Abstract
Cholesterol is an essential component of higher eukaryotic membranes and plays a crucial role in membrane organization, dynamics and function. The G-protein coupled receptors (GPCRs) are the largest class of molecules involved in signal transduction across membranes, and represent major targets in the development of novel drug candidates in all clinical areas. Membrane cholesterol has been reported to have a modulatory role in the function of a number of GPCRs. Two possible mechanisms have been previously suggested by which membrane cholesterol could influence the structure and function of GPCRs (i) through a direct/specific interaction with GPCRs, or (ii) through an indirect way by altering membrane physical properties in which the receptor is embedded, or due to a combination of both. Recently reported crystal structures of GPCRs have shown structural evidence of cholesterol binding sites. Against this backdrop, we recently proposed a novel mechanism by which membrane cholesterol could affect structure and function of GPCRs. According to our hypothesis, cholesterol binding sites in GPCRs could represent ‘nonannular’ binding sites. Interestingly, previous work from our laboratory has demonstrated that membrane cholesterol is required for the function of the serotonin1A receptor (a representative GPCR), which could be due to specific interaction of the receptor with cholesterol. Based on these results, we envisage that there could be specific/nonannular cholesterol binding site(s) in the serotonin1A receptor. We have analyzed putative cholesterol binding sites from protein databases in the serotonin1A receptor. Our analysis shows that cholesterol binding sites are inherent characteristic features of serotonin1A receptors and are conserved through natural evolution. Progress in deciphering molecular details of the GPCR-cholesterol interaction in the membrane would lead to better insight into our overall understanding of GPCR function in health and disease, thereby enhancing our ability to design better therapeutic strategies to combat diseases related to malfunctioning of GPCRs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- G-protein coupled receptor
- Membrane cholesterol
- Nonannular binding site
- Serotonin1A receptor
- Specific interaction
- Specific cholesterol binding site
16.1 Introduction
Biological membranes are complex two-dimensional, non-covalent assemblies of a diverse variety of lipids and proteins. They impart an identity to the cell and its organelles and represent an ideal milieu for the proper function of a diverse set of membrane proteins. Membrane proteins mediate a wide range of essential cellular processes such as signaling across the membrane, cell-cell recognition, and membrane transport. About 30% of all open reading frames (ORFs) are predicted to encode membrane proteins and almost 50% of all proteins encoded by eukaryotic genomes are membrane proteins (Liu et al., 2002; Granseth et al., 2007). Importantly, they represent prime candidates for the generation of novel drugs in all clinical areas (Drews, 2000; Dailey et al., 2009) owing to their involvement in a wide variety of cellular processes. Since a significant portion of integral membrane proteins remains in contact with the membrane (Lee, 2003), and reaction centers in them are often buried within the membrane, the function of membrane proteins depends on the surrounding membrane lipid environment. Work spanning several years from a number of groups has contributed to our understanding of the requirement of specific lipids and/or the membrane environment for maintaining the proper topology, structure and function of membrane proteins (Opekarová and Tanner, 2003; Lee, 2004; Palsdottir and Hunte 2004; Nyholm et al., 2007). These effects have been attributed either to specific interactions of lipids with amino acids in proteins or to bulk properties of membranes. Considering the diverse array of lipids in natural membranes, it is believed that physiologically relevant processes occurring in membranes involve a precise coordination of multiple lipid-protein interactions. Such lipid-protein interactions are of particular importance because cells possess the ability to vary the lipid composition of their membranes in response to a variety of stresses and stimuli, thereby changing the environment and the activity of the proteins in their membranes. Insights into the structure of membrane proteins and specific lipid-protein interactions required for their function are therefore of considerable interest and physiological relevance.
16.2 Cholesterol in Biological Membranes: A Tale of Two Faces
Cholesterol is an essential and representative lipid in higher eukaryotic cellular membranes and is crucial in membrane organization, dynamics, function, and sorting (Liscum and Underwood, 1995; Simons and Ikonen, 2000; Mouritsen and Zuckermann, 2004). Cholesterol is a predominantly hydrophobic molecule comprising a near planar tetracyclic fused steroid ring and a flexible isooctyl hydrocarbon tail (see Fig. 16.1a). The basic hydrocarbon skeleton of cholesterol (and other sterols found in eukaryotes) is sterane (Fig. 16.1b). Since sterane resists microbial attack and is stable over long periods of time, sterols have emerged as important fossil markers for paleontologists (Kodner et al., 2008). The 3β-hydroxyl moiety provides cholesterol its amphiphilic character and helps cholesterol to orient and anchor in the membrane (Villalaín, 1996). The tetracyclic nucleus and isooctyl side chain create the bulky wedge-type shape of the cholesterol molecule. Interestingly, the planar tetracyclic ring system of cholesterol is asymmetric about the ring plane. The sterol ring has a flat and smooth side with no substituents (the α face) and a rough side with methyl substitutions (the β face; see Fig. 16.1c). The smooth α face of the sterol nucleus helps in favorable van der Waals interaction with the saturated fatty acyl chains of phospholipids (Lange and Steck, 2008). The α face of cholesterol contains only axial hydrogen atoms. The absence of any bulky group in this face facilitates close contact between the sterol nucleus and phospholipid chains. The bumpiness of the β face of cholesterol molecule is due to the protruding methyl groups at positions C18, C19 and C21. The molecular structure of cholesterol has been exceedingly fine-tuned over a very long time scale of natural evolution. This is exemplified by the recent report that removal of methyl groups from cholesterol results in altered tilt angle which affects ordering and condensing effects, as shown by atomic scale molecular dynamics simulations (Róg et al., 2007; Pöyry et al., 2008). Molecular simulation approaches have earlier shown that the α face of cholesterol promoted a stronger ordering effect on saturated alkyl chains compared to the β face (Róg and Pasenkiewicz-Gierula, 2001). In addition, molecular dynamics simulation has shown that cholesterol orients its smooth α face toward saturated chains and its uneven β face toward unsaturated chains of phospholipids (Pandit et al., 2004), or with a bumpy transmembrane domain of an integral membrane protein (see Fig. 16.1d).
Chemical structure and membrane orientation of cholesterol: (a) Structure of cholesterol showing the individual rings (A–D). Three structurally distinct regions are shown as shaded boxes: the 3β-hydroxyl group, the rigid steroid ring, and the flexible alkyl chain. The 3β-hydroxyl moiety is the only polar group in cholesterol thereby contributing to its amphiphilic character and it helps cholesterol to orient and anchor in the membrane. Reproduced from Paila et al. (2009). (b) Chemical structure of sterane. Sterane is the basic hydrocarbon skeleton of cholesterol and other sterols found in eukaryotes. (c) Two faces of cholesterol. Cholesterol is characterized by a flat and smooth α face, and a rough β face. The α face of cholesterol contains only axial hydrogen atoms. The roughness of the β face is due to the protruding bulky methyl groups. (d) Schematic orientation of cholesterol in relation to a phospholipid molecule in a lipid bilayer. The smooth α face of the sterol nucleus helps in favorable van der Waals interaction with the saturated fatty acyl chains of phospholipids. α and β faces of cholesterol can simultaneously interact with a saturated fatty acyl chain of phospholipids and uneven transmembrane domain of an integral membrane protein, respectively. Cholesterol is shown to align in bilayers with its 3β-hydroxyl group in the vicinity of the ester carbonyls of phospholipids and its tetracyclic ring immersed in the bilayer interior, in close contact with a part of the phospholipid fatty acyl chain. Since the length of the cholesterol molecule including the isooctyl tail in all-trans energy minimum conformation is ∼20 Å, a single cholesterol molecule can traverse each leaflet of a bilayer composed of phospholipids typically found in eukaryotic plasma membranes. It should be noted that the effective length of cholesterol molecule in membranes could vary, depending on the nature of the phospholipids. See text for other details
Cholesterol is oriented in the membrane bilayer with its long axis perpendicular to the plane of the membrane (Fig. 16.1d), so that it’s polar hydroxyl group encounters the aqueous environment and the hydrophobic steroid ring is oriented parallel to and immersed in the hydrophobic fatty acyl chains of the phospholipids (Yeagle, 1985). It has been previously shown using X-ray and neutron diffraction that cholesterol is aligned in bilayers with its 3β-hydroxyl group in the proximity of the ester bonds of phospholipids and its tetracyclic ring buried in the bilayer interior, in close contact with a part of the phospholipid fatty acyl chains (Villalaín, 1996; Bittman, 1997). It should be mentioned here that although the hydroxyl group of cholesterol is shown to be aligned at the level of sn-2 ester carbonyl group of the phospholipid in Fig. 16.1d, unambiguous experimental evidence supporting the interaction (hydrogen bonding) between the hydroxyl group of cholesterol and the lipid carbonyl group is lacking. Since the length of the cholesterol molecule including the isooctyl tail in all-trans energy minimum conformation is ∼20 Å, a single cholesterol molecule can traverse one leaflet of a bilayer composed of phospholipids (Bittman, 1997), typically found in eukaryotic plasma membranes (Fig. 16.1d). In fact, cholesterol has previously shown to exist as transbilayer (‘tail-to-tail’) dimers spanning the two leaflets of the membrane bilayer at low concentrations (Harris et al., 1995; Mukherjee and Chattopadhyay, 1996; Loura and Prieto, 1997). Interestingly, the transbilayer dimer arrangement of cholesterol was shown to be sensitive to the membrane surface curvature and is stringently controlled by a narrow window of membrane thickness (Rukmini et al., 2001). The environment around the cholesterol dimers appears to be more rigid (Mukherjee and Chattopadhyay, 2005) and the dimer population exhibits relatively slow lateral diffusion (Pucadyil et al., 2007). Importantly, such transbilayer tail-to-tail cholesterol dimers have been implicated in atherogenesis (Tulenko et al., 1998) and in human ocular lens fiber cell plasma membranes, especially in cataractous condition (Jacob et al., 1999; 2001; Mason et al., 2003).
Cholesterol is often found distributed non-randomly within domains found in biological and model membranes (Liscum and Underwood, 1995; Schroeder et al., 1995; Simons and Ikonen, 1997, 2000; Xu and London, 2000; Mukherjee and Maxfield, 2004). Many of these domains (sometimes termed as ‘lipid rafts’) are believed to be important for the maintenance of membrane structure and function. The idea of such specialized membrane domains assumes significance in cell biology since physiologically important functions such as membrane sorting and trafficking (Simons and van Meer, 1988), signal transduction processes (Simons and Toomre, 2000), and the entry of pathogens (Simons and Ehehalt, 2002; Riethmüller et al., 2006; Pucadyil and Chattopadhyay, 2007) have been attributed to these domains. Importantly, cholesterol plays a vital role in the function and organization of membrane proteins and receptors (Burger et al., 2000; Pucadyil and Chattopadhyay, 2006).
16.3 Role of Membrane Cholesterol in the Function of G-Protein Coupled Receptors
The G-protein coupled receptor (GPCR) superfamily is the largest and most diverse protein family in mammals, involved in signal transduction across membranes (Pierce et al., 2002; Perez, 2003; Rosenbaum et al., 2009). Cellular signaling by GPCRs involves their activation by ligands present in the extracellular environment and the subsequent transduction of signals to the interior of the cell through concerted changes in their transmembrane domain structure (Gether, 2000). GPCRs are prototypical members of the family of seven transmembrane domain proteins and include >800 members which together constitute ∼1–2% of the human genome (Fredriksson and Schiöth, 2005). GPCRs dictate physiological responses to a diverse array of stimuli that include endogenous ligands such as biogenic amines, peptides, glycoproteins, lipids, nucleotides, Ca2+ ions and various exogenous ligands for sensory perception such as odorants, pheromones, and even photons. As a consequence, these receptors mediate multiple physiological processes such as neurotransmission, cellular metabolism, secretion, cellular differentiation, growth, inflammatory and immune responses. It is therefore only natural that GPCRs have emerged as major targets for the development of novel drug candidates in all clinical areas (Nature reviews drug discovery GPCR questionnaire participants 2004; Jacoby et al., 2006; Schlyer and Horuk, 2006; Insel et al., 2007; Heilker et al., 2009). Interestingly, although GPCRs represent 30–50% of current drug targets, only a small fraction of all GPCRs are presently targeted by drugs (Lin and Civelli, 2004). This points out the exciting possibility that the receptors which are not yet recognized could be potential drug targets for diseases that are difficult to treat by currently available drugs.
GPCRs are integral membrane proteins with a significant portion of the protein embedded in the membrane. In the case of rhodopsin, molecular dynamics simulation studies have estimated that the lipid-protein interface corresponds to ∼38% of the total surface area of the receptor (Huber et al., 2004). This raises the obvious possibility that the membrane lipid environment could be an important modulator of receptor structure and function (Lee, 2004). The importance of a membrane lipid environment for optimal function of membrane proteins in general, and GPCRs in particular, is evident from the adverse effects of delipidation on receptor function (Kirilovsky and Schramm, 1983; Jones et al., 1988). Importantly, membrane cholesterol has been shown to modulate the function of a number of GPCRs. From the available data on the role of cholesterol on GPCR function (see Table 16.1), it appears that there is a lack of consensus on the manner in which cholesterol modulates receptor function. For example, while cholesterol is found to be essential for the proper function of several GPCRs, the function of rhodopsin has been shown to be inhibited in the presence of cholesterol. This calls for a detailed mechanistic analysis of the effect of cholesterol on any given receptor. What follows is a critical analysis of the available literature on the role of membrane cholesterol in GPCR function, with an overall objective to distinguish specific and general effects.
16.3.1 Effect of Membrane Cholesterol on the Function of GPCRs: General Effect or Specific Interaction ?
The mechanism underlying the effect of cholesterol on the structure and function of integral membrane proteins and receptors is complex and as yet no general consensus has emerged (Burger et al., 2000; Pucadyil and Chattopadhyay, 2006; Paila and Chattopadhyay, 2009). It has been proposed that cholesterol can modulate the function of GPCRs in two ways: (i) by a direct/specific interaction with the GPCR, which could induce a conformational change in the receptor (Gimpl et al., 2002a,b), or (ii) through an indirect way by altering the membrane physical properties in which the receptor is embedded (Ohvo-Rekilä et al., 2002; Lee, 2004) or due to a combination of both. There could be yet another mechanism by which membrane cholesterol could affect structure and function of membrane proteins. This mechanism invokes the concept of ‘nonannular’ binding sites of membrane lipids (Lee et al., 1982; Simmonds et al., 1982). We recently proposed that cholesterol binding sites in GPCRs could represent nonannular binding sites (Paila et al., 2009; see below). A comprehensive discussion on the representative GPCRs, for which the mechanism of cholesterol-dependence of function has been addressed, is provided below.
16.3.1.1 Rhodopsin
Rhodopsin, the photoreceptor of retinal rod cells, undergoes a series of conformational changes upon exposure to light. The light-activated receptor exists in equilibrium with various intermediates, collectively termed metarhodopsins. The state of equilibrium is sensitive to the presence of cholesterol in the membrane (Straume and Litman, 1988; Mitchell et al., 1990; Bennet and Mitchell, 2008). An increase in the amount of cholesterol in the membrane shifts this equilibrium toward the inactive conformation of the protein. The inhibitory effect of cholesterol on rhodopsin function has been explained by direct (see below) as well as indirect modes of action. The indirect mode of action has been rationalized on the basis of the free-volume theory of membranes, which relates the alteration in membrane physical properties due to the presence of cholesterol, to receptor function (Mitchell et al., 1990). The conversion of the photointermediates, metarhodopsin I to metarhodopsin II, upon exposure to light involves an expansion of the protein in the plane of the bilayer (Attwood and Gutfreund, 1980), which occupies the available partial free volume from the surrounding bilayer. The presence of cholesterol in the membrane has been reported to inhibit the formation of metarhodopsin II, due to its role in reducing the partial free volume in the membrane (Niu et al., 2002). Importantly, fluorescence resonance energy transfer (FRET) measurements have indicated an inherent property of rhodopsin to partition out of cholesterol-rich regions of the membrane (Polozova and Litman, 2000). These results have been reinforced by molecular dynamics simulation with rhodopsin in membranes containing a mixture of cholesterol and polyunsaturated phospholipids (Pitman et al., 2005).
16.3.1.2 Oxytocin and Cholecystokinin Receptors
Oxytocin and cholecystokinin (CCK) receptors have been shown to require membrane cholesterol for their function (Fahrenholz et al., 1995; Klein et al., 1995; Gimpl et al., 1995, 1997, 2002b; Harikumar et al., 2005). Interestingly, while the interaction between the oxytocin receptor and cholesterol is believed to be specific, the function of the CCK receptor appears to be dependent on the physical properties of membranes, which are a function of cholesterol content. This is demonstrated by the fact that these receptors exhibited different types of correlation, when fluorescence anisotropy of the membrane probe DPH was correlated with ligand binding activity. In case of the CCK receptor, ligand binding showed linear increase with measured anisotropy values (Gimpl et al., 1997). On the other hand, the ligand binding activity of the oxytocin receptor showed a slight reduction upon cholesterol depletion followed by a sharp decline, when the membrane cholesterol content reached a certain critical level (∼57% of the original cholesterol content). This shows that membrane cholesterol could affect the ligand binding activity of the oxytocin receptor by a cooperative mechanism. Hill analysis of cholesterol content versus ligand binding revealed that the oxytocin receptor binds several molecules of cholesterol (n ≥ 6) in a positive cooperative manner (Burger et al., 2000; Gimpl et al., 2002b). These conclusions were reinforced by structure-activity analysis of the oxytocin and CCK receptor using a variety of cholesterol analogues (Gimpl et al., 1997). In order to examine the specific molecular features of cholesterol required to maintain the high-affinity state of the oxytocin receptor, MβCD was used to replenish cholesterol-depleted membranes with a broad range of cholesterol analogues that are subtly different from cholesterol in the headgroup, the steroid ring, or in the hydrocarbon tail. Interestingly, ligand binding of the oxytocin receptor could be restored only with certain analogues, thereby indicating a specific molecular feature in cholesterol to support receptor function. Although cholesterol depletion reduces ligand binding to the CCK receptor, this effect could be reversed with most analogues of cholesterol that could restore membrane order. The ligand binding of the CCK receptor therefore was supported by each of the cholesterol analogues and was well correlated with the corresponding fluorescence anisotropy values. However, similar effects on the oxytocin receptor could be demonstrated only with certain analogues that structurally resembled cholesterol in some critical features. Taken together, these data provide support for a specific molecular interaction between the oxytocin receptor and cholesterol. In addition, molecular modeling studies have indicated a putative docking site (involving residues on the surface of transmembrane segments 5 and 6) for cholesterol in the oxytocin receptor that is absent in the CCK receptor (Politowska et al., 2001). Further, it has been reported that cholesterol stabilizes oxytocin receptor against thermal inactivation and protects the receptor from proteolytic degradation (Gimpl and Fahrenholz, 2002).
16.3.1.3 Galanin Receptors
Membrane cholesterol has been shown to be required for ligand binding and intracellular signaling of the subtype 2 galanin receptor (GalR2) (Pang et al., 1999). The role of membrane cholesterol in modulating ligand binding to the galanin receptor was monitored by treating membranes with MβCD or by culturing cells expressing the receptor in lipoprotein-deficient serum. These studies revealed a marked reduction in galanin binding to the receptor in cholesterol-deficient membranes. Importantly, replenishment of cholesterol to cholesterol-depleted membranes restored galanin binding to normal levels. This interaction appears to be specific, as only a limited number of cholesterol analogues were able to rescue galanin binding. In addition, treatment of membranes either with filipin (which binds cholesterol) or with cholesterol oxidase markedly reduced galanin binding. Hill analysis suggested that several molecules of cholesterol (n ≥ 3) could bind in a positively cooperative manner to GalR2 (Pang et al., 1999).
16.4 Nonannular Lipids in the Function of Membrane Proteins
It has been proposed for the nicotinic acetylcholine receptor (which requires cholesterol for its function) that cholesterol could be present at the ‘nonannular’ sites of the receptor (Jones and McNamee, 1988). Early evidence for the presence of nonannular lipids was obtained from experiments monitoring effects of cholesterol and fatty acids on the Ca2+/Mg2+-ATPase (Lee et al., 1982; Simmonds et al., 1982). Integral membrane proteins are surrounded by a shell or annulus of lipid molecules, which mimics the immediate layer of solvent surrounding soluble proteins (Jost et al., 1973; Lee, 2003). These are termed ‘annular’ lipids surrounding the membrane protein. After several years of moderate controversy surrounding the interpretation of spectroscopic data, it later became clear that the annular lipids are exchangeable with bulk lipids (Devaux and Seigneuret, 1985). The rate of exchange of lipids between the annular lipid shell and the bulk lipid phase was shown to be approximately an order of magnitude slower than the rate of exchange of bulk lipids, resulting from translational diffusion of lipids in the plane of the membrane. It therefore appears that exchange between annular and bulk lipids is relatively slow, since lipid-protein interaction is favorable compared to lipid-lipid interaction. However, the difference in interaction energy is modest, consistent with the observation that lipid-protein binding constants (affinity) depend weakly on lipid structure (Lee, 2003). Interestingly, the two different types of lipid environments (annular and bulk) can be readily detected using electron spin resonance (ESR) spectroscopy (Marsh, 1990). In addition to the annular lipids, there is evidence for other lipid molecules in the immediate vicinity of integral membrane proteins. These are termed as ‘nonannular’ lipids. Nonannular sites are characterized by lack of accessibility to the annular lipids, i.e., these sites cannot be displaced by competition with annular lipids. This is evident from analysis of fluorescence quenching of intrinsic tryptophans of membrane proteins by phospholipids or cholesterol covalently labelled with bromine (Simmonds et al., 1982; Jones and McNamee, 1988), which acts as a quencher due to the presence of the heavy bromine atom (Chattopadhyay, 1992). These results indicate that nonannular lipid binding sites remain vacant even in the presence of annular lipids around the protein (Marius et al., 2008). The exchange of lipid molecules between nonannular sites and bulk lipids would be relatively slow compared to the exchange between annular sites and bulk lipids (although this has not yet been shown experimentally), and binding to the nonannular sites is considered to be more specific compared to annular binding sites (Lee, 2003).
The location of the postulated nonannular sites merits comment. It has been suggested that the possible locations for the nonannular sites could be either inter or intramolecular (interhelical) protein interfaces, characterized as deep clefts (or cavities) on the protein surface (Simmonds et al., 1982; Marius et al., 2008). For example, in the crystal structure of the potassium channel KcsA from S. lividans, a negatively charged lipid molecule was found to be bound as ‘anionic nonannular’ lipid at each of the protein-protein interface in the homotetrameric structure (Marius et al., 2005). These nonannular sites show high selectivity for anionic lipids over zwitterionic lipids, and it has been proposed that the change in the nature of the nonannular lipid leads to a change in packing at the protein-protein interface which modulates the open channel probability and conductance. Interestingly, the relationship between open channel probability of KcsA and negative phospholipid content exhibits cooperativity. This is consistent with a model in which the nonannular sites in the KcsA homotetramer have to be occupied by anionic lipids for the channel to remain open (Marius et al., 2008). This example demonstrates the crucial requirement of nonannular lipids in the function of membrane proteins and the stringency associated with regard to specificity of nonannular lipids.
In the context of GPCRs, it is interesting to note that many GPCRs are believed to function as oligomers (Park et al., 2004). More importantly, cholesterol has been shown to improve stability of GPCRs such as the β2-adrenergic receptor (Yao and Kobilka, 2005), and appears to be a necessary component for crystallization of the receptor since it facilitates receptor-receptor interaction and consequent oligomerization (Cherezov et al., 2007). Since a possible location of the nonannular sites is inter-protein interfaces (Simmonds et al., 1982; Jones and McNamee, 1988), it is possible that cholesterol molecules located between individual receptor molecules (see below, Fig. 16.2b) occupy nonannular sites and modulate receptor structure and function.
Presence of tightly bound cholesterol molecules in the transmembrane regions in the crystal structures of metarhodopsin I (panel a) and human β2-adrenergic receptor (panels b and c). Panel (a) shows side view of metarhodopsin I showing cholesterol between transmembrane helices. Notice the close proximity of tryptophan residues (W161 and W265) to cholesterol, independently confirmed by FRET studies (see text for more details). Panel (b) depicts the structure of the human β2-adrenergic receptor (shown in blue) bound to the partial inverse agonist carazolol (in green) embedded in a lipid bilayer. Cholesterol molecules between two receptor molecules are shown in orange. Panel (c) shows the Cholesterol Consensus Motif (CCM) in the β2-adrenergic receptor (bound to the partial inverse agonist timolol) crystal structure. The side chain positions of the β2-adrenergic receptor and two bound cholesterol molecules are shown. Residues at positions 4.39–4.43 fulfill the CCM requirement (if one or more of these positions contains an arginine or lysine residue) and constitute site 1 (shown in blue) toward the cytoplasmic end of transmembrane helix IV. Site 2 (in cyan) represents the most important site at position 4.50 on transmembrane helix IV since it is the most conserved site with tryptophan occupying this position in 94% of class A GPCRs. The other choice of amino acid for this site is tyrosine. Site 3 (in green) at position 4.46 on transmembrane helix IV satisfies the CCM requirement if isoleucine, valine, or leucine occupy the position. Site 4 (in orange) on transmembrane helix II is at position 2.41 and can be either phenylalanine or tyrosine. Reproduced from Paila et al. (2009)
16.4.1 Presence of Specific (Nonannular?) Cholesterol binding Sites in the Crystal Structures of GPCRs
16.4.1.1 Rhodopsin
Specific interaction between rhodopsin and cholesterol has been monitored utilizing FRET between tryptophan residues of rhodopsin (donor) and cholestatrienol (acceptor) (Albert et al., 1996). Cholestatrienol is a naturally occurring fluorescent cholesterol analogue and has been reported to be a faithful mimic of cholesterol (Gimpl and Gehrig-Burger, 2007; Wüstner, 2007). In the aforementioned work (Albert et al., 1996), replenishment of cholesterol or ergosterol into cholesterol-depleted rod outer segment disk membranes was carried out and their ability to inhibit the quenching of donor tryptophan fluorescence was monitored. Interestingly, cholesterol was able to inhibit tryptophan quenching, whereas in presence of ergosterol, quenching was observed due to energy transfer between tryptophan residues of rhodopsin and cholestatrienol, indicating a specific interaction between rhodopsin and cholesterol. In addition, it was postulated that one cholesterol molecule per rhodopsin monomer would be present at the lipid-protein interface (Albert et al., 1996). This has been supported by the crystal structure of a photo-stationary state, highly enriched in metarhodopsin I, which shows a cholesterol molecule between two rhodopsin monomers, which could possibly represent a nonannular site for cholesterol binding (Ruprecht et al., 2004; see Fig. 16.2a). These authors also reported that cholesterol could improve the reliability and yield of crystallization. In this structure, cholesterol is shown to be oriented with its tetracyclic ring aligned normal to the membrane bilayer. Interestingly, these authors proposed that some of the tryptophans in transmembrane helices would be able to interact with the cholesterol tetracyclic ring. Recent crystallographic structures of the β2-adrenergic receptor have shown similar interactions (see below).
16.4.1.2 β2-Adrenergic Receptor
Lipid molecules that are resolved in crystal structures of membrane proteins are believed to be tightly bound. These lipid molecules, which are preserved even in the crystal structure, are often localized at protein-protein interfaces in multimeric proteins and belong to the class of nonannular (sometimes termed as ‘co-factor’) lipids (Lee, 2003, 2005). Cholesterol has been shown to improve stability of the β2-adrenergic receptor (Yao and Kobilka, 2005), and appears to be necessary for crystallization of the receptor (Cherezov et al., 2007). The cholesterol analogue, cholesterol hemisuccinate, has recently been shown to stabilize the β2-adrenergic receptor against thermal inactivation (Hanson et al., 2008). Since a possible location of the nonannular sites is at inter-protein interfaces (Simmonds et al., 1982; Jones and McNamee, 1988), it is possible that cholesterol molecules located between individual receptor molecules (see Fig. 16.2b) occupy nonannular sites and modulate receptor structure and function. Importantly, the recent crystal structure of the β2-adrenergic receptor has revealed structural evidence of a specific cholesterol binding site (Fig. 16.2c, Hanson et al., 2008). The crystal structure shows a cholesterol binding site between transmembrane helices I, II, III and IV with two cholesterol molecules bound per receptor monomer. The cholesterol binding site appears to be characterized by the presence of a cleft located at the membrane interfacial region. Both cholesterol molecules bind in a shallow surface groove formed by segments of the above mentioned helices (I–IV), thereby providing an increase in the intramolecular occluded surface area, a parameter often correlated to the enhanced thermal stability of proteins (DeDecker et al., 1996). Calculation of packing values of various helices in the β2-adrenergic receptor which are involved in the cholesterol interacting site showed that the packing of transmembrane helices II and IV increases upon cholesterol binding, which would restrict their mobility rendering greater thermal stability to the protein (Hanson et al., 2008).
Earlier literature suggests that there are several structural features of proteins that are believed to result in preferential association with cholesterol (Epand, 2006). In many cases, proteins interacting with cholesterol have a characteristic stretch of amino acids, termed the cholesterol recognition/interaction amino acid consensus (CRAC) motif (Li and Papadopoulos, 1998). Another important cholesterol interacting domain is the sterol-sensing domain (SSD). The SSD is relatively large and consists of five transmembrane segments and is involved in cholesterol biosynthesis and homeostasis (Kuwabara and Labouesse, 2002; Brown and Goldstein, 1999). It has been recently proposed that cholesterol binding sequence or motif should contain at least one aromatic amino acid, which could interact with ring D of cholesterol (Hanson et al., 2008) and a positively charged residue (Epand et al., 2006; Jamin et al., 2005), capable of participating in electrostatic interactions with the 3β-hydroxyl group. In the crystal structure of the β2-adrenergic receptor, three amino acids in transmembrane helix IV, along with an amino acid in transmembrane helix II, have been suggested to constitute a cholesterol consensus motif (CCM, see Fig. 16.2c). The aromatic Trp 1584.50 [according to the Ballesteros-Weinstein numbering system (Ballesteros and Weinstein, 1995)] is conserved to a high degree (∼94%) among rhodopsin-like GPCRs and appears to contribute the most significant interaction with ring D of cholesterol (see Fig. 16.1a; Hanson et al., 2008). In this structure, the hydrophobic residue Ile1544.46 would interact with rings A and B of cholesterol and is largely conserved (∼60%) in rhodopsin family GPCRs. The aromatic residue Tyr702.41 in transmembrane helix II could interact with ring A of cholesterol and with Arg1514.43 of transmembrane helix IV through hydrogen bonding. The criterion of specific residues in CCM (as described above) could be somewhat broadened by conservative substitutions of amino acids (see legend to Fig. 16.2c).
The above description of CCM in the recently reported crystal structure of the β2-adrenergic receptor raises the interesting possibility of the presence of putative nonannular binding sites in transmembrane inter-helical locations in GPCRs in general. It was previously proposed, from quenching analysis of intrinsic tryptophan fluorescence in the nicotinic acetylcholine receptor by brominated phospholipids and cholesterol analogues, that there could be 5–10 nonannular sites per ∼250 kDa monomer of the receptor (Jones and McNamee, 1988). This is consistent with the above proposal of two putative nonannular sites per ∼50 kDa monomer of the β2-adrenergic receptor.
16.5 The Serotonin1A Receptor: A Representative Member of the GPCR Superfamily in the Context of Membrane Cholesterol Dependence for Receptor Function
The serotonin1A (5-HT1A) receptor is an important neurotransmitter receptor and is the most extensively studied of the serotonin receptors for a number of reasons (Pucadyil et al., 2005a; Kalipatnapu and Chattopadhyay, 2007). Serotonin receptors have been classified into at least 14 subtypes on the basis of their pharmacological responses to specific ligands, sequence similarities at the gene and amino acid levels, gene organization, and second messenger coupling pathways (Hoyer et al., 2002). The serotonin1A receptor was the first among all types of serotonin receptors to be cloned as an intronless genomic clone (G-21) of the human genome, which cross-hybridized with a full length β-adrenergic receptor probe at reduced stringency (Kobilka et al., 1987; Pucadyil et al., 2005a). Sequence analysis of this genomic clone (later identified as the serotonin1A receptor gene) showed ∼43% amino acid similarity with the β2-adrenergic receptor in the transmembrane domain. The serotonin1A receptor was therefore initially discovered as an ‘orphan’ receptor and was identified (‘deorphanized’) later (Fargin et al., 1988). The human gene for the receptor encodes a protein of 422 amino acids (see Fig. 16.3). Considering the presence of three consensus sequences for N-linked glycosylation in the amino terminus, and the homology of the receptor with β-adrenergic receptor, it is predicted that the receptor is oriented in the plasma membrane with the amino terminus facing the extracellular region and the carboxy terminus facing the intracellular cytoplasmic region (Raymond et al., 1999; Pucadyil et al., 2005a; Kalipatnapu and Chattopadhyay, 2007; see Fig. 16.3). The transmembrane domains (TM1–TM7) of the receptor are connected by hydrophilic sequences of three extracellular loops (EC1, EC2, EC3) and three intracellular loops (IC1, IC2, IC3). Such an arrangement is typical of the G-protein coupled receptor superfamily (Gether and Kobilka, 1998). Although the structure of the serotonin1A receptor has not yet been experimentally determined, mutagenesis studies have helped in identifying amino acid residues important for ligand binding and G-protein coupling of the serotonin1A receptor (reviewed in Pucadyil et al., 2005a). Among the predicted structural features of the serotonin1A receptor, palmitoylation status of the receptor has been confirmed in a recent report (Papoucheva et al., 2004). An interesting aspect of this study is that palmitoylation of the serotonin1A receptor was found to be stable and independent of stimulation by the agonist. This is unusual for GPCRs, which undergo repeated cycles of palmitoylation and depalmitoylation (Milligan et al., 1995). It has therefore been proposed that stable palmitoylation of the receptor could play an important role in maintaining the receptor structure (Papoucheva et al., 2004).
A schematic representation of the membrane embedded human serotonin1A receptor showing its topological and other structural features. The membrane is shown as a bilayer of phospholipids and cholesterol, representative of typical eukaryotic membranes. The transmembrane helices of the receptor were predicted using TMHMM2. Seven transmembrane stretches, each composed of ∼22 amino acids, are depicted as putative α-helices. The exact boundary between the membrane and the aqueous phase is not known and therefore the location of the residues relative to the membrane bilayer is putative. The amino acids in the receptor sequence are shown as circles and are marked for convenience. The potential sites (shown in lavender) for N-linked glycosylation (depicted as branching trees in red) on the amino terminus are shown. A putative disulfide bond between Cys109 and Cys187 is shown. The transmembrane domains contain residues (shown in cyan) that are important for ligand binding. The putative cholesterol binding site (see text) is highlighted (in orange). The receptor is stably palmitoylated (shown in blue) at residues Cys417 and/or Cys420 (shown in green). Light blue circles represent contact sites for G-proteins. Light pink circles represent sites for protein kinase mediated phosphorylation. Further structural details of the receptor are available in (Pucadyil et al., 2005a; Pucadyil and Chattopadhyay, 2006). Reproduced from Paila et al. (2009). It is probable, based on comparison with known crystal structures of similar GPCRs such as rhodopsin and β2-adrenergic receptor, that there are motionally restricted water molecules that could be important in inducing conformational transitions in the transmembrane portion of the receptor (see, for example, Angel et al., 2009)
Serotonergic signaling plays a key role in the generation and modulation of various cognitive, developmental and behavioral functions. The serotonin1A receptor agonists and antagonists have been shown to possess potential therapeutic effects in anxiety-or stress-related disorders (Pucadyil et al., 2005a). As a result, the serotonin1A receptor serves as an important target in the development of therapeutic agents for neuropsychiatric disorders such as anxiety and depression. Interestingly, mutant (knockout) mice lacking the serotonin1A receptor exhibit enhanced anxiety-related behavior, and represent an important animal model for genetic vulnerability to complex traits such as anxiety disorders and aggression in higher animals (Toth, 2003; Gardier, 2009). Taken together, the serotonin1A receptor is a central player in a multitude of physiological processes, and an important drug target.
Seminal work from our laboratory has comprehensively demonstrated the requirement of membrane cholesterol in the function of the serotonin1A receptor (Pucadyil and Chattopadhyay, 2004, 2006). We demonstrated the crucial modulatory role of membrane cholesterol on the ligand binding activity and G-protein coupling of the hippocampal serotonin1A receptor using a number of approaches such as treatment with (i) MβCD, which physically depletes cholesterol from membranes (Pucadyil and Chattopadhyay, 2004, 2005) (ii) the sterol-complexing detergent digitonin (Paila et al., 2005), and (iii) the sterol-binding antifungal polyene antibiotic nystatin (Pucadyil et al., 2004). Interestingly, while treatment with MβCD physically depletes membrane cholesterol, treatment with other agents merely modulates the availability of membrane cholesterol without physical depletion. The common message from these observations is that it is the non-availability of membrane cholesterol, rather than the manner in which its availability is modulated, is crucial for ligand binding of the serotonin1A receptor. Importantly, replenishment of membrane cholesterol using MβCD-cholesterol complex resulted in recovery of ligand binding activity to a considerable extent. However, it was not clear from these results whether the effect of membrane cholesterol on the function of the serotonin1A receptor is due to specific interaction of membrane cholesterol with the receptor, or general effect of cholesterol on the membrane bilayer, or a combination of both.
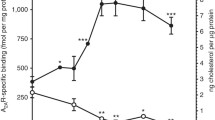
In order to further examine the mechanism of cholesterol-dependent function of the serotonin1A receptor and monitor the stringency of the process, membranes were treated with cholesterol oxidase, which catalyzes the oxidation of cholesterol to cholestenone. These results showed that oxidation of membrane cholesterol led to inhibition of the ligand binding activity of the serotonin1A receptor without altering overall membrane order (Figs. 16.4a and 16.5; Pucadyil et al., 2005b). Based on these results, it was proposed that there could be specific interaction between membrane cholesterol and the serotonin1A receptor. Toward this effect, we have recently generated a cellular model of the Smith-Lemli-Opitz Syndrome (SLOS), using cells stably expressing the human serotonin1A receptor (Paila et al., 2008). SLOS is a congenital and developmental malformation syndrome associated with defective cholesterol biosynthesis in which the immediate biosynthetic precursor of cholesterol (7-dehydrocholesterol or 7-DHC) is accumulated (Porter, 2008). We have recently shown that the effects of 7-DHC and cholesterol on membrane organization and dynamics are considerably different (Shrivastava et al., 2008). The cellular model of SLOS was generated by metabolically inhibiting the biosynthesis of cholesterol, utilizing a specific inhibitor (AY 9944) of the enzyme required in the final step of cholesterol biosynthesis. Importantly, AY 9944 treatment has previously been shown to generate animal (rat) models of SLOS (Wolf et al., 1996; Gaoua et al., 2000). SLOS serves as an appropriate condition to ensure the specific effect of membrane cholesterol in the function of the serotonin1A receptor, since the two aberrant sterols that are accumulated in SLOS, i.e., 7- and 8-DHC, differ with cholesterol only by a double bond. Our results showed a progressive and drastic reduction in specific ligand binding with increasing concentrations of AY 9944 (Paila et al., 2008). In addition, these results show that the G-protein coupling and downstream signaling of serotonin1A receptors are impaired in SLOS-like condition, although the membrane receptor level does not exhibit any reduction. Importantly, metabolic replenishment of cholesterol using serum partially restored the ligand binding activity of the serotonin1A receptor under these conditions.
(a) Effect of replenishment of 7-DHC and cholesterol into cholesterol-depleted membranes on the specific binding of [3H]8-OH-DPAT to the hippocampal serotonin1A receptor. Cholesterol depletion in native hippocampal membranes was achieved using MβCD followed by replenishment with 7-DHC or cholesterol. In addition, this panel shows the effect of oxidation of membrane cholesterol on the specific binding of [3H]8-OH-DPAT to the hippocampal serotonin1A receptor. Membrane cholesterol was oxidized using cholesterol oxidase (CO). Values (means ± standard error) are expressed as percentages of specific binding obtained in native membranes. (b) Effect of replenishment of 7-DHC or cholesterol into solubilized membranes (denoted as SM) on specific binding of [3H]8-OH-DPAT to the hippocampal serotonin1A receptor. Solubilized membranes were replenished with 7-DHC or cholesterol, using the corresponding sterol:MβCD complex. Values (means ± standard error) are expressed as percentages of specific ligand binding obtained in native membranes. Adapted and modified from Pucadyil et al. (2005b) and Paila and Chattopadhyay (2009)
Effect of replenishment of 7-DHC or cholesterol into cholesterol-depleted, solubilized membranes on fluorescence anisotropy (means ± standard error) of the membrane probe DPH. Cholesterol depletion was carried out using MβCD. Fluorescence anisotropy of cholesterol-oxidase treated membranes is also shown. Cholesterol was oxidized using cholesterol oxidase (CO). Membranes (cholesterol-depleted or solubilized) were replenished with 7-DHC or cholesterol, using the corresponding sterol:MβCD complex. Adapted and modified from Pucadyil et al. (2005b) and Paila and Chattopadhyay (2009)
Figure 16.4a shows that cholesterol depletion from native hippocampal membranes followed by replenishment with 7-DHC did not result in restoration of the ligand binding to the serotonin1A receptor, in spite of recovery of membrane order (Fig. 16.5) (Singh et al., 2007). In addition, solubilization of the hippocampal serotonin1A receptor is accompanied by loss of membrane cholesterol, which results in a reduction in specific ligand binding activity and overall membrane order (Chattopadhyay et al., 2005, 2007). It is important to note here that the loss in ligand binding of the serotonin1A receptor is not necessarily related to the reduction in overall membrane order. For example, solubilized membranes retained higher ligand binding compared to cholesterol-depleted membranes (Fig. 16.4), although overall membrane order appears to be lower in solubilized membranes (Fig. 16.5). This implies that general effects may not be an important factor.
Replenishment of cholesterol to solubilized membranes restores the cholesterol content of the membrane and significantly enhances specific ligand binding activity (Fig. 16.4b) and overall membrane order (Fig. 16.5). Importantly, replenishment of solubilized hippocampal membranes with 7-DHC does not result in restoration of ligand binding activity of the serotonin1A receptor (Fig. 16.4b), in spite of recovery of membrane order (Fig. 16.5). We therefore conclude that the requirement for maintaining ligand binding activity is more stringent than the requirement for maintaining membrane order. Taken together, these results indicate that the molecular basis for the requirement of membrane cholesterol in maintaining the ligand binding activity of serotonin1A receptors could be specific interaction, although global bilayer effects may not be ruled out (Prasad et al., 2009). In the light of these results, it is indeed interesting to note that there are reported cholesterol binding sites (possibly nonannular) in the crystal structure of a closely related receptor i.e., the β2-adrenergic receptor, as discussed above.
16.5.1 Cholesterol binding Motif(s) in Serotonin1A Receptors?
In the overall context of the presence of CCM in the recently reported crystal structure of the β2-adrenergic receptor (Hanson et al., 2008), it is tempting to consider whether there is a similar CCM(s) present in the serotonin1A receptor and if present, whether it is conserved during the natural evolution of the receptor. This is particularly relevant in view of the similarity between the serotonin1A and β2-adrenergic receptors (∼43% amino acid similarity in the transmembrane domain) (Kalipatnapu and Chattopadhyay, 2007), and the reported cholesterol dependence of serotonin1A receptor function (Pucadyil and Chattopadhyay, 2006). In order to examine the evolution of specific cholesterol binding site(s) of the serotonin1A receptor over various phyla, we analyzed amino acid sequences of the serotonin1A receptor from available databases (see Fig. 16.6). Partial, duplicate and other non-specific sequences were removed from the set of sequences obtained. The amino acid sequences used for the analysis belong to diverse taxons that include insects, fish and other marine species, amphibians and extending up to mammals. Initial alignment was carried out using ClustalW. It is apparent from this alignment that the cholesterol binding motif, which includes Tyr73 in the putative transmembrane helix II and Arg151, Ile157 and Trp161 in the putative transmembrane helix IV (Figs. 16.3 and 16.6), is conserved in most species. Realignment with ClustalW (after eliminating the relatively divergent parts of the receptor) resulted in conservation of the motif across all phyla analyzed, except in organisms such as T. adhaerens and S. purpuratus. Interestingly, pairwise alignment of the human serotonin1A receptor with the human β2-adrenergic receptor and rhodopsin exhibited conservation of the motif in all sequences. Cholesterol binding sites may therefore represent an inherent characteristic feature of serotonin1A receptors, which are conserved during the course of evolution. It is interesting to note here that cholesterol binding sites appear to be present even in organisms which are not capable of biosynthesis of cholesterol. Organisms which lack cholesterol biosynthesis could, however, acquire cholesterol through diet (Bloch, 1983). Organisms such as insects possess sterols that are different from cholesterol, and which have diverged from cholesterol during the evolution of the sterol pathway (Clark and Bloch, 1959). The presence of CCM in these organisms could be due to binding of closely related sterols or dietary cholesterol to CCM.
Multiple alignment of the serotonin1A receptor around the CCM of interest with the conserved residues highlighted. As evident from panel (a), Trp161 is the most conserved residue, except in S. purpuratus. The sequences of T. adhaerens, M. sexta and A. gambiae are putative serotonin1A receptors whereas those of S. pupuratus, B. taurus, O. anatinus, D. rerio, M. domestica, M. mulatta and T. guttata are predicted by homology. The sequence of C. elegans belongs to the serotonin receptor family. The sequences of C. porcellus, A. uniparens and A. inornata are partial. The numbers of amino acid residues in respective sequences are mentioned in parentheses. Amino acid sequences of serotonin1A receptors are from NCBI and Expasy databases. Panel (b) is a graphical representation displaying the quality of alignment, with lighter shades representing higher quality. Adapted and modified from Paila et al. (2009)
16.6 Conclusion and Future Perspectives
Previous work from our laboratory has comprehensively demonstrated that membrane cholesterol is required for the function of the serotonin1A receptor, which could be due to specific interaction of the receptor with cholesterol. Based on these results, we envisage that there could be specific/nonannular cholesterol binding site(s) in the serotonin1A receptor. Mutation of the amino acid residues involved in the cholesterol binding site of the serotonin1A receptor, followed by functional and organizational analyses of the receptor, are likely to provide further insight into the membrane cholesterol-dependence of receptor function.
GPCRs are involved in a multitude of physiological functions and represent important drug targets. Although the pharmacological and signaling features of GPCRs have been extensively studied, aspects related to their interaction with membrane lipids have been addressed in very few cases. In this context, the realization that lipids such as cholesterol could influence the function of GPCRs has remarkably transformed our ideas regarding the function of this important class of membrane proteins. With progress in deciphering molecular details on the nature of this interaction, our overall understanding of GPCR function in health and disease would improve significantly, thereby enhancing our ability to design better therapeutic strategies to combat diseases related to malfunctioning of these receptors. A comprehensive understanding of GPCR function in relation to the membrane lipid environment is important, in view of the enormous implications of GPCR function in human health (Jacoby et al., 2006; Schlyer and Horuk, 2006), and the observation that several diagnosed diseases are attributed to altered lipid-protein interactions (Pavlidis et al., 1994; Chattopadhyay and Paila, 2007).
Abbreviations
- 5-HT1A receptor:
-
5-hydroxytryptamine-1A receptor
- 7-DHC:
-
7-dehydrocholesterol
- 8-OH-DPAT:
-
8-hydroxy-2(di-N-propylamino)tetralin
- CCK:
-
cholecystokinin
- CCM:
-
cholesterol consensus motif
- DPH:
-
1,6-diphenyl-1,3,5-hexatriene
- FRET:
-
fluorescence resonance energy transfer
- GPCR:
-
G-protein coupled receptor
- MβCD:
-
methyl-β-cyclodextrin
- SLOS:
-
Smith-Lemli-Opitz syndrome
References
Albert, A.D., Young, J.E., Yeagle, P.L., 1996, Rhodopsin-cholesterol interactions in bovine rod outer segment disk membranes. Biochim. Biophys. Acta 1285: 47–55.
Albert, A.D., Boesze-Battaglia, K., 2005, The role of cholesterol in rod outer segment membranes. Prog. Lipid Res. 44: 99–124.
Angel, T.E., Chance, M.R., Palczewski, K., 2009, Conserved waters mediate structural and functional activation of family A (rhodopsin-like) G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 106: 8555–8560.
Attwood, P.V., Gutfreund, H., 1980, The application of pressure relaxation to the study of the equilibrium between metarhodopsin I and II from bovine retinas. FEBS Lett. 119: 323–326.
Ballesteros, J.A., Weinstein, H., 1995, Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 25: 366–428.
Bari, M., Battista, N., Fezza, F., Finazzi-Agrò, A., Maccarrone, M., 2005a, Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J. Biol. Chem. 280: 12212–12220.
Bari, M., Paradisi, A., Pasquariello, N., Maccarrone, M., 2005b, Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. J. Neurosci. Res. 81: 275–283.
Ben-Arie, N., Gileadi, C., Schramm, M., 1988, Interaction of the β-adrenergic receptor with Gs following delipidation. Specific lipid requirements for Gs activation and GTPase function. Eur. J. Biochem. 176: 649–654.
Bennet, M.P., Mitchell, D.C., 2008, Regulation of membrane proteins by dietary lipids: effects of cholesterol and docosahexanoic acid acyl chain-containing phospholipids on rhodopsin stability and function. Biophys. J. 95: 1206–1216.
Bittman, R., 1997, Has nature designed the cholesterol side chain for optimal interaction with phospholipids ? Subcell. Biochem. 28: 145–171.
Bloch, K.E., 1983, Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14: 47–92.
Brown, M.S., Goldstein, J.L., 1999, A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA 96: 11041–11048.
Burger, K., Gimpl, G., Fahrenholz, F., 2000, Regulation of receptor function by cholesterol. Cell. Mol. Life Sci. 57: 1577–1592.
Chattopadhyay, A., 1992, Membrane penetration depth analysis using fluorescence quenching: a critical review. In: Biomembrane Structure and Function: The State of The Art (Gaber, B.P. and Easwaran, K.R.K., eds.), Adenine Press, Schenectady, NY, pp. 153–163.
Chattopadhyay, A., Jafurulla, Md., Kalipatnapu, S., Pucadyil, T.J., Harikumar, K.G., 2005, Role of cholesterol in ligand binding and G-protein coupling of serotonin1A receptors solubilized from bovine hippocampus. Biochem. Biophys. Res. Commun. 327: 1036–1041.
Chattopadhyay, A., Paila, Y.D., 2007, Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem. Biophys. Res. Commun. 354: 627–633.
Chattopadhyay, A., Paila, Y.D., Jafurulla, Md., Chaudhuri, A., Singh, P., Murty, M.R.V.S., Vairamani, M., 2007, Differential effects of cholesterol and 7-dehydrocholesterol on ligand binding of solubilized hippocampal serotonin1A receptors: Implications in SLOS. Biochem. Biophys. Res. Commun. 363: 800–805.
Cherezov, V., Rosenbaum, D.M., Hanson, M.A., Rasmussen, S.G.F., Thian, F.S., Kobilka, T.S., Choi, H.-J., Kuhn, P., Weis, W.I., Kobilka, B.K., Stevens, R.C., 2007, High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318: 1258–1265.
Clark, A.J., Bloch, K., 1959, The absence of sterol synthesis in insects. J. Biol. Chem. 234: 2578–2582.
Colozo, A.T., Park, P.S.-H., Sum, C.S., Pisterzi, L.F., Wells, J.W., 2007, Cholesterol as a determinant of cooperativity in the M2 muscarinic cholinergic receptor. Biochem. Pharmacol. 74: 236–255.
Dailey, M.M., Hait, C., Holt, P.A., Maguire, J.M., Meier, J.B., Miller, M.C., Petraccone, L., Trent, J.O., 2009, Structure-based drug design: From nucleic acid to membrane protein targets. Exp. Mol. Pathol. 86:141–150.
DeDecker, B.S., O’Brien, R., Fleming, P.J., Geiger, J.H., Jackson, S.P., Sigler, P.B., 1996, The crystal structure of a hyperthermophilic archaeal TATA-box binding protein. J. Mol. Biol. 264: 1072–1084.
Devaux, P.F., Seigneuret, M., 1985, Specificity of lipid-protein interactions as determined by spectroscopic techniques. Biochim. Biophys. Acta 822: 63–125.
Drews, J., 2000, Drug discovery: a historical perspective. Science 287: 1960–1964.
Epand, R.F., Thomas, A., Brasseur, R., Vishwanathan, S.A., Hunter, E., Epand, R.M., 2006, Juxtamembrane protein segments that contribute to recruitment of cholesterol into domains. Biochemistry 45: 6105–6114.
Epand, R.M., 2006, Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 45: 279–294.
Eroglu, C., Cronet, P., Panneels, V., Beaufils, P., Sinning, I., 2002, Functional reconstitution of purified metabotropic glutamate receptor expressed in the fly eye. EMBO Rep. 3: 491–496.
Eroglu, C., Brugger, B., Wieland, F., Sinning, I., 2003, Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts. Proc. Natl. Acad. Sci. USA 100: 10219–10224.
Fahrenholz, F., Klein, U., Gimpl, G., 1995, Conversion of the myometrial oxytocin receptor from low to high affinity state by cholesterol. Adv. Exp. Med. Biol. 395: 311–319.
Fargin, A., Raymond, J.R., Lohse, M.J., Kobilka, B.K., Caron, M.G., Lefkowitz. R.J., 1988, The genomic clone G-21 which resembles a β-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature 335: 358–360.
Fredriksson, R., Schiöth, H.B., 2005, The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 67: 1414–1425.
Gaoua, W., Wolf, C., Chevy, F., Ilien, F., Roux, C., 2000, Cholesterol deficit but not accumulation of aberrant sterols is the major cause of the teratogenic activity in the Smith-Lemli-Opitz syndrome animal model. J. Lipid Res. 41: 637–646.
Gardier, A.M., 2009, Mutant mouse models and antidepressant drug research: focus on serotonin and brain-derived neurotrophic factor. Behav. Pharmacol. 20: 18–32.
Gether, U., 2000, Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21: 90–113.
Gether, U., Kobilka, B.K., 1998, G protein-coupled receptors. II. Mechanism of agonist activation. J. Biol. Chem. 273:17979–17982.
Gimpl, G., Klein, U., Reiländer, H., Fahrenholz, F., 1995, Expression of the human oxytocin receptor in baculovirus-infected insect cells: high-affinity binding is induced by a cholesterol-cyclodextrin complex. Biochemistry 34: 13794–13801.
Gimpl, G., Burger, K., Fahrenholz, F., 1997, Cholesterol as modulator of receptor function. Biochemistry 36: 10959–10974.
Gimpl, G., Fahrenholz, F., 2002, Cholesterol as stabilizer of the oxytocin receptor. Biochim. Biophys. Acta 1564: 384–392.
Gimpl, G., Burger, K., Fahrenholz, F., 2002a, A closer look at the cholesterol sensor. Trends Biochem. Sci. 27: 596–599.
Gimpl, G., Wiegand, V., Burger, K., Fahrenholz, F., 2002b, Cholesterol and steroid hormones: modulators of oxytocin receptor function. Prog. Brain Res. 139: 43–55.
Gimpl, G., Gehrig-Burger, K., 2007, Cholesterol reporter molecules. Biosci. Rep. 27: 335–358.
Granseth, E., Seppälä, S., Rappi, M., Daleyi, D.O., von Heijne, G., 2007, Membrane protein structural biology-How far can the bugs take us? Mol. Membr. Biol. 24: 329–332.
Hanson, M.A., Cherezov, V., Griffith, M.T., Roth, C.B., Jaakola, V.-P., Chien, E.Y.T., Velasquez, J., Kuhn, P., Stevens, R.C., 2008, A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure 16: 897–905.
Harikumar, K.G., Puri, V., Singh, R.D., Hanada, K., Pagano, R.E., Miller, L.J., 2005, Differential effects of modification of membrane cholesterol and sphingolipids on the conformation, function, and trafficking of the G protein-coupled cholecystokinin receptor. J. Biol. Chem. 280: 2176–2185.
Harris, J.S., Epps, D.E., Davio, S.R., Kezdy, F.J., 1995, Evidence for transbilayer, tail-to-tail cholesterol dimers in dipalmitoylglycerophosphocholine liposomes. Biochemistry 34: 3851–3857.
Heilker, R., Wolff, M., Tautermann, C.S., Bieler, M., 2009, G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov. Today 14: 231–240.
Hoyer, D., Hannon, J.P., Martin, G.R., 2002, Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 71: 533–554.
Huang, P., Xu, W., Yoon, S.-I., Chen, C., Chong, P.L.-G., Liu-Chen, L.-Y., 2007, Cholesterol reduction by methyl-β-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem. Pharmacol. 73: 534–549.
Huber, T., Botelho, A.V., Beyer, K., Brown, M.F., 2004, Membrane model for the G-protein-coupled receptor rhodopsin: hydrophobic interface and dynamical structure. Biophys. J. 86: 2078–2100.
Insel, P.A., Tang, C.M., Hahntow, I., Michel, M.C., 2007, Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim. Biophys. Acta 1768: 994–1005.
Jacob, R.F., Cenedella, R.J., Mason, R.P., 1999, Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. J. Biol. Chem. 274: 31613–31618.
Jacob, R.F., Cenedella, R.J., Mason, R.P., 2001, Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. J. Biol. Chem. 276: 13573–13578.
Jacoby, E., Bouhelal, R., Gerspacher, M., Seuwen, K., 2006, The 7TM G-protein-coupled receptor target family. Chem. Med. Chem. 1: 760–782.
Jamin, N., Neumann, J.M., Ostuni, M.A., Vu, T.K., Yao, Z.X., Murail, S., Robert, J.C., Giatzakis, C., Papadopoulos, V., Lacapere, J.J., 2005, Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol. Endocrinol. 19: 588–594.
Jones, O.T., Eubanks, J.H., Earnest, J.P., McNamee, M.G., 1988, A minimum number of lipids are required to support the functional properties of the nicotinic acetylcholine receptor. Biochemistry 27: 3733–3742.
Jones, O.T., McNamee, M.G., 1988, Annular and nonannular binding sites for cholesterol associated with the nicotinic acetylcholine receptor. Biochemistry 27: 2364–2374.
Jost, P.C., Griffith, O.H., Capaldi, R.A., Vanderkooi, G., 1973, Evidence for boundary lipids in membranes. Proc. Natl. Acad. Sci. USA 70: 480–484.
Kalipatnapu, S., Chattopadhyay, A., 2007, Membrane organization and function of the serotonin1A receptor. Cell. Mol. Neurobiol. 27: 1097–1116.
Kirilovsky, J., Schramm, M., 1983, Delipidation of a β-adrenergic receptor preparation and reconstitution by specific lipids. J. Biol. Chem. 258: 6841–6849.
Kirilovsky, J., Eimerl, S., Steiner-Mordoch, S., Schramm, M., 1987, Function of the delipidated β-adrenergic receptor appears to require a fatty acid or a neutral lipid in addition to phospholipids. Eur. J. Biochem. 166: 221–228.
Klein, U., Gimpl, G., Fahrenholz, F., 1995, Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34: 13784–13793.
Kobilka, B.K., Frielle, T., Collins, S., Yang-Feng, T., Kobilka, T.S., Francke, U., Lefkowitz, R.J., Caron, M.G., 1987, An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature 329: 75–79.
Kodner, R.B., Summons, R.E., Pearson, A., King, N., Knoll, A.H., 2008, Sterols in unicellular relative of the metazoans. Proc. Natl. Acad. Sci. USA 105: 9897–9902.
Kuwabara, P.E., Labouesse, M., 2002, The sterol-sensing domain: multiple families, a unique role. Trends Genet. 18: 193–201.
Lagane, B., Gaibelet, G., Meilhoc, E., Masson, J.-M., Cézanne, L., Lopez, A., 2000, Role of sterols in modulating the human μ-opioid receptor function in Saccharomyces cerevisiae. J. Biol. Chem. 275: 33197–33200.
Lange, Y., Steck, T.L., 2008, Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog. Lipid Res. 47: 319–332.
Lee, A.G., East, J.M., Jones, O.T., McWhirter, J., Rooney, E.K., Simmonds, A.C., 1982, Interaction of fatty acids with the calcium-magnesium ion dependent adenosinetriphosphatase from sarcoplasmic reticulum. Biochemistry 21: 6441–6446.
Lee, A.G., 2003, Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612: 1–40.
Lee, A.G., 2004, How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 1666: 62–87.
Lee, A.G., 2005, How lipids and proteins interact in a membrane: a molecular approach. Mol. BioSyst. 1: 203–212.
Levitt, E.S., Clark, M.J., Jenkins, P.M., Martens, J.R., Traynor, J.R., 2009, Differential effect of membrane cholesterol removal on μ and δ opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase. J. Biol. Chem. 284: 22108–22122.
Li, H., Papadopoulos, V., 1998, Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 139: 4991–4997.
Lin, S.H., Civelli, O., 2004, Orphan G protein-coupled receptors: targets for new therapeutic interventions. Ann. Med. 36: 204–214.
Liscum, L., Underwood, K.W., 1995, Intracellular cholesterol transport and compartmentation. J. Biol. Chem. 270: 15443–15446.
Liu, Y., Engelman, D.M., Gerstein, M., 2002, Genomic analysis of membrane protein families: abundance and conserved motifs. Genome Biol. 3: 1–12.
Loura, L.M.S., Prieto, M., 1997, Dehydroergosterol structural organization in aqueous medium and in a model system of membranes. Biophys. J. 72: 2226–2236.
Marius, P., Alvis, S.J., East, J.M., Lee, A.G., 2005, The interfacial lipid binding site on the potassium channel KcsA is specific for anionic phospholipids. Biophys. J. 89: 4081–4089.
Marius, P., Zagnoni, M., Sandison, M.E., East, J.M., Morgan, H., Lee, A.G., 2008, Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys. J. 94: 1689–1698.
Marsh, D., 1990, Lipid-protein interactions in membranes. FEBS Lett. 268: 371–375.
Mason, R.P., Tulenko, T.N., Jacob, R.F., 2003, Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim. Biophys. Acta 1610: 198–207.
Milligan, G., Parenti, M., Magee, A.I., 1995, The dynamic role of palmitoylation in signal transduction. Trends Biochem. Sci. 20: 181–187.
Mitchell, D.C., Straume, M., Miller, J.L., Litman, B.J., 1990, Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry 29: 9143–9149.
Monastyrskaya, K., Hostettler, A., Buergi, S., Draeger, A., 2005, The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J. Biol. Chem. 280: 7135–7146.
Mouritsen, O.G., Zuckermann, M.J., 2004, What’s so special about cholesterol? Lipids 39:1101–1113.
Mukherjee, S., Chattopadhyay, A., 1996, Membrane organization at low cholesterol concentrations: a study using 7-nitrobenz-2-oxa-1,3-diazol-4-yl-labeled cholesterol. Biochemistry 35: 1311–1322.
Mukherjee, S., Maxfield, F.R., 2004, Membrane domains. Annu. Rev. Cell Dev. Biol. 20: 839–866.
Mukherjee, S., Chattopadhyay, A., 2005, Monitoring cholesterol organization in membranes at low concentrations utilizing the wavelength-selective fluorescence approach. Chem. Phys. Lipids 134: 79–84.
Nature reviews drug discovery GPCR questionnaire participants, The state of GPCR research in 2004. Nat. Rev. Drug Discov. 3: 577–626.
Nguyen, D.H., Taub, D., 2002a, CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168: 4121–4126.
Nguyen, D.H., Taub, D., 2002b, Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood 99: 4298–4306.
Nguyen, D.H., Taub, D.D., 2003, Inhibition of chemokine receptor function by membrane cholesterol oxidation. Exp. Cell Res. 291: 36–45.
Niu, S.L., Mitchell, D.C., Litman, B.J., 2002, Manipulation of cholesterol levels in rod disk membranes by methyl-β-cyclodextrin: effects on receptor activation. J. Biol. Chem. 277: 20139–20145.
Nyholm, T.K.M., Özdirekcan, S., Killian, J.A., 2007, How transmembrane segments sense the lipid environment. Biochemistry 46: 1457–1465.
Ohvo-Rekilä, H., Ramstedt, B., Leppimäki, P., Slotte, J.P., 2002, Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41: 66–97.
Opekarová, M., Tanner, W., 2003, Specific lipid requirements of membrane proteins – a putative bottleneck in heterologous expression. Biochim. Biophys. Acta 1610: 11–22.
Paila, Y.D., Pucadyil, T.J., Chattopadhyay, A., 2005, The cholesterol-complexing agent digitonin modulates ligand binding of the bovine hippocampal serotonin1A receptor. Mol. Membr. Biol. 22: 241–249.
Paila, Y.D., Murty, M.R.V.S., Vairamani, M., Chattopadhyay, A., 2008, signaling by the human serotonin1A receptor is impaired in cellular model of Smith-Lemli-Opitz Syndrome. Biochim. Biophys. Acta 1778: 1508–1516.
Paila, Y.D., Chattopadhyay, A., 2009, The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction? Glycoconj. J. 26: 711–720.
Paila, Y.D., Tiwari, S., Chattopadhyay, A., 2009, Are specific nonannular cholesterol binding sites present in G-protein coupled receptors ? Biochim. Biophys. Acta 1788: 295–302.
Palsdottir, H., Hunte, C., 2004, Lipids in membrane protein structures. Biochim. Biophys. Acta 1666: 2–18.
Pandit, S.A., Jakobsson, E., Scott, H.L., 2004, Simulation of the early stages of nano-domain formation in mixed bilayers of sphingomyelin, cholesterol, and dioleylphosphatidylcholine. Biophys. J. 87: 3312–3322.
Pang, L., Graziano, M., Wang, S., 1999, Membrane cholesterol modulates galanin-GalR2 interaction. Biochemistry 38: 12003–12011.
Papoucheva, E., Dumuis, A., Sebben, M., Richter, D.W., Ponimaskin, E.G., 2004, The 5-hydroxytryptamine1A receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi protein. J. Biol. Chem. 279:3280–3291.
Park, P.S., Filipek, S., Wells, J.W., Palczewski, K., 2004, Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry 43: 15643–15656.
Pavlidis, P., Ramaswami, M., Tanouye, M.A., 1994, The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell 79: 23–33.
Perez, D.M., 2003, The evolutionarily triumphant G-protein-coupled receptor. Mol. Pharmacol. 63: 1202–1205.
Pierce, K.L., Premont, R.T., Lefkowitz, R.J., 2002, Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3: 639–650.
Pitman, M.C., Grossfield, A., Suits, F., Feller, S.E., 2005, Role of cholesterol and polyunsaturated chains in lipid-protein interactions: molecular dynamics simulation of rhodopsin in a realistic membrane environment. J. Am. Chem. Soc. 127: 4576–4577.
Politowska, E., Kazmierkiewicz, R., Wiegand, V., Fahrenholz, F., Ciarkowski, J., 2001, Molecular modeling study of the role of cholesterol in the stimulation of the oxytocin receptor. Acta Biochim. Pol. 48: 83–93.
Polozova, A., Litman, B.J., 2000, Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J. 79: 2632–2643.
Porter, F.D., 2008, Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 16: 535–541.
Pöyry, S., Róg, T., Karttunen, M., Vattulainen, I., 2008, Significance of cholesterol methyl groups. J. Phys. Chem. B 112: 2922–2929.
Prasad, R., Singh, P., Chattopadhyay, A., 2009, Effect of capsaicin on ligand binding activity of the hippocampal serotonin1A receptor. Glycoconj. J. 26: 733–738.
Pucadyil, T.J., Chattopadhyay, A., 2004, Cholesterol modulates the ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim. Biophys. Acta 1663: 188–200.
Pucadyil, T.J., Shrivastava, S., Chattopadhyay, A., 2004, The sterol-binding antibiotic nystatin differentially modulates ligand binding of the bovine hippocampal serotonin1A receptor. Biochem. Biophys. Res. Commun. 320: 557–562.
Pucadyil, T.J., Chattopadhyay, A., 2005, Cholesterol modulates the antagonist-binding function of bovine hippocampal serotonin1A receptors. Biochim. Biophys. Acta 1714: 35–42.
Pucadyil, T.J., Kalipatnapu, S., Chattopadhyay, A., 2005a, The serotonin1A receptor: A representative member of the serotonin receptor family. Cell. Mol. Neurobiol. 25: 553–580.
Pucadyil, T.J., Shrivastava, S., Chattopadhyay, A., 2005b, Membrane cholesterol oxidation inhibits ligand binding function of hippocampal serotonin1A receptors. Biochem. Biophys. Res. Commun. 331: 422–427.
Pucadyil, T.J., Chattopadhyay, A., 2006, Role of cholesterol in the function and organization of G-protein coupled receptors. Prog. Lipid Res. 45: 295–333.
Pucadyil, T.J., Chattopadhyay, A., 2007, Cholesterol: a potential therapeutic target in Leishmania infection? Trends Parasitol. 23: 49–53.
Pucadyil, T.J., Mukherjee, S., Chattopadhyay, A., 2007, Organization and dynamics of NBD-labeled lipids in membranes analyzed by fluorescence recovery after photobleaching. J. Phys. Chem. B 111: 1975–1983.
Raymond, J.R., Mukhin, Y.V., Gettys, T.W., Garnovskaya, M.N., 1999, The recombinant 5-HT1A receptor: G protein coupling and signaling pathways. Br. J. Pharmacol. 27: 1751–1764.
Riethmüller, J., Riehle, A., Grassmé, H., Gulbins, E., 2006, Membrane rafts in host-pathogen interactions. Biochim. Biophys. Acta 1758: 2139–2147.
Róg, T., Pasenkiewicz-Gierula, M., 2001, Cholesterol effects on the phosphatidylcholine bilayer nonpolar region: A molecular simulation study. Biophys. J. 81: 2190–2202.
Róg, T., Pasenkiewicz-Gierula, M., Vattulainen, I., Karttunen, M., 2007, What happens if cholesterol is made smoother: importance of methyl substituents in cholesterol ring structure on phosphatidylcholine-sterol interaction. Biophys. J. 92: 3346–3357.
Rosenbaum, D.M., Rasmussen, S.G.F., Kobilka, B.K., 2009, The structure and function of G-protein-coupled receptors. Nature 459: 356–363.
Rukmini, R., Rawat, S.S., Biswas, S.C., Chattopadhyay, A., 2001, Cholesterol organization in membranes at low concentrations: effects of curvature stress and membrane thickness. Biophys. J. 81: 2122–2134.
Ruprecht, J.J., Mielke, T., Vogel, R., Villa, C., Schertler, G.F., 2004, Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 23: 3609–3620.
Schlyer, S., Horuk, R., 2006, I want a new drug: G-protein-coupled receptors in drug development. Drug Discov. Today 11: 481–493.
Schroeder, F., Woodford, J.K., Kavecansky, J., Wood, W.G., Joiner, C., 1995, Cholesterol domains in biological membranes. Mol. Membr. Biol. 12: 113–119.
Shrivastava, S., Paila, Y.D., Dutta, A., Chattopadhyay, A., 2008, Differential effects of cholesterol and its immediate biosynthetic precursors on membrane organization. Biochemistry 47: 5668–5677.
Simmonds, A.C., East, J.M., Jones, O.T., Rooney, E.K., McWhirter, J., Lee, A.G., 1982, Annular and nonannular binding sites on the (Ca2+ + Mg2+)-ATPase. Biochim. Biophys. Acta 693: 398–406.
Simons, K., van Meer, G., 1988, Lipid sorting in epithelial cells. Biochemistry 27: 6197–6202.
Simons, K., Ikonen, E., 1997, Functional rafts in cell membranes. Nature 387: 569–572.
Simons, K., Ikonen, E., 2000, How cells handle cholesterol. Science 290: 1721–1725.
Simons, K., Toomre, D., 2000, Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39.
Simons, K., Ehehalt, R., 2002, Cholesterol, lipid rafts, and disease. J. Clin. Invest. 110: 597–603.
Singh, P., Paila, Y.D., Chattopadhyay, A., 2007, Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin1A receptors: Implications in SLOS. Biochem. Biophys. Res. Commun. 358: 495–499.
Sjögren, B., Hamblin, M.W., Svenningsson, P., 2006, Cholesterol depletion reduces serotonin binding and signaling via human 5-HT7(a) receptors. Eur. J. Pharmacol. 552: 1–10.
Straume, M., Litman, B.J., 1988, Equilibrium and dynamic bilayer structural properties of unsaturated acyl chain phosphatidylcholine-cholesterol-rhodopsin recombinant vesicles and rod outer segment disk membranes as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry 27: 7723–7733.
Toth, M., 2003, 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur. J. Pharmacol. 463: 177–184.
Tulenko, T.N., Chen, M., Mason, P.E., Mason, R.P., 1998, Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid Res. 39: 947–956.
Villalaín, J., 1996, Location of cholesterol in model membranes by magic-angle-sample-spinning NMR. Eur. J. Biochem. 241: 586–593.
Wolf, C., Chevy, F., Pham, J., Kolf-Clauw, M., Citadelle, D., Mulliez, N., Roux, C., 1996, Changes in serum sterols of rats treated with 7-dehydrocholesterol-Δ7-reductase inhibitors: comparison to levels in humans with Smith-Lemli-Opitz syndrome. J. Lipid Res. 37: 1325–1333.
Wüstner, D., 2007, Fluorescent sterols as tools in membrane biophysics and cell biology. Chem. Phys. Lipids 146: 1–25.
Xu, W., Yoon, S.-I., Huang, P., Wang, Y., Chen, C., Chong, P.L.-G., Liu-Chen, L.-Y., 2006, Localization of the κ opioid receptor in lipid rafts. J. Pharmacol. Exp. Ther. 317: 1295–1306.
Xu, X., London, E., 2000, The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39: 843–849.
Yao, Z., Kobilka, B., 2005, Using synthetic lipids to stabilize purified β2 adrenoceptor in detergent micelles. Anal. Biochem. 343: 344–346.
Yeagle, P.L., 1985, Cholesterol and the cell membrane. Biochim. Biophys. Acta 822: 267–287.
Acknowledgements
Work in A.C.’s laboratory was supported by the Council of Scientific and Industrial Research, Department of Biotechnology, Life Sciences Research Board, and the International Society for Neurochemistry. Y.D.P. thanks the Council of Scientific and Industrial Research for the award of a Senior Research Fellowship. A.C. is an Adjunct Professor at the Special Centre for Molecular Medicine of Jawaharlal Nehru University (New Delhi, India), and Honorary Professor of the Jawaharlal Nehru Centre for Advanced Scientific Research (Bangalore, India). A.C. gratefully acknowledges J.C. Bose Fellowship (Dept. Science and Technology, Govt. of India). We thank Sourav Haldar for helpful discussions, Roopali Saxena for help with the figures, Shrish Tiwari for his help in generating Fig. 16.6 and members of our laboratory for critically reading the manuscript
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Paila, Y.D., Chattopadhyay, A. (2010). Membrane Cholesterol in the Function and Organization of G-Protein Coupled Receptors. In: Harris, J. (eds) Cholesterol Binding and Cholesterol Transport Proteins:. Subcellular Biochemistry, vol 51. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-8622-8_16
Download citation
DOI: https://doi.org/10.1007/978-90-481-8622-8_16
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-8621-1
Online ISBN: 978-90-481-8622-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)