Abstract
Arbuscular mycorrhizal fungi (AMF) are obligate biotrophs, living symbiotically in the roots of most land plants. They form spores in the soil, which are able to germinate and grow, but are unable to complete their life cycle without establishing a functional symbiosis with a host plant. In this chapter, results of recent studies providing new insights into the main developmental switches occurring in the fungal organism, from the relief of spore dormancy to the development of germlings and growth arrest in the absence of the host, are reviewed. The knowledge of environmental, cytological, biochemical and molecular events involved in early stages of AMF life cycle may reveal how these obligate symbionts compensate for the lack of host-regulated spore germination, possibly representing a strong selective disadvantage. Diverse scientific approaches showed multiple survival strategies, active during pre-symbiotic mycelial growth, contributing to the survival of AM fungal individuals and populations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Arbuscular mycorrhizal fungi
- Spore dormancy
- AMF life cycle
- Spore germination
- Pre-symbiotic growth
- Germling growth arrest
- Host signals
- Survival strategies
- Ancient asexuals
- Gene expression
1 Introduction
Arbuscular mycorrhizal (AM) fungi (AMF) are obligate biotrophs, which live symbiotically in the roots of about 80% of plant species. Most AMF form spores in the soil which are able to germinate and grow from a quiescent-like state in response to different edaphic and environmental conditions, but are unable to produce extensive mycelia and to complete their life cycle without establishing a functional symbiosis with a host plant (Mosse 1959; Hepper and Smith 1976). The key developmental switches occurring in the fungal organism, from the germination of an individual spore to the formation of an extensive hyphal network in the soil, involve a sequence of morphogenetic events represented by: spore germination and pre-symbiotic mycelial growth, differential hyphal branching pattern in the presence of host roots, appressorium formation, root colonization, arbuscule development, extraradical mycelial growth and spore production (Giovannetti 2000).
The lack of host-regulated spore germination, contrary to what happens with many pathogenic biotrophic fungi, could have represented a strong selective disadvantage. Nevertheless, AMF are considered evolutionary successful “living fossils”, having survived and evolved for 460 millions years, their ancestral nature having been shown by diverse fossil records and DNA sequence data (Simon et al. 1993; Remy et al. 1994; Phipps and Taylor 1996; Redecker et al. 2000a, b). Their persistence indicates that they must have evolved efficient strategies to overcome the lack of spore germination regulation and to allow the survival of individuals and populations (Logi et al. 1998; Giovannetti et al. 2000; Giovannetti 2002).
The aim of this chapter is to review recent developments which contributed to our understanding of cellular and molecular events involved in the early stages of the life cycle of AMF, from relieving spore dormancy and triggering spore germination to germling growth and growth arrest in the absence of the host.
2 Spore Dormancy
The phenomenon of spore dormancy has concerned researchers since Godfrey’s early studies on spore germination (Godfrey 1957). As early as 1959, Barbara Mosse suggested the storage of collected spores on damp filter paper at 5°C for 6 weeks in order to obtain the regular germination of resting spores of an Endogone sp. (presumably Glomus mosseae) (Mosse 1959). Eighty percent of spores treated in this way germinated within 3–4 days. The problem of erratic spore germination has been mentioned in many reports, and in 1983 Tommerup gave a clear-cut definition of spore dormancy, making a distinction between dormancy and quiescence (Tommerup 1983a). A dormant spore was defined as one failing to germinate when exposed to physical and chemical conditions which support germination of apparently identical spores, defined as quiescent spores. Differences in cytoplasmic organization between young and old resting spores were described in Acaulospora laevis and in Glomus species: in dormant spores the oil globules enlarged at the expense of the cytoplasm, which was restricted to small interstitial spaces (Mosse 1970a, b; Meier and Charvat 1992; Maia and Kimbrough 1998). A fine network of cytoplasmic material interlaced between large lipid droplets was also described by Sward (1981a) in dormant spores of Gigaspora margarita.
The relief of dormancy by storage was reported by many authors. Hepper and Smith (1976) found that spores of G. mosseae from freshly harvested sporocarps germinated slowly compared to spores detached from sporocarps and stored at 6°C for 5 weeks. The same results were obtained with a North American isolate of G. mosseae, which showed a marked difference in germinability between freshly isolated and 10°C-stored spores (Daniels and Graham 1976). Diverse species of the genus Glomus exhibited spore dormancy, such as Glomus intraradices, Glomus clarum, Glomus caledonium, Glomus monosporum (Hepper 1979; Tommerup 1983b; Louis and Lim 1988; Douds and Schenck 1991; Juge et al. 2002). Other species, such as Glomus coronatum, showed erratic germination even after cold treatments lasting 1 year (Giovannetti et al. 1991).
A marked dormancy was shown by spores of A. laevis, which germinated after 6 months storage in two different experimental conditions (Tommerup 1983a; Gazey et al. 1993). Other species within the genus Acaulospora exhibited the same behaviour: in a laboratory experiment only a small proportion of spores stored for 2 months germinated, while most spores germinated well after storage for 4–6 months (Gazey et al. 1993). Similarly, Acaulospora longula showed complete relief of dormancy after 8 weeks storage at 23°C in soil (Douds and Schenck 1991).
Not all the species and genera of AMF show spore dormancy. Spores of Gigaspora gigantea collected throughout the year from sand dunes did not show any dormancy, and were able to germinate as early as 1 day after incubation, either when they had been surface sterilized or not (Koske 1981a), while newly formed spores showed a period of endogenous dormancy (Gemma and Koske 1988). Germ tubes of G. margarita emerged after 72 h incubation on water agar or within 3–5 days on agar media without any storage treatment (Sward 1981c; Siqueira et al. 1982). Similarly, spores of Scutellospora fulgida and Scutellospora persica did not possess any dormancy, showing mycelial growth and the formation of auxiliary cells after 2 weeks in the dark at 24°C (Turrini et al. 2008).
Propagule dormancy may contribute to the survival of AMF in adverse environments, but despite many different experimental reports on spore dormancy of many species of AMF a complete understanding of the phenomenon has not been obtained. We still do not know whether dormancy is more species or genus than isolate correlated, because experiments have often been carried out on different isolates. Moreover, no studies have been performed on the molecular bases of dormancy: we ignore whether it may be affected by the presence of compounds in young spores, which inhibit germination, or by the occurrence of compounds in mature, old spores, which enhance germination.
3 Triggers for Spore Germination
The molecular signals which relieve spore dormancy and activate the cell cycle still remain unknown, though different environmental conditions triggering the initiation of germination in genera and species of AMF have been investigated. In fact, resting spores of many AM fungal species germinate both in soil and in agar under adequate physical, chemical and microbiological conditions.
Many germination factors have been identified which play important roles in growth activation of quiescent spores. Although complex interactions among different factors probably play the most important role in spore germination in nature, many investigators have studied germination factors such as pH, temperature, moisture, mineral and organic nutrients, host plants, and microorganisms as if they were independent triggers, and as such they will be considered here.
3.1 pH
Differences in spore germination among species and genera are often related to the environment where the endophytes live and to which they are ecologically adapted (Sylvia and Williams 1992; Clark 1997). For example, spore population surveys from different sites showed that A. laevis is the predominant AM fungus in low pH soils (Abbott and Robson 1977), or even the only species in soils at pH < 4.9 (Nicolson and Schenck 1979). Also data from INVAM (International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi, http://invam.caf.wvu.edu/) collection showed that 88.5% of Acaulospora isolates live at soil pH < 6.0 (Morton et al. 1993). Accordingly, results obtained in experimental conditions demonstrated that spore germination of A. laevis is strongly regulated by soil pH, being optimum between 4 and 5, decreasing at pH 6, and declining to less than 10% between pH 6.5 and 8 (Hepper 1984b). Similar results were exhibited by Gigaspora coralloidea and Gigaspora heterogama isolated from acidic soils, which germinated best at pH from 4 to 6 (Green et al. 1976). Other authors reported that G. margarita is less sensitive to acidic conditions than G. mosseae (Siqueira et al. 1984).
An isolate of G. mosseae, collected from a wheat field, showed a pH optimum for spore germination between 6 and 9 in water or in soil extract agar, and was not able to form germ tubes at pH 4 and 5 (Green et al. 1976). Another strain of the same species, isolated from an agricultural soil, failed to germinate at pH 4.5 (Mosse and Hepper 1975). Other species of the genus Glomus germinated best at pH ranging from 6 to 8 and were capable of producing root infection and multiplying in very alkaline soils (Daniels and Trappe 1980; Giovannetti 1983; Douds 1997).
The thorough surveys of Sieverding (1991) confirmed that G. mosseae does not occur in natural tropical soils with pH < 5.5. Thus, it may be that edaphic factors related to the environment from which the different species of glomeromycotan fungi were originally isolated play an important role in spore germination and pre-symbiotic hyphal growth (Giovannetti and Gianinazzi-Pearson 1994). However, it may also be that the optimal pH values attributed to each species are actually characteristic of the isolates used in each experiment and cannot be applied to all the isolates of the species. Each isolate originating from a specific environment could in fact represent an ecotype adapted to peculiar soil characteristics. This could apply in particular to G. mosseae, which has been shown to occur in 55 different countries throughout all continents and biomes (Avio et al. 2009). Different geographic isolates of the same species should be used to obtain new evidence on this point.
3.2 Temperature
The germination of AM fungal spores is greatly affected by temperature and the limits for germination exhibited by different species have been ascribed to their fundamental dissimilarity. Tommerup (1983b) reported that three fungal species isolated from the same source possessed different temperature limits for germination: A. laevis germinated best at temperature ranges of 15–25°C, Gigaspora calospora at 10–30°C, and G. caledonium at 10–25°C.
Some studies have suggested that many differences among glomeromycotan fungi in temperature ranges affecting spore germination reflect the differences in the environments from which the fungi were isolated. Accordingly, two Florida isolates of G. coralloidea and G. heterogama germinated best at 34°C, while G. mosseae, isolated from more northern latitudes, showed maximum germination at 20°C and failed to germinate at 34°C (Schenck et al. 1975). Also, an isolate of Glomus epigaeum from cool climates showed maximum germination at 22°C (Daniels and Trappe 1980). Most rapid germination of spores of G. gigantea was obtained at 30°C, whereas no germination occurred at 15°C, and only 6% spores germinated at 35°C (Koske 1981a).
It is interesting to note that the lethal exposure times to 60°C for G. caledonium and A. laevis spores were 5 min and 1 min, respectively (Tommerup and Kidby 1980). Viability of G. intraradices and G. mosseae was nil beyond 60°C, and that of Glomus deserticola beyond 54°C (Nemec 1987). Interestingly, an isolate of G. intraradices showed a great tolerance to 45°C for up to 24 h (Bendavid-Val et al. 1997).
Temperature optima for germination may be related to the environment to which each endophyte is indigenous. The demonstration of this requires the germination of different strains of the same species isolated from geographical areas with very dissimilar climates.
3.3 Moisture
Soil water content can have variable effects upon spore germination of species and genera of AMF. G. margarita spores germinated independently of soil water content, while germination of G. intraradices, G. mosseae and A. longula was strongly inhibited by matric potentials between −0.50 and −2.20 MPa (Douds and Schenck 1991). Other authors reported that spore germination of G. epigaeum and G. gigantea was increased at soil moisture near field capacity or above (Daniels and Trappe 1980; Koske 1981a). Three other Glomus species, Glomus macrocarpum, G. clarum and G. etunicatum, showed tolerance to soil drying, maximum germination occurring at matric potential of −0.01 MPa (Sylvia and Schenck 1983). G. epigaeum spores germinated well when soil moisture content ranged from field capacity to soil saturation, and no germination was observed below −3.4 MPa (Daniels and Trappe 1980), while G. gigantea showed delayed germination at −1.0 Mpa (Koske 1981a).
As noted above for pH and temperature, differences in spore germination of AM fungal species and genera are often related to the moisture conditions of the environment to which they are ecologically adapted. No general conclusions can be made without knowing germination responses of several isolates of a species, each from environments with widely different moisture regimes, to different soil matric potentials. Moreover, it is probable that soil wetting and drying cycles are the most important factors affecting survival, germination and thus infectivity of AMF in nature; in particular in Mediterranean climates where glomeromycotan spores survive the hot and dry summers to colonize young emerging plants during the following seasons (Braunberger et al. 1996).
3.4 Mineral and Organic Nutrients
The germination of AM fungal spores is inconsistently affected by mineral nutrient content of soil. G. gigantea spores germinated at the same rate regardless of phosphorus concentrations (5–500 ppm) in sand plates (Koske 1981a). Germination of G. mosseae and G. caledonium spores was not affected by phosphorus concentrations in agar up to 30 mM, while above this level germination was reduced by 56% or more (Hepper 1983). Similar results were obtained with G. margarita, whose spores germinated well up to 16 mM phosphate solution (Tawaraya et al. 1996a) and with G. epigaeum spores, whose germination was not influenced by increasing levels of NH4NO3 and K2SO4, up to 200 ppm (Daniels and Trappe 1980). However, when phosphorus was added to soil, spore germination of different species of AMF decreased with soil P increments (De Miranda and Harris 1994). Investigations on the role of inorganic sulphur-containing compounds on the growth of G. caledonium mycelium showed that it was stimulated by the presence of thiosulphate, metabisulphite, sulphite and sulphate in the medium (Hepper 1984b).
Some inorganic ions completely inhibit spore germination of AMF. Hepper and Smith (1976) found that the inhibitory effects of different agar media on germination of G. mosseae spores was due to Mn and Zn. Toxicity of heavy metals such as Cu, Mn and Zn also affected spore germination of G. caledonium (Hepper 1979).
Germination and hyphal growth of different AM fungal species, evaluated in acidic soils with varying Al saturation, showed that most species of Gigaspora and Scutellospora were more tolerant than Glomus species (Bartolome-Esteban and Schenck 1994). However, no generalization is possible, since different isolates within a species showed varying responses to heavy metals (Gildon and Tinker 1981; Weissenhorn et al. 1993).
Studies on the effects of salinity on AM fungal spores showed inhibition of germination and hyphal growth by increasing concentrations of NaCl (Hirrel 1981; Estaun 1989; Juniper and Abbott 1993, 2006; McMillen et al. 1998). However, Koske et al. (1996) reported on the ability of G. gigantea spores to retain germinability upon exposure to natural conditions of immersion in sea water and stressed the importance of this feature for the dispersal of the species in coastal waters.
A range of organic substrates such as glucose, fructose, sucrose, L-arabinose and aspartic, succinic, malic, pyruvic acids, reduced germination and germ tube growth of G. mosseae spores (Siqueira et al. 1982). Accordingly, their germination was inhibited by excess nutrients, such as those provided by Potato Dextrose or Nutrient Broth Agars (Daniels and Graham 1976). However, hyphal growth of G. mosseae was stimulated by tartaric acid (Mosse 1959), and peptone, yeast extract, thiamine, cystine, glycine and lysine showed great growth promoting effects on G. caledonium hyphae (Hepper 1979; Hepper and Jakobsen 1983). Recent findings also reported that a growth stimulant isolated from the brown alga Laminaria japonica increased hyphal growth of G. margarita sterile spores germinated in vitro (Kuwada et al. 2006).
3.5 Host/Non Host Plants
Early germination trials showed that AMF are able to germinate in axenic culture in the absence of the host (Godfrey 1957; Mosse 1959; Hepper and Smith 1976; Powell 1976; Koske 1981a). Thus, host-derived signals do not represent essential factors for spore germination of AMF. Accordingly, the presence of growing host roots did not trigger the relief of spore dormancy in different AM fungal species (Tommerup 1983a). Nevertheless, host roots, crude or purified root exudates and compounds derived by their fractioning positively affected spore germination and germling growth in different experimental conditions, depending on both plant and AM fungal species (Graham 1982; Bécard and Piché 1989; Gianinazzi-Pearson et al. 1989; Nair et al. 1991; Tsai and Phillips 1991; Giovannetti et al. 1993a, 1994, 1996; Suriyapperuma and Koske 1995; Tawaraya et al. 1996b; Buée et al. 2000; Nagahashi and Douds 2000; Scervino et al. 2006). The key compounds exuded from host roots able to induce hyphal branching in AMF, strigolactones, stimulated spore germination in Gigaspora rosea, G. intraradices and Glomus claroideum, and increased mitochondrial density and respiration in G. intraradices (Tamasloukht et al. 2003; Besserer et al. 2006). Modulation of AM fungal spore germination was also reported in the presence of exudates of mycorrhizal and non mycorrhizal host roots and of their differential flavonoid components, which showed species-specific effects (Scervino et al. 2005a, b). Detailed information on fungal responses to host-derived signals is given later in this volume (Chapters 2 and 4).
It is interesting to note that transgenic plants may or may not affect AMF life cycle, since the experimental works showed different results depending on the type of genetic modification and gene product expressed. Elfstrand et al. (2005) reported that the constitutive 35S-driven expression of Mtchit 3-3, a class III chitinase gene in Medicago truncatula root-organ cultures, was associated with stimulation of spore germination of G. intraradices and Glomus constrictum, suggesting that the Mtchit 3-3 gene product might directly act on the walls of AM fungal spores. Actually, one gene belonging to class III chitinases was specifically induced in mycorrhizal M. truncatula (Salzer et al. 2000; Bonanomi et al. 2001).
Root exudates of both non host and ectomycorrhizal plants often showed no effects on spore germination (Daniels and Trappe 1980; Azcón and Ocampo 1984; El-Atrach et al. 1989; Gianinazzi-Pearson et al. 1989). Nevertheless, contradictory fungal behaviours were reported, in in vitro and in vivo experiments (Ocampo et al. 1980; Glenn et al. 1985; Parra-Garcia et al. 1992; Schreiner and Koide 1993a, b; Giovannetti and Sbrana 1998). The release of inhibitory compounds by non-hosts was reported by different authors (Vierheilig et al. 2000; Roberts and Anderson 2001; Oba et al. 2002; Bainard et al. 2009), and a heat-labile factor able to reduce G. gigantea and G. intraradices germination and growth was detected in root exudates of a non mycorrhizal tomato mutant (David-Schwartz et al. 2001, 2003; Gadkar et al. 2003).
3.6 Microorganisms
Although several species of AMF germinate well in axenic culture, some growth stimulation by soil and rhizosphere microorganisms has been reported (Mosse 1959; Watrud et al. 1978; Daniels and Trappe 1980; Azcón-Aguilar et al. 1986; Azcón 1987, 1989; Gryndler et al. 2000; Scervino et al. 2008; Pivato et al. 2009). The mechanisms of such activity remain unknown. Many laboratory experiments indicated that different bacterial isolates may affect spore germination and hyphal extension. For example, Streptomyces orientalis stimulated germination of G. mosseae (Mugnier and Mosse 1987), diverse field isolates of Streptomyces spp. increased germination of G. margarita by production of volatile compounds (Carpenter-Boggs et al. 1995; Tylka et al. 1991), and Klebsiella pneumoniae increased hyphal extension in G. deserticola germlings (Will and Sylvia 1990).
Differential effects of factors released by Bacillus subtilis, Mesorhizobium mediterraneum and a PGPR strain on G. mosseae and G. rosea spore germination and growth was reported by Requena et al. (1999). G. mosseae spore germination was not affected by bacteria, whereas a fungistatic effect was evidenced in G. rosea when challenged with a strain of B. subtilis, although such strain was able to induce hyphal growth enhancement in G. mosseae.
Several saprophytic fungi isolated from G. mosseae sporocarps decreased or did not affect germination of G. mosseae spores on water agar (Fracchia et al. 1998). By contrast, the soil fungus Trichoderma spp. enhanced the development of mycelium from germinating spores of G. mosseae (Calvet et al. 1992). A recent study reported the increase of G. rosea hyphal length in the presence of exudates of Drechslera sp., a common fungal endophyte isolated by the inner cortical cells of the grass Lolium multiflorum (Scervino et al. 2009).
Gram-positive bacteria (Paenibacillus spp. and Bacillus spp.) were found associated or attached to fungal hyphae (Artursson and Jansson 2003), and among them Paenibacillus validus induced the production of new spores of G. intraradices grown in plates in dual culture in the absence of the host (Hildebrandt et al. 2002, 2006).
Different taxa of microbes are associated with spores collected from the field, which may remain contaminated even after surface disinfestation procedures (Mayo et al. 1986; Ames et al. 1989; Walley and Germida 1996). Investigations on the role played by such resident microbial populations are very interesting. For example, spore-associated bacteria, including Pseudomonas and Corynebacterium, enhanced germination of Glomus versiforme spores in vitro, confirming that this fungal species germinates best under non-sterile conditions (Mayo et al. 1986). Other bacteria were intimately associated with the outer spore wall of G. clarum (Walley and Germida 1996), or embedded in the electron-dense spore wall of Glomus species (Filippi et al. 1998; Maia and Kimbrough 1998), confirming previous reports on the occurrence of chitin-decomposing microorganisms in washed, healthy spores of G. macrocarpum (Ames et al. 1989). Recent PCR-DGGE analyses showed that bacterial species associated with spores of Glomus geosporum and G. constrictum belonged to taxonomic groups known to degrade biopolymers (Cellvibrio, Chondromyces, Flexibacter, Lysobacter, and Pseudomonas) (Roesti et al. 2005), suggesting that such microbes, being able to digest the outer walls of AMF, mainly composed of chitin, may aid spore germination.
In the family Gigasporaceae, spores originating from different geographic areas were shown to harbour intracellular symbionts belonging to β-proteobacteria (Bianciotto et al. 2000, 2003), which could possibly affect germination, since an isolate of G. margarita, cured of its endobacteria, showed delayed germling growth (Lumini et al. 2007). Actually, previous results showed that germination frequency of G. decipiens spores was significantly enhanced by diverse intracellular strains of Burkholderia vietnamiensis, but not by Burkholderia pseudomallei (Levy et al. 2003).
4 Modes of Spore Germination
Glomeromycotan fungi germinate in different ways depending on the genus. Spores of most Glomus species germinate by regrowth from the end of hyphal attachments (Godfrey 1957; Mosse 1959). Many germ tubes may emerge from the old subtending hypha, as in G. clarum, or a single one, as in G. mosseae and in G. caledonium. Some Glomus species, such as Glomus viscosum, germinate after forming a balloon-shaped swelling at the broken end of the subtending hypha (Godfrey 1957; Walker et al. 1995). By contrast, germ tubes of Gigaspora, Scutellospora and Acaulospora species emerge directly through the spore wall. Though, different germination structures can be formed depending on the genus. A simple structure is produced by Gigaspora spores, which germinate after a papillate layer has formed in the inner part of the spore wall. Light and electron microscopy studies of this mode of germination were performed in G. margarita (Becker and Hall 1976; Sward 1981b, c). In other genera inner (or germinal) walls are involved in germination, with the formation of specialised structures usually on the outer surface of the innermost wall. Species of Scutellospora develop germination shields (Walker and Sanders 1986) whose morphology has been recently used to taxonomically revise the family Gigasporaceae (Oehl et al. 2008). A different structure, described in some species of Acaulospora and Kuklospora, was termed “germination orb” (Spain 1992) since it differs morphologically from Scutellospora shields, while persisting after germination. The cellular events leading to spore germination in A. laevis were monitored at the ultrastructural level, by means of sequential sampling of spores incubated in conditions allowing germination (Mosse 1970a, b). Germination structures were described as dense peripheral compartments, containing cytoplasm and many nuclei, from which germ tubes arose and pushed through the outer layers of the spore wall. Some Pacispora species are known to develop Glomus-like spores with germination structures morphologically similar to Scutellospora shields, but differing from them, since they are delicate, deteriorating over time and therefore difficult to discern (Walker et al. 2004; da Silva et al. 2008). Distinctive simpler germination structures occur in spores of Archaeospora trappei and some Ambispora species (Spain 2003; Spain et al. 2006; Goto et al. 2008).
Multiple germination can be defined as the abilily of fungal spores to germinate several times by producing successive germ tubes when those formed previously are severed from the parent spores (Koske 1981b). This capacity was described in spores of a Glomus sp. (Mosse 1959), and later studied in G. gigantea, whose spores were able to germinate up to ten times over a period of 50 days, after their germ tubes had been severed (Koske 1981b). Multiple germination may be considered an additional strategy to increase the probability of successful infection of a host root by germinating spores of AMF.
5 Development of Pre-symbiotic Mycelium
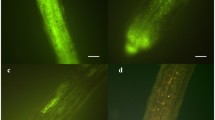
After germination, hyphae generally follow a forward, linear growth, with a strong apical dominance and regular, right-angled branches. Hyphae are thick-walled, aseptate, about 5–10 µm wide, and contain many nuclei (Fig. 1a, b).
Micrographs showing differential stainings of mycelium originated by Glomus mosseae spores growing in the absence of the host. (a, b) DAPI-stained mycelium showing nuclear distribution along hyphae and in secondary spores. Scale bars = 130 and 40 µm, respectively; (c) Haematoxylin-stained anastomosing hyphae showing protoplasm continuity in the hyphal bridge. Scale bar = 13 µm; (d) Succinate dehydrogenase localisation and Trypan blue staining of an incompatible interaction between hyphae belonging to geographically different isolates. Scale bar = 10 µm
Cytoplasm and nuclei can be easily observed migrating along two directions in hyphae originating from spores during germination (Mosse 1959). Ultrastructural studies confirmed these early observations, obtaining clear evidence of a swirling motion of the cytoplasm, and suggested redistribution of spore cytoplasm into the germ tube (Sward 1981c). Two-photon fluorescence microscopy and video-enhanced microscopy allowed the detection of nuclei moving along hyphae originating from germinated spores of G. rosea and G. caledonium, respectively (Bago et al. 1998; Logi et al. 1998). Such movement could be a microtubules (MT)-dependent process, since in G. mosseae germlings nuclei were always detected in close association with MT, as visualised by indirect immunofluorescence microscopy (Astrom et al. 1994), confirming previous observations on the growth of G. margarita germ-tubes (Sward 1981c).
The elongating germ tubes give rise to a mycelial network whose extension is highly variable between individuals. Even when growing in the most suitable media, hyphal growth of AMF is poor. For example, mycelial length in G. caledonium reached 30–50 mm after 10–15 days growth on water agar, and the mean growth rate of the mycelium during the early phase was 1.97 ± 0.39 µm/min (Logi et al. 1998). Accordingly, hyphal growth rate in G. mosseae growing in the absence of root factors ranged between 1.65 and 2.7 µm/min (Mosse 1959; Giovannetti et al. 1993b). New hyphae of G. clarum extended up to 8 mm after 10 days incubation (Louis and Lim 1988). Hyphal length of G. margarita after 9 days growth ranged between 18 and 25 mm (Bécard and Piché 1989; Gianinazzi-Pearson et al. 1989), while that of G. gigantea reached 54.4 cm after 15 days growth in vitro (Douds et al. 1996).
Studies on transgenic plants designed to constitutively express the insecticidal toxin from Bacillus thuringiensis reported diverse effects on hyphal growth of G. mosseae germinated sporocarps, which was lower in the presence of Bt corn 176 than in the presence of Bt 11 or non-transgenic plants. By contrast, hyphal length of G. mosseae did not show differences when grown in soil samples containing Bt and non-Bt plant residues (Turrini et al. 2004a; Castaldini et al. 2005). Root exudates of aubergine plants transformed to express the antimicrobial Dm-AMP1 defensin from Dahlia merckii did not affect hyphal growth of G. mosseae, as compared with non transgenic plants (Turrini et al. 2004b).
Fungal hyphae expanding from the primary mycelium or from branches meet frequently and often fuse, by means of hyphal fusions (anastomoses), when growing on agar or on membranes (Fig. 1c). The occurrence of anastomosis in AMF was mentioned by some authors who did not report any quantitative data on the frequency of hyphal fusions in the different species or on the cytological events involved (Godfrey 1957; Mosse 1959; Tommerup 1988). In 1999 for the first time anastomoses between living hyphae of individually germinated spores of AMF were monitored via a combination of time-lapse and video-enhanced light microscopy, image analysis, and epifluorescence microscopy (Giovannetti et al. 1999) The percentage of contacts leading to anastomosis ranged from 35% to 69% in hyphae from the same germling and from 34% to 90% in hyphae from different germlings of the same isolate of G. mosseae, G. caledonium, G. intraradices. By contrast, no anastomoses were detected between hyphae from the same or different germlings of G. rosea and Scutellospora castanea. Such differential behaviour of AM fungal species belonging to Glomeraceae and Gigasporaceae families was later confirmed by other authors (de Souza and Declerck 2003; de la Providencia et al. 2005).
Spatiotemporal studies made it possible to monitor anastomosis formation: complete fusion of hyphal walls and the establishment of cytoplasmic flow in the fusion bridge took about 35 min after a hyphal tip showed directed growth towards another hypha, both in G. caledonium and in G. mosseae mycelia. Protoplasmic continuity, the distinctive mark of true anastomoses, was evidenced by SDH activity in hyphal bridges, where cellular organelles moved at the speed of 1.8 µm/s (Giovannetti et al. 1999). Nuclear migration through fusion bridges suggested that genetic exchange could occur by means of anastomosis between hyphae derived from genetically different individuals. Accordingly, other studies demonstrated that geographically and genetically different G. mosseae isolates were unable to fuse (Giovannetti et al. 2003) (Fig. 1d), while genetic exchange occurred, by means of anastomosis, between genetically distinct isolates of one population of G. intraradices from the same field (Croll et al. 2009). Such nuclear exchange may represent a fundamental mechanism allowing the maintenance of genetic diversity in AMF, hitherto regarded as ancient asexuals.
6 Biochemical Changes During Germination and Pre-symbiotic Growth
The germination of AM fungal spores is characterized by increased activity of the cytoplasm, involving essential biochemical changes for the switching from a metabolically quiescent state to active metabolism.
Early studies on biochemical events that take place during germination and growth of germlings in G. caledonium reported that kinetics of radioactive leucine and uracil incorporation was suggestive of RNA and protein synthesis being operative by 35 min after imbibition (Beilby and Kidby 1982). The response of ungerminated and pregerminated spores to inhibitors of nucleic acid synthesis suggested that the synthesis of mRNA, unnecessary for germination of G. caledonium spores, was required for germling growth, and that mitochondrial DNA was synthesized during germination and hyphal growth (Hepper 1979; Beilby 1983). However, production of detectable amounts of ribosomal and mRNAs during imbibition and cold storage was shown in ungerminated spores of G. rosea (Franken et al. 1997). Other authors were not able to demonstrate the occurrence of DNA synthesis in vitro, during and after germination of G. margarita spores, by using cell cycle inhibitors or direct labelling of nuclear DNA (Burggraaf and Beringer 1989). By contrast, the capability of DNA replication was reported to occur in a small nuclear population of germlings of the same species (Bianciotto and Bonfante 1993). More evidences of DNA replication and transcription during germination and early stage of fungal growth are reported in Section 7.
Protein synthesis was demonstrated to be essential for spore germination and germling growth by studying the effects of the protein synthesis inhibitor cycloheximide (Hepper 1979) and later confirmed by using radioactive leucine and the same metabolic inhibitor (Beilby 1983). The early report, based on 14C labeled acetate, that amino acid biosinthetic pathway were operating within 35 min of imbibition in G. caledonium (Beilby and Kidby 1982), has been recently confirmed by 15N labeling experiments and gene expression studies, which showed the ability of G. intraradices and G. mosseae to synthesize aminoacids from endogenous reserves (Breuninger et al. 2004; Gachomo et al. 2009).
A net synthesis of lipids was observed during germination and germ-tube growth of G. caledonium spores, with an increase of free fatty acids and polar lipids and a decrease in neutral lipids (Beilby and Kidby 1980). Total lipid content increased from 45% of dry weight in ungerminated spores to 55% and 75% of dry weight in 7 and 14 days old germinated spores, respectively. However, in other experiments, using 13C-labeled substrates and nuclear magnetic resonance spectroscopy, no detectable labeling of lipids was reported (Bago et al. 1999a), suggesting the lack of lipid biosynthesis in G. intraradices germinating spores. Later experiments, using 13C labeled glycerol or 14C acetate furtherly supported this hypothesis (Bago et al. 2002b; Trépanier et al. 2005). On the other hand, the occurrence of labeled 18- and 20-carbon fatty acids but not of 16-carbon fatty acids in germinating spores of G. intraradices and G. rosea, suggested that germlings could elongate and desaturate palmitic acid even in the absence of fatty acid synthase activity (Trépanier et al. 2005).
Other biosynthetic abilities of AMF during spore germination and germling growth have been demonstrated in G. caledonium and G. intraradices, which were able to synthesize sterols (Beilby and Kidby 1980; Fontaine et al. 2001a, b), as confirmed by the use of sterol biosynthesis inhibitors (Zocco et al. 2008).
The biosynthesis of polyamines, important regulators of fungal growth and differentation (Walters 1995), was studied in G. mosseae and G. rosea in order to assess the effects on AMF of polyamine biosynthesis inhibitors used to control plant disease. An increase in polyamines levels was observed after germination in G. mosseae, although enhanced germling growth in the presence of exogenous putrescine and spermidine suggested a low, growth limiting level of their endogenous concentrations (El Gachtouli et al. 1996). Interestingly, polyamine biosynthesis seems to occur only via the ornithine decarboxylase in G. mosseae, while in G. rosea the alternative pathway using arginine decarboxylase was active (Sannazzaro et al. 2004).
As for carbohydrate metabolism, cytochemical studies and isozyme staining performed on spores or germ tubes showed the occurrence of many enzymes of central metabolic pathways such as glycolysis, tricarboxylic acid cycle (TCA), pentose phosphate pathway and gluconeogenesis (Macdonald and Lewis 1978; Hepper et al. 1986; Saito 1995), many of which were operative 35 min after hydration. A rapid increase in spore ATP concentration after 45 min was evidence of the presence of an active respiratory system in G. caledonium germlings (Beilby and Kidby 1982).
A thorough survey of biochemical potentiality of germinating spores of G. intraradices was performed by using 13C-labeled substrates and nuclear magnetic resonance spectroscopy. The labeling patterns observed were consistent with significant carbon fluxes via various pathways, confirming that gluconeogenesis, TCA, glycolysis, and pentose phosphate pathway are operational in germlings, and supporting the important role played by glyoxylate cycle and non-photosynthetic one-carbon metabolism during germination (Bago et al. 1999a).
Since triacylglycerols (TAG) and free fatty acids may represent a large proportion of AMF spores’ weight (Beilby and Kidby 1980; Gaspar et al. 1994), their degradation is central to the process of spores germination and germling growth. Actually, the breakdown of TAG was assessed 5 days after germination in G. versiforme spores, probably by an active lipase (Gaspar et al. 1994, 1997).
Ultrastructural data on the movement and disappearance of lipid globules in hyphae originating from germinating spores (Maia and Kimbrough 1998; Bago et al. 2002a, b) support the hypothesis that storage lipids are used to provide precursors for anabolism through glyoxylate pathway and gluconeogenesis, and to fuel respiratory chains by β-oxidation and TCA, as confirmed by labeling experiments (Bago et al. 1999a; Lammers et al. 2001). Such hypothesis was also confirmed by the detection of isocitrate lyase and malate synthase genes involved in the glyoxylate cycle and of an acyl CoA dehydrogenase involved in fatty acid β-oxidation in G. intraradices and G. rosea spores (Lammers et al. 2001; Bago et al. 2002b).
Interestingly, trehalose was detected in spores of G. etunicatum in small quantities, decreasing during the early germination stage, suggesting its role as a source of energy before the start of lipid breakdown (Bécard et al. 1991).
Electron microscope observations of membrane-bound crystals in spores of G. margarita and A. laevis suggested the occurrence of protein storage material, observed in different stages of apparent breakdown in G. margarita germlings (Bonfante et al. 1994; Mosse 1970b; Sward 1981a, b). Native and denatured protein profiles of G. mosseae showed the presence of bands whose intensity decreased during spore germination, supporting the hypothesis of the existence of storage proteins in AM fungal spores (Avio and Giovannetti 1998; Samra et al. 1996). In addition to storage proteins, spores may utilize N stored in the form of aminoacids, especially asparagine, which was present in high concentration in quiescent spores of G. intraradices and G. caledonium (Beilby and Kidby 1982; Gachomo et al. 2009).
Transmembrane electric potential differences and ion fluxes in AM fungal hyphae showed a generally weak polarization of germ tubes growing in the absence of host derived signals, confirming a basal metabolic activity with low ATP consumption (Berbara et al. 1995; Ayling et al. 2000; Ramos et al. 2008).
In summary, AMF spores possess a large pool of enzymes allowing them to germinate and grow. Though, in the absence of host roots germling growth is arrested, even before depletion of spore reserves (see Section 8), while a boost of metabolism, primarily an increase of respiration (Tamasloukht et al. 2003; Bücking et al. 2008), occurs in the presence of root exudates. Interestingly, analyses of electric potential differences and H+ ion flux profile in AM fungal hyphae showed a strong influence of host derived signals, which induced ion fluxes enhancement depending on the specific hyphal domains, suggesting a differential activation and distribution of electrogenic H+-pump isoforms through plasma membrane (Ayling et al. 2000; Ramos et al. 2008).
7 Cytological and Genetic Changes During Germination and Pre-symbiotic Growth
Early evidence of cell cycle activation in AMF growing in the absence of the host was reported by Mosse, who described the development of dense regions containing normal cytoplasm and many dividing nuclei in spores of A. laevis prior to germination (Mosse 1970a). Also, Sward (1981b) observed a large number of nuclei with highly condensed chromatin and prominent nucleoli in G. margarita spores after 24 h of incubation on water agar. Cytological studies showed that nuclei from quiescent spores of G. versiforme were in the GO/G1 phase, whereas nuclei from mycorrhizal roots were in the synthetic and G2/M phases (Bianciotto et al. 1995). Mitotic spindles were also detected in germinated spores of G. mosseae by tubulin immunostaining, confirming the occurrence of DNA replication during presymbiotic growth (Requena et al. 2000). In the latter work, the gene GmTOR2, encoding a protein with high homology to the C terminus of Saccharomyces cerevisiae TOR2 (controlling cell cycle), was characterised. Under treatment with the anti-inflammatory drug rapamycin, which interferes with TOR2 by arresting S. cerevisiae cell cycle in G1 phase, G. mosseae spore germination was unaffected, whereas hyphal growth decreased, suggesting that nuclear replication in the pre-symbiotic stage is only necessary for hyphal growth (Requena et al. 2000).
EST sequencing from germinated spores of G. intraradices and G. rosea revealed putative homologues to cell cycle and meiosis-specific genes from other fungi, such as chromatin assembly factor, ubiquitin-encoding genes (Stommel et al. 2001) and Neurospora crassa NDT80, known to control exit from pachytene phase of meiosis (Jun et al. 2002). Furthermore, a putative gene involved in the biosynthesis of new nucleotides was detected in germinated spores of G. intraradices (Jun et al. 2002).
The occurrence of nuclear division was inferred in non symbiotic mycelium by using image analysis counts of the number of nuclei (Bécard and Pfeffer 1993), which decreased from 2,000 to 800 in individual spores during the early days of germination, suggesting the migration of nuclei from spores to hyphae. This was confirmed by data on the occurrence of cytoskeletal components, both microtubules and microfilaments, in the mycelium originating from germinating spores of G. mosseae and G. caledonium (Astrom et al. 1994; Logi et al. 1998). The presence of such components is consistent with the role of cytoskeleton in the migration of nuclei and cellular organelles during active growth. Expression of β-tubulins in germinating AM fungal spores (Franken et al. 1997; Butehorn et al. 1999) was confirmed by the detection of sequences putatively encoding other cytoskeletal proteins, such as α-tubulin, β-actin, dynein and actin-related protein, possibly involved in nuclear and nutrient movements, in G. intraradices germinated spores (Jun et al. 2002). Recently, full-length β-tubulin gene has been sequenced from G. gigantea and G. clarum, showing some peculiar traits compared to fungi other than glomeromycota (Msiska and Morton 2009).
Nuclear division in G. rosea hyphae was also detected in the presence of host root exudates or of the synthetic strigolactone GR24, which induced an accumulation of nuclei in the apical area of treated hyphae (Buée et al. 2000; Besserer et al. 2008).
Early experiments showed that inhibitors of mRNA translation hindered AM fungal spore germination (Hepper 1979; Beilby 1983). Accordingly, differential display analysis of G. rosea did not show changes in RNA accumulation patterns during hyphal development, suggesting that in this phase proteins are produced only by translating transcripts synthesized prior and during spore germination (Franken et al. 2000).
Many expressed genes detected in germinating AM fungal spores showed homology to those encoding for proteins involved in translation, protein processing, primary metabolism and transport processes (Franken et al. 1997; Lammers et al. 2001; Stommel et al. 2001; Jun et al. 2002; Bago et al. 2002a, 2003). The identification of genes putatively codifying for several enzymes involved in carbon metabolism and lipid breakdown often confirmed biochemical data.
An interesting gene, G. mosseae GmGIN1, was highly and specifically expressed in non symbiotic mycelium, whereas it was silenced during the symbiosis, both in the intraradical structures and the extraradical mycelium (Requena et al. 2002). Interestingly, several genes with homology to the N-terminus of GmGIN1, sequenced from Magnaporthe grisea, N. crassa, Gibberella zeae and Aspergillus nidulans, encode for a family of proteins playing an essential role in polarized growth, septal formation and hyphal morphological changes in the phytopathogenic fungus Ustilago maydis and in the ectomycorrhizal fungus Suillus bovinus (Gorfer et al. 2001; Weinzierl et al. 2002).
A 14-3-3 protein encoding gene, known to be involved in modulation of cell ion pumps and channels, was detected in G. intraradices mycelium (Porcel et al. 2006). This finding suggests an important role of this gene in controlling the activity of P-type H+-ATPases, detected in G. intraradices and G. mosseae (Requena et al. 2003; Corradi and Sanders 2006), which are responsible of the maintenance of hyphal ionic gradient during polarized growth (Ramos et al. 2008).
Interestingly, a sequence showing strong similarity to an endonuclease involved in lateral transfer of an rDNA intron has been detected in G. intraradices germinated spores, suggesting the occurrence of lateral gene transfer during nuclear exchange between anastomosing hyphae belonging to genetically different AMF (Jun et al. 2002; Croll et al. 2009).
Induction of genes encoding for putative pyruvate carboxylase and mitochondrial ADP/ATP translocase, involved in respiration enhancement activity, has been observed in G. rosea and G. intraradices during early responses to host root factors, before hyphal branching (Tamasloukht et al. 2003, 2007). The expression of the former gene could explain the stimulatory effects exerted by CO2 on AM fungal growth (Bécard and Piché 1989), whereas the expression of the latter gene could be necessary for the delivery of large quantity of ATP produced at high respiration rates (Requena et al. 2003). Activation of such genes and oxygen consumption were induced by host root exudates after 0.5–3 h, when no morphological change in hyphal growth pattern was detectable yet. On the contrary, no differences in the expression of key metabolic genes during the first 48 h after strigolactone analogue GR24 treatment were observed in G. rosea, which showed strong enhancement in transcript levels after 2 days of incubation, independently of GR24 treatment (Besserer et al. 2008). These findings suggest that other unknown signal molecules may be active and that strigolactone-induced mitochondrial activity is due to post-translational regulation of key enzymes (Delano-Frier and Tejeda-Sartorius 2008; Rani et al. 2008). The need of host-derived signals for developmental stages following spore germination can be inferred by results obtained with the pmi mutants of Solanum lycopersicum, which are regularly colonised by extraradical mycelium and mycorrhizal roots but are not susceptible to colonisation by hyphal germlings (David-Schwartz et al. 2001, 2003).
AM fungal spores germinating in the absence of host-derived factors constitutively release unknown compounds which are perceived as signals by host plants and are able to elicit recognition responses, such as a transient cytoplasmic calcium induction in soybean cells (Navazio et al. 2007) and the accumulation of starch in Lotus japonicus roots (Gutjahr et al. 2009). Ca2+-mediated signaling was also suggested by expression of genes involved in Ca2+-mediated signal transduction in M. truncatula roots in the presence of a diffusible factor released by G. mosseae (Weidmann et al. 2004). Previous studies had reported the release of a diffusible signal by G. mosseae, G. rosea, G. gigantea, G. margarita and G. intraradices growing in the presence of host plants (Chabaud et al. 2002; Kosuta et al. 2003). The perception of such signals by M. truncatula induced root expression of the early nodulin gene MtENOD11, which was related, both spatially and temporally, with the appearance of hyphal branching enhancement. Moreover, factors released by G. margarita and G. intraradices mycelium growing nearby M. truncatula plant roots were able to induce lateral root formation (Olah et al. 2005) and those released by G. intraradices branching hyphae elicited root calcium-spiking responses (Kosuta et al. 2008). No information is still available on the chemical nature of AM fungal factor(s).
Although many studies reported germling growth improvement by different microorganisms, little is known about the molecular mechanisms of such phenomenon. Changes in AM fungal gene expression in response to the perception of microbial derived factors were detected by Requena et al. (1999) during co-culture of G. mosseae with a strain of the rhizobacterium B. subtilis, inducing mycelial growth increases. In particular, down-regulation of the putative gene GmFOX2, encoding a protein involved in long-chain fatty acids catabolism, was evidenced. It is not known which is the signaling pattern between bacteria and fungi, although it has been hypothesized that an increase in fungal cAMP, due to the perception of flavonoid/estrogen bacterial signals, could be responsible for the glucose repression stage that down-regulates GmFOX2 (Requena et al. 1999).
8 Growth Arrest in the Absence of the Host
Although spores of AMF are able to germinate in vitro in response to different edaphic and environmental conditions, they are not capable of extensive independent hyphal growth, and, in the absence of the host, germlings cease growth within 8–20 days (Mosse 1959; Daniels and Graham 1976; Beilby and Kidby 1980; Koske 1981a; Hepper 1984b; Bécard and Piché 1989; Giovannetti et al. 1993b; Schreiner and Koide 1993b; Logi et al. 1998) (Fig. 2).
Microchambers allowing continuous observation of living mycelium over a period of several hours, showed that when no host-derived signals from the surrounding environment were perceived by G. caledonium and G. rosea germlings, hyphae entered a state of developmental arrest. Cytoplasm, nuclei and cellular organelles were retracted from the tips and from peripheral hyphae and retraction septa were produced, separating viable from empty hyphal segments (Logi et al. 1998). In vivo two-photon microscopy, carried out on G. rosea germlings, showed differences in the organization and distribution of nuclei between actively growing hyphae and those undergoing septation (Bago et al. 1998, 1999b). Protoplasmic flow rate, measured in actively growing germlings on the basis of the movement of cell particles – nuclei, small vacuoles, mitochondria, fat droplets, tiny organelles – ranged from 2.98 to 4.27 µm/s in living hyphae of G. caledonium (Giovannetti et al. 2000). Microchambers and two-photon microscopy studies revealed that neither protoplasm streaming nor nuclear movements occurred in protoplasm-retracting hyphae and that progressively enlarged vacuoles led to the formation of empty areas where a cross wall was eventually formed (Bago et al. 1998, 1999b; Giovannetti et al. 2000) (Fig. 3a, b).
Micrographs showing protoplasm retraction during growth arrest in hyphae originating from Glomus mosseae spores. (a) DAPI staining, evidencing nuclar occurrence in retracting protoplasm. Scale bar = 7 µm; (b) Haematoxylin staining showing a viable hyphal compartment below a septum isolating the empty hyphal tip. Scale bar = 10 µm
Metabolic activity was still detectable in G. caledonium 6-month-old hyphae proximal to the mother spore, which was able to retain infectivity, suggesting that such resource reallocation is functional to long-term maintenance of viability, allowing survival of fungal propagules in the absence of host plants (Logi et al. 1998; Giovannetti et al. 2000).
The reasons for such behaviour have been investigated with the aim of determining whether vital metabolic pathways may be blocked. The main results have been considered earlier in this chapter (see Section 6), and they indicate that germinating spores do possess the metabolic machinery for hyphal growth and that spore reserves are not totally depleted during germling growth (Hepper 1979; Beilby and Kidby 1980; Koske 1981b). Germinating AM fungal spores showed low respiratory activity and reduced resource utilization, allowing limited biosynthesis, whereas higher respiration rates and use of C sources, sustaining growth and morphogenesis, were detected after the perception of host root factors. Respiratory metabolism seems a suitable control target for non symbiotic growth arrest, which has been suggested to represent a strategic mechanism preventing spore reserves consumption in the absence of host-regulated germination.
9 Concluding Remarks
Several survival strategies are supposed to have affected the evolutionary history of AMF, allowing them to overcome their obligate biotrophic status. The first survival strategy is represented by the wide host range – ∼80% of land plant species –, which increases the possibility of individually germinated spores to come into contact and colonise host roots: such strategy, relying wholly on chance, appears a weak explanation for 460 million years continued existence. A second evolutionary mechanism allows the survival of spores germinated in the absence of host roots by mycelial growth arrest, which is accompanied by peripheral protoplasm withdrawal and resource reallocation towards mother spores, functional to retaining long-term colonisation ability. Challenges remain concerning factors triggering the onset of growth arrest and the molecular mechanisms involved. Further energy-saving mechanisms allow the unequivocal discrimination of host from non host roots, since AM fungal hyphae undergo a biochemical switch and a distinctive pattern of hyphal morphogenesis only after perceiving host-derived signals. Recently, we obtained data on the ability of AMF germlings to plug into a compatible mycorrhizal mycelium by means of anastomoses, thus gaining access to plant-derived carbon before undergoing growth arrest, enhancing their survival chances. The ability of AM fungal mycelium to form anastomosis and to discriminate self from nonself may represent a fundamental additional survival strategy. These strategies may compensate for the lack of host-regulated spore germination, an apparently inconsistent behaviour for obligate symbionts, and contribute to the survival of individuals and populations of AMF.
References
Abbott LK, Robson AD (1977) The distribution and abundance of vesicular-arbuscular endophytes in some Western Australian soils. Aust J Bot 25:515–522
Ames RN, Mihara KL, Bayne HG (1989) Chitin-decomposing actynomycetes associated with a vesicular-arbuscular mycorrhizal fungus from a calcareous soil. New Phytol 111:67–71
Artursson V, Jansson JK (2003) Use of bromodeoxyuridine immunocapture to identify active bacteria associated with arbuscular mycorrhizal hyphae. Appl Environ Microbiol 69:6208–6215
Astrom H, Giovannetti M, Raudaskoski M (1994) Cytoskeletal components in the arbuscular mycorrhizal fungus Glomus mosseae. Mol Plant Microbe Interact 7:309–312
Avio L, Giovannetti M (1998) The protein pattern of spores of arbuscular mycorrhizal fungi: comparison of species, isolates and physiological stages. Mycol Res 102:985–990
Avio L, Cristani C, Strani P, Giovannetti M (2009) Genetic and phenotypic diversity of geographically different isolates of Glomus mosseae. Can J Microbiol 55:242–253
Ayling SM, Smith SE, Smith FA (2000) Transmembrane electric potential difference of germ tubes of arbuscular mycorrhizal fungi responds to external stimuli. New Phytol 147:631–639
Azcón R (1987) Germination and hyphal growth of Glomus mosseae in vitro. Effect of rhizosphere bacteria and cell-free culture media. Soil Biol Biochem 19:417–419
Azcón R (1989) Selective interaction between free-living rhizosphere bacteria and vesicular-arbuscular mycorrhizal fungi. Soil Biol Biochem 21:639–644
Azcón R, Ocampo JA (1984) Effect of root exudation on VA mycorrhizal infection at early stages of plant growth. Plant Soil 82:133–138
Azcón-Aguilar C, Diaz-Rodriguez RM, Barea JM (1986) Effect of soil micro-organisms on spore germination and growth of the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Trans Br Mycol Soc 86:337–340
Bago B, Zipfel W, Williams RM, Chamberland H, Lafontaine JG, Webb WW, Piche Y (1998) In vivo studies on the nuclear behavior of the arbuscular mycorrhizal fungus Gigaspora rosea grown under axenic conditions. Protoplasma 203:1–15
Bago B, Pfeffer PE, Douds DD, Brouillette J, Becard G, Shachar-Hill Y (1999a) Carbon metabolism in spores of the arbuscular mycorrhizal fungus Glomus intraradices as revealed by nuclear magnetic resonance spectroscopy. Plant Physiol 121:263–271
Bago B, Zipfel W, Williams RM, Piche Y (1999b) Nuclei of symbiotic arbuscular mycorrhizal fungi as revealed by in vivo two-photon microscopy. Protoplasma 209:77–89
Bago B, Pfeffer PE, Zipfel W, Lammers P, Shachar-Hill Y (2002a) Tracking metabolism and imaging transport in arbuscular mycorrhizal fungi. Metabolism and transport in AM fungi. Plant Soil 244:189–197
Bago B, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, Pfeffer PE, Shachar-Hill Y (2002b) Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol 128:108–124
Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, Douds DD, Lammers PJ, Shachar-Hill Y (2003) Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol 131:1496–1507
Bainard LD, Brown PD, Upadhyaya MK (2009) Inhibitory effect of tall hedge mustard (Sisymbrium loeselii) allelochemicals on rangeland plants and arbuscular mycorrhizal fungi. Weed Sci 57:386–393
Bartolome-Esteban H, Schenck NC (1994) Spore germination and hyphal growth of arbuscular mycorrhizal fungi in relation to soil aluminum saturation. Mycologia 86:217–226
Becker WN, Hall IR (1976) Gigaspora margarita, a new species in the Endogonaceae. Mycotaxon 4:155–160
Beilby JP (1983) Effects of inhibitors on early protein, RNA, and lipid synthesis in germinating vesicular-arbuscular mycorrhizal fungal spores of Glomus caledonium. Can J Microbiol 29:596–601
Beilby JP, Kidby DK (1980) Biochemistry of ungerminated and germinated spores of the vesicular-arbuscular mycorrhizal fungus, Glomus caledonium: changes in neutral and polar lipids. J Lipid Res 21:739–750
Beilby JP, Kidby DK (1982) The early synthesis of RNA, protein, and some associated metabolic events in germinating vesicular-arbuscular mycorrhizal fungal spores of Glomus caledonium. Can J Microbiol 28:623–628
Bendavid-Val R, Rabinowitch HD, Katan J, Kapulnik Y (1997) Viability of VA-mycorrhizal fungi following soil solarization and fumigation. Plant Soil 195:185–193
Berbara RLL, Morris BM, Fonseca HMAC, Reid B, Gow NAR, Daft MJ (1995) Electrical currents associated with arbuscular mycorrhizal interactions. New Phytol 129:433–438
Besserer A, Puech-Pages V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Becard G, Sejalon-Delmas N (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4:e226
Besserer A, Becard G, Jauneau A, Roux C, Sejalon-Delmas N (2008) GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol 148:402–413
Bécard G, Piché Y (1989) Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl Environ Microbiol 55:2320–2325
Bécard G, Doner LW, Rolin DB, Douds DD, Pfeffer PE (1991) Identification and quantification of trehalose in vesicular-arbuscular mycorrhizal fungi by in vivo C-13 NMR and HPLC analyses. New Phytol 118:547–552
Bécard G, Pfeffer PE (1993) Status of nuclear division in arbuscular mycorrhizal fungi during in vitro development. Protoplasma 174:62–68
Bianciotto V, Bonfante P (1993) Evidence of DNA replication in an arbuscular mycorrhizal fungus in the absence of the host plant. Protoplasma 176:100–105
Bianciotto V, Barbiero G, Bonfante P (1995) Analysis of the cell cycle in an arbuscular mycorrhizal fungus by flow cytometry and bromodeoxyuridine labelling. Protoplasma 188:161–169
Bianciotto V, Lumini E, Lanfranco L, Minerdi D, Bonfante P, Perotto S (2000) Detection and identification of bacterial endosymbionts in arbuscular mycorrhizal fungi belonging to the family Gigasporaceae. Appl Environ Microbiol 66:4503–4509
Bianciotto V, Lumini E, Bonfante P, Vandamme P (2003) ‘Candidatus Glomeribacter gigasporarum’ gen. nov., sp nov., an endosymbiont of arbuscular mycorrhizal fungi. Int J Syst Evol Microbiol 53:121–124
Bonanomi A, Wiemken A, Boller T, Salzer P (2001) Local induction of a mycorrhiza-specific class III chitinase gene in cortical root cells of Medicago truncatula containing developing or mature arbuscules. Plant Biol 3:194–199
Bonfante P, Balestrini R, Mendgen K (1994) Storage and secretion processes in the spore of Gigaspora margarita Becker & Hall as revealed by high-pressure freezing and freeze substitution. New Phytol 128:93–101
Braunberger PG, Abbott LK, Robson AD (1996) Infectivity of arbuscular mycorrhizal fungi after wetting and drying. New Phytol 134:673–684
Breuninger M, Trujillo CG, Serrano E, Fischer R, Requena N (2004) Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet Biol 41:542–552
Bücking H, Abubaker J, Govindarajulu M, Tala M, Pfeffer PE, Nagahashi G, Lammers P, Shachar-Hill Y (2008) Root exudates stimulate the uptake and metabolism of organic carbon in germinating spores of Glomus intraradices. New Phytol 180:684–695
Buée M, Rossignol M, Jauneau A, Ranjeva R, Bécard G (2000) The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol Plant Microbe Interact 13:693–698
Burggraaf JP, Beringer JE (1989) Absence of nuclear DNA synthesis in vesicular-arbuscular mycorrhizal fungi during in vitro development. New Phytol 111:25–33
Butehorn B, Gianinazzi-Pearson V, Franken P (1999) Quantification of beta-tubulin RNA expression during asymbiotic and symbiotic development of the arbuscular mycorrhizal fungus Glomus mosseae. Mycol Res 103:360–364
Calvet C, Barea JM, Pera J (1992) In vitro interactions between the vesicular-arbuscular mycorrhizal fungus Glomus mosseae and some saprophytic fungi isolated from organic substrates. Soil Biol Biochem 24:775–780
Carpenter-Boggs L, Loynachan TE, Stahl PD (1995) Spore germination of Gigaspora margarita stimulated by volatiles of soil-isolated actinomycetes. Soil Biol Biochem 27:1445–1451
Castaldini M, Turrini A, Sbrana C, Benedetti A, Marchionni M, Mocali S, Fabiani A, Landi S, Santomassimo F, Pietrangeli B (2005) Impact of Bt corn on rhizospheric and soil eubacterial communities and on beneficial mycorrhizal symbiosis in experimental microcosms. Appl Environ Microbiol 71:6719–6729
Chabaud M, Venard C, Defaux PA, Becard G, Barker DG (2002) Targeted inoculation of Medicago truncatula in vitro root cultures reveals MtENOD11 expression during early stages of infection by arbuscular mycorrhizal fungi. New Phytol 156:265–273
Clark RB (1997) Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisition at low pH. Plant Soil 192:15–22
Corradi N, Sanders IR (2006) Evolution of the P-type II ATPase gene family in the fungi and presence of structural genomic changes among isolates of Glomus intraradices. BMC Evol Biol 6:21
Croll D, Giovannetti M, Koch AM, Sbrana C, Ehinger M, Lammers PJ, Sanders IR (2009) Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 181:924–937
da Silva DKA, Freitas ND, Cuenca G, Maia LC, Oehl F (2008) Scutellospora pernambucana, a new fungal species in the Glomeromycetes with a diagnostic germination orb. Mycotaxon 106:361–370
Daniels BA, Graham SO (1976) Effects of nutrition and soil extracts on germination of Glomus mosseae spores. Mycologia 68:108–116
Daniels BA, Trappe JM (1980) Factors affecting spore germination of the vesicular-arbuscular mycorrhizal fungus, Glomus epigaeus. Mycologia 72:457–471
Schwartz RD, Badani H, Smadar W, Levy AA, Galili G, Kapulnik Y (2001) Identification of a novel genetically controlled step in mycorrhizal colonization: plant resistance to infection by fungal spores but not extra-radical hyphae. Plant J 27:561–569
David Schwartz R, Gadkar V, Wininger S, Bendov R, Galili G, Levy AA, Kapulnik Y (2003) Isolation of a premycorrhizal infection (pmi2) mutant of tomato, resistant to arbuscular mycorrhizal fungal colonization. Mol Plant Microbe Interact 16:382–388
de la Providencia IE, de Souza FA, Fernandez F, Delmas NS, Declerck S (2005) Arbuscular mycorrhizal fungi reveal distinct patterns of anastomosis formation and hyphal healing mechanisms between different phylogenic groups. New Phytol 165:261–271
de Miranda JCC, Harris PJ (1994) Effects of soil phosphorus on spore germination and hyphal growth of arbuscular mycorrhizal fungi. New Phytol 128:103–108
de Souza FA, Declerck S (2003) Mycelium development and architecture, and spore production of Scutellospora reticulata in monoxenic culture with Ri T-DNA transformed carrot roots. Mycologia 95:1004–1012
Delano-Frier JP, Tejeda-Sartorius M (2008) Unraveling the network: novel developments in the understanding of signaling and nutrient exchange mechanisms in the arbuscular mycorrhizal symbiosis. Plant Signal Behav 3:936
Douds DD, Schenck NC (1991) Germination and hyphal growth of VAM fungi during and after storage in soil at five matric potentials. Soil Biol Biochem 23:177–183
Douds DD, Nagahashi G, Abney GD (1996) The differential effects of cell wall associated phenolics, cell walls, and cytosolic phenolics of host and non host roots on the growth of two species of AM fungi. New Phytol 133:289–294
Douds DD (1997) A procedure for the establishment of Glomus mosseae in dual culture with Ri T-DNA-transformed carrot roots. Mycorrhiza 7:57–61
El-Atrach F, Vierheilig H, Ocampo JA (1989) Influence of non-host plants on vesicular-arbuscular mycorrhizal infection of host plants and on spore germination. Soil Biol Biochem 21:161–163
El Gachtouli N, Paynot M, Morandi D, Gianinazzi S (1996) Effect of polyamines on endomycorrhizal infection of Pisum sativum and spore germination of Glomus mosseae. In: Azcón-Aguilar C, Barea JM (eds) Mycorrhizas in integrated Systems: from genes to plant development. European Commission, Luxembourg
Elfstrand M, Feddermann N, Ineichen K, Nagaraj VJ, Wiemken A, Boller T, Salzer P (2005) Ectopic expression of the mycorrhiza-specific chitinase gene Mtchit 3-3 in Medicago truncatula root-organ cultures stimulates spore germination of glomalean fungi. New Phytol 167:557–570
Estaun V (1989) Effect of sodium chloride and mannitol on germination and hyphal growth of the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Agric Ecosyst Environ 29:123–129
Filippi C, Bagnoli G, Citernesi AS, Giovannetti M (1998) Ultrastructural spatial distribution of bacteria associated with sporocarps of Glomus mosseae. Symbiosis 24:1–12
Fontaine J, Grandmougin FA, Hartmann MA, Sancholle M (2001a) Sterol biosynthesis by the arbuscular mycorrhizal fungus Glomus intraradices. Lipids 36:1357–1363
Fontaine J, Grandmougin FA, Sancholle M (2001b) Lipid metabolism of the endomycorrhizal fungus Glomus intraradices. CR Acad Sci III-Vie 324:847–853
Fracchia S, Mujica MT, Garcia Romera I, Garcia Garrido JM, Martin J et al (1998) Interactions between Glomus mosseae and arbuscular mycorrhizal sporocarp-associated saprophytic fungi. Plant Soil 200:131–137
Franken P, Lapopin L, MeyerGauen G, Gianinazzi-Pearson V (1997) RNA accumulation and genes expressed in spores of the arbuscular mycorrhizal fungus Gigaspora rosea. Mycologia 89:293–297
Franken P, Requena N, Bütehorn B, Krajinski F, Kuhn G, Lapopin L, Mann P, Rhody D, Stommel M (2000) Molecular analysis of the arbuscular mycorrhiza symbiosis. Arch Agric Soil Sci 45:271–286
Gachomo E, Allen JW, Pfeffer PE, Govindarajulu M, Douds DD, Jin H, Nagahashi G, Lammers PJ, Shachar-Hill Y, Bücking H (2009) Germinating spores of Glomus intraradices can use internal and exogenous nitrogen sources for de novo biosynthesis of amino acids. New Phytol 184:399-411
Gadkar V, David SR, Nagahashi G, Douds DD, Wininger S, Kapulnik Y (2003) Root exudate of pmi tomato mutant M161 reduces AM fungal proliferation in vitro. FEMS Microbiol Lett 223:193–198
Gaspar ML, Pollero RJ, Cabello MN (1994) Triacylglycerol consumption during spore germination of vesicular-arbuscular mycorrhizal fungi. J Am Oil Chem Soc 71:449–452
Gaspar ML, Pollero R, Cabello M (1997) Partial purification and characterization of a lipolytic enzyme from spores of the arbuscular mycorrhizal fungus Glomus versiforme. Mycologia 89:610–614
Gazey C, Abbott LK, Robson AD (1993) VA mycorrhizal spores from three species of Acaulospora – Germination, longevity and hyphal growth. Mycol Res 97:785–790
Gemma JN, Koske RE (1988) Seasonal variation in spore abundance and dormancy of Gigaspora gigantea and in mycorrhizal inoculum-potential of a dune soil. Mycologia 80:211–216
Gianinazzi-Pearson V, Branzanti B, Gianinazzi S (1989) In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis 7:243–255
Gildon A, Tinker PB (1981) A heavy metal tolerant strain of a mycorrhizal fungus. Trans Br Mycol Soc 77:648–649
Giovannetti M (1983) Establishment and growth effects of Glomus mosseae on the legume Hedysarum coronarium L. growing in poor alkaline soils. Soil Biol Biochem 15:385–387
Giovannetti M (2000) Spore germination and pre-symbiotic mycelia growth. In: Kapulnik Y, Douds DD Jr (eds) Arbuscular mycorrhizas: physiology and function. Kluwer, Dordrecht, The Netherlands
Giovannetti M (2002) Survival strategies in arbuscular mycorrhizal symbionts. In: Sechback J (ed) Symbiosis mechanisms and model systems. Kluwer, Dordrecht, The Netherlands
Giovannetti M, Gianinazzi-Pearson V (1994) Biodiversity in arbuscular mycorrhizal fungi. Mycol Res 98:705–715
Giovannetti M, Sbrana C (1998) Meeting a nonhost: the behaviour of arbuscular mycorrhizal symbionts. Mycorrhiza 8:123–130
Giovannetti M, Avio L, Salutini L (1991) Morphological, cytochemical, and ontogenetic characteristics of a new species of a vesicular-arbuscular mycorrhizal fungus. Can J Bot 69:161–167
Giovannetti M, Avio L, Sbrana C, Citernesi AS (1993a) Factors affecting appressorium development in the vesicular- arbuscular mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.)Gerd. & Trappe. New Phytol 123:114–122
Giovannetti M, Sbrana C, Avio L, Citernesi AS, Logi C (1993b) Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during pre-infection stages. New Phytol 125:587–594
Giovannetti M, Sbrana C, Logi C (1994) Early processes involved in host recognition by arbuscular mycorrhizal fungi. New Phytol 127:703–709
Giovannetti M, Sbrana C, Citernesi AS, Avio L (1996) Analysis of factors involved in fungal recognition responses to host derived signals by arbuscular mycorrhizal fungi. New Phytol 133:65–71
Giovannetti M, Azzolini D, Citernesi AS (1999) Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl Environ Microbiol 65:5571–5575
Giovannetti M, Sbrana C, Logi C (2000) Microchambers and video-enhanced light microscopy for monitoring cellular events in living hyphae of arbuscular mycorrhizal fungi. Plant Soil 226:153–159
Giovannetti M, Sbrana C, Strani P, Agnolucci M, Rinaudo V, Avio L (2003) Genetic diversity of isolates of Glomus mosseae from different geographic areas detected by vegetative compatibility testing and biochemical and molecular analysis. Appl Environ Microbiol 69:616–624
Glenn MG, Chew FS, Williams PH (1985) Hyphal penetration of Brassica (Cruciferae) roots by a vesicular-arbuscular mycorrhizal fungus. New Phytol 99:463–472
Godfrey RM (1957) Studies on British species of Endogone. III. Germination of spores. Trans Br Mycol Soc 40:203–210
Gorfer M, Tarkka MT, Hanif M, Pardo AG, Laitiainen ER (2001) Characterization of small GTPases Cdc42 and Rac and the relationship between Cdc42 and actin cytoskeleton in vegetative and ectomycorrhizal hyphae of Suillus bovinus. Mol Plant Microbe Interact 14:135–144
Goto BT, Maia LC, Oehl F (2008) Ambispora brasiliensis, a new ornamented species in the arbuscular mycorrhiza-forming Glomeromycetes. Mycotaxon 105:11–18
Graham JH (1982) Effect of citrus exudates on germination of chlamydospores of the vesicular-arbuscular mycorrhizal fungus, Glomus epigaeum. Mycologia 74:831–835
Green NE, Graham JH, Schenck NC (1976) The influence of pH on the germination of vesicular-arbuscular mycorrhizal spores. Mycologia 68:929–934
Gryndler M, Hrselova H, Striteska D (2000) Effect of soil bacteria on hyphal growth of the arbuscular mycorrhizal fungus Glomus claroideum. Folia Microbiol 45:545–551
Gutjahr C, Novero M, Guether M, Montanari O, Udvardi M, Bonfante P (2009) Presymbiotic factors released by the arbuscular mycorrhizal fungus Gigaspora margarita induce starch accumulation in Lotus japonicus roots. New Phytol 183:53–61
Hepper CM, Smith GA (1976) Observation on the germination of Endogone spores. Trans Br Mycol Soc 66:189–194
Hepper CM (1979) Germination and growth of Glomus caledonium spores: the effects of inhibitors and nutrients. Soil Biol Biochem 11:269–277
Hepper CM (1983) Effect of phosphate on germination and growth of vesicular-arbuscular mycorrhizal fungi. Trans Br Mycol Soc 80:487–490
Hepper CM (1984a) Inorganic sulphur nutrition of the vesicular-arbuscular mycorrhizal fungus Glomus caledonium. Soil Biol Biochem 16:669–671
Hepper CM (1984b) Regulation of spore germination of the vesicular-arbuscular mycorrhizal fungus Acaulospora laevis by soil pH. Trans Br Mycol Soc 83:154–156
Hepper CM, Jakobsen I (1983) Hyphal growth from spores of the mycorrhizal fungus Glomus caledonius: effect of amino acids. Soil Biol Biochem 15:55–58
Hepper CM, Sen R, Maskall CS (1986) Identification of vesicular-arbuscular mycorrhizal fungi in roots of leek (Allium porrum L.) and maize (Zea mays L.) on the basis of enzyme mobility during polyacrylamide gel electrophoresis. New Phytol 102:529–539
Hildebrandt U, Janetta K, Bothe H (2002) Towards growth of arbuscular mycorrhizal fungi independent of a plant host. Appl Environ Microbiol 68:1919–1924
Hildebrandt U, Ouziad F, Marner FJ, Bothe H (2006) The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett 254:258–267
Hirrel MC (1981) The effect of sodium and chloride salts on the germination of Gigaspora margarita. Mycologia 73:610–617
Juge C, Samson J, Bastien C, Vierheilig H, Coughlan A, Piche Y (2002) Breaking dormancy in spores of the arbuscular mycorrhizal fungus Glomus intraradices: a critical cold-storage period. Mycorrhiza 12:37–42
Jun J, Abubaker J, Rehrer C, Pfeffer PE, Shachar-Hill Y, Lammers PJ (2002) Expression in an arbuscular mycorrhizal fungus of genes putatively involved in metabolism, transport, the cytoskeleton and the cell cycle. Plant Soil 244:141–148
Juniper S, Abbott LK (1993) Vesicular-arbuscular mycorrhizas and soil salinity. Mycorrhiza 4:45–57
Juniper S, Abbott LK (2006) Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16:371–379
Koske R, Bonin C, Kelly J, Martinez C (1996) Effects of sea water on spore germination of a sand-dune-inhabiting arbuscular mycorrhizal fungus. Mycologia 88:947–950
Koske RE (1981a) Gigaspora gigantea: observations on spore germination of a VA-mycorrhizal fungus. Mycologia 73:288–300
Koske RE (1981b) Multiple germination by spores of Gigaspora gigantea. Trans Br Mycol Soc 76:328–330
Kosuta S, Chabaud M, Lougnon G, Gough C, Denarie J, Barker DG, Becard G (2003) A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol 131:952–962
Kosuta S, Hazledine S, Sun J, Miwa H, Morris RJ, Downie JA, Oldroyd GE (2008) Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc Natl Acad Sci USA 105:9823–9828
Kuwada K, Kuramoto M, Utamura M, Matsushita I, Ishii T (2006) Isolation and structural elucidation of a growth stimulant for arbuscular mycorrhizal fungus from Laminaria japonica Areschoug. J Appl Phycol 18:795–800
Lammers PJ, Jun J, Abubaker J, Arreola R, Gopalan A, Bago B, Hernandez Sebastia C, Allen JW, Douds DD, Pfeffer PE, Shachar-Hill Y (2001) The glyoxylate cycle in an arbuscular mycorrhizal fungus. Carbon flux and gene expression. Plant Physiol 127:1287–1298
Levy A, Chang BJ, Abbott LK, Kuo J, Harnett G, Inglis TJJ (2003) Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl Environ Microbiol 69:6250–6256
Logi C, Sbrana C, Giovannetti M (1998) Cellular events involved in survival of individual arbuscular mycorrhizal symbionts growing in the absence of the host. Appl Environ Microbiol 64:3473–3479
Louis I, Lim G (1988) Effect of storage of inoculum on spore germination of a tropical isolate of Glomus clarum. Mycologia 80:157–161
Lumini E, Bianciotto V, Jargeat P, Novero M, Salvioli A, Faccio A, Becard G, Bonfante P (2007) Presymbiotic growth and sporal morphology are affected in the arbuscular mycorrhizal fungus Gigaspora margarita cured of its endobacteria. Cell Microbiol 9:1716–1729
Macdonald RM, Lewis M (1978) Occurrence of some acid-phosphatases and dehydrogenases in vesicular-arbuscular mycorrhizal fungus Glomus mosseae. New Phytol 80:135–141
Maia LC, Kimbrough JW (1998) Ultrastructural studies of spores and hypha of a Glomus species. Int J Plant Sci 159:581–589
Mayo K, Davis RE, Motta J (1986) Stimulation of germination of spores of Glomus versiforme by spore-associated bacteria. Mycologia 78:426–431
McMillen BG, Juniper S, Abbott LK (1998) Inhibition of hyphal growth of a vesicular-arbuscular mycorrhizal fungus in soil containing sodium chloride limits the spread of infection from spores. Soil Biol Biochem 30:1639–1646
Meier R, Charvat I (1992) Germination of Glomus mosseae spores: procedure and ultrastructural analysis. Int J Plant Sci 153:541–549
Morton JB, Bentivenga SP, Wheeler WW (1993) Germplasm in the International collection of arbuscular and vesicular-arbuscular mycorrhizal fungi (INVAM) and procedures for culture development, documentation and storage. Mycotaxon 48:491–528
Mosse B (1959) The regular germination of resting spores and some observations on the growth requirements of an Endogone sp. causing vesicular- arbuscular mycorrhiza. Trans Br Mycol Soc 42:273–286
Mosse B (1970a) Honey-coloured sessile Endogone spores. I. Life history. Arch Microbiol 70:167–175
Mosse B (1970b) Honey-coloured sessile Endogone spores. II. Changes in fine structure during spore development. Arch Microbiol 74:129–145
Mosse B, Hepper CM (1975) Vesicular-arbuscular mycorrhizal infections in root organ cultures. Physiol Plant Pathol 5:215–223
Msiska Z, Morton JB (2009) Phylogenetic analysis of the Glomeromycota by partial beta-tubulin gene sequences. Mycorrhiza 19:247–254
Mugnier J, Mosse B (1987) Spore germination and viability of a vesicular arbuscular mycorrhizal fungus, Glomus mosseae. Trans Br Mycol Soc 88:411–413
Nagahashi G, Douds DD (2000) Partial separation of root exudate components and their effects upon the growth of germinated spores of AM fungi. Mycol Res 104(Part 12):1453–1464
Nair MG, Safir GR, Siqueira JO (1991) Isolation and identification of vesicular-arbuscular mycorrhiza- stimulatory compounds from clover (Trifolium repens) roots. Appl Environ Microbiol 57:434–439
Navazio L, Moscatiello R, Genre A, Novero M, Baldan B, Bonfante P, Mariani P (2007) A diffusible signal from arbuscular mycorrhizal fungi elicits a transient cytosolic calcium elevation in host plant cells. Plant Physiol 144:673–681
Nemec S (1987) Effect of storage temperature and moisture on Glomus species and their subsequent effect on Citrus rootstock seedling growth and mycorrhiza development. Trans Br Mycol Soc 89:205–212
Nicolson TH, Schenck NC (1979) Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71:178–198
Oba H, Tawaraya K, Wagatsuma T (2002) Inhibition of pre-symbiotic hyphal growth of arbuscular mycorrhizal fungus Gigaspora margarita by root exudates of Lupinus spp. Soil Sci Plant Nutr 48:117–120
Ocampo JA, Martin J, Hayman DS (1980) Influence of plant interactions on vesicular-arbuscular mycorrhizal infection.I.Host and non-host plants grown together. New Phytol 84:23–25
Oehl F, de Souza FA, Sieverding E (2008) Revision of Scutellospora and description of five new genera and three new families in the arbuscular mycorrhiza-forming Glomeromycetes. Mycotaxon 106:311–360
Olah B, Briere C, Becard G, Denarie J, Gough C (2005) Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J 44:195
Parra-Garcia MD, Lo Giudice V, Ocampo JA (1992) Absence of VA colonization in Oxalis pes-caprae inoculated with Glomus mosseae. Plant Soil 145:298–300
Phipps CJ, Taylor TN (1996) Mixed arbuscular mycorrhizae from the Triassic of Antarctica. Mycologia 88:707–714
Pivato B, Offre P, Marchelli S, Barbonaglia B, Mougel C, Lemanceau P, Berta G (2009) Bacterial effects on arbuscular mycorrhizal fungi and mycorrhiza development as influenced by the bacteria, fungi, and host plant. Mycorrhiza 19:81–90
Porcel R, Aroca R, Cano C, Bago A, Ruiz-Lozano JM (2006) Identification of a gene from the arbuscular mycorrhizal fungus Glomus intraradices encoding for a 14-3-3 protein that is up-regulated by drought stress during the AM symbiosis. Microb Ecol 52:575–582
Powell CL (1976) Development of mycorrhizal infections from Endogone spores and infected root fragments. Trans Br Mycol Soc 66:439–445
Ramos AC, Facanha AR, Feijo JA (2008) Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 178:177–188
Rani K, Zwanenburg B, Sugimoto Y, Yoneyama K, Bouwmeester HJ (2008) Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol Biochem 46:617–626
Redecker D, Kodner R, Graham LE (2000a) Glomalean fungi from the Ordovician. Science 289:1920–1921
Redecker D, Morton JB, Bruns TD (2000b) Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol Phylogenet Evol 14:276–284
Remy W, Taylor TN, Hass H, Kerp H (1994) Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA 91:11841–11843
Requena N, Fuller P, Franken P (1999) Molecular characterization of GmFOX2, an evolutionarily highly conserved gene from the mycorrhizal fungus Glomus mosseae, down-regulated during interaction with rhizobacteria. Mol Plant Microbe Interact 12:934–942
Requena N, Mann P, Franken P (2000) A homologue of the cell cycle check point TOR2 from Saccharomyces cerevisiae exists in the arbuscular mycorrrhizal fungus Glomus mosseae. Protoplasma 212:89–98
Requena N, Mann P, Hampp R, Franken P (2002) Early developmentally regulated genes in the arbuscular mycorrhizal fungus Glomus mosseae: identification of GmGIN1, a novel gene with homology to the C-terminus of metazoan hedgehog proteins. Plant Soil 244:129–139
Requena N, Breuninger M, Franken P, Ocon A (2003) Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiol 132:1540–1549
Roberts KJ, Anderson RC (2001) Effect of garlic mustard [Alliaria petiolata (Beib. Cavara and Grande)] extracts on plants and arbuscular mycorrhizal (AM) fungi. Am Midl Nat 146:146–152
Roesti D, Ineichen K, Braissant O, Redecker D, Wiemken A, Aragno M (2005) Bacteria associated with spores of the arbuscular mycorrhizal fungi Glomus geosporum and Glomus constrictum. Appl Environ Microbiol 71:6673–6679
Saito M (1995) Enzyme activities of the internal hyphae and germinated spores of an arbuscular mycorrhizal fungus, Gigaspora margarita Becker & Hall. New Phytol 129:425–431
Salzer P, Bonanomi A, Beyer K, Vogeli LR, Aeschbacher RA, Lange J, Wiemken A, Kim D, Cook DR, Boller T (2000) Differential expression of eight chitinase genes in Medicago truncatula roots during mycorrhiza formation, nodulation, and pathogen infection. Mol Plant Microbe Interact 13:763–777
Samra A, Dumas-Gaudot E, Gianinazzi-Pearson V, Gianinazzi S (1996) Soluble proteins and polypeptide profiles of spores of arbuscular mycorrhizal fungi. Interspecific variability and effects of host (myc(+)) and non-host (myc(−)) – Pisum sativum root exudates. Agronomie 16:709–719
Sannazzaro AI, Alvarez CL, Menendez AB, Pieckenstain FL, Alberto EO, Ruiz OA (2004) Ornithine and arginine decarboxylase activities and effect of some polyamine biosynthesis inhibitors on Gigaspora rosea germinating spores. FEMS Microbiol Lett 230:115–121
Scervino JM, Ponce MA, Erra-Bassells R, Vierheilig H, Ocampo JA, Godeas A (2005a) Flavonoids exclusively present in mycorrhizal roots of white clover exhibit a different effect on arbuscular mycorrhizal fungi than flavonoids exclusively present in non-mycorrhizal roots of white clover. J Plant Interact 1:15–22
Scervino JM, Ponce MA, Erra-Bassells R, Vierheilig H, Ocampo JA, Godeas A (2005b) Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of Gigaspora and Glomus. Mycol Res 109:789–794
Scervino JM, Ponce MA, Erra-Bassells R, Bompadre MJ, Vierheilig H, Ocampo JA, Godeas A (2006) Glycosidation of apigenin results in a loss of its activity on different growth parameters of arbuscular mycorrhizal fungi from the genus Glomus and Gigaspora. Soil Biol Biochem 38:2919–2922
Scervino JM, Sampedro I, Ponce MA, Rodriguez MA, Ocampo JA, Godeas A (2008) Rhodotorulic acid enhances root colonization of tomato plants by arbuscular mycorrhizal (AM) fungi due to its stimulatory effect on the pre-symbiotic stages of the AM fungi. Soil Biol Biochem 40:2474–2476
Scervino JM, Gottlieb A, Silvani VA, Pergola M, Fernandez L, Godeas AM (2009) Exudates of dark septate endophyte (DSE) modulate the development of the arbuscular mycorrhizal fungus (AMF) Gigaspora rosea. Soil Biol Biochem 41:1753–1756
Schenck NC, Graham SO, Green NE (1975) Temperature and light effect on contamination and spore germination of vesicular-arbuscular mycorrhizal fungi. Mycologia 67:1189–1192
Schreiner RP, Koide RT (1993a) Mustards, mustard oils and mycorrhizas. New Phytol 123:107–113
Schreiner RP, Koide RT (1993b) Stimulation of vesicular-arbuscular mycorrhizal fungi by mycotrophic and nonmycotrophic plant root systems. Appl Environ Microbiol 59:2750–2752
Sieverding E (1991) Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Deutsche Gesellschaft Technische Zusammenarbeit GmbH, Eschborn
Simon L, Bousquet J, Levesque RC, Lalonde M (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363:67–69
Siqueira JO, Hubbell DH, Schenck NC (1982) Spore germination and germ tube growth of a vesicular-arbuscular mycorrhizal fungus in vitro. Mycologia 74:952–959
Siqueira JO, Hubbell DH, Mahmud AW (1984) Effect of liming on spore germination, germ tube growth and root colonization by vesicular-arbuscular mycorrhizal fungi. Plant Soil 76:115–124
Spain JL (1992) Patency of shields in water mounted spores of 4 species in Acaulosporaceae (Glomales). Mycotaxon 43:331–339
Spain JL (2003) Emendation of Archaeospora and of its type species, Archaeospora trappei. Mycotaxon 87:109–112
Spain JL, Sieverding E, Oehl F (2006) Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97:163–182
Stommel M, Mann P, Franken P (2001) EST-library construction using spore RNA of the arbuscular mycorrhizal fungus Gigaspora rosea. Mycorrhiza 10:281–285
Suriyapperuma SP, Koske RE (1995) Attraction of germ tubes and germination of spores of the arbuscular mycorrhizal fungus gigaspora gigantea in the presence of roots of maize exposed to different concentrations of phosphorus. Mycologia 87:772–778
Sward RJ (1981a) The structure of the spores of Gigaspora margarita. I. The dormant spore. New Phytol 87:761–768
Sward RJ (1981b) The structure of the spores of Gigaspora margarita. II. Changes accompanying germination. New Phytol 88:661–666
Sward RJ (1981c) The structure of the spores of Gigaspora margarita. III. Germ tube emergence and growth. New Phytol 88:667–673
Sylvia DM, Schenck NC (1983) Germination of chlamidospores of three Glomus species as affected by soil matric potential and fungal contamination. Mycologia 75:30–35
Sylvia D, Williams SE (1992) Vesicular-arbuscular mycorrhizae and environmental stresses. In: Bethlenfalvay GJ, Linderman RG (eds) Mycorrhizae in sustainable agriculture. Agronomy Society of America, Madison, WI
Tamasloukht M, Sejalon DN, Kluever A, Jauneau A, Roux C, Becard G, Franken P (2003) Root factors induce mitochondrial-related gene expression and fungal respiration during the developmental switch from asymbiosis to presymbiosis in the arbuscular mycorrhizal fungus Gigaspora rosea. Plant Physiol 131:1468–1478
Tamasloukht M, Waschke A, Franken P (2007) Root exudate-stimulated RNA accumulation in the arbuscular mycorrhizal fungus Gigaspora rosea. Soil Biol Biochem 39:1824–1827
Tawaraya K, Saito M, Morioka M, Wagatsuma T (1996a) Effect of concentration of phosphate on spore germination and hyphal growth of arbuscular mycorrhizal fungus, Gigaspora margarita Becker & Hall. Soil Sci Plant Nutr 42:667–671
Tawaraya K, Watanabe S, Yoshida E, Wagatsuma T (1996b) Effect of onion (Allium cepa) root exudates on the hyphal growth of Gigaspora margarita. Mycorrhiza 6:57–59
Tommerup IC (1983a) Spore dormancy in vesicular-arbuscular mycorrhizal fungi. Trans Br Mycol Soc 81:37–45
Tommerup IC (1983b) Temperature relations of spore germination and hyphal growth of vesicular-arbuscular mycorrhizal fungi in soil. Trans Br Mycol Soc 81:381–387
Tommerup IC (1988) The vesicular-arbuscular mycorrhizas. Adv Plant Pathol 6:81–91
Tommerup IC, Kidby DK (1980) Production of aseptic spores of vesicular-arbuscular endophytes and their viability after chemical and physical stress. Appl Environ Microbiol 39:1111–1119
Trépanier M, Bécard G, Moutoglis P, Willemot C, Gagne S, Avis TJ, Rioux JA (2005) Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl Environ Microbiol 71:5341–5347
Tsai SM, Phillips DA (1991) Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro. Appl Environ Microbiol 57:1485–1488
Turrini A, Sbrana C, Nuti MP, Pietrangeli BM, Giovannetti M (2004a) Development of a model system to assess the impact of genetically modified corn and aubergine plants on arbuscular mycorrhizal fungi. Plant Soil 266:69–75
Turrini A, Sbrana C, Pitto L, Castiglione MR, Giorgetti L, Briganti R, Bracci T, Evangelista M, Nuti MP, Giovannetti M (2004b) The antifungal Dm-AMP1 protein from Dahlia merckii expressed in Solanum melongena is released in root exudates and differentially affects pathogenic fungi and mycorrhizal symbiosis. New Phytol 163:393–403
Turrini A, Avio L, Bedini S, Giovannetti M (2008) In situ collection of endangered arbuscular mychorrhizal fungi in a Mediterranean UNESCO Biosphere Reserve. Biodivers Conserv 17:643–657
Tylka GL, Hussey RS, Roncadori RW (1991) Axenic germination of vesicular-arbuscular mycorrhizal fungi: effects of selected Streptomyces species. Phytopathology 81:754–759
Vierheilig H, Bennett R, Kiddle G, Kaldorf M, Ludwig MJ (2000) Differences in glucosinolate patterns and arbuscular mycorrhizal status of glucosinolate-containing plant species. New Phytol 146:343–352
Walker C, Sanders FE (1986) Taxonomic concepts in the Endogonaceae: III. The separation of Scutellospora gen. nov. from Gigaspora Gerd. & Trappe. Mycotaxon 27:169–182
Walker C, Giovannetti M, Avio L, Citernesi AS, Nicolson TH (1995) A new fungal species forming arbuscular mycorrhizas: Glomus viscosum. Mycol Res 99:1500–1506
Walker C, Blaszkowski J, Schwarzott D, Schussler A (2004) Gerdemannia gen. nov., a genus separated from Glomus, and Gerdemanniaceae fam. nov., a new family in the Glomeromycota. Mycol Res 108:707–718
Walley FL, Germida JJ (1996) Failure to decontaminate Glomus clarum NT4 spores is due to spore wall-associated bacteria. Mycorrhiza 6:43–49
Walters DR (1995) Inhibition of polyamine biosynthesis in fungi. Mycol Res 99:129–139
Watrud LS, Heithaus JJ, Jaworski EG (1978) Evidence for production of inhibitor by vesicular arbuscular mycorrhizal fungus Gigaspora margarita. Mycologia 70:821–828
Weidmann S, Sanchez L, Descombin J, Chatagnier O, Gianinazzi S, Gianinazzi Pearson V (2004) Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 gene in Medicago truncatula. Mol Plant Microbe Interact 17:1385–1393
Weinzierl G, Leveleki L, Hassel A, Kost G, Wanner G, Bolker M (2002) Regulation of cell separation in the dimorphic fungus Ustilago maydis. Mol Microbiol 45:219–231
Weissenhorn I, Leyval C, Berthelin J (1993) Cd-tolerant arbuscular mycorrhizal (AM) fungi from heavy- metal polluted soils. Plant Soil 157:247–256
Will ME, Sylvia DM (1990) Interaction of rhizosphere bacteria, fertilizer, and vesicular-arbuscular mycorrhizal fungi with sea oats. Appl Environ Microbiol 56:2073–2079
Zocco D, Fontaine J, Lozanova E, Renard L, Bivort C et al (2008) Effects of two sterol biosynthesis inhibitor fungicides (fenpropimorph and fenhexamid) on the development of an arbuscular mycorrhizal fungus. Mycol Res 112:592–601
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Giovannetti, M., Avio, L., Sbrana, C. (2010). Fungal Spore Germination and Pre-symbiotic Mycelial Growth – Physiological and Genetic Aspects. In: Koltai, H., Kapulnik, Y. (eds) Arbuscular Mycorrhizas: Physiology and Function. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9489-6_1
Download citation
DOI: https://doi.org/10.1007/978-90-481-9489-6_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9488-9
Online ISBN: 978-90-481-9489-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)