Abstract
The book starts with the brief review of chapters. In this chapter heavy metals have been redefined as those trace elements that have ≥ 3 g/cm3 densities and may cause harmful biological effects. The chapter arrived to this definition by clarifying first the light elements on the basis of their electronic configurations and compatibility with those of bioelements (CHNOPS group) in constructing biomolecules. As compatibility criteria the chemical bond formation between s–p electrons and p–p electrons were taken, allowing the tetrahedral three dimensional construction of biological compounds with four bonding partners. The compatibility range ended at 1s22s22p63s23p64s2 electronic configuration corresponding to calcium, which is the 20th element in the periodic table. From element 21 (Sc) the wide range of redox behavior, high reactivity, rich coordination chemistry and complex formation of transition metals is due to the outer d and f electron subshells and explain their important catalytic role in enzyme reactions and toxicity at higher cellular concentrations. The chapter describes the most important cellular effects of heavy metals. The advantages of changing from in vivo to in vitro cellular systems have been pointed out. The methods for the detection and determination of heavy metals in cells are summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
- Definition of heavy metals
- Compatibility of bioelements
- Cellular effects
- Cellular systems
- Detection of heavy metals
Introduction
Why Another Book on Heavy Metals?
The short answer to this question is that cellular and subcellular functions of heavy metals have neither been described in any detail nor summarized in a book. To the contrary the effect of heavy metals on organs and organisms has been intensively studied. This is indicated by a fast search in PubMed by entering the words “heavy metals” and getting more than 338,000 publications (as of January 2011). After justifying the requirement of a book dealing with the cellular effects of heavy metals one can omit lengthy discussions of general toxic effects, comprehensive reviews of heavy metals dealing with the physiology, including nutrition, intestinal absorption, heavy metal poisoning, excretion, homeostasis and their role in the function of different organs (heart, muscle, liver, lung, brain, kidney etc.).
Brief Review of Chapters
Before going into the details of Chap. 1, a brief outline of Chaps. 2–16 is given.
Chapter 2. The aim of this chapter is the biotechnological evaluation of data accumulated in the last decade on the molecular background of the toxic metal/metalloid tolerance of fungi.
Chapter 3 describes that although, Cr(VI) reduction itself may proceed outside the cell, it is now generally accepted that Cr(VI)-induced DNA damage and genotoxicity takes place intracellularly. The extracellular reduction of Cr(VI) to Cr(III) is regarded as a detoxification process, as Cr(III) crosses the cell membrane at a much slower rate than Cr(VI). There are certain Cr(V) and Cr(III) complexes generated extracellularly that have high permeabilities and consequently may penetrate into the cell and cause intracellular damage. The reduction of Cr(VI) to Cr(III) with different antioxidants is not only a detoxification reaction, but also increases the viability of the budding yeast Saccharomyces ceravisiae.
Chapter 4. The arsenic tolerance mechanism of yeast cells is elucidated by reviewing the molecular biology of arsenic tolerance in budding yeast, focusing on arsenic sensing, signalling and detoxification mechanisms and how these pathways are regulated.
Chapter 5. Heavy metal toxicity of methyl-HgCl, HgCl2 and CdCl2 in the Aedes albopictus insect cell line is discussed. Short treatment of Aedes albopictus cells with sublethal doses of CdCl2 and HgCl2 induced abnormal microtubular polymerization giving the cells a neuron-like appearance, while MeHgCl was not able to induce such neurite-like processes. Viability and proliferation assays showed clear distinction among the toxicities of Cd, Hg and MeHg reflecting differences of their mechanisms of action.
Chapters 6–9. The effect of heavy metal ions (Cd2+, Ni2+, Cr6+, Ag+) on chromatin structure was a neglected field so far. Chromatin distorsions are visualized in these chapters. These chapters confirm the notion that cells similarly to organisms die in many ways and the genotoxic cell death is dependent on the heavy metal or other toxic agent.
Chapter 6. Oxidative DNA damages of Cd have apoptotic biochemical and morphological consequences. Low concentrations of CdCl2 (0.5–5 mM) cause biochemical (strand breaks, carcinogenic indicator, DNA replication, DNA repair) and morphological (chromatin) changes in CHO and murine preB cells.
In Chap. 7 chromatotoxicity was visualized by time-lapse video microscopy designed to follow the motility of single cells under physiological and genotoxic conditions. Ni-treated cells moved faster than control cells, but this motion was less intense than the so called “apoptotic dance” preceding cell death observed recently after Pb-treatment of HaCaT cells.
Chapter 8. With regard to carcinogenicity two heavy metals, chromium(VI) and arsenic(III) are considered human carcinogens sharing common properties. That chromium ions, especially Cr(VI) interfere with the chromatin structure and cellular functions of Schisosaccharomyces pombe are discussed in Chap. 8. Results show that subtoxic levels of Cr(VI) (<1 mM) did not cause significant chromatin changes in S. pombe cells. Early signs of apoptotic cytotoxicity were observed at 10 mM Cr(VI)concentration. Nuclear changes caused by 10 and 50 mM Cr(VI) were characterized by apoptosis seen as broken nuclei and apoptotic bodies. High concentration of Cr(VI) ions (75–200 mM) initiated necrotic nuclear changes. MIC50 values of Cr(VI) and Cr(III) show that Cr(VI) is ~15-times more toxic than Cr(III).
Chapter 9. The most typical chromatin change upon AgNO3 treatment of HaCaT and K562 cells was chromatin tail formation that could be accounted for by a decrease in chromatin supercoiling related to a dose dependent reduction of ATP content.
Chapter 10. In this chapter, two of the more recent additions to the weapons arena, depleted uranium and heavy metal tungsten-induced genotoxic and carcinogenic properties are detailed. The toxicological and genotoxic properties derived from in vitro and in vivo studies and the health effects of known human exposures are discussed.
Chapter 11. The possible role of oxidative damage in metal-induced carcinogenesis is reviewed in this chapter presenting evidence of the possible mechanistic involvement of oxidative DNA and protein damage in metal-induced carcinogenesis. The strongest association of oxidative damage with carcinogenesis comes from the mutagenicity of DNA. Oxidative damage to nuclear proteins affects chromatin structure, gene expression, whereas damage to regulatory proteins disturbs cell cycle and induces apoptosis.
Chapter 12. Proteins as targets of heavy metals and metalloids are discussed in this chapter. The interference of heavy metal ions (Cd2+, Hg2+ and Pb2+), metalloid arsenic(III) species inhibit the refolding of denatured proteins and may lead to the formation of proteotoxic aggregates of misfolded proteins.
Chapter 13 describes that ubiquitin ligases play a key role in the cadmium induced stress response. Murine embryonic fibroblast cells exposed to cadmium, methylmercury and arsenic induce oxidative stress, disrupt the ubiquitin proteasomal system, cell cycle regulation and may affect key cell stress pathways.
Chapter 14. The heavy metal-induced disruption of ubiquitin proteasomal system plays a critical role in cellular mechanisms such as cell cycle regulation and apoptosis.
Chapter 15: As far as the cellular effect of heavy metals is concerned lead poisoning is known to cause the formation of small red blood cells. As a consequence of lead poisoning megaloblastic anemia/pernicious anemia may occur inducing elevated serum iron level. Blood level-Pb is regarded as a biological marker to environmental lead exposure.
Chapter 16: The best known way to inactivate heavy metals is by chelation, without removing them from the environment. The last chapter of the book provides a solution for the removal of heavy metals as their highly insoluble sulfides and the oxidation of toxic contaminants.
Definition of Heavy Metals
The term heavy metals has been generally used by referring to a group of metals and metalloids (semimetals), and to their contamination that is often causing toxicity and ecological problems. The available lists of heavy metals significantly differ from one another raising the question how heavy metals could be more precisely defined. Since there is no such clear definition, let us define first the light metals. Looking at the periodic table we see a gradual increase in the atomic number of elements starting from hydrogen. But which element should be the upper limit of the light elements. The electronic configurations is a reasonable orientation point. The basic principle is to go as far in the electronic configuration of elements as possible without disturbing the compatibility of the bioelements known as the CHNOPS group. In the mnemonic CHNOPS the letters stand for the symbols of carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur. Worthwhile to notice that the elements of the CHNOPS group belong to the first three periods and these elements contain exclusively s and p electrons, but no d or f electrons (Table 1.1). The s sublevel can hold two electrons, so 1s in the first period is filled at helium (1s 2). The p sublevel in the second and third periods can hold six electrons, in the d-block the atoms of the elements have between 1 and 10 d electrons and the f sublevel 14 electrons. Periods four and five can hold 18, period six can hold 32 and period seven 16 elements.
Although, there are more than 30 elements that can be found in cells, the elements of the CHNOPS group are the most abundant. These six bioelements make up nearly 98% of humans. Although, calcium is not among the bioelements, it contributes to 1.5% body weight at least in humans. Most of the calcium is present as bones where Ca is in homeostatic equilibrium with the calcium level of the blood. In Table 1.1 the first 20 elements are indeed light, as their density is less than 3 g/cm3. The 21st element in the periodic table is scandium (Sc) with a density of nearly 3 g/cm3 and an electron configuration of 1s22s22p63s23p64s23d1. Its properties are intermediate between those of Al and Y, which are other eka-boron elements (lying under boron in the periodic table), similarly to the properties of Ca which is intermediate between those of Mg and Sr. There is also a diagonal relationship between Mg and Sc, just as there is between Be and Al. Scandium is not only a transition element, but also a rare earth element. The atoms of transition metals have incomplete d-sub-shells or can give rise to cations with incomplete d-sub-shells. Groups 3 through 12 of the periodic table are called “transition metals” with valence electrons, or the electrons they use to combine with other elements, present in more than one shell. The three noteworthy elements in the transition metals family are iron, cobalt, and nickel, which are able to produce a magnetic field. Of the 38 transition elements here only those are mentioned which also belong to the microelements i.e. they have been found in cells or are frequently mentioned among toxic agents: Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Zr, Mo, Ag, Cd, Hg, B. Post-transition metals are: Zn, Cd, Hg and UUb of group 12. The 30 rare earth elements that are special transition metals at the bottom of the periodic table belong to the lanthanide and actinide series found in group 3 of the periodic table and in the 6th and 7th periods. Some of the trans-uranium elements have been synthesized by man. Sixteen of the rare earth elements are not as rare as their name would suggest. They are found in nature in much higher quantities than the precious metals gold or platinum with a yearly rare earth element production of nearly 150,000 t. Actinides are all radioactive including the best known and most dangerous actinides of uranium (U), thorium (Th), neptunium (Np) and the transuranium plutonium (Pu, artificial). Environmental radioactivity and pollution is not limited solely to actinides. There are non-actinides such as radium (Ra) and radon (Rn) which is the radioactive daughter from the decay of uranium or cobalt-60 (Co60) and strontium-90 (Sr90).
Due to the number of properties shared by the transition elements it is reasonable to classify them as heavy metals. But is it right to draw the dividing line between light and heavy metals at ~3 g/cm3? The term heavy metals was regarded as meaningless as scientists were unable to come up with a consensus. Moreover, the term heavy metal has never been defined by IUPAC, thus it was suggested that any idea of defining heavy metals on the basis of density should be abandoned as it was yielding nothing but confusion (Duffus 2002). This conclusion came from definitions modified by various authors classifying heavy metals with different specific gravity values (Bjerrum 1936; Van Nostrand 1964; Grant and Grant 1987; Parker 1989; Lozet and Mathieu 1991; Morris 1992; Streit 1994; Thornton 1995; Falbe and Regitz 1996; Srivastava and Majumder 2008). The general tendency in these earlier definitions was that the elemental specific gravity values of heavy metals decreased from ≥ 7 to ≥ 3.5 g/cm3 between 1936 and 1996. Heavy metal is meaningless in the sense that metals are available to plant and animal cells in solution and once such a chemical compound is formed the density of the metal is of less importance (Appenroth 2010). The main reason why the name “heavy metal” cannot be abandoned is that the term is associated with elements that have harmful biological effects or are expected to be toxic. In plant science, the term heavy metal is so widely accepted that it would be difficult to eliminate it (Appenroth 2010). As a typical example the accumulation of heavy metals in plants is mentioned. There is a significant difference in heavy metal accumulation in plant roots versus shoots between accumulator and nonaccumulator plants indicating that metal accumulation and tolerance is a complex trait, requiring the coordinated expression of specific metal transport-related genes in different cell types (Klein et al. 2008). Specific metal tolerance found at cellular level in hyperaccumulating plants reflected specific adaptations to heavy metals (Ni, Zn, Cd) not only in the whole plant but also in cells (Marquès et al. 2004). The metal/metalloid tolerance in fungi is discussed in the next chapter.
As we can neither abandon the use of “heavy metals” nor wait till this term becomes obsolete, it is better to redefine it. It is suggested to set the boundary between light and heavy metals at the lowest specific gravity value (≥ 3 g/cm3). The most important heavy metals include (in alphabetic order): antimony (Sb, atomic number 51), arsenic (As, 33), barium (Ba, 56), bismuth (Bi, 83), cadmium (Cd, 48), cerium (Ce, 58), chromium (Cr, 24), cobalt (Co, 27), copper (Cu, 29), gallium (Ga, 31), germanium (Ge, 32), gold (Au, 79), iron (Fe, 26), lead (Pb, 82), manganese (Mn, 25), mercury (Hg, 80), nickel (Ni, 28), platinum (Pt, 78), rubidium (Rb, 37) silver (Ag, 47), strontium (Sr, 38), tellurium (Te, 52), thallium (Th, 81), tin (Sn, 50), titanium (Ti, 22), uranium (U, 92), vanadium (V, 23), zinc (Zn, 30), zirconium (Zr, 40).
At 3 g/cm3 and higher density values the chemical properties of transition or d-metals have several features in common: most transition metals have variable valences, with more than one possible oxidation- or valence-state causing significant incompatibility with bioelements. The covalent bonding of bioelements requires the consideration of valence shell atomic orbitals. For second period elements such as carbon, nitrogen and oxygen, these orbitals have been designated 2s, 2p x, 2p y and 2p z. The spatial distribution of electrons occupying each of these orbitals is shown in the diagram below reflecting the axial symmetry of single bond formation between electrons and the tetrahedral geometry of s–p and p–p bond formation with a tetrahedral bond angel of 109.5° with four bonding partners (Fig. 1.1), trigonal configuration (120°) with three and linear arrangement (180°) with two bonding partners. The bond angles of water (H2O, 104.5°) and ammonia (NH3, 107.3°) are somewhat distorted relative to the tetrahedral bond angel and make these molecules polar.
Due to the presence of d electrons the atoms of transition metals can form nine sp3d hybrid bond orbitals either as neutral atoms (Co, Rh, Ir) or after taking up an electron (Fe, Ru, Os). To demonstrate how much such structures differ from the tetrahedral building blocks of the CHNOPS group, the involvement of enneacovalence (formation of nine covalent bonds) is taken as an example. In enneacovalent structures with nine hydrogens, these atoms lie at the corners of a trigonal prism with three lateral caps (Abrahams et al. 1964). Enneacovalence of iron can be achieved by transferring an electron to the iron atom. Enneacovalent iron and cobalt structures are compatible with the electroneutrality principle of covalent bonds, but their molecular shape is quite different. The electroneutrality principle states that the resultant electric charge on each atom is close to zero (in the range of ±1).
If we would not consider their biological impact and would take only the specific gravity to define heavy metals, then all metals above 3 g/cm3 would belong to the heavy metals. To come closer to an acceptable definition we have to make a selection and specify that heavy metals have higher than 3 g/cm3 densities, have biological effects at low concentrations and are toxic when present in cells at higher than the tolerable physiological level.
Trace Metal Elements
Here only the analytical and biochemical meaning of trace metal elements is dealt with, while the geochemical composition of trace metals is neglected. To denote the relative proportions, a trace metal element is present in a sample of less than 100 parts per million (ppm, 10− 6) i.e. less than 100 mg/g. Such minute quantities of trace metals are needed for the proper growth, development, and physiology of the cell or organism (Bowen 1976). Trace metal elements in nutrition are also referred to as micronutrients. Major criteria for a micronutrient trace element are: (a) its presence in healthy tissues, (b) appearance in fetus and newborns, (c) homeostatic control of uptake in the blood, (d) control of excretion, (e) known biological function. The essential microelements that meet all these criteria include cobalt (Co, atomic number 27), copper (Cu, 29), chromium (Cr, 24), fluorine (F, 9), iron (Fe, 26), iodine (I, 53), manganese (Mn, 25), molybdenum (Mo, 42), selenium (Se, 34) and zinc (Zn, 30). Less stringent criteria would allow the additional incorporation of nickel (Ni, 28), tin (Sn, 50), vanadium (V, 23), silicon (Si, 14), boron (B, 5) as important micronutrients, whereas aluminum (Al, 13), arsenic (As, 33), barium (Ba, 56), bismuth (Bi, 83), bromine (Br, 35), cadmium (Cd, 48), germanium (Ge, 32), gold (Au, 79), lead (Pb, 82), lithium (Li, 3), magnesium (Mg, 12), mercury (Hg, 80), rubidium (Rb, 37), silver (Ag, 47), strontium (Sr, 38), titanium (Ti, 22) and zirconium (Zr, 40) have been found in plant and animal tissues (Fig. 1.2), but their biological importance is still to be clarified. The comparison of heavy metals with trace elements shows that most of the heavy metals are trace elements some of them with known, others with unknown biological function. Trace elements which are not heavy metals i.e. their density is less than 3 g/cm3 are: Li, B, Mg, Al and Si. Trace amounts of lithium are present in the oceans and in some marine organisms, without apparent vital biological function. Lithium, despite of its simple structure, has numerous biological effects. It was reported to have a therapeutic effect in the prophylactic treatment of manic depression (Shastry 2005). Boron is a plant nutrient, its lack causes boron deficiency, whereas high soil boron concentration is toxic to plants. It is regarded as an ultratrace element at least for rats, without known biological function. Magnesium ions play an important role in the chemical reactions of biological phosphate compounds such as ATP, DNA, and RNA. Many enzymes require magnesium ions in order to function. Magnesium is the metallic ion at the center of chlorophyll. Aluminum has no known biological function, but may play a role in pathology. Silicium is ingested with tap water as silicon. Silicon present in the body as silicic acid may have a role in protection against aluminium toxicity (Parry et al. 1998). Looking at the periodic table we can see that in periods 1–4 only the noble gases (He, Ne, Ar, Kr) and scandium (Sc) have no biological function. In the right side of periods 5 and 6 we find primarily heavy metals belonging to transition metals (Ag, Au), post-transition metals (Cd and Hg), metals belonging to main group elements (Rb, Sr, Ba, Sn, Pb, Bi) and the metalloids along the stair-step line that distinguishes metals from non-metals drawn from between boron and aluminum to the border between polonium and astatine (Sb, Te, Po).
Trace elements of the periodic table. These include elements from the 2nd to the 6th periods: Li, B, F, Mg, Al, Si, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Rb, Sr, Zr, Mo, Ag, Cd, Sn, I, Ba, Au, Hg, Pb, Bi (boxed and indicated by asterisk in the periodic table). The staircase-shaped dividing line between metalloids from boron to astatine is indicated by the white dotted line. In periods 1–4 only the noble gases (He, Ne, Ar, Kr) and scandium (Sc) have no known biological function
After reviewing briefly the trace elements, one can define heavy metals as:
-
1.
metals that have ≥ 3 g/cm3 density,
-
2.
those trace metal elements that are found in cells and are known as microelements: including elements from the 2nd to the 6th periods: Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Rb, Sr, Zr, Mo, Sn, I, Ba, Pb, Bi, with the exception of those trace elements which are not heavy, i.e. their density is less than 3 g/cm3: Li, B, Mg, Al and Si,
-
3.
trace elements that may form amphoteric oxides (Al, Ga, In, Tl, Sn, Pb, Sb, Po), include metalloids (Ge, As, Te), that are transition metals (Ag, Au), post-transition metals (Al, Ga, In, Sn, Th, Pb, Bi), rare earth element (Sc),
-
4.
transition metals mentioned among the trace elements.
As a conclusion of the introduction heavy metals are defined as trace elements with ≥ 3 g/cm3 densities, have some biological functions at low concentration and cause toxic effects at higher than physiological concentrations.
Cellular Effects of Heavy Metals
Biological toxicity may originate from inorganic (metals, metalloids, non-metal compounds) and organic (phosphorus, nitrogen, aldehyde containing) compounds, drugs, biological venoms, toxins, poisonous food, plant, fungi. Here only the cellular effects of inorganic compounds particularly those exerted by metals and metalloids will be dealt with. Minerals occurring in small quantities in cells are important for normal biological function. Minerals play a homeostatic role in organisms controlling nerve function, muscle contraction and metabolism, regulate electrolyte and hormone production. Among the heavy metals trace amounts of cobalt, copper, iron, manganese, molybdenum, vanadium, strontium, selenium and zinc are essential for cells.
Cobalt: The reactive C–Co bond of vitamin B12 (cyanocobalamin) participates in enzyme catalyzed reactions (Voet and Voet 1995).
Copper is found in the copper centers of cytochrome c oxidase, in the Cu–Zn containing enzyme superoxide dismutase and in the oxygen carrying pigment hemocyanin. More than a dozen enzymes have been found to contain copper.
Iron has two vital functions in the cells of all mammals: (1) hemoglobin is limited to oxygen transport in erythrocytes where it is in ferrous state (Fe2+), and (2) the other cellular function of iron is mitochondrial oxidation where it is involved in iron-cytochrome-c reductase catalysing the chemical oxydoreduction of:
Cyanide is poisoning the cytochrome function, forming a stable complex with another cytochrome, namely the ferric form of cytochrome oxidase. This enzyme promotes the transfer of electrons in the mitochondria of cells during the synthesis of ATP. Without cytochrome oxidase function, cells cannot utilize the oxygen present in the blood, resulting in cytotoxic hypoxia or cellular asphyxiation, respiratory arrest and death.
Manganese is necessary for energy production, bone and blood formation, nerve function, protein, lipid, and glucose metabolism Mn is involved in the production of cholesterol in the metabolism of lipids and glucose.
Molybdenum as a trace element helps to regulate the iron stores and is involved in the carbohydrate, lipid and urine metabolism. Mo plays a crucial role in the catalytic activation of xanthine oxidase (uric acid synthesis) aldehyde and sulfite oxidation.
Vanadium seems to be essential to growth and is involved in lipid metabolism. Its deficiency may increase blood cholesterol and triglyceride levels.
Stroncium is promoting calcium uptake into bones at moderate supplementation, but contributes to rachitis at higher Sr levels (Nielsen 2004).
Selenium containing enzymes are involved in the hormonal effects of thyroid hormones (T3, T4) and are efficient antioxidant peroxidases and in combination with antioxidant vitamins (vitamin C, E) help the production of antibodies, aid the function of pancreas and provide defence against oxidation. Over 70 metalloenzymes are known to require zinc for their functions. (http://www.diagnose-me.com/cond/C15891.html#G368).
Non-essential Harmful Heavy Metals
These metals include cadmium, antimony, chromium, mercury, lead, and arsenic, the last three of them being the most toxic. The toxicity of metals on multicellular organisms is not the subject of this book. Signs and symptoms of heavy metal poisoning are only mentioned but not discussed as they belong to the organismal level of toxicity. Heavy metals (Fe, Mn, Cd, Cu, Hg) originating from dental amalgams are suspicious of being implicated in the development of Parkinson’s disease. Toxic exposure to heavy metals generating high blood and brain levels, is believed to play a role in the development of Alzheimer’s disease. Heavy metals such as mercury, cadmium, lead and thallium poison the glucose metabolism resulting in hypoglycemia manifested as lack of concentration, hyperactivity, impulsive, unpredictable or depressive behavior. Arsenic and lead may cause neuritis accompanied by nerve pain (neuralgia).
Cellular Toxicity of Heavy Metals
As far as the cellular effects of heavy metals are concerned lead poisoning is known to cause the formation of small red blood cells. As a consequence of lead poisoning megaloblastic anemia/pernicious anemia may occur inducing elevated serum iron level. Blood level-Pb as a biological marker to environmental lead exposure will be detailed in Chap. 15.
At the cellular level the wide range of redox behavior, higher reactivity and complex formation of transition metals is based on the outer d electron subshell and explains their important catalytic role in enzyme reactions. It has been recently described that such pluridentate protein–metal complexes interfere severely with the formation of the native protein conformation (Sharma et al. 2008; Ramadan et al. 2009). That heavy metal ions (e.g. Cd2+, Hg2+ and Pb2+) and metalloid arsenic(III) species efficiently inhibit the refolding of chemically denatured proteins will be discussed in Chap. 12.
Cell injury is likely to be initiated by the formation of stable complex with a protein, receptor, cofactor, or via the formation of highly reactive species (Bridges et al. 2006). All ionic heavy metals significantly contribute to the production of free radicals. A free radical is an atom or a compound which has an odd number of electrons in its outer orbital, living this electron unpaired and reactive. Cells constantly encounter low levels of free radicals. These reactive species indiscriminately pick up electrons from other atoms in their neighborhood and convert those into secondary free radicals setting up a chain reaction causing random biological damage in the cell. This process is now considered a major cause of aging. One of the most reactive transition metal ions is iron, present at low levels in biological systems that catalyzes the Haber–Weiss reaction generating hydroxyl (•OH) and superoxide (•O2 −) radicals. Iron catalyzed by hydrogen peroxide generates the production of hydroxy (OH−) ions and hydroxyl radical (•OH) known as the Fenton’s reaction. The Haber–Weiss reaction and the following Fenton reaction have been widely postulated to account for the in vivo generation of hydroxyl radical, known as the most reactive oxygen species (Haber and Weiss 1932; Kehrer 2000; Koppenol 2001).
The reason why heavy metals entering cells multiply free radicals from thousand to million times is that when free radicals collide with toxic metals many new free radicals are generated initiating a chain-reaction. Environmental agents significantly contribute to an increase of free radicals. Among them lead, cadmium, pesticides, ionizing radiation, alcohol and cigarette smoking initiate most frequently free radicals. Chapter 11 presents and discusses evidence of the involvement of oxidative DNA and protein damage in metal-induced carcinogenesis.
Detoxification of Heavy Metals
Known means in heavy metal detoxification include garlic and cilantro (coriander leaves), vegetables, fruits and their juices. Vegetable juice is an important source of raw food and has the advantage that it does not raise insulin levels like fruit juice. The loaded sugar content is a significant drawback to drinking soda and is even worse than fruit juicing resulting in weight gain. Antioxidants (e.g. vitamin C, vitamin E, beta carotene, coenzyme Q10) are able to prevent the formation of reactive oxygen species, but do not eliminate metals from cells. The best known way to remove toxic heavy metals is chelation using N-acetyl-cystein or EDTA. The removal of heavy metals from water as sulfides and toxic contaminants will be described in the last chapter of this book.
There are specific enzymes adapted to catalyze the detoxification of superoxide radicals to oxygen and peroxide. These superoxide dismutases provide an effective antioxidant defense mechanism in most of the cells exposed to molecular oxygen. The general chemical reactions catalyzed by dismutases:
where M is the metal (Cu, n = 1; Mn, n = 2; Fe, n = 2; Ni, n = 2), \(^{{\bullet}}{\kern -1pt}{{\rm{O}}_2}^ - \)is the superoxide radical and SOD stands for superoxide dismutase.
Detection of Cellular Toxicity of Heavy Metals
Replacing In Vivo Animal Studies with In Vitro Systems
The classical approach to determine the toxicity of a compound aimed to predict whether a chemical could be harmful to man was done by using different animal species and increasing doses in short-term (acute) and long-term (chronic) toxicity tests. Long-term animal studies served as conclusive evidence for the carcinogeneity of a chemical. The lowest short-term toxic dose of a potential drug in the most sensitive animal was regarded as the highest applicable dose to be given to man. This approach has been abandoned as such a theoretical external dose could have significantly differed from the internal level measured in the blood or specific target organs due to adsorption, distribution, metabolization or elimination in the organism. To bypass most of these possible influences it is advised to monitor toxic changes in vitro as in such contained systems it is easier to follow cellular and metabolic alterations. After this recognition many in vitro systems have been developed for testing purposes.
Bacterial, Fungal and Mammalian In Vitro Systems
The biological end points of in vitro systems for the assesment of the genotoxic potential of a compound include: induction of mutation, DNA damage, chromosomal and chromatin alterations. Mutations are detected mainly as phenotypic changes and structural alterations of DNA (deletion, insertion, substitution, frameshift, translocation). To measure such mutational changes bacteria and fungi turned out to be the most sensitive and easy to handle test organisms (Dunkel 2006). The best known and most widely accepted gene assay is the Aimes test that uses Salmonella typhimurium to assess the mutagenic potential of a chemical (Ames 1971). As bacteria do not contain cytochromes, they are unable to metabolize and eventually convert procarcinogenic compounds to carcinogens, thus this test was complemented by the inclusion of an exogeneous mammalian metabolic activation system containing liver microsomal fraction (Ames et al. 1973). The microsomal fraction contains artifactual vesicles formed from the endoplasmic reticulum when cells are disrupted.
The problem with other less frequently used microbial systems utilizing Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae and Neurospora crassa is the impermeability of the cell wall preventing the entry of chemicals into these cells. Moreover, fungi require higher concentrations of mutagens than bacteria due to their more effective detoxification capability and repair systems (Dunkel 2006). The more effective detoxification and repair systems of mammalian cells is reflected by their mechanisms of recovery during early molecular changes before irreversible changes including cell death would occur (Bridges et al. 2006).
Mammalian Cell Cultures
When the toxic effects of heavy metals are studied in mammalian cells, cell cultures might be useful. In such cell culture systems cell viability and cellular growth data should be supported by additional metabolic parameters reflecting toxicity (De Ruiter et al. 1985). During the screening of toxic compounds including heavy metals in mammalian cell cultures, it turned out that most agents (~80%) had a similar toxicity in vitro and in vivo. This observation suggested an interference in man with basal functions common to all specialized human tissues as well as cultured cells. In vitro tests can be used: (a) to screen an unlimited number of chemicals and their extracts for potential basal cytotoxicity, (b) to supplement animal tests in acute toxicity examinations and (c) to study the mechanism of cytotoxicity of a wide variety of chemicals (Ekwall 1983). However, this does not mean that the results of cytotoxicity tests are sufficient by themselves. They should be compared with toxicity data in vivo to evaluate the systemic cytotoxicity of chemicals (Ekwall 1980).
Permeability Changes Caused by Heavy Metals
Early observations of studies related to the cellular toxicity of heavy metals were reported back in 1936 and 1938. These experiments have shown that some heavy metals caused the loss of potassium from rabbit erythrocytes (Henriques and Orskov 1936; Davson and Danielli 1938). The slow exchange of K+ between erythrocytes and the surrounding fluid was demonstrated with radioactive tracer experiments (Mullins et al. 1941). Heavy metals caused a net loss of K+ from rabbit erythrocytes in the order of Pb2 + > Au+ > Hg2+ (Joyce et al. 1954). Passow and Rothstein found that Hg2+ treatment led to the complete loss of K+ and that the mercury induced loss of K+ from yeast cells was an all-or-none effect. Cu2+ caused loss of K+ from baker’s yeast, but similar concentration of Pb2+ or Zn2+ did not have any effect (Passow and Rothstein 1960). The comparison of heavy metal toxicity in an insect cell line (Aedes albopictus) made a further distinction among three metal species (organomercurial, mercury and cadmium chlorides) tested and decreased viability to different extents (MeHgCl > HgCl2 > CdCl2). Low concentrations of methylmercury chloride caused both cell death and inhibition of cell proliferation; HgCl2 primarily disrupted the plasma membrane, whereas the primary effect of CdCl2 was exerted on cell proliferation (Braeckman et al. 1997). Heavy metal toxicity of methyl-HgCl, HgCl2 and CdCl2 in the Aedes albopictus insect cell line will be the subject of Chap. 5.
In 1965 McBrien and Hassall studied the toxic effects of copper on respiration, photosynthesis and growth of the single-celled green alga, Chlorella vulgaris belonging to the Chlorella genus and the phylum Chlorophyta. They found that potassium normally low of permeability was released by the graded response of the barrier, due to increasing concentration of bound copper (McBrien and Hassal 1965). This increase of permeability of the cells was considered to be the primary toxic effect of copper. Potassium-depleted cells remained viable if subsequently transferred to a copper-free medium. This experiment can be regarded as one of the early attempts to reversibly permeabilize cells. With plant cells not only the loss of K+, but also the abolition of photosynthesis are among the known results of heavy metal poisoning (Overnell 1975). Reversible permeabilization is a technique for the introduction of nucleotides (Banfalvi et al. 1984) and oligonucleotides into cells (Lesh et al. 1995) or to induce nuclear reprogramming (Miyamoto et al. 2008).
In animal cells the rapid interaction of mercury or copper with the membrane could be followed by the penetration into the interior of the muscle cell (Demis and Rothstein 1955). A physiological function associated with the action of heavy metals on the membrane is the uptake of glucose (Passow and Rothstein 1960). Another function associated with the interior of the cell is respiration. It is likely that in different animal cells a similar sequence of events occurs. Some of the effects on membrane function include: changes in the permeability to glycerol generated by copper (Jacobs 1950) and by mercury (Wilbrandt 1941); losses of K+ from red blood cells produced by lead, mercury, gold, and silver (Joyce et al. 1954); inhibition of surface-bound invertase in the yeast cell by silver and mercury (Myrbäck 1957) and inhibitory effects on absorptive activities of kidney cells with organic mercurials. Effects of metals might include the inhibition of metabolic activities (respiration and glycolysis) and the block of cell division (Passow and Rothstein 1960). Mercury, cadmium, lead and thallium are known to poison glucose metabolism in cells.
Oxidative Damages Caused by Heavy Metals
Heavy metals generate oxidizing radicals through Fenton chemistry and by the Haber–Weiss reaction leading to the conclusion that metal carcinogenesis is mediated primarily by the elevated level of free radicals (Kasprzak 1995). When cells were exposed to mercury a sequence of interactions was observed starting at the cell membrane and proceeding inward. The first reaction was between the metal and the ligands of the cell membrane for which the metal possesses chemical affinity. In addition the metal passed through the membrane into the cytoplasm reacting with subcellular constituents the kinetics of which is governed by the rate of entry, mixing and chemical reactions (Passow and Rothstein 1960). The possible role of oxidative damage in metal-induced carcinogenesis will be reviewed in Chap. 11. That ubiquitin ligases play a key role in the cadmium induced stress response will be discussed in Chap. 13. The heavy metal-induced disruption of ubiquitin proteasomal system plays a critical role in cellular mechanisms such as cell cycle regulation and apoptosis (Chap. 14).
Lipid Peroxidation
Cell membranes made of unsaturated lipids are particularly susceptible to free radicals also referred to as reactive oxygen species (ROS) initiate uncontrolled chain reactions in cells. The oxidative damage by lipid peroxidation causes the hardening of lipids constituting the cell membrane. The damaged cell membrane changes the uptake of nutrients, the cell signaling system and many other cellular functions. Inside the cell informational macromolecules (DNA, RNA, proteins) are also susceptible to oxidative damage.
Oxidative DNA Damage
The most susceptible macromolecule in the eukaryotic cell is mitochondrial DNA. In mitochondria the citric acid cycle converts the energy of fuel carbon atoms of acetate to CO2 and reducing equivalents (3 NADH + H+ and 1 FADH2) by hydrolytic oxidation using two molecules of water (Banfalvi 1991). In turn the increased reducing power represented by the reducing power is released in the mitochondrial respiratory chain producing water and ATP. As in the terminal oxidation of hydrogen to water in the presence of molecular oxygen (O2) many steps are involved it is unavoidable to generate radicals. The other reason why most of the radicals affect mitochondrial DNA is that the mitochondrial DNA has no repair system. To the contrary cellular (nuclear) DNA damages are effectively removed. Oxidative DNA damages with apoptotic biochemical and morphological consequences induced by cadmium are dealt with in Chap. 6
Estimation of Toxic Effects of Heavy Metals
Tumorigenic Potential of Heavy Metals
With regard to carcinogenicity two heavy metals, chromium(VI) and arsenic(III) are considered human carcinogens sharing common properties. That chromium ions, especially Cr(VI) interfere with the cellular functions and chromatin structure of Schisosaccharomyces pombe are discussed in Chaps. 3 and 8. The arsenic tolerance mechanism of yeast cells will be elucidated in Chap. 4.
To estimate the tumorigenic potential of heavy metals, the 18FDG glucose analog uptake and the expression of facilitative glucose transporters have been suggested (Rastogi et al. 2007). Of the estimated twelve GLUT transporters the GLUT-1 and GLUT-3 take up more 18FDG than other GLUT transporters, suggesting that 18FDG uptake of cultured tumor cells is governed by GLUT expression, and is a distinct characteristic of the neoplastic process (Waki et al. 1998). Several lines of studies indicate that malignant cells with increased uptake of 18FDG have overexpressed GLUT-1 and/or GLUT-3 (Yamamoto et al. 1990; Nishioka et al. 1992; Brown and Wahl 1993; Waki et al. 1998; Rastogi et al. 2007). Increased expression of GLUT-1 and GLUT-3 has been reported in rat tumor cancers and is associated with poor survival (Trencsenyi et al. 2010). The heavy metal-induced toxicological changes depleted uranium and heavy metal tungsten-induced genotoxic and carcinogenic properties will be detailed in Chap. 10.
Metabolic Parameters
To support basal cytotoxicity data such as cell viability, growth and different staining methods have been developed. Biochemical changes in cells caused by the loss of total protein content can be measured by lactate dehydrogenase leakage, lowered lysosomal activity and succinate dehydrogenase activity. The production of nitric oxide (NO) plays multiple roles in defence reactions under abiotic stresses, including heavy metal load.
Beside the loss of ions and proteins heavy metal ions such as Cd2+, Hg2+ and Pb2+ as well as metalloid arsenic(III) species inhibit efficiently the refolding of chemically denatured proteins. Proteins as targets of heavy metals and metalloids will be discussed in Chap. 12.
Cytoskeletal and Nucleoskelatal Changes
For the detection of the toxic effects of heavy metals on the cytoskeleton, fluorescein isothiocyanate (FITC)-labelled phalloidin is regularly used for staining F-actin, whereas conjugated monoclonal anti-tubulin antibody could be applied to stain tubulin.
Genotoxicity. There are two major cytogenetic endpoints applied for genotoxicity studies and biomonitoring purposes: chromosome aberrations and micronuclei (Mateuca et al. 2006). If the process of cell division is disturbed the distribution of the genetic material can be affected and DNA is not incorporated into a new nucleus but may form a small nucleus (“micronucleus”), visible under microscope. However, we have observed many times in different mammalian cells that during regular chromatin condensation the extruded chromatin turns around itself forming the head portion (micronucleus) seen during early S phase when chromatin structures are succeeded by linearly arranged, distinguishable chromosomes. Based on these observations the micronucleus is regarded as a regularly occurring element of chromatin condensation (Banfalvi, unpublished results).
Age related cytotoxicity of stannic chloride (SnCl4) affected mitotic index, damaged cells, caused chromosome aberration, and micronuclei formation as endpoints (Ganguly 1993). Less attention has been paid to chromosomal changes taking place in the early stages of chromosome condensation in interphase nuclei of eukaryotic cells. Such chromatin changes will be visualized after Cd(II), Ni(II), Cr(VI) and Ag(I) treatment in Chaps. 6–9.
Chromosomal and Chromatin Changes Induced by Heavy Metals
Human lymphocytes treated for 3 h with lead, cadmium, and zinc acetate separately and in combinations of two or three metal salts between 10− 3 and 10− 8 M was followed by chromosome analysis and revealed higher incidences of chromatid-type aberrations and gaps only for cultures exclusively treated with cadmium (Gasiorek and Bauchinger 1981). The heavy metal load of small rodents increased the frequency of chromosomal aberrations and pathological changes in erythrocytes has shown that mercury is a strong damaging factor for chromosomes and red blood cell apparatus (Topashka-Ancheva et al. 2003).
Due to its diagnostic significance we have started to systematize the chromatin changes of toxic agents including heavy metals (Banfalvi 2009). The most characteristic changes caused by cadmium were large extensive disruptions and holes in the nuclear membrane, sticky and incompletely folded chromosomes at the end of the S phase (Banfalvi et al. 2005, 2007). These alterations are in agreement with chromatid-type aberrations observed by others (Gasiorek and Bauchinger 1981).
Cellular changes of mercuric ions were characterized by their properties of causing reduced cellular motility (10–50 mM), and complete lack of cellular movement at higher concentrations (100–1,000 mM). In K562 erythroleukemia cells upon low concentration of mercuric acetate (£1 mM): (a) chromatin changes were the earliest signs of cytotoxicity, (b) highly condensed supercoiled and decondensed veil-like chromatin appeared, (c) decondensed chromosomes were rejected as clustered puffs and (d) often the nuclear material was broken down to apoptotic bodies. In the concentration range between 10 and 50 mM of Hg(II) acetate chromatin changes were characterized by apoptosis seen as broken nuclei and apoptotic bodies. High concentration of Hg2+ ions (100 mM) initiated necrotic nuclear changes, with enlarged leaky or opened nuclei (Farkas et al. 2010).
Detection of Apoptotic and Necrogenic Chromatin Changes
Such changes can be detected by cytotoxic assays, staining methods, in situ end labeling, flow cytometry, light scattering flow cytometry, fluorescence activated cell sorting (FACS), TUNEL assay, comet assay, immunological detection of low molecular weight DNA, detection of mono- and oligonucleosomes, apoptotic proteins and enzymes (Banfalvi 2009).
Detection and Determination of Heavy Metals in Cells
Several instrumental methods have been used to determine heavy metals in aqueous samples, some of which needed preconcentration when the sensitivity of direct analysis was insufficient. For the selective solvent extraction of heavy metals (e.g. Ag, Cu, Hg) macrocyclic extractants have been applied. The separated heavy metals have been determined either by means of atomic absorption spectrometry, spectrometry, X-ray fluorescence analysis, thin layer chromatography in combination with visual or densitometric determination, protein based biosensors, isotope-labelled chelates, X-ray fluorescence, inductively coupled plasma atomic emission spectroscopy, indirect detection of heavy metals as unithiol complexes, disposable cuvette test.
Spectroscopy, Spectrometry
Atomic Absorption Spectrophotometry
The organic matter in samples can be digested by wet, dry or microwave digestion and the heavy metals (As, Cd, Pb, Hg) determined by using graphite furnace atomic absorption spectrophotometer (GF-AAS) and flow injection analysis system-atomic absorption spectrophotometer (FIAS-AAS) (http://www.aseansec.org/MRA-Cosmetic/Doc-3.pdf).
Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES)
This turned out to be one of the most reliable analytical techniques for the determination of heavy metal (Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, and Zn) concentrations in plants after digestion in a closed microwave system using HNO3, and ICP-AES was applicable to different environmental samples (Kos et al. 1996).
X-ray Fluorescence
Small amounts of heavy metals have been determined by the technique of X-ray fluorescence (XRF) in natural waters and pure salts (Eksperiandova et al. 1998).
Backscatter Electron (BSE) Imaging and Energy Dispersive Spectroscopy
Backscatter electron (BSE) imaging (20 kV) was used to discern metal particles within tissues. Energy dispersive spectroscopy (EDS) (15 kV) verified the specific elements present. This allowed for the spatial characterization of the nanoparticles within the tissues but quantification of the amount of metal was not possible (Ayers et al. 2007).
Amperometric Detection of Unithiol Complexes of Heavy Metals
Pb(II), Cd(II), Hg(II), Ni(II), Co(II), and Cu(II) were indirectly determined as their unithiol complexes by amperometric detection under static and HPLC conditions (Osipova et al. 2000).
Isotope Techniques
Isotope Dilution Mass Spectrometric Method (IDMS)
IDMS has been developed for the simultaneous determination of the complexes of heavy metals (Ag, Cd, Cu, Mo, Ni, Pb, Tl, U, W, Zn and Zr) by coupling high pressure liquid chromatography (HPLC) with inductively coupled plasma mass spectroscopy (ICP-MS) and applying the on-line isotope dilution technique (Vogl and Heumann 1997).
Tritium-Labelled Chelates
Such compounds have been used for the radiometric determination of Pb, Cd and Hg (Zolotov et al. 1987).
Whole-Cell-Sensing Systems
Protein Based Bisosensors
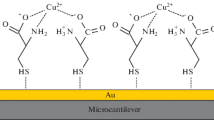
These biosensors have been applied to detect femtomolar (10− 15 M) levels of heavy metal ions. Heavy metal binding proteins were overexpressed in Escherichia coli, purified, and immobilized to a self-assembled thiol layer on a gold electrode placed as the working electrode in a potentiostatic arrangement in a flow analysis system (Bontidean et al. 1998).
Construction of Metal Detection Circuits in E. coli
Bacterial whole-cell biosensors are useful for toxicity measurements of various samples. Simple bacterial biosensors were constructed for the quantitative and rapid measurements of arsenite and arsenate in potable water. These bisosensors are based on the natural resistance of E. coli against arsenite and arsenate, and on three reporter proteins: bacterial luciferase, beta-galactosidase and green fluorescent protein (GFP) (Stocker et al. 2003). The introduction of new reporter genes and refined detection equipment, could lead to the extensive use of stress-responsive biosensors for toxicity estimations (Sørensen et al. 2006).
Luminescence-Based Whole-Cell-Sensing Systems using Genetically Engineered Bacteria
Genetically engineered cells, tailored to respond by a dose-dependent signal to the presence of toxic chemicals, are useful tools for the environmental monitoring of toxic substances (Elad et al. 2008). Biosensing cells can be classified into two groups in terms of their biosensing mechanisms—constitutive expression and stress- or chemical-specific inducible expression (Gu et al. 2004). For the detection of heavy metals inducible gene promoters have been utilized. Systems responding to cadmium and lead ions have been designed and developed using genetically engineered bacteria. These systems take advantage of the ability of certain bacteria to survive in polluted environments. In Escherichia coli harboring two plasmids (pYSC1 and pYS2/pYSG1) the expression of the reporters beta-galactosidase and red-shifted green fluorescent protein (rs-GFP) was controlled by CadC, the regulatory protein of the cad operon. This heavy metal sensing bacterial systems responds to cadmium, lead, and zinc ions, but has no significant response to nickel, copper, manganese, and cobalt (Shetty et al. 2003). A genetically engineered bacterial system was developed for the electrochemical sensing of antimonite and arsenite (Scott et al. 1997). Of the five cysteinyl residues of the cadCA operon in Staphylococcus aureus three (Cys-7, Cys-58, and Cys-60) are involved in sensing Pb(II), Cd(II), and Zn(II) by the plasmid pI258 CadC repressor and could be used for the detection of these soft metals (Sun et al. 2001).
Whole-Cell Heavy Metal Detecting Yeast System using Cadmium-Inducible Gene Promoter
The acquisition of metals (e.g. iron, copper, and zinc) by yeasts is tightly regulated. High affinity uptake systems are induced under metal-limiting conditions to maintain an adequate supply of these essential nutrients (Waters and Eide 2002). Metal-responsive transcription factors exist in yeast to modulate expression of genes that encode proteins involved in cellular uptake of iron, copper and zinc. These signal transduction pathways are involved in the homeostasis of the intracellular concentration of free metal ions. A second component of the equilibrium is the regulation of metal-ion binding through protein-mediated metallation (Winge et al. 1998). Fungi detect changes in metal ion levels using unique metallo-regulatory factors whose activity is responsive to the cellular metal ion status (Bird 2008). The genome-wide gene expression profiling of the methylotrophic yeast Hansenula polymorpha exposed to cadmium (Cd) allowed the identification of novel genes responsive to Cd treatment. The HpSEO1 promoter of H. polymorpha as a bioelement in whole-cell biosensors is expected to monitor heavy metal contamination, particularly Cd (Park et al. 2007).
The detection of three heavy metals has been reported recently by the team KU_Seoul successfully applied metal detection circuits in E. coli and constructed a heavy metal detecting machine. Such machines are now commercially available. In the KU_Seoul instrument the integrated Zn detecting promoter used red fluorescence protein (RFP) as a reporter and worked at the range of 1~2 mM concentration. The arsenic detector applied green fluorescent protein (GFP) as a reporter and worked at the range of 0.15~1 mM concentration. Their cadmium detector utilized aryl acylamidase (AMD) as a reporter and worked in the range of 0.2–0.4 mM concentration (Ko et al. 2009).
Heavy Metal Toxicity Detected by Cardiac Cell-Based Biosensor
Among the many types of whole-cell bacterial biosensors that have been developed using recombinant DNA technology cardiac cell-based biosensors turned out to be of clinical importance (Yagi 2007). A novel biosensor for monitoring electrophysiological activity was developed by light-addressable potentiometric sensor (LAPS). After exposing cardiomyocytes to 10 mM concentration of different heavy metal ions (Hg, Pb, Cd, Fe, Cu, Zn) for 15 min the characteristic changes in beating frequency, amplitude and duration suggested that with physiological monitoring acute and chronic toxicities induced by heavy metals can be studied in a non-invasive way (Liu et al. 2007).
Antibody-Based Sensors for Heavy Metal Ions
Competitive immunoassays for Cd(II), Co(II), Pb(II) and U(VI) were developed using two different assay formats, a competitive microwell format and an immunosensor format (Blake et al. 2001).
Porphyrin Test
To measure mercury that was absorbed into the cells the Porphyrin Analysis test requires urine samples that are subjected to laboratory analysis. If Coproporphyria III is high, this indicates that mercury is present. Altered porphyrin metabolism is a biomarker of mercury exposure and toxicity (Woods 1996).
Disposable Cuvette Test for the Enzymatic Determination of Heavy Metals
An optical cuvette test for the determination of total heavy metals was reported. The test is based on the inhibition of the enzyme urease by metals ions including silver(I), mercury(II), copper(II), nickel(II), cobalt(II), and cadmium(II) (Wolfbeis and Preininger 1995).
References
Abrahams SC, Ginsberg P, Knox K (1964) Transition metal-hydrogen compounds. II. The crystal and molecular structure of potassium rhenium hydride, K2ReH9. Inorg Chem 3:558–567
Ames BN (1971) The detection of chemical mutagens with enteric bacteria. In: Hollaender A (ed) Chemical mutagens, principles and methods for their detection, vol 1. Plenum Press, New York, pp 267–282
Ames BN, Durston WE, Yamasaki E, Lee FD (1973) Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A 70:2281–2285
Appenroth K-J (2010) Definition of “Heavy Metals” and their role in biological systems. In: Sherameti A, Verma A (eds) Soil heavy metals. Springer, Berlin, pp 19–29
Ayers R, High W, Chandler J, Ranville J (2007) The detection and characterization of nanoparticulate heavy metals in epithelial tissues in patients with nephrogenic fibrosing dermopathy. MRS Fall Meeting. Symposium 00, 1063-0010-06. MRS Website: http://www.mrs.org/s_mrs/sec_subscribe.asp?CID = 11306&DID = 212403&action=detail
Banfalvi G (1991) Conversion of oxidation energy to reductive power in the citrate cycle. Biochem Educ 19:24–26
Banfalvi G (2009) Apoptotic chromatin changes. Springer, Holland, pp 269–293
Banfalvi G, Sooki-Toth A, Sarkar N, Csuzi S, Antoni F (1984) Nascent DNA chains synthesized in reversibly permeable cells of mouse thymocytes. Eur J Biochem 139:553–559
Banfalvi G, Gacsi M, Nagy G, Kiss ZB, Basnakian AG (2005) Cadmium induced apoptotic changes in chromatin structure and subphases of nuclear growth during the cell cycle in CHO cells. Apoptosis 10:631–642
Banfalvi G, Ujvarosi K, Trencsenyi G, Somogyi C, Nagy G, Basnakaian AG (2007) Cell culture density dependent toxicity and chromatin changes upon cadmium treatment in murine pre-B-cells. Apoptosis 12:1219–1228
Bird AJ (2008) Metallosensors, the ups and downs of gene regulation. Adv Microb Physiol 53:231–267
Bjerrum N (1936) Bjerrum’s inorganic chemistry, 3rd Danish edn. Heinemann, London
Blake DA, Jones RM, Blake RC II, Pavlov AR, Darwish IA, Yu H (2001) Antibody-based sensors for heavy metal ions. Biosensors Bioelectronics 16:799–809
Bontidean I, Berggren C, Johansson G, Csöregi E, Mattiasson B, Lloyd JR, Jakeman KJ, Brown NL (1998) Detection of heavy metal ions at femtomolar levels using protein-based biosensors. Anal Chem 70:4162–4169
Bowen HJM (1976) Trace elements in biochemistry, 2nd edn. Academic, London
Braeckman B, Raes H, Van Hoye D (1997) Heavy-metal toxicity in an insect cell line. Effects of cadmium chloride, mercuric chloride and methylmercuric chloride on cell viability and proliferation in Aedes albopictus cells. Cell Biol Toxicol 13:389–397
Bridges JW, Benford DJ, Hubbard SA (2006) Mechanism of toxic injury. Ann N Y Acad Sci 407:42–63
Brown RS, Wahl RL (1993) Overexpression of GLUT-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer 72:2979–2985
Davson H, Danielli JF (1938) Studies on the permeability of erythrocytes. Factors in cation permeability. Biochem J 32:991–1001
De Ruiter N, Mailänder V, Kappus H (1985) Effect of heavy metals on cellular growth, metabolism and integrity of cultured Chinese hamster kidney cells. Xenobiotica 15:665–671
Demis DJ, Rothstein A (1955) Relationship of the cell surface to metabolism. XII. Effect of mercury and copper on glucose uptake and respiration of rat diaphragm. Am J Physiol 180:566–574
Duffus JH (2002) Heavy metals—a meaningless term? Pure Appl Chem 74:793–807
Dunkel VC (2006) Biological significance of end points. Ann N Y Acad Sci 407:34–41
Eksperiandova LP, Makarovska YN, Blank AB (1998) Determination of small quantities of heavy metals in water-soluble salts and natural water by X-ray fluorescence. Anal Chim Acta 371:1105–1108
Ekwall B (1980) Screening of toxic compounds in tissue culture. Toxicology 17:127–142
Ekwall B (1983) Screening of toxic compounds in mammalian cell cultures. Ann N Y Acad Sci 407:64–77
Elad T, Benovich E, Magrisso S, Belkin S (2008) Toxicant identification by a luminescent bacterial bioreporter panel: application of pattern classification algorithms. Environ Sci Technol 42:8486–8491
Falbe JM, Regitz M (eds) (1996) Roempp Chemie Lexikon, Georg Thieme, Weinheim
Farkas E, Ujvarosi K, Nagy G, Posta J, Banfalvi G (2010) Apoptogenic and necrogenic effects of mercuric acetate on the chromatin structure of K562 human erythroleukemia cells. Toxicol in Vitro 24:267–275
Ganguly BB (1993) Cell division, chromosomal aberration, and micronuclei formation in human peripheral blood lymphocytes. Biol Trace Elem Res 38:55–62
Gasiorek K, Bauchinger M (1981) Chromosome changes in human lymphocytes after separate and combined treatment with divalent salts of lead, cadmium, and zinc. Environ Mutagen 3:513–518
Grant R, Grant C (eds) (1987) Grant and Hackh’s chemical dictionary, 5th edn. McGraw-Hill, New York
Gu MB, Mitchell RJ, Kim BC (2004) Whole-cell-based biosensors for environmental biomonitoring and application. Adv Biochem Eng Biotechnol 87:269–305
Haber F, Weiss J (1932) On the catalysis of hydroperoxide. Naturwissenschaften 20:948–950
Henriques V, Orskov SL (1936) Untersuchungen fiber die Schwankungen des Kationgehaltes.der roten Blutkörperchen. Skand Arch Physiol 74:63–78
Jacobs MH (1950) Surface properties of the erythrocyte. Ann N Y Acad Sci 50:824–834
Joyce CRB, Moore H, Weatherall M (1954) The effects of lead, mercury, and gold on the potassium turnover of rabbit blood cells. Br J Pharmacol Chemother 9:463–470
Kasprzak KS (1995) Possible role of oxidative damage in metalinduced carcinogenesis. Cancer Invest 13:411–430
Kehrer JP (2000) The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149:43–50
Klein MA, Sekimoto H, Milner MJ, Kochian LV (2008) Investigation of heavy metal hyperaccumulation at the cellular level: development and characterization of Thlaspi caerulescens suspension cell lines. Plant Physiol 147:2006–2016
Ko H, Roh H, Kim S, Choi C, Jang J, Shin J, Byun YS, Choi I-G (2009) KU Seoul team: integrated heavy metal detection system. http://2009.igem.org/files/poster/KU_Seoul.pdf
Koppenol WH (2001) The Haber–Weiss cycle—70 years later. Redox Report 6:229–234
Kos V, Budie B, Hudnik V, Lobnik F, Zupan M (1996) Determination of heavy metal concentrations in plants exposed to different degrees of pollution using ICP-AES. Fresenius J Anal Chem 354:648–652
Lesh RE, Somlyo AP, Owens GK, Somlyo AV (1995) Reversible permeabilization. A novel technique for the intracellular introduction of antisense oligodeoxynucleotides into intact smooth muscle. Circ Res 77:220–230
Liu Q, Cai H, Xu Y, Xiao L, Yang M, Wang P (2007) Detection of heavy metal toxicity using cardiac cell-based biosensor. Biosens Bioelectron 22:3224–3229
Lozet J, Mathieu C (1991) Dictionary of soil science, 2nd edn. Balkema, Rotterdam
Marquès L, Cossegal M, Bodin S, Czernic P, Lebrun M (2004) Heavy metal specificity of cellular tolerance in two hyperaccumulating plants, Arabidopsis halleri and Thlaspi caerulescens. New Phytologist 164:289–295
Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M (2006) Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie 88:1515–1531
McBrien DCH, Hassal KA (1965) The effect of toxic doses of copper upon respiration, photosynthesis and growth of Chlorella vulgaris. Physiol Plant 20:113–117
Miyamoto K, Yamashita T, Tsukiyama T, Kitamura N, Minami N, Yamada M, Imai H (2008) Reversible membrane permeabilization of mammalian cells treated with digitonin and its use for inducing nuclear reprogramming by Xenopus egg extracts. Cloning Stem Cells 10:535–542
Morris C (ed) (1992) Academic press dictionary of science and technology. Academic Press, San Diego
Mullins LJ, Fenn WO, Noonan TR, Haege L (1941) Permeability of erythrocytes to radioactive potassium. Am J Physiol 135:93–101
Myrbäck K (1957) Inhibition of yeast invertase (saccharase) by metal ions. V. Inhibition by mercury compounds. Ark Kemi 11:471–479
Nielsen SP (2004) The biological role of strontium. Bone 35:583–588
Nishioka T, Oda Y, Seino Y, Yamamoto T, Inagaki N, Yano H, Imura H, Shigemoto R, Kikuchi H (1992) Distribution of the glucose transporters in human brain tumors. Cancer Res 52:3972–3979
Osipova EA, Shapovalova EN, Ofitserova MN, Podlesnykh SV (2000) Amperometric detection of unithiol complexes of heavy metals in high-performance liquid chromatography. J Anal Chem 55:52–57
Overnell J (1975) The effect of heavy metals on photosynthesis and loss of cell potassium in two species of marine algae, Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar Biol 29:99–103
Park JN, Sohn MJ, Oh DB, Kwon O, Rhee SK, Hur CG, Lee SY, Gellissen G, Kang HA (2007) Identification from transcriptome analysis and application to whole-cell heavy metal detection systems of the cadmium-inducible Hansenula polymorpha SEO1 gene promoter. Appl Environ Microbiol 73:5990–6000
Parker SP (ed) (1989) McGraw-Hill dictionary of scientific and technical terms, 4th edn. McGraw-Hill, New York
Parry R, Plowman D, Delves HT, Roberts NB, Birchall JD, Bellia JP, Davenport A, Ahmad R, Fahal I, Altmann P (1998) Silicon and aluminium interactions in haemodialysis patients. Nephrol Dial Transplant 13:1759–1762
Passow H, Rothstein A (1960) The binding of mercury by the yeast cell in relation to changes in permeability. J Gen Physiol 43:621–633
Ramadan D, Rancy PC, Nagarkar RP, Schneider JP, Thorpe C (2009) Arsenic(III) species inhibit oxidative protein folding in vitro. Biochemistry 48:424–432
Rastogi S, Banerjee S, Chellappan S, Simon GR (2007) GLUT-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett 257:244–251
Scott DL, Ramanathan S, Shi W, Rosen BP, Daunert S (1997) Genetically engineered bacteria: electrochemical sensing systems for antimonite and arsenite. Anal Chem 69:16–20
Sharma SK, Goloubinoff P, Christen P (2008) Heavy metal ions are potent inhibitors of protein folding. Biochem Biophys Res Commun 372:341–345
Shastry BS (2005) On the functions of lithium: the mood stabilizer. Bioessays 19:199–200
Shetty RS, Deo SK, Shah P, Sun Y, Rosen BP, Daunert S (2003) Luminescence-based whole-cell-sensing systems for cadmium and lead using genetically engineered bacteria. Anal Bioanal Chem 376:11–17
Sørensen SJ, Burmølle M, Hansen LH (2006) Making bio-sense of toxicity: new developments in whole-cell biosensors. Curr Opin Biotechnol 17:11–16
Srivastava NK, Majumder CB (2008) Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater 151:1–8
Stocker J, Balluch D, Gsell M, Harms H, Feliciano J, Daunert S, Malik KA, van der Meer JR (2003) Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ Sci Technol 37:4743–4750
Streit B (1994) Lexikon der Okotoxikologie. VCH, Weinheim
Sun Y, Wong MD, Rosen BP (2001) Role of cysteinyl residues in sensing Pb(II), Cd(II), and Zn(II) by the plasmid pI258 CadC repressor. J Biol Chem 276:14955–14960
Thornton I (1995) Metals in the global environment—facts and misconceptions. ICME, Ottawa
Topashka-Ancheva M, Metcheva R, Teodorova S (2003) A comparative analysis of the heavy metal loading of small mammals in different regions of Bulgaria II: chromosomal aberrations and blood pathology. Ecotoxicol Environ Saf 54:188–193
Trencsenyi G, Juhasz T, Bako F, Marian T, Pocsi I, Kertai P, Hunyadi J, Banfalvi G (2010) Comparison of the tumorigenic potential of liver and kidney tumors induced by N-nitrosodimethylamine. Histol Histopathol 25:309–320
Van Nostrand’s International Encyclopaedia of Chemical Science (1964) Van Nostrand, Princetown
Voet JG, Voet D (1995) Biochemistry. Wiley, New York, p 675
Vogl J, Heumann KG (1997) Determination of heavy metal complexes with humic substances by HPLC/ICP-MS coupling using on-line isotope dilution technique. Fresenius J Anal Chem 359:438–441
Waki A, Kato H, Yano R, Sadato N, Yokoyama A, Ishii Y, Yonekura Y, Fujibayashi Y (1998) The importance of glucose transport activity as the rate-limiting step of 2-eoxyglucose uptake in tumor cells in vitro. Nucl Med Biol 25:593–597
Waters BM, Eide DJ (2002) Combinatorial control of yeast FET4 gene expression by iron, zinc, and oxygen. J Biol Chem 277:33749–33757
Wilbrandt W (1941) Die Wirkung von Schwermetallsalzen auf die Erythrocyten-permeabilität für Glycerin. Arch Ges Physiol (Pjlilgers). 244:637–643
Winge DR, Jensen LT, Srinivasan C (1998) Metal-ion regulation of gene expression in yeast. Curr Opin Chem Biol 2:216–221
Wolfbeis OS, Preininger C (1995) Disposable cuvette test for enzymatic determination of heavy metals. In: Vo-Dinh T (ed) Environmental monitoring and hazardous waste site remediation, Proceedings SPIE, p 140
Woods JS (1996) Altered porphyrin metabolism as a biomarker of mercury exposure and toxicity. Can J Physiol Pharmacol 74:210–215
Yagi K (2007) Applications of whole-cell bacterial sensors in biotechnology and environmental science. Appl Microbiol Biotechnol 73:1251–1258
Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H (1990) Overexpression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 170:223–230
Zolotov YA, Malofeeva GI, Petrukhin OM, Timerbaev AR (1987) New methods for preconcentration and determination of heavy metals in natural water. Pure Appl Chem 59:497–504
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Bánfalvi, G. (2011). Heavy Metals, Trace Elements and Their Cellular Effects. In: Banfalvi, G. (eds) Cellular Effects of Heavy Metals. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0428-2_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-0428-2_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0427-5
Online ISBN: 978-94-007-0428-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)