Abstract
Adherens junctions are the most common junction type found in animal epithelia. Their core components are classical cadherins and catenins, which form membrane-spanning complexes that mediate intercellular binding on the extracellular side and associate with the actin cytoskeleton on the intracellular side. Junctional cadherin–catenin complexes are key elements involved in driving animal morphogenesis. Despite their ubiquity and importance, comparative studies of classical cadherins, catenins and their related molecules suggest that the cadherin/catenin-based adherens junctions have undergone structural and compositional transitions during the diversification of animal lineages. This chapter describes the molecular diversities related to the cadherin–catenin complex, based on accumulated molecular and genomic information. Understanding when and how the junctional cadherin–catenin complex originated, and its subsequent diversification in animals, promotes a comprehensive understanding of the mechanisms of animal morphological diversification.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
All multicellular animals composed of differentiated tissues, such as epithelia, are referred to as metazoans. The shaping of their bodies relies on cell–cell adhesion and its regulation. An understanding of how the mechanisms of cell–cell adhesion originated and evolved in animals is required to understand the mechanisms that regulate the morphological diversification of animals.
Metazoan cells adhere to each other using specialized membrane structures termed intercellular junctions. Cells in the differentiated epithelia of vertebrates typically have a junctional complex consisting of a tight junction , an adherens junction and a desmosome (Fig. 2.1a). However, this junction organization is not universal, even within the phylum Chordata. Desmosomes are unique to vertebrates, and tight junctions are unique to vertebrates and urochordates (e.g., ascidians). In the epithelia of cephalochordates (e.g., amphioxus), adherens junctions are the only junction type expressed (Lane et al. 1987). Junctional complexes consisting of an adherens junction and septate junction are widely observed in the epithelia of non-chordate metazoan animals including arthropods, echinoderms and cnidarians (Fig. 2.1b, c). The various junction types show distinct phylogenetic distributions; the most common junction type is the adherens junction (Oda and Takeichi 2011), which is found in all metazoan phyla including the Porifera.
Transmission electron micrographs of intercellular junctions in metazoan epithelia. a A junctional complex consisting of a tight junction (top), an adherens junction (middle) and a desmosome (bottom) in the epithelium of a mouse small intestine (Courtesy of Dr. Tomohiro Haruta, JEOL Ltd.). b A junctional complex consisting of an adherens junction and a septate junction in the epithelium of a Drosophila salivary gland (Courtesy of Dr. Tomohiro Haruta, JEOL Ltd.). c A junctional complex consisting of an adherens junction and a septate junction in the epithelium of an Asterina (starfish) midgastrula (Copyright 1995 Wiley-Liss Inc. Used with permission from Dan-Sohkawa et al. (1995)). In all panels, the apical end of the lateral cell–cell contact is at the top, and the arrows sandwich an adherens junction
Classical cadherins and catenins in vertebrate and non-vertebrate model species. a Schematic illustrations showing the domain structures of classical cadherins and catenins in Mus musculus (mouse) and those of their homologues in Drosophila melanogaster (fruit fly) and Caenorhabditis elegans (nematode worm). The regions that are responsible for interactions between the classical cadherin, β-catenin, αE-catenin and actin are also shown. PM, plasma membrane. b, c Amino acid sequence alignments for the p120-catenin- (b) and β-catenin- (c) binding motifs of the cadherins shown in (a). Residues that are identical among four or more of the proteins are colored. HMR-1 is truncated at the C-terminus
Molecular and genetic studies of vertebrates, Drosophila melanogaster and Caenorhabditis elegans have revealed that the adherens junctions of these animals share common molecular compositions and organizations, providing strong support for homology of this junction type across the Bilateria (Knust and Bossinger 2002). The core components of adherens junctions are cadherins and catenins, which form complexes that mediate cell–cell adhesion and the association of adherens junctions with the actin cytoskeleton. The cadherin–catenin complex is a basic molecular machinery involved in various morphogenetic processes including cell migration, cell rearrangement, epithelial folding and epithelial-to-mesenchymal transitions.
Despite the ubiquity of cadherin/catenin-based adherens junctions and their importance in shaping animal bodies, comparative studies of cadherins, catenins and related molecules suggest that the adherens junctions have undergone structural and compositional transitions during the diversification of animal lineages. This chapter does not cover the functional and mechanistic details of the cadherin–catenin complex, but instead focuses on describing the molecular diversities related to its components.
2 Cadherin and Catenins at Adherens Junctions
2.1 Classical Cadherins in Vertebrates
The first molecule to be termed “cadherin” was Mus musculus (mouse) E-cadherin (Yoshida-Noro et al. 1984), although it is also referred to as uvomorulin and CDH1 (Hyafil et al. 1981). This cadherin was identified using antibodies that were capable of inhibiting calcium -dependent cell–cell adhesion and cell compaction (Kemler et al. 1977; Hyafil et al. 1981; Yoshida and Takeichi 1982; Nagafuchi et al. 1987; Ringwald et al. 1987). L-CAM, a cell adhesion molecule independently identified in chickens, is an ortholog of mouse E-cadherin (Brackenbury et al. 1981; Gallin et al. 1987). E-cadherin is enriched in adherens junctions in a wide range of epithelial tissues (Boller et al. 1985; Takeichi 1988). N-cadherin (also referred to as CDH2) is abundant in mesodermal and neural tissues and serves as a major adhesion molecule at the adherens junctions in these tissues in the place of E-cadherin (Volk and Geiger 1984; Hatta and Takeichi 1986). VE-cadherin (also referred to as CDH5) is another representative cadherin, which is specifically expressed by endothelial tissues (Heimark et al. 1990) and functions at endothelial adherens junctions. E-, N-, VE- and other cadherins share a common structure and form a molecular family; they consist of five extracellular cadherin domains (ECs) , a transmembrane domain and a cytoplasmic domain (Fig. 2.2a). Their cytoplasmic domains have highly conserved sequences and bind p120- and β-catenins at the juxtamembrane and C-terminal regions, respectively (Fig. 2.2b, c). Each cadherin subtype preferentially binds to the same subtype in a homotypic fashion (Nagafuchi et al. 1987; Nose et al. 1988). The most membrane-distal EC (EC1 ) contains amino acid sites that are critical in determining the binding specificities of the cadherins (Nose et al. 1990). Another common feature of the cadherins is the presence of a precursor domain that is removed during the maturation process to activate the cadherin (Shirayoshi et al. 1986; Ozawa and Kemler 1990).
In later studies, an increasing number of genes containing ECs has been found in vertebrate and non-vertebrate animals, but many of these ECs are structurally distinct from the original cadherins. Therefore, although the general term “cadherin” refers to any member of the cadherin superfamily (molecules having ECs), members of the first identified cadherin family are referred to as “classical cadherins.” Typical ECs contain conserved amino acid sequence motifs, such as “DxD,” “DRE” and “DxNDN,” which are involved in the Ca2+ binding that is necessary for protease resistance and interdomain rigidification (Ozawa et al. 1990; Overduin et al. 1995; Nagar et al. 1996).
2.2 Drosophila Homologs of Classical Cadherins
The first non-vertebrate member of the cadherin superfamily identified was the product of the Drosophila fat gene; mutations of this gene cause tumor-like overgrowth of the larval imaginal discs without disrupting the multicellular and epithelial organization (Mahoney et al. 1991). Fat is a single-pass transmembrane protein that contains 34 ECs, four epidermal growth factor-like repeat domains (EGFs) and two laminin A globular domains (LmGs) in its extracellular region (Fig. 2.3a). The amino acid sequence of the cytoplasmic domain of Fat largely differs from those of the classical cadherins.
Major subfamilies of the cadherin superfamily in metazoans. a Schematic illustrations showing the varied domain structures of selected cadherin superfamily members. Domains and motifs are indicated as in Fig. 2.2. Conserved tryptophan (W) residues at the N-terminal regions of type I, type II and desmosomal cadherins are also shown. Abbreviations of species are as follows: Mm, Mus musculus (mouse); Gg, Gallus gallus (chicken); Dm, Drosophila melanogaster (fruit fly); Ta, Trichoplax adhaerens; Bb, Branchiostoma belcheri (amphioxus); and Sp, Strongylocentrotus purpuratus (sea urchin). Abbreviations of taxa in parentheses are as follows: Ver, Subphylum Vertebrata (Phylum Chordata); Hex, Superclass Hexapoda (Phylum Arthropoda); Pla, Phylum Placozoa; Cep, Subphylum Cephalochordata (Phylum Chordata); and Ech, Phylum Echinodermata. b Partial sequence similarities in the cytoplasmic domains of classical cadherin (E-cadherin), FAT1, FAT3 and PCDH9. c Partial sequence similarities in the cytoplasmic domains of the classical cadherin (cadherin-11), DCHS1 and sea urchin Dachsous. d Sequence similarities in the cytoplasmic domains of classical and desmosomal cadherins. Amino acid sequences derived from the regions indicated by green arrows in a are aligned in b to d. Residues that are identical among two or more of the proteins are colored
DE-cadherin , a second cadherin in Drosophila , was identified as a glycoprotein that forms a complex with Armadillo and D α-catenin, the Drosophila homologs of β- and α-catenin, respectively (Fig. 2.2a; Oda et al. 1994). The amino acid sequence of the DE-cadherin cytoplasmic domain exhibits 33–37% identity with the mouse E- and N-cadherin cytoplasmic domains. The p120-catenin and β-catenin binding sequence motifs are conserved in DE-cadherin. However, despite the strong conservation of the cytoplasmic domain, the extracellular region of DE-cadherin exhibits a domain organization distinct from that of the classical cadherins; it has seven ECs followed by an EGF and an LmG. The presence of EGF and LmG is a structural feature shared with the Fat cadherin. Another unique feature of DE-cadherin is that it is proteolytically cleaved at a site between the EC7 and the EGF (between residues 1,010 and 1,011) (Oda and Tsukita 1999). Mature DE-cadherin consists of two fragments that are bound to each other probably by non-covalent interactions between the regions near the cleaved ends. DE-cadherin is a gene product of the shotgun locus (Tepass et al. 1996; Uemura et al. 1996), and is enriched, together with Armadillo and D α-catenin, at the adherens junctions in essentially all epithelial cells. Genetic evidence suggests that DE-cadherin is the functional counterpart of mammalian E-cadherin .
DN-cadherin (CadN) is also a cadherin in Drosophila with a cytoplasmic domain that interacts with Armadillo and D α-catenin (Iwai et al. 1997). It is structurally similar to, but much larger than, DE-cadherin (Fig. 2.2a) and has at least 16 ECs in its membrane-distal extracellular region , and 4 EGFs and 2 LmGs in its membrane-proximal extracellular region. The final EC is followed by a domain that is homologous to the DE-cadherin proteolytic cleavage site and the flanking regions. Since immunochemical data suggest that DN-cadherin consists of two fragments as well (Iwai et al. 1997), the proteolytic cleavage is likely to be conserved in DN-cadherin. Like vertebrate N-cadherin , DN-cadherin is expressed in mesodermal and neural tissues. The functions of DN-cadherin are also similar to the functions of the vertebrate N-cadherin (Takeichi 2007). Thus, the relationship between DE- and DN-cadherin is analogous to the relationship between E and N-cadherin, despite the structural differences existing between Drosophila and vertebrate cadherins.
A DN-cadherin -like gene (CadN2) exists next to the DN-cadherin gene in the Drosophila genome. However, this cadherin exhibits no detectable adhesion activity, and CadN2-null mutants are viable, although subtle functions for CadN2 are detectable (Prakash et al. 2005; Yonekura et al. 2007).
2.3 C. elegans Homologs of Classical Cadherins
A study of C. elegans identified three genes, hmp-1 , hmp-2 and hmr-1 , that are related to α-catenin, β-catenin/Armadillo and classical cadherin, respectively (Fig. 2.2a; Costa et al. 1998). In the C. elegans genome, hmr-1 is the sole cadherin gene related to classical cadherins. The products of hmp-1, hmp-2 and hmr-1 localize to hypodermal (or epidermal) adherens junctions and their activities are required for hypodermal ventral closure during mid-embryogenesis; however, none of these products are essential for cell–cell adhesion and cell shape regulation before and during gastrulation. Even at early stages, HMR-1 functions in blastoderm compaction and gastrulation, but these functions are redundant with those of an immunoglobulin domain adhesion molecule, SAX-7 (Grana et al. 2010).
hmr-1 encodes two isoforms, HMR-1A and HMR-1B, which have 2 and 13 ECs, respectively, followed by DN-cadherin -like membrane-proximal extracellular domains (Broadbent and Pettitt 2002). Whereas the HMR-1A transcript is expressed to play a role in hypodermal morphogenesis, the HMR-1B transcript is transcribed by an alternative, neuron-specific promoter, and subjected to alternative splicing. HMR-1B and DN-cadherin resemble each other in their domain organizations and in vivo functions, and the relationship between HMR-1A and HMR-1B is analogous to the relationships between DE- and DN-cadherin and between E- and N-cadherin.
2.4 β-Catenin/Armadillo
β-catenin/Armadillo functions as a part of the cadherin–catenin complex in cell–cell adhesion (McCrea et al. 1991; Peifer et al. 1992), and as a signal transducer in the canonical Wnt /Wingless signaling pathway. Armadillo was originally identified as a product of one of the Drosophila segment polarity class genes (Riggleman et al. 1989). Vertebrate β-catenin and Drosophila Armadillo exhibit essentially the same overall structure; they are divided into three domains, the N-terminal domain, the central domain and the C-terminal domain (Fig. 2.2a). The central domain consists of 12 repeats of ~ 42 amino acid residues, referred to as Armadillo repeats (ArmR1–ArmR12). These ArmRs each form three α-helices, tightly packed against one another to form a superhelical structure that serves as a scaffold for the binding of the classical cadherin cytoplasmic domain (Huber and Weis 2001). The α-catenin binding site is a 29-amino-acid region of β-catenin that encompasses the junction of the N-terminal domain and ArmR1 (Aberle et al. 1996).
The signaling function of β-catenin depends on the regulation of its stability in the cytoplasm (Peifer and Polakis 2000; Tolwinski and Wieschaus 2004; Brembeck et al. 2006). Binding of Wnt ligands induces the stabilization of the cytoplasmic pool of β-catenin, allowing β-catenin to translocate to the nucleus and to there act as a transcriptional activator in conjunction with DNA-binding proteins, T cell factor (TCF ), lymphoid enhancer factor-1 (LEF-1) and Pangolin (Pan). In the absence of Wnt signal input, cytoplasmic β-catenin is efficiently degraded by a destruction complex consisting of the tumor suppressor gene product adenomatous polyposis coli (APC), axin, glycogen synthase kinase 3-beta (GSK-3β) and casein kinase (CKI). The ArmR domain in β-catenin, when free from cadherin and α-catenin, interacts with either components of the destruction complex or TCF/LEF-1/Pan. The N-terminal and C-terminal domains in β-catenin have essential roles in its signaling function. Unlike vertebrates and Drosophila , C. elegans has three diverged β-catenin genes with separate roles (Eisenmann 2005): hmp-2 , which is involved in cadherin-mediated adhesion, and wrm-1 and bar-1, which are involved in Wnt signaling.
Plakoglobin (or γ-catenin), a component of adherens junctions and desmosomes, is closely related to β-catenin and only found in vertebrates (Fig. 2.2a). Although plakoglobin and β-catenin exhibit less conservation in their C-terminal domain, their ArmR domains share high sequence identity (approximately 80%), which accounts for the ability of plakoglobin to bind to the classical cadherin cytoplasmic domain. However, plakoglobin also binds to the cytoplasmic domains of other cadherin types, desmoglein and desmocollin, that are responsible for desmosome assembly. Compared to plakoglobin, β-catenin exhibits weaker binding to desmoglein-1, which partly accounts for the specific participation of β-catenin in adherens junction assembly (Choi et al. 2009).
2.5 α-Catenin
α-Catenin mediates regulatory interactions between the cadherin-β-catenin complex and the cytoskeleton. Vertebrates have two subtypes of α-catenin, αE- and αN-catenins, which are expressed in epithelial and neural tissues (Nagafuchi et al. 1991; Herrenknecht et al. 1991; Hirano et al. 1992), whereas Drosophila and C. elegans have a single α-catenin homolog (D α-catenin and HMP-1, respectively) (Fig. 2.2a). These α-catenins share essentially the same structural features, including three vinculin-homology domains , VH1, VH2 and VH3, in their N-terminal, middle and C-terminal regions, respectively. αE-catenin binds β-catenin through its N-terminal region (Huber et al. 1997; Koslov et al. 1997; Obama and Ozawa 1997; Nieset et al. 1997) and without β-catenin, it forms homodimers using the same N-terminal region (Koslov et al. 1997; Pokutta and Weis 2000; Drees et al. 2005). The αE-catenin homodimer can bind and bundle F-actin using the C-terminal regions (Rimm et al. 1995). The middle region of αE -catenin binds to other actin-binding proteins, such as vinculin and α-actinin (Kobielak and Fuchs 2004). Unlike mammalian αE-catenin, recombinant full-length HMP-1 is a monomer. The actin-binding ability of the C-terminal region of HMP-1 is usually suppressed by its other regions (Kwiatkowski et al. 2010).
2.6 p120-Catenin
p120-Catenin was originally identified as a tyrosine kinase substrate for which tyrosine phosphorylation was induced by transformation with Src in mammalian cells (Reynolds et al. 1989). It was later found to directly bind to the juxtamembrane region of classical cadherins (Fig. 2.2a, b; Reynolds et al. 1994; Daniel and Reynolds 1995; Lampugnani et al. 1997; Yap et al. 1998). p120-catenin has been functionally characterized as a key regulator of classical cadherin stability (Ireton et al. 2002; Davis et al. 2003; Xiao et al. 2003). p120-catenin is an ArmR-containing protein, like β-catenin and plakoglobin, but belongs to a distinct subgroup referred to as the p120-catenin family, whose members share a conserved central domain comprised of 9 ArmRs. Four members of the vertebrate p120-catenin family, p120-catenin, ARVCF (Armadillo repeat gene deleted in velo-cardio-facial syndrome), δ-catenin and p0071, can bind classical cadherin via their ArmR domains in a mutually exclusive manner (Daniel and Reynolds 1995; Hatzfeld and Nachtsheim 1996; Mariner et al. 2000; Paulson et al. 2000). The vertebrate p120-catenin family also includes components of desmosomes known as plakophilins. In contrast to vertebrates, Drosophila and C. elegans each possess only one member of the p120-catenin family (p120ctn and JAC-1 , respectively). Ablation or depletion of p120-catenin in vertebrate embryos causes severe morphogenetic defects (Fang et al. 2004; Davis and Reynolds 2006); however, the sole p120-catenin of Drosophila and C. elegans is not essential for the viability and morphogenesis of the animals, although these molecules modulate cadherin-mediated adhesion (Pacquelet et al. 2003; Myster et al. 2003; Pettitt et al. 2003).
3 Cadherin Superfamily
3.1 Major Subfamilies in Metazoans
A single mammalian genome encodes more than one hundred members of the cadherin superfamily. The Drosophila and C. elegans genomes include 17 and 12 cadherin genes, respectively (Fung et al. 2008; Pettitt 2005). The sea urchin Strongylocentrotus purpuratus genome has fewer cadherin genes than the Drosophila and C. elegans genomes (Whittaker et al. 2006). Recent advances in genome sequencing have made genomic information available on cadherins from many other metazoan species, including Ciona intestinalis (urochordate ascidian), Branchiostoma floridae (cephalochordate amphioxus), Nematostella vectensis (cnidarian sea anemone) and Trichoplax adhaerens (placozoan). The accumulated information indicates that there are at least eight major subfamilies distributed across two or more metazoan phyla (Nollet et al. 2000; Whittaker et al. 2006; Hulpiau and van Roy 2009, 2010), including the classical cadherin, Fat, Fat-like, Dachsous, Flamingo, protocadherin, PCDH15 and CDH23 subfamilies (Fig. 2.3a).
3.2 Classical Cadherin
Cadherins with cytoplasmic domains that are closely homologous to those of vertebrate classical cadherins are distributed widely in Metazoans, although a great diversity is seen in their extracellular structures. Irrespective of the species and the extracellular domain, classical cadherin has been re-defined as a molecule having a conserved cytoplasmic domain that interacts (or is expected to interact) with p120-catenin and β-catenin (Oda and Takeichi 2011).
The classical cadherin 5-EC organization has only been observed in the vertebrate and urochordate subphyla, while association of ECs with EGF and LmG is a common feature of all classical cadherins identified in non-chordate metazoans. Another feature shared by most nonchordate classical cadherins is the presence of an extracellular region that is homologous to the DE-cadherin extracellular proteolytic cleavage site and its flanking regions, which are referred to as the primitive classical cadherin proteolytic site domain (PCPS; Oda and Tsukita 1999; Oda and Takeichi 2011). Because some PCPSs show weakly detectable partial similarities to ECs, this domain type might have diverged from an EC. However, to avoid potential confusion, the PCPS will hereafter not be considered an EC. The classical cadherins of bilaterian species contain between 2 and 17 ECs, with the exception of the unique molecules described below. In contrast, 25 or more ECs are encoded by the classical cadherin genes found in the genomes of cnidarian and placozoan species (Chapman et al. 2010; Hulpiau and Van Roy 2010; Fahey and Degnan 2010). Expressions and functions of these huge classical cadherins have not been studied.
Bb1- and Bb2-cadherins are a pair of exceptional “cadherins” that have been reported in the amphioxus Branchiostoma belcheri, a chordate belonging to the cephalochordate subphylum (Oda et al. 2002, 2004). These molecules each possess a well-conserved classical cadherin cytoplasmic domain, but their extracellular regions consist of no ECs, only LmGs and EGFs. Despite the lack of ECs, Bb1- and Bb2-cadherins can mediate homophilic cell–cell adhesion and cell sorting in vitro, although their activities are independent of calcium ions. Importantly, Bb1- and Bb2-cadherins, together with an amphioxus β-catenin homolog, are enriched at adherens junctions in various epithelial tissues (Fig. 2.4e). Bb1- and Bb2-cadherins are not formally included in the cadherin superfamily, but appear to be derivatives of a classical cadherin subfamily member. The sequenced genome of another amphioxus species, Branchiostoma floridae, contains orthologs of the Bb1- and Bb2-cadherin genes, as well as an additional gene encoding AmphiCDH, a DN-cadherin -like nonchordate-type classical cadherin (Hulpiau and Van Roy 2010).
A “lineage-specific domain loss” model for classical cadherin extracellular structure diversification. a–e Localization of various classical cadherins at epithelial adherens junctions (indicated by arrows). In each panel, immunostaining for classical cadherin is shown in green, and staining for DNA is in blue. a Ap-cadherin (type III) expression in a midgastrula of the starfish Asterina pectinifera; b At-cadherin (type III) expression in a germ-disc stage embryo of the spider Achaearanea tepidariorum; c DE-cadherin (type IV) expression in a Drosopohila gastrula; d Af1-cadherin (type IV) expression in a nauplius larva of the branchiopod Artemia franciscana; e Bb1-cadherin (EC-lacking type) expression in a neurula of the amphioxus B. belcheri. Bars, 50 µm. f Reconstruction of evolutionary transitions (indicated by circles in various colors) that diversified the extracellular domain structures of classical cadherins at the epithelial adherens junctions, based on comparative studies (Oda et al. 2002, 2005; Hulpiau and Van Roy 2010). Gaps are introduced to highlight homologous regions between distinct classical cadherins. Taxa in which the same or similar conditions have been observed are shown in the right column. In this model, type III cadherin represents the ancestral form of classical cadherin for bilaterians. Distinct N-terminal truncations and internal deletions (indicated by broken back lines) in type III cadherin occurred in different bilaterian lineages. The epithelial adherens junctions in “ancestral” animals, such as starfish and spider, use type III cadherin, and the epithelial adherens junctions in “derived” animals use structurally reduced forms of classical cadherin such as types I/II and IV. The establishment of type III cadherin may have been preceded by N-terminal truncations. a, No expression data is available for Ta-cadherin and other non-bilaterian classical cadherins. PM, plasma membrane; AJ, adherens junction
3.3 Fat, Fat-Like and Dachsous
The extracellular regions of members of the Fat and Fat-like subfamilies typically have an array of ~ 34 ECs , which is followed by EGFs and LmGs. Drosophila Fat appears to be orthologous to mammalian FAT4, whereas Drosophila Fat-like has a closer relationship to mammalian FAT1, FAT2 and FAT3 (Castillejo-López et al. 2004; Hulpiau and van Roy 2009). The Drosophila and mammalian Dachsous cadherins, which have 27 ECs with no EGF and LmG, heterophilically bind the corresponding Fat cadherins in vitro (Matakatsu and Blair 2004; Ishiuchi et al. 2009), and the Drosophila Dachsous-Fat pair and possibly mouse FAT4 function in a signaling pathway that regulates tissue growth, planar cell polarity and tissue patterning (Reddy and Irvine 2008; Saburi et al. 2008). In Drosophila larval epithelial cells, Fat and Dachsous cadherins are concentrated in a subapical region of cell–cell contact, which is more apical than the adherens junction (Ma et al. 2003). In mouse embryonic neuroepithelial cells, a similar pattern of subcellular localization has been observed for Fat4 and Dachsous1 (Ishiuchi et al. 2009).
Despite being phylogenetically separated from the classical cadherin subfamily, Drosophila Fat and Dachsous, mammalian FAT1 and FAT3, and an echinoderm homolog of Dachsous have been reported to contain cytoplasmic sequences that exhibit faintly detectable similarities to part of the β-catenin-binding sequence motif of classical cadherins (Fig. 2.3a–c; Clark et al. 1995; Whittaker et al. 2006; Hulpiau and van Roy 2009).
3.4 Flamingo
Drosophila Flamingo (also known as Starry night) and its vertebrate counterparts, Celsrs (cadherin, EGF -like, laminin A globular, seven-pass receptor), are seven-pass transmembrane proteins categorized as adhesion G protein-coupled receptors (Usui et al. 1999). They each have 8 or 9 ECs and two LmGs together with several EGFs. Irrespective of the phylogenetic distances, the echinoderm, C. elegans , cnidarian and placozoan members of the Flamingo subfamily exhibit markedly similar domain organization (Whittaker et al. 2006; Hulpiau and Van Roy 2010). Like classical cadherins, Flamingo exhibits homophilic binding in vitro. Notably, this Drosophila protein and the vertebrate Celsrs have similar functions in regulating planar cell polarity (Usui et al. 1999; Curtin et al. 2003; Formstone and Mason 2005; Carreira-Barbosa et al. 2008).
3.5 Protocadherin
The term “protocadherin” is often confusing, since it is used to refer to many non-classical cadherins without phylogenetic considerations. In vertebrates, cadherins with six or seven ECs are considered to constitute a phylogenetic group, which is termed the protocadherin subfamily (Morishita and Yagi 2007). This subfamily is divided, based on genomic organization, into two subgroups, i.e., the clustered and non-clustered protocadherins. In the mouse, clustered protocadherins, each of which contains six ECs, are encoded by three tandemly aligned gene clusters (α, β and γ) and are predominantly expressed in the nervous system; their in vivo functions are poorly understood. Non-clustered protocadherins (e.g., PCDH9) have two unique amino acid sequence motifs in their cytoplasmic regions and are also referred to as δ-protocadherins. Notably, this type of protocadherin is found in a wider range of metazoans including Nematostella (Whittaker et al. 2006; Hulpiau and Van Roy 2010) but is missing in Drosophila and C. elegans . Three vertebrate members of the δ-protocadherin subfamily, paraxial protocadherin, OL-protocadherin (PCDH10) and PCDH19, are known to cooperate with classical cadherins to promote cell sorting and/or cell movements (Chen and Gumbiner 2006; Nakao et al. 2008; Biswas et al. 2010).
3.6 PCDH15 and CDH23
Mammalian PCDH15 (protocadherin 15) and CDH23 (cadherin 23) have 11 and 27 ECs, respectively, and interact heterophilically to facilitate mechanosensing in the stereocilia of the mammalian inner ear (Kazmierczak et al. 2007). Mutations in these genes cause hearing loss, termed Usher syndrome. A Drosophila homolog of PCDH15, termed Cad99C, regulates microvilli length (D’Alterio et al. 2005). However, Drosophila has no CDH23 counterpart. Nonetheless, in the cnidarian sea anemone tentacle, a CDH23-related polypeptide has been detected between the mechanosensory stereocilia (Watson et al. 2008).
3.7 Desmosomal Cadherin
The desmosomal cadherins have 4 or 5 ECs and constitute a vertebrate-specific subfamily of the cadherin superfamily and are divided into two types, desmocollins and desmogleins, which bind heterophilically, probably via their EC1 domains. The cytoplasmic binding partners of desmosomal cadherins include plakophilins, plakoglobin and desmoplakin, which play roles in desmosome assembly and intermediate filament anchorage. The cytoplasmic domains of desmosomal cadherins have amino acid sequences that are related to, but significantly divergent from, the β-catenin-binding sequence motif of classical cadherins (Fig. 2.3a, d; Troyanovsky et al. 1994; Hulpiau and van Roy 2009); these sequences appear to be bound to plakoglobin, a close relative of β-catenin, and are required for the anchoring of intermediate filaments by the desmosomal plaque. Amino acid sequences that are partially similar to the p120-catenin -binding sequence motif of classical cadherins are detectable in the juxtamembrane regions of desmogleins and desmocollins (Hulpiau and van Roy 2009) and an association has been shown between p120-catenin and desmoglein 3 (Kanno et al. 2008).
4 Evolution of Classical Cadherins
4.1 Type I and Type II Cadherins
The major subfamilies of the cadherin superfamily are varied in the number of their ECs, the polypeptide length and the domain composition and organization. However, within each subfamily, domain organization tends to be conserved across the metazoans. In this respect, the classical cadherin subfamily is exceptional. Members of this subfamily exhibit a great structural diversity in their extracellular regions. Reconstruction of the processes responsible for the generation of the structural diversity of classical cadherins may facilitate an understanding of the evolution of adherens junctions.
Classical cadherins that have been identified in the vertebrate subphylum are classified into type I (e.g., E- and N-cadherins), type II (e.g., VE-cadherin and cadherin-11) and type III (e.g., cHz-cadherin) (Fig. 2.3a). The details of type III cadherins are described below. A shared recent origin of type I, type II and desmosomal cadherins is strongly supported by the presence of the prodomain , which is processed for activation, the extracellular 5-EC organization and the exon-intron structures (Greenwood et al. 1997; Nollet et al. 2000). The differences between type I and type II cadherins are apparent at the amino acid sequence level. The HAV sequence, which is conserved in the EC1 domains of type I cadherins, is missing in type II cadherins. In contrast to type I cadherins, which have a single conserved tryptophan residue at the N-terminal region, most type II cadherins have two tryptophan residues at their N-terminal regions. A crystallographic study has proposed distinct structural mechanisms for adhesion mediated by type I and type II cadherins (Patel et al. 2006). Whereas type I cadherins exhibit broad tissue distribution, type II cadherins tend to be expressed in more restricted cell populations and types. For example, the type I N-cadherin is expressed broadly in the mesodermal and neural tissues, including endothelial cells, but the type II VE-cadherin is expressed only in the endothelial cell populations of the mesoderm (Salomon et al. 1992; Navarro et al. 1998).
In the human genome, at least four genes encode type I classical cadherins, and at least 14 genes encode type II classical cadherins. Many of these genes form clusters. The largest cluster, which is located on the long arm of chromosome 16, comprises two type I cadherin genes, including E-cadherin, three type II cadherin genes, including VE-cadherin , and a non-classical cadherin (Ksp-cadherin) gene (Kremmidiotis et al. 1998). The urochordate Ciona intestinalis genome has only two classical cadherin genes; one is related to the type I cadherins, and the second is related to the type II cadherins (Sasakura et al. 2003). Neither type I nor type II cadherins have been discovered outside of the vertebrate and urochordate subphyla. The complexity of type I and type II cadherins increased due to gene duplications in the vertebrate lineage after it diverged from the urochordate lineage. In addition, vertebrates, but not non-vertebrate animals, have desmosomal cadherins and other cadherins that have ECs closely related to the ECs of type I/type II cadherins but lack the classical cadherin cytoplasmic domain. These cadherins include T-cadherin (CDH13 and H-cadherin), Ksp-cadherin (CDH16) and LI-cadherin (CDH17) (Vestal and Ranscht 1992; Wendeler et al. 2006). Type I and/or type II cadherin genes may have acted as a source of such vertebrate-specific non-classical cadherins during evolution.
4.2 Type III Cadherin
Chicken cHz-cadherin was first regarded as a type III cadherin (Tanabe et al. 2004). Despite its vertebrate source, cHz-cadherin is markedly similar to Drosophila DN-cadherin with respect to its domain organization. Genes encoding classical cadherins with similar domain organization are also found in other arthropods, echinoderms, amphioxus and fish. These classical cadherins have 14–17 ECs , a PCPS, multiple EGFs and two LmGs arranged specifically in their extracellular regions. Importantly, their mutually similar domain organization has been suggested to result from conservation, not convergence (Oda et al. 2005; Hulpiau and Van Roy 2010). This domain conservation defines the type III cadherins. The only classical cadherin gene in the genome of the sea urchin Strongylocentrotus purpuratus encodes a type III cadherin (Whittaker et al. 2006). It is likely that the last common ancestor of all bilaterian animals possessed a type III cadherin. However, the type III cadherin gene is absent from the ascidian and placental mammalian genomes (Tanabe et al. 2004; Hulpiau and Van Roy 2010), suggesting that this cadherin type was lost secondarily at multiple separate points of bilaterian evolution.
The tissue distributions of type III cadherins vary depending on species. cHz-cadherin is expressed in horizontal cells, one of the basic cell types of the chicken retina (Tanabe et al. 2004). Type III cadherins of hexapods (e.g., cricket) and branchiopods (e.g., brine shrimp) are widely expressed in embryonic mesodermal and neural tissues (Oda et al. 2005; Hsu et al. 2009), similar to DN-cadherin . In contrast, the type III cadherins of malacostracan crustaceans (e.g., shrimp), chelicerates (e.g., spider) and echinoderms (e.g., starfish) are localized at the adherens junctions in the embryonic epithelia (Fig. 2.4a, b), although the arthropod cadherins are also found in the neural tissues. These observations suggest that the roles of type III cadherins have been altered in a lineage-specific way during evolution.
4.3 Type IV Cadherin
DE-cadherin and its orthologs are grouped as type IV cadherins, and have 7 ECs, a PCPS, an EGF and an LmG in their extracellular regions. Type IV cadherins have only been found within the branchiopods and hexapods. These cadherins exhibit conserved expression at the adherens junctions in the embryonic epithelia (Fig. 2.4c, d; Oda et al. 2005), and this finding is potentially correlated with the absence of type III cadherin from these tissues in the branchiopods and hexapods. It appears that the domain structure and role of type IV cadherin in adherens junction assembly in the epithelia have been stably maintained during branchiopod and hexapod evolution, indicating the rarity of evolutionary transitions in the extracellular architecture of adherens junctions.
4.4 Lineage-Specific Domain Loss
The assumption that type III cadherin represents the bilaterian ancestral form of classical cadherin may facilitate the understanding of not only the wide phylogenetic distribution of this cadherin type, but also the processes that contributed to the structural diversification of classical cadherins. BLAST-based comparisons between individual domains of type IV and type III cadherins and between those of type I/II and type III cadherins have identified homologous regions between the different cadherin types (Oda et al. 2005; Hulpiau and Van Roy 2010; Oda and Takeichi 2011). For example, the membrane-distal 6-EC region of type IV cadherins appears to be homologous to the internal 6-EC region of type III cadherins that is separated from the PCPS by four ECs, and the entire extracellular region of type I/II cadherins appears to be homologous to the membrane-proximal 5-EC region of type III cadherins (Fig. 2.4f). In addition, the region covering the last EC and the single LmG in type IV cadherins is homologous to the region covering the last EC and the first LmG in type III cadherins. Thus, losses of distinct combinations of domains from the type III cadherin may account for the establishment of the type I/II and type IV domain organizations in the branchiopod/hexapod and urochordate/vertebrate lineages.
The “lineage-specific domain loss” model is potentially also applicable to understanding the variously reduced forms of classical cadherin that are observed in other bilaterian animal lineages, including the short form in C. elegans and the EC-lacking form in amphioxus. In the echinoderm lineage, sea urchin LvG-cadherin lacks an EC that corresponds to EC2 of starfish Ap-cadherin (Oda et al. 2005). In the hemichordate lineage, which is considered to be a sister lineage to the echinoderms, Ptychodera flava Pf1-cadherin has a reduced number of ECs (eight) and a small membrane-proximal deletion (~ 40 amino acid residues) in its extracellular region (Oda et al. 2002). The validity of the “lineage-specific domain loss” model needs to be tested by collecting more extensive information about the structures of classical cadherin genes from various species. Such effort will also contribute to a better general understanding of the deep phylogenies of animal lineages.
The genomes of cnidarian and placozoan (non-bilaterian eumetazoan) species encode putative classical cadherins that resemble type III cadherins, although they all have many more ECs (Chapman et al. 2010; Hulpiau and Van Roy 2010). Surprisingly, there is a detectable collinear homology between the entire EC region of type III cadherins and the membrane-proximal EC region of the very large classical cadherins of Trichoplax and Nematostella, implying that size reduction by loss of membrane-distal ECs preceded the establishment of the type III cadherin (Hulpiau and Van Roy 2010). The length and domain composition of the non-bilaterian eumetazoan classical cadherins resemble those of Fat and Fat-like cadherins. The entire extracellular region of δ-protocadherin appears to be derived from the membrane-distal 7-EC region of Fat cadherin (Hulpiau and Van Roy 2010). Size reduction through domain loss is a common strategy in the structural diversification of the cadherin superfamily.
4.5 Functional Relevance of Structural Transitions at the Adherens Junction
A remarkable feature of classical cadherin diversification is that structurally reduced-derived forms of classical cadherin tend to be used in the epithelial adherens junctions in “derived” animal lineages (Fig. 2.4f). Assuming that the embryonic surface epithelia are homologous across the eumetazoans, adherens junctions in this tissue type must have undergone distinct transitions in their extracellular architecture in the respective derived lineages. It is yet to be resolved how the classical cadherins with their highly varied sizes are accommodated in the similar intercellular spaces (15–25 nm) of the adherens junctions of different metazoan species (Fig. 2.1). The large non-classical cadherins, PCDH15 and CDH23, form helical filaments bridging the large spaces between stereocilia (150–300 nm) (Kazmierczak et al. 2007; Elledge et al. 2010), a structural mechanism that appears to be advantageous for interciliary force transduction, but that would not be suitable for the large classical cadherins at the adherens junctions.
Particularly, in the vertebrate/urochordate lineage, the PCPS-LmG region was entirely lost in the cadherin responsible for epithelial adherens junction formation. An experimental study using DE-cadherin suggests that the EC7/PCPS-LmG region is not essential for type IV cadherins to exhibit strong cell–cell adhesion activity (Haruta et al. 2010). This study also showed that this region, which covers about half of the entire extracellular region of DE-cadherin , is unlikely to be the major factor that determines the intercellular space of the adherens junction. Interestingly, early Drosophila embryos expressing DE-cadherin that lack the EC7/PCPS-LmG region form the normal blastoderm epithelium but exhibit defects in the apical constriction of presumptive mesodermal cells. An important implication of this work is that an abrupt loss of all non-EC domains in the extracellular region of a functional cadherin at the non-chordate adherens junction can occur without disrupting the ability of the animal to form epithelia, although this domain loss may affect morphogenetic processes.
5 Ancestry of the Cadherin–Catenin Complex
5.1 Poriferans
Poriferans (sea sponges) are the key phylum for exploring the evolutionary origins of intercellular junctions. This phylum comprises four lineages, the Calcispongiae, Demospongiae, Hexactinellida and Homoscleromorpha. Of these four lineages, the Homoscleromorpha lineage is the only lineage in which intercellular junctions resembling the bilaterian adherens junctions have been observed (Ereskovsky et al. 2009). However, molecular information is scarce for the Homoscleromorpha at present. On the other hand, the genome of Amphimedon queenslandica, a species of the Demospongiae, has been completely sequenced, revealing the presence of a classical cadherin-like gene, AmqCadherin1, and β-, α-, and p120-catenin gene homologs (Fig. 2.5a; Sakarya et al. 2007; Abedin and King 2008; Fahey and Degnan 2010). AmqCadherin1 encodes a single-pass transmembrane protein with 14 ECs followed by 13 EGFs and 2 LmGs (Fig. 2.5b). However, sequence similarities between the cytoplasmic domains of AmqCadherin1 and classical cadherins are limited. It has not yet been tested whether AmqCadherin1 physically interacts with the catenin homologs, and it is curious why the extracellular domain structure of AmqCadherin1 is dissimilar from those of the non-bilaterian eumetazoan classical cadherins.
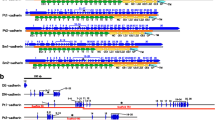
Cadherins and catenins in primitive metazoans and non-metazoans. a The presence/absence of cadherin, catenin and vinculin genes in the sequenced genomes as reported by Abedin and King 2008; Fahey and Degnan 2010 and Dickinson et al. 2011. b The domain structures of AmqCadherin1, a classical cadherin-like protein from A. queenslandica, and MBCDH21, a cadherin with EGF and LmG in M. breviollis. c A comparison of the domain structures of β-catenin in the mouse and a β-catenin homolog in D. discoideum, Aardvark (adapted from Dickinson et al. 2011). d A comparison of the domain structures of αE-catenin and vinculin in the mouse and an α-catenin homolog in D. discoideum. (adapted from Dickinson et al. 2011)
5.2 Choanoflagellates
Choanoflagellates are the only non-metazoan organisms in which the presence of ECs has been described (Fig. 2.5a). The genome of the choanoflagellate Monosiga brevicollis, a unicellular animal close to the metazoans, contains up to 23 cadherin genes (Abedin and King 2008). However, there are no sequences related to the classical cadherin cytoplasmic domain in the Monosiga brevicollis genome. In accordance with this finding, the Monosiga genome has no β-catenin gene homolog, although an α-catenin gene homolog is present (Fig. 2.5a; Dickinson et al. 2011). MBCDH21 is the only choanoflagellate gene that represents the combination of EC, EGF and LmG (Fig. 2.5a, b); however, this cadherin is highly diverged from any metazoan cadherins that contain EGF and LmG. MBCDH21 has 45 ECs that are preceded by an LmG and EGFs in the extracellular region, and a protein tyrosine phosphatase domain in the cytoplasmic region. No significant precursor genes for metazoan classical cadherins have been discovered outside of the metazoans.
5.3 Slime Molds
Although β-catenin-binding sequence motifs characteristic of classical cadherin cytoplasmic domains have been only found within the metazoans, genes related to β-catenin, as well as those related to α-catenin, show a wider phylogenetic distribution (Fig. 2.5a; Coates 2003; Dickinson et al. 2011). Studies of the cellular slime mold Dictyostelium discoideum have provided insights into the evolutionary origins of adherens junctions. This organism grows as a unicellular amoeba, and when starved, D. discoideum develops into a multicellular structure termed the fruiting body. Tip cells surrounding the stalk of the fruiting body organize into a simple epithelium (Grimson et al. 2000; Dickinson et al. 2011). Between these tip cells, actin-enriched intercellular junctions resembling the metazoan adherens junctions have been observed by electron microscopy (Grimson et al. 2000). D. discoideum has a α β-catenin homolog, referred to as Aardvark, which has fewer ArmRs and a truncated C-terminus compared to the metazoan β-catenins (Fig. 2.5c; Grimson et al. 2000). Importantly, however, it retains an α-catenin-binding sequence motif and is localized at the epithelial cell junctions. Consistent with these facts, an α-catenin homolog, referred to as Dd α-catenin, exists in D. discoideum (Fig. 2.5d; Dickinson et al. 2011). Although α-catenins and vinculins form a molecular family, sequences specific to vinculins have only been found within the metazoans. The ancestral form for this family appears to be α-catenin-like.
Ddα-catenin binds to Aardvark and mouse β-catenin in vitro and localizes to regions of cell–cell contact in an Aardvark-dependent manner in vivo (Dickinson et al. 2011). Purified Dd α-catenin, unlike mammalian αE-catenin, does not form homodimers but it is capable of bundling F-actin. Both Dd α-catenin and Aardvark are required to organize and polarize the tip epithelium. However, they are not essential for the formation of the D. discoideum tip cell junctions, and Dd α-catenin is not concentrated at the junctional regions. It is still unclear whether the ArmR domain of Aardvark interacts with an adhesion molecule. Commonalities and differences in the molecular compositions and interactions for metazoan adherens junctions and the D. discoideum cell junctions require further investigation.
6 Summary and Future Perspectives
Functional interactions between β-catenin, α-catenin and the actin cytoskeleton predate the origin of cadherin. Because of the early diversification of the cadherin superfamily, a cadherin containing EGFs and LmGs achieved the ability to interact with β-catenin and p120/δ-catenin. This origination of cadherin–catenin interactions was followed by diversification of the extracellular domain organization of classical cadherins. Classical cadherins prior to the origin of bilaterians must have been very large in size, like the current Fat and Fat-like cadherins. Comparative studies suggest that step-by-step size reductions through lineage-specific domain losses resulted in variations in the forms of classical cadherins among metazoans, implying that the extracellular architecture of adherens junctions in the epithelia underwent distinct alterations depending on the lineage. For example, in the vertebrate/urochordate lineage, the 5-EC domain organization for classical cadherin was established, and this was followed by a further diversification of classical and non-classical cadherins and an increase in the repertoire of the catenins. This relatively recent diversification of the cadherin–catenin system and its derivatives may have contributed to vertebrate-specific morphological complications.
Many questions regarding the evolution of cadherins and catenins are emerging and remain to be answered. Unicellular lineages exist between the metazoans and slime molds, but it is difficult to reconstruct evolutionary transitions between unicellular and multicellular life; non-metazoan β- and α-catenins are rare clues to this issue. The phylogenetic distribution of classical cadherins appears to be restricted to metazoans, whereas cadherins predate the last common ancestor of metazoans and non-metazoan choanoflagellates. Biochemical and cell biological studies of non-bilaterian metazoans, poriferans in particular, are increasingly important for exploring the origin of cadherin-based intercellular junctions. One exciting goal of these studies is to determine the functions of ancient cadherins prior to their being co-opted for junction formation. After being co-opted for junction formation, the cadherin–catenin complex, the extracellular structure of classical cadherin in particular, experienced distinct changes in different metazoan lineages. Did such changes contribute to innovations of morphogenetic mechanisms in the respective lineages? More specifically, what happened to the junctional systems in the earliest chordates? It remains unclear whether the unique conditions of the classical cadherin forms and junction organization in the amphioxus represent the ancestral state for all extant chordates. Analyses of the functional and mechanistic aspects of structural and compositional transitions at the adherens junctions is required to determine the relationships between the diversification of the junctional cadherin–catenin complex and the morphological diversification in animals.
References
Abedin M, King N (2008) The premetazoan ancestry of cadherins. Science 319:946–948. doi:10.1126/science1151084
Aberle H, Schwartz H, Hoschuetzky H, Kemler R (1996) Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J Biol Chem 271:1520–1526. doi:10.1074/jbc.271.3.1520
Biswas S, Emond MR, Jontes JD (2010) Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. J Cell Biol 191:1029–1041. doi:10.1083/jcb.201007008
Boller K, Vestweber D, Kemler R (1985) Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol 100:327–332
Brackenbury R, Rutishauser U, Edelman GM (1981) Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A 78:387–391
Brembeck FH, Rosário M, Birchmeier W (2006) Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr Opin Genet Dev 16:51–59. doi:10.1016/j.gde.2005.12.007
Broadbent ID, Pettitt J (2002) The C. elegans hmr-1 gene can encode a neuronal classic cadherin involved in the regulation of axon fasciculation. Curr Biol 12:59–63. doi:10.1016/S0960-9822(01)00624-8
Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, Fujita Y, Wilson SW, Tada M (2008) Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development 136:383–392. doi:10.1242/dev.026542
Castillejo-López C, Arias WM, Baumgartner S (2004) The fat-like gene of Drosophila is the true orthologue of vertebrate Fat cadherins and is involved in the formation of tubular organs. J Biol Chem 279:24034–24043. doi:10.1074/jbc.M313878200
Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T, Rattei T, Balasubramanian PG, Borman J, Busam D, Disbennett K, Pfannkoch C, Sumin N, Sutton GG, Viswanathan LD, Walenz B, Goodstein DM, Hellsten U, Kawashima T, Prochnik SE, Putnam NH, Shu S, Blumberg B, Dana CE, Gee L, Kibler DF, Law L, Lindgens D, Martinez DE, Peng J, Wigge PA, Bertulat B, Guder C, Nakamura Y, Ozbek S, Watanabe H, Khalturin K, Hemmrich G, Franke A, Augustin R, Fraune S, Hayakawa E, Hayakawa S, Hirose M, Hwang JS, Ikeo K, Nishimiya-Fujisawa C, Ogura A, Takahashi T, Steinmetz PR, Zhang X, Aufschnaiter R, Eder MK, Gorny AK, Salvenmoser W, Heimberg AM, Wheeler BM, Peterson KJ, Böttger A, Tischler P, Wolf A, Gojobori T, Remington KA, Strausberg RL, Venter JC, Technau U, Hobmayer B, Bosch TC, Holstein TW, Fujisawa T, Bode HR, David CN, Rokhsar DS, Steele RE (2010) The dynamic genome of Hydra. Nature 464:592–596. doi:10.1038/nature08830
Chen X, Gumbiner BM (2006) Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol 174:301–313. doi:10.1083/jcb.200602062
Choi H J, Gross JC, Pokutta S, Weis WI (2009) Interactions of plakoglobin and β-catenin with desmosomal cadherins: basis of selective exclusion of α- and β-catenin from desmosomes. J Biol Chem 284:31776–31788. doi:10.1074/jbc.M109.047928
Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M (1995) Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev 9:1530–1542. doi:10.1101/gad.9.12.1530
Coates JC (2003) Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol 13:463–471. doi:10.1016/S0962-8924(03)00167-3
Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR (1998) A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol 141:297–308. doi:10.1083/jcb.141.1.297
Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN (2003) Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13:1129–1133. doi:10.1016/S0960-9822(03)00374-9
D’Alterio C, Tran DD, Yeung MW, Hwang MS, Li MA, Arana CJ, Mulligan VK, Kubesh M, Sharma P, Chase M, Tepass U, Godt D (2005) Drosophila melanogaster Cad99C, the orthologue of human Usher cadherin PCDH15, regulates the length of microvilli. J Cell Biol 171:549–558. doi:10.1083/jcb.200507072
Dan-Sohkawa MDS, Kaneko HK, Noda KN (1995) Paracellular, transepithelial permeation of macromolecules in the body wall epithelium of starfish embryos. J Exp Zool 271:264–272. doi:10.1002/jez.1402710404
Daniel JM, Reynolds AB (1995) The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or α-catenin. Mol Cell Biol 15:4819–4824
Davis MA, Reynolds AB (2006) Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev. Cell 10:21–31. doi:10.1016/j.devcel.2005.12.004
Davis MA, Ireton RC, Reynolds AB (2003) A core function for p120-catenin in cadherin turnover. J Cell Biol 163:525–534. doi:10.1083/jcb.200307111
Dickinson DJ, Nelson WJ, Weis WI (2011) A polarized epithelium organized by b- and α-catenin predates cadherin and metazoan origins. Science 331:1336–1339. doi:10.1126/science.1199633
Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI (2005) α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123:903–915. doi:10.1016/j.cell.2005.09.021
Eisenmann DM (2005) Wnt signaling. WormBook 25:1–17
Elledge HM, Kazmierczak P, Clark P, Joseph JS, Kolatkar A, Kuhn P, Müller U (2010) Structure of the N terminus of cadherin 23 reveals a new adhesion mechanism for a subset of cadherin superfamily members. Proc Natl Acad Sci U S A 107:10708–10712. doi:10.1073/pnas.1006284107
Ereskovsky AV, Borchiellini C, Gazave E, Ivanisevic J, Lapébie P, Perez T, Renard E, Vacelet J (2009) The Homoscleromorph sponge Oscarella lobularis, a promising sponge model in evolutionary and developmental biology: model sponge Oscarella lobularis. Bioessays 31:89–97. doi:10.1002/bies.080058
Fahey B, Degnan BM (2010) Origin of animal epithelia: insights from the sponge genome. Evol Dev 12:601–617. doi:10.1111/j.1525-142X.2010.00445.x
Fang X, Ji H, Kim SW, Park JI, Vaught TG, Anastasiadis PZ, Ciesiolka M, McCrea PD (2004) Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J Cell Biol 165:87–98. doi:10.1083/jcb.200307109
Formstone CJ, Mason I (2005) Combinatorial activity of Flamingo proteins directs convergence and extension within the early zebrafish embryo via the planar cell polarity pathway. Dev Biol 282:320–335. doi:10.1016/j.ydbio.2005.03.026
Fung S, Wang F, Chase M, Godt D, Hartenstein V (2008) Expression profile of the cadherin family in the developing Drosophila brain. J Comp Neurol 506:469–488. doi:10.1002/cne.21539
Gallin WJ, Sorkin BC, Edelman GM, Cunningham BA (1987) Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A 84:2808–2812
Grana TM, Cox EA, Lynch AM, Hardin J (2010) SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during C. elegans gastrulation. Dev Biol 344:731–744. doi:10.1016/j.ydbio.2010.05.507
Greenwood MD, Marsden MD, Cowley CM, Sahota VK, Buxton RS (1997) Exon-intron organization of the human type 2 desmocollin gene (DSC2): desmocollin gene structure is closer to “classical” cadherins than to desmogleins. Genomics 44:330–335. doi:10.1006/geno.1997.4894
Grimson MJ, Coates JC, Reynolds JP, Shipman M, Blanton RL, Harwood AJ (2000) Adherens junctions and β-catenin-mediated cell signalling in a non-metazoan organism. Nature 408:727–731 doi:10.1038/35047099
Haruta T, Warrior R, Yonemura S, Oda H. (2010) The proximal half of the Drosophila E-cadherin extracellular region is dispensable for many cadherin-dependent events but required for ventral furrow formation. Genes Cells 15:193–208. doi:10.1111/j.1365-2443.2010.01389.x
Hatta K, Takeichi M (1986) Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320:447–449. doi:10.1038/320447a0
Hatzfeld M, Nachtsheim C (1996) Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: evidence for a subfamily of closely related proteins. J Cell Sci 109:2767–2778
Heimark RL, Degner M, Schwartz SM (1990) Identification of a Ca2+-dependent cell-cell adhesion molecule in endothelial cells. J Cell Biol 110:1745–1756
Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R (1991) The uvomorulin-anchorage protein α catenin is a vinculin homologue. Proc Natl Acad Sci U S A 88:9156–9160
Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M (1992) Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell 70:293–301. doi:10.1016/0092-8674(92)90103-J
Hsu SN, Yonekura S, Ting CY, Robertson HM, Iwai Y, Uemura T, Lee CH, Chiba A (2009) Conserved alternative splicing and expression patterns of arthropod N-cadherin. PLoS Genet 5(4):e1000441. doi:10.1371/journal.pgen.1000441
Huber AH, Weis WI (2001) The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 105:391–402. doi:10.1016/S0092-8674(01)00330-0
Huber O, Krohn M, Kemler R (1997) A specific domain in α-catenin mediates binding to β-catenin or plakoglobin. J Cell Sci 110:1759–1765
Hulpiau P, van Roy F (2009) Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol 41:349–369. doi:10.1016/j.biocel.2008.09.027
Hulpiau P, van Roy F (2010) New Insights into the Evolution of Metazoan Cadherins. Mol Biol Evol 28:647–657. doi:10.1093/molbev/msq233
Hyafil F, Babinet C, Jacob F (1981) Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell 26:447–454. doi:10.1016/0092-8674(81)90214-2
Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB (2002) A novel role for p120 catenin in E-cadherin function. J Cell Biol 159:465–476. doi:10.1083/jcb.200205115
Ishiuchi T, Misaki K, Yonemura S, Takeichi M, Tanoue T (2009) Mammalian Fat and Dachsous cadherins regulate apical membrane organization in the embryonic cerebral cortex. J Cell Biol 185:959–967. doi:10.1083/jcb.200811030
Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T (1997) Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron 19:77–89. doi:10.1016/S0896-6273(00)80349-9
Kanno M, Isa Y, Aoyama Y, Yamamoto Y, Nagai M, Ozawa M, Kitajima Y (2008) p120-catenin is a novel desmoglein 3 interacting partner: identification of the p120-catenin association site of desmoglein 3. Exp Cell Res 314:1683–1692. doi:10.1016/j.yexcr.2008.01.031
Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449:87–91. doi:10.1038/nature06091
Kemler R, Babinet C, Eisen H, Jacob F (1977) Surface antigen in early differentiation. Proc Natl Acad Sci U S A 74:4449–4452
Knust E, Bossinger O (2002) Composition and formation of intercellular junctions in epithelial cells. Science 298:1955–1959. doi:10.1126/science.1072161
Kobielak A, Fuchs E (2004) Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol 5:614–625. doi:10.1038/nrm1433
Koslov ER, Maupin P, Pradhan D, Morrow JS, Rimm DL (1997) α-catenin can form asymmetric homodimeric complexes and/or heterodimeric complexes with β-catenin. J Biol Chem 272:27301–27306. doi:10.1074/jbc.272.43.27301
Kremmidiotis G, Baker E, Crawford J, Eyre HJ, Nahmias J, Callen DF (1998) Localization of human cadherin genes to chromosome regions exhibiting cancer-related loss of heterozygosity. Genomics 49:467–471. doi:10.1006/geno.1998.5281
Kwiatkowski AV, Maiden SL, Pokutta S, Choi HJ, Benjamin JM, Lynch AM, Nelson WJ, Weis WI, Hardin J (2010) In vitro and in vivo reconstitution of the cadherin-catenin-actin complex from Caenorhabditis elegans. Proc Natl Acad Sci U S A 107:14591–14596. doi:10.1073/pnas.1007349107
Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E (1997) Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci 110:2065–2077
Lane NJ, Dallai R, Martinucci GB, Burighel P (1987) Cell junctions in amphioxus (Cephalochordata): a thin section and freeze-fracture study. Tissue Cell 19:399–411. doi:10.1016/0040-8166(87)90035-8
Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD (2003) Fidelity in planar cell polarity signalling. Nature 421:543–547. doi:10.1038/nature01366
Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS (1991) The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 67:853–868. doi:10.1016/0092-8674(91)90359-7
Mariner DJ, Wang J, Reynolds AB (2000) ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120ctn in E-cadherin complexes. J Cell Sci 113:1481–1490
Matakatsu H, Blair SS (2004) Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development 131:3785–3794. doi:10.1242/dev.01254
McCrea PD, Turck CW, Gumbiner B (1991) A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1359–1361. doi:10.1126/science.1962194
Morishita H, Yagi T (2007) Protocadherin family: diversity, structure, and function. Curr Opin Cell Biol 19:584–592. doi:10.1016/j.ceb.2007.09.006
Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M (2003) Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol 160:433–449. doi:10.1083/jcb.200211083
Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M (1987) Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 329:341–343
Nagafuchi A, Takeichi M, Tsukita S (1991) The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell 65:849–857. doi:10.1016/0092-8674(91)90392-C
Nagar B, Overduin M, Ikura M, Rini JM (1996) Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380:360–364. doi:10.1038/380360a0
Nakao S, Platek A, Hirano S, Takeichi M (2008) Contact-dependent promotion of cell migration by the OL-protocadherin-Nap1 interaction. J Cell Biol 182:395–410. doi:10.1083/jcb.200802069
Navarro P, Ruco L, Dejana E (1998) Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140:1475–1484. doi:10.1083/jcb.140.6.1475
Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ (1997) Characterization of the interactions of α-catenin with a-actinin and β-catenin/plakoglobin. J Cell Sci 110:1013–1022
Nollet F, Kools P, van Roy F (2000) Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol 299:551–572. doi:10.1006/jmbi.2000.3777
Nose A, Nagafuchi A, Takeichi M (1988) Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54:993–1001. doi:10.1016/0092-8674(88)90114-6
Nose A, Tsuji K, Takeichi M (1990) Localization of specificity determining sites in cadherin cell adhesion molecules. Cell 61:147–155. doi:10.1016/0092-8674(90)90222-Z
Obama H, Ozawa M (1997) Identification of the domain of α-catenin involved in its association with β-catenin and plakoglobin (γ-catenin). J Biol Chem 272:11017–11020
Oda H, Takeichi M (2011) Evolution: Structural and functional diversity of cadherin at the adherens junction. J Cell Biol 193:1137–1146. doi:10.1083/jcb.201008173
Oda H, Tsukita S (1999) Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev Biol 216:406–422. doi:10.1006/dbio.1999.9494
Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M (1994) A Drosophila homolog of cadherin associated with Armadillo and essential for embryonic cell-cell adhesion. Dev. Biol 165:716–726. doi:10.1006/dbio.1994.1287
Oda H, Wada H, Tagawa K, Akiyama-Oda Y, Satoh N, Humphreys T, Zhang S, Tsukita S (2002) A novel amphioxus cadherin that localizes to epithelial adherens junctions has an unusual domain organization with implications for chordate phylogeny. Evol Dev 4:426–434. doi:10.1046/j.1525-142X.2002.02031.x
Oda H, Akiyama-Oda Y, Zhang S (2004) Two classic cadherin-related molecules with no cadherin extracellular repeats in the cephalochordate amphioxus: distinct adhesive specificities and possible involvement in the development of multicell-layered structures. J Cell Sci 117:2757–2767. doi:10.1242/jcs.01045
Oda H, Tagawa K, Akiyama-Oda Y (2005) Diversification of epithelial adherens junctions with independent reductive changes in cadherin form: identification of potential molecular synapomorphies among bilaterians. Evol Dev 7:376–389. doi:10.1111/j.1525-142X.2005.05043.x
Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M (1995) Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science 267:386–389. doi:10.1126/science.7824937
Ozawa M, Kemler R (1990) Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol 111:1645–1650
Ozawa M, Engel J, Kemler R (1990) Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function. Cell 63:1033–1038. doi:10.1016/0092-8674(90)90506-A
Pacquelet A, Lin L, Rorth P (2003) Binding site for p120/δ-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol 160:313–319. doi:10.1083/jcb.200207160
Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L (2006) Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124:1255–1268. doi:10.1016/j.cell.2005.12.046
Paulson AF, Mooney E, Fang X, Ji H, McCrea PD (2000) Xarvcf, Xenopus member of the p120 catenin subfamily associating with cadherin juxtamembrane region. J Biol Chem 275:30124–30131. doi:10.1074/jbc.M003048200
Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287:1606–1609. doi:10.1126/science.287.5458.1606
Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM (1992) The vertebrate adhesive junction β-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol 118:681–691
Pettitt J (2005) The cadherin superfamily. WormBook 29:1–9
Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J (2003) The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol 162:15–22. doi:10.1083/jcb.200212136
Pokutta S, Weis WI (2000) Structure of the dimerization and β-catenin-binding region of α-catenin. Mol Cell 5:533–543. doi:10.1016/S1097-2765(00)80447-5
Prakash S, Caldwell JC, Eberl DF, Clandinin TR (2005) Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci 8:443–450. doi:10.1038/nn1415
Reddy B V, Irvine KD (2008) The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development 135:2827–2838. doi:10.1242/dev.020974
Reynolds AB, Roesel DJ, Kanner SB, Parsons JT (1989) Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol 9:629–638
Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z (1994) Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14:8333–8342
Riggleman B, Wieschaus E, Schedl P (1989) Molecular analysis of the armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev 3:96–113. doi:10.1101/gad.3.1.96
Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS (1995) α 1(E)-catenin is an actin-binding and—bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A 92:8813–8817
Ringwald M, Schuh R, Vestweber D, Eistetter H, Lottspeich F, Engel J, Dölz R, Jähnig F, Epplen J, Mayer S (1987) The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J 6:3647–3653
Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H (2008) Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40:1010–1015. doi:10.1016/j.ceb.2005.08.011
Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, Degnan BM, Oakley TH, Kosik KS (2007) A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE 2(6):e506. doi:10.1371/journal.pone.0000506
Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B (1992) Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci 102:7–17
Sasakura Y, Shoguchi E, Takatori N, Wada S, Meinertzhagen IA, Satou Y, Satoh N (2003) A genomewide survey of developmentally relevant genes in Ciona intestinalis. X. Genes for cell junctions and extracellular matrix. Dev Genes Evol 213:303–313. doi:1007/s00427-003-0320-1
Shirayoshi Y, Hatta K, Hosoda M, Tsunasawa S, Sakiyama F, Takeichi M (1986) Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J 5:2485–2488
Takeichi M (1988) The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102:639–655
Takeichi M (2007) The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci 8:11–20. doi:10.1038/nrn2043
Tanabe K, Takeichi M, Nakagawa S (2004) Identification of a nonchordate-type classic cadherin in vertebrates: chicken Hz-cadherin is expressed in horizontal cells of the neural retina and contains a nonchordate-specific domain complex. Dev Dyn 229:899–906. doi:10.1002/dvdy.10493
Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Török T, Hartenstein V (1996) shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev 10:672–685. doi:10.1101/gad.10.6.672
Tolwinski NS, Wieschaus E (2004) Rethinking WNT signaling. Trends Genet 20:177–181. doi:10.1016/j.tig.2004.02.003
Troyanovsky SM, Troyanovsky RB, Eshkind LG, Krutovskikh VA, Leube RE, Franke WW (1994) Identification of the plakoglobin-binding domain in desmoglein and its role in plaque assembly and intermediate filament anchorage. J Cell Biol 127:151–160
Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M (1996) Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev 10:659–671. doi:10.1101/gad.10.6.659
Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T (1999) Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98:585–595. doi:10.1016/S0092-8674(00)80046-X
Vestal DJ, Ranscht B (1992) Glycosyl phosphatidylinositol—anchored T-cadherin mediates calcium-dependent, homophilic cell adhesion. J Cell Biol 119:451–461
Volk T, Geiger B (1984) A 135-kd membrane protein of intercellular adherens junctions. EMBO J 3:2249–2260
Watson GM, Pham L, Graugnard EM, Mire P (2008) Cadherin 23-like polypeptide in hair bundle mechanoreceptors of sea anemones. J Comp. Physiol. A Neuroethol. Sens. Neural Behav Physiol 194:811–820. doi:10.1007/s00359-008-0352-0
Wendeler MW, Jung R, Himmelbauer H, Gessner R (2006) Unique gene structure and paralogy define the 7D-cadherin family. Cell Mol Life Sci 63:1564–1573. doi:10.1007/s00018-006-6014-x
Whittaker CA, Bergeron KF, Whittle J, Brandhorst BP, Burke RD, Hynes RO (2006) The echinoderm adhesome. Dev Biol 300:252–266. doi:10.1016/j.ydbio.2006.07.044
Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP (2003) Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 163:535–545. doi:10.1083/jcb.200306001
Yap AS, Niessen CM, Gumbiner BM (1998) The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol 141:779–789. doi:10.1083/jcb.141.3.779
Yonekura S, Xu L, Ting CY, Lee CH (2007) Adhesive but not signaling activity of Drosophila N-cadherin is essential for target selection of photoreceptor afferents. Dev Biol 304:759–770. doi:10.1016/j.ydbio.2007.01.030
Yoshida C, Takeichi M (1982) Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell 28:217–224. doi:10.1016/0092-8674(82)90339-7
Yoshida-Noro C, Suzuki N, Takeichi M (1984) Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol 101:19–27. doi:10.1016/0012-1606(84)90112-X
Acknowledgments
I would like to thank Shicui Zhang for having supported my access to amphioxus embryos; Tomohiro Haruta for his permission to use unpublished electron micrographs; and Yasuko Akiyama-Oda for her comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Hiroki, O. (2012). Evolution of the Cadherin–Catenin Complex. In: Harris, T. (eds) Adherens Junctions: from Molecular Mechanisms to Tissue Development and Disease. Subcellular Biochemistry, vol 60. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4186-7_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-4186-7_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4185-0
Online ISBN: 978-94-007-4186-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)