Abstract
Along with the number of potential applications for gold nanoparticles (AuNP) especially for medical and scientific purposes, the interest in possible toxic effects of such particles is rising. The general perception views nanosized gold colloids as relatively inert towards biological systems. However, a closer analysis of pertinent studies reveals a more complex picture. While the chemical compound of which the nanoparticles consists plays an important role, further biocompatibility determining aspects have been made out. The vast majority of trials concerning AuNP-toxicity were performed using somatic cell culture lines. The results show a considerable dependency of toxic effects on size, zeta potential and surface functionalisation. In vivo studies on this subject are still rare. Based on the existing data it can be assumed, that a dosage of under <400 µg Au/kg showed no untoward effects. If higher amounts were applied toxicity depended on route of administration and particle size. Since nanoparticles have been shown to cross reproduction-relevant biological barriers such as the blood-testicle and the placental barrier the question of their reprotoxicity arises. Yet data concerning this subject is far from adequate. Regarding gametes, recent experiments showed a dose-dependent sensitivity of spermatozoa towards AuNP. Oocytes have not yet been tested in that respect. Interestingly, so far no effects were detected on embryos after gold nanoparticle exposure. In conclusion, the biocompatibility of gold nanoparticles depends on a range of particle specific aspects as well as the choice of target tissue. Further clarification of such matters are subject to ongoing research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gold nanoparticles
- Toxicity
- Reprotoxicity
- Ligand-free
- Pulsed laser ablation in liquids
- Cell culture
- Mouse
- Gametes
- Embryos
12.1 Introduction
Gold nanoparticles have triggered an emerging interest for medical and scientific purposes because of their outstanding characteristics due to their electronic, optical, magnetic and catalytic properties when compared with corresponding bulk material. Among the most popular application areas within life sciences are selective coupling (Sokolov et al. 2003) and sensing (Wang and Ma 2009) of target molecules, localized cancer therapy by plasmonic heating of malignant tissue (Gannon et al. 2008) and delivery of effector molecules to specific receptors or areas of interest (Han et al. 2007). However, since many potential applications for gold nanoparticles are likely to be performed on living cells or organisms, the question of their biocompatibility is of high relevance.
Unintended effects of nanoscaled particles seem to derive mainly from their higher mass-specific surface area, which renders them more biologically active than larger particles of the same chemistry, with a surface-specific dose–response (Faux et al. 2003; Oberdörster et al. 2005.). The underlying mechanisms for nanoparticle-related cellular damage suggested in recent literature are the production of reactive oxygen species (ROS) (Oberdörster et al. 2005) and interaction with DNA (Singh et al. 2009). The interactions at the nano-bio interface, which ultimately determine the toxic potential of any given nanoparticle, are driven by a multitude of parameters (Nel et al. 2009). Some of them are predestined by the nanoparticle itself, such as chemical composition, surface functionalization, size, shape and polarity. Others are influenced by the suspending medium, which most importantly applies to the surface charge. Therefore, it is little surprising that the results of gold nanoparticle biocompatibility studies display a certain amount of heterogeneity, since the used particles, even though they were all based on gold nanoparticles, differed in many aspects, like size and surface functionalization. The influence of AuNP on cell cultures has been examined most extensively and therefore shows exemplarily the diverse effect such particles can potentially have. However, while in somatic cells insults derived from nanoparticle exposure may cause inflammation or even malignant transformation, in case of germline cells, either defect might lead to impaired fertility and/or congenital defects in the offspring. Thus, the current knowledge about reproductive nanotoxicology shall also be summarized.
12.2 Effect of Gold Nanoparticles on Somatic Cells
12.2.1 Cell Culture Studies
Looking at the results of different cell culture studies, the considerable influence of the above mentioned individual particle properties on the harmful potential of gold nanoparticles could be sensed. In most of the trials the effect of differently composed or sized gold nanoparticles were compared with each other. The observed toxicity ranged from negligible, regardless of the used particle type (Shenoy et al. 2006; Salmaso et al. 2009) to intermediate (Thomas and Klibanov 2003; Connor et al. 2005; Massich et al. 2010; Taylor et al. 2010b) and even severe (Pan et al. 2007; Patra et al. 2007; Ding et al. 2010) (Table 12.1). The study performed by Pan et al. (2007) is a distinct example how the effect of nanoparticle on cells can be driven by size, showing that even a moderate decrease in size (from 1.8 nm to 1.4 nm) can make particles four to six times more noxious (Pan et al. 2007). Trials conducted by Ding et al. (2010) exemplify nicely the impact of the nanoparticles zeta potential, i.e. its electric potential at the particle-liquid-interface, on the outcome of the study, elucidating that cytotoxicity correlated with an increasing in positive charge. The experiments from Massich et al. (2010) indicate the influence of surface functionalization, detecting a cytotoxic effect of gold nanoparticle in conjunction with citrate, a common stabilizing agent in chemically derived nanoparticles, which is quite remarkable, because the employed gold nanoparticle dosage was with 10 nM gold fairly low. Only in two studies nanoparticles entirely without any surface modification were used. In these cases the nanoparticles were produced by pulsed laser ablation in liquids (PLAL) without the need for any stabilizing agent. Salmaso et al. (2009) did not observe any toxicity with such particles up to a concentration of 0.74 nM gold. However, Taylor et al. (2010b) noticed a cytotoxic effect, but only at concentrations five magnitudes higher than the ones tested by Salmaso et al. (2009) (Fig. 12.1).
(a) Representative laser scanning microscope images of bovine endothelial cells (GM7373) (3D – projections of 10 optical sections (1 μm each)) after 48 h co-incubation with AuNPs (50 μM Au). (b) Diagram displaying the results of the XTT Proliferation Assay with bovine endothelial cells (GM7373) as a percentage of living cells (negative control = 100%) against the AuNP concentration on a logarithmic scale (Taylor et al. 2010b)
12.2.2 In-Vivo Response to Gold Nanoparticles
In-vivo toxicity studies with gold nanoparticles are not as numerous yet. All trials were performed with chemically produced particles. But despite the relative similarity of the particles, the results still give very different indications. Low doses (<400 μg/kg) in general seem to cause no appreciable toxicity in mice (Lasagna-Reeves et al. 2010; Zhang et al. 2010). At higher concentrations, reports start to differ, which most probably is due to variations in the experimental set up regarding animal, material and frequency as well as route of administration. The latter is highlighted in the study by Zhang et al. (2010), where the highest level of toxicity was detected if the particles were given orally or injected into the peritoneum, while intravenous injection of gold nanoparticles proved to elicit the least damage (Zhang et al. 2010). Worth noticing is also a mouse study performed by Chen et al. (2009), which observed a drastic effect of gold nanoparticles after intraperitoneal injection, only depending on particle size (Chen et al. 2009). Particles with a diameter of 3, 5, 50 and 100 nm did not show any harmful effects, while sizes between 8 and 37 nm induced severe sickness and shortend the survival time on average to 21 days. This finding once again implies the impact of particle properties other the chemical composition on the potential of nanoparticles to have detrimental effects. No in-vivo trials were run so far comparing the influence other aspects such as surface charge. However, Cho et al. (2009) tested PEG-coated gold nanoparticles and found that they induced acute liver inflammation after a single intravenous injection of 850 μg/kg (Cho et al. 2009).
12.3 Reproductive Toxicology of Gold Nanoparticles
12.3.1 Effect of Gold Nanoparticles on Gametes
So far, there have only been two studies published concerning the impact of gold nanoparticles on gametes. However, both trials concentrated on the male side, i.e. the effect of AuNP on spermatozoa. In each study, one working with chemically derived gold nanoparticles (Wiwanitkit et al. 2009), the other using laser-generated ligand-free particles (Taylor et al. 2010c) a decrease in sperm motility was observed after gold nanoparticle exposure. In the former study, which also reported severe morphological defects in treated spermatozoa, no information has been provided concerning the particle concentration. However, in the latter the particle concentration needed to actually show an effect was 50 μM, which is so high, that it exceeds by far the amount of gold nanoparticles necessary for scientific or medical applications. Moreover, in this case the decrease in motility was not accompanied by an increase in abnormal sperm morphology or impaired membrane integrity (Fig. 12.2). A possible explanation for the apparently more severe toxicity of chemically derived nanoparticles could be that the observed effect is actually due to remnants of the reducing or stabilizing agents used during production, not the nanoparticles themselves. Additionally, current trials indicate an influence of ligand-free AuNP also on the fertilising capability of spermatozoa (Taylor et al., unpublished).
(a) Percentage of morphologically abnormal spermatozoa after co-incubation with increasing AuNP concentrations. (b) Percentage of membrane intact spermatozoa after co-incubation with increasing AuNP concentrations. (c) Percentage of motile spermatozoa after co-incubation with increasing AuNP concentrations (*p < 0.05) (Taylor et al. 2010c)
Up to date there are no studies available concerning the impact of gold nanoparticles on oocytes.
12.3.2 Translocation of Gold Nanoparticles to Reproduction-Relevant Sites
Not many studies concerning AuNP biodistribution have examined their ability to pass through reproduction-relevant physiological barriers. So far no information can be given about their ability to enter ovarian follicles. Concerning the crossing of the blood-testicle barrier Balasubramanian et al. (2010) reported the nanoparticle accumulation of AuNP 1 month in the testis thus showing that nanoparticles can potentially cross the blood-testis barrier (Balasubramanian et al. 2010). Translocation of AuNP across the placenta, which acts as another major barrier in reproduction, has been examined after intravenous injection in rodent models. Two of these studies could indeed confirm nanoparticle transfer through the placental membranes (Takahashi and Matsuoka 1981; Semmler-Behnke et al. 2007). Interestingly, another two studies could not find any gold nanoparticles passing through the placental barrier (Challier et al. 1973; Sadauskas et al. 2007), neither did an author who used human placenta in an ex vivo model to investigate the transplacental trafficking of AuNP (Myllynen et al. 2008). Due to the variations in study outcome it is difficult to draw any final conclusions concerning this subject. Therefore, more research is needed to clarify this important matter.
12.3.3 Gold Nanoparticle Impact on Embryo Development
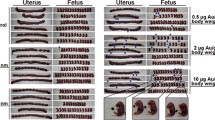
Another crucial aspect is the developmental toxicity of gold nanoparticles. This subject has been addressed in studies using zebrafish (Bar-Ilan et al. 2009; Browning et al. 2009) and chicken (Zielinska et al. 2009, Sawosz et al. 2010) embryos in conjunction with chemically derived particles as well as murine embryos (Taylor et al. 2010a) employing laser-generated nanoparticles. No detrimental effects were noted, even though the presence of AuNP inside the embryos was proven (Bar-Ilan et al. 2009; Browning et al. 2009; Taylor et al. 2010a). However, the data currently available is by far not adequate to properly assess the influence of gold nanoparticles on embryo development because the variety of particle compositions tested is still way to narrow. Furthermore, as yet no long-term in vivo studies have been performed investigating the effect of AuNP on the developing embryo or fetus, respectively. Especially regarding the fact that particle translocation through the placenta cannot be ruled out, there should be an ongoing effort to thoroughly deduce the effect of gold nanoparticles on such a vulnerable organism.
12.4 Conclusion
From the provided data it is rather difficult to depict clear trends regarding the biocompatibility of gold nanoparticles. Compared to other metal nanoparticles such as silver (Bar-Ilan et al. 2009), the general toxicity is certainly rather low. However, most studies did observe adverse effects from a certain dosage onwards. Unfortunately, it is rather difficult to compare between studies especially because the information given concerning the dosage are very diverse. It would be recommendable to find a common notion on how to express nanoparticle dosage. One option would be to calculate the particle surface exposed to the cells or the organism as suggested by Oberdörster et al. (2005), since it combines particle number and size and has shown to fit very well in dose–response curves.
References
Balasubramanian, S. K., Jittiwat, J., Manikandan, J., Ong, C. N., Yu, L. E., & Ong, W. Y. (2010). Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials, 31, 2034–2042.
Bar-Ilan, O., Albrecht, R. M., Fako, V. E., & Furgeson, D. Y. (2009). Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small, 5(16), 1897–1910.
Browning, L. M., Lee, K. J., Huang, T., Nallathamby, P. D., Lowman, J. E., & Xu, X.-H. N. (2009). Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale, 1, 138–152.
Challier, J. C., Panigel, M., & Meyer, E. (1973). Uptake of colloidal 198Au by fetal liver in rat, after direct intrafetal administration. International Journal of Nuclear Medicine and Biology, 1, 103–106.
Chen, Y. S., Hung, Y. C., Liau, I., & Huang, G. S. (2009). Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Research Letters, 4, 858–864.
Cho, W. S., Cho, M., Jeong, J., Choi, M., Cho, H. Y., Han, B. S., Kim, S. H., Kim, H. O., Lim, Y. T., & Chung, B. H. (2009). Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicology and Applied Pharmacology, 236, 16–24.
Connor, E. E., Mwamuka, J., Gole, A., Murphy, C. J., & Wyatt, M. D. (2005). Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small, 1, 325–327.
Ding, Y., Bian, X. C., Yao, W., Li, R. T., Ding, D., Hu, Y., Jiang, X. Q., & Hu, Y. Q. (2010). Surface-potential-regulated transmembrane and cytotoxicity of chitosan/gold hybrid nanospheres. ACS Applied Materials & Interfaces, 2, 1456–1465.
Faux, S. P., Tran, C. L., Miller, B. G., Jones, A. D., Montellier, C., & Donaldson, K. (2003). In vitro determinants of particulate toxicity: The dosemetric for poorly soluble dusts. Sudbury/Suffolk: HSE Books.
Fu W., Shenoy D., Li J., Crasto C., Jones G., DiMarzio C., Sridhar S., Amiji M. (2005). Biomedical applications of gold nanoparticles functionalized using hetero-bifunctional poly (ethylene glycol) spacer. Proceedings of Material Research Symposium, 845.
Gannon, C. J., Patra, C. R., Bhattacharya, R., Mukherjee, P., & Curley, S. A. (2008). Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. Journal of Nanobiotechnology, 6, 2.
Han, G., Ghosh, P., & Rotello, V. M. (2007). Functionalized gold nanoparticles for drug delivery. Nanomedicine-UK, 2, 113–123.
Lasagna-Reeves, C., Gonzalez-Romero, D., Barria, M. A., Olmedo, I., Clos, A., Ramanujam, V. M. S., Urayama, A., Vergara, L., Kogan, M. J., & Soto, C. (2010). Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochemical Biophysical Research Communications, 393, 649–655.
Massich, M. D., Giljohann, D. A., Schmucker, A. L., Patel, P. C., & Mirkin, C. A. (2010). Cellular response of polyvalent oligonucleotide-gold nanoparticle conjugates. ACS Nano, 4, 5641–5646.
Myllynen, P. K., Loughran, M. J., Howard, C. V., Sormunen, R., Walsh, A. A., & Vahakangas, K. H. (2008). Kinetics of gold nanoparticles in the human placenta. Reproductive Toxicology, 26, 130–137.
Nel, A. E., Madler, L., Velegol, D., Xia, T., Hoek, E. M. V., Somasundaran, P., Klaessig, F., Castranova, V., & Thompson, M. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials, 8, 543–557.
Oberdörster, G., Oberdorster, E., & Oberdorster, J. (2005). Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives, 113, 823–839.
Pan, Y., Neuss, S., Leifert, A., Fischler, M., Wen, F., Simon, U., Schmid, G., Brandau, W., & Jahnen-Dechent, W. (2007). Size-dependent cytotoxicity of gold nanoparticles. Small, 3, 1941–1949.
Patra, H. K., Banerjee, S., Chaudhuri, U., Lahiri, P., & Dasgupta, A. K. (2007). Cell selective response to gold nanoparticles. Nanomedicine-Nanotechnology, 3, 111–119.
Sadauskas, E., Wallin, H., Stoltenberg, M., Vogel, U., Doering, P., Larsen, A., & Danscher, G. (2007). Kupffer cells are central in the removal of nanoparticles from the organism. Particle and Fibre Toxicology, 4, 10.
Salmaso, S., Caliceti, P., Amendola, V., Meneghetti, M., Magnusson, J. P., Pasparakis, G., & Alexander, C. (2009). Cell up-take control of gold nanoparticles functionalized with a thermoresponsive polymer. Journal of Materials Chemistry, 19, 1608–1615.
Sawosz, E., Grodzik, M., Lisowski, P., Zwierzchowski, L., Niemiec, T., Zielinska, M., Szmidt, M., & Chwalibog, A. (2010). Influence of hydro-colloids of Ag, Au and Ag/Cu alloy nanoparticles on the inflammatory state at transcriptional level. B Vet I Pulawy, 54, 81–85.
Semmler-Behnke M., Fertsch S., Schmid G., Wenk A., Kreyling W. (2007). Uptake of 1.4 nm versus 18 nm gold nanoparticles by secondary target organs is size dependent in control and pregnant rats after intertracheal or intravenous application. EuroNanoForum 2007, 102–104.
Shenoy, D., Fu, W., Li, J., Crasto, C., Jones, G., DiMarzio, C., Sridhar, S., & Amiji, M. (2006). Surface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and delivery. International Journal of Nanomedicine, 1, 51–57.
Singh, N., Manshian, B., Jenkins, G. J. S., Griffiths, S. M., Williams, P. M., Maffeis, T. G. G., Wright, C. J., & Doak, S. H. (2009). NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials, 30, 3891–3914.
Sokolov, K., Aaron, J., Mack, V., Collier, T., Coghlan, L., Gillenwater, A., Follen, M., & Richards-Kortum, R. (2003a). Vital molecular imaging of carcinogenesis with gold bioconjugates. Medical Physics, 30, 1539–1539.
Sokolov, K., Follen, M., Aaron, J., Pavlova, I., Malpica, A., Lotan, R., & Richards-Kortum, R. (2003b). Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Research, 63, 1999–2004.
Takahashi, S., & Matsuoka, O. (1981). Cross placental-transfer of Au-198-colloid in near term rats. Journal of Radiation Research, 22, 242–249.
Taylor, U., Garrels, W., Petersen, S., Barcikowski, S., Klein, S., Kues, W., Lucas-Hahn, A., Niemann, H., & Rath, D. (2010a). Development of murine embryos after injection of uncoated gold and silver nanoparticles. Reproduction, Fertility and Development, 22, 240–241.
Taylor, U., Klein, S., Petersen, S., Kues, W., Barcikowski, S., & Rath, D. (2010b). Nonendosomal cellular uptake of ligand-free, positively charged gold nanoparticles. Cytometry. Part A, 77(5), 439–446.
Taylor, U., Petersen, S., Barchanski, A., Mittag, A., Barcikowski, S., & Rath, D. (2010c). Influence of gold nanoparticles on vitality parameters of bovine spermatozoa. Reproduction in Domestic Animals, 45, 60–60.
Thomas, M., & Klibanov, A. M. (2003). Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. Proceedings of the National Academy of Science of the United States of America, 100, 9138–9143.
Wang, Z. X., & Ma, L. N. (2009). Gold nanoparticle probes. Coordination Chemistry Reviews, 253, 1607–1618.
Wiwanitkit, V., Sereemaspun, A., & Rojanathanes, R. (2009). Effect of gold nanoparticles on spermatozoa: The first world report. Fertility and Sterility, 91, e7–e8.
Zhang, X. D., Wu, H. Y., Wu, D., Wang, Y. Y., Chang, J. H., Zhai, Z. B., Meng, A. M., Liu, P. X., Zhang, L. A., & Fan, F. Y. (2010). Toxicologic effects of gold nanoparticles in vivo by different administration routes. International Journal of Nanomedicine, 5, 771–781.
Zielinska A. K., Sawosz E., Grodzik M., Chwalibog A., & Kamaszewski M. (2009). Influence of nanoparticles of gold on chicken embryos’ development. Annals of Warsaw University of Life Sciences - SGGW, Animal Science, 249–253.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Taylor, U. et al. (2012). Toxicity of Gold Nanoparticles on Somatic and Reproductive Cells. In: Zahavy, E., Ordentlich, A., Yitzhaki, S., Shafferman, A. (eds) Nano-Biotechnology for Biomedical and Diagnostic Research. Advances in Experimental Medicine and Biology, vol 733. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2555-3_12
Download citation
DOI: https://doi.org/10.1007/978-94-007-2555-3_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-2554-6
Online ISBN: 978-94-007-2555-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)