q fever. Clin Microbiol Rev 12:518–553
McCaughey C, McKenna J, McKenna C et al (2008) Human seroprevalence to Coxiella burnetii (Q fever) in Northern Ireland. Zoonoses Public Health 55:189–194
Meekelenkamp JCE, Notermans DW, Rietveld A et al (2009) Seroprevalence of Coxiella burnetii in pregnant women in the province of Noord-Brabant in 2007. Infectieziekten Bull 20:57–61 [in Dutch]. http://www.rivm.nl/cib/publicaties/bulletin/jaargang_20/bull2002/prevalentie-coxiella-burnetii.jsp. Accessed May 2011
Mertens K, Samuel JE, Raoult D et al (2007) Bacteriology of Coxiella. In: Raoult D, Parola P (eds) Rickettsial diseases. Informa Healthcare, New York
Morroy G, Peters JB, van Nieuwenhof M et al (2011) The health status of Q-fever patients after long-term follow-up. BMC Infect Dis 11:97. doi:10.1186/1471-2334-11-97
Munster JM, Leenders AC, van der Hoek W et al (2010a) Cost-effectiveness of a screening strategy for Q fever among pregnant women in risk areas: a clustered randomized controlled trial. BMC Womens Health 10:32
Munster JM, Leenders ACAP, Aarnoudse JG et al (2010b) A screening strategy for Q fever during pregnancy. Poster presentation at the 2010 European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE) – 11–13 Nov 2010, Lisbon, Portugal
Parker NR, Barralet JH, Bell AM (2006) Q fever. Lancet 367:679–688
Penttila IA, Harris RJ, Storm P et al (1998) Cytokine dysregulation in the post-Q-fever fatigue syndrome. QJM 91:549–560
Peter O, Dupuis G, Bee D et al (1988) Enzyme-linked immunosorbent assay for diagnosis of chronic Q fever. J Clin Microbiol 26:1978–1982
Peters JB, Daudey L, Heijdra YF et al (2009) Development of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: the Nijmegen Clinical Screening Instrument. Qual Life Res 18:901–912
Porten K, Rissland J, Tigges A et al (2006) A super-spreading ewe infects hundreds with Q fever at a farmers’ market in Germany. BMC Infect Dis 6:147
Raoult D, Tissot-Dupont H, Foucault C et al (2000) Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 79:109–123
Rey D, Obadia Y, Tissot-Dupont H et al (2000) Seroprevalence of antibodies to Coxiella burnetti among pregnant women in South Eastern France. Eur J Obstet Gynecol Reprod Biol 93:151–156
Richardus JH, Schaap GJ, Donkers A et al (1984a) Q fever in the Netherlands; a description of 33 case reports observed between 1979 and 1983. Ned Tijdschr Geneeskd 128:2253–2258
Richardus JH, Donkers A, Schaap GJ et al (1984b) Serological study of the presence of antibodies against Coxiella burnetti and Brucella abortus in veterinarians in the Netherlands. Tijdschr Diergeneeskd 109:612–615
Richardus JH, Dumas AM, Huisman J et al (1985) Q fever in infancy: a review of 18 cases. Pediatr Infect Dis 4:369–373
Richardus JH, Donkers A, Dumas AM et al (1987) Q fever in the Netherlands: a sero-epidemiological survey among human population groups from 1968 to 1983. Epidemiol Infect 98:211–219
Rimes KA, Chalder T (2005) Treatments for chronic fatigue syndrome. Occup Med (Lond) 55:32–39
Roest HI, Tilburg JJ, van der Hoek W et al (2011) The Q fever epidemic in The Netherlands: history, onset, response and reflection. Epidemiol Infect 139:1–12
Schimmer B, Dijkstra F, Vellema P et al (2009) Sustained intensive transmission of Q fever in the south of the Netherlands, 2009. Euro Surveill 14(19)
Schimmer B, ter Schegget R, Wegdam M et al (2010) The use of a geographic information system to identify a dairy goat farm as the most likely source of an urban Q-fever outbreak. BMC Infect Dis 10:69
Schimmer B, Notermans DW, Harms MG et al (2011) Low seroprevalence of Q fever in The Netherlands prior to a series of large outbreaks. Epidemiol Infect. doi:10.1017/S0950268811000136 1-9
Schneeberger P, Schimmer B, Dijkstra F et al (2010a) Epidemiology and control of Q fever in the Netherlands, 2007–2009 (Abstract 2151) European Congress of Clinical Microbiology and Infectious Diseases, Vienna Austria, 10–13 Apr 2010
Schneeberger PM, Hermans MH, van Hannen EJ et al (2010b) Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol 17:286–290
Setiyono A, Ogawa M, Cai Y et al (2005) New criteria for immunofluorescence assay for Q fever diagnosis in Japan. J Clin Microbiol 43:5555–5559
Statistics Netherlands (CBS) STATLINE: landbouw; gewassen, dieren, grondgebruik naar region. http://www.cbs.nl/nl-NL/menu/home/default.htm. Accessed May 2011
Stein A, Raoult D (1998) Q fever during pregnancy: a public health problem in southern France. Clin Infect Dis 27:592–596
Testa MA, Simonson DC (1996) Assesment of quality-of-life outcomes. N Engl J Med 334:835–840
Tilburg JJ, Melchers WJ, Pettersson AM et al (2010) Interlaboratory evaluation of different extraction and real-time PCR methods for detection of Coxiella burnetii DNA in serum. J Clin Microbiol 48:3923–3927
Tissot Dupont H, Thirion X, Raoult D (1994) Q fever serology: cutoff determination for microimmunofluorescence. Clin Diagn Lab Immunol 1:189–196
Tissot Dupont H, Vaillant V, Rey S et al (2007) Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 44:232–237
van den Wijngaard CC, Dijkstra F, van Pelt W et al (2011) In search of hidden Q-fever outbreaks: linking syndromic hospital clusters to infected goat farms. Epidemiol Infect 139:19–26
van der Hoek W, Dijkstra F, Wijers N et al (2010a) Three years of Q fever in the Netherlands: faster diagnosis. Ned Tijdschr Geneeskd 154:A1845
van der Hoek W, Dijkstra F, Schimmer B et al (2010b) Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 15(12):pii–19520
van der Hoek W, Hunink J, Vellema P et al (2011a) Q fever in the Netherlands: the role of local environmental conditions. Int J Environ Health Res. doi:10.1080/09603123.2011.574270
van der Hoek W, Meekelenkamp JCE, Leenders ACAP et al (2011b) Antibodies against Coxiella burnetii and pregnancy outcome during the 2007–2008 Q fever outbreaks in the Netherlands. BMC Infect Dis 11:44
van der Hoek W, Versteeg B, Meekelenkamp J et al (2011c) Follow-up of 686 acute Q fever patients and detection of chronic infection. Clin Infect Dis 52(12):1431–1436
van der Klis FR, Mollema L, Berbers GA et al (2009) Second national serum bank for population-based seroprevalence studies in the Netherlands. Neth J Med 67:301–308
Van Gageldonk-Lafeber AB, Koopmans MPG, Bosman A et al (2003) Het vóórkomen van Q-koorts in Nederland. Infectieziekten Bulletin [in Dutch] 14:173–177
van Steenbergen JE, Morroy G, Groot CA et al (2007) An outbreak of Q fever in The Netherlands–possible link to goats. Ned Tijdschr Geneeskd 151:1998–2003
Vercoulen JH, Daudey L, Molema J et al (2008) An Integral assessment framework of health status in chronic obstructive pulmonary disease (COPD). Int J Behav Med 15:263–279
Waag D, Chulay J, Marrie T et al (1995) Validation of an enzyme immunoassay for serodiagnosis of acute Q fever. Eur J Clin Microbiol Infect Dis 14:421–427
Wegdam-Blans M, Verduin C, Wulf M et al (2009) The role of complement fixations tests in the diagnosis of acute Q fever: is PCR always necessary? Ned Tijdschr Med Microbiol 17:28
Wegdam-Blans MC, Nabuurs-Franssen MN, Horrevorts AM et al (2010) Laboratory diagnosis of acute Q fever. Ned Tijdschr Geneeskd 154:A2388
Wegdam-Blans MCA, Kampschreur LM, Nabuurs-Franssen MH et al (2011) Dutch consensus chronic Q fever. Tijdschrift voor Infectieziekten 6:71–73
Westra SA, Lopes CE, ten Berg J (1958) The first cases of Q-fever in the Netherlands. Ned Tijdschr Geneeskd 102:69–72
Whelan J, Schimmer B, Schneeberger P et al (2011) Q fever among culling workers, the Netherlands, 2009–2010. Emerg Infect Dis 17:1719–1723
Wildman MJ, Smith EG, Groves J et al (2002) Chronic fatigue following infection by Coxiella burnetii (Q fever): ten-year follow-up of the 1989 UK outbreak cohort. QJM 95:527–538
Wolff JW, Kouwenaar W (1954) Investigation on occurrence of Q fever in Netherlands. Ned Tijdschr Geneeskd 98:2726–2732
Wouda W, Dercksen DP (2007) Abortion and stillbirth among dairy goats as a consequence of Coxiella burnetii. Tijdschr Diergeneeskd 132:908–911
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
van der Hoek, W. et al. (2012). Epidemic Q Fever in Humans in the Netherlands. In: Toman, R., Heinzen, R., Samuel, J., Mege, JL. (eds) Coxiella burnetii: Recent Advances and New Perspectives in Research of the Q Fever Bacterium. Advances in Experimental Medicine and Biology, vol 984. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4315-1_17
Download citation
DOI: https://doi.org/10.1007/978-94-007-4315-1_17
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4314-4
Online ISBN: 978-94-007-4315-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)