Abstract
Hemiclonal hybrids of Western Palearctic water frogs of the Rana esculenta complex transmit only one parental genome to their offspring without recombination (hybridogenesis). Such genomes are thus prone to accumulate deleterious mutations. The frog complex is unique among hybridogens in that hemiclonal hybrids occur in both sexes. This provides the opportunity of using experimental crosses to produce offspring possessing two clonal genomes of various origins and thereby study their homozygous and heterozygous effects on fitness. Here we review work that made use of this possibility to assess the evolutionary consequences of clonal inheritance in water frogs. Overall, these studies indicate that clonally transmitted genomes bear a substantial load of fixed deleterious mutations, yet these mutations appear to have minor effects on fitness in the heterozygous state. We also point out potential mechanisms for episodic recombination by which otherwise clonal genomes may be purged of deleterious alleles, and we present evidence for such episodic recombination to occur in natural populations of hybridogenetic frogs. Finally, we provide an outlook on work in progress that exploits the peculiarities of this system to obtain relevant estimates of the frequency of segregating lethal mutations in sexual populations of water frogs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
20.1 Hybridogenesis
The term hybridogenesis was introduced by Schultz (1969) to describe a remarkable type of non-Mendelian inheritance that was detected in interspecific hybrids of fishes from the genus Poeciliopsis in Mexico (see also Chapter 19). These all-female hybrids form distinct taxa that are somatically intermediate between the parental species, but they routinely exclude the entire paternal chromosome set from the germline to produce haploid eggs only containing the unrecombined maternal genome. For their reproduction, hybrids depend entirely on backcrossing with males of their paternal species, with which they are thus always sympatric. Because hybridogens always possess one sexually and one clonally transmitted genome, individuals sharing the same clonal genome can be referred to as a hemiclone (Vrijenhoek et al. 1977).
Since Schultz’ original description of the phenomenon, hybridogenesis has been found in other fishes, Iberian minnows of the Squalius alburnoides complex (Carmona et al. 1997) and in Korean fish of the genus Cobitis (Saitoh et al. 2004), stick insects of the genus Bacillus (Mantovani and Scali 1992; Tinti et al. 1995), and – the topic of this review – Western Palearctic water frogs of the genus Rana Footnote 1 (Tunner 1974). However, this unusual mode of reproduction may well have remained undetected in other species complexes with frequent hybridization. Hybridogenesis can arise as a spontaneous consequence of interspecific hybridization, as evidenced by the successful “de novo” formation of hybridogens in Poeciliopsis and Rana (Schultz 1973; Hotz et al. 1985; Vorburger and Reyer 2003). The molecular and cytological mechanisms by which genome exclusion is achieved in hybridogens are still poorly understood and may well differ among the different taxa. What is important for this review is the genetic consequence: Hybridogenesis leads to the clonal transmission of a haploid genome.
20.2 The Special Case of Water Frogs
It was an extensive series of crossing experiments performed by Leszek Berger in Poland that uncovered the fact that Rana esculenta, described by Linnaeus and until then considered a proper species, was in fact a hybrid between R. ridibunda Pallas and R. lessonae Camerano (Berger 1967, 1968, 1970). Rana lessonae is the smallest of the Western Palearctic water frogs (females up to 80 mm snout-to-vent length); it preferentially breeds in shallow, small and vegetated ponds and overwinters terrestrially. Rana ridibunda, on the other hand, is Europe’s largest amphibian (females up to 140 mm snout-to-vent length) and occurs in larger, deeper, more open water bodies, in which it also overwinters (Plötner 2005). The hybrid R. esculenta is intermediate in size as well as in traits relevant for mate choice like advertisment calls and coloration. Its habitat preferences are also intermediate yet very broad, almost encompassing both parental niches. This may at least in part explain its wide distribution and high abundance in Europe.
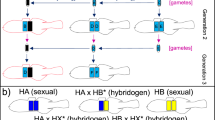
The striking non-Mendelian inheritance of morphological traits and of serological markers led Tunner (1973) to realize that only one parental chromosome set is transmitted by hybrids, and he consequentially applied the term hybridogenesis to R. esculenta’s mode of reproduction (Tunner 1974). Unlike all other known hybridogens, R. esculenta is bisexual, and both sexes reproduce by hybridogenesis, offering unique research opportunities that will be discussed later on. Over much of central Europe, R. esculenta occurs outside the range of R. ridibunda and forms mixed populations with only one of its parental species, R. lessonae. This is referred to as the L-E system (Uzzell and Berger 1975) and is illustrated in Fig. 20.1. In such populations, R. esculenta excludes the lessonae genome from the germline and clonally transmits the ridibunda genome. Hybrids persist by backcrossing with R. lessonae every generation anew to produce R. esculenta offspring that again exclude the lessonae genome from the germline. Rana esculenta is thus a sexual parasite of R. lessonae. Matings among hybrids also occur in the L-E system. Such matings would produce R. ridibunda offspring, but these are typically inviable and die at an early larval stage (Fig. 20.1). Another interesting characteristic of the L-E system is that hybrid males – with a few exceptions – only produce daughters. The reason for this is that these frogs exhibit X–Y sex determination (Berger et al. 1988), and that for behavioural reasons, primary hybridizations tend to occur between females of R. ridibunda and males of R. lessonae. As a consequence, the clonally transmitted ridibunda genome in the hybrid R. esculenta contains an X chromosome and male hybrids thus produce X-bearing sperm.
Gamete production, possible mating combinations and resulting offspring in the L-E system, i.e., mixed populations of Rana lessonae (LL) and R. esculenta (RL), a hemiclonal hybrid between R. ridibunda (RR) and R. lessonae. Reproduced with permission from Vorburger (2001c)
The sensational discovery of their hemiclonal reproduction raised interest in the water frogs and subsequent field surveys revealed a very complex picture of different population systems across Europe (reviewed in Graf and Polls Pelaz 1989; Günther 1990; Plötner 2005). A second hybridogen, R. grafi, occurs in southern France and north-eastern Spain. It is a hybrid between R. ridibunda and R. perezi, with which it coexists to form the so called P-G system. A third hybridogen, a little confusingly referred to as R. hispanica, occurs in central and southern Italy. This taxon is a hybrid between R. ridibunda and R. bergeri, a water frog of the Italian peninsula with a somewhat uncertain taxonomic status. The mirror image of the L-E system, the R-E system, occurs in parts of north-eastern Europe (Uzzell and Berger 1975; Uzzell et al. 1977). There, R. esculenta males form mixed populations with R. ridibunda and often exclude the ridibunda rather than the lessonae genome from the germline. Finally and most remarkably, all-hybrid populations exist in northern Europe (Ebendal 1979; Günther et al. 1979). These populations consist of diploid and triploid hybrids with different combinations of parental genomes and different types of genome exclusion. Yet by far the most widespread and best studied is the L-E system, to which we will restrict ourselves for the rest of this chapter.
20.3 Hemiclonality – A Predisposition to Mutation Accumulation
Hybridogenesis obviously serves the selfish interests of the clonally transmitted genome very well, but it also seems like the perfect recipe for its rapid mutational degradation. Clonal populations of finite size suffer from the accumulation of deleterious mutations through Muller’s ratchet (Muller 1964). In the haploid case, it has been shown that the rate of fixation of deleterious alleles equals the rate of accumulation, such that over time, a large part of the mutations in the population are shared by all the individuals of the population (Higgs and Woodcock 1995; Charlesworth and Charlesworth 1997). The rate at which this fixation occurs depends on the deleterious mutation rate U, on the average selection coefficient against deleterious alleles s, and on the population size N. We have no reason to assume that U would be higher for clonally transmitted genomes than for sexually transmitted genomes in the L-E system. In fact, U may even be lower as explained later on. Selection against deleterious mutations, on the other hand, will be weak. This is because clonal ridibunda genomes are constantly sheltered by sexual lessonae genomes in R. esculenta, preventing their occurrence in the homozygous state and reducing selection against deleterious alleles by their dominance coefficient h. Thus, deleterious mutations with very low h are invisible to selection and can accumulate roughly at the rate they occur. What about population size? Water frogs are common at permanent water bodies and tend to occur in large populations. Yet with respect to mutation accumulation, the hybrids’ population history may be of particular relevance. The immigration of R. esculenta from areas of sympatry of the parental species into Central and Western Europe may have been accompanied by many colonization events, each possibly representing a bottleneck of only few founders. Founder events promote genetic drift and thus the fixation of deleterious mutations (e.g., Chao 1990). A second source of bottlenecks on a more regular basis is the fact the R. esculenta relies on matings with R. lessonae for its persistence. Depending on the ecological characteristics of the breeding habitat, water frog populations vary widely in their composition (Rybacki and Berger 1994, 2001; Holenweg Peter et al. 2002). Commonly, they are dominated by R. esculenta and in some cases contain less than 5% of R. lessonae (e.g., Blankenhorn et al. 1973; Rybacki and Berger 1994, 2001). At such sites, the number of successfully breeding hybrids may only be a fraction of the total population.
Finally, a particularly intriguing possibility is that mutation accumulation on clonally transmitted genomes may be promoted by sexual selection. While male R. lessonae do not appear to discriminate (and may not even benefit from doing so, see Schmeller et al. 2005a), female R. lessonae have been shown to exhibit a preference for males of their own species (Abt and Reyer 1993). This preference is crucial for the stability of the L-E system (Guex et al. 1993; Hellriegel and Reyer 2000; Som et al. 2000). If a loss-of-function mutation in a gene affecting a male trait used in female choice occurs on a clonally transmitted ridibunda genome, it may cause a more “lessonae-like” phenotype in R. esculenta males bearing it and thus increase their chances of mating with females of the sexual host. Although undoubtedly deleterious in a homospecific background, such mutations could be beneficial to hybrids and thus even be under positive selection within an L-E system.
20.4 Mutational Load in Rana esculenta
Based on these considerations, the expectation is that ridibunda genomes in natural L-E systems suffer from a high mutational load. The first and most manifest evidence that this may indeed be so comes from the very fact that defines an L-E system – the lack of adult R. ridibunda in the population. It cannot be explained by the lack of R. esculenta × R. esculenta matings that give rise to R. ridibunda offspring. Although female R. esculenta prefer male R. lessonae over hybrid males in two-choice situations (Abt and Reyer 1993; Roesli and Reyer 2000; Engeler and Reyer 2001), this preference is not always realized in more complex situations (Bergen et al. 1997). Abt Tietje and Reyer (2004) further showed that a substantial fraction of clutches found in natural ponds are the result of matings between two hybrids. Thus, R. ridibunda are produced in L-E systems but are apparently inviable. This was confirmed by numerous crossing experiments during early investigations of the system (e.g., Berger 1967; Blankenhorn et al. 1971; Heusser and Blankenhorn 1973). These findings were consistent with a high mutational load on clonal ridibunda genomes, but could not reveal whether the inviability is caused (i) by the homozygosity of recessive deleterious alleles at particular loci or (ii) by the cumulative load from the general deterioration of the non-recombining ridibunda genomes, independent of homozygosity. Although not mutually exclusive, these hypotheses create different predictions. Under the first hypothesis, matings between different hemiclones, i.e., between R. esculenta possessing evolutionarily independent ridibunda genomes from different primary hybridizations, should produce viable offspring, because it is very unlikely that different clonal lineages are just by chance fixed for the same mutations. Under the second hypothesis, all hybrid × hybrid matings should produce inviable progeny, with a possible correlation between the age of a clonal lineage and the severity of observed genetic defects (although that might be impossible to quantify, because a tadpole cannot be more than dead). In the following two paragraphs, we summarize studies that explicitly addressed these predictions and provided support for the first hypothesis, although with some very informative inconsistencies.
Vorburger (2001a) performed a diallele crossing experiment with R. esculenta parents from three different populations in Switzerland. The two northern populations (Elliker Auen and Alpnach) were separated from the southern population (Seseglio) by the Alps, which represent an insurmountable dispersal barrier for water frogs. Allozyme analysis revealed that the parents from Seseglio and Alpnach each belonged to a single but different hemiclone, while parents from Elliker Auen comprised three hemiclones, one of which was indistinguishable from the one in Alpnach. The clearest result was provided by the crosses within and between populations Alpnach and Seseglio: All within-population crosses produced inviable progeny, whereas all crosses between populations – using the same parents – produced viable tadpoles that successfully completed metamorphosis. Importantly, tadpoles of within-population crosses from Alpnach suffered from different developmental abnormalities than tadpoles from Seseglio (Fig. 20.2). This pattern clearly supports the hypothesis that the two clonal ridibunda genomes from the two localities are fixed for different recessive deleterious mutations that are responsible for the observed inviability of hybrid × hybrid crosses in the natural populations. Crosses within population Elliker Auen and between Elliker Auen and Alpnach produced less consistent results. It was not surprising that many of the crosses between the two populations were inviable, because one hemiclone occurred at both sites. It was also not too surprising that some within-population crosses from Elliker Auen survived, because the parents belonged to three different hemiclones. However, there were also two viable crosses between parents belonging to the same hemiclone, and five inviable crosses between parents belonging to different hemiclones. While the former could be explained by the limited resolution of the allozyme markers used, the latter seems to contradict the hypothesis that offspring inviability is caused by homozygosity for recessive deleterious alleles, unless the assumption is unjustified that different clonal ridibunda genomes found in the Elliker Auen population are evolutionarily independent (see below). All Swiss hemiclones were also backcrossed with sexual R. ridibunda to produce offspring possessing one clonal and one sexual ridibunda genome. These crosses were generally viable and larval life-history traits were comparable to those of normal, sexually produced R. ridibunda tadpoles, suggesting that the heterozygous fitness effects of fixed mutations on the clonally transmitted ridibunda genomes are minor.
Lateral and dorsal view of representative tadpoles from Rana esculenta × R. esculenta crosses between Alpnach and Seseglio (A), within population Alpnach (B), and within population Seseglio (C). Drawings were produced from photographs taken approximately three weeks after hatching. Reproduced with permission from Vorburger (2001a)
In a second study by Guex et al. (2002), crosses were performed within and among two different hemiclones from a Swiss population (Gütighausen) and a single hemiclone from Sicily. The inclusion of the Sicilian hemiclone added a temporal dimension; while ridibunda genomes found in Swiss hemiclones may plausibly have persisted without recombination for about 5,000 or fewer generations (Guex et al. 2002), paleogeography indicates that the Sicilian ridibunda genome must have experienced a minimum of about 20,000 generations of clonal transmission (Santucci et al. 1996). As expected, all crosses within the Sicilian hemiclone were inviable. Yet despite the age of the Sicilian hemiclone, it produced viable offspring when crossed with the two Swiss hemiclones, also indicating that homozygosity for particular recessive deleterious alleles rather than the cumulative mutational load is responsible for the observed inviabilities in natural populations. All crosses among Swiss R. esculenta, on the other hand, were inviable, even those between the two different hemiclones occurring at Gütighausen. Again, this finding is inconsistent with the homozygosity hypothesis, unless the two ridibunda genomes from Gütighausen are not evolutionarily independent. Backcrosses of Swiss and Sicilian hemiclones with R. ridibunda were viable, with at most minor reductions in larval performance compared to offspring with two R. ridibunda parents.
Both of the above studies thus suggest that when in the heterozygous state, the negative effects of fixed deleterious alleles on non-recombining ridibunda genomes are very small. This seems to be supported by the high fitness and competitive ability of R. esculenta reported in many studies (e.g., Semlitsch 1993; Rist et al. 1997). However, this evidence is weak because comparisons with sexual R. ridibunda reported above are based on only a small number of crosses, and because the high fitness of R. esculenta may be a consequence of heterosis observed in these hybrids (Hotz et al. 1999), compensating for the ridibunda genome’s mutational load. A valid test must therefore uncouple clonal inheritance from hybridity. Vorburger (2001b) thus used artificial fertilizations to cross each of several R. ridibunda females with six males, three R. ridibunda males from three different populations, and three R. esculenta males from three populations. The resulting offspring were raised under benign (high food) and stressful (low food) conditions. In both treatments, tadpoles with R. esculenta fathers performed just as well and for one trait (size at metamorphosis) even better than tadpoles with R. ridibunda fathers, confirming that clonal ridibunda genomes in the heterozygous state have little, if any, negative effects on fitness. Because the evolutionary fate of the hybrid taxon R. esculenta depends primarily on these heterozygous effects, it may not be as gloomy as suggested by Milinski (1994).
In the following sections, we discuss mechanisms by which clonal ridibunda genomes may be able to purge deleterious alleles through episodic recombination and thus maintain a bearable load. However, it is also worth mentioning that their deleterious mutation rate may be reduced from the outset. The reason is that clonal ridibunda genomes in L-E systems contain an X chromosome and are therefore passed on through females more often than through males (Som and Reyer 2006). As mutation rates are generally higher in male than in female germlines (Redfield 1994; Hurst and Ellegren 1998; Ellegren and Fridolfsson 2003), clonal autosomes may overall experience a lower per-generation input of deleterious mutations than sexually transmitted autosomes, which on average spend equal amounts of time in both sexes. This effect may even be stronger in hybridogenetic systems other than water frogs, because there clonal genomes occur exclusively in females (Som et al. 2007).
20.5 Lost Load: Occasional Recombination Between Hemiclones
The studies by Vorburger (2001a) and Guex et al. (2002) both found that self-incompatible hemiclones can produce viable R. ridibunda offspring when crossed with different hemiclones, thus supporting the homozygosity hypothesis. Interestingly, both studies also shared the same inconsistency, namely that this outcome was entirely predictable for hemiclones that were widely separated geographically, but not for hemiclones occurring within the same population. We believe there is a common reason for this similarity: the potential for episodic recombination among clonal ridibunda genomes, resulting from the presence of both sexes in hybridogens.
Hotz et al. (1992) found a subpopulation of R. ridibunda females within an L-E system at Trubeschloo, Switzerland, and used mtDNA RFLPs to demonstrate conclusively that these females were produced by matings among R. esculenta. Apparently, compatible hemiclones came into contact at this site. Similarly, Vorburger (2001c) detected numerous R. ridibunda metamorphs in another Swiss L-E population (Elliker Auen – the multiclonal population also used in one of the crossing experiments described above). The allozyme genotypes of these froglets were consistent with combinations of clonal genomes present at the site and they all developed into females, also proving that they were formed by matings among R. esculenta. Such successful hybrid × hybrid matings cannot found independently reproducing populations of R. ridibunda, because all offspring are female. However, these females are expected to exhibit normal Mendelian meiosis and thus recombine the two clonal genomes they inherited from their hybrid parents. Subsequent matings with syntopic R. lessonae males may then found new hemiclones possessing recombined ridibunda genomes. This potential for occasional recombination, illustrated in Fig. 20.3, led Schmidt (1993) to compare hybridogenetic water frogs with cyclical parthenogens. As a consequence of this process, coexisting hemiclones would no longer be evolutionarily independent. Deleterious mutations present in the original ridibunda genomes may either be purged (Som and Reyer 2007) or else become linked to new combinations or marker alleles, making it impossible to predict offspring viability from the combination of parental hemiclones. We believe that this is the reason for what we called “informative inconsistencies” of the crossing experiments described above. These findings also urge some caution in the use of R. esculenta as a model to study the long-term consequences of the lack of recombination, because many ridibunda genomes found in L-E systems today may not have an uninterrupted history of clonal inheritance.
Proposed mechanism for occasional recombination between otherwise clonally transmitted ridibunda genomes in L-E systems, mediated by viable R. ridibunda females produced by matings among two different, genetically compatible hemiclones of R. esculenta. Reproduced with permission from Vorburger (2001c)
20.6 Lost Load Continued: Hybridogens as Vehicles for Gene Transfer
An alternative mechanism of genetic exchange for clonal genomes in hybridogenetic water frogs is provided when hybridogenesis is “leaky”, i.e., when occasional recombination between parental genomes is possible in hybrids (Uzzell et al. 1977). Such exchange has far-reaching consequences because hybridogens can then act as vehicles for gene transfer among the parental species (introgression). This is common in hybrid zones with fertile hybrids (e.g., Szymura and Barton 1991), but typically precluded in hybridogens by the complete elimination of one parental genome prior to meiosis. However, deviations from this standard model of hybridogenesis are known to occur (reviewed by Schmeller 2004), particularly in populations where both parental species are present (i.e., outside the L-E system) or in “disturbed” L-E systems into which R. ridibunda has been introduced by humans, often from multiple origins (Pagano et al. 2003). This may be due to the fact that genomes of R. ridibunda vary geographically in their ability to induce hybridogenesis (Hotz et al. 1985). The evidence for recombination among parental genomes in hybridogenetic water frogs is mainly inferred from observed introgression of species-specific alleles in population studies employing allozyme electrophoresis (Schmeller 2004). Direct evidence for recombination from comparisons of parent and offspring genotypes is still virtually lacking. This is not surprising given that the genetic signature of a presumably rare event may be detected more easily in large population samples than the event itself in a limited number of breeding experiments. Vorburger and Reyer (2003) found just a single R. esculenta female exhibiting recombination in the germline among a large number of frogs from a “disturbed” L-E system (i.e., one also containing introduced R. ridibunda) used in experimental crosses. But even if rare, this process should not be ignored (Pagano and Schmeller 1999). It may provide a way for expanding hemiclones to acquire locally adapted alleles (Schmeller et al. 2005b), and it may allow for purging of deleterious mutations from clonally transmitted genomes. This, again, urges caution in the use of hemiclonal water frogs to study the long-term consequences of clonal inheritance.
Maybe not of direct relevance for purging, but worth mentioning here, is another form of introgression in which hybrids act as vehicles for gene transfer: the introgression of lessonae mtDNA into R. ridibunda. As explained earlier, primary hybridizations occur between females of R. ridibunda and males of R. lessonae, thus producing R. esculenta lineages that possess ridibunda mtDNA. In L-E systems, this would remain so as long as female R. esculenta mated with male R. lessonae, which is the more common combination. However, the reciprocal mating combination between females of R. lessonae and males of R. esculenta also occurs (Fig. 20.1), and such matings generate R. esculenta progeny possessing lessonae mtDNA. In the absence of syntopic R. ridibunda, this transfer of lessonae mtDNA into a hemiclonal hybrid lineage is irreversible, which explains why R. esculenta in natural L-E systems are indeed found to possess the mtDNA of their local host rather than ridibunda mtDNA (Spolsky and Uzzell 1986; Hotz et al. 1992). Within the range of R. ridibunda, such hemiclonal hybrid lineages can act as a vehicle for directional introgression of lessonae mtDNA into R. ridibunda, because matings between R. esculenta females bearing lessonae mtDNA and male R. ridibunda produce R. ridibunda progeny with mtDNA derived from R. lessonae (Spolsky and Uzzell 1984). Indeed, 42% of R. ridibunda in central Poland have been found to carry such introgressed lessonae mtDNA (Spolsky and Uzzell 1984).
20.7 Open Questions and Outlook: Spontaneous Mutational Load in Natural Populations of Rana ridibunda
The crossing experiments reported above provide evidence that non-recombining ridibunda genomes occurring in L-E systems bear a substantial mutational load, but they cannot distinguish between mutations that have accumulated over generations of clonal transmission and mutations that were already present in the original population of R. ridibunda and became “frozen” in clonal genomes through hybridization. In fact, the experiments only prove that clonal ridibunda genomes in L-E systems contain at least one lethal equivalent, which may well be the case for any ridibunda genome even in a sexual population. To assess the relative importance of the two processes requires a quantitative estimate in terms of lethal equivalents of the spontaneous mutational load in sexual populations of R. ridibunda. Such an estimate is currently lacking, but the amphisexuality of R. esculenta lineages together with the relative ease to generate hemiclonally-reproducing F1 hybrids provides a unique opportunity to obtain this information. This work is in progress and includes four steps:
-
(1)
Crosses between R. ridibunda from particular regions such as central Poland and R. lessonae spontaneously generate hemiclonal offspring (Hotz et al. 1985). Such crosses are used to obtain F1 individuals containing a “frozen” ridibunda haplotype.
-
(2)
Each of these haplotypes is perpetuated in many copies by crossing such F1 hybrids with R. lessonae, producing a set of hemiclonal lineages that all contain the same, clonally transmitted ridibunda genome.
-
(3)
The hybrids so obtained are crossed back with sexual R. ridibunda to produce R. ridibunda offspring that inherit a “frozen” ridibunda haplotype from their hybrid parent and a recombined ridibunda genome from their R. ridibunda parent. Such offspring exhibit normal Mendelian recombination.
-
(4)
Finally, these backcrossed R. ridibunda are crossed with hybrids that possess the same ridibunda haplotype as their hybrid parent, generating offspring that are on average homozygote for 50% of their genome. Their viability compared to control crosses provides an estimate of the number of lethal equivalents on this ridibunda haplotype. Survival will be reduced to 0.5 for one lethal equivalent and to 0.5n for n lethal equivalents.
Such estimates of the spontaneous mutational load in sexual populations of R. ridibunda will provide a plausible null hypothesis for testing the operation and magnitude of Muller’s ratchet in natural hemiclones. The estimates will be even more important in their own right. Spontaneous deleterious mutations are an issue of central focus in evolutionary genetics because they critically influence a large array of biological phenomena such as extinction risk of small populations, ploidy level, Y chromosome degeneration, senescence, inbreeding avoidance and mate choice, as well as the maintenance of sexual reproduction (reviewed by Charlesworth and Charlesworth 1998). Yet relevant estimates of such loads are still scarce and such data are therefore badly needed (McCune et al. 2002; Halligan and Keightley 2003).
Notes
- 1.
Please note that according to the latest revision of amphibian classification (Frost et al. 2006), the Western Palearctic water frogs are now contained in the genus Pelophylax, formerly considered a subgenus of Rana (Dubois 1992). The new classification is likely to take hold (Vences 2007). For consistency with the literature we review, however, we adhere to the old classification in this chapter.
References
Abt G, Reyer H-U (1993) Mate choice and fitness in a hybrid frog: Rana esculenta females prefer Rana lessonae males over their own. Behav Ecol Sociobiol 32: 221–228
Abt Tietje G, Reyer H-U (2004) Larval development and recruitment of juveniles in a natural population of Rana lessonae and Rana esculenta. Copeia 3: 638–646
Bergen K, Semlitsch RD, Reyer H-U (1997) Hybrid female matings are directly related to the availability of Rana lessonae and Rana esculenta males in experimental populations. Copeia 2: 275–283
Berger L (1967) Embryonal and larval development of F1-generation of green frogs different combinations. Acta Zool Cracov 12: 123–160
Berger L (1968) Morphology of F1-generation of various crosses within Rana esculenta-complex. Acta Zool Cracov 13: 301–324
Berger L (1970) Some characteristics of the crosses within Rana esculenta complex in postlarval development. Ann Zool 27: 373–416
Berger L, Uzzell T, Hotz H (1988) Sex determination and sex ratios in wstern Palearctic water frogs: XX and XY female hybrids in the Pannonian basin? Proc Acad Nat Sci Phila 140:220–239
Blankenhorn HJ, Heusser H, Notter P (1973) Zur Verbreitung von Rana esculenta Linnaeus und Rana lessonae Camerano im Zürcher Oberland. Rev Suisse Zool 80: 662–666
Blankenhorn HJ, Heusser H, Vogel P (1971) Drei Phänotypen von Grünfröschen aus dem Rana esculenta-Komplex in der Schweiz. Rev Suisse Zool 78: 1242–1247
Carmona JA, Sanjur OI, Doadrio I, Machordom A, Vrijenhoek RC (1997) Hybridogenetic reproduction and maternal ancestry of polyploid Iberian fish: the Tropidophoxinellus alburnoides complex. Genetics 146: 983–993
Chao L (1990) Fitness of RNA virus decreased by Muller’s ratchet. Nature 348: 454–455
Charlesworth B, Charlesworth D (1997) Rapid fixation of deleterious alleles can be caused by Muller’s ratchet. Genet Res 70: 63–73
Charlesworth B, Charlesworth D (1998) Some evolutionary consequences of deleterious mutations. Genetica 102/103: 3–19
Dubois A (1992) Notes sur la classification des Ranidae (amphibiens anoures). Bull Mens Soc Linn Lyon 61: 305–352
Ebendal T (1979) Distribution, morphology and taxonomy of the Swedisch green frogs (Rana esculenta complex). Mitt Zool Mus Berl 55: 143–152
Ellegren H, Fridolfsson AK (2003) Sex-specific mutation rates in salmonid fish. J Mol Evol 56: 458–463
Engeler B, Reyer HU (2001) Choosy females and indiscriminate males: mate choice in mixed populations of sexual and hybridogenetic water frogs (Rana lessonae, Rana esculenta). Behav Ecol 12: 600–606
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad DFB, de Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297: 1–370
Graf J-D, Polls Pelaz M (1989) Evolutionary genetics of the Rana esculenta complex. In: Dawley RM, Bogart JP (eds) Evolution and ecology of unisexual vertebrates. Bulletin 466, New York State Museum, Albany, New York, pp. 289–301
Guex G-D, Beerli P, Barbour AD, Hotz H (1993) A dynamic model to describe equilibrium conditions in mixed populations of a hemiclonal hybrid and its sexual host in European water frogs. In: Catzeflis FM, Gautier M (eds) Evolution 93. 4th congress of the European society for evolutionary biology. Université Montpellier II, Montpellier, pp. 158
Guex GD, Hotz H, Semlitsch RD (2002) Deleterious alleles and differential viability in progeny of natural hemiclonal frogs. Evolution 56: 1036–1044
Günther R (1990) Die Wasserfrösche Europas (Anura – Froschlurche). A. Ziemsen Verlag, Wittenberg Lutherstadt
Günther R, Uzzell T, Berger L (1979) Inheritance patterns in triploid Rana “esculenta” (Amphibia, Salientia). Mitt Zool Mus Berl 55: 35–57
Halligan DL, Keightley PD (2003) How many lethal alleles? Trends Genet 19: 57–59
Hellriegel B, Reyer H-U (2000) Factors influencing the composition of mixed populations of a hemiclonal hybrid and its sexual host. J Evol Biol 13: 906–918
Heusser H, Blankenhorn HJ (1973) Crowding-Experimente mit Kaulquappen aus homo- und heterotypischen Kreuzungen der Phänotypen esculenta, lessonae und ridibunda (Rana esculenta-Komplex, Anura, Amphibia). Rev Suisse Zool 80: 543–569
Higgs PG, Woodcock G (1995) The accumulation of mutations in asexual populations and the structure of genealogical trees in the presence of selection. J Math Biol 33: 677–702
Holenweg Peter AK, Reyer HU, Tietje GA (2002) Species and sex ratio differences in mixed populations of hybridogenetic water frogs: The influence of pond features. Ecoscience 9: 1–11
Hotz H, Beerli P, Spolsky C (1992) Mitochondrial DNA revelas formation of nonhybrid frogs by natural matings between hemiclonal hybrids. Mol Biol Evol 9: 610–620
Hotz H, Mancino G, Bucci-Innocenti S, Ragghianti M, Berger L, Uzzell T (1985) Rana ridibunda varies geographically in inducing clonal gametogenesis in interspecies hybrids. J Exp Zool 236: 199–210
Hotz H, Semlitsch RD, Gutmann E, Guex G-D, Beerli P (1999) Spontaneous heterosis in larval life-history traits of hemiclonal frog hybrids. Proc Natl Acad Sci USA 96: 2171–2176
Hurst LD, Ellegren H (1998) Sex biases in the mutation rate. Trends Genet 14: 446–452
Mantovani B, Scali V (1992) Hybridogenesis and androgenesis in the stick insect Bacillus rossius-grandii benazzii (Insecta, Phasmatodea). Evolution 46: 783–796
McCune AR, Fuller RC, Aquilina AA, Dawley RM, Fadool JM, Houle D, Travis J, Kondrashov AS (2002) A low genomic number of recessive lethals in natural populations of bluefin killifish and zebrafish. Science 296: 2398–2401
Milinski M (1994) Hybridogenetic frogs on an evolutionary dead end road. Trends Ecol Evol 9:62
Muller HJ (1964) The relation of recombination to mutational advance. Mutat Res 1: 2–9
Pagano A, Dubois A, Lesbarrères D, Lodé T (2003) Frog alien species: a way for genetic invasion? C R Biol 326: S85–S92
Pagano A, Schmeller D (1999) Is recombination less negligible than previously described in hybridogenetic water frogs? In: Miaud C, Guyétant R (eds) 9th OG meeting – current studies in Herpetology. SEH, Le Bourget du Lac, France, pp. 351–356
Plötner J (2005) Die westpaläarktischen Wasserfrösche. Laurenti-Verlag, Bielefeld
Redfield RJ (1994) Male mutation rates and the cost of sex for females. Nature 369: 145–147
Rist L, Semlitsch RD, Hotz H, Reyer H-U (1997) Feeding behaviour, food consumption, and growth efficiency of hemiclonal and parental tadpoles of the Rana esculenta complex. Funct Ecol 11: 735–742
Roesli M, Reyer H-U (2000) Male vocalization and female choice in the hybridogenetic Rana lessonae/Rana esculenta complex. Anim Behav 60: 745–755
Rybacki M, Berger L (1994) Distribution and ecology of water frogs in Poland. Zoologica Poloniae 39: 293–303
Rybacki M, Berger L (2001) Types of water frog populations (Rana esculenta complex) in Poland. Mitt Mus Naturkd Berlin, Zool Reihe 77: 51–57
Saitoh K, Kim IS, Lee EH (2004) Mitochondrial gene introgression between spined loaches via hybridogenesis. Zool Sci 21: 795–798
Santucci F, Nascetti G, Bullini L (1996) Hybrid zones between two genetically differentiated forms of the pond frog Rana lessonae in southern Italy. J Evol Biol 9: 429–450
Schmeller DS (2004) Tying ecology and genetics of hemiclonally reproducing waterfrogs (Rana, Anura). Ann Zool Fenn 41: 681–687
Schmeller DS, O’Hara R, Kokko H (2005a) Male adaptive stupidity: male mating pattern in hybridogenetic frogs. Evol Ecol Res 7: 1039–1050
Schmeller DS, Seitz A, Crivelli A, Veith M (2005b) Crossing species’ range borders: interspecies gene exchange mediated by hybridogenesis. Proc R Soc Lond B 272: 1625–1631
Schmidt BR (1993) Are hybridogenetic frogs cyclical parthenogens? Trends Ecol Evol 8: 271–273
Schultz RJ (1969) Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am Nat 103:605–619
Schultz RJ (1973) Unisexual fish – laboratory synthesis of a species. Science 179: 180–181
Semlitsch RD (1993) Asymmetric competition in mixed populations of tadpoles of the hybridogenetic Rana esculenta complex. Evolution 47: 510–519
Som C, Anholt BR, Reyer H-U (2000) The effect of assortative mating on the coexistence of a hybridogenetic waterfrog and its sexual host. Am Nat 156: 34–46
Som C, Bagheri HC, Reyer HU (2007) Mutation accumulation and fitness effects in hybridogenetic populations: a comparison to sexual and asexual systems. BMC Evol Biol 7
Som C, Reyer HU (2006) Variation in sex ratio and evolutionary rate of hemiclonal Rana esculenta populations. Evol Ecol 20: 159–172
Som C, Reyer HU (2007) Hemiclonal reproduction slows down the speed of Muller’s ratchet in the hybridogenetic frog Rana esculenta. J Evol Biol 20: 650–660
Spolsky C, Uzzell T (1984) Natural interspecies transfer of mitochondrial DNA in amphibians. Proc Natl Acad Sci USA 81: 5802–5805
Spolsky C, Uzzell T (1986) Evolutionary history of the hybridogenetic hybrid frog Rana esculenta as deduced from mtDNA analyses. Mol Biol Evol 3: 44–56
Szymura JM, Barton NH (1991) The genetic structure of the hybrid zone between the fire-bellied toads Bombina bombina and B. variegata – comparisons between transects and between loci. Evolution 45: 237–261
Tinti F, Mantovani B, Scali V (1995) Reproductive features of homospecific hybridogenetically-derived stick insects suggest how unisexuals can evolve. J Evol Biol 8: 81–92
Tunner HG (1973) Das Albumin und andere Bluteiweisse bei Rana ridibunda Pallas, Rana lessonae Camerano, Rana esculenta Linné und deren Hybriden. Z Zool Syst Evol 11: 219–233
Tunner HG (1974) Die klonale Struktur einer Wasserfroschpopulation. Z Zool Syst Evol 12:309–314
Uzzell T, Berger L (1975) Electrophoretic phenotypes of Rana ridibunda, Rana lessonae, and their hybridogenetic associate, Rana esculenta. Proc Acad Nat Sci Phila 127: 13–24
Uzzell T, Günther R, Berger L (1977) Rana ridibunda and Rana esculenta– a leaky hybridogenetic system (Amphibia-Salientia). Proc Acad Nat Sci Phila 128: 147–171
Vences M (2007) The amphibian tree of life: Ideologie, Chaos oder biologische Realität? Z Feldherpetol 14: 153–162
Vorburger C (2001a) Fixation of deleterious mutations in clonal lineages: Evidence from hybridogenetic frogs. Evolution 55: 2319–2332
Vorburger C (2001b) Heterozygous fitness effects of clonally transmitted genomes in waterfrogs. J Evol Biol 14: 602–610
Vorburger C (2001c) Non-hybrid offspring from matings between hemiclonal hybrid waterfrogs suggest occasional recombination between clonal genomes. Ecol Lett 4: 628–636
Vorburger C, Reyer HU (2003) A genetic mechanism of species replacement in European waterfrogs? Conserv Genet 4: 141–155
Vrijenhoek RC, Angus RA, Schultz RJ (1977) Variation and heterozygosity in sexually vs. clonally reproducing populations of Poeciliopsis. Evolution 31: 767–781
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Vorburger, C., Schmeller, D.S., Hotz, H., Guex, GD., Reyer, HU. (2009). Masked Damage: Mutational Load in Hemiclonal Water Frogs. In: Schön, I., Martens, K., Dijk, P. (eds) Lost Sex. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-2770-2_20
Download citation
DOI: https://doi.org/10.1007/978-90-481-2770-2_20
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-2769-6
Online ISBN: 978-90-481-2770-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)