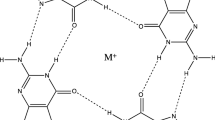
Abstract
Metal ions stabilize quadruplex nucleic acids by coordinating the O6 guanine atoms from G-quartets. These quartets form the basic motif of quadruplex structures. This article systematically surveys the available crystallographic data on native quadruplexes, their ligand complexes and (in one instance) a protein complex. Three categories of quadruplex are examined, tetramolecular, bimolecular, and intramolecular: all are formed by telomeric nucleic acid sequences from human or ciliate organisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction – The Basics of Quadruplex Structure
Polynucleotides and oligonucleotides containing long repeats of guanosine nucleotides have long been known to have a marked tendency to self-associate and aggregrate into gel-like materials. Analysis of the fibres formed from gels of guanosine monophosphate [1] led to a structural model with four strands held together by a hydrogen-bonding arrangement of four guanines – the G-quartet (Figure 1). This very stable motif can self-associate and layers of G-quartets are then formed. The structures of polynucleotides containing purely guanosines are thus four-stranded analogues of the Watson-Crick AT and GC base-paired double helices [2].
Short lengths of nucleic acid can also form structures based on the G-quartet motif under certain circumstances. These quadruplex nucleic acids can be formed from one, two or four strands of DNA or RNA sequences that contain a number of short tracts of guanine nucleotides. Each G-tract should contain optimally 3–5 consecutive guanosines, with a typical sequence being:
where m is the number of G residues in each short G-tract, which are usually directly involved in G-quartet interactions. Xn, Xo, and Xp can be any combination of residues, including G, forming the linkers between G-quartets. This notation has all the G-tracts of equal length. However, this is not a necessary condition since if one or more of the short G tracts is longer than the others, some G residues will be most likely located in the linker regions. Also, the presence of G residues within connecting sequences can give rise to more complex arrangements in which such individual G residues become inserted into the G-quartet stack and displace the G-tract G residues. The structures and topologies of a number of telomeric quadruplexes DNAs in particular, have been determined by X-ray crystallographic and NMR methods; the reader is referred to the extensive reviews on the subject for further detail [3–5].
In principle, tetramolecular (tetrameric) and bimolecular (dimeric) quadruplexes can be formed from the association of non-equal sequences, although almost all bimolecular quadruplexes reported to date are formed by the association of two identical sequences XnGmXoGmXp, where n and p may or may not be zero. Tetramolecular quadruplexes are formed by four XnGmXo strands associating together, where Xn and Xo may be terminal sequences of zero or non-zero length.
The connecting sequences intervening between successive G-tracts can link stacked G-quartets in a number of distinct ways, so that a wide variety of quadruplex topologies can be formed. These connectors can form loops, of which there are three principal types: diagonal, lateral or propeller (also sometimes termed chain-reversal). The particular type formed is dependent on the number of G-quartets comprising the stem of a quadruplex, on loop length and sequence and sometimes on the nature of the alkali metal ion. Propeller loops necessarily connect two strands in the same parallel orientation, linking the bottom G-quartet with the top G-quartet in a G-stack whereas diagonal and lateral loops connect chains in opposing, anti-parallel orientations. Lateral (sometimes termed edge-wise) loops join adjacent G-strands. Two lateral loops can be located either on the same or opposite faces of a quadruplex, corresponding to head-to-head or head-to-tail, respectively, when in bimolecular quadruplexes. Strand polarities can vary, with one being a head-to-tail lateral loop dimer in which all adjacent strands are anti-parallel, and the other is a head-to-head hairpin quadruplex with one adjacent strand parallel and the other is anti-parallel. The second type of anti-parallel loop is the diagonal loop, which joins opposite G-strands. In this instance the directionalities of adjacent strands must alternate between parallel and anti-parallel, and are arranged around a core of stacked G-quartets. All-parallel quadruplexes have all guanine glycosidic angles in the anti conformation, whereas anti-parallel quadruplexes have equal numbers of the guanosine nucleotides in syn and anti conformations, arranged in a way that is particular for a given topology. All quadruplex structures have four grooves, defined as the cavities bounded by the phosphodiester backbones.
2 The Role of Metal Ions
Early models for four-fold helices and quadruplex oligonucleotide structures based on the G-quartet motif [6,7] suggested the existence of a central channel into which water molecules or cations could be placed. This interpretation was subsequently confirmed and extended by a large number of biophysical and structural studies on G-quartet-containing nucleic acid sequences (reviewed in [8]). This central channel is a universal and unique feature of all quadruplex structures, distinguishing them from other types of nucleic acid arrangements, not least nucleic acid double helices. Of particular significance have been the findings that the stability of these four-stranded structures depends on the presence of physiological concentrations of the alkali metals sodium or potassium with the order of stabilizing ability being K+ > Na+.
The precise location of an ion in the channel would be expected to depend on its ionic radius. K+ ions are too large to be accommodated in the plane of a G-quartet, whereas Na+ ions are small enough to be coordinated in-plane with all four guanine bases in a G-quartet. Several other ions can stabilize quadruplexes, including those of divalent heavier metals such as rubidium and cesium (Rb+, Cs+), strontium (Sr3+), thallium (Tl+), calcium (Ca2+), lead (Pb2+), barium (Ba2+), though all to a lesser extent than K+. Metal ions in the channel are always located in crystal structures. Metal ion coordination patterns observed in X-ray crystallographic analyses generally concur with findings from several multinuclear NMR studies, which have been able to directly locate Na+, K+, Rb+, and Ca2+ ions [9]. The presence of particular ions in the channel can also change quadruplex conformation and fold, an effect that has been most extensively documented for human telomeric quadruplexes, reviewed in [5].
The integral role that metal cations play in G-quadruplex structures was directly observed when the first crystal structure of a quadruplex, the tetramolecular d(TGGGGT), was reported [10]. The crystal structures of bimolecular and intramolecular human telomeric G-quadruplex [11], also showing central channel metal ion arrangements. Here, a novel structure, with an all-parallel topology, was revealed to be the form crystallizing in potassium-containing experimental conditions. Surprisingly, this was fundamentally distinct from an earlier structure reported using NMR techniques [12]. The latter was reported for the identical G-quadruplex-forming sequence in a sodium-containing environment and showed an anti-parallel topology (Figure 2). Earlier studies on the bimolecular quadruplex formed by d(GGGGTTTTGGGG) had suggested that here too, NMR and crystallography were in disagreement [13,14], although a subsequent crystallographic analysis [15] showed that the NMR assignment of topology was correct and that the initial crystal structure [13] was not. G-quadruplex structures are also sensitive to flanking nucleotides, concentration, and molecular crowding conditions [16,17]. In general, metal cations coordinate to the O6 guanine substituent groups lining the central channel of a quadruplex. The positively-charged cations counter the negative electrostatic effect of the carbonyl groups, thus enhancing quadruplex stabilization (Figure 2).
The aim of this review is to survey all the available crystal structure data on the geometry of metal ions in quadruplex crystal structures, as these constitute the most accurate and reliable source of geometric information on ion environments in quadruplexes. We have conducted a systematic search of the Protein Data Bank Database (PDB) [18] using the key search words quadruplex or tetraplex DNA or RNA crystal structures, which resulted in 151 hits (May 2011). The list was further edited by omitting structures that do not represent biologically relevant sequences. The final list contained 36 structures. All 36 structures are from sequences that are of telomeric origin. These have been further categorized into three subgroups in respect of the organism from which the telomeric G-quadruplex-forming sequence originated: Tetrahymena, Oxytricha nova, and human. Each structure was visualized and metal coordination distances measured and recorded.
A variety of metal ions have been observed in the central quadruplex channel in these crystal structures. For example, mono-cations such as sodium, potassium and thallium and di-cations such as strontium and calcium have all been reported. The observed ions either coordinate to a single G-quartet in (or close to) a square-planar arrangement or between two stacking G-quartets in a bipyramidal antiprismatic arrangement. The interplay between specific quadruplex sequence, cation type and size and other factors affecting the coordination arrangement are discussed below.
3 Metal Ions in Quadruplex Structures
3.1 Tetrahymena Telomeric Quadruplexes
The quadruplex crystal structure formed from the telomeric sequence in Tetrahymena is tetramolecular. Four strands, each comprising the sequence d(TGGGGT), associate together, adopting the same orientation in the 5’ to 3’ direction to form a parallel quadruplex (Figure 3). This parallel quadruplex arrangement is maintained in both of the RNA and the DNA forms. The ions that have been observed in various crystal structures of this quadruplex are: sodium, calcium, thallium, strontium or potassium.
Both sodium and thallium ions are able to adopt either the square planar or bipyramidal antiprismatic arrangement. The preference for forming one coordination arrangement compared to another is a consequence of the position of the cation in the ion channel. It is notable that quadruplexes can stack in the crystal structures in a manner such as to allow a continuous one-dimensional array of ions in the ion channel to be formed, proceeding from one quadruplex to the next. At the point where this continuity is broken, for example when just two quadruplexes are stacked together (a typical motif), the ion channel ends. The ion channel hence resembles a linear tube with a center and two ends. The ends are represented by an open G-quartet that does not have a further quadruplex stacking onto it. So, where the ions are coordinating to an “end” G-quartet, sodium and thallium ions adopt a square planar coordination arrangement, and a bipyramidal antiprismatic arrangement where they are positioned away from the “end” G-quartet and closer to the centre of the ion channel (Figure 4).
Views of the structures of the tetramolecular quadruplexes listed in Table 1, showing the ion coordination in the channels and the relevant PDB codes. Sodium ions are colored mauve.
On the other hand, calcium, strontium and potassium ions show a marked preference for the bipyramidal antiprismatic coordination arrangement. These ions are always positioned between the stacked G-quartets and never within a single G-quartet (Figure 5). The ionic radius for strontium and potassium ions are similar, 1.18 Å and 1.38 Å, respectively. These ions are larger than sodium and thallium (ionic radii of 1.02 Å and 0.89 Å, respectively). It is apparent that the larger size ions simply may not be accommodated except between the stacking G-quartets, for purely steric reasons. Calcium ions, on the other hand, have an ionic radius of 1.00 Å (closer to that of sodium and thallium) but still show a tendency to coordinate between the G-quartets rather than be in-plane or close to them. It may be that the two positive charges of the calcium ion are sufficient to counter the negative electrostatic character of eight O6 carbonyl groups from two consecutive stacked G-quartets and thus calcium ions are observed to be positioned between two G-quartets in all the crystal structures where it is present.
The coordination distances have a smaller distribution of values for the square planar arrangement, ranging between 2.3 to 2.4 Å compared to the range seen with bipyramidal antiprismatic coordination, of 2.3 to 3.6 Å (Table 1). In a square-planar arrangement, coordination distances are limited by the linear dimensions of the relatively small and flat area in the center of the G-quartet where the cation has to fit in order to maintain the cation-G-quartet square planar geometry. In contrast, a larger area is available between two stacked G-quartets for an ion to be fitted in the bipyramidal antiprismatic arrangement.
The range of distances for cations at the interface between two separate G-quadruplexes is 2.6 Å to 3.2 Å (Table 1), with the ions adopting bipyramidal antiprismatic coordination in all instances. This is not surprising since the ion is positioned between two continuous ion channels.
3.2 Oxytricha nova Telomeric Quadruplexes
The quadruplex crystal structures determined for the telomeric sequence in Oxytricha nova are all bimolecular. Two strands, each formed of the sequence d(GGGGTTTTGGGG), associate together adopting an anti-parallel quadruplex conformation where the strands run in opposite direction relative to each other in the 5’ to 3’ direction (Figure 3) and the d(TTTT) sequence forms a diagonal loop. The quadruplex fold is not affected by ion type or presence of bound ligands or protein (Table 2). It is also not affected by the crystal form or space group (i.e. crystal packing); all of the native (ligand-free) structures have this identical topology (PDB IDs 1JPQ, 1JRN, 2GWQ and 2GWE) (Table 2).
In this quadruplex type, both potassium and thallium ions always adopt bipyramidal antiprismatic coordination between two consecutive stacked G-quartets despite the large difference in their ionic radius (Figure 6). The coordination distances to O6 atoms range between 2.4 Å and 3.3 Å. However, sodium ions (even though they are slightly larger than thallium ions) are coordinated in square planar arrangements (Figure 6) within the G-quartets, with coordination distances to O6 atoms ranging between 2.1 Å and 2.5 Å (Table 2).
In contrast to the Tetrahymena quadruplex structures, individual Oxytricha nova bimolecular quadruplexes cannot stack on one another in the crystal structures due to the presence of the diagonal T4 loop (Figure 3) capping each terminal G-quartet face and thus preventing the continuation of the ion channel from one quadruplex to the next. No crystal structure of an intramolecular Oxytricha nova quadruplex is currently available, but it will be interesting to see what geometries are shown by the channel ions.
3.3 Human Telomeric Quadruplexes
The available database of human telomeric crystal structures consists of eight structures; six DNA quadruplexes, two of which are ligand-free and four ligand-bound, together with two RNA quadruplexes, one ligand-free and one ligand-bound (Table 3).
All the structures reported to date contain potassium ions only in the ion channel, irrespective of the ions present in the experimental conditions used in the crystallization setup – typically a number of different ions are employed simultaneously in crystallization trials. This is a consequence of the higher affinity of potassium ions for quadruplexes relative to any other ion. In all the structures in the database, the potassium ions adopt bipyramidal antiprismatic coordination (Figure 7) with distances ranging between 2.4 Å to 3.3 Å. Similarly, the potassium ion involved in linking two stacking quadruplexes adopts the same bipyramidal antiprismatic coordination with distances in a similar range: 2.6 Å to 3.0 Å (Table 3).
4 Conclusions
All non-human telomeric quadruplex crystal structures reported to date consistently show that the fold of a specific quadruplex sequence is not affected by the ionic conditions used in the crystallization experiment or the coordination of the ions in the ion channel. All these structures are highly hydrated and the crystal lattice environments are crowded with ions, solvent and quadruplex molecules. The relevance of the human telomeric quadruplex crystal structures to biological environments is still controversial [35,36], but whatever the form of these quadruplexes, the ion channel is retained as an essential structural motif.
Smaller ions, such as sodium and thallium, are more inherently likely to exhibit dynamic behavior within the ion channel but they are trapped in a particular position by coordinating within a G-quartet in a square planar arrangement and hence are observable by X-ray crystallography. It is significant that none of the quadruplex crystal structures show any evidence for disordered sodium ions in the channels, which would indicate high mobility. Similarly sized ions that possess greater net positive charge (such as calcium), may also in principle be able to move along the ion channel, though again they are only observed with bipyramidal antiprismatic coordination between two stacked G-quartets and not within individual channels. This may be due to a preference for their larger net positive charge to simultaneously counter the negative electrostatics of the O6 carbonyl groups in two stacked G-quartets.
Note added in proof
A very recent crystal structure (PDB id 3QXR: D. Wei, G. N. Parkinson, S. Neidle, to be published) has shown that potassium and magnesium ions can be bound in loops and clefts of an intramolecular quadruplex, suggesting that some metal ions have important additional stabilizing roles in these structures over and above their presence in quadruplex ion channels.
References
M. Gellert, M. N. Lipsett, D. R. Davies, Proc. Natl. Acad. Sci. USA 1962, 48, 2013-2018.
S. Arnott, R. Chandrasekaran, C. M. Marttila, Biochem. J. 1974, 141, 537-543.
J. T. Davies, Angew. Chem. Int. Ed. 2004, 43, 668-698.
S. Burge, G. N. Parkinson, P. Hazel, A. K. Todd, S. Neidle, Nucleic Acids Res. 2006, 34, 5402-5415.
S. Neidle, Therapeutic Applications of Quadruplex Nucleic Acids. Academic Press, San Diego, USA, 2011.
F. B. Howard, H. T. Miles, Biochemistry 1982, 21, 6736-6745.
P. Balagurumoorthy, S. K. Brahmachari, J. Biol. Chem. 1994, 269, 21858-21869
N. V. Hud, J. Plavec, in Quadruplex Nucleic Acids, Eds S. Neidle, S. Balasubramanian, Royal Society of Chemistry, Cambridge, UK, 2006.
R. Ida, G. Wu, J. Amer. Chem. Soc. 2008, 130, 3590-3602.
G. Laughlan, A. I. Murchie, D. G. Norman, M. H. Moore, P. C. Moody, D. M. Lilley, B. Luisi, Science 1994, 265, 520-524; K. Phillips, Z. Dauter, A. I. Murchie, D. M. Lilley, B. Luisi, J. Mol. Biol. 1997, 273, 171-182.
G. N. Parkinson, M. P. H. Lee, S. Neidle, Nature 2002, 417, 876-880.
Y. Wang, D. J. Patel, Structure 1993, 1, 263-282.
C. Kang, X. Xhang, R. Ratliff, R. Moysis, A. Rich, Nature 1992, 356, 126-131.
P. Schultze, N. V. Hud, F. W. Smith, J. Feigon, Nucleic Acids Res. 1994 , 27, 3018-3028; P. Schultze, F. W. Smith, J. Feigon, Structure 1994 , 2, 221-233.
S. M. Haider, G. N. Parkinson, S. Neidle, J. Mol. Biol. 2002, 320, 189-200.
S. Neidle, Curr. Opin. Struct. Biol 2009, 19, 239-250
S. Neidle, G. N. Parkinson, Biochimie 2008, 90, 1184-1196.
H. M. Berman, T. Battistuz, T. N. Bhat, W. F. Bluhm, P. E. Bourne, K. Burkhardt, Z. Feng, G. L. Gilliland, L. Iype, S. Jain, P. Fagan, J. Marvin, D. Padilla, V. Ravichandran, B. Schneider, N. Thanki, H. Weissig, J. D. Westerbrook, C. Zardecki, Acta Crystallogr. 2002, D58, 899-907.
G. R. Clark, P. D. Pytel, C. J. Squire, S. Neidle, J. Amer. Chem. Soc. 2003, 125, 4066-4067.
C. Creze, B. Rinaldi, R. Haser, P. Bouvet, P. Gouet, Acta Crystallogr. 2007, D63, 682-688.
M. P. H. Lee, G. N. Parkinson, P. Hazel, S. Neidle, J. Amer. Chem. Soc. 2007, 129, 10106-10107.
C. Cáceres, G. Wright, C. Gouyette, G. Parkinson, J. A. Subirana, Nucleic Acids Res. 2004, 32, 1097-1102.
J. Deng, Y. Xiong, M. Sundaralingam, Proc. Natl. Acad. Sci. USA 2001, 98, 13665-13670.
B. Pan, K. Shi, M. Sundaralingam, J. Mol. Biol. 2006, 363, 451-459.
N. H. Campbell, D. L. Smith, A. P. Reszka, S. Neidle, D. O’Hagan, Org. Biomol. Chem. 2011, 9, 1328-1331.
M. L. Gill, S. A. Strobel, J. P. Loria, Nucleic Acids Res. 2006, 34, 4506-4514.
S. M. Haider, G. N. Parkinson, S. Neidle, J. Mol. Biol. 2003, 326, 117-125.
N. H. Campbell, M. Patel, A. B. Tofa, R. Ghosh, G. N. Parkinson, S. Neidle, Biochemistry 2009, 48, 1675-1680.
M. P. Horvath, S. C. Schultz, J. Mol. Biol. 2001, 310, 367-377.
G. N. Parkinson, R. Ghosh, S. Neidle, Biochemistry 2007, 46, 2390-2397.
G. N. Parkinson, F. Cuenca, S. Neidle, J. Mol. Biol. 2008, 381, 1145-1156.
N. H. Campbell, G. N. Parkinson, A. P. Reszka, S. Neidle, J. Amer. Chem. Soc. 2008, 130, 6722-6724.
G. W. Collie, S. M. Haider, S. Neidle, G. N. Parkinson, Nucleic Acids Res. 2010, 38, 5569-5580.
G. W. Collie, S. Sparapani, G. N. Parkinson, S. Neidle, J. Amer. Chem. Soc. 2011, 133, 2721-2728.
D. Renčiuk, I. Kejnovská, P. Školáková, K. Bednářová, J. Motlová, M. Vorlíčková, Nucleic Acids Res. 2009, 37, 6625-6634.
Y. Xue, Z. Y. Kan, O. Wang, Y. Yao, J. Liu, Y. H. Hao, Z. Tan, J. Amer. Chem. Soc. 2007, 129, 11185-11191.
Acknowledgments
This work was supported by a CRUK Programme Grant to S. Neidle.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Campbell, N.H., Neidle, S. (2012). G-Quadruplexes and Metal Ions. In: Sigel, A., Sigel, H., Sigel, R. (eds) Interplay between Metal Ions and Nucleic Acids. Metal Ions in Life Sciences, vol 10. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2172-2_4
Download citation
DOI: https://doi.org/10.1007/978-94-007-2172-2_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-2171-5
Online ISBN: 978-94-007-2172-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)