Abstract
Oribatid mites (Acari, Oribatida) are an extraordinarily old and speciose group of chelicerate arthropods that probably originated in Silurian times. A high number (∼10%) of oribatid mite species reproduces via parthenogenesis, presumably by terminal fusion automixis with holokinetic chromosomes and an inverted sequence of meiotic divisions. Several of the old taxa of oribatid mites likely have radiated while being parthenogenetic. Many species of those parthenogenetic clusters are morphologically distinct – this distinctness contrasts with high genetic variance, as has been confirmed by molecular studies, e.g. for Platynothrus peltifer and species of the genus Tectocepheus. Platynothrus peltifer comprises at least seven distinct molecular lineages which are geographically separated and may be recognized as cryptic species. Stable isotope ratios (15N/14N and 13C/12C) of oribatid mite species indicate that they occupy distinct trophic niches; however, the exact nature of these niches is unknown. One of the few microhabitats colonized by specific oribatid mite species is the bark of trees. The tree-inhabiting genus Crotonia re-evolved sexual reproduction from parthenogenetic ancestors, potentially while colonizing trees. Understanding the high degree of parthenogenetic reproduction in soil living oribatid mites allows the dissection of the functional role and evolution of sexual reproduction, and the factors responsible for the long-term survival and radiation of parthenogenetic species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 General Biological Aspects of Oribatid Mites
12.1.1 Overview
Oribatid mites (Acari, Oribatida; Fig. 12.1) are a speciose group: about 10,000 species are now described (Subias 2004) and 100,000 may actually exist (Schatz 2002). While mainly soil-living, several groups also live on trees or in aquatic systems. They range in length mostly between 200 and 800 μm, and are unusual among chelicerates for two reasons. Firstly, they are principally particle-feeding saprophages and microbivores, rather than fluid-feeders (Heethoff and Norton 2009). Secondly, as adults, they display a great range of physical and chemical defense mechanisms (Sanders and Norton 2004; Schmelzle et al. 2008), the most common of which is heavy sclerotization or mineralization of the cuticle that results in the common names “beetle mites”, “armoured mites” and “box mites”. An interesting feature of oribatid mites is that parthenogenesis is one or two orders of magnitude more common than in most other eukaryotic groups: about 10% of all species reproduce parthenogenetically (Norton and Palmer 1991; Norton et al. 1993).
12.1.2 Geological Age
Oribatid mites have seemingly existed since the first complex terrestrial communities evolved. The first indisputable fossil record is from the Devonian (380 million years; Shear et al. 1984; Norton et al. 1988), although the origin of the group probably dates back to 400–440 million years (Lindquist 1984). Based on specific patterns of occurrence, low dispersal power and genetic distances, the distribution of oribatid mite species seems to largely reflect vicariance associated with continental drift, rather than dispersal (Hammer and Wallwork 1979; Heethoff et al. 2007a). From biogeographic inferences, some species predated the breakup of the great landmass of Pangea about 200 million years ago yet kept their distinct morphology (Hammer and Wallwork 1979).
12.1.3 Population Density
The population density of oribatid mites is predictable within broad ranges (Maraun and Scheu 2000). In acidic boreal forests, they reach densities of up to 400,000 ind/m² whereas in calcareous forests, densities are usually somewhere between 20,000 and 40,000 ind/m². In tropical lowland and mountain rainforests densities are also rather low, e.g. in a tropical Brazilian lowland about 10,000 ind/m² (Badejo et al. 2002) and in a tropical mountain rainforest about 15,000 ind/m² (Maraun et al. 2008). There is little seasonal fluctuation of oribatid mite densities, indicating that the communities are in equilibrium conditions.
One of the main factors regulating the density of oribatid mites is the substrate: however, this affects oribatid mites indirectly by triggering, for example, the presence of macrofauna decomposers such as earthworms and diplopods. These reach high density and biomass in calcareous forests, and by feeding and removing litter material they destroy the habitat of litter-living mesofauna, thereby detrimentally affecting oribatid mite communities. The density of oribatid mites in base-rich forests therefore is low. Exceptions are some Canadian forests which are base-rich but virtually devoid of macrodecomposers, in particular earthworms, due to glaciation. In these forests (e.g. in the mountain ranges of western Alberta, Canada), oribatid mite densities are high. However, recent invasion by European earthworm species has transformed these systems by incorporating the ectorganic matter into the mineral soil thereby damaging the habitat of litter-living invertebrates; this in turn has caused the density of oribatid mites to decline strongly (Migge-Kleian et al. 2006; Eisenhauer et al. 2007).
Laboratory and field experiments with European soil showed that mechanical disturbance by earthworms is one of the main factors responsible for low densities of oribatid mites (Maraun et al. 2001). The low densities in tropical mountain rainforests cannot be explained by high densities of macrodecomposers since, at these sites, the density of such macrodecomposers is low; presumably other factors including low resource quality are responsible for these low densities of oribatid mites in tropical forest ecosystems (Maraun et al. 2008). Overall, the low density of oribatid mites in calcareous forest soils presumably is mainly due to macrofauna activity, whereas their density in more acidic forest soils probably is limited by the availability of high quality resources (bottom-up control; Salamon et al. 2006). Predation (top-down control) is likely to be of little importance as a regulatory factor for adult oribatid mites since these are well-defended (enemy free space hypothesis; Peschel et al. 2006). However, predation on more vulnerable juvenile oribatid mites has not been studied and may be an important factor regulating oribatid mite density.
12.1.4 Niche Differentiation and Feeding Biology
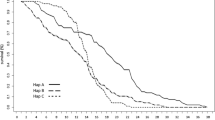
Anderson (1975) hypothesized that trophic niche differentiation contributes to the high diversity of soil animal species. However, laboratory feeding choice experiments indicate that soil-living microarthropods such as adult oribatid mites are generalist feeders, suggesting that trophic niche differentiation is poor. They feed on a variety of substrates when offered the choice, including algae, lichens, different species of saprophytic and mycorrhizal fungi and dead organic material (Maraun et al. 1998a, 2003a; Schneider and Maraun 2005; Schneider et al. 2005; Heethoff et al. 2007b). In contrast, natural variations in stable isotope ratios (15N/14N, 13C/12C) of oribatid mites indicate distinct trophic niches that collectively span a wide range of trophic levels, including phytophagous species feeding on algae, primary decomposers feeding on detritus, secondary decomposers feeding on fungi, predators presumably feeding on nematodes or necrophages feeding on small dead invertebrates (Schneider et al. 2004). Stable isotope signatures (δ15N) of soil and bark-living oribatid mite species span over about 20 δ units – equivalent to about six trophic levels (cf. Minagawa and Wada 1984; Post 2002) – which is unique for a single animal taxon (Schneider et al. 2004; Erdmann et al. 2007; Fig. 12.2). The contradiction between field data (which indicate narrow trophic niches) and laboratory data (which indicate generalist feeding) is unresolved and deserves further attention. Possibly, immature oribatid mites are more specialized feeders and change to a wider food spectrum when adult.
Variation of δ15N values of oribatid mites from litter (open symbols) and from the bark of trees (filled symbols; species names in bold) of different beech forests. Data for litter are calibrated to the L/F litter material of the Solling (L/F layer); data for bark are calibrated to the average δ15N signature of the outer bark layer of the four forest types. Single measurements (without SD) and means of 2–5 replicates with SD. Species were ordered according to increasing δ15N value. Underlined species occurred in the litter and also on the bark of trees (modified after Schneider et al. 2004 and Erdmann et al. 2007).
The high diversity of soil animals, especially of soil microarthropods, has been considered enigmatic (Anderson 1975; Maraun et al. 2003a), mainly for two reasons. First, the spatial structure of the soil system appears to be rather homogeneous compared with above-ground habitats. Second, co-evolutionary processes between decomposer soil invertebrates and their resources are probably weak; there is no selection pressure for dead organic matter to “defend” itself against detritivores, and co-evolution between microbivorous microarthropods and their prey species may have been prevented by difficulty in physically isolating individual microbial species for ingestion (Scheu and Setälä 2002). Like many soil animal taxa, few oribatid mite species inhabit specific physical niches (microhabitats) such as earthworm burrows or specific litter types (Migge et al. 1998; Maraun et al. 1999; Hansen 2000). While soil-associated microhabitats like mosses and decaying wood might contain oribatid mite species with strong substrate affiliations, these species often are also present at other places. If there is microhabitat specificity it is mostly found in the immature instars, many of which burrow in rather specific types of living (fungal fruiting bodies, lichens) or decomposing (needles, twigs, seeds, wood) substrates. One exception may be arboreal oribatid mite species which, even as adults, almost exclusively occur on the bark or in the canopy of trees (Erdmann et al. 2006, 2007; Lindo and Winchester 2006).
12.1.5 Functioning
As a combined result of feeding on decomposing organic matter and their high density, oribatid mites are important decomposers in forest ecosystems, fallows, fields and meadows (Hansen 1999; Maraun and Scheu 2000). In particular in acidic forests, they are among the major decomposer groups (along with collembolans, enchytraeids and dipteran larvae) and drive mineralization processes and humus formation. The fossil record of oribatid mites indicates that this was already the case in Palaeozoic forests (Labandeira et al. 1997). Despite this perceived ancient importance of oribatid mites for terrestrial ecosystems, their function in the soil system and their role in aboveground – belowground interactions have been little studied. The main reason for this probably is their slow reproduction rate which makes rearing for experimental purposes difficult. The few available studies indicate that oribatid mites affect the composition of microbial communities via dispersal of fungal spores (Maraun et al. 1998b). These can be dispersed in the gut of the animals via their faeces but also attached to the body surface (Behan and Hill 1978; Renker et al. 2005). Besides these indirect effects on decomposition processes via modification of the microbial community, oribatid mites also affect decomposition processes directly by feeding on dead plant material. For example, box mites (Ptyctima) and Adoristes ovatus (Koch) feed inside decomposing needles and leaves (Harding and Stuttard 1974; Lions and Gourbiere 1988).
Due to their wide ecological tolerance, their limited reaction after disturbances and their broad feeding habits, oribatid mites are of limited use as bioindicators in forest ecosystems (Lindo and Visser 2004). However, they may be used as bioindicators during ecosystem succession, especially in early successional stages of agroecosystems (Behan-Pelletier 1999).
12.2 Reproductive and Developmental Biology
12.2.1 General Aspects
Mites are without peer among chelicerates with respect to their reproductive potential and the diversity of their reproductive strategies, genetic systems and ontogenies (Norton et al. 1993; Walter and Proctor 1999). Oribatid mites are diplodiploid organisms (usually with 2n=18; Heethoff et al. 2006) in which fertilization is usually indirect, via spermatophores deposited by males in the absence of females; alternatively, parthenogenetic development occurs. They retain the presumed ancestral developmental series of Acari: embryological development terminates in a regressive prelarva which is succeeded by the larva, protonymph, deuteronymph, tritonymph and adult. As in most acariform mites, the first active instar is the hexapod larva; the prelarva is inactive (calyptostase) and remains inside the egg shell in all known species, unlike some other groups with active prelarvae in primitive species (Otto 1997).
Egg laying strategies range from iteroparity (repeated production of a few eggs) to semelparity (single production of many eggs). Eggs are usually laid in crevices at an early developmental stage (embryo or prelarva), but larviparity also occurs (Walter and Proctor 1999). With some exceptions, oribatid mites tend to have low reproductive outputs, and long developmental times of 50 weeks or more are common for species of temperate zones (Norton 1994; Walter and Proctor 1999, Sovik and Leinaas 2003; Heethoff et al. 2007b).
12.2.2 Female System and Reproductive Strategies
The morphology of the female reproductive system in the Acari is variable (Evans 1992). Depending on the group, the ovary can be unpaired, paired or divided into germinal and nutritive regions (Evans 1992; Alberti and Coons 1999; Bergmann et al. 2008). Oribatid mites usually have an unpaired ovary from which two oviducts originate and proceed through the opisthosoma until they fuse at the vagina (Alberti and Coons 1999; Heethoff et al. 2007b; Bergmann et al. 2008). Oviposition is accomplished with an extrusible ovipositor (Fig. 12.3), but the mechanism of extrusion is poorly understood. It has been assumed that haemolymph pressure extends the ovipositor and that muscles attached to its wall retract it (Michael 1884; Grandjean 1956). However, more probably, direct muscular action is also responsible for ovipositor extrusion (U. Kurz and M. Heethoff, unpubl.). Wallwork (1977) further suggested that muscles inserting on the pleated wall are responsible for oviposition, although he did not provide a mechanism.
12.2.3 Parthenogenesis
Parthenogenesis is widespread among oribatid mites, but the cytological mechanism involved is poorly understood. Available data suggest that automixis is the rule (Taberly 1987; Heethoff et al. 2006; see also Chapter 4). Taberly (1987) performed the first cytological study of meiosis in parthenogenetic oribatid mites. He observed that the parthenogenetic species Platynothrus peltifer (Koch) and Trhypochthonius tectorum (Berlese) restore diploidy by terminal fusion (fusion of the egg pronucleus with the second polar nucleus), a mechanism expected to produce homozygous offspring (Wrensch et al. 1994). This mechanism was also suggested for Archegozetes longisetosus (Heethoff et al. 2006; Laumann et al. 2008). However, using isozyme techniques, Palmer and Norton (1992) found fixed heterozygosity, absence of complete homozygosity and absence of recombination in nine parthenogenetic oribatid mite species. They proposed that apomixis or central fusion automixis may be common in parthenogenetic oribatid mites, even if neither mechanism had yet been discovered. However, Wrensch et al. (1994) suggested inverted meiosis as an alternative mechanism – made possible by holokinetic chromosomes of oribatid mites – in which the sequence of meiotic divisions (reductional and equational) is inverted compared to normal meiosis, leading to a reversal in effects of terminal and central fusion. Fixed heterozygosity and terminal fusion would thus be compatible observations (Wrensch et al. 1994; Heethoff et al. 2006; Laumann et al. 2008; Fig. 12.4).
Effects of inverted meiosis on the genetic reconstitution of the embryo under the aspect of terminal fusion automixis (exemplified by one homologous chromosome pair under the absence of recombination). A normal meiosis with monocentric chromosomes. B inverted meiosis with holokinetic chromosomes. a: diploid cell. b: chromosomes duplicated by replication prior to the initiation of meiosis. c: meiosis I: separation of homologous chromatids in normal meiosis (A reductional division) and sister chromatids in inverted meiosis (B equational division) leading to haploid cells (A) and diploid cells (B). d: meiosis II: equational division in normal meiosis (A), reductional division in inverted meiosis (b). e: the genetic constitution of the embryo as compared to the mother’s genome dramatically differs in (A) and (B): A – embryo constitutes a diploid chromosome number containing sister chromatids of one of the initiating chromosomes of the mother, B – embryo has the same genetic constitution as the mother (and hence is in effect a clone)
We studied oogenesis of the parthenogenetic oribatid mite Archegozetes longisetosus Aoki, a widely used model organism for Chelicerata with mother-daughter genetic fidelity, i.e. no recombination of heterozygous isoenzyme genotypes (Palmer and Norton 1992). Oocytes grow inside the rhodoid of the ovary, vitellogenesis takes place in the meros of the ovary and afterwards, eggs enter the oviducts via an ovarial bulb (Bergmann et al. 2008). Meiotic divisions probably occur in the meros of the ovary, the first polar body seems to be expelled (Laumann et al. 2008). The first three to five embryonic cleavages are holoblastic and occur in the beginning/middle of the oviduct (M. Laumann, P. Bergmann and M. Heethoff, unpubl.).
Clearly, the chromosomal kinetics during meiotic divisions still has to be investigated in more detail since Schaefer et al. (2006) showed that the so-called “Meselson effect” (diverging allelic sequences in absence of recombination; Mark Welch and Meselson 2000), expected to occur in this scenario, does not occur in parthenogenetic oribatid mites even with the probable absence of genetic recombination. These authors used partial sequences of the genes of the elongation factor-1α (ef-1α) and the heat shock protein 82 (hsp82) to test the existence of the “Meselson effect” for putative ancient parthenogenetic oribatid mite lineages. Unexpectedly, the intra-individual divergence of parthenogenetic lineages was rather low and resembled that in sexual species. Additionally, strong selection pressure may keep both the ef-1α and the hsp82 gene functioning. No evidence for recombination or gene conversion was found for sexual or parthenogenetic oribatid mite species in the hsp 82 gene supporting the assumption that homogenizing mechanisms prevent the accumulation of sequence divergences, i.e. the “Meselson effect”.
The sex determination mechanism in oribatid mites is unknown (Heethoff et al. 2006). In diplodiploid systems, sex determination is often accomplished by sex chromosomes and the sex ratio is assumed to be close to unity, i.e. 1:1 (Fisher 1930). Sex determination may also be influenced by external factors, such as temperature (Ewert et al. 1994), or by hormonal or pheromonal control (White 1973). Oribatid mites lack sex chromosomes despite their diplodiploidy (Sokolov 1954; Norton et al. 1993; Wrensch et al. 1994; Heethoff et al. 2006). Nevertheless, the sex ratio of sexual oribatid mite species is close to unity, whereas in parthenogenetic species, males are rare (spanandric) and sterile. Spermatophores are non-functional, as spermatogenesis is incomplete (Taberly 1988), and there is no evidence for recombination or incorporation of paternal genetic material into the offspring (Palmer and Norton 1992; see also Cianciolo and Norton 2006; Schaefer et al. 2006). A genetic mechanism based on inverted meiosis with terminal fusion automixis does not explain the occurrence of these spanandric males. Environmental sex determination is also unlikely because sexual and parthenogenetic oribatid mite species coexist under a wide variety of conditions, i.e. they are exposed to similar external stimuli. Further efforts are necessary to uncover the mechanism for the sporadic production of sterile males in parthenogenetic oribatid mite species (Heethoff et al. 2006).
12.2.4 Endosymbiotic Bacteria
In recent years, much has been written about the ability of endosymbiotic bacteria (Wolbachia, Cardinium) to control the reproductive biology of hosts, particularly arthropods, and the induction of parthenogenesis is one of several possible manifestations of infection (Stouthamer et al. 1999). The first use of DNA techniques to search for Wolbachia in parthenogenetic oribatid mites was negative for all species tested (Perrot-Minnot and Norton 1997), but Wolbachia was detected in an unidentified oribatid mite by Cordaux et al. (2001) and the parthenogenetic species Oppiella nova (Oudemans) can serve as host for both Wolbachia and Cardinium (Weeks et al. 2003). Ongoing research (A. Weeks, R. Stouthamer and R. A. Norton, unpubl.) suggests that both bacteria are present in a small range of oribatid mite species. While bacterial phenotypes remain unproven, the distribution of endosymbionts in relation to mite reproductive mode does not support inducement of parthenogenesis.
12.3 Phylogeny of Parthenogenetic Lineages
12.3.1 General Phylogeny
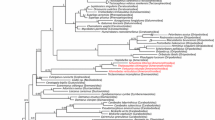
A morphology-based classification of oribatid mites that reflects “natural” phylogenetic groups was first established by Grandjean (1953, 1965, 1969). He grouped oribatid mites into six taxa, including (1) the basal Palaeosomata, with few species; (2) the Enarthronota, including e.g. the species-rich Brachychthonioidea and Hypochthonioidea; (3) the small group Parhyposomata; (4) the “Mixonomata”, which includes the species-rich box mites (Phthiracaroidea and Euphthiracaroidea); (5) the “Desmonomata”, with several species-rich groups; and (6) the diverse, species-rich Circumdehiscentiae (=Brachypylina). As indicated by quotes, two of these groups are probably paraphyletic (Haumann 1991; Norton et al. 1993; Weigmann 1996). Maraun et al. (2004) and Weigmann (2006) summarized a common view of oribatid mite phylogeny based on morphological characters. Phylogenetic studies using molecular markers (18S rDNA, 28S rDNA, hsp82, elongation factor 1 α) have largely confirmed this view (Maraun et al. 2004; I. Schaefer et al. unpubl.; Fig. 12.5), but efforts to refine phylogenetic hypotheses continue.
Neighbour-joining tree of 26 genera of oribatid mites with Trachytes sp. (Mesostigmata, Uropodina) as outgroup taxon, constructed using the 18S rDNA sequences and a part of the heat shock protein (hsp82) region
Numbers at nodes represent percentages of 1000 bootstrap replicates (only values above 50% are reported; I. Schaefer, K. Domes, S. Scheu and M. Maraun; unpubl. data).
12.3.2 Radiation of Parthenogenetic Lineages
The existence of ancient parthenogenetic taxa questions the necessity of sexual reproduction for the evolution and diversification of lineages into discrete genetic and morphological entities (Barraclough et al. 2003; see also Lushai et al. 2003 and Chapter 10). A single parthenogenetic population may display a tree-like ancestry and give the appearance of discrete taxa, but the degree of diversification is determined by the time elapsed since the split of the last common ancestor. Diversification at this level could be achieved through isolation in different geographic areas or adaptive divergence in a heterogeneous environment (Barraclough et al. 2003; Laumann et al. 2007). Diversifying selection and geographic isolation drive speciation in sexual species and are expected to have similar effects in parthenogenetic species.
The radiation and diversification of ancient taxa of parthenogenetic species has empirically been little studied. It challenges theories on the evolution and maintenance of sex, and ancient parthenogenetic taxa therefore have been termed “evolutionary scandals” (Maynard Smith 1978; Judson and Normark 1996). It is generally assumed that parthenogenetic lineages are “dead ends” in evolution for various reasons. They are believed to be unable to keep up in the evolutionary arms race with pathogens and predators (cf. Red Queen hypothesis; van Valen 1973; Hamilton 1980; see also Chapter 7), they may be unable to adapt to different ecological niches (cf. Tangled bank hypothesis; Ghiselin 1974; Bell 1982), and they may accumulate deleterious mutations (cf. Muller’s ratchet; Muller 1964; cf. Mutation load reduction theory; Kondrashov 1993; Crow 1994; see also Chapter 5).
Despite the theoretical arguments, there is increasing empirical evidence that oribatid mites comprise many clades of various size, extant members of which reproduce exclusively via parthenogenesis (Norton and Palmer 1991; Norton et al. 1993; Maraun et al. 2003b, 2004; Heethoff et al. 2007a; Laumann et al. 2007). These groups presumably radiated without sexual reproduction. The largest clades are within early to middle-derivative groups, such as Enarthronota (Brachychthoniidae; 102 spp.; Lohmanniidae; 156 spp.) and “Desmonomata” (Nanhermanniidae, 56 spp.; Malaconothridae, 104 spp.; Trhypochthoniidae, 68 spp.; Camisiidae, 92 spp.; and the genus Nothrus (Nothridae, 54 spp.)). Within the “Desmonomata”, sexual reproduction reevolved in the family Crotoniidae. This evolutionary recovery of a complex trait (sexual reproduction) is a unique evolutionary scenario in the animal kingdom and was termed a “breaking of Dollo’s law” (Domes et al. 2007). Violation of Dollo’s law has been controversial (Goldberg and Igic 2008) but recent phylogenetic studies have demonstrated that the re-evolution of complex features occurs more often than previously assumed. If the underlying developmental pathway is retained after the loss of a certain character, it may be regained quickly when needed (Collin and Miglietta 2008).
Molecular evidence further suggests the existence of parthenogenetic speciation for several groups of oribatid mites. Maraun et al. (2003b) used comparisons of sequence divergences of the 28S rDNA D3-region of closely related parthenogenetic oribatid mite species to show that parthenogenesis is an ancient phenomenon for the genus Tectocepheus (Brachypylina) and lineages within the “Desmonomata”. Phylogenetic analyses of the D3-region also suggest the existence and radiation of multiple parthenogenetic and diverse lineages, such as Nanhermanniidae, Malaconothridae, Camisiidae and the genus Tectocepheus (Maraun et al. 2004). Laumann et al. (2007) analysed four nuclear genes of three controversial species of Tectocepheus and showed that T. velatus (Michael), T. sarekensis (Träghård) and T. minor (Berlese), although similar in morphological aspects, have distinct genotypes and likely split into separate species while being parthenogenetic. Heethoff et al. (2007a) found seven distinct genetic lineages (which can be termed cryptic species) for the mitochondrial gene cytochrome oxidase 1 (cox1) of Platynothrus peltifer (Camisiidae), corresponding to their geographical origin (North America, Northern/Central Europe, Southern Europe, Northern Tyrolia, Eastern Europe and Japan).
The 4X rule is a quantitative measurement for speciation in parthenogentic species based on genetic distances and is used to identify species under the Evolutionary Genetic Species Concept (EGSC; W. Birky, pers. comm.; see also Chapter 10 in this book). The seven lineages of Platynothrus peltifer fulfill the conditions of the 4X rule and thus may be considered evolutionary genetic species. High genetic distances corresponded to divergence times up to 64 million years and it was concluded that the current distribution of lineages result from vicariance rather than dispersal. This supports the old theory that the general distribution of oribatid mites is largely related to patterns of continental drift (Hammer and Wallwork 1979).
Besides the phylogenetically clustered, ancient parthenogenetic lineages of oribatid mites, there are also recent and phylogenetically isolated parthenogenetic lineages with close sexual congeners (Cianciolo and Norton 2006). Of the proposed explanations for the ecological distribution of parthenogenetic oribatid mites, three were examined by Cianciolo and Norton (2006), namely a correlation with overall biotic uncertainty (as generated by competitors and predators); a lack of ecological correlation consistent with Muller’s ratchet and the existence of general purpose genotypes (Lynch 1984; see also Chapter 6) among ancient parthenogenetic lineages and recent parthenogenetic lineages. They rejected biotic uncertainty as an explanatory mechanism for either ancient parthenogenetic lineages or recent parthenogenetic lineages; further, there was no evidence for the existence of general purpose genotypes in the two groups, although this clearly deserves further investigation. However, while Muller’s ratchet (Muller 1964) seems unimportant for ancient parthenogenetic lineages, it may influence the distribution of recent parthenogenetic lineages (Cianciolo and Norton 2006). This is surprising since Muller’s ratchet acts in the long-term and should therefore apply more strongly to ancient parthenogenetic lineages than to recent parthenogenetic lineages.
The large number of parthenogenetic lineages, the spectrum-like distribution of reproductive modes (ancient parthenogenetic lineages to recent parthenogenetic lineages) and the unusual mode of reproduction (terminal fusion automixis and possibly inverted meiosis) make oribatid mites a unique model system to analyse the ecological and evolutionary advantages and disadvantages of parthenogenetic reproduction. It is a challenge for future studies to elucidate the mechanisms that allow the presence and persistence of numerous parthenogenetic oribatid mite clades that vary widely in size and, presumably, in age.
References
Alberti G, Coons LB (1999) Acari: Mites. In: Harrison FW, Foelix RF (eds) Microscopic Anatomy of Invertebrates. Wiley-Liss Inc., New York, pp. 515–1215
Anderson JM (1975) The enigma of soil animal species diversity. Proc. 5th Int Coll Soil Zool, Prague, 1973, pp. 51–58
Badejo MA, Espindola JAA, Guerra JGM, De Aquino AM, Correa MEA (2002) Soil oribatid mite communities under three species of legumes in an ultisol in Brazil. Exp Appl Acarol 27: 283–296
Barraclough TG, Birky CW Jr, Burt A (2003) Diversification in sexual and asexual organisms. Evolution 57: 2166–2172
Bell G (1982) The Masterpiece of Nature. The Evolution and Genetics of Sexuality. University of California Press, Berkeley, CA
Behan VM, Hill SB (1978) Feeding-habits and spore dispersal of oribatid mites in the North-American arctic. Rev Ecol Biol Sol 15: 497–516
Behan-Pelletier VM (1999) Oribatid mite biodiversity in agroecosystems: role for bioindication. Agric Ecosys Environ 74: 411–423
Bergmann M, Laumann M, Cloetens P, Heethoff M (2008) Morphology of the internal reproductive organs of Archegozetes longisetosus Aoki (Acari, Oribatida). Soil Org 80: 171–195
Cianciolo JM, Norton RA (2006) The ecological distribution of reproductive mode in oribatid mites, as related to biological complexity. Exp Appl Acarol 40: 1–25
Collin R, Miglietta MP (2008) Reversing opinions on Dollo’s law. Trends Ecol Evol 23: 602–609
Cordaux R, Michel-Salzat A, Bouchon D (2001) Wolbachia infection in crustaceans: novel hosts and potential routes for horizontal transmission. J Evol Biol 14: 237–243
Crow JF (1994) Advantages of sexual reproduction. Dev Gen 15: 205–213
Domes K, Norton RA, Maraun M, Scheu S (2007) Reevolution of sexuality breaks Dollo’s law. Proc Natl Acad Sci USA 104: 7139–7144
Eisenhauer N, Partsch S, Parkinson D, Scheu S (2007) Invasion of a deciduous forest by earthworms: changes in soil chemistry, microflora, microarthropods and vegetation. Soil Biol Biochem 39: 1099–1110
Erdmann G, Floren A, Linsenmair KE, Scheu S, Maraun M (2006) Effect of forest age on oribatid mites from the bark of trees. Pedobiologia 50: 433–441
Erdmann G, Otte V, Langel R, Scheu S, Maraun M (2007) The trophic structure of bark-living oribatid mite communities analysed with stable isotopes (15N; 13C) indicates strong niche differentiation. Exp Appl Acarol 41: 1–10
Evans GO (1992) Principles of Acarology. CAB International, Wallingford
Ewert MA, Jackson DR, Nelson CE (1994) Patterns of the temperature-dependant sex determination in turtles. J Exp Zool 270: 3–15
Fisher RA (1930) The genetical theory of sexual selection. Clarendon Press
Ghiselin MT (1974) The Economy of Nature and the Evolution of Sex. University of California Press, Berkeley, CA
Goldberg EE, Igic B (2008) On phylogenetic tests of irreversible evolution. Evolution 62: 2727–2741
Grandjean F (1953) Essai de classification des Oribates (Acariens). Bull Soc Zool France 78: 421–446
Grandjean F (1956) Caracteres chitineux de l’ovipositeur, en structure normale chez les Oribates (Acariens). Arch Zool Exp Gen Paris 93: 96–106
Grandjean F (1965) Complément a mon travail de 1953 sur la classification des oribates. Acarologia 7: 713–734
Grandjean F (1969) Considérations sur le classement des oribates: leur division en 6 groupes majeurs. Acarologia 11: 127–153
Hamilton WD (1980) Sex versus non-sex versus parasites. Oikos 35: 282–290
Hammer M, Wallwork JA (1979) A review of the world distribution of oribatid mites (Acari: Cryptostigmata) in relation to continental drift. Biol Skr Dan Vid Selsk 22: 1–31
Hansen RA (1999) Red oak litter promotes a microarthropod functional group that accelerates its decomposition. Plant Soil 209: 37–45
Hansen RA (2000) Effects of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology 81: 1120–1132
Harding DJL, Stuttard RA (1974) Microarthropods. In: Dickinson CH, Pugh GJF (eds) Biology of Plant Litter Decomposition. Acad Press, London, pp. 489–532
Haumann G (1991) Zur Phylogenie primitiver Oribatiden (Acari: Oribatida). Verlag Technische Univ, Graz
Heethoff M, Bergmann P, Norton RA (2006) Karyology and sex determination of oribatid mites. Acarologia 46: 127–131
Heethoff M, Domes K, Laumann M, Maraun M, Norton RA, Scheu S (2007a) High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). J Evol Biol 20: 392–402
Heethoff M, Laumann M, Bergmann P (2007b) Adding to the reproductive biology of the parthenogenetic oribatid mite Archegozetes longisetosus (Acari, Oribatida, Trhypochthoniidae). Turk J Zool 31: 151–159
Heethoff M, Norton RA (2009) Role of musculature during defecation in a particle-feeding arachnid, Archegozetes longisetosus (Acari, Oribatida). J Morphol 270: 1–13
Judson PO, Normark BB (1996) Ancient asexual scandals. Trends Ecol Evol 11: 41–46
Kondrashov AS (1993) Classification of hypotheses on the advantage of amphimixis. J Hered 84: 372–387
Labandeira CC, Phillips TL, Norton RA (1997) Oribatid mites and the decomposition of plant tissues in Palaeozoic coal-swamp forests. Palaios 12: 319–353
Laumann M, Norton RA, Weigmann G, Scheu S, Maraun M, Heethoff M (2007) Speciation in the parthenogenetic oribatid mite genus Tectocepheus (Acari, Oribatida) as indicated by molecular phylogeny. Pedobiologia 51: 111–122
Laumann M, Bergmann P, Heethoff M (2008) Some remarks on the cytogenetics of oribatid mites. Soil Org 80: 223–232
Lindo Z, Visser S (2004) Forest floor microarthropod abundance and oribatid mite (Acari: Oribatida) composition following partial and clearcut harvesting in the mixed wooded boreal forest. Can J Forest Res 34: 998–1006
Lindo Z, Winchester NN (2006) A comparison of microarthropod assemblages with emphasis on oribatid mites in canopy suspended soils and forest floors associated with ancient western redcedar trees. Pedobiologia 50: 31–41
Lindquist EE (1984) Current theories on the evolution of major groups of Acari and on their relationships with other groups of Arachnida, with consequent implications for their classification. In: Griffith DA, Bowman CE (eds) Acarology VI. Ellis Horwood Publ, Chichester, pp. 28–62
Lions JC, Gourbiere F (1988) Adult and immature populations of Adoristes ovatus (Acari, Oribatida) in the litter needles of Abies alba. Rev Ecol Biol Sol 25: 343–352
Lushai G, Loxdale HD, Allen JA (2003) The dynamic clonal genome and its adaptive potential. Biol J Linn Soc 79: 193–208
Lynch M (1984) Destabilizing hybridization, general purpose genotypes and geographic parthenogenesis. Quart Rev Biol 59: 257–290
Maraun M, Migge S, Schaefer M, Scheu S (1998a) Selection of microfungal food by six oribatid mite species (Oribatida, Acari) from two different beech forests. Pedobiologia 42: 232–240
Maraun M, Visser S, Scheu S (1998b) Oribatid mites enhance the recovery of the microbial community after a strong disturbance. Appl Soil Ecol 9: 179–186
Maraun M, Alphei J, Bonkowski M, Buryn R, Migge S, Peter M, Schaefer M, Scheu S (1999) Middens of the earthworm Lumbricus terrestris (Lumbricidae): microhabitats for micro- and mesofauna in forest soil. Pedobiologia 43: 276–287
Maraun M, Scheu S (2000) The structure of oribatid mite communities (Acari, Oribatida): patterns, mechanisms and implications for future research. Ecography 23: 374–383
Maraun M, Alphei J, Beste P, Bonkowski M, Buryn R, Peter M, Migge S, Schaefer M, Scheu S (2001) Indirect effects of carbon and nutrient amendments on the soil meso- and microfauna of a beechwood. Biol Fertil Soil 34: 222–229
Maraun M, Martens H, Migge M, Theenhaus A, Scheu S (2003a) Adding to the ‘enigma of soil animal diversity’: fungal feeders and saprophagous soil invertebrates prefer similar food substrates. Eur J Soil Biol 39: 85–95
Maraun M., Heethoff M, Scheu S, Norton RA, Weigmann G, Thomas RH (2003b) Radiation in sexual and parthenogenetic oribatid mites (Oribatida, Acari) as indicated by genetic divergence of closely related species. Exp Appl Acarol 29: 265–277
Maraun M, Heethoff M, Schneider K, Scheu S, Weigmann G, Cianciolo J, Thomas RH, Norton RA (2004) Molecular phylogeny of oribatid mites (Oribatida, Acari): evidence for multiple radiations of parthenogenetic lineages. Exp Appl Acarol 33: 183–201
Maraun M, Illig J, Sandmann D, Krashevska V, Norton RA, Scheu S (2008) Soil Fauna. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R (eds) Gradients in a Tropical Mountain Ecosystem of Ecuador. Ecological Studies 198, Springer, Berlin, pp. 181–192.
Mark Welch D, Meselson M (2000) Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288: 1211–1215
Maynard Smith J (1978) The Evolution of Sex. Cambridge University Press, Cambridge, UK
Michael AD (1884) British Oribatidae, Vol. 1, Ray Society, London
Migge S, Maraun M, Scheu S, Schaefer M (1998) The oribatid mite community (Acarina) on pure and mixed stands of beech (Fagus sylvatica) and spruce (Picea abies) at different age. Appl Soil Ecol 9: 119–126
Migge-Kleian S, McLean MA, Maerz JC, Heneghan L (2006) The influence of invasive earthworms on indigenous fauna in ecosystems previously uninhabited by earthworms. Biol Invasions 8: 1275–1285
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relationship between δ15N and animal age. Geochim Cosmochim Acta 48: 1135–1140
Muller HJ (1964) The relation of recombination to mutational advance. Mutat Res 1: 2–9
Norton RA, Bonamo PM, Grierson JD, Shear WA (1988) Oribatid mite fossils from a terrestrial Devonian deposit near Gilboa, New York. J Paleontol 62: 421–499
Norton RA, Palmer SC (1991) The distribution, mechanisms and evolutionary significance of parthenogenesis in oribatid mites. In: Schuster R, Murphy W (eds) The Acari: Reproduction, Development and Life-History Strategies. Chapman and Hall, London, pp. 107–136
Norton RA, Kethley JB, Johnston DE, OConnor BM (1993) Phylogenetic perspectives on genetic systems and reproductive modes of mites. In: Wrensch DL, Ebbert MA (eds) Evolution and Diversity of Sex Ratios. Chapman and Hall, New York, pp. 8–99
Norton RA (1994) Evolutionary aspects of oribatid mite life histories and consequences for the origin of the Astigmata. In: Houck MA (ed) Mites: Ecological and Evolutionary Studies of Life-History Patterns. Chapmann and Hall, New York, pp. 99–135
Otto J (1997) Observations on prelarvae in Anystidae and Tenerifiidae. In: Mitchell R, Horn DJ, Needham GR, Welbourn WC (eds) Acarology IX. Proc Vol. 1. Ohio Biological Survey, Columbus, OH, pp. 343–354
Palmer SC, Norton RA (1992) Genetic diversity in thelytokous oribatid mites (Acari; Acariformes: Desmonomata). Biochem Syst Ecol 20: 219–231
Perrot-Minnot MJ, Norton RA (1997) Obligate thelytoky in oribatid mites: no evidence for Wolbachia-inducement. Can Entomol 129: 691–698
Peschel K, Norton RA, Scheu S, Maraun M (2006) Do oribatid mites live in enemy-free space? Evidence from feeding experiments with the predatory mite Pergamasus septentrionalis. Soil Biol Biochem 38: 2985–2989
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718
Renker C, Otto P, Schneider K, Zimdars B, Maraun M, Buscot F (2005) Oribatid mites as potential vectors for soil microfungi: study of mite-associated fungal species. Microbiol Ecol 50: 518–528
Salamon JA, Alphei J, Ruf A, Schaefer M, Scheu S, Schneider K, Sührig A, Maraun M (2006) Transitory dynamic effects in the soil invertebrate community in a temperate deciduous forest: effects of resource quality. Soil Biol Biochem 38: 209–221
Sanders FH, Norton RA (2004) Anatomy and function of the ptychoid defensive mechanism in the mite Euphthiracarus cooki (Acari: Oribatida). J Morphol 259: 119–154
Schaefer I, Domes K, Heethoff M, Schneider K, Schön I, Norton RA, Scheu S, Maraun M (2006) No evidence for the ‘Meselson effect’ in parthenogenetic oribatid mites (Acari, Oribatida). J Evol Biol 19: 184–193
Schatz H (2002) Die Oribatidenliteratur und die beschriebenen Oribatidenarten (1758–2001) – eine Analyse. Abh Ber Naturkundemus Görlitz 72: 37–45
Scheu S, Setälä H (2002) Multitrophic interactions in decomposer food webs. In: Hawkins BA, Tscharntke T (eds) Multitrophic Level Interactions. Cambridge University Press, Cambridge, UK, pp. 223–264
Schmelzle S, Norton RA, Helfen L, Heethoff M (2008) The ptychoid defensive mechanism in Euphthiracaroidea (Acari: Oribatida): a comparison of exoskeletal elements. Soil Org 80: 233–247
Schneider K, Migge S, Norton RA, Scheu S, Langel R, Reineking A, Maraun M (2004) Trophic niche differentiation in oribatid mites (Oribatida, Acari): evidence from stable isotope ratios (15N/14N). Soil Biol Biochem 36: 1769–1774
Schneider K, Maraun M (2005) Feeding preferences among dark pigmented fungi (Dematiacea) indicate trophic niche differentiation of oribatid mites. Pedobiologia 49: 61–67
Schneider K, Renker C, Scheu S, Maraun M (2005) Oribatid mite feeding on ectomycorrhizal fungi. Mycorrhiza 16: 67–72
Shear WA, Bonamo M, Grierson JD, Rolfe WDI, Smith EL, Norton RA (1984) Early land animals on North America: evidence from Devonian age arthropods from Gilboa, New York. Science 224: 492–494
Sokolov II (1954) The chromosome complex of mites and its importance for systematics and phylogeny. Tr O Estestvoispyt 72: 124–159
Sovik G, Leinaas HP (2003) Long life cycle and high adult survival in an arctic population of the mite Ameronothrus lineatus (Acari, Oribatida) from Svalbard. Polar Biol 26: 500–508
Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53: 71–102
Subias LS (2004) Listado sistimatico, sininimico y biogeografico de los Acaros Oribatidos (Acariformes, Oribatida) del mundo (1748–2002). Graellsia 60: 3–305
Taberly G (1987) Recherches sur la parthéogenèse thélytoque de deux espèces d’acariens oribatides: Trypochthonius tectorum (Berlese) et Platynothrus peltifer (Koch). III. Etude anatomique, histologique et cytologique des femelles parthénogenétiques. Acarologia 28: 389–403
Taberly G (1988) Recherches sur la parthénogenèse thélytoque de deux espèces d’acariens oribatides: Trypochthonius tectorum (Berlese) et Platynothrus peltifer (Koch). IV. Observation sur les males ataviques. Acarologia 29: 95–107
Van Valen LM (1973) A new evolutionary law. Evol Theory 1: 1–30
Wallwork JA (1977) The structure of the ovipositor and the mechanisms of oviposition in the oribatid mite Machadobelba symmetrica Bal. (Acari: Cryptostigmata). Acarologia 19: 149–154
Walter DE, Proctor HC (1999) Mites. Ecology, Evolution and Behaviour. CAB International, Oxon
Weeks AR, Velten R, Stouthamer R (2003) Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc Lond B 270: 1857–1865
Weigmann G (1996) Hypostome morphology of Malaconothridae and phylogenetic conclusions on primitive Oribatida. In: Mitchell R, Horn DJ, Needham GJ, Welbourn WC (eds) Acarology IX, vol 1, Proc, Ohio Biological Survey, Columbus, OH, pp. 273–276
Weigmann G (2006) Hornmilben (Oribatida). Goecke and Evers, Keltern
White MJD (1973) Animal Cytology and Evolution. Cambridge University Press, Cambridge, UK
Wrensch DL, Kethley JB, Norton RA (1994) Cytogenetics of holokinetic chromosomes and inverted meiosis: keys to the evolutionary success of mites, with generalizations on eukaryotes. In: Houck MA (ed) Mites: Ecological and Evolutionary analyses of life-history pattern. Chapman and Hall, New York, pp. 282–343
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
- Hysterosoma:
-
the hind body division of Acari consisting of the opisthosoma and the metopodosoma (segments bearing the two hind legs)
- Meros:
-
distal part of the ovary in Acari with vitellogenetic oocytes
- Proterosoma:
-
the fore body division of Acari consisting of the gnathosoma (mouthparts) and the fore two segments with walking legs
- Rhodoid:
-
central part of the ovary in Acari with previtellogenetic oocytes
- Vitellogenesis:
-
process of yolk deposition and formation via nutrients being deposited in the oocyte
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Heethoff, M., Norton, R.A., Scheu, S., Maraun, M. (2009). Parthenogenesis in Oribatid Mites (Acari, Oribatida): Evolution Without Sex. In: Schön, I., Martens, K., Dijk, P. (eds) Lost Sex. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-2770-2_12
Download citation
DOI: https://doi.org/10.1007/978-90-481-2770-2_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-2769-6
Online ISBN: 978-90-481-2770-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)