Abstract
TRPV5 and TRPV6 are unique members of the TRP super family. They are highly selective for Ca2+ ions with multiple layers of Ca2+-dependent inactivation mechanisms, expressed at the apical membrane of Ca2+ transporting epithelia, and robustly responsive to 1,25-dihydroxivitamin D3. These features are well suited for their roles as Ca2+ entry channels in the first step of transcellular Ca2+ transport pathways, which are involved in intestinal absorption, renal reabsorption of Ca2+, placental transfer of Ca2+ to fetus, and many other processes. While TRPV6 is more broadly expressed in a variety of tissues such as esophagus, stomach, small intestine, colon, kidney, placenta, pancreas, prostate, uterus, salivary gland, and sweat gland, TRPV5 expression is relatively restricted to the distal convoluted tubule and connecting tubule of the kidney. There is only one TRPV6-like gene in fish and birds in comparison to both TRPV5 and TRPV6 genes in mammals, indicating TRPV5 gene was likely generated from duplication of TRPV6 gene during the evolution of mammals to meet the needs of complex renal function. TRPV5 and TRPV6 are subjected to vigorous regulations under physiological, pathological, and therapeutic conditions. The elevated TRPV6 level in malignant tumors such as prostate and breast cancers makes it a potential therapeutic target. TRPV6, and to a lesser extent TRPV5, exhibit unusually high levels of single nucleotide polymorphisms (SNPs) in African populations as compared to other populations, indicating TRPV6 gene was under selective pressure during or after humans migrated out of Africa. The SNPs of TRPV6 and TRPV5 likely contribute to the Ca2+ conservation mechanisms in African populations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
TRPV5 and TRPV6 are unique members of the TRP super family with three major common features that distinguish them from other members: highly selective for Ca2+, distributing to apical membranes in Ca2+ transporting epithelial cells, and highly responsive to 1, 25-dihydroxyvitamin D3 (1,25[OH]2D3). These features also make them suitable for their roles as Ca2+ entry channels in the first step of the transcellular pathway, which is involved in many processes such as Ca2+ absorption in the intestine and reabsorption in the kidney. In this chapter, I will summarize the evolution of the TRPV5 and TRPV6 genes, the features of TRPV5 and TRPV6 in function and regulation to meet the needs for Ca2+ entry channels in the transcellular Ca2+ transport pathways, their regulation under pathophysiological and therapeutic conditions, and the unusual high allele frequency of TRPV6 (and to a lesser extent TRPV5) single nucleotide polymorphisms (SNPs) in African populations. Due to the scope of this chapter, it is impossible to cover all important work. Readers are directed to relevant chapters of this series and recent reviews [1–12] for further details about various aspects of TRPV5 and TRPV6.
14.1 Identification – Results of Seeking Ca 2+ Transporters in Transcellular Pathways
TRPV5 and TRPV6 were identified as results of searching for genes responsible for Ca2+ transport in the transcellular pathway in the kidney [13] and intestine [14], respectively. Until the end of the twentieth century, calbindins had remained to be the main molecules in the transcellular Ca2+ transport pathway with little information available about the apical entry mechanism. To tackle this problem, an expression cloning approach with Xenopus laevis oocytes was employed to identify Ca2+ transport proteins by two groups independently. Using mRNA isolated from connecting tubule and cortical collecting duct cells immunodissected from rabbit kidney, Hoenderop, Bindels and colleagues [13] constructed a cDNA library and identified a cDNA clone by functional screening of the library using radiotracer Ca2+ uptake assay. The resultant cDNA encodes a protein of 730 amino acids, which was named ECaC (Epithelial Ca2+ channel) [13]. In contrast, we chose intestinal epithelial tissues as the source to clone an intestinal Ca2+ transporter [14]. We observed significant increases in Ca2+ uptake in oocytes injected with mRNAs from cecum or duodenum from rats fed on Ca2+ deficient diet for 2 weeks. A 10–20% reduction in serum Ca2+ was observed in these rats. From the rat duodenal cDNA library, we identified a cDNA encoding a protein of 727 amino acids, which we named CaT1 (Ca2+ transport protein subtype 1). The two proteins share 74% amino-acid identity. ECaC and CaT1 were renamed later on as TRPV5 and TRPV6, respectively. Other alternative names include CaT2 [15] and ECaC1 [16] for TRPV5, and CaT-L [17] and ECaC2 [18] for TRPV6. Only two cloned proteins share primary sequence similarity to TRPV5 and TRPV6 at the time of cloning, the capsaicin receptor VR1 (TRPV1), a heat activated channel in the pain pathway [19] and OSM-9, a C. elegans protein involved in olfaction, mechanosensation, and olfactory adaptation [20]. The subsequent identification of VRL-1 (TRPV2) [21, 22], OTRPC4/VR-OAC/Trp12/VRL-2 (TRPV4) [23–26] and TRPV3 [27–29], completed the TRPV family. TRPV5 and TRPV6 share approximately 40–45% amino-acid identity with the other 4 members and are the only calcium selective channels in the family; the other four members are non-selective cation channels, they serves as sensors for heat (TRPV1-3) or osmolarity (TRPV4).
Phylogenetic tree generated from alignment of TRPV5 and TRPV6 proteins from various species. Clustal W method was used to align the proteins with MegAlign program in DNASTAR Lasergene software (DNASTAR Inc., Madison, WI). TRPV6-like proteins are present in fish and birds, whereas both TRPV5 and TRPV6 are present in mammals
TRPV5 and TRPV6 and their flanking genes in zebrafish, chicken, mouse, chimpanzee, and human genomes. OnlyTRPV6 is present in zebrafish and chicken genomes, whereas both TRPV5 and TRPV6 are present in mouse, chimpanzee and human genomes. Cd209e, CD209E antigen-like protein E-like, current official gene symbol LOC563797; Ephb2, ephrin (EPH) receptor B2-like, current official gene symbol LOC572411; KEL, Kell blood group, metallo-endopeptidase; EPHB6, EPH receptor B6
14.2 Evolution of the Genes: From One to Two
No proteins similar to TRPV members were identified in E. coli or yeast. Five TRPV proteins were identified in C. elegans, namely osm-9, ocr-1, ocr-2, ocr-3 and ocr-4 [30]. Among them, ocr-4 is closer to TRPV5 and TRPV6 (25.9 and 25.4% identity, respectively) than other TRPV proteins in C. elegans (20.3–23.6% identity). Ocr-4 is also closer to TRPV5 and TRPV6 among all the 6 mammalian TRPV members. This indicates that TRPV5 and TRPV6 in vertebrates were likely evolved from Ocr-4 in invertebrate.
The phylogenic tree of TRPV5 and TRPV6 from vertebrates is shown in Fig. 14.1. One interesting observation is that both TRPV5 and TRPV6 are present in mammals, but only one of the two, i.e., TRPV6, is present in birds and fish based on the current available databases (Fig. 14.1). It has been noted by Qiu and Hogstrand that there is only one TRPV5/6-like gene in pufferfish genome [31]. This is not a single incident because the same is true in zebrafish (Fig. 14.2). Furthermore, in the chicken genome, only TRPV6 gene is present. In contrast, both TRPV5 and TRPV6 genes are present in mouse, chimpanzee, and humans (Fig. 14.2). All mammals examined so far have both TRPV5 and TRPV6 genes in their genomes. In human and other mammal genomes, TRPV5 and TRPV6 genes situate side by side (Fig. 14.2). The two genes are completely conserved in the exon size in the coding region [32]. Thus, it is likely TRPV5 gene was generated by duplication of TRPV6 gene. As both birds and mammals are evolved from reptiles, it is likely that gene duplication event occurred during evolution of mammals from reptiles. TRPV6 has a broader tissue expression pattern whereas TRPV5 expression is relatively restricted to the kidney (see Sections 14.3.1 and 14.4), therefore, the difference in complex kidney function between reptiles, birds and mammals [33] is likely the driving force behind the generation of the kidney specific TRPV5 gene.
14.3 Roles as Ca 2+ Entry Channel in Active Intestinal/Renal Ca 2+ Re/absorption
Transcellular Ca2+ transport pathway is a 3-step process: entry across the apical membrane, transfer of Ca2+ from the site of entry to the site of extrusion, and extrusion of Ca2+ across the basolateral membrane. This active pathway is involved in intestinal Ca2+ absorption, renal Ca2+ reabsorption, and many other processes. While vitamin D responsive Ca2+ binding protein calbindins may facilitate intracellular Ca2+ transfer and the Na+-Ca2+ exchanger and Ca2+ ATPase mediate the extrusion of Ca2+, TRPV5 and TRPV6 serve the role for Ca2+ entry as the first step of the transcellular pathway.
14.3.1 Expression in Ca2+ Transporting Epithelia
The major Ca2+ transporting epithelia reside in the intestine and kidney, where Ca2+ absorption and reabsorption take place, respectively. In the intestine, TRPV6 is the major Ca2+ entry channel whereas TRPV5 is barely detectable. Due to the high sequence similarity between TRPV5 and TRPV6, strong signals could be detected in the rat or rabbit duodenum using TRPV5 cDNA probe in Northern blot analyses [13, 15], but this is likely due to cross hybridization with TRPV6 mRNA [15]. In small intestine, TRPV6 mRNA expression decreases from duodenum to ileum in rats [14] and humans [34]; TRPV6 mRNA is also detectable in large intestine in rats and humans [14, 34]. TRPV6 protein is localized to the apical membrane of intestinal and colonic epithelial cells [35]. Even though TRPV6 mRNA is barely detectable in the ileum in mice and rats, immunostaining with an antibody against TRPV6 indicates the presence of TRPV6 protein in ileum [35]. It is worth noting that in sheep, TRPV6 mRNA and protein level are more abundant in jejunum than in duodenum [36]; higher expression of TRPV6 mRNA in proximal jejunum than duodenum was also documented in horse [37], suggesting a more important role of jejunum in active Ca2+ absorption in some animals. TRPV6 mRNA and protein are also weakly expressed in ovine rumen [36], where active Ca2+ absorption also occurs in sheep and goats [38].
In mice and rats, TRPV5 mRNA is much more abundant than that of TRPV6 in the kidney [39, 40]. TRPV5 mRNA level is approximately 10 to 20 times higher than that of TRPV6 in mouse kidney [39]. In humans, we have observed much higher mRNA level of TRPV6 than TRPV5 in the kidney [32]. Similarly, in horse kidney, TRPV6 is also much abundant than TRPV5 [37]. Thus, TRPV6 may play more significant roles in some species such as humans and horses.
TRPV5 is localized to the apical membrane of the tubular cells in the late segment of distal convoluted tubule and connecting tubule in mouse kidney [41]. In the rabbit, much TRPV5 is distributed in the connecting tubule [13]. With regards to TRPV6, the tubular distribution is less clear and controversial. Using an RT-PCR approach with microdissected mouse kidney tubules, TRPV6 signal was detected in the medullary thick ascending limb [42]. Due to its low level of expression and the lack of efficient antibodies for TRPV6, TRPV6 protein is difficult to detect in rodent kidney; however, it has been reported that TRPV6 is distributed to mouse distal convoluted tubules (DCT2), connecting tubules, and cortical and medullary collecting ducts using an immunohistochemistry approach [43]. We found a higher mRNA level of TRPV6 in outer medulla than cortex of human kidney, opposite to that of TRPV5 (unpublished observations). Using a TRPV6 specific antibody, we found TRPV6 protein is more abundant in the outer medulla, much less in the cortex, little or none in inner medulla (Zhang et al., unpublished observations). This is consistent with TRPV6 protein being mostly distributed to the thick ascending limb and/or the proximal tubule in the kidney.
14.3.2 Function and Regulation as Ca2+ Entry Channels
TRPV5 and TRPV6 share very similar functional properties. Being identified by 45Ca2+ uptake assay, TRPV5 and TRPV6 mediate Ca2+ influx in the absence of any exogenous ligand, altered membrane potential, mechanical stress, or other cues. This is different from other members of the TRPV family, including TRPV1-4, which are activated by ligands, heat, and/or reduction of osmolarity. At macroscopic level, this constitutive Ca2+ transport activity makes them act as facilitative transporters that transport solute down its concentration gradient. The Ca2+ uptake mediated by TRPV5 or TRPV6 is saturable, with apparent K m values at sub-millimolar range, which are well suited for the luminal environment in the intestine and the distal tubule [13–15, 34].
At microscopic level, TRPV5 and TRPV6 exhibit single channel activities. The single channel activity could only be detected in the absence of extracellular divalent cation, such as Ca2+ and Mg2+, using Na+ or K+ as charge carrier [44–46]. Single channel activity could not be detected with Ca2+ as charge carrier due to very small conductance. One common feature is that TRPV5 and TRPV6 are highly selective for Ca2+, with permeability ratio between Ca2+ and Na+ higher than 100:1 [45, 47]. This sets TRPV5 and TRPV6 apart from other TRPV members, which are non-selective cation channels.
TRPV5 and TRPV6 likely function as tetramers [48]. Five ankyrin repeats are present in the N-terminal region of TRPV5 and TRPV6, and it is believed that the third ankyrin repeat initiates a molecular zippering process necessary for the assembly of TRPV6 channels [49]. Regions in N- and C-terminal of TRPV5 were also found important to TRPV5 assembly [50]. However, the crystal structure of ankyrin repeats of TRPV6 indicates that ankyrin repeat domain of TRPV6 does not form a tetramer, challenging its role in channel assembly [51].
TRPV6 outer pore structure is likely similar to that of K+ channel with a single filter preceded by a pore helix [52]. An aspartate residue at 542 (D542) from each subunit of the TRPV5/6 tetramer likely forms the filter for Ca2+ [53–55]. This residue is also responsible for the Mg2+ blockade [53, 56].
TRPV5 and TRPV6 exhibit Ca2+-dependent inactivation as feedback control mechanisms via intrinsic channel structure, interaction with Ca2+ binding proteins, and phosphatidylinositol 4,5-bisphosphate (PIP2). TRPV6 activity is tightly regulated by intracellular Ca2+ [57]. It has a fast inactivation mechanism compared to TRPV5 [58]. The intracellular loop between transmembrane domains 2 and 3 is the molecular determinant of this mechanism [58]. A CaM binding site is present in the C-terminal of TRPV6 [59], and it mediates a slow phase of Ca2+-dependent inactivation of TRPV6 [59–61]. The interaction between Ca2+/CaM and TRPV6 increases dynamically as intracellular Ca2+ level rises [60]. Calbindin-D28 k, a vitamin D regulated Ca2+ binding protein, buffers Ca2+ entered through TRPV5 to prevent inactivation of the channel [62]. TRPV5 activity is also regulated by a Ca2+ sensor 80 K-H [63]. In addition, PIP2 increases TRPV5 and TRPV6 activity and prevents their Ca2+-dependent inactivation [64–67]. The activation of phospholipase C by Ca2+ depletes PIP2 and this contributes to the Ca2+-dependent inactivation of the channel [65]. ATP stabilizes TRPV6 and prevents it from rundown, and this action is counteracted by PKC βII [68].
TRPV5 and TRPV6 are also regulated by extracellular cues. Both TRPV5 and TRPV6 activities increase at alkaline pH [13, 14, 69]. Glutamate residues in the pore helix and in the extracellular domain close to the pore of TRPV5 sense the pH and alter channel activity via conformational changes of the pore [70–73]. In addition, extracellular pH also regulates the dynamic of plasma membrane delivery of functional TRPV5 proteins from intracellular vesicles [74]. Furthermore, tissue kallikrein, a serine protease secreted in the connecting tubule, enhances TRPV5 plasma membrane abundance by delaying its retrieval via the PLC/DAG/PKC pathway upon activating the bradykinin 2 receptor [75]. Finally, TRPV5 and TRPV6 can be stabilized at the plasma membrane by deglycosylation via Klotho (see Section 14.3.3).
The activity, stability and trafficking of TRPV5 and TRPV6 are also regulated by associated proteins [for reviews see [7, 8]].
14.3.3 Robust Regulation by Calcitrophic Hormones
14.3.3.1 Vitamin D
TRPV6 gene is one of the genes most responsive to 1,25(OH)2D3. TRPV6 mRNA was up-regulated as early as 2 h after administration of 0.1 μM of 1,25(OH)2D3 in intestinal cell line Caco-2 and a 10-fold increase was achieved by 24 h [76]. Compared to calbindin-D9 k, a vitamin D responsive Ca2+ binding protein, the response of TRPV6 to 1,25(OH)2D3 was more rapid and robust [76]. In vitamin D receptor (VDR) knockout mice, Duodenal TRPV6 mRNA was reduced by 90%; however, TRPV5 mRNA level in the kidney appeared to be unchanged. In contrast, TRPV5 mRNA and protein abundance was decreased in vitamin D depleted rats [77]. A single injection of 1,25(OH)2D3 in vitamin D deficient mice induced a ~10-fold increase of duodenal mRNA of TRPV6 which peaked at 6 h after injection, and ~three fold to fourfold increase in TRPV5 and TRPV6 mRNA in the kidney that peaked at 12 h after injection [39]. This suggests that TRPV5 and TRPV6 may be differently regulated by vitamin D in the kidney and intestine, respectively.
In humans, duodenal TRPV6 transcript was initially found not significantly correlated with vitamin D metabolites [18]; but later studies by Walter and colleagues indicate that duodenal TRPV6 level was positively correlated with plasma 1,25(OH)2D3 in a VDR genotype group [78], in men and likely in younger women, but not in older women [79]. This is likely due to the lower level of VDR in older women [79]. In isolated human duodenal specimens, TRPV6 transcript was very responsive to 1,25(OH)2D3 [80, 81]. TRPV6 was also responsive to 25-hydroxyvitamin D [25(OH)D], both in Caco-2 cells [82] and in human duodenal specimens [81]. This is likely due to the expression of 25(OH)D 1α-hydroxylase (CYP27B1), which converts 25(OH)D to 1,25(OH)2D3 [81, 82].
The most unequivocal evidence that TRPV6 is a direct target of vitamin D action came from the identification of vitamin D responsive elements (VDREs) from TRPV6 promoter by Pike and colleagues [83, 84]. Three active VDREs were identified in a 7 kb TRPV6 promoter at –1.2, –2.1 and –4.3 kb from the transcriptional start site [83]. Similar sites are present in mouse Trpv6 promoter [84]. VDR/RXR (retinoid X receptor) heterodimer binds to TRPV6 promoter, and then recruits steroid receptor coactivator 1 and RNA polymerase II; and this is accompanied by a broad change in histone 4 acetylation [83, 84]. The presence of VDREs in TRPV6 gene was also confirmed by another study [85]. With regards to TRPV5, it is suggested that there exist VDREs in human TRPV5 promoter [77]; however, experimental evidence is yet to be provided for these potential VDREs [86].
Finally, evidence exist to support the involvement of TRPV6 in the non-genomic action of 1,25(OH)2D3 to stimulate Ca2+ absorption in chicken intestinal cells [87]. 1,25(OH)2D3 stimulated β-glucuronidase secretion in a protein kinase A (PKA) dependent pathway. In response to β-glucuronidase administration, TRPV6 was translocated to the brush border membrane where it mediates Ca2+ influx. The non-genomic effect of 1,25(OH)2D3 on Ca2+ uptake was blocked by siRNA for either β-glucuronidase or TRPV6 [87]. Thus, 1,25(OH)2D3 appears to stimulate TRPV6 expression and trafficking via genomic and non-genomic pathways, respectively. TRPV6 remains the most vitamin D-responsive Ca2+ channel identified thus far.
14.3.3.2 Parathyroid Hormone
Parathyroid hormone (PTH) is well known to regulate renal Ca2+ reabsorption. The regulation of TRPV5 by PTH is twofold: expression and activity. In parathyroidectomized rats and calcimimetic compound NPS R-467 infused mice that had a lower PTH level, renal TRPV5 (calbindin-D28 k, and NCX1) level was decreased; and the PTH supplementation restored the level of TRPV5 and other Ca2+ transport proteins [88]. Inhibition of TRPV5-mediated Ca2+ influx by ruthenium red decreased the levels of other Ca2+ transport proteins, suggesting the major effect of PTH on Ca2+ transport machinery is to enhance TRPV5 expression, and the expression of other Ca2+ transport proteins depends on TRPV5-mediated Ca2+ influx [88].
PTH is also capable of increasing Ca2+ transport in the distal tubule acutely [89]. Both PKA and protein kinase C (PKC) are involved in this regulation [90]. Indeed, activation of TRPV5 by PTH via both PKA and PKC has been reported [91, 92]. PTH activated the cAMP-PKA signaling cascade and increased TRPV5 open probability via phosphorylation of threonine-709 of TRPV5 [91]. This regulation required a strong intracellular buffering of intracellular Ca2+. However, the PKA phorsphorylation site in TRPV5 (threonine-709) is not conserved in human TRPV5. To what extent this mechanism is applicable to humans remains to be determined.
In another study, the increase of TRPV5 activity by heterogeneously expressed PTH receptor was prevented by PKC inhibitor [92]. Mutation of PKC phosphorylation sites serine-299/serine-654 in TRPV5 abolished the regulation. Caveolae-mediated endocytosis of TRPV5 appears to be inhibited via PKC-dependent pathway in response to PTH [92].
14.3.3.3 Calcitonin
Calcitonin affects renal Ca2+ reabsorption through the thick ascending limb [93, 94]. Thus, it is not surprising that this action is independent of TRPV5 because its localization to distal convoluted tubule and connecting tubule. This was confirmed using the Trpv5 KO mice, as the effects of calcitonin on urinary excretion of Ca2+ (and Na+ and K+) was not different between wild-type (WT) and Trpv5 KO mice [95]. To what extent TRPV6 is involved in calcitonin-mediated regulation of Ca2+ reabsorption is yet to be examined.
14.3.3.4 Klotho
KL gene product Klotho functions to suppress aging process [96]. The two forms of Klotho, membrane and secreted forms, function distinctively (see [97] for a recent review). While the membrane Klotho interacts with fibroblast growth factor (FGF) receptors to form a high affinity receptor for FGF23, a hormone regulating phosphate excretion, the secreted form acts as a hormonal factor that regulates the stability of TRPV5/6 [98–100] and renal outer medullary potassium (ROMK) channel [101]. Klotho is expressed in the distal convoluted tubule where it colocalizes with TRPV5 [98]. Because Klotho exhibits β-glucuronidase activity [102], it was thought that Klotho modifies TRPV5 glycan through this activity [98]. Like Klotho, β-glucuronidase also activated TRPV5 and TRPV6 but not related TRP channels TRPV4 and TRPM6 [99]. Alternatively, Klotho removed terminal sialic acids from their glycan chains of TRPV5 and exposes disaccharide galactose-N-acetylglucosamine, which bound to galactoside-binding lectin galectin-1 [100]. Galectin-1 linked TRPV5 proteins are likely resistant to endocytosis, but the underlying mechanism is not well understood. However, the removal of N-glycan by endoglycosidase-F, which completely removes N-glycans, also increased TRPV5 and TRPV6 activity [99]. Under this condition, galectin-1 may not be able to bind to TRPV5. Although the mechanisms are still to be clarified, the regulation of TRPV5/6 by Klotho represents a novel area of ion channel regulation. The dysregulation of TRPV5/6 may be responsible for the increased excretion of Ca2+ in KL deficient mice, in which PTH stimulated Ca2+ reabsorption in the connecting tubule is impaired [103].
14.3.4 Regulation Under Physiological Conditions
14.3.4.1 Low Dietary Ca2+
The biosynthesis of TRPV6 has been shown to be the most responsive to the body’s need for Ca2+ under conditions such as low Ca2+ diet, pregnancy, and lactation. Restriction of dietary Ca2+ drastically increases duodenal TRPV6 level, and to a less extent, renal TRPV5 and TRPV6 in mice [39, 40], and in rats [104]. At least part of the effect of low Ca2+ diet to up-regulate TRPV6 is independent of VDR [105].
14.3.4.2 Pregnancy and Lactation
During pregnancy, duodenal TRPV6 transcript level was up-regulated by 12-fold in either WT or VDR KO mice, whereas calbindin-D9 k and Ca2+-ATPase PMCA1 were only moderately increased in WT mice [106]. This observation was confirmed with global microarray analysis in pregnant VDR KO mice [107]. Similarly, a 13-fold increase in duodenal TRPV6 transcript was observed in both mouse lines during lactation [106]. The increase of prolactin during lactation causes a positive genomic effect on the TRPV6 transcription along with paracellular claudins; however, TRPV6 appears not to be involved in the nongenomic effect of suckling induced prolactin surge on intestinal Ca2+ absorption [108].
14.3.4.3 Sex Hormones
TRPV5 and TRPV6 levels are also controlled by sex hormones. In estrogen receptor β KO mice, TRPV6 level was not altered, however, a 55% reduction of duodenal TRPV6 mRNA level was observed in estrogen receptor α KO mice. Pharmacological doses of estrogens increased TRPV6 mRNA by fourfold and eightfold in ovariectomized WT and VDR KO mice, respectively [106]. At the same level of 1,25(OH)2D3, female mice had a higher Ca2+ absorption and TRPV6 mRNA level than male mice. The response of duodenal TRPV6 mRNA to 1,25(OH)2D3 in female mice was twofold of that in male mice even though the VDR mRNA level was the same [109]. In ovariectomized rats, both TRPV5 mRNA and protein levels were increased by pharmacological doses of 17β-estradiol and this effect was independent of 1,25(OH)2D3 [110]. In aromatase deficient (ArKO) mice, deficiency in estrogen caused hypercalciuria together with decreased levels of renal TRPV5 and TRPV6 [111]. Additionally, 17β-estradiol exerted a nongenomic effect to enhance TRPV6 activity in human colonic T84 cells [112] and TRPV5 activity in rat cortical collecting duct cells [113].
We have previously observed that TRPV6 expression was negatively regulated by androgen in LNCaP cells [114]. Whether this effect of androgen on TRPV6 is related to the lowered intestinal Ca2+ absorption and TRPV6 level in male mice is unclear [109]. Interestingly, male mice also have a higher urinary Ca2+ excretion and a lower renal TRPV5 level than female mice [115]. TRPV5 level was increased in orchidectomized mice, which excreted less Ca2+ in their urine [115]. Males in general have a higher bone mineral density than females; therefore, a higher intestinal Ca2+ absorption is expected for males.
14.3.4.4 Exercise
Endurance swimming stimulated Ca2+ transport in the small intestine and cecum and decreased that in the proximal colon in rats [116]. The increased Ca2+ absorption were associated with increased mRNA levels of VDR and Ca2+ transport proteins (such as TRPV6 and TRPV5) in transcellular and paracellular pathways [116]. In contrast, TRPV6 and TRPV5 mRNA levels dropped along with decreased 1,25(OH)2D3 level in immobilized rats, leading to a reduction in intestinal Ca2+ absorption [117]. These studies suggest that TRPV6 (and to a lesser extent TRPV5) participate in exercise-induced increase in Ca2+ absorption in a vitamin D-dependent manner.
14.3.4.5 Aging
Decreased Ca2+ absorption and increased urinary Ca2+ excretion are associated with aging. TRPV6 (and also TRPV5) mRNA level in adult (12-month old) rats was less than half of that in young (2-month old) rats [104]. In mice, intestinal absorption decreased and urinary Ca2+ excretion increased with age; this was associated with decreased TRPV6 and TRPV5 in the duodenum and kidney, respectively [118]. The same is true for VDR [118]. In women, both TRPV6 and VDR transcripts were significantly decreased in older age (> 50) [79]. Thus, reduction in TRPV5 and TRPV6 may contribute to a negative Ca2+ balance as one ages.
In addition to the above physiological conditions, TRPV6 expression is up-regulated by short-chain fatty acids, fermentation products of indigestible oligosaccharides in rat large intestine [119]. This is likely involved in the increase of Ca2+ absorption by the indigestible oligosaccharides such as fructooligosaccharides [120].
14.3.5 Gene Knockout Studies
The roles of TRPV5 or TRPV6 in vitamin D-regulated Ca2+ transport pathways have been evaluated by gene knockout studies. The major phenotypes of Trpv5 and Trpv6 KO mice are listed in Table 14.1. The common features of the Trpv5 and Trpv6 KO mice include elevated serum 1,25(OH)2D3, increased urinary Ca2+ excretion, and some degree of deficiency in the bone [121, 122]. Urinary Ca2+ excretion increased by sixfold in Trpv5 KO mice [122]. Intestinal Ca2+ absorption is decreased in Trpv6 KO mice [121], increased in Trpv5 KO mice as duodenal TRPV6 and calbindin-D9 k mRNAs are increased due to an elevated 1,25(OH)2D3 level [122]. However, the serum Ca2+ levels are largely normal, even though the PTH level is elevated in Trpv6 KO mice [121], and in older Trpv5 KO mice [118].
It is worth noting, however, active Ca2+ transport did occur in the absence of TRPV6 alone or together with calbindin-D9 k [123]; and the increase of intestinal Ca2+ absorption in response to 1,25(OH)2D3 appeared to be intact in the absence of TRPV6 [124]. While Ca2+ balance studies are needed to verify the results, it is not surprising as transcellular Ca2+ transport contributes only a small fraction of total Ca2+ absorbed when the dietary Ca2+ level is adequate or high due to the short sojourn time of digested food in the duodenum [125]. The transcellular pathway plays a significant role only when Ca2+ in diet is low. The main driving forces for Ca2+ absorption through the paracellular pathway are transepithelial potential and Ca2+ concentration gradient. As Ca2+ concentration in the plasma is around 1 mM, the driving force for Ca2+ absorption via paracellular pathway would be decreased when dietary Ca2+ level is low. In contrast, there is a huge (10,000-fold normally) Ca2+ gradient across the brush border membrane, even when Ca2+ in the diet is scarce, the transcellular pathway will still function properly. When dietary Ca2+ is limited, 1,25(OH)2D3 will be elevated in the body, which in turn will induce the transcription of TRPV6 and related Ca2+ transport proteins [39]. Low dietary Ca2+ also increases TRPV6 transcription independent of the genomic action of vitamin D [105]. Thus, TRPV6 would be an important component for Ca2+ absorption when dietary Ca2+ is restricted; when the dietary Ca2+ is adequate, it may play a regulatory role to fine tune the level of Ca2+ absorption. This role is also indispensable because 1,25(OH)2D3 level is elevated in the absence of TRPV6.
14.4 Roles Beyond Intestinal/Renal Ca 2+ Re/absorption
14.4.1 TRPV6 in Maternal-Fetal Ca 2+ Transport
Both TRPV5 and TRPV6 are all expressed in the placenta [17, 34, 130–132], however, TRPV6 mRNA level is ~1,000 times higher than that of TRPV5 [32]. TRPV6 is expressed in the trophoblasts and syncytiotrophoblasts of human placenta [17]. In human syncyiotrophoblasts, TRPV6 and TRPV5 were detected in both apical and basal membranes [130]. Ca2+ transport in human syncyiotrophoblasts was insensitive to voltage and L-type Ca2+ channel modulators but is sensitive to TRPV5/6 blocker Mg2+ and ruthenium red [132]. In cultured human trophoblasts isolated from term placenta, TRPV6 and TRPV5 expression correlated with the Ca2+ uptake potential along the differentiation of the trophoblasts [131]. Cyclophilin B, a member of immunophilin family, was associated with TRPV6 in human syncytiotrophoblasts and increased TRPV6 activity in vitro [133].
The role of TRPV6 in maternal-fetal Ca2+ transport was confirmed by the Trpv6 KO model [134]. TRPV6 mRNA and protein were detected mainly in intraplacental and the visceral layer of extraplacental yolk sac, where maternal-fetal Ca2+ transport takes place in mice. A 14-fold increase was observed in mouse placenta in the last 4 days of gestation to meet the need of Ca2+ required for fetal bone mineralization. The maternal-fetal Ca2+ transport was 40% lower in Trpv6 KO fetuses than in WT. As a result, Trpv6 KO fetuses exhibited low blood Ca2+ level and low ash weight [134]. Thus, TRPV6 represents an important Ca2+ entry route for maternal-fetal Ca2+ transport pathway. A significant reduction of Ca2+ transport along with decreases of TRPV5, TRPV6 and other Ca2+ transport proteins was found in primary preeclamptic syncytiotrophoblasts, suggesting that TRPV5 and TRPV6 are involved in the abnormal placental Ca2+ transport in preeclampsia [135].
14.4.2 TRPV6 Expression and Regulation in the Uterus
The expression of TRPV6 in uterine tissue was studied in mice [136, 137], rats [137, 138], and pigs [139]. TRPV6 mRNA was detected in rat uterine endometrium and glandular endometrium and was highly up-regulated at diestrus compared with proestrus [138]. In mice, uterine TRPV6 mRNA increased in mid- and late-pregnancy, and TRPV6 mRNA was responsive to 17β-estradiol in the luminal and glandular epithelia [136]. In rats, uterine TRPV6 was regulated by progesterone via its receptor [138], whereas TRPV6 appears to be dependent on estrogen receptors in mice [137]. Uterine and placenta TRPV6 levels changed cyclically during pregnancy, suggesting a role of TRPV6 in the embryo implantation process [137]. TRPV6 mRNA was only expressed in placenta-unattached areas of the uterus, and in the labyrinth and spongy zone of placenta. In pig uterus TRPV6 was localized mainly to luminal epithelial cells and to a lesser extent to glandular epithelial cells and chorionic membrane during pregnancy [139]. TRPV6 was higher during pregnancy than in estrous cycle [139]. These observations indicate that TRPV6 plays a role in establishing and maintaining pregnancy as well as in Ca2+ transfer between embryo and placenta [137, 139].
14.4.3 TRPV5 in Bone Resorption
TRPV5 was shown to be expressed in the ruffled border membrane in mouse osteoclasts [140]. In the absence of TRPV5, there were increased osteoclast numbers and osteoclast area; however, urinary bone resorption marker deoxypyridinoline was reduced [140]. This is consistent with the impaired bone resorption by osteoclasts derived from Trpv5 KO mice in an in vitro bone marrow culture system [140]. However, bone resorption inhibiter alendronate normalized the reduced bone thickness in Trpv5 KO mice, even though it specifically increased bone TRPV5 expression in mice [128]. This indicates that although TRPV5 may play a role in bone resorption, this process is still functional in the absence of TRPV5. Thus the defect of bone in Trpv5 KO mice is largely due to other compensatory mechanisms as a result of urinary Ca2+ wasting.
14.4.4 TRPV5 and TRPV6 in Maintaining Ca 2+ Gradient in the Inner Ear
The low Ca2+ concentration of mammalian endolymph in the inner ear is required for normal hearing and balance. Marcus and colleagues showed that TRPV5 and TRPV6 may play a role in the function of inner ear [141–144]. Both TRPV5 and TRPV6 were detected in primary cultures of semicircular canal duct (SCCD) epithelial cells from neonatal rats, and TRPV5 transcript was responsive to 1,25(OH)2D3 [144], however, the protein level was not up-regulated [141]. TRPV5 and TRPV6 were detected in native SCCD, cochlear lateral wall and stria vascularis (TRPV5 only) of adult rats along with other Ca2+ transport proteins [141]. TRPV5 protein was localized close to the apical membrane of strial marginal cells; and both TRPV5 and TRPV6 were found in outer and inner sulcus cells of the cochlea and in the SCCD of the vestibular system [141]. TRPV5 and TRPV6 immunostaining was detected in mouse inner ear as well, and the levels of TRPV5 and TRPV6 decreased in older mice [145]. Mutations in pendrin (Slc26a4, an anion exchanger) cause the most common form of syndromic deafness. Reduced pH and utricular endolymphatic potential and increased Ca2+ concentration were found in pendrin KO mice [142, 143]. The reduced pH likely blocks the activity of TRPV5 and TRPV6, whose Ca2+ transport activity is reduced at low pH, similar to what was observed in primary SCCD cells [143]. The elevation of endolymphatic Ca2+ level in pendrin KO mice may inhibit sensory transduction necessary for hearing and promote the degeneration of the sensory hair cells, which is necessary for the development of normal hearing [142]. Similar mechanisms may be responsible for loss of hearing in patients with Pendred syndrome.
14.4.5 TRPV6 in Exocrine Organs
Human TRPV6 transcripts are most abundant in exocrine organs including pancreas, prostate, mammary, salivary and sweat glands [17, 34, 35]. In pancreas, TRPV6 protein is prominently distributed to the apical membrane and granules of the secretory pole in both mice and humans [35]. In human mammary glands, TRPV6 was strongly expressed in the apical membrane of ductal epithelial cells. Apical membrane localization of TRPV6 was also observed in human prostate epithelial cells [35]. In human sweat gland, TRPV6 appears to be expressed in both apical and basolateral membranes [35]. TRPV6 mRNA is expressed in human parotid and submandibular glands, and TRPV6 protein is localized to the basolateral plasma membrane of acinar cells [146]. While TRPV6 might be involved in salivary Ca2+ secretion which is important in mineralization of dental enamel and exposed dentin [146], details of TRPV6 in exocrine function are largely unavailable although it is well known that Ca2+ plays important role in secretion. It is possible that TRPV6-mediated Ca2+ influx stimulates exocytosis; more likely, TRPV6 may replenish cellular Ca2+ by apical re-uptake of released Ca2+ following the secretory events, or from extracellular fluid via the basolaterial membrane.
In addition, TRPV6 may play a role in Ca2+ induced keratinocyte differentiation [147]. Both TRPV5 and TRPV6 are expressed in retina pigment epithelium and they may mediate Ca2+ influx from subretinal space to regulate the Ca2+ level in light/dark transitions [148].
14.5 Regulation Under Pathological and Therapeutic Conditions
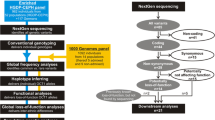
TRPV5 and TRPV6 undergo changes in response to diseases and therapies. Tables 14.2 and 14.3 summarize the changes in TRPV5 and TRPV6 mRNA or protein level under disease and therapeutic conditions, respectively. The regulation of TRPV6 and TRPV5 in cancers, pseudohypoaldosteronism Type II (PHA II), and under treatments of glucocorticoids and diuretics are summarized in the following.
14.5.1 TRPV6 in Cancer
TRPV6 level is elevated in prostate, breast, thyroid, colon and ovarian carcinomas [35]. In prostate cancer samples, TRPV6 mRNA level increases as tumor grade advances as defined by Gleason score [17, 114, 149]. Furthermore, TRPV6 expression also correlates with pathological stage and extraprostatic extension; and androgen-insensitive tumors exhibited decreased TRPV6 levels compared to untreated tumors [149]. Thus, TRPV6 represents a prognostic marker for advanced prostate cancer.
TRPV6 appears to be a component of the store-operated Ca2+ channels in prostate LNCaP cells [150, 151] while evidence exists against this [152]. Ca2+ store depletion induced the expression of TRPC1, TRPC3, and TRPV6 in LNCaP cells, but these channels were not sufficient to stimulate store-operated Ca2+ entry [153]. The store-operated current was down-regulated when LNCaP cells differentiated to androgen-insensitive, apoptotic-resistant neuroendocrine phenotype [149]. TRPV6 expression activated Ca2+/NFAT pathways that enables LNCaP cells to proliferate at high rate and to become resistant to apoptosis [154]. The expression of intermediate-conductance Ca2+-activated K+ channels, which are preferentially expressed in human prostate cancer tissues, hyperpolarized the cells to drive TRPV6-mediated Ca2+ entry, and in turn, proliferation of cancer cells [155]. Reducing Ca2+ entry via blocking the K+ channels prevented proliferation [155]. Therefore, TRPV6 represents a potential therapeutic target to treat prostate cancer.
The potential role of TRPV6 in breast cancer was recently put forward by Hediger and colleagues [156, 157]. Seven out of 12 patients exhibited 2–15-fold increases of TRPV6 mRNA in breast cancer tissues over normal tissues as control [156]. Interestingly, estrogen receptor antagonist tamoxifen not only partially blocked TRPV6 expression in breast cancer cell line T47D, but also inhibited TRPV6-mediated Ca2+ uptake activity in Xenopus oocytes [156]. In MCF-7 breast cancer cells, TRPV6 activity was blocked by tamoxifen in the presence of estrogen receptor antagonist and the effect of tamoxifen on TRPV6 remained intact in estrogen receptor-negative MDA-MB-231 cells [157]. Activation of PKC blocked the effect of tamoxifen [157]. Thus, tamoxifen-mediated inhibition of TRPV6 might be part of the therapeutic effects of tamoxifen in both estrogen sensitive and insensitive breast cancers.
In a Citrobacter rodentium-induced transmissible murine colonic hyperplasia model, a 10- to 20-fold increase of TRPV6 mRNA was observed during the induction of colonic hyperplasia, while VDR, calcium sensing receptor, calbindin-D9 k and TRPV5 mRNA levels were unaltered [158]. High Ca2+ diet abrogated TRPV6 expression and hyperplasia [158]. Elevated TRPV6 expression was associated with early-stage colon cancer in humans, and blocking TRPV6 expression by siRNA inhibited colon cancer cell proliferation and induced apoptosis [158]. Thus, elevated TRPV6 expression likely contributes to colonic hyperplasia and colon cancer cell proliferation and TRPV6 represents a therapeutic target for colon cancer.
Capsaicin, an ingredient of chili pepper and TRPV1 activator, induces apoptosis in some cancer cells. Interestingly, gastric cancer cells were more susceptible to capsaicin-induced apoptosis because of the high TRPV6 expression in cancer cells [159]. Overexpression of TRPV6 in normal cells increased capsaicin-induced apoptosis and knockdown of TRPV6 in cancer cells suppressed it [159]. This suggests a strategy to selectively induce cancer cell apoptosis based on its high level TRPV6 expression. The potential roles of TRPV6 as a cancer marker and therapeutic target are still to be further explored.
14.5.2 TRPV5 and TRPV6 in Pseudohypoaldosteronism Type II
Pseudohypoaldosteronism type II (PHAII), also known as familial hyperkalemia and hypertension (FHH) or Gordon syndrome, is a genetic disorder caused by mutations in With No lysine (K) (WNK) kinases 1 and 4 [160]. Patients carrying WNK4 mutation exhibit marked sensitivity to thiazides, hypercalciuria and low bone mineral density, indicating a defect in Ca2+ reabsorption [161, 162]. To understand potential roles of TRPV5 and TRPV6 in this process, we investigated their regulation by WNK kinases [163–165]. In Xenopus oocytes expression system, WNK4 selectively up-regulated TRPV5 while exerted little effect on TRPV6 [163]. WNK1 showed no significant effect on either of them [163]. In contrast, WNK3 was capable of up-regulating both [164]. The effects of WNK3 and WNK4 on TRPV5 are at least in part via enhancing the forward trafficking of TRPV5 to the plasma membrane through the secretory pathway [164, 165]. While the disease causing mutants of WNK4 regulated TRPV5 to similar extent as WT, the presence of the thiazide-sensitive Na+-Cl– cotransporter, which was down-regulated by WNK4 [166, 167], blocked the effect of WNK4 on TRPV5 in a dose-dependent manner. The blocking effect of NCC was further strengthened when WT WNK4 was replaced by the PHAII-causing Q565E mutant, suggesting a reduction of TRPV5-mediated Ca2+ influx under this condition [163]. This is consistent with the observation of hypercalciuria and low bone mineral density in patients carrying WNK4 Q565E mutation.
In the Wnk4 D561A/+ knock-in mouse model of human PHAII, TRPV5 protein level appears to be similar to the WT littermate, but a significant increase in TRPV6 and calbindin-D28 k was observed, along with attenuated level of NKCC2, and increased abundance of NCC and ENaC [168]. In addition, urine Ca2+ excretion rate was not increased by furosemide in Wnk4 D561A/+ mice as was in WT mice [168]. These data indicate decreased Ca2+ reabsorption in the thick ascending loop of Wnk4 D561A/+ mice, and the enhanced level of TRPV6 and calbindin-D28 k represents a secondary adaptive mechanism in the distal nephron [168]. The effects of WNK4 mutation on NKCC2 and TRPV6 are consistent with the high abundance of WNK4 in the outer medulla [169]. Since TRPV6 is predominantly expressed in the thick ascending limb [42](our unpublished observation), the effect of WNK4 on TRPV6 expression (or indirectly via NKCC2) warrants further studies. The increased NCC level is also consistent with a potential decrease of TRPV5-mediated Ca2+ reabsorption through NCC-mediated inhibitory effect on the positive action of WNK4 on TRPV5 as noted earlier [163].
14.5.3 Glucocorticoids
Glucocorticoids are a class of hormones that are used widely as an anti-inflammatory and immunosuppressive drug. However, they have side effects such as inducing osteoporosis. Reduced intestinal Ca2+ absorption and renal Ca2+ reabsorption may be part of the mechanisms of glucocorticoid-induced osteoporosis. Regulation of TRPV5 and TRPV6 by glucocorticoids may occur through two mechanisms, expression regulation [170–172] and activity regulation via glucocorticoid inducible protein kinase SGK1 [173–176].
Oral application of prednisolone for a week in mice reduced intestinal Ca2+ absorption and TRPV6 mRNA level and calbindin-D9 k mRNA and protein levels but renal TRPV5 and calbindin-D28 k and NCX1 mRNA levels remained unaltered [172]. In calbindin-D9 k and calbindin-D28 k KO mice, compensatory elevation of TRPV6 mRNA in the duodenum was blocked by 5-day dexamethasone treatment [171]. The renal TRPV5 mRNA in calbindin-D9 k KO mice was elevated while the renal TRPV6 mRNA was reduced in calbindin-D28 k KO mice after dexamethasone administration [171]. These regulations appear to be related to the decreased duodenal VDR level by dexamethasone [171]. The effects of dexamethasone were time dependent: at day one it increased duodenal TRPV6 mRNA and renal TRPV5 mRNA while decreased renal TRPV6 mRNA; after 5-day treatment, only TRPV6 in the duodenum was robustly reduced [170]. Thus, it appears intestinal TRPV6 is the target of glucocorticoids action and this action appears to be associated with decreased VDR level in the intestine.
The activity of TRPV5 and TRPV6 could be regulated by glucocorticoid inducible SGK kinases. In Xenopus oocyte system, SGK1 and SGK3 up-regulated TRPV5 activity in the presence of Na+/H+ exchanger (NHE) regulating factor 2 (NHERF2), and this effect could not be reproduced by active form of protein kinase B (PKB) and SGK2 [175]. The surface abundance of TRPV5 was increased by SGK1 and NHERF2 [174]. TRPV5 C-terminal tail interacts with NHERF2, and deletion of the second PDZ domain in NHERF2 abrogates the stimulating effect of SGK1/NHERF2 on TRPV5 [174]. In contrast, TRPV6 was also up-regulated by SGK1, SGK3 and PKB/Akt; however, neither NHERF1 nor NHERF2 was required for this regulation [173, 176]. The effect of SGK1 on TRPV6 was augmented in the presence of phosphatidylinositol-3-phosphate-5-kinase PIKfyve (PIP5K3), a kinase generating phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] [176]. TRPV6 expression at or close to the Xenopus oocytes surface was significantly increased by the co-expression of SGK1 and PIKfyve [176]. PIKfyve alone did not affect TRPV6, and S318APIKfyve lacking the SGK1 phosphorylation site could not further increase the positive effect of SGK1 on TRPV6 [176]. Thus, PIKfyve requires its phosphorylation by SGK1 to act on TRPV6.
The effect of SGK1 on Ca2+ reabsorption and TRPV5 was evaluated in Sgk1 KO mice [177]. Membrane expression of TRPV5 protein and cytosolic calbindin-D28 k protein were all decreased in the KO mice, suggesting that SGK1 play a role in the regulation of TRPV5 trafficking as observed in previous in vitro studies [174, 175, 177]. However, fractional excretion of Ca2+ was lower in the Sgk1 KO mice than in the WT mice, despite normal 1,25(OH)2D3 and PTH levels [177]. The lowered Ca2+ excretion is likely due to the increased reabsorption of Ca2+ in the thick ascending limb because NKCC2 inhibitor furosemide dissipated the difference in Ca2+ excretion between the KO and WT mice [177]. Whether TRPV6 is altered in the Sgk1 KO mice is yet to be determined.
14.5.4 Diuretics
Hypocalciuria is a well known effect of thiazide diuretics, which blocks the Na+-Cl– cotransporter NCC. A number of studies have been undertaken to dissect the molecular mechanisms of this effect [178–181]; however, the mechanisms of thiazides-induced hypocalciuria are controversial. Application of high dose of hydrochlorothiazide (HCTZ), at 12 mg/day for a week, significantly reduced TRPV5, calbindin-D28 k, NCX1 and NCC mRNA and protein levels and the number of tubules expressing calbindin-D28 k and NCC in rat kidneys [181]. Since Na+ repletion prevented both volume contraction and hypocalciuria in HCTZ treated rats, it is suggested volume contraction play a critical role in thiazide induced hypocalciuria [181]. At lower dose, acute application of chlorothiazide (CTZ), at either 25 or 50 mg/kg but not at 100 mg/kg, significantly increased TRPV5 expression in mouse kidneys [180]. Chronic treatment with CTZ at 25 mg/kg twice daily for 3 days caused no change in TRPV5, TRPV6 and calbindins, but salt supplement in drinking water alone or with CTZ significant increased the expression of these Ca2+ transport proteins [180]. This study agrees that under volume contraction, increased Ca2+ reabsorption via paracellular pathway in the proximal tubule plays the major role; however, without volume contraction, hypocalciuria is probably achieved through increased Ca2+ reabsorption in the distal convoluted tubule by up-regulating the transcellular pathway [180].
Subsequent studies with the Trpv5 KO mice indicate that enhanced passive Ca2+ transport in the proximal tubule rather than active Ca2+ transport in distal convolution is the cause of thiazide-induced hypocalciuria [179]. HCTZ-induced hypocalciuria remained unaltered in Trpv5 KO mice, and the reabsorption of Na+ and Ca2+ was increased in the proximal tubule and the Ca2+ reabsorption in the distal convolution was apparently unaffected in micropuncture experiments [179]. In a recent study that evaluates the effects of HCTZ on different transporters in proximal tubule, thick ascending limb and distal convoluted tubule under high salt or high Ca2+ diet induced hypercalciuric rats, TRPV5, NHE3, and NKCC2 proteins were decreased in all hypercalciuric rats, whereas increased TRPV5 protein was associated with hypocalciuric effect induced by HCTZ [178]. These results indicate that thiazide-induced hypocalciuria is a result of coordinated alterations of Ca2+ reabsorption mechanisms in all tubular segments involved in Ca2+ reabsorption, including proximal tubule, thick ascending limb, and the distal convoluted and connecting tubule.
In contrast to hypocalciuric effect of thiazide diuretics, loop diuretics such as furosemide promote urinary Ca2+ excretion [182]. Furosemide inhibits NKCC2 in the thick ascending limb and diminishes the negative luminal potential needed to drive Ca2+ and Mg2+ reabsorption through the paracellular pathway. Lien and colleagues [183] found that acute and chronic application of furosemide increased both TRPV5 and TRPV6 levels along with that of calbindins; similar increases of TRPV5 and TRPV6 were found in hypercalciuria induced by gentamicin [183] and in streptozotocin-induced diabetes mellitus [184]. The up-regulation of Ca2+ transporters in these situations is likely due to the increased Ca2+ load to the segments [183, 184], consistent with Ca2+ load-dependent expression of TRPV5 in the kidney [185].
14.6 Unusual High Frequencies of SNPs in African Populations
TRPV6 and TRPV5 have been shown to have high frequency of SNPs in African populations. By analyzing SNP data of 132 genes from 24 African–Americans and 23 European–Americans, Akey and colleagues [191] identified 4 contiguous genes under demographically robust selection, including EPHB6, TRPV6, TRPV5, and KEL4, in a 115-kb region in chromosome 7q34-35 exhibiting features of a recent selective sweep. Similarly, using data from 151 genes, Stajich and Hahn [192] found that TRPV6 gene shows a population-specific pattern of positive selection. All these studies utilized the public available database from SeatleSNPs programs (http://pga.mbt.washington.edu/), in which approximately 200 genes from 24 African–Americans and 23 European–Americans are sequenced. The visual SNP genotype of TRPV6 (http://pga.gs.washington.edu/data/trpv6/trpv6.prettybase.png) clearly shows that there are many rare alleles in African–Americans whereas very few in European–Americans. The same is true for TRPV5. Subsequent studies [193, 194] also support that TRPV6 locus has undergone positive selection. The selective scenario put forward by Stajich and Hahn [192] is that there were many mutations in TRPV6 locus in early humans in Africa; as early humans migrated out of Africa, a preexisting mutation in the ancestral African population became advantageous in a new environment and rose to high frequency. Akey and colleagues found that the haplotype defined by 3 nonsynonymous SNPs (C157R, M378 V and M681T, Allele frequency ~0.5 in African–Americans, ~0.02 in European–Americans from SeattleSNPs) are nearly fixed for the derived alleles in non-African populations based on genotyping data of 1,064 individuals from 52 populations [195]. Hughes and colleagues [193] further provided evidence for independent, parallel selection on TRPV6 locus in Europeans and Asians.
Which one of the 4 genes in chromosome 7q34-35 was under the selective pressure outside of Africa is unclear; however, TRPV6 is a good candidate as suggested by Akey and colleagues [191, 195]. What drove the selection of TRPV6 haplotye outside of Africa is unknown; however, it might be associated with the alteration of dietary Ca2+ availability and the change of vitamin D status in earlier humans. Akey and colleagues [191, 195] suggested that patterns of TRPV6 sequence variation may have been influenced by availability of dairy products due to the domestication of milk-producing animals approximately 10,000 years ago, similar to positive selection for lactase persistence [196]. As early humans migrated away from the equator their vitamin D level decreased due to the reduction of ultraviolet light exposure [197]. Because TRPV6 is a highly vitamin D-responsive gene, the alteration of vitamin D status may be a factor. Another possibility is that the derived TRPV6 allele may be advantageous in resistant to a pathogen humans encountered after migrated outside of Africa [195]. TRPV6 is expressed in the brush border membrane of intestinal epithelium, thus it is likely that TRPV6 could be a receptor for certain pathogens. Consistent with this hypothesis, M378 V variation in the first extracellular loop of TRPV6 is in direct contact with digested food and potential pathogens. The 378 V residue is conserved in many species. If the extracellular loop of TRPV6 interacts with a contagious pathogen, this pathogen is potentially transferable between animals and humans. Thus, the 378 M mutation could potentially make TRPV6 resistant to this pathogen and provide an advantage for the 378 M carriers to survive disastrous infectious diseases. In this case, the derived TRPV6 allele may not necessarily be beneficial for Ca2+ absorption. Due to the increased Ca2+ intake from the dairy products and the increased availability of vitamin D by the development of fishery, the role of TRPV6-meidated Ca2+ absorption may not be as critical for human survival outside of Africa. This hypothesis is consistent with the fact that African–Americans have higher intestinal Ca2+ absorption compared to Caucasians [198].
The nonsynonymous SNPs in the ancestral haplotype (157R, 378 V, 681T) may have an impact on TRPV6 function; however, the results from different groups are controversial [193, 199]. In one study the Ca2+ uptake activity of the ancestral TRPV6 protein (157R, 378 V, 681T) was twofold that of the derived protein (157C, 378 M, 681 M) when expressed in Xenopus oocytes; and 378 V, located to the first extracellular loop, appeared to be responsible for the increase in Ca2+ uptake activity [199]. In another study, however, no significant differences in channel function were identified for the ancestral TRPV6 [193]. We also failed in observing any functional difference between the two forms of TRPV6 (unpublished observations). An interesting observation is that ATP binds to TRPV6 and prevents rundown of channel activity and 2 arginine residues R153R154 close to 157R are involved in ATP binding [68]. The 157R in the ancestral TRPV6 is located to N-terminal region between ankyrin repeats 3 and 4. The S155 becomes a potential phosphorylation site (S155P156R157) in the ancestral TRPV6 and phosphorylation of S155 by PKC is likely involved in the slower rundown of TRPV6 ancestral protein in the presence of ATP [68]. However, the difference is very small and may not result in significant difference in overall channel activity under normal condition.
Four nonsynonymous SNPs in TRPV5 were identified by SeattleSNPs: three of them (A8V, A563T and L712F) were only present in African–Americans, not in European–Americans; R154H is common in both populations. In contrast to the nonsynonymous SNP variations in TRPV6 ancestral haplotype that are mostly conserved in other species, the variations in TRPV5 are newly derived as they are not commonly present in other species surveyed including chimpanzee, dog, rat and mouse, with the exceptions of 563T in dog and 8 V in rat [200]. In addition, the nonsynonymous SNPs of TRPV5 are not associated with each other as are those in TRPV6. Thus, these variations more likely alter the function of TRPV5. By expression in Xenopus oocytes, we found that 2 of the SNPs, A563T and L712F, significantly increased TRPV5-mediated Ca2+ uptake by approx. 50 and 25%, respectively [200]. For A563T variant, the increased Ca2+ uptake activity was not associated with increased protein abundance in the plasma membrane; rather it was associated with increase apparent K m for Ca2+ and increased sensitivity to extracellular Mg2+, suggesting increased permeation of Ca2+ in the cation translocation pathway of the channel [200]. The location of A563T in the last transmembrane domain of TRPV5, 20 amino-acid residues away from the D542 that forms Ca2+ filter in the pore, is likely close to the cation translocation path in a 3D structure.
Africans exhibit Ca2+ conservation mechanisms. African–Americans have lowered urinary Ca2+ excretion than Caucasians [198, 201, 202], and the risk of kidney stone in African–American is lower than that in Caucasians [203, 204]. African–Americans have higher bone mass [205], and lower incidence of osteoporosis related fractures than whites [206]. Because of the high allele frequencies of TRPV5 and TRPV6 SNPs in African populations, these SNPs may contribute to the Ca2+ conservation mechanisms in African populations. This is yet to be tested in population studies.
The roles of TRPV6 or TRPV5 SNPs have been evaluated in kidney stone disease and hypercalciuria. In a study of 170 kidney stone formers, the prevalence of the TRPV6 ancestral haplotype in stone-forming patients was significantly higher than that of non-stone-forming patients [199]. The only kidney stone patient carrying the homozygous ancestral TRPV6 haplotype exhibited more stone episodes, much higher urinary Ca2+ excretion and lower plasma PTH level, suggesting a potential role of ancestral TRPV6 protein in the enhanced Ca2+ absorption in this patient [199]. In a study involving 20 renal hypercalciuria patients, non-synonymous variation of TRPV5 (A8V, R154H and A561T) and synonymous variations were identified among these patients [207]. Although A561T is very close to A563T, which has significant effect on Ca2+ permeation [200], no functional difference was identified between A561T variant and control TRPV5. This result, together with the previous screening that failed to identify TRPV5 mutations in patients with autosomal dominant idiopathic hypercalciuria [208], indicates that TRPV5 may not play a primary role in hypercalciuria. However, the role of TRPV6 variations in hypercalciuria warrants further investigation.
TRPV6 expression level positively correlates with tumor grade in prostate cancer. Mortality from prostate cancer is two to three times greater in African–Americans than in Caucasians [209, 210]. It is not clear whether the high prostate cancer mortality in African–Americans is relevant to the high prevalence of ancestral TRPV6 allele in African–Americans. However, in a recent study the frequency of TRPV6 ancestral allele in 142 Caucasian prostate adenocarcinoma samples was not different from that in 169 Caucasian healthy control subjects [211]. The TRPV6 ancestral allele frequency did not correlate with the onset of prostate cancer, the Gleason score, or the tumor stage [211]. Because of the low frequency of ancestral alleles in the Caucasian subjects (only one patient with homozygous TRPV6 ancestral allele) of this study, a conclusion on a relation between TRPV6 genotype and prostate cancer should be drawn with caution. Studies in African or African–American populations will likely provide more conclusive results.
14.7 Perspectives
Over 10 years after the identification of TRPV5 and TRPV6, a much better understanding of the apical Ca2+ entry mechanisms in the transcellular transport pathway has been achieved. TRPV5 and TRPV6 provide a tightly regulated selective Ca2+ entry in the transcellular Ca2+ transport pathway that is responsible for the fine tuning of Ca2+ absorption and reabsorption, even though this pathway does not contribute greatly to the bulk Ca2+ absorption and reabsorption as does the paracellular pathway. TRPV5 and TRPV6 certainly are not the sole mediator of vitamin D regulated Ca2+ transport; however, without either of them, the body appears to be in a negative Ca2+ balance as indicated by the elevated 1,25(OH)2D3 and PTH levels. The roles of TRPV5 and TRPV6 in tuning Ca2+ homeostasis are well indicated by their robust responses to the body’s need for Ca2+ under physiological, pathological and therapeutic conditions. Notably, TRPV6 participates in cell proliferation and apoptosis and is emerging as a potential cancer marker and therapeutic target for cancers.
The study in renal Ca2+ transport has been mostly focused on TRPV5 in the past 10 more years. However, Trpv6 KO mice also lose Ca2+ in the urine, even though intestinal absorption of Ca2+ is decreased. Although TRPV6 mRNA level is much lower than TRPV5 in rodent kidney, in humans and horses, renal TRPV6 mRNA is much higher than TRPV5, indicating a more important role of TRPV6 in renal function in these species. TRPV5 and TRPV6 are not always regulated in the same way in the kidney, indicating their overlapping yet distinct roles.
While TRPV6 mediates Ca2+ uptake in many tissues, TRPV5 is relatively specific to the kidney. The need of a specific Ca2+ transporter for the complex mammalian distal tubule likely resulted in the generation of TRPV5 by duplication of TRPV6. The changed source of dietary Ca2+ and vitamin D status during or after humans migrated out of Africa may be part of the reasons for further evolution of the TRPV6 gene. TRPV6 genotype is a good genetic maker in African populations. African populations could be separated into 3 groups based on their TRPV6 genotype because of their ~50% allele frequency of the 3 TRPV6 nonsynonymous SNPs. The involvement of TRPV6 and TRPV5 SNPs in the health issues related to Ca2+, salt and water homeostasis in African descents will likely become a hot spot of investigation in the near future.
References
Suzuki Y, Landowski CP, Hediger MA (2008) Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu Rev Physiol 70:257–271
Boros S, Bindels RJ, Hoenderop JG (2009, May) Active Ca2+ reabsorption in the connecting tubule. Pflugers Arch 458(1):99–109
De GT, Bindels RJ, Hoenderop JG (2008, Nov) TRPV5: an ingeniously controlled calcium channel. Kidney Int 74(10):1241–1246
Hoenderop JG, Bindels RJ (2008, Feb) Calciotropic and magnesiotropic TRP channels. Physiology (Bethesda) 23:32–40
van de Graaf SF, Bindels RJ, Hoenderop JG (2007) Physiology of epithelial Ca2+ and Mg2+ transport. Rev Physiol Biochem Pharmacol 158:77–160
Topala CN, Bindels RJ, Hoenderop JG (2007, July) Regulation of the epithelial calcium channel TRPV5 by extracellular factors. Curr Opin Nephrol Hypertens 16(4):319–324
Schoeber JP, Hoenderop JG, Bindels RJ (2007, Feb) Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem Soc Trans 35(Pt 1):115–119
van de Graaf SF, Hoenderop JG, Bindels RJ (2006, June) Regulation of TRPV5 and TRPV6 by associated proteins. Am J Physiol Renal Physiol 290(6):F1295–F1302
Bodding M (2007, Mar) TRP proteins and cancer. Cell Signal 19(3):617–624
Perez AV, Picotto G, Carpentieri AR, Rivoira MA, Peralta Lopez ME, Tolosa de Talamoni NG (2008) Minireview on regulation of intestinal calcium absorption. Emphasis on molecular mechanisms of transcellular pathway. Digestion 77(1):22–34
Vriens J, Appendino G, Nilius B (2009, June) Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75(6):1262–1279
Vennekens R, Owsianik G, Nilius B (2008) Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des 14(1):18–31
Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ (1999, Mar) Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274(13):8375–8378
Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA (1999, Aug) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274(32):22739–22746
Peng JB, Chen XZ, Berger UV, Vassilev PM, Brown EM, Hediger MA (2000, Sep) A rat kidney-specific calcium transporter in the distal nephron. J Biol Chem 275(36): 28186–28194
Muller D, Hoenderop JG, Meij IC, van den Heuvel LP, Knoers NV, den Hollander AI, Eggert P, Garcia-Nieto V, Claverie-Martin F, Bindels RJ (2000, July) Molecular cloning, tissue distribution, and chromosomal mapping of the human epithelial Ca2+ channel (ECAC1). Genomics 67(1):48–53
Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V (2001, June) Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem 276(22):19461–19468
Barley NF, Howard A, O’Callaghan D, Legon S, Walters JR (2001, Feb) Epithelial calcium transporter expression in human duodenum. Am J Physiol Gastrointest Liver Physiol 280(2):G285–G290
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius. D (1997, Oct) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Colbert HA, Smith TL, Bargmann CI (1997, Nov) OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17(21):8259–8269
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999, Apr) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398(6726):436–441
Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I (1999, July) Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1(3):165–170
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000, Oct) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2(10):695–702
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (October 2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103(3):525–535
Wissenbach U, Bodding M, Freichel M, Flockerzi V (2000, Nov) Trp12, a novel Trp related protein from kidney. FEBS Lett 485(2–3):127–134
Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN (2001, Jan) Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics 4(3):165–174
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB (2002, July) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418(6894):186–190
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE (2002, July) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418(6894):181–186
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A (2002, June) A heat-sensitive TRP channel expressed in keratinocytes. Science 296(5575):2046–2049
Kahn-Kirby AH, Bargmann. CI (2006) TRP channels in C. elegans. Annu Rev Physiol 68:719–736
Qiu A, Hogstrand C (2004, Nov) Functional characterisation and genomic analysis of an epithelial calcium channel (ECaC) from pufferfish, Fugu rubripes. Gene 342(1):113–123
Peng JB, Brown EM, Hediger MA (2001, Aug) Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76(1–3):99–109
Dantzler WH, Braun EJ (1980, Sep) Comparative nephron function in reptiles, birds, and mammals. Am J Physiol 239(3):R197–R213
Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA (2000, Nov) Human calcium transport protein CaT1. Biochem Biophys Res Commun 278(2):326–332
Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR (2002, Dec) Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82(12):1755–1764
Wilkens MR, Kunert-Keil C, Brinkmeier H, Schroder B (2009, Nov) Expression of calcium channel TRPV6 in ovine epithelial tissue. Vet J 182(2):294–300
Rourke KM, Coe S, Kohn CW, Rosol TJ, Mendoza FJ, Toribio RE (2010, May) Cloning, comparative sequence analysis and mRNA expression of calcium transporting genes in horses. Gen Comp Endocrinol 167(1):6–10
Schroder B, Vossing S, Breves G (1999, Oct) In vitro studies on active calcium absorption from ovine rumen. J Comp Physiol B 169(7):487–494
Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S (2003, Sep) Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 144(9):3885–3894
Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van HE, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G (2001, Nov) Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98(23):13324–13329
Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B (2001, Dec) Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281(6): F1021–F1027
Suzuki M, Ishibashi K, Ooki G, Tsuruoka S, Imai M (2000, Aug) Electrophysiologic characteristics of the Ca-permeable channels, ECaC and CaT, in the kidney. Biochem Biophys Res Commun 274(2):344–349
Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ (2003, Nov) Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14(11):2731–2740
Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G (2000, Sep) Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol 527(Pt 2):239–248
Yue L, Peng JB, Hediger MA, Clapham DE (2001, Apr) CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410(6829):705–709
Vassilev PM, Peng JB, Hediger MA, Brown EM (2001, Nov) Single-channel activities of the human epithelial Ca2+ transport proteins CaT1 and CaT2. J Membr Biol 184(2):113–120
Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ (2000, Feb) Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275(6):3963–3969
Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ (2003, Feb) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22(4):776–785
Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA (2004, Aug) Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem 279(33):34456–34463
Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, Hoenderop JG (2004, Dec) Molecular determinants in TRPV5 channel assembly. J Biol Chem 279(52):54304–54311
Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R (2008, Feb) Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47(8): 2476–2484
Voets T, Janssens A, Droogmans G, Nilius B (2004, Apr) Outer pore architecture of a Ca2+-selective TRP channel. J Biol Chem 279(15):15223–15230
Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ (2001, Jan) The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem 276(2):1020–1025
Dodier Y, Banderali U, Klein H, Topalak O, Dafi O, Simoes M, Bernatchez G, Sauve R, Parent L (2004, Feb) Outer pore topology of the ECaC-TRPV5 channel by cysteine scan mutagenesis. J Biol Chem 279(8):6853–6862
Dodier Y, Dionne F, Raybaud A, Sauve R, Parent L (2007, Dec) Topology of the selectivity filter of a TRPV channel: rapid accessibility of contiguous residues from the external medium. Am J Physiol Cell Physiol 293(6):C1962–C1970
Voets T, Janssens A, Prenen J, Droogmans G, Nilius B (2003, Mar) Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol 121(3): 245–260
Bodding M, Wissenbach U, Flockerzi V (2002, Sep) The recombinant human TRPV6 channel functions as Ca2+ sensor in human embryonic kidney and rat basophilic leukemia cells. J Biol Chem 277(39):36656–36664
Nilius B, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Weidema AF, Droogmans G, Bindels RJ (2002, Aug) Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J Biol Chem 277(34):30852–30858
Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C (2001, Mar) Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci USA 98(6):3600–3605
Derler I, Hofbauer M, Kahr H, Fritsch R, Muik M, Kepplinger K, Hack ME, Moritz S, Schindl R, Groschner K, Romanin C (2006, Nov) Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation. J Physiol 577(Pt 1):31–44
Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ (2004, July) Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+-sensor calmodulin. J Biol Chem 279(28):28855–28861
Lambers TT, Mahieu F, Oancea E, Hoofd L, de LF, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ (2006, July) Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J 25(13):2978–2988
Gkika D, Mahieu F, Nilius B, Hoenderop JG, Bindels. RJ (2004, June) 80 K-H as a new Ca2+ sensor regulating the activity of the epithelial Ca2+ channel transient receptor potential cation channel V5 (TRPV5). J Biol Chem 279(25):26351–26357
Thyagarajan B, Benn BS, Christakos S, Rohacs T (2009, Mar) Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels: implications in active intestinal Ca2+ transport. Mol Pharmacol 75(3):608–616
Thyagarajan B, Lukacs V, Rohacs T (2008, May) Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem 283(22):14980–14987
Rohacs T, Nilius B (2007, Oct) Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch 455(1):157–168
Lee J, Cha SK, Sun TJ, Huang CL (2005, Nov) PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+ . J Gen Physiol 126(5):439–451
Al-Ansary D, Bogeski I, Disteldorf BM, Becherer U, Niemeyer BA (2010, Feb) ATP modulates Ca2+ uptake by TRPV6 and is counteracted by isoform-specific phosphorylation. FASEB J 24(2):425–435
Bonny O, Rubin A, Huang CL, Frawley WH, Pak CY, Moe OW (2008, Aug) Mechanism of urinary calcium regulation by urinary magnesium and pH. J Am Soc Nephrol 19(8): 1530–1537
Yeh BI, Yoon J, Huang CL (2006) On the role of pore helix in regulation of TRPV5 by extracellular protons. J Membr Biol 212(3):191–198
Yeh BI, Kim YK, Jabbar W, Huang CL (2005, Sep) Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J 24(18):3224–3234
Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL (2003, Dec) Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem 278(51):51044–51052
Cha SK, Jabbar W, Xie J, Huang CL (2007, Dec) Regulation of TRPV5 single-channel activity by intracellular pH. J Membr Biol 220(1–3):79–85
Lambers TT, Oancea E, de GT, Topala CN, Hoenderop JG, Bindels RJ (2007, Feb) Extracellular pH dynamically controls cell surface delivery of functional TRPV5 channels. Mol Cell Biol 27(4):1486–1494
Gkika D, Topala CN, Chang Q, Picard N, Thebault S, Houillier P, Hoenderop JG, Bindels RJ (2006, Oct) Tissue kallikrein stimulates Ca2+ reabsorption via PKC-dependent plasma membrane accumulation of TRPV5. EMBO J 25(20):4707–4716
Wood RJ, Tchack L, Taparia S (2001) 1,25-Dihydroxyvitamin D3 increases the expression of the CaT1 epithelial calcium channel in the Caco-2 human intestinal cell line. BMC Physiol 1:11
Hoenderop JG, Muller D, van der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, van Os CH, Bindels RJ (2001, July) Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 12(7):1342–1349
Walters JR, Barley NF, Khanji M, Rhodes-Kendler O (2004, May) Duodenal expression of the epithelial calcium transporter gene TRPV6: is there evidence for Vitamin D-dependence in humans? J Steroid Biochem Mol Biol 89–90(1–5):317–319
Walters JR, Balesaria S, Chavele KM, Taylor V, Berry JL, Khair U, Barley NF, van Heel DA, Field J, Hayat JO, Bhattacharjee A, Jeffery R, Poulsom R (2006, Nov) Calcium channel TRPV6 expression in human duodenum: different relationships to the vitamin D system and aging in men and women. J Bone Miner Res 21(11):1770–1777
Walters JR, Balesaria S, Khair U, Sangha S, Banks L, Berry JL (2007, Mar) The effects of Vitamin D metabolites on expression of genes for calcium transporters in human duodenum. J Steroid Biochem Mol Biol 103(3–5):509–512
Balesaria S, Sangha S, Walters JR (2009, Dec) Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol 297(6):G1193–G1197
Taparia S, Fleet JC, Peng JB, Wang XD, Wood RJ (2006, June) 1,25-Dihydroxyvitamin D and 25-hydroxyvitamin D–mediated regulation of TRPV6 (a putative epithelial calcium channel) mRNA expression in Caco-2 cells. Eur J Nutr 45(4):196–204
Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW (2006, June) The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20(6):1447–1461
Meyer MB, Zella LA, Nerenz RD, Pike JW (2007, Aug) Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem 282(31):22344–22352
Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH (2005, Nov) Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 19(11):2685–2695
Pike JW, Zella LA, Meyer MB, Fretz JA, Kim S (2007, Dec) Molecular actions of 1,25-dihydroxyvitamin D3 on genes involved in calcium homeostasis. J Bone Miner Res 22(Suppl 2):V16–V19
Khanal RC, Peters TM, Smith NM, Nemere I (2008, Nov) Membrane receptor-initiated signaling in 1,25(OH)2D3-stimulated calcium uptake in intestinal epithelial cells. J Cell Biochem 105(4):1109–1116
van AM, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ (2005, Oct) Coordinated control of renal Ca2+ transport proteins by parathyroid hormone. Kidney Int 68(4):1708–1721
Bacskai BJ, Friedman. PA (1990, Sep) Activation of latent Ca2+ channels in renal epithelial cells by parathyroid hormone. Nature 347(6291):388–391
Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA (1996, Jan) Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving protein kinase A and protein kinase C. Endocrinology 137(1):13–20
de GT, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG (2009, Aug) Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20(8):1693–1704
Cha SK, Wu T, Huang CL (2008, May) Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294(5):F1212–F1221
Elalouf JM, Roinel N, de RC (1984, Feb) ADH-like effects of calcitonin on electrolyte transport by Henle’s loop of rat kidney. Am J Physiol 246(2 Pt 2):F213–F220
Di SA, Wittner M, Nitschke R, Braitsch R, Greger R, Bailly C, Amiel C, Roinel N, de RC (1990, Oct) Effects of parathyroid hormone and calcitonin on Na+, Cl-, K+, Mg2+ and Ca2+ transport in cortical and medullary thick ascending limbs of mouse kidney. Pflugers Arch 417(2):161–167
Hsu YJ, Dimke H, Hoenderop JG, Bindels RJ (2010, May) Calcitonin-stimulated renal Ca2+ reabsorption occurs independently of TRPV5. Nephrol Dial Transplant 25(5):1428–1435
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997, Nov) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51
Kuro-o M (2010, Jan) Klotho. Pflugers Arch 459(2):333–343
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005, Oct) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310(5747):490–493
Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG (2008, Nov) The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant 23(11):3397–3402
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro O, Huang CL (2008, July) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105(28):9805–9810
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009, July) Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76(1):38–46
Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y (2004, Mar) Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem 279(11):9777–9784
Tsuruoka S, Nishiki K, Ioka T, Ando H, Saito Y, Kurabayashi M, Nagai R, Fujimura A (2006, Oct) Defect in parathyroid-hormone-induced luminal calcium absorption in connecting tubules of Klotho mice. Nephrol Dial Transplant 21(10):2762–2767
Brown AJ, Krits I, Armbrecht HJ (2005, May) Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch Biochem Biophys 437(1):51–58
Song Y, Kato S, Fleet JC (2003, Feb) Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr 133(2):374–380
Van Cromphaut SJ, Rummens K, Stockmans I, Van HE, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G (2003, Oct) Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 18(10):1725–1736
Fudge NJ, Kovacs CS (2010, Mar) Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 151(3): 886–895
Charoenphandhu N, Nakkrasae LI, Kraidith K, Teerapornpuntakit J, Thongchote K, Thongon N, Krishnamra N (2009, Sep) Two-step stimulation of intestinal Ca2+ absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge. Am J Physiol Endocrinol Metab 297(3):E609–E619
Song Y, Fleet JC (2004, Aug) 1,25 dihydroxycholecalciferol-mediated calcium absorption and gene expression are higher in female than in male mice. J Nutr 134(8):1857–1861
van AM, Hoenderop JG, Dardenne O, St Arnaud R, van Os CH, Van Leeuwen HJ, Bindels RJ (2002, Aug) 1,25-dihydroxyvitamin D(3)-independent stimulatory effect of estrogen on the expression of ECaC1 in the kidney. J Am Soc Nephrol 13(8):2102–2109
Oz OK, Hajibeigi A, Howard K, Cummins CL, van AM, Bindels RJ, Word RA, Kuro-o M, Pak CY, Zerwekh JE (2007, Dec) Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice *. J Bone Miner Res 22(12):1893–1902
Irnaten M, Blanchard-Gutton N, Harvey BJ (2008, Nov) Rapid effects of 17beta-estradiol on epithelial TRPV6 Ca2+ channel in human T84 colonic cells. Cell Calcium 44(5): 441–452
Irnaten M, Blanchard-Gutton N, Praetorius J, Harvey BJ (2009, Aug) Rapid effects of 17beta-estradiol on TRPV5 epithelial Ca2+ channels in rat renal cells. Steroids 74(8): 642–649
Peng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR (2001, Apr) CaT1 expression correlates with tumor grade in prostate cancer. Biochem Biophys Res Commun 282(3):729–734
Hsu YJ, Dimke H, Schoeber JP, Hsu SC, Lin SH, Chu P, Hoenderop JG, Bindels RJ (2010, Apr) Testosterone increases urinary calcium excretion and inhibits expression of renal calcium transport proteins. Kidney Int 77(7):601–608
Teerapornpuntakit J, Dorkkam N, Wongdee K, Krishnamra N, Charoenphandhu N (2009, Apr) Endurance swimming stimulates transepithelial calcium transport and alters the expression of genes related to calcium absorption in the intestine of rats. Am J Physiol Endocrinol Metab 296(4):E775–E786
Sato T, Yamamoto H, Sawada N, Nashiki K, Tsuji M, Nikawa T, Arai H, Morita K, Taketani Y, Takeda E (2006) Immobilization decreases duodenal calcium absorption through a 1,25-dihydroxyvitamin D-dependent pathway. J. Bone Miner Metab 24(4):291–299
van AM, Huybers S, Hoenderop JG, van der Kemp AW, van Leeuwen, JP, Bindels RJ (2006, Dec) Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6. Am J Physiol Renal Physiol 291(6):F1177–F1183
Fukushima A, Aizaki Y, Sakuma K (2009, Jan) Short-chain fatty acids induce intestinal transient receptor potential vanilloid type 6 expression in rats and Caco-2 cells. J Nutr 139(1):20–25
Fukushima A, Ohta A, Sakai K, Sakuma K (2005, Dec) Expression of calbindin-D9k, VDR and Cdx-2 messenger RNA in the process by which fructooligosaccharides increase calcium absorption in rats. J Nutr Sci Vitaminol (Tokyo) 51(6):426–432
Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA (2007, Feb) Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22(2):274–285
Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ (2003, Dec) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112(12):1906–1914
Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S (2008, June) Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149(6):3196–3205
Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, Deluca HF (2008, Dec) TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci USA 105(50):19655–19659
Bronner F (2009, Feb) Recent developments in intestinal calcium absorption. Nutr Rev 67(2):109–113
Renkema KY, Nijenhuis T, van der Eerden BC, van der Kemp AW, Weinans H, van Leeuwen JP, Bindels RJ, Hoenderop JG (2005, Nov) Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J Am Soc Nephrol 16(11): 3188–3195
Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG (2009, Aug) The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20(8):1705–1713
Nijenhuis T, van der Eerden BC, Hoenderop JG, Weinans H, van Leeuwen JP, Bindels. RJ (2008, Nov) Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5–/–mice. J Bone Miner Res 23(11):1815–1824
Lieben L, Benn BS, Ajibade D, Stockmans I, Moermans K, Hediger MA, Peng JB, Christakos S, Bouillon R, Carmeliet G (2010, Aug) Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone 47(2):301–308
Bernucci L, Henriquez M, Diaz P, Riquelme. G (2006, Nov) Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta 27 (11–12):1082–1095
Moreau R, Hamel A, Daoud G, Simoneau L, Lafond J (2002, Nov) Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol Reprod 67(5):1473–1479
Moreau R, Daoud G, Bernatchez R, Simoneau L, Masse A, Lafond J (2002, Aug) Calcium uptake and calcium transporter expression by trophoblast cells from human term placenta. Biochim Biophys Acta 1564(2):325–332
Stumpf T, Zhang Q, Hirnet D, Lewandrowski U, Sickmann A, Wissenbach U, Dorr J, Lohr C, Deitmer JW, Fecher-Trost C (2008, June) The human TRPV6 channel protein is associated with cyclophilin B in human placenta. J Biol Chem 283(26):18086–18098
Suzuki Y, Kovacs CS, Takanaga H, Peng JB, Landowski CP, Hediger MA (2008, Aug) Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J Bone Miner Res 23(8):1249–1256
Hache S, Takser L, Lebellego F, Weiler H, Leduc L, Forest JC, Giguere Y, Masse A, Barbeau B, Lafond J (2010, Feb) Alteration of calcium homeostasis in primary preeclamptic syncytiotrophoblasts: effect on calcium exchange in placenta. J Cell Mol Med [Epub ahead of print]
Lee GS, Jeung EB (2007, July) Uterine TRPV6 expression during the estrous cycle and pregnancy in a mouse model. Am J Physiol Endocrinol Metab 293(1):E132–E138
Lee BM, Lee GS, Jung EM, Choi KC, Jeung EB (2009) Uterine and placental expression of TRPV6 gene is regulated via progesterone receptor- or estrogen receptor-mediated pathways during pregnancy in rodents. Reprod Biol Endocrinol 7:49
Kim HJ, Lee GS, Ji YK, Choi KC, Jeung EB (2006, Aug) Differential expression of uterine calcium transporter 1 and plasma membrane Ca2+ ATPase 1b during rat estrous cycle. Am J Physiol Endocrinol Metab 291(2):E234–E241
Choi Y, Seo H, Kim M, Ka H (2009, Dec) Dynamic expression of calcium-regulatory molecules, TRPV6 and S100G, in the uterine endometrium during pregnancy in pigs. Biol Reprod 81(6):1122–1130
van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP (2005, Nov) The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102(48):17507–17512
Yamauchi D, Nakaya K, Raveendran NN, Harbidge DG, Singh R, Wangemann P, Marcus DC (2010) Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol 10:1
Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC (2007, May) Loss of cochlear. Am J Physiol Renal Physiol 292(5): F1345–F1353
Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC (May 2007) Lack of pendrin. Am J Physiol Renal Physiol 292(5):F1314–F1321
Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, Marcus DC (2005, June) Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun 331(4):1353–1357
Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M (February 2009) Age-dependent changes in the expression of klotho protein, TRPV5 and TRPV6 in mouse inner ear. Acta Otolaryngol 25:1–11
Homann V, Kinne-Saffran E, Arnold WH, Gaengler P, Kinne RK (May 2006) Calcium transport in human salivary glands: a proposed model of calcium secretion into saliva. Histochem Cell Biol 125(5):583–591
Lehen’kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, Prevarskaya N (2007, Aug) TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem 282(31):22582–22591
Kennedy BG, Torabi AJ, Kurzawa R, Echtenkamp SF, Mangini NJ (2010) Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in retinal pigment epithelium. Mol Vis 16:665–675
Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H (2003, Oct) Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene 22(49):7858–7861
Vanden Abeele F, Lemonnier L, Thebault S, Lepage G, Parys JB, Shuba Y, Skryma R, Prevarskaya N (2004, July) Two types of store-operated Ca2+ channels with different activation modes and molecular origin in LNCaP human prostate cancer epithelial cells. J Biol Chem 279(29):30326–30337
Vanden Abeele F, Roudbaraki M, Shuba Y, Skryma R, Prevarskaya N (2003, Apr) Store-operated Ca2+ current in prostate cancer epithelial cells. Role of endogenous Ca2+ transporter type 1. J Biol Chem 278(17):15381–15389
Bodding M, Fecher-Trost C, Flockerzi V (2003, Dec) Store-operated Ca2+ current and TRPV6 channels in lymph node prostate cancer cells. J Biol Chem 278(51):50872–50879
Pigozzi D, Ducret T, Tajeddine N, Gala JL, Tombal B, Gailly P (2006, May) Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium 39(5):401–415
Lehen’kyi V, Flourakis M, Skryma R, Prevarskaya N (2007, Nov) TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene 26(52):7380–7385
Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackiere F, Bidaux G, Urbain R, Gosset P, Delcourt P, Fleurisse L, Slomianny C, Dewailly E, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N (2009, Apr) Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 28(15):1792–1806
Bolanz KA, Hediger MA, Landowski CP (2008, Feb) The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther 7(2):271–279
Bolanz KA, Kovacs GG, Landowski CP, Hediger MA (2009, Dec) Tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol Cancer Res 7(12):2000–2010
Peleg S, Sellin JH, Wang Y, Freeman MR, Umar S (2010, Sep) Suppression of aberrant transient receptor potential cation channel, subfamily V, member 6 expression in hyperproliferative colonic crypts by dietary calcium. Am J Physiol Gastrointest Liver Physiol 299(3):G593–601
Chow J, Norng M, Zhang J, Chai J (2007, Apr) TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells–Mechanisms behind a possible new hot cancer treatment. Biochim Biophys Acta 1773(4):565–576
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001, Aug) Human hypertension caused by mutations in WNK kinases. Science 293(5532):1107–1112
Mayan H, Munter G, Shaharabany M, Mouallem M, Pauzner R, Holtzman EJ, Farfel Z (2004, Aug) Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: description of a large family with the Q565E WNK4 mutation. J Clin Endocrinol Metab 89(8):4025–4030
Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z (2002, July) Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87(7):3248–3254
Jiang Y, Ferguson WB, Peng JB (2007, Feb) WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol 292(2):F545–F554
Zhang W, Na T, Peng JB (2008, Nov) WNK3 positively regulates epithelial calcium channels TRPV5 and TRPV6 via a kinase-dependent pathway. Am J Physiol Renal Physiol 295(5):F1472–F1484
Jiang Y, Cong P, Williams SR, Zhang W, Na T, Ma HP, Peng JB (2008, Oct) WNK4 regulates the secretory pathway via which TRPV5 is targeted to the plasma membrane. Biochem Biophys Res Commun 375(2):225–229
Yang CL, Angell J, Mitchell R, Ellison DH (2003, Apr) WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111(7):1039–1045
Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP (2003, Jan) Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100(2):680–684
Yang SS, Hsu YJ, Chiga M, Rai T, Sasaki S, Uchida S, Lin SH (2010, Apr) Mechanisms for Hypercalciuria in Pseudohypoaldosteronism Type II-Causing WNK4 Knock-In Mice. Endocrinology 151(4):1829–1836
O’Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW (2006, Sep) Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17(9):2402–2413
Kim MH, Lee GS, Jung EM, Choi KC, Jeung EB (2009, July) The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder. Life Sci 85(3–4):146–152
Kim MH, Lee GS, Jung EM, Choi KC, Oh GT, Jeung EB (2009, Jan) Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-D9k and -D28k knockout mice. Exp Physiol 94(1):138–151
Huybers S, Naber TH, Bindels RJ, Hoenderop JG (2007, Jan) Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol 292(1):G92–G97
Bohmer C, Palmada M, Kenngott C, Lindner R, Klaus F, Laufer J, Lang F (2007, Dec) Regulation of the epithelial calcium channel TRPV6 by the serum and glucocorticoid-inducible kinase isoforms SGK1 and SGK3. FEBS Lett 581(29):5586–5590
Palmada M, Poppendieck S, Embark HM, van de Graaf SF, Boehmer C, Bindels RJ, Lang F (2005) Requirement of PDZ domains for the stimulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase SGK1. Cell Physiol Biochem 15(1–4):175–182
Embark HM, Setiawan I, Poppendieck S, van de Graaf SF, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun CC, Bindels RJ, Lang F (2004) Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem 14(4–6):203–212
Sopjani M, Kunert A, Czarkowski K, Klaus F, Laufer J, Foller M, Lang F (2010, Feb) Regulation of the Ca2+ channel TRPV6 by the kinases SGK1, PKB/Akt, and PIKfyve. J Membr Biol 233(1–3):35–41
Sandulache D, Grahammer F, Artunc F, Henke G, Hussain A, Nasir O, Mack A, Friedrich B, Vallon V, Wulff P, Kuhl D, Palmada M, Lang F (2006, July) Renal Ca2+ handling in sgk1 knockout mice. Pflugers Arch 452(4):444–452
Jang HR, Kim S, Heo NJ, Lee JH, Kim HS, Nielsen S, Jeon US, Oh YK, Na KY, Joo KW, Han JS (2009, Jan) Effects of thiazide on the expression of TRPV5, calbindin-D28K, and sodium transporters in hypercalciuric rats. J Korean Med Sci 24(Suppl):S161–S169
Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ (2005, June) Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115(6):1651–1658
Lee CT, Shang S, Lai LW, Yong KC, Lien YH (2004, Dec) Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am J Physiol Renal Physiol 287(6):F1164–F1170
Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ (2003, Aug) Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int 64(2):555–564
Friedman PA, Bushinsky DA (1999, Nov) Diuretic effects on calcium metabolism. Semin Nephrol 19(6):551–556
Lee CT, Chen HC, Lai LW, Yong KC, Lien YH (2007, Oct) Effects of furosemide on renal calcium handling. Am J Physiol Renal Physiol 293(4):F1231–F1237
Lee CT, Lien YH, Lai LW, Chen JB, Lin CR, Chen HC (2006, May) Increased renal calcium and magnesium transporter abundance in streptozotocin-induced diabetes mellitus. Kidney Int 69(10):1786–1791
Hoenderop JG, Dardenne O, van AM, van der Kemp AW, van Os CH, St Arnaud R, Bindels RJ (2002, Sep) Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J 16(11):1398–1406
Ignat M, Teletin M, Tisserand J, Khetchoumian K, Dennefeld C, Chambon P, Losson R, Mark M (2008, Feb) Arterial calcifications and increased expression of vitamin D receptor targets in mice lacking TIF1alpha. Proc Natl Acad Sci USA 105(7):2598–2603
Huybers S, Apostolaki M, van der Eerden BC, Kollias G, Naber TH, Bindels RJ, Hoenderop JG (2008, June) Murine TNF(DeltaARE) Crohn’s disease model displays diminished expression of intestinal Ca2+ transporters. Inflamm Bowel Dis 14(6):803–811
Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Gunzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Muller D (2010, Feb) Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol [Epub ahead of print]
Nijenhuis T, Hoenderop JG, Bindels RJ (2004, Mar) Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15(3):549–557
Lee GS, Byun HS, Kim MH, Lee BM, Ko SH, Jung EM, Gwak KS, Choi IG, Kang HY, Jo HJ, Lee HJ, Jeung EB (2008, Nov) The beneficial effect of the sap of Acer mono in an animal with low-calcium diet-induced osteoporosis-like symptoms. Br J Nutr 100(5):1011–1018
Akey JM, Eberle MA, Rieder MJ, Carlson CS, Shriver MD, Nickerson DA, Kruglyak L (2004, Oct) Population history and natural selection shape patterns of genetic variation in 132 genes. PLoS Biol 2(10):e286
Stajich JE, Hahn MW (2005, Jan) Disentangling the effects of demography and selection in human history. Mol Biol Evol 22(1):63–73
Hughes DA, Tang K, Strotmann R, Schoneberg T, Prenen J, Nilius B, Stoneking M (2008) Parallel selection on TRPV6 in human populations. PLoS One 3(2):e1686
Soejima M, Tachida H, Koda Y (2009, Feb) Sequence analysis of human TRPV6 suggests positive selection outside Africa. Biochem Genet 47(1–2):147–153
Akey JM, Swanson WJ, Madeoy J, Eberle M, Shriver MD (2006, July) TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum Mol Genet 15(13):2106–2113
Bersaglieri T, Sabeti PC, Patterson N, Vanderploeg T, Schaffner SF, Drake JA, Rhodes M, Reich DE, Hirschhorn JN (2004, June) Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet 74(6):1111–1120
Holick MF, MacLaughlin JA, Doppelt SH (1981, Feb) Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science 211(4482):590–593
Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM (2007, June) Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr 85(6):1657–1663
Suzuki Y, Pasch A, Bonny O, Mohaupt MG, Hediger MA, Frey FJ (2008, June) Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum Mol Genet 17(11):1613–1618
Na T, Zhang W, Jiang Y, Liang Y, Ma HP, Warnock DG, Peng JB (2009, May) The A563T variation of the renal epithelial calcium channel TRPV5 among African Americans enhances calcium influx. Am J Physiol Renal Physiol 296(5):F1042–F1051
Pratt JH, Manatunga AK, Peacock M (1996, Jan) A comparison of the urinary excretion of bone resorptive products in white and black children. J Lab Clin Med 127(1):67–70
Taylor EN, Curhan GC (2007, Feb) Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18(2):654–659
Sarmina I, Spirnak JP, Resnick MI (1987, July) Urinary lithiasis in the black population: an epidemiological study and review of the literature. J Urol 138(1):14–17
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC (2003, May) Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63(5):1817–1823
Bell NH, Shary J, Stevens J, Garza M, Gordon L, Edwards J (1991, July) Demonstration that bone mass is greater in black than in white children. J Bone Miner Res 6(7):719–723
Bohannon AD (1999, June) Osteoporosis and African American women. J Womens Health Gend Based Med 8(5):609–615
Renkema KY, Lee K, Topala CN, Goossens M, Houillier P, Bindels RJ, Hoenderop JG (2009, June) TRPV5 gene polymorphisms in renal hypercalciuria. Nephrol Dial Transplant 24(6):1919–1924
Muller D, Hoenderop JG, Vennekens R, Eggert P, Harangi F, Mehes K, Garcia-Nieto V, Claverie-Martin F, Os CH, Nilius B, Bindels JM (2002, Sep) Epithelial Ca2+ channel (ECAC1) in autosomal dominant idiopathic hypercalciuria. Nephrol Dial Transplant 17(9):1614–1620
Burks DA, Littleton RH (1992) The epidemiology of prostate cancer in black men. Henry Ford Hosp Med J 40(1–2):89–92
Powell IJ (1997, May) Prostate cancer and African–American men. Oncology (Williston Park) 11(5):599–605
Kessler T, Wissenbach U, Grobholz R, Flockerzi V (2009) TRPV6 alleles do not influence prostate cancer progression. BMC Cancer 9:380
Acknowledgments
I thank my colleagues Drs. Yi Jiang, Wei Zhang, Tao Na, Guojin Wu, and Haiyan Jing for their contributions to the research projects in our group and their participation in regular literature review. The research of our lab was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072154), and American Heart Association National Center (0430125 N) and Greater Southeast Affiliate (09GRNT2160024).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Peng, J.B. (2011). TRPV5 and TRPV6 in Transcellular Ca2+ Transport: Regulation, Gene Duplication, and Polymorphisms in African Populations. In: Islam, M. (eds) Transient Receptor Potential Channels. Advances in Experimental Medicine and Biology, vol 704. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0265-3_14
Download citation
DOI: https://doi.org/10.1007/978-94-007-0265-3_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0264-6
Online ISBN: 978-94-007-0265-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)