Abstract
Proteinase activated receptors (PARs), a small subfamily of G protein-coupled receptors with four members, PAR1, PAR2, PAR3 and PAR4, are expressed in various tumours from epithelial origin and can play an important role in tumour progression and metastasis. Within the complex intracellular PAR signaling networks triggered by PARs, an elevation in intracellular free calcium ion concentrations represents a key second messenger system. In this review, we summarize current information about the mechanisms whereby PARs can signal via intracellular calcium in the setting of cancer and we discuss possibilities for using the PAR-[Ca2+]i signaling pathway as a target for the therapy of epithelial cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Proteinase activated receptors
- PARs
- Thrombin receptor
- PAR1
- PAR2
- PAR3
- PAR4
- Signal transduction
- Calcium signaling
- Intracellular free calcium ion
- Carcinogenesis
- Cancer progression
Proteinase Activated Receptors: A Specialized Subfamily of G Protein Coupled Receptors with Complex Intracellular Signal Transduction Pathways
Proteinase activated receptors (PARs) comprise a unique subfamily of G protein-coupled receptors (GPCRs) with four subtypes, PAR1, PAR2, PAR3 and PAR4 [for reviews see: [1–4]]. Although PARs share basic structural features of all GPCRs, including seven putative hydrophobic transmembrane-spanning alpha helices, they exhibit a novel mechanism of activation that distinguishes them from all other GPCRs. While most GPCRs are activated reversibly by small hydrophilic molecules to elicit cellular responses [5], PAR activation occurs through an irreversible proteolytic mechanism that involves the recognition and cleavage of the receptor by a proteinase at a specific ‘cleavage-activation’ site located at the extracellular amino-terminus (Fig. 45.1).
Model for activation and dis-arming of PAR 2 . The scheme illustrates activation of the intact receptor (left-hand panel) by two distinct mechanisms: either (I) by proteolysis and unmasking of the tethered ligand sequence (middle Panel: green sequence, SLIGRL, also seen in the intact receptor) or (II) by a receptor-derived peptide (SLIGRL-NH2: right-hand panel) that activates signaling without the need for receptor proteolysis. The scheme also shows the ‘disarming’ site for the receptor, where cleavage removes the tethered ligand sequence and the ‘cleavage-activation’ site’, where a ‘blocking antibody’ can prevent proteolytic activation of the receptor (Redrawn from Hollenberg and Compton, Ref. [6])
This cleavage exposes a cryptic N-terminal domain that acts as a ’tethered ligand’ that binds to the receptor extracellular domains to trigger receptor signalling [3, 6, 7].
This cleavage exposes a cryptic N-terminal domain that acts as a ’tethered ligand’ that binds to the receptor extracellular domains to trigger receptor signaling [3, 6–8]. Remarkably, short synthetic peptides modelled on the sequences of the proteolytically-exposed tethered ligand sequences are capable of binding to PARs 1, 2 and 4, mimicking the actions of agonist proteinases [right-hand portion, Fig. 45.1; [9, 10]]. However, the proteolytically exposed N-terminal sequence of PAR3 and its corresponding synthetic peptides appear to be incapable of causing PAR3 signaling and instead are able to activate PAR1 and PAR2 [11, 12]. As an alternative, a proteinase may cleave a PAR downstream of the ‘tethered ligand sequence’ (e.g. red arrow, left-hand portion, Fig. 45.1), to as to ‘dis-arm’ and prevent activation of the PAR by an enzyme that would otherwise expose the tethered ligand. Thus, PARs have a variety of both endogenous ‘tethered ligand-exposing’ proteinase agonists as well as a number of endogenous proteolytic ‘antagonists’ that can ‘silence’ receptor activation by other proteinases. Therefore, in the setting of a tumour, both tumour-derived and non-tumour-derived proteinases in the microenvironment can play roles as either PAR agonists or antagonists.
During the last few years it has become evident that PARs, which are triggered by endogenous serine proteinases, mediate hormone-like cellular responses. PAR1 [9, 13], PAR3 [14] and PAR4 [15, 16] are targeted not only by the coagulation cascade proteinases including thrombin, factor Xa and activated protein C, but also by other proteinases including cathepsin and matrix metalloproteinase-I [17–19]. PAR2 [20] can be activated by trypsin, mast cell tryptase, neutrophil proteinase 3, tissue factor/factor VIIa/factor Xa, human kallikrein-related peptidases, membrane-tethered serine proteinase-1/matriptase 1 and by parasite cysteine proteinases, but not by thrombin [2, 3, 21, 22].
The PAR family is able to stimulate a variety of intracellular signaling pathways which can be either overlapping or distinct for the different PARs, depending on the PAR subtype and the phenotype or stage of differentiation of its specific cellular ‘host’ [e.g. platelets vs. hepatocytes: for reviews see: [4, 22–28]]. Like other ‘GPCRs’, the PARs signal via a variety of heterotrimeric guanyl nucleotide-binding proteins (G proteins), including Gq, Gi, G12/13 but not directly via Gs [7, 29]. In addition, PAR2 and possibly the other PARs are able to signal via a non-G-protein mechanism that involves the beta-arrestin-mediated internalization of a PAR2-beta-arrestin signaling scaffold [30–35]. The coupling of the PARs to either the G-proteins or arrestins is driven by ligand-triggered changes of receptor conformation that for other GPCRs is thought to involve the putative transmembrane helices 3 and 6 of the receptor [36, 37]. Of importance, different agonists are in principle capable of driving different conformational changes in the receptor to result in selective interactions with different downstream ‘effectors’. This principle was outlined by the ‘floating’ or ‘mobile’ receptor paradigm some time ago [38, 39]. More recently, the concept has evolved to encompass the concept of ‘biased receptor signaling’ or ‘functional selectivity’ as outlined in detail elsewhere [40]. For G-protein-mediated signaling, the receptor acts as a ligand-triggered guanine nucleotide exchange factor (GEF), stimulating the exchange of GTP for GDP in the Gα subunit of the heterotrimeric G-protein oligomer. This exchange enables the ‘release’ of the Gα subunit from its tight binding to the Gβγ dimer subunit. Each of the G-protein moieties (Gα-GTP and Gβγ) is then independently able to interact with other select downstream signaling effectors like ion channels (Gβγ) or phospholipase C-β (Gq). This ‘dual effector’ signaling resulting in principle from the same PAR-activated G-protein heterotrimer (e.g. Gq Gβγ) can converge for complex downstream signaling, for instance leading to NF-κB activation and ICAM-1 transcription by the engagement of parallel Gq/PKC- and Gi/PI3-kinase pathways that converge [41, 42]. Alternatively, via a ‘biased signaling’ process, PARs can be activated to affect selectively, MAPKinase signaling via a G12/13-triggered process, without causing a Gq-mediated calcium signaling event [31]. This kind of selective signaling can depend not only on the agonist per se [e.g. thrombin or activated protein-C (APC)] but also upon the membrane environment in which a PAR is localized. For instance, triggering of PAR1 localized in the caveolae by APC can signal via a distinct set of downstream effectors that differ from those regulated when thrombin activates PAR1 in a non-caveolar environment [29]. The PAR1 signal pathways activated in these distinct membrane environments lead to a diametrically opposed set of responses that either increase or decrease endothelial barrier integrity. Thus in principle, it is possible to activate and/or inhibit selectively one or other of the downstream signaling pathways activated by PARs (e.g. calcium vs. MAPKinase signals).
PARs Are Involved in Cancer Progression
Local and systemic coagulation is a hallmark of cancer [review: [43]]. In this complicated scenario, tissue factor (TF) induces the formation of the complex TF-VIIa. Both the complex TF-VIIa-Xa and thrombin (factor IIa) can activate proteinase activated receptors. Thrombin can activate PAR1 and PAR4 [44], whereas the binary TF-VIIa enzyme complex is able to activate PAR2 but not PAR1 [45, 46]. However, as a TF-VIIa-Xa complex, factor Xa efficiently cleaves PAR2 as well as PAR1 [47, 48]. In addition, a variety of other proteinases may also be important in the tumour microenvironment, where both stromal and tumour-derived cells can produce PAR-regulating proteinases. Such enzymes can either, like tumour-derived tissue kallikreins [49–51], activate PAR2, or alternatively proteinases of tissue origin can ‘dis-arm’ a PAR, by cleaving downstream of the ‘tethered ligand’ domain (Fig. 45.1, left), thereby silencing a PAR from activation by its target proteinase (e.g. disarmed PAR1 can no longer respond to thrombin). Moreover tumour-derived proteinases like matrix metalloproteinase-I can cleave the N-terminal domain of a PAR to unmask a ‘non-canonical’ tethered activating sequence different from the one revealed by serine proteinases [19, 52]. The ability of thrombin to act via PARs was highlighted by the demonstration of the ability of PAR1 to stimulate tumour invasion [53, 54] by its expression in carcinosarcoma and melanoma cells [55]. The extensive work in this field related to tumour tissue done over the past decade has therefore focused primarily on PAR1 for which the expression and signaling at the cellular level have been characterized in tumour cells from different tumour entities including cancers of the larynx [56], pancreas [57], glioma [58, 59], glioblastoma [60, 61], meningioma [62], prostate [63] and colon [64]. In addition, PAR1 activation has been observed to cause (I) increased tumour cell adhesion to the endothelium, extracellular matrix and platelets, (II) enhanced metastatic capacity of tumour cells, (III) activated cell growth and (IV) increased angiogenesis [65–67]. In breast and pancreatic carcinoma cells, the level of PAR1 expression has been correlated with the degree of invasiveness [54, 68]. Furthermore, transfection of B16F10 melanoma cells with PAR1, compared with non-transfected cells, leads to a 2.5-fold enhanced thrombin-induced tumour cell adhesion to fibronectin and a 39-fold increase in pulmonary metastasis [69]. At present there is substantial evidence that thrombin acting via PAR1 contributes to the metastatic process of certain epithelial tumours including breast [53, 54, 70], colon [64], kidney [71] and liver [72]. However, PAR1 is not the only functional receptor for thrombin in tumour cells since several reports have demonstrated that PAR1 can cooperate with the other thrombin target, PAR4, to act as a ‘dual receptor system’ in human astrocytoma cells [73] and in cells from liver cancer [72]. In addition to PAR1, PAR2 is also known to be expressed in a variety of epithelial tumour cells from different origins [32, 74–82] and to act as an upstream activator of promigratory signaling pathways [34, 75, 80, 83] resulting in an enhancement of tumour progression.
Multiple Effects of PAR Activation on Cancer Cells
Studies dealing with a variety of tumour-related cells have observed important effects of PAR activation, several examples of which will be outlined in this paragraph. Seminal work from the Bar-Shavit laboratory has demonstrated the key role that PAR1 may exhibit in breast cancer cell invasion [53, 54, 70] and recently Gonda et al. provided impressive data showing movements of breast cancer cells and PAR1 during metastasis in vivo using a highly sophisticated nano-imaging technique [84]. In breast carcinoma cells PAR1 mediates both migratory and invasive effects [85]. These PAR1-mediated actions occur in cooperation with alpha-vbeta 5 integrin [53] and with the involvement of increases in intracellular calcium [86]. In 1321N1 astrocytoma cells, Blum and colleagues demonstrated that PAR1-stimulated ATP release is Ca2+-dependent and that concurrent Rho signaling markedly potentiates this effect [87]. In keratinocyte-related HaCaT cells, PAR2 activation by matriptase, a membrane-tethered serine proteinase released from the cell surface, has been shown to induce intracellular calcium mobilization and to inhibit proliferation. Based on this information, a role for PAR2 signaling in skin cancer has been suggested [82]. A substantial amount of data also exist pointing to a role for PARs in colon cancer. In cells from this tumour entity, PAR1 and PAR2 have been demonstrated to signal via [Ca2+]i and to induce migratory and proliferative effects that also involve both activation of p42/p44-MAPKinase and trans-activation of the receptor for epidermal growth factor (EGFR) [64, 74, 88, 89]. In addition, PAR4 has recently surfaced as a new important player in the regulation of colon tumour-derived cells. In colon carcinoma cells activation of PAR4 has been found to be involved in stimulating mitogenesis. This stimulation is observed to occur in the setting of PAR4-induced increases intracellular calcium and activation of p42/p44 MAPKinase along with trans-activation of ErbB-2, a member of the epidermal growth factor receptor B-2 receptor family, but not via trans-activation of the EGF-Erb-B1 homodimer receptor itself [90]. Since PAR4 does not mediate an increase in cytoplasmic free Ca2+ in hepatocellular carcinoma cells [72], but does so in colon carcinoma cells, the ability of PAR4 to stimulate increases in intracellular calcium appears to be dependent upon the cellular context in which the receptor is expressed. Thus, different tumours with their unique expression of GPCR-regulated effectors have the potential to respond to PAR activation in a unique way that may or may not depend on calcium signaling.
PARs Are Relevant in Different Cells from the Tumour Microenvironment
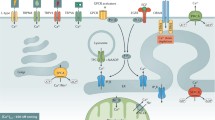
Relatively recently, oncologists have begun to focus on the tumour microenvironment as a major contributing factor to the development of cancer. Thus, in the setting of a tumour, both the resident non-tumour cells as well as the tumour cells can engage in signaling cross-talk (tumour cell to stromal cell and back) that changes the phenotype of the stromal cells and alters the growth and metastatic potential of the tumour cell [91–93]. This cross-talk communication between tumour and stromal cells is mediated by a variety of hormone-like regulators, including secreted growth factors and proteinases [94–96]. PAR expression and function in different cell types found in the stromal elements of the tumour microenvironment, including fibroblasts, inflammatory leukocytes, platelets, macrophages, endothelial cells and smooth muscle cells has been documented in other contexts [reviewed: [1–4]]. Thus, the potential function of PARs in these stromal bystander cells is directly relevant to the malignant process and is currently under close scrutiny [97–102]. For example, in the setting of hepatocellular carcinoma (HCC), one of the leading malignancies worldwide, recently published data suggest that activated stromal hepatic stellate cells (HSCs) in the tumour microenvironment may contribute to the promotion of HCC tumorigenicity [103]. As illustrated in Fig. 45.2 PAR2 mediates calcium signaling in HSCs that could readily occur in the setting of a hepatic tumour.
PAR 2 mediates [Ca 2+ ] i increase in LX-2 hepatic stellate cells. LX-2 cells grown on Lab Tek chambered borosilicate coverglass were loaded with fluo-4-AM (0.5 μM). For calcium measurements, an inverted confocal laser scanning microscope LSM 510 was used. Fluorescence was monitored at 488 nm. Upper part: Fluorescence images, in pseudocolor, from an individual LX-2 cell preloaded with fluo-4-AM dye and stimulated with the PAR2-activating peptide, 2-furoyl-LIGRLO-NH2 (10 μM). The time sequence of three panels shows a transient fluorescence increase 30 s after PAR2-AP addition (0 s: time of addition of PAR2-AP), with a return to baseline fluorescence at 1 min. Lower part: Time course of calcium response induced by the PAR2-activating peptide, 2-furoyl-LIGRLO-NH2 (10 μM). The intracellular calcium concentration was calculated using the equation [Ca2+]i = 345 (F–Fmin)/(Fmax−F) [104]. The Ca2+ affinity of fluo-4 (Kd) is 345 nM [105]. Fmax was obtained by addition of 10 μM ionomycin (+6 mM CaCl2), Fmin by addition of 10 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA). Data represent the mean ± SE from calcium measurements in 20 single cells. (LX-2 cells were a gift from Prof. Scott L. Friedman, Division of Liver Diseases, Mount Sinai School of Medicine, New York)
Intracellular Calcium – A Key Secondary Messenger in Cancer and a Potential Target for Therapy
Ca2+ is a ubiquitous intracellular signaling molecule that is involved in the regulation of almost all cellular functions including gene transcription, metabolism, proliferation and apoptosis [reviewed: [106–110]]. Since cancer growth is based on increased proliferation, decreased differentiation and decreased apoptosis, all of which processes involve a regulation of intracellular calcium concentrations, Ca2+-homeostasis has become an important topic in current cancer research. Apart from the ‘calcium-sensing receptor’ [111, 112], G-protein-coupled receptor mechanisms involving Gq-stimulated phospholipase Cβ and growth factor receptor mechanisms that trigger phospholipase Cγ represent key receptor mechanisms that regulate intracellular calcium. These mechanisms are in addition to the voltage-regulated and other ion channel mechanisms that regulate the entry of calcium from the extracellular environment. The current knowledge in this area is well documented by several detailed and comprehensive review articles that are cited in the following text. Here, only a brief overview is provided that is relevant for understanding the rationale for targeting PAR-mediated Ca2+ signaling as a possible therapeutic option for the treatment of cancer.
It is well known that an elevation of cytoplasmic [Ca2+]i can result either from Ca2+-influx from the extracellular space through a variety of plasma membrane ion channels or from Ca2+-release from intracellular stores. More specifically, voltage- and ligand-gated Ca2+ channels in the plasma membrane, along with intracellular ryanodine receptors and inositol (1,4,5)-triphosphate (InsP3) receptors in the endoplasmic reticulum as well as mitochondrial voltage-dependent anion channels and calcium ion exchangers provide fluxes of Ca2+ to the cytoplasm [106–108].
It has become evident that during the multistage process of carcinogenesis, the transformation of a normal cell into the malignant state is associated with a major change in the organization and expression of Ca2+ pumps, Na/Ca exchangers and Ca2+ channels. These changes occur in a setting that leads to the enhanced proliferation and impaired ability of the cancer cell to die [109]. In addition, work in this area done over the past decade has shown that altered intracellular Ca2+ signaling stimulated by G-protein coupled receptors via Gq [113–115] and involving tumour-associated changes in calcium release depots like the ryanodine receptor [116] can play a role in various tumourigenic pathways [117–120]. Thus, modulation of [Ca2+]i signaling is a potential therapeutic option in cancer. In this regard, strategies can include specific blockade of membrane-localized calcium channels and targeting calcium release mechanisms via the InsP3 and ryanodine receptors. Since many of these targets are expressed in a large number of cell types and organs where they may have essential functions, targeting specific Ca2+ channels or pumps with restricted tissue distribution, altered expression in cancer and/or a role in the regulation of tumourigenic pathways are a potential way to specifically disrupt intracellular Ca2+ homeostasis in cancer cells wherein different pharmacological strategies are possible [117–120]. One approach makes use of a bystander enzyme mechanism that results in the metabolic conversion of a pro-drug to an active moiety specifically at a site of restricted expression of that enzyme. For instance, since the human kallikrein-related serine peptidase-3 (KLK3, also known as ‘prostate-specific antigen’) is highly restricted in its expression to prostate tissue, it has proved possible to target the conversion of a thapsigargin ‘prodrug’ for activation in prostate cancer tissue, where the released thapsigargin can block the sarcoplasmic/endoplasmic Ca2+ pump. This ‘smart-bomb’ targeting method has the ability to induce cell death in prostate cancer cells [121]. A second pharmacological approach involves the direct targeting of specific isoforms of Ca2+ channels or pumps associated with a specific cancer type. There is yet another aspect in Ca2+ signaling that makes Ca2+ channels and pumps highly attractive as therapeutic targets in cancer. While the Ca2+ signal in differentiated non malignant cells is spatially and temporally highly regulated [106–110], in tumour cells there is a shift to a more global elevation of intracellular calcium with a sustained elevation of intracellular calcium. Therefore, cancer cells and their calcium-regulated signaling pathways may be more susceptible than normal cells to modulation of their Ca2+ channels and pumps [117–120]. Taken together the information obtained over the past decade, including quite recent data [122–124] suggest that the intracellular calcium-regulating machinery may represent a promising target for cancer therapy.
[Ca2+]i Is Involved in PAR Signaling in Cancer
As outlined above, one of the main cell signaling pathways triggered by activation of distinct PARs is the Gq/11–mediated activation of phospholipase Cβ. This activation, leads to the formation of inositol (1,4,5)-triphosphate and diacylglycerol that in turn cause the elevation of intracellular Ca2+ (illustrated for LX-2 hepatic stellate cells in Fig. 45.2) and activation of protein kinase C. Indeed, the ability of the PAR1 receptor for thrombin to mobilize intracellular calcium was instrumental in its cloning via an oocyte expression system [9].
This Gq/11 calcium signaling pathway activated by PARs has been observed in a variety of cancer cell types as seen by the activation of calcium signaling by thrombin in glioma cells ostensibly via PAR1 [125]. The documentation of PAR-mediated calcium signaling in cancer-derived cells was greatly facilitated by the use of PAR subtype selective peptide agonists based on the sequences of the revealed PAR tethered ligands and PAR1-targeted antagonists (see Table 45.1 for PAR-selective agonists and antagonists).
The presence of a specific PAR in a target cancer cell and its ability to increase intracellular calcium can be established using a receptor cross-desensitization protocol with PAR-selective agonists and appropriate PAR-inactive ‘control’ peptides [126]. This approach that uses fluorimetric methods to monitor calcium transients with different calcium sensitive fluorescence dyes has documented PAR-mediated increases in [Ca2+]i in cells from various malignancies including those from brain [53, 56, 57, 109], colon [64, 74], pancreas [127], kidney[128], breast [19], larynx [56], prostate [112] and liver [72]. Although all of PARs 1, 2 and 4 can couple with Gq to elevate intracellular calcium in all PAR-expressing cells so far examined, the precise downstream consequences of elevated calcium per se have not been established in any detail. Further, as already mentioned, upon enzyme or peptide agonist activation the PARs can activate multiple G-proteins leading not only to elevations of intracellular calcium but also to (I) a Gi-mediated inhibition of adenylyl cyclase, (II) activation of MAPKinase [both Gi-dependent as well as G-protein independent via beta-arrestin interactions: review: [35]] and (III) a G12/13-mediated activation of Rho and its downstream targets. Thus, singling out the PAR-triggered signal pathways that are uniquely calcium-mediated represents a considerable challenge.
Ca2± and PAR2-Triggered p42/p44 MAPKinase Signaling
Increases in intracellular calcium result in a complex signaling network that includes p42/p44 MAPKinases as an intracellular effector system critically related to cell growth and transcriptional regulation [129, 130]. For prostate cancer PC3 cells it has been shown that kallikrein related peptidase 4 (KLK4), one of the 15 members of the human KLK family and a trypsin-like prostate cancer-associated serine protease, initiates Ca2+ signaling via PAR1 and PAR2. Stimulation of PAR2 by KLK4 also results in p42/p44 MAPKinase activation [131]. Very recently, for hepatocellular carcinoma where altered Ca2+ signaling contributes to cancer development and progression [132], a PAR2 dependent calcium-p42/p44 MAPKinase signaling axis was defined [133]. Since p42/p44 MAPKinases are established key players in HCC progression and invasive growth [134–137], and more specifically, since they contribute to a PAR2-mediated effect on HCC cell invasion, the results suggest a role for both Ca2+ and p42/p44 MAPKinase-driven signaling as an invasive axis in HCC cells. What is difficult to sort out is the signaling route whereby MAPKinase is activated in the HCC cells. Activation of p42/p44 MAPKinase could be (I) directly downstream of Ca2+ signaling as a consequence of the activation of protein kinase C, (II) independent of Gq/11 Ca2+ signaling, via a G12/13-Rho kinase mechanism or (III) via a G-protein-independent mechanism triggered by a beta-arrestin-internalized signal scaffold [35]. In principle, all three mechanisms could result in the activation of MAPKinase signaling pathways in cancer cells. However, it is likely that the downstream effects of MAPKinase activation by these three distinct mechanisms will be found to differ (e.g. increase in transcription vs. activation of cytosolic phospholipase-A2 or changes in cell motility). Thus, identifying those events that result uniquely from elevations in intracellular calcium will be of much interest in the setting of tumour cell behaviour. To sum up, although Ca2+ plays a central role in regulating cancer cell behaviour, it has not yet proved possible to single out the impact on tumourigenesis of blocking Ca2+ signaling selectively, without affecting other PAR-triggered signaling events.
PAR-Mediated Increases in Cytoplasmic Free Ca2±: Involvement of Both Extracellular and Intracellular Calcium
For numerous GPCRs it has been shown, as outlined above, that receptor-triggered increases in free intracellular calcium ion concentration can result from both influx of Ca2+ across the plasma membrane and the release of Ca2+ from intracellular stores [106, 138]. For PAR2 this dual mechanism has been suggested for hepatocellular carcinoma cells, where PAR2-stimulated increases in intracellular calcium can be reduced either by removing extracellular Ca2+ with the use of EGTA or by depletion of internal Ca2+ stores with thapsigargin [133]. This ‘dual mechanism’ for calcium signaling very likely also occurs for PARs 1 and 4. Thus, to block calcium signaling completely in cancer cells, it may be necessary to inhibit not only the Gq-triggered calcium signal that involves intracellular stores but also the receptor-mediated calcium entry process that occurs via receptor-regulated channels.
Intracellular Calcium Oscillations in Cancer-Derived Cells
Most of the knowledge about the effects of receptor agonists on [Ca2+]i has come from studies on cell suspensions. In such experiments, the estimated [Ca2+]i value represents the average value of [Ca2+]i in all cells in the sample being explored. That response is represented by a peak of intracellular calcium that occurs within a minute of cell activation and a return to baseline calcium concentrations over a 2–5-min time frame, as calcium is first released and then rapidly taken back up into intracellular stores. However, at the single cell level, agonists can also trigger persistent oscillations in intracellular calcium ion concentrations that wax and wane with time. Agonist-induced oscillations in intracellular calcium concentrations have been observed in many excitable and non-excitable cells, wherein a number of mechanisms have been proposed [for reviews see e.g.:[107, 138–140]]. As an example, such oscillations have been observed in response to PAR1 activation in glioblastoma cells. The oscillatory response was observed after treatment with either thrombin or by the dual PAR1–2 activating peptide, SFLLRN-NH2 [60]. The relevance of these oscillating intracellular calcium concentrations to tumour cell behaviour has yet to be determined.
Can PAR-Mediated Calcium Signaling Be Selectively Blocked?
Given that PAR-triggered calcium signaling can be of importance for the oncogenic process, an important question to deal with is: Can PAR-mediated calcium signaling be selectively blocked? Studies with human PAR2 have identified a C-terminal domain that is directly involved in the ability of this receptor to stimulate elevations in intracellular calcium [141]. Thus, when activated by trypsin, a mutant PAR2 missing a key C-terminal domain was able to stimulate MAPKinase and JNK, but not an elevation in intracellular calcium. In principle, this region of PAR2 can thus be targeted as a ‘calcium regulating domain’ for the development of receptor-selective antagonists that will potentially affect calcium transients only in PAR2-expressing tumour cells. A similar situation was found for the activation of PAR1. It has been shown that the C-terminal part of PAR1 is a critical site for receptor coupling to phospholipase C activation and thus for Ca2+-signaling, while the third intracellular loop of PAR1 is implicated in PAR1 coupling to MAPkinase activation. Therefore, a strategy specifically targeting Ca2+ signaling might be possible not only for PAR2 but also for the other PAR subtypes [142].
One may readily ask: How might such signal-selective antagonists be developed? The answer lies in making use of (I) the concept of ‘biased’ signaling and (II) cell-penetrating peptides. For instance, the PAR2 antagonist, K-14585 can block PAR2- stimulated elevations of intracellular calcium and a concurrent activation of p42/44 MAPKinase, but cannot block increases in p38 MAPKinase activation [143]. This compound therefore exhibits ‘biased’ antagonism for PAR2. In principle more potent antagonists of this kind can be developed to block calcium signaling selectively. The concurrent blockade of both MAPkinase and calcium signaling may be particularly attractive in terms of targeting cancer cells.
“Pepducins” are cell-penetrating palmitoylated peptides based on sequences of the intracellular loops of G protein-coupled receptors. Due to the ability of their lipid moiety to anchor to the lipid bilayer of the plasma membrane these lipopeptides are thought to act by being internalized and then targeting the receptor-G protein interface [144, 145]. “Pepducins” based on the third intracellular loop of proteinase activated receptors have been successfully used for inhibition of PAR-mediated effects on signaling and cellular level [146–149]. The ‘pepducin GPCR antagonist’ approach provides an excellent platform technology for the design of a variety of other PAR inhibiting cell-penetrating peptide variants corresponding to sequences of the intracellular receptor domains that are important for G protein coupling of GPCRs [150, 151]. It is known that for GPCRs, the C-terminus appears to be only of modest relevance for interacting with some G proteins [152–154]. However, as outlined above, a sequence in the C-terminus of PAR2 has been shown to be important for calcium signaling [141]. This C-terminal domain can be a target for palmitoylation that results in a potential ‘8th helix’ and a ‘fourth intracellular loop’ in G-protein-coupled receptors. Of particular note, a synthetic pepducin, termed jF5, targeted to this domain of GPCRs, including PAR1 and the alpha-2A adrenoceptor, can selectively block GPCR-triggered calcium signaling, but not signaling via Gα12 [155]. It can be predicted that jF5 would also affect PAR2 calcium signaling, which is dependent on a homologous sequence that can be a target for palmitoylation [141]. Finally, this ‘lipopeptide concept’ could also be expanded in principle to target PAR sequences within the transmembrane helical domains 3 and 6 that are also known to regulate GPCR G-protein coupling [36, 37].
Possible Impact of PAR-Triggered Calcium Signaling in Cancer Therapy
Data describing the PAR-induced effects in cancer published over the last 15 years clearly highlight PARs as possible targets in cancer treatment [156]. Given that PAR1 is an attractive therapeutic target for thromboembolic disease, a number of receptor-targeted antagonists have been developed. Two PAR1-targeted antagonists, SCH 530348 and E5555 or Atopaxar are currently in Phase III clinical trials for treating acute coronary syndrome [157–159]. Whether these antagonists will prove of value in the clinic for cardiovascular disease is yet to be determined. The compounds may, however be considered for use in the prevention of cancer metastasis and invasion. In addition, novel PAR2 antagonists containing nonpeptidic moieties have been developed very recently [160]. Their therapeutic potential should also be tested for epithelial carcinoma. Since PAR stimulation does activate calcium signaling and because calcium signaling per se can affect cancer cell migration and invasion, agents that also target intracellular Ca2+-signaling like those used in cardiovascular disease [for reviews see e.g.: [161–164]] may prove of value in the setting of cancer along with PAR antagonists. This possibility has yet to be considered.
Over the past decade there has been substantial success in targeting signal transduction pathways for treating cancer [165–167]. Impressive success can be seen in the use of the Abl-kinase-targeted imatinib-like inhibitors and their analogues, and a ‘multitarget drug’ that affects a number of signal pathways, sorafenib (BAY-43-9006), a bis-aryl urea-type inhibitor that blocks several kinases involved in tumour proliferation and angiogenesis. This inhibitor can affect Raf, vascular endothelial growth factor receptor (VEGFR) and platelet derived growth factor receptor (PDGFR) signaling [168]. Data from several patient studies indicate that sorafenib seems to be a promising drug for the treatment of various epithelial cancers including those from breast, colon, kidney and liver [for review see e.g.: [169]]. Since targeting multiple signal pathways rather than a single enzyme may be advantageous in treating cancer, it can be suggested that in combination with other therapeutic agents, the selective blockade of PAR-mediated calcium signaling may be worthy of consideration for dealing with epithelial carcinoma.
References
Ossovskaya V, Bunnett N (2004) Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84:579–621
Ramachandran R, Hollenberg M (2008) Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol 153(Suppl 1):S263–S282
Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger T, Hollenberg M (2005) Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev 26:1–43
Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD (2011) Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 130(3):248–282
Wettschureck N, Offermanns S (2005) Mammalian G proteins and their cell type specific functions. Physiol Rev 85:1159–1204
Hollenberg M, Compton S (2002) International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev 54:203–217
Coughlin SR (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3:1800–1814
Gandhi PS, Chen Z, Di Cera E (2010) Crystal structure of thrombin bound to the uncleaved extracellular fragment of PAR1. J Biol Chem 285:15393–15398
Vu T, Hung D, Wheaton V, Coughlin S (1991) Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64:1057–1068
Scarborough RM, Naughton MA, Teng W, Hung DT, Rose J, Vu TK, Wheaton VI, Turck CW, Coughlin SR (1992) Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem 267:13146–13149
Hansen KK, Saifeddine M, Hollenberg MD (2004) Tethered ligand-derived peptides of proteinase-activated receptor 3 (PAR3) activate PAR1 and PAR2 in Jurkat T cells. Immunology 112:183–190
Kaufmann R, Schulze B, Krause G, Mayr LM, Settmacher U, Henklein P (2005) Proteinase-activated receptors (PARs)–the PAR3 Neo-N-terminal peptide TFRGAP interacts with PAR1. Regul Pept 125:61–66
Rasmussen UB, Vouret-Craviari V, Jallat S, Schlesinger Y, Pagès G, Pavirani A, Lecocq JP, Pouysségur J, Van Obberghen-Schilling E (1991) cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett 288:123–128
Ishihara H, Connolly A, Zeng D, Kahn M, Zheng Y, Timmons C, Tram T, Coughlin S (1997) Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 386:502–506
Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC (1998) Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA 95:6642–6646
Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Tam C, Coughlin SR (1998) A dual thrombin receptor system for platelet activation. Nature 394:690–694
Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR (2000) Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem 275:6819–6823
Schuepbach RA, Riewald M (2010) Coagulation factor Xa cleaves protease-activated receptor-1 and mediates signaling dependent on binding to the endothelial protein C receptor. J Thromb Haemost 8:379–388
Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120:303–313
Nystedt S, Emilsson K, Wahlestedt C, Sundelin J (1994) Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA 91:9208–9212
Grab D, Garcia-Garcia J, Nikolskaia O, Kim Y, Brown A, Pardo C, Zhang Y, Becker K, Wilson B, de Lima A, Scharfstein J, Dumler J (2009) Protease activated receptor signaling is required for African trypanosome traversal of human brain microvascular endothelial cells. PLoS Negl Trop Dis 3:e479
McCoy KL, Traynelis SF, Hepler JR (2010) PAR1 and PAR2 couple to overlapping and distinct sets of G proteins and linked signaling pathways to differentially regulate cell physiology. Mol Pharmacol 77:1005–1015
Sekiguchi F, Takaoka K, Kawabata A (2007) Proteinase-activated receptors in the gastrointestinal system: a functional linkage to prostanoids. Inflammopharmacology 15:246–251
Macfarlane S, Plevin R (2003) Intracellular signalling by the G-protein coupled proteinase-activated receptor (PAR) family. Drug Dev Res 59:367–374
Coelho A, Ossovskaya V, Bunnett N (2003) Proteinase-activated receptor-2: physiological and pathophysiological roles. Curr Med Chem Cardiovasc Hematol Agents 1:61–72
Kawabata A, Kawao N (2005) Physiology and pathophysiology of proteinase-activated receptors (PARs): PARs in the respiratory system: cellular signaling and physiological/pathological roles. J Pharmacol Sci 97:20–24
Soh UJ, Dores MR, Chen B, Trejo J (2010) Signal transduction by protease-activated receptors. Br J Pharmacol 160:191–203
Hollenberg MD (2005) Physiology and pathophysiology of proteinase-activated receptors (PARs): proteinases as hormone-like signal messengers: PARs and more. J Pharmacol Sci 97:8–13
Russo A, Soh UJ, Trejo J (2009) Proteases display biased agonism at protease-activated receptors: location matters! Mol Interv 9:87–96
Chen CH, Paing MM, Trejo J (2004) Termination of protease-activated receptor-1 signaling by beta-arrestins is independent of receptor phosphorylation. J Biol Chem 279:10020–10031
Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, Defea K, Hollenberg MD (2009) Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol 76:791–801
Ge L, Shenoy SK, Lefkowitz RJ, DeFea K (2004) Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem 279:55419–55424
Wang P, Jiang Y, Wang Y, Shyy JY, DeFea KA (2010) Beta-arrestin inhibits CAMKKbeta-dependent AMPK activation downstream of protease-activated-receptor-2. BMC Biochem 11:36
Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA (2007) Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem 282:20634–20646
Defea K (2008) Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol 153(Suppl 1):S298–S309
Weis WI, Kobilka BK (2008) Structural insights into G-protein-coupled receptor activation. Curr Opin Struct Biol 18:734–740
Gether U, Kobilka BK (1998) G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem 273:17979–17982
de Haën C (1976) The non-stoichiometric floating receptor model for hormone sensitive adenylyl cyclase. J Theor Biol 58:383–400
Jacobs S, Cuatrecasas P (1976) The mobile receptor hypothesis and “cooperativity” of hormone binding. Application to insulin. Biochim Biophys Acta 433:482–495
Kenakin T, Miller LJ (2010) Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62:265–304
Hung DT, Wong YH, Vu TK, Coughlin SR (1992) The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J Biol Chem 267:20831–20834
Rahman A, True AL, Anwar KN, Ye RD, Voyno-Yasenetskaya TA, Malik AB (2002) Galpha(q) and Gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ Res 91:398–405
ten Cate H, Falanga A (2008) Overview of the postulated mechanisms linking cancer and thrombosis. Pathophysiol Haemost Thromb 36:122–130
Coughlin SR (2000) Thrombin signalling and protease-activated receptors. Nature 407:258–264
Rao LV, Pendurthi UR (2005) Tissue factor-factor VIIa signaling. Arterioscler Thromb Vasc Biol 25:47–56
Belting M, Ahamed J, Ruf W (2005) Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol 25:1545–1550
Riewald M, Ruf W (2001) Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA 98:7742–7747
Schaffner F, Ruf W (2009) Tissue factor and PAR2 signaling in the tumor microenvironment. Arterioscler Thromb Vasc Biol 29:1999–2004
Borgoño CA, Diamandis EP (2004) The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer 4:876–890
Oikonomopoulou K, Diamandis EP, Hollenberg MD (2010) Kallikrein-related peptidases: proteolysis and signaling in cancer, the new frontier. Biol Chem 391:299–310
Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, Scarisbrick I, Andrade-Gordon P, Cottrell GS, Bunnett NW, Diamandis EP, Hollenberg MD (2006) Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem 281:32095–32112
Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A (2009) Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 137:332–343
Even-Ram SC, Maoz M, Pokroy E, Reich R, Katz BZ, Gutwein P, Altevogt P, Bar-Shavit R (2001) Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem 276:10952–10962
Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R (1998) Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 4:909–914
Wojtukiewicz MZ, Tang DG, Ben-Josef E, Renaud C, Walz DA, Honn KV (1995) Solid tumor cells express functional “tethered ligand” thrombin receptor. Cancer Res 55:698–704
Kaufmann R, Schafberg H, Rudroff C, Nowak G (1997) Thrombin receptor activation results in calcium signaling and protein kinase C-dependent stimulation of DNA synthesis in HEp-2g laryngeal carcinoma cells. Cancer 80:2068–2074
Rudroff C, Schafberg H, Nowak G, Weinel R, Scheele J, Kaufmann R (1998) Characterization of functional thrombin receptors in human pancreatic tumor cells (MIA PACA-2). Pancreas 16:189–194
Kaufmann R, Lindschau C, Höer A, Henklein P, Adomeit A, Haller H, Liebmann C, Oberdisse E, Nowak G (1996) Signaling effects of alpha-thrombin and SFLLRN in rat glioma C6 cells. J Neurosci Res 46:641–651
Schafberg H, Nowak G, Kaufmann R (1997) Thrombin has a bimodal effect on glioma cell growth. Br J Cancer 76:1592–1595
Kaufmann R, Patt S, Schafberg H, Kalff R, Neupert G, Nowak G (1998) Functional thrombin receptor PAR1 in primary cultures of human glioblastoma cells. Neuroreport 9:709–712
Zieger M, Tausch S, Henklein P, Nowak G, Kaufmann R (2001) A novel PAR-1-type thrombin receptor signaling pathway: cyclic AMP-independent activation of PKA in SNB-19 glioblastoma cells. Biochem Biophys Res Commun 282:952–957
Kaufmann R, Patt S, Kraft R, Zieger M, Henklein P, Neupert G, Nowak G (1999) PAR 1-type thrombin receptors are involved in thrombin-induced calcium signaling in human meningioma cells. J Neurooncol 42:131–136
Chay CH, Cooper CR, Gendernalik JD, Dhanasekaran SM, Chinnaiyan AM, Rubin MA, Schmaier AH, Pienta KJ (2002) A functional thrombin receptor (PAR1) is expressed on bone-derived prostate cancer cell lines. Urology 60:760–765
Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M (2003) Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol 162:1503–1513
Nierodzik ML, Bain RM, Liu LX, Shivji M, Takeshita K, Karpatkin S (1996) Presence of the seven transmembrane thrombin receptor on human tumour cells: effect of activation on tumour adhesion to platelets and tumor tyrosine phosphorylation. Br J Haematol 92:452–457
Helland IB, Klementsen B, Jørgensen L (1997) Addition of both platelets and thrombin in combination accelerates tumor cells to adhere to endothelial cells in vitro. In Vitro Cell Dev Biol Anim 33:182–186
Klementsen B, Jørgensen L (1997) Distribution of adhesion molecules on HeLa cells, platelets and endothelium in an in vitro model mimicking the early phase of metastasis. An immunogold electron microscopic study. APMIS 105:546–558
Rudroff C, Seibold S, Kaufmann R, Zetina CC, Reise K, Schäfer U, Schneider A, Brockmann M, Scheele J, Neugebauer EA (2002) Expression of the thrombin receptor PAR-1 correlates with tumour cell differentiation of pancreatic adenocarcinoma in vitro. Clin Exp Metastasis 19:181–189
Nierodzik ML, Chen K, Takeshita K, Li JJ, Huang YQ, Feng XS, D’Andrea MR, Andrade-Gordon P, Karpatkin S (1998) Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood 92:3694–3700
Henrikson KP, Salazar SL, Fenton JW, Pentecost BT (1999) Role of thrombin receptor in breast cancer invasiveness. Br J Cancer 79:401–406
Bergmann S, Junker K, Henklein P, Hollenberg MD, Settmacher U, Kaufmann R (2006) PAR-type thrombin receptors in renal carcinoma cells: PAR1-mediated EGFR activation promotes cell migration. Oncol Rep 15:889–893
Kaufmann R, Rahn S, Pollrich K, Hertel J, Dittmar Y, Hommann M, Henklein P, Biskup C, Westermann M, Hollenberg M, Settmacher U (2007) Thrombin-mediated hepatocellular carcinoma cell migration: cooperative action via proteinase-activated receptors 1 and 4. J Cell Physiol 211:699–707
Kaufmann R, Patt S, Zieger M, Kraft R, Tausch S, Henklein P, Nowak G (2000) The two-receptor system PAR-1/PAR-4 mediates alpha-thrombin-induced [Ca(2+)](i) mobilization in human astrocytoma cells. J Cancer Res Clin Oncol 126:91–94
Darmoul D, Gratio V, Devaud H, Laburthe M (2004) Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem 279:20927–20934
Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, Rao L (2004) Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood 103:3029–3037
Jikuhara A, Yoshii M, Iwagaki H, Mori S, Nishibori M, Tanaka N (2003) MAP kinase-mediated proliferation of DLD-1 carcinoma by the stimulation of protease-activated receptor 2. Life Sci 73:2817–2829
Shi X, Gangadharan B, Brass L, Ruf W, Mueller B (2004) Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res 2:395–402
Shimamoto R, Sawada T, Uchima Y, Inoue M, Kimura K, Yamashita Y, Yamada N, Nishihara T, Ohira M, Hirakawa K (2004) A role for protease-activated receptor-2 in pancreatic cancer cell proliferation. Int J Oncol 24:1401–1406
Rattenholl A, Seeliger S, Buddenkotte J, Schön M, Schön M, Ständer S, Vergnolle N, Steinhoff M (2007) Proteinase-activated receptor-2 (PAR2): a tumor suppressor in skin carcinogenesis. J Invest Dermatol 127:2245–2252
Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J (2006) Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res 66:307–314
Versteeg H, Schaffner F, Kerver M, Petersen H, Ahamed J, Felding-Habermann B, Takada Y, Mueller B, Ruf W (2008) Inhibition of tissue factor signaling suppresses tumor growth. Blood 111:190–199
Bocheva G, Rattenholl A, Kempkes C, Goerge T, Lin C, D’Andrea M, Ständer S, Steinhoff M (2009) Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol 129:1816–1823
Kaufmann R, Oettel C, Horn A, Halbhuber KJ, Eitner A, Krieg R, Katenkamp K, Henklein P, Westermann M, Bohmer FD, Ramachandran R, Saifeddine M, Hollenberg MD, Settmacher U (2009) Met receptor tyrosine kinase transactivation is involved in proteinase-activated receptor-2-mediated hepatocellular carcinoma cell invasion. Carcinogenesis 30:1487–1496
Gonda K, Watanabe TM, Ohuchi N, Higuchi H (2010) In vivo nano-imaging of membrane dynamics in metastatic tumor cells using quantum dots. J Biol Chem 285:2750–2757
Su S, Li Y, Luo Y, Sheng Y, Su Y, Padia RN, Pan ZK, Dong Z, Huang S (2009) Proteinase-activated receptor 2 expression in breast cancer and its role in breast cancer cell migration. Oncogene 28:3047–3057
Kamath L, Meydani A, Foss F, Kuliopulos A (2001) Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res 61:5933–5940
Blum AE, Joseph SM, Przybylski RJ, Dubyak GR (2008) Rho-family GTPases modulate Ca(2+) -dependent ATP release from astrocytes. Am J Physiol Cell Physiol 295:C231–C241
Darmoul D, Marie JC, Devaud H, Gratio V, Laburthe M (2001) Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer 85:772–779
Darmoul D, Gratio V, Devaud H, Peiretti F, Laburthe M (2004) Activation of proteinase-activated receptor 1 promotes human colon cancer cell proliferation through epidermal growth factor receptor transactivation. Mol Cancer Res 2:514–522
Gratio V, Walker F, Lehy T, Laburthe M, Darmoul D (2009) Aberrant expression of proteinase-activated receptor 4 promotes colon cancer cell proliferation through a persistent signaling that involves Src and ErbB-2 kinase. Int J Cancer 124:1517–1525
De Wever O, Mareel M (2003) Role of tissue stroma in cancer cell invasion. J Pathol 200:429–447
Micke P, Ostman A (2004) Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer 45(Suppl 2):S163–S175
Ostman A, Augsten M (2009) Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev 19:67–73
Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B (1998) Tumor induction of VEGF promoter activity in stromal cells. Cell 94:715–725
Vitolo D, Ciocci L, Cicerone E, Rossi C, Tiboni F, Ferrauti P, Gallo A, Baroni CD (2001) Laminin alpha2 chain (merosin M chain) distribution and VEGF, FGF(2), and TGFbeta1 gene expression in angiogenesis of supraglottic, lung, and breast carcinomas. J Pathol 195:197–208
Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR (2002) Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res 62:3298–3307
D’Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P (2001) Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol 158:2031–2041
Blackburn JS, Brinckerhoff CE (2008) Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol 173:1736–1746
Wang W, Zhang X, Mize G, Takayama T (2008) Protease-activated receptor-I upregulates fibroblast growth factor 7 in stroma of benign prostatic hyperplasia. Prostate 68:1064–1075
Al-Ani B, Hewett P, Cudmore M, Fujisawa T, Saifeddine M, Williams H, Ramma W, Sissaoui S, Jayaraman P, Ohba M, Ahmad S, Hollenberg M, Ahmed A (2010) Activation of proteinase-activated receptor 2 stimulates soluble vascular endothelial growth factor receptor 1 release via epidermal growth factor receptor transactivation in endothelial cells. Hypertension 55(3):689–697
Nakanuma S, Tajima H, Okamoto K, Hayashi H, Nakagawara H, Onishi I, Takamura H, Kitagawa H, Fushida S, Tani T, Fujimura T, Kayahara M, Ohta T, Wakayama T, Iseki S, Harada S (2010) Tumor-derived trypsin enhances proliferation of intrahepatic cholangiocarcinoma cells by activating protease-activated receptor-2. Int J Oncol 36:793–800
Zhang X, Wang W, True L, Vessella R, Takayama T (2009) Protease-activated receptor-1 is upregulated in reactive stroma of primary prostate cancer and bone metastasis. Prostate 69:727–736
Amann T, Bataille F, Spruss T, Mühlbauer M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff A, Hellerbrand C (2009) Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci 100:646–653
Grynkiewicz G, Poenie M, Tsien R (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Gee K, Brown K, Chen W, Bishop-Stewart J, Gray D, Johnson I (2000) Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium 27:97–106
Bootman M, Collins T, Peppiatt C, Prothero L, MacKenzie L, De Smet P, Travers M, Tovey S, Seo J, Berridge M, Ciccolini F, Lipp P (2001) Calcium signalling–an overview. Semin Cell Dev Biol 12:3–10
Berridge M, Bootman M, Roderick H (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529
Clapham D (2007) Calcium signaling. Cell 131:1047–1058
Carafoli E (2002) Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA 99:1115–1122
Rizzuto R, Pozzan T (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86:369–408
Brown EM, Pollak M, Hebert SC (1998) The extracellular calcium-sensing receptor: its role in health and disease. Annu Rev Med 49:15–29
Brown EM, Pollak M, Chou YH, Seidman CE, Seidman JG, Hebert SC (1995) Cloning and functional characterization of extracellular Ca(2+)-sensing receptors from parathyroid and kidney. Bone 17:7S–11S
Gutkind JS (1998) Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene 17:1331–1342
Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE (2003) Insights into G protein structure, function, and regulation. Endocr Rev 24:765–781
Spiegelberg BD, Hamm HE (2007) Roles of G-protein-coupled receptor signaling in cancer biology and gene transcription. Curr Opin Genet Dev 17:40–44
Abdul M, Ramlal S, Hoosein N (2008) Ryanodine receptor expression correlates with tumor grade in breast cancer. Pathol Oncol Res 14:157–160
Jaffe LF (2005) A calcium-based theory of carcinogenesis. Adv Cancer Res 94:231–263
Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ (2007) Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7:519–530
Capiod T, Shuba Y, Skryma R, Prevarskaya N (2007) Calcium signalling and cancer cell growth. Subcell Biochem 45:405–427
Roderick HL, Cook SJ (2008) Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer 8:361–375
Denmeade SR, Isaacs JT (2005) The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther 4:14–22
Kaddour-Djebbar I, Choudhary V, Brooks C, Ghazaly T, Lakshmikanthan V, Dong Z, Kumar MV (2010) Specific mitochondrial calcium overload induces mitochondrial fission in prostate cancer cells. Int J Oncol 36:1437–1444
McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Ferland RA, Steelman LS (2010) Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul 50:285–307
Lin J, Denmeade S, Carducci MA (2009) HIF-1alpha and calcium signaling as targets for treatment of prostate cancer by cardiac glycosides. Curr Cancer Drug Targets 9:881–887
Turner JS, Redpath GT, Humphries JE, Gonias SL, Vandenberg SR (1994) Plasmin modulates the thrombin-evoked calcium response in C6 glioma cells. Biochem J 297(Pt 1): 175–179
Kawabata A, Saifeddine M, Al-Ani B, Leblond L, Hollenberg MD (1999) Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1-targeted ligands. J Pharmacol Exp Ther 288:358–370
Kaufmann R, Schafberg H, Nowak G (1998) Proteinase-activated receptor-2-mediated signaling and inhibition of DNA synthesis in human pancreatic cancer cells. Int J Pancreatol 24:97–102
Kaufmann R, Junker U, Nuske K, Westermann M, Henklein P, Scheele J, Junker K (2002) PAR-1- and PAR-3-type thrombin receptor expression in primary cultures of human renal cell carcinoma cells. Int J Oncol 20:177–180
Kanno H, Horikawa Y, Hodges R, Zoukhri D, Shatos M, Rios J, Dartt D (2003) Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol 284:C988–C998
Hodges R, Horikawa Y, Rios J, Shatos M, Dartt D (2007) Effect of protein kinase C and Ca(2+) on p42/p44 MAPK, Pyk2, and Src activation in rat conjunctival goblet cells. Exp Eye Res 85:836–844
Ramsay AJ, Dong Y, Hunt ML, Linn M, Samaratunga H, Clements JA, Hooper JD (2008) Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J Biol Chem 283:12293–12304
Gearhart T, Bouchard M (2010) The hepatitis B virus X protein modulates hepatocyte proliferation pathways to stimulate viral replication. J Virol 84:2675–2686
Kaufmann R, Mußbach F, Henklein P, Settmacher U (2011) Proteinase-activated receptor 2-mediated calcium signaling in hepatocellular carcinoma cells. J Cancer Res Clin Oncol 137(6):965–973
Huynh H, Nguyen T, Chow K, Tan P, Soo K, Tran E (2003) Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol 3:19
Tsuboi Y, Ichida T, Sugitani S, Genda T, Inayoshi J, Takamura M, Matsuda Y, Nomoto M, Aoyagi Y (2004) Overexpression of extracellular signal-regulated protein kinase and its correlation with proliferation in human hepatocellular carcinoma. Liver Int 24:432–436
Klein P, Schmidt C, Wiesenauer C, Choi J, Gage E, Yip-Schneider M, Wiebke E, Wang Y, Omer C, Sebolt-Leopold J (2006) The effects of a novel MEK inhibitor PD184161 on MEK-ERK signaling and growth in human liver cancer. Neoplasia 8:1–8
Calvisi D, Pascale R, Feo F (2007) Dissection of signal transduction pathways as a tool for the development of targeted therapies of hepatocellular carcinoma. Rev Recent Clin Trials 2:217–236
Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signalling. Nature 341:197–205
Berridge MJ, Rapp PE (1979) A comparative survey of the function, mechanism and control of cellular oscillators. J Exp Biol 81:217–279
Berridge MJ (2007) Inositol trisphosphate and calcium oscillations. Biochem Soc Symp 74:1–7
Seatter MJ, Drummond R, Kanke T, Macfarlane SR, Hollenberg MD, Plevin R (2004) The role of the C-terminal tail in protease-activated receptor-2-mediated Ca2+ signalling, proline-rich tyrosine kinase-2 activation, and mitogen-activated protein kinase activity. Cell Signal 16:21–29
Chen X, Berrou J, Vigneau C, Rondeau E (2001) Role of the third intracellular loop and of the cytoplasmic tail in the mitogenic signaling of the protease-activated receptor 1. Int J Mol Med 8:309–314
Goh FG, Ng PY, Nilsson M, Kanke T, Plevin R (2009) Dual effect of the novel peptide antagonist K-14585 on proteinase-activated receptor-2-mediated signalling. Br J Pharmacol 158:1695–1704
Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A (2002) Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA 99:643–648
Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A (2011) Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol Biol 683:259–275
Yang E, Boire A, Agarwal A, Nguyen N, O’Callaghan K, Tu P, Kuliopulos A, Covic L (2009) Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res 69:6223–6231
Covic L, Misra M, Badar J, Singh C, Kuliopulos A (2002) Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med 8:1161–1165
Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G, Sharifi S, Kuliopulos A (2008) Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther 7:2746–2757
Sevigny LM, Zhang P, Bohm A, Lazarides K, Perides G, Covic L, Kuliopulos A (2011) Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci USA 108(20):8491–8496
Iida-Klein A, Guo J, Takemura M, Drake MT, Potts JT, Abou-Samra A, Bringhurst FR, Segre GV (1997) Mutations in the second cytoplasmic loop of the rat parathyroid hormone (PTH)/PTH-related protein receptor result in selective loss of PTH-stimulated phospholipase C activity. J Biol Chem 272:6882–6889
Cotecchia S, Ostrowski J, Kjelsberg MA, Caron MG, Lefkowitz RJ (1992) Discrete amino acid sequences of the alpha 1-adrenergic receptor determine the selectivity of coupling to phosphatidylinositol hydrolysis. J Biol Chem 267:1633–1639
Estall JL, Koehler JA, Yusta B, Drucker DJ (2005) The glucagon-like peptide-2 receptor C terminus modulates beta-arrestin-2 association but is dispensable for ligand-induced desensitization, endocytosis, and G-protein-dependent effector activation. J Biol Chem 280:22124–22134
Budd DC, McDonald J, Emsley N, Cain K, Tobin AB (2003) The C-terminal tail of the M3-muscarinic receptor possesses anti-apoptotic properties. J Biol Chem 278:19565–19573
Wess J, Bonner TI, Brann MR (1990) Chimeric m2/m3 muscarinic receptors: role of carboxyl terminal receptor domains in selectivity of ligand binding and coupling to phosphoinositide hydrolysis. Mol Pharmacol 38:872–877
Dowal L, Sim DS, Dilks JR, Blair P, Beaudry S, Denker BM, Koukos G, Kuliopulos A, Flaumenhaft R (2011) Identification of an antithrombotic allosteric modulator that acts through helix 8 of PAR1. Proc Natl Acad Sci USA 108:2951–2956
García-López MT, Gutiérrez-Rodríguez M, Herranz R (2010) Thrombin-activated receptors: promising targets for cancer therapy? Curr Med Chem 17:109–128
O’Donoghue ML, Bhatt DL, Wiviott SD, Goodman SG, Fitzgerald DJ, Angiolillo DJ, Goto S, Montalescot G, Zeymer U, Aylward PE, Guetta V, Dudek D, Ziecina R, Contant CF, Flather MD, Investigators obotLA (2011) Safety and tolerability of atopaxar in the treatment of patients with acute coronary syndromes: the lessons from antagonizing the cellular effects of thrombin-acute coronary syndromes trial. Circulation 123(17):1843–1853
Leonardi S, Tricoci P, Mahaffey KW (2012) Promises of PAR-1 inhibition in acute coronary syndrome. Curr Cardiol Rep 14(1):32–39
Wiviott SD, Flather MD, O’Donoghue ML, Goto S, Fitzgerald DJ, Cura F, Aylward P, Guetta V, Dudek D, Contant CF, Angiolillo DJ, Bhatt DL, Investigators obotLC (2011) Randomized trial of atopaxar in the treatment of patients with coronary artery disease: the lessons from antagonizing the cellular effect of thrombin-coronary artery disease trial. Circulation 123(17):1854–1863
Barry GD, Suen JY, Le GT, Cotterell A, Reid RC, Fairlie DP (2010) Novel agonists and antagonists for human protease activated receptor 2. J Med Chem 53:7428–7440
Ghali J, Smith W, Torre-Amione G, Haynos W, Rayburn B, Amato A, Zhang D, Cowart D, Valentini G, Carminati P, Gheorghiade M (2007) A phase 1–2 dose-escalating study evaluating the safety and tolerability of istaroxime and specific effects on electrocardiographic and hemodynamic parameters in patients with chronic heart failure with reduced systolic function. Am J Cardiol 99:47A–56A
Triposkiadis F, Parissis JT, Starling RC, Skoularigis J, Louridas G (2009) Current drugs and medical treatment algorithms in the management of acute decompensated heart failure. Expert Opin Investig Drugs 18:695–707
Talukder MA, Zweier JL, Periasamy M (2009) Targeting calcium transport in ischaemic heart disease. Cardiovasc Res 84:345–352
Duncan RS, Goad DL, Grillo MA, Kaja S, Payne AJ, Koulen P (2010) Control of intracellular calcium signaling as a neuroprotective strategy. Molecules 15:1168–1195
Roberts L, Gores G (2005) Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis 25:212–225
Beeram M, Patnaik A (2002) Targeting intracellular signal transduction. A new paradigm for a brave new world of molecularly targeted therapeutics. Hematol Oncol Clin North Am 16:1089–1100
Levitzki A, Klein S (2010) Signal transduction therapy of cancer. Mol Aspects Med 31:287–329
Wilhelm S, Chien DS (2002) BAY 43-9006: preclinical data. Curr Pharm Des 8:2255–2257
Sharma PS, Sharma R, Tyagi T (2011) VEGF/VEGFR pathway inhibitors as anti-angiogenic agents: present and future. Curr Cancer Drug Targets 11(5):624–653
Acknowledgements
Work in the author’s laboratories is supported by grants from German Cancer Aid (RK) and German Research Foundation (RK), the Canadian Institutes for Health Research (MDH) and the Heart & Stroke Foundation of Alberta and Nunavut (MDH). We are grateful for the referee’s comments which have helped with the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Kaufmann, R., Hollenberg, M.D. (2012). Proteinase-Activated Receptors (PARs) and Calcium Signaling in Cancer. In: Islam, M. (eds) Calcium Signaling. Advances in Experimental Medicine and Biology, vol 740. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2888-2_45
Download citation
DOI: https://doi.org/10.1007/978-94-007-2888-2_45
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-2887-5
Online ISBN: 978-94-007-2888-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)