Abstract
Infrared thermography is highly suitable for the detection of disease-induced changes in plant transpiration and water status. Depending on the host-pathogen system diseases can be detected at various stages of development. Pathogens attacking plant roots or colonizing the vascular system affect water uptake and translocation within the plant and cause a decrease in transpiration associated with an increase in leaf temperature . Diseases causing early malfunction of stomatal regulation produce pre-symptomatic modifications in transpiration, some affect cuticular transpiration when visible symptoms appear or only in later stages when tissue is severely damaged. Diseases without or with only minor effects on transpiration cannot be detected thermographically. In some host-pathogen systems a close relationship between disease severity and thermal effect exist which may be used for disease quantification. The low specificity of the signal limits the use of thermography for disease identification, however, this may be compensated by the use of patterns of leaf temperature. IR remote sensing has a large potential in disease forecasting and the definition of management zones because of its high sensitivity to changes in plant water relationships.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
With shrinking resources for arable land and water, the optimization of crop productivity by early detection of biotic (and abiotic) stress factors and the remediation of these perturbations by effective disease control become more and more important. Technical sensors for non-destructive detection, identification, quantification, and monitoring of biotic plant stress(ors), therefore, are highly needed.
The visual assessment of plant diseases is often tedious and expensive and is fraught with variations among assessors. Technical sensors for non-destructive remote sensing are more objective and should improve accuracy as well as sensitivity of disease assessment as sensors are not limited to the visible range of the electromagnetic spectrum. Non-destructive, pre-visual detection and quantification of diseases can contribute to facilitate and limit the timely application of appropriate control activities to the specific sites where needed.
Digital infrared thermography permits remote recording of plant surface temperature as affected by disease development on different scales – leaf, shoot, crop canopy, field, and region – without interfering with plants. Imaging thermography is able to produce spatial and temporal patterns of plant temperature. Its – potential – use in plant pathology and crop protection is summarized.
2 Temperature of Plants
The temperature of plants largely depends on temperature of the environment; only a few thermogenic species – members of the Araceae, such as Philodendron selloum , Symplocarpus foetidus and Dracunculus vulgaris and non-aroids such as Nelumbo nucifera – are able to actively increase tissue temperature well above air temperature by a mitochondrial respiratory pathway that is distinct from the cytochrome chain (Wagner et al. 2008); volatile compounds are released by the inflorescences in order to attract insects for pollination. In all other plants, the metabolic activity is described to have no effect on leaf temperature (Chaerle and Van der Straeten 2000) and transpiration reduces tissue temperature below air temperature.
Transpiration rate has been shown to be negatively correlated to leaf temperature (Inoue et al. 1990) physically linked to its stomatal resistance (= 1/stomatal conductance ). Leaf temperature results from the incoming irradiation, the water status of the plant and the functionality of the epidermal layer (cuticle and stomata) to regulate the transpiration of leaves, as well as from environmental conditions like air temperature, relative humidity (RH) and wind speed (Jones 1992). Unintentional transpiration is prevented mainly by the coverage of leaf surfaces with the cuticle which is an effective barrier against water loss (Schönherr 1982) whereas the water status of the shoot tissue determines the temperature of plants via stomatal transpiration. Leaf temperature increases as transpiration rate decreases. There is a correlation between leaf temperature and water status (Farquhar and Sharkey 1982, Cohen et al. 2005, Jones and Schofield 2008).
Leaf temperature is a highly sensitive indicator of stomatal aperture as latent heat loss is a large component of the overall leaf energy balance that determines leaf temperature (Jones 1992). The transition of liquid water into water vapor requires a high amount of energy because of the high latent heat of vaporization of water – the energy required for the vaporization of 1 mg H2O is able to cool 600 mg H2O by 1 Kelvin (K). Transpiration at the cell walls below stomata, therefore, is accompanied by a significant cooling of the plant tissue and the surface. As leaf temperature is directly related to the rate of evapotranspiration from the canopy surface, infrared sensing of the canopy temperature may be used to monitor the transpiration rate of plants (Jones 1999, Jones et al. 2002, Merlot et al. 2002). Digital infrared thermography allows the quantitative analysis of spatial and dynamic physiological information on the plant status at the canopy and leaf level without interfering with plants (Jones 2004). In plant sciences, the method has been applied to study the relationship between stomatal conductivity and leaf temperature, to visualize temperature stress of plants or plant parts, the drying of fruits and for yield estimations as well as for scheduling irrigation.
Control of transpirational water loss through stomata on plant leaves is an important mechanism for maintaining leaf surface temperature. Stomatal transpiration accounts for 94–99.7 % of the total gas exchange of leaves under normal conditions (Körner 1994). The transpiration rate of astomatous cuticles isolated from the adaxial leaf surface of Prunus laurocerasus was two to three orders of magnitude lower than that of the abaxial leaf side (Schreiber and Riederer 1996). Furthermore, the cuticular permeance of astomatous and stomatous leaf surfaces has been reported to differ by a factor of 11 in Hedera helix, indicating pronounced differences in barrier properties between cuticles (Santrucek et al. 2004).
In addition to the abiotic environment, pathogenic organisms may affect both cuticular and stomatal conductance of plant tissue resulting in significant modifications of leaf temperature (Ayres and Jones 1975, Smith et al. 1986, Wright et al. 2000, Chaerle et al. 2001, Bassanezi et al. 2002). Depending on the site of attack – roots, vascular system, photosynthetic active tissue – water relations of crops are affected directly or indirectly, localized to (some parts of) organs or the whole plant. Perturbations of transpiration may be used as cues for the development of plant diseases affecting stomatal aperture and functionality of cuticle integrity.
3 Principles of Infrared Thermography and Instrumentation
All objects above 0 K emit electromagnetic radiation, especially infrared (IR) radiation allowing the measurement of their surface temperature. IR radiation is part of the electromagnetic spectrum and spans a range – 0.75–1,000 μm – between the visible light and radio waves. It may be divided into several bands of interest to disease detection: NIR (near infrared , IR-A) 0.75–1.4 μm; SWIR (short-wavelength infrared , IR-B) 1.4–3 μm; MWIR (mid-wavelength infrared , IR-C) 3–8 μm (3–5 μm, atmospheric window used by ‘heat-seeking’ missiles); LWIR (long-wavelength infrared , IR-C) 8–15 μm (thermal infrared TIR); FIR (far infrared) 15–1,000 μm (according to International Commission on Illumination).
According to Planck’s Law and Wien’s displacement Law the wavelength at which the maximum amount of energy is emitted increases from hot to colder objects (Fig. 11.1). Sensors for biological samples in their natural environment, therefore, should have a maximum sensitivity in the range from 9.5 to 10 μm. Stephan Boltzmann Law allows calculation of the object’s temperature from its total radiated energy. Atmospheric attenuation of radiation caused by water vapor and other gases strongly depends on the wavelength; for thermography the mid-wave window (3–5 μm) and the long-wave window (8–14 μm) may be used.
Emissivity is the ability of a material to emit or absorb thermal radiation; it ranges from 0.0 – no emission − to 1.0 – complete emission (of a Black Body). Infrared radiation of real-world objects tends to be less than the actual temperature; the rate between infrared radiation and contact temperature of an object is its emissivity. For plant physiological experiments emissivity of plant tissue is often set to fixed values from 0.95 to 1. However, differing emissivities of various plant tissues and reflections from other surfaces – like the soil – interferes with IR measurements.
Non-contact thermometers (pyrometer , radiothermometer ) have been applied in agricultural sciences since the 1980s. Their use is limited as they provided no spatial information and comparative measurements are affected by transient changes in environmental conditions. Originally developed for military use – forward looking infrared (FLIR) imaging technology –, thermographic cameras (thermal imaging = thermography) have a wide area of application ranging from firefighting, assessment of thermal insulation of buildings to industrial applications like online quality control.
Thermographic cameras detect electromagnetic radiation in the range of 3–15 μm and produce images of that radiation. Thermal energy is emitted by all objects based on their temperatures. As the amount of radiation increases with temperature variations in temperature in space and time may be detected. Thermal sensors are specialized focal plane arrays (FPAs) and non-cooled microbolometers. FPAs with low thermal resolution require cryogenic cooling by liquid nitrogen or a miniature Sterling cycle refrigerator.
The imaging system is a scanner – a single detector in combination of rotating mirrors or oscillating refractive elements which scan the field of view (FOV) in horizontal and vertical directions – or a focal plane array – a matrix of detectors to resolve the FOV (Meola and Carlomagno 2004). The detectors are photon detectors requiring cooling by liquid nitrogen or Stirling coolers for rapid scanning, high sensitivity and low noise. Infrared systems may use the mid-wave (MW, 3–5 μm) and the long-wave (LW, 8–12 μm) IR range with mercury cadmium telluride (HgCdTe) photon detectors being used for both ranges (Table 11.1). Sensors may be used on handheld systems, ground-based equipment or may be airborne.
The performance of an infrared camera is expressed in terms of thermal sensitivity, scan speed, image resolution and intensity resolution. Thermal sensitivity – expressed as noise equivalent temperature difference (NETD) – is typically 80–200 mK for uncooled detectors and approaches 10 mK for cooled photon detectors. The rate at which an image is acquired may be higher than 1,600 Hz for new systems. Pixel resolution may reach 15 μm in microscope applications and the number of pixels per image is often 640 × 512. Modern systems provide 14-bit recording for a broad dynamic range (= intensity resolution). New detector systems may be also used for spectral analysis of radiation resulting in multispectral IR signatures.
In passive thermography the object is measured in its environment without any additional influence; in active thermography, an additional energy source is used and the thermal response of the object to this energy is recorded.
4 Detection of Disease Symptoms
4.1 Use of Radiometers in the Field
Early reports on the use of hand-held IR thermometers for quantifying the effect of root and vascular diseases on field crops have been summarized by Nilsson (1995). He pointed out that the radiometric response of crops to diseases may be divided into two groups: (I) a modification of the plant–water relationship, and (II) the expression of symptoms of senescence. Various diseases caused by bacteria, fungi, oomycetes, and nematodes affect the water supply of crops and result in an increase of canopy temperature, often in the range from 1 to 4 K, however, sometimes exceeding 10 K above air temperature (Pinter et al. 1979, Nicolas et al. 1991, Nilsson 1991). In contrast, stripe rust caused by P. striiformis reduced leaf temperature of wheat in early disease stages by 0.2–1.0 K (Smith et al. 1986).
The results obtained for several pathosystems made it difficult to quantify an attack in a reliable way, but led locally to good relationships between remote sensing data and indicators of the severity of the attack (Lili et al. 1991, Duchesne et al. 1992).
4.2 Infrared Imaging
As radiothermometers provide no spatial information, their use is limited because of influences of environmental conditions like sunlight, wind, soil, etc. (West et al. 2003). The development of thermal imaging systems, although more expensive, has increased considerably the potential of IR thermography in plant stress detection. The investigations have focused on the – sometimes pre-symptomatic – detection of diseases caused by viruses, bacteria, oomycetes and fungi infecting leaves. Imaging systems allow, however, the assessment of spatial heterogeneities on various scales, from the leaf level to canopies and landscapes.
4.2.1 Leaf Level
Localized changes in leaf temperature have been used as indicator of several biotic stresses (Chaerle and Van der Straeten 2000, Chaerle et al. 2001, 2006). Hypersensitive reaction of tobacco to tobacco mosaic virus (TMV) infection was preceded by a local, rapidly expanding increase in tissue temperature because of the accumulation of salicylic acid, a pivotal compound in plant resistance to pathogens, also known for its stomatal closing activity (Chaerle et al. 1999). During establishment of hypersensitive response of Nicotiana sylvestris to Erwinia amylovora 3–4 h after harpin-infiltration, tissue temperature decreased associated to stomatal opening. The marked drop in temperature reached 2 K and preceded necrotic symptoms for several hours (Boccara et al. 2001). Toxins released by pathogens are also described to alter stomatal behavior (Chaerle and Van der Straeten 2001); they may also cause tissue degradation associated to a localized decrease in tissue temperature in early stages of Cercospora leaf spot of sugar beet (Chaerle et al. 2004).
The oomycete Pseudoperonospora cubensis causing downy mildew of cucumber and fungi of the genus Phyllosticta acting on two tree species caused an increase in overall leaf temperature at early stages of infection (Lindenthal et al. 2005, Aldea et al. 2006, Oerke et al. 2006). Leaf spot due to Phyllosticta sp. increased temperature of surrounding leaf tissue of Quercus velutina, in contrast to a cynipid gall wasp which resulted in a spatially very limited decrease of temperature (Aldea et al. 2006). Transient decreases in temperature of infected leaf areas due to the evaporation of leaf water resulting from damage of plant cuticle or degradation of cells have been also described for downy mildew of cucumber, late stages of TMV infection and apple scab (Chaerle et al. 1999, Lindenthal 2005, Oerke et al. 2005).
In greenhouse experiments Plasmopara viticola caused a pre-symptomatic increase in leaf temperature at the site of infection in irrigated grapevine, whereas drought-stressed plants showed a localized drop in temperature 2–3 days before typical symptoms of downy mildew appeared (Stoll et al. 2008b). Spatial and temporal analysis of leaf temperature improved the differentiation between healthy and infected leaves irrespective of their water status (Stoll et al. 2008a).
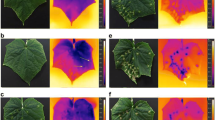
Single mature rust colonies may reduce temperature by up to 0.7 K compared to the non-diseased surrounding tissue (Lenthe 2005). The cool spots have a clear temperature gradient with the center displaying the lowest temperature. This thermal signal can be detected only when cuticular transpiration is largely uncontrollable because of the cuticle perforation by the urediniospores (Fig. 11.2). Healthy and diseased plants may differ also in the pattern of leaf temperature. Bean rust due to Uromyces phaseoli caused a greater heterogeneity in small-scale temperature variability in later stages of the disease (Lenthe 2005). Dense colonies of powdery mildew, in contrast, were associated with a temperature only slightly lower (0.2 K) than healthy tissue. In later stages, powdery mildew tends to slightly increase tissue temperature because of reduced water potential of diseased leaf areas (Fig. 11.3). These results also reflect the subtle way biotroph pathogens maintain the functionality of host tissue they are living from.
The fungus Venturia inaequalis causes scab of apple, a hypostomatous plant species. Despite of the limitation of this pathogen to subcuticular colonization of leaf tissue, scab symptoms on one leaf side significantly reduced temperature of both, adaxial and abaxial leaf surface. Scab lesions are associated with a localized increase in intercellular free water which results in a significant increase of stomatal conductivity and a spatially limited cooling effect for the complete leaf profile. This effect stresses the correlation between leaf temperature and water status – water is the primary source of infrared absorption in plant tissue (Kümmerlen et al. 1999).
Root diseases due to bacteria, oomycetes, fungi or nematodes may be detected by an increase in shoot temperature as all these pests affect water uptake and transport, ultimately reducing transpiration of shoots (Jones 2004, Schmitz et al. 2004). Mechanical wounding and tissue loss to chewing arthropods feeding on crops may result in a short temperature increase, followed by a localized decrease due to water loss from damaged cells, that progressively disappears again upon wound healing (Chaerle et al. 2002, Aldea et al. 2006).
The leaf area assessed thermographically for disease may be larger than that with visible symptoms. The leaf area affected by fungal colonization and its effect on host plant physiology – transpiration – often exceed the size of the visible damage. For other diseases like rusts thermographic anomalies linked to the impairment of cuticle function become only detectable after the appearance of symptoms. As powdery mildews seem to have only a very limited effect on stomatal transpiration and almost no effect on cuticular transpiration, thermography proved to be rather insensitive for this type of disease (see Table 11.2).
An effect of disease stage on leaf temperature – decrease for colonized, but living tissue, temperature rise (above the level of non-diseased tissue) for necrotic tissue – has been reported for various host-pathogen interactions (Chaerle et al. 2004, Lindenthal et al. 2005, Oerke et al. 2005).
Measurements of absolute temperatures are inappropriate for disease assessment, especially under varying environmental conditions. The use of the temperature difference between air temperature and leaf tissue (= transpirational cooling) and the assessment of spatial heterogeneity of temperature within leaves are more suitable. Similar, the maximum temperature difference (= range, MTD) of a leaf may be used as a sensitive parameter for an early detection (Lindenthal et al. 2005). MTD of leaves is more sensitive to pathogen-induced alterations than the mean, minimum or maximum of leaf temperature because this parameter assesses spatially restricted rare modifications in early disease stages. As it is less dependent on environmental conditions and does not significantly exceeds 1 K in healthy leaves, MTD may be used as a threshold level for disease detection (Lindenthal et al. 2005, Oerke et al. 2006).
Oerke et al. (2006) showed a correlation between disease severity (= percentage of leaf area affected) and MTD with a maximum at about 60–70% both for downy mildew of cucumber and apple scab (Fig. 11.4). MTD of leaves not only increased with the size of scab lesions but also with their number per leaf indicating that the probability of large lesions increases with their frequency. The increase of MTD with colony size may be explained by the high thermal conductivity of water. Small lesions have a very limited effect on transpiration/increase of leaf water content and the cooling effect dissipates very soon because of high lateral thermal flow which restricts the effect on tissue temperature in magnitude and size. With large lesions, the cooling effect is greater and even the largest lesions displayed a temperature gradient from the margin to the center of colonies.
Spatial patterns of temperature abnormalities due to pathogen development may be used for the differentiation of stress factors affecting the plant.
4.2.2 Canopy Level
The detection of primary disease spots in the field is one prerequisite for an efficient disease control. Hatfield (1990) postulated that patterns of reflectance or (thermal) radiation may be used for the identification of disease-induced changes that have within-field heterogeneity. In field experiments, the severity of Septoria tritici infection was associated with a decrease of the normalized difference vegetation index (NDVI) and an increase of the canopy surface temperature (<1 K) relative to reference plots (Nicolas 2004). Sensors for the optical range, however, proved to give better results for decision making of fungicide timing.
The complexity and heterogeneity of crop canopies due to varying plant densities, different leaf layers, leaf orientation, etc., restrict the informative value of temperature measurements as well as spatial resolution does. Early detection of primary disease foci on lower leaf levels is impeded by the upper leaf layers, high relative humidity (RH) at lower levels reducing overall transpiration, and the preponderance of healthy tissue often resulting in mixed pixels for diseased tissue. Significant thermal effects, therefore, can be obtained from crops only at later stages of disease development.
Under specific conditions, however, these obstacles play a minor role. In inoculation experiments – for resistance screening of crop varieties, tests on fungicide efficacy, etc. – disease intensity has to be assessed later in the growth period for a large number of plots. In this mono-factorial experiments disease severity may be quantified by areal thermography (Fig. 11.5). Earlier, Eyal and coworkers (1989) successfully used a hand-held thermometer for the differentiation of varieties’ response to Septoria leaf blotch of wheat. Also diseases affecting only plant parts at the top of the canopy may be localized and quantified by thermographic imaging (Fig. 11.6). Thermograms were used to localize Fusarium infected ears in wheat and may be also used for the quantification of Fusarium head blight severity in screening plot experiments.
Assessment of plant vitality of 10 wheat varieties depending on the frequency of fungicide applications (none, low, moderate, high). Reflectance image (a), thermographic image (b) taken at growth stage 75 from a helicopter; relationship between temperature difference between untreated and fungicide-treated plots and yield increase due to fungicide activity (c, different colors depict different varieties) (Lenthe 2005)
Schmitz et al. (2004) were able to differentiate between sugar beet varieties susceptible and resistant to the nematode Heterodera schachtii by using aerial thermography. Only the susceptible variety exhibited a significant correlation between canopy temperature and the density of nematodes in the soil.
5 Canopy Temperature and Management Zones
On the field level digital thermography has been evaluated for its potential in the definition of management zones (Lenthe 2005, Stenzel et al. 2007). Canopy temperature is the result of the temperature of the crop – the evaporation increases with crop biomass – and the environment, notably air and soil. Dense canopies with high biomass have lower temperatures than sparse crops when the soil is rather dry (Fig. 11.7). In contrast, when the soil is wet and cool and a reasonable proportion of soil is included in the thermogram, dense crops result in higher temperatures than poor plant stands. The differences in biomass depended on soil type and quality and were rather stable over time.
Assessment of plant vitality and crop density of wheat before flowering (GS 59) and at soft dough (GS 75) by thermography from a helicopter (Lenthe 2005). Thermographic patterns display a high level of similarity
Even with relative homogenous soil conditions the microclimate within crop canopies may be heterogeneous. Comparing canopy temperature before and after rainfall, a promising degree of spatial similarity between leaf wetness and wheat temperature was detected by Lenthe et al. (2007). It may be possible in the near future to monitor spatial heterogeneity in crop temperature and leaf wetness (duration), factors related to the incidence and spread of diseases in fields, by using thermograhic imaging.
6 Conclusions and Perspectives
Digital infrared thermography is suitable for the detection and quantification of diseases directly affecting plant transpiration by their activity on stomatal functionality and the integrity of the cuticle. Modifications in the plant’s water status may be detected several days before the appearance of visible symptoms. The method, however, is less sensitive to ectoparasitic diseases like powdery mildews and diseases rupturing the leaf cuticle only in late stages of development (like rusts). It largely lacks diagnostic potential because of the uniformity of the stomatal response of plant tissue to various pathogens (and abiotic stressors) and its modulation/variation during various stages of the disease. Environmental factors, especially air temperature, irradiation, relative humidity and wind speed, affect leaf temperature complicating the interpretation of results, notably for images taken under field conditions. Abiotic stressors like water deficit resulting in stomatal closure may interfere with effects of diseases and arthropod pests affecting leaf temperature and compromise the detection and quantification of the primary stress factor. The large diversity of factors affecting stomatal aperture highlights the challenge of identifying and differentiating the cause.
Additional information is required for the identification of the cause. Temporal and spatial dynamics of (canopy) temperature may give additional information – areal thermographs taken at noon and in the evening were used to differentiate permanent wilting of sugar beet due to nematode attack from transient wilting due to circadian water shortage, stresses on leaf level tend to have a more heterogeneous response pattern than root level stresses (Chaerle et al. 2009) – nevertheless, non-specificity of temperature is limiting its application in disease detection and quantification.
Thermal imagery has potential for use in early disease detection which is very important for efficient and environmental friendly disease control since late detection of plant diseases may result in ineffective control reducing the quantity and quality of crop yield. In inoculation experiments when the pathogen is known, IR sensor systems may be used also for the quantification of diseases. Moreover, the technology can be used for the definition of management zones and the monitoring of environmental factors – temperature, leaf wetness – at the canopy level. It has also the potential to assess the quality of spray coverage within a canopy, hence optimizing pesticide application efficiency (Stoll et al. 2008b).
References
Aldea M, Hamilton JG, Resti et al (2006) Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 149: 221–232
Ayres PG, Jones P (1975) Increased transpiration and the accumulation of root absorbed 86Rb in barley leaves infected by Rhynchosporium secalis (leaf blotch). Physiol Plant Pathol 7: 49–58
Bassanezi RB, Amorim L, Bergamin FA et al (2002) Gas exchange and emission of chlorophyll fluorescence during the monocycle of rust, angular leaf spot and anthracnose on bean leaves as a function of their trophic characteristics. J Phytopathol 150:37–47
Boccara M, Boue C, Garmier M et al (2001) Infra-red thermography revealed a role for mitochondria in presymptomatic cooling during harpin-induced hypersensitive response. Plant J 28:663–670
Chaerle L, De Boever F, Van der Straeten D (2002) Infrared detection of early biotic stress in plants. Thermology Int 12:100–106
Chaerle L, De Boever F, van Montagu M et al (2001) Thermographic visualization of cell death in tobacco and Arabidopsis. Plant Cell Environ 24:15–25
Chaerle L, Hagenbeek D, De Bruyne E et al (2004) Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol 45:887–896
Chaerle L, Lenk S, Leinonen I et al (2009) Multi-sensor imaging of plant stresses: towards the development of a stress-catalogue for stress diagnosis. Biotechol J. Published online: Jun 25 2009. doi:10.1002/biot.200800242
Chaerle L, Pineda M, Romero-Aranda R et al (2006) Robotized thermal and chlorophyll fluorescence imaging of pepper mild mottle virus infection in Nicotiana benthamiana. Plant Cell Physiol 47:1323–1336
Chaerle L, Van Caeneghem W, Messens E et al (1999) Presymptomatic visualization of plant-virus interactions by thermography. Nature Biotechnol 17:813–816
Chaerle L, Van der Straeten D (2000) Imaging techniques and the early detection of plant stress. Trends Plant Sci 5:495–501
Chaerle L, Van der Straeten D (2001) Seeing is believing: imaging techniques to monitor plant health. Biochim Biophys Acta 1519:153–166
Cohen Y, Alchanatis V, Meron M et al (2005) Estimation of leaf water potential by thermal imagery and spatial analysis. J Exp Bot 56:1843–1852
Duchesne J, Lili Z, Nicolas H et al (1992) Elaboration of a pathological stress index from thermal infrared measurements. AAB Symposium, Rennes, 8–10 Sept, pp 49–50
Eyal Z, Wise GS, Blum A (1989) Canopy temperature as a correlative measure for assessing host response to Septoria tritici blotch of wheat. Plant Dis 73:468–471
Farquhar GD, Sharkey TD (1982) Stomata conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Hatfield JL (1990) Remote detection of crop stress: Application to plant pathology. Phytopathology 80:37–39
Inoue Y, Kimball BA, Jackson RD et al (1990) Remote estimation of leaf transpiration rate and stomatal resistance based on infrared thermometry. Agric For Meteorol 51:21–33
Jones HG (1992) Plant and microclimate, 2nd edn. Cambridge University Press, Cambridge
Jones HG (1999) Use of infrared thermography for estimation of stomatal conductance in irrigation scheduling. Agric For Meteorol 95:135–149
Jones HG (2004) Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Adv Bot Res 41:107–163
Jones HG, Schofield P (2008) Thermal and other remote sensing of plant stress. Gen Appl Plant Physiol 34(1–2), 19–32
Jones HG, Stoll M, Santoa T et al (2002) Use of infrared thermography for monitoring stomatal closure in the field: application to grapevine. J Exp Bot 53:2249–2260
Körner C (1994) Scaling from species to vegetation: the usefulness of functional groups. In: Schulze ED, Mooney HA (eds) Biodiversity and ecosystem function. Springer, Berlin, pp 119–140
Kümmerlen B, Dauwe S, Schmundt D, Schurr U (1999) Thermography to measure water relations of plant leaves. In: Jähne B (ed) Handbook of computer vision and applications, vol 3. Academic Press, London, pp 636–637
Lenthe JH (2005) Erfassung befallsrelevanter Klimafaktoren in Weizenbeständen mit Hilfe digitaler Infrarot-Thermografie. PhD thesis, University of Bonn, Bonn
Lenthe JH, Oerke EC, Dehne HW (2007) Digital infrared thermography for monitoring canopy health of wheat. Prec Agric 8:15–26
Lili Z, Duchesne J, Nicolas H et al (1991) Détection infrarouge thermique des maladies du blé d’hiver. Bull OEPP 21:659–672
Lindenthal M (2005) Visualisierung der Krankheitsentwicklung von Falschem Mehltau an Gurken durch Pseudoperonospora cubensis mittels Thermografie. PhD thesis, University of Bonn, Bonn
Lindenthal M, Steiner U, Dehne HW et al (2005) Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology 95:233–240
Meola C, Carlomagno GM (2004) Recent advances in the use of infrared thermography. Meas Sci Technol 15:R27–R58
Merlot S, Mustilli AC, Genty B et al (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30:601–609
Nicolas H (2004) Using remote sensing to determine of the date of a fungicide application on winter wheat. Crop Prot 23:853–863
Nicolas H, Rivoal R, Duchesne J et al (1991) Detection of Heterodera avenae infestations on winter wheat by radiothermometry. Revue Nematol 14:285–290
Nilsson HE (1991) Hand-held radiometry and IR-thermography of plant diseases in field plots experiments. Int J Remote Sens 12:545–557
Nilsson HE (1995) Remote sensing and image analysis in plant pathology. Annu Rev Phytopathol 33:489–527
Oerke E-C, Lindenthal M, Fröhling P et al (2005) Digital infrared thermography for the assessment of leaf pathogens. In: Stafford JV (ed) Precision agriculture ’05. Proceedings of 5th European Conference on Precision Agriculture. Wageningen Academic Publishers, Wageningen, pp 91–98
Oerke EC, Steiner U, Dehne HW, Lindenthal M (2006) Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J Exp Bot 57:2121–2132
Pinter PJ, Stanghellini ME, Reginato RJ et al (1979) Remote detection of biological stress in plants with thermography. Science 205:585–587
Santrucek J, Simanova E, Karbulkova J et al (2004) A new technique for measurement of water permeability of stomatous cuticular membranes isolated from Hedera helix leaves. J Exp Bot 55:1411–1422
Schmitz A, Kiewnick S, Schlang J et al (2004) Use of high resolution digital thermography to detect Heterodera schachtii infestation in sugar beets. Comm Appl Biol Sci 69:359–363
Schönherr J (1982) Resistance of plant surfaces to water loss: transport properties of cutin, suberin and associated lipids. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, vol 2B. Springer, Berlin, pp 153–179
Schreiber L, Riederer M (1996) Ecophysiology of cuticular transpiration: comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 107:426–432
Smith RCG, Heritage AD, Stapper M et al (1986). Effect of stripe rust (Puccinia striiformis West.) and irrigation on the yield and foliage temperature of wheat. Field Crops Res 14:39–51
Stenzel I, Steiner U, Dehne HW, Oerke EC (2007) Occurrence of fungal leaf pathogens in sugar beet fields monitored with digital infrared thermography. In: Stafford JV (ed) Precision agriculture ’07. Papers presented at the 6th European Conference on Precision Agriculture. Wageningen Academic Publishers, pp 529–535
Stoll M, Schultz HR, Baecker G et al (2008b) Early pathogen detection under different water status and the assessment of spray application in vineyards through the use of thermal imagery. Prec Agric 9:407–417
Stoll M, Schultz HR, Berkelmann-Loehnertz B (2008a) Exploring the sensitivity of thermal imaging for Plasmopara viticola pathogen detection under different water status. Funct Plant Biol 35:281–288
Wagner AM, Krab K, Wagner MJ, Moore AL (2008) Regulation of thermogenesis in flowering Araceae: The role of the alternative oxidase. Biochim Biophys Acta 1777:993–1000
West JS, Bravo C, Oberti R et al (2003) The potential of optical canopy measurement for targeted control of field crop diseases. Annu Rev Phytopathol 41:593–614
Wright KN, Duncan GH, Pradel KS et al (2000). Analysis of the N gene hypersensitive response induced by a fluorescently tagged tobacco mosaic virus. Plant Physiol 123:1375–1385
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Oerke, EC., Steiner, U. (2010). Potential of Digital Thermography for Disease Control. In: Oerke, EC., Gerhards, R., Menz, G., Sikora, R. (eds) Precision Crop Protection - the Challenge and Use of Heterogeneity. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9277-9_11
Download citation
DOI: https://doi.org/10.1007/978-90-481-9277-9_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9276-2
Online ISBN: 978-90-481-9277-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)