Abstract
The first stem cells described in the biological literature were those of hydroid cnidarians; their detection by Weismann in 1883 gave rise to his germ line and “germ plasm” theory (with “germ plasm” meaning what is called genome today). Somatic cells preserving properties of eggs (called Stammzellen, i.e. stem cells, by him) were considered by him to be the cellular source of regeneration and reproduction. Weismann’s studies have been the foundation of modern cnidarian stem cell research. In the latter, hydroid stem cells have been referred to as interstitial cells (shortly i-cells), and have mostly been studied in two cnidarian genera: the freshwater polyp Hydra and the colonial marine hydroid Hydractinia. In these animals, i-cells constitute a complex system of multipotent (in Hydra) or totipotent (in Hydractinia) stem cells and their derivatives. I-cells’ potencies have been investigated by specific elimination of stem cells and reintroduction of i-cells from donors. The complement of stem cells confers potential immortality to the genetic individual. Cnidarians’ cells in general have an unmatched capability of re- and transdifferentiation. Isolated, fully differentiated striated muscle cells of hydroid medusae may resume features of multipotent stem cells and give rise to almost all cell types including germ cells. Reverse development of adult stages back into juveniles is a further manifestation of cnidarian developmental plasticity. Typical i-cells have not been described in other cnidarian groups. In these taxa the source of new nematocytes nerve and germ cells may be differentiated cells that preserve plasticity. Following a historical perspective we review recent advances in hydroid i-cell research, and discuss the potential of invertebrate stem cell work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cnidaria
- Hydra
- Hydractinia
- Podocoryne
- Germ cells
- Germ plasm theory
- Nematocytes
- Nerve cells
- Reverse development
- Redifferentiation
- Transdifferentiation
3.1 Introduction
Hydroid cnidarians were the first animals in which stem cells have been described. These cells, which today are referred to as interstitial cells, or shortly, i-cells, constitute a pool of migratory, undifferentiated cells, capable of self-renewal as well as of differentiation into specialized cell types. I-cells are lodged in interstitial spaces of epithelial cells (mostly ectoderm), that form their niche, and hence their name.
Surprisingly, the presence of i-cells, and stem cells altogether, in other cnidarians has been unclear (see Section 3.8). Even within the Hydrozoa most i-cell research has been conducted on only two genera, the solitary freshwater polyp, Hydra, and the colonial marine hydroid, Hydractinia. These studies (see refs below) revealed the complexity, developmental potencies and some of the molecular mechanisms that control i-cell fate. Although still in its infancy, i-cell research at the molecular level provides exciting perspectives for both basic as well as applied biomedical research. First evidences (e.g. Teo et al. 2006; Khalturin et al. 2007 suggest that many components of the genetic networks controlling i-cell fate are shared with vertebrate stem cell systems. With the rapidly growing cnidarian developmental biology community, and the newly established resources, such as full genome sequences, transgenic and knockdown technologies now available, it is anticipated that the coming years will bring great advances to the field.
In this chapter we review studies done on i-cells in some hydroid species. After a historical view, we discuss different aspects of i-cell biology. In particular we concentrate on morphology, staining properties, developmental potencies, gene expression, and the molecular mechanisms controlling their fate. Finally, we address the perspectives of i-cell research in basic biology and biomedicine.
3.2 Historical View
It was the physician and zoologist August Weismann (1834–1914) from the University of Freiburg (Germany) who first detected stem cells in the animal kingdom, and these were the interstitial stem cells in hydroids (Weismann 1883). The term “Stammzellen” (stem cells) in the sense of committed precursors with capacity to multiply appears in paragraphs dealing with the origin of germ cells in Hydractinia echinata (Weismann 1883, p. 82), and Coryne pusilla (Weismann 1883, p. 238). In Coryne “stem cells of the Glockenkern (the jellyfish’s entocodon tissue) would migrate out into the endoderm, and would do this singly” (translations by WAM). These putative stem cells were thought to include the germ cell precursors. In a paragraph related to Corydendrium the term “interstitielle Zellen” (interstitial cells) refers to the precursors of nematocytes (Weismann 1883, p. 38).
Weismann’s (1883) extensive investigations on the origin of germ cells in many colonial hydroids aimed at detecting the first identifiable “Stammzellen der Keimzellen” (stem cells of germ cells, p. 239) for which he coined the new term “Ur-Keimzellen” or “Urkeimzellen” (primordial germ cells; p. 230). He stated “histologically differentiated cells” never transform into germ cells but “only cells having preserved embryonic characteristics”. He described their presence in the blastostyles (gonozoids, sexual polyps/hydranths) and in the stolonal compartment of marine, colonial species such as Coryne pulsilla, Eudendrium racemosum, Hydractinia echinata and Podocoryne carnea (and many others). Weismann (1904) described the location of germ cell precursors at the base of epithelial cells contacting the basal lamina (mesoglea), their migration into budding medusae, and their final residence in the manubrium (feeding organ) of the medusae in Eudendrium and Podocoryne, or in the phylogenetic derivatives of medusae, the gonophores in Hydractinia (Fig. 3.1).
Origin of germ cell precursors in a typified hydrozoan according to Weismann 1883, 1904. In terms of phylogeny, the origin of germ cell precursors (kz) is shifted from the manubrium (Mst) of the medusa (M) into the gonophore bud (Gph K) and further into the stalk (St) of the polyp and eventually into the stolon (A). GH gastral cavity. The drawing shown is reproduced from Weismann 1904, p. 94. In retrospect, one correction should be made: The precursors in the stolons are not strictly germ line- restricted but multipotent
Weismann concluded that “there are distinct generations of cells, determined in advance, that undergo transformation into germ cells”. For Weismann this observation was the incentive to infer the existence of a particular germ line separated from mortal somatic cell lineages in animals in general and to propose his influential but disputed “Keimplasmatheorie” (germ plasm theory, Weismann 1885, 1892a,b).
It may appear trivial that in multicellular organisms a cell line exists leading from the fertilized egg through series of cell divisions to new primordial germ cells. The question is when in ontogeny a particular cell line separates from cell lines leading to terminally differentiated somatic cell types such as nerve cells or muscle cells. Corroborating Weismann’s expositions, recent reviews (Extavour and Akam 2003) and textbooks tell of two different modes of germ line separation: In some animals, represented by the classical model organisms Drosophila, Caenorhabditis and Xenopus, primordial germ cells are specified very early in embryogenesis by maternal cytoplasmatic determinants stored in the egg and allocated during cleavage to one or few daughter cells. These cells are definitively programmed to become primordial germ cells and set aside for future use in the adult animal. In other animals, represented by the annelid Platynereis (Rebscher et al. 2007), by a parasitic arthropod barnacle (Shukalyuk et al. 2006), and the mouse embryo, specification of separate germ lines occurs later in the development (Extavour and Akam 2003), probably by inductive signals emitted by the surrounding tissues. It is ironic that the animals in which the existence of germ line-restricted stem cells was first proposed (i.e. hydroids) probably do not have an early committed germ line, but rather, germ cells differentiate continuously from multi- or totipotent stem cells that can also give rise to somatic cells.
Weismann hypothesized that in the evolution of the hydroids the time point of separation moved gradually back from the gonad of the medusa into to the polyp and, in colonial forms such as Hydractinia, into the stolons (as exposed below in Section 2.3, the origin of the multipotent or totipotent interstitial stem cells is even more shifted back into the gastrula). Weismann also described the transitory migration of the germ cell precursors from the ectoderm into the endoderm in the germ zone of the hydranth (polyp) that produces medusae or gonophores, and their eventual emigration from the endoderm to their final location (Figs. 3.1 and 3.2). As Weismann already stated, in other animals a separate germ line can be traced back to a few cells in the embryo. It was Weismann who coined the term “Polzellen” (pole cells) to designate the primordial germ cells in the early embryo of certain dipterans (later also found in Drosophila).
Primordial germ cells in the sexual polyp. (A) Blastostyle (gonozoid, sexual polyp) in a colony of Hydractinia echinata. Keimzone = germ zone. Gon.-spross-zone = gonophore budding zone. Gonophores are the ball-shaped containers of the germ cells (here of eggs). In terms of phylogeny, they are reduced, sessile medusae. In light-induced spawning the wall of gonophores opens at a prespecified spot to release the mature eggs. Detail from a drawing by Müller 1964. (B) Intersexual germ zone of a blastostyle of Hydractinia echinata. The multiplying spermatogonia (Spg) displace the oocytes (Oo); therefore, oocytes do not mature and the polyp will funtionally be male. Cc = cnidocyte (nematocyte). From Müller 1964
What determines cells to become germ cell precursors? In the model organisms mentioned before, i.e. Drosophila, Caenorhabditis and Xenopus, the determinants are called “germ plasm”. This consists of non-vesicular aggregates of various types of RNAs including mitochondrial RNA in the cytoplasm, and/or of RNA bound to accumulations of fibrous material near the nucleus, known as nuage (French for cloud). The germ plasm also embodies transcripts of vasa. The term “Keimplasma” (germ plasm) was coined by Weismann (1885, 1892a,b, 1904) but the meaning of his term was very different from its present usage. In his theory, proposed before the dawn of classic genetics, germ plasm means the entirety of the putative “Vererbungssubstanzen” (hereditary substances), consisting of different material units, which he called “Iden”. When chromosomes and their behavior in cell division and meiosis were described, Weismann did not hesitate to attribute his “Vererbungssubstanz” to these structures. He equated his Iden with chromosomes. Thus, his germ plasm is identical with the whole set of chromosomes. In his germ plasm theory he proposed that the cells of the germ line always are supplied with the entire set of chromosomes and thus with the whole complement of determinants, now known as genome.
However, in the further elaboration of his theory, Weismann made a wrong prediction. He thought cellular differentiation in the somatic cell lines would result from the discriminate distribution of the different “Iden” (chromosomes) among the various somatic cell precursors. “Biophores” would then export different information from the chromosomes of the nucleus into the cytoplasm. Asexual reproduction by budding and regeneration would be possible by cells, which inherit more chromosomes from the egg cells. This reserve supply of chromosomes would be silent until activated by external influences such as wounding. Although the idea of cells equipped with varying numbers of chromosomes soon turned out to be invalid (except for certain nematodes such as Parascaris and a few other invertebrates), the idea of multipotent stem cells preserving features of embryonic cells was born. From now on, stem cells with properties not much different from those of fertilized eggs were considered to be the source for regeneration and asexual reproduction.
Weismann himself applied this hypothesis to regeneration in hydroid polyps (and other animals). Stem cells distributed among the entire body would harbor a large complement of hereditary determinants enabling renewal and restoration. But only the germ line would retain the whole complement (Weismann 1904).
The origin of germ cells from interstitial stem cells was inferred by Weismann from the interpretation of histological preparations only and was described in more detail by a contemporary study (Bunting 1894). Experimentally, the origin of germ cells from i-cells was first shown by Hauenschild (1956, 1957) who transplanted i-cells into asexual clones of the medusa Eleutheria dichotoma. The recipients were enabled to produce germ cells. This pioneering study was followed by transplantation studies in Hydractinia echinata, a species of reference for Weismann (Müller 1964, 1967; Müller et al. 2004a). In the genus Hydra subpopulations of interstitial cells were shown to be stem cells with potencies limited to the germ line by introducing few stem cells in stem cell-depleted recipients (Littlefield and Bode 1986; Bosch and David 1987; Littlefield 1991; Nishimiya-Fujisawa and Sugiyama 1993). The experimental setup for eliminating i-cells is described below.
3.3 Hydroid Interstitial Stem Cells: Morphology, Cellular Properties, and Gene Expression
3.3.1 Staining and Morphology
In hydroids, interstitial stem cells have traditionally been identified by their morphology and staining properties. The i-cells of Hydractinia echinata (see Fig. 3.3 for life cycle) are shown in Fig. 3.4. I-cells are small, rounded or spindle-shaped, and approximately 7–10 μm in diameter. Their nuclei are large, taking a significant proportion of the cells’ volumes, and the chromatin is less dense than in differentiated somatic cells. By contrast, their cytoplasm is very dense and filled with ribosomes (Lentz 1965, 1966; Weis et al. 1985; Martin and Archer 1986, 1997). This high content of nucleic acids confers stainability with basic dyes. I-cells can be stained with toluidine blue or methylene blue and particularly set off from the surrounding tissue after a consecutive staining first with eosine-methylene blue solution after May-Grünwald followed by an azur-eosine-methylene blue staining after Giemsa (Müller 1964). Labeling the cytoplasm with acridine orange allows observing migrating cells for short periods of time. Over long periods and distances migrating cells can be traced by labeling cycling i-cells with BrdU and detection with anti-BrdU antibodies (Plickert and Kroiher 1988; Plickert et al. 1988; Kroiher et al. 1990; Müller et al. 2004a). Transgenic interstitial stem cells expressing GFP can be traced in living animals (Miljkovic et al. 2002; Wittlieb et al. 2006; Kalthurin et al. 2007; Plickert, unpubl).
Morphology and location of i-cells in Hydractinia. (A) Structure of the stolon plate of a mature colony with i-cells between two ectodermal epithelial layers. The upper ectodermal epithelium is translucent and allows detection of i-cells in whole mount preparations. (B) Detail of a stolon plate stained with blue basic dyes (May Grünwald + Giemsa). Source: Müller et al. (2004a)
3.3.2 Cell Cycle
Labeling with 3H-thymidine enables measurement of the duration of the cell cycle and its phases. This has been carried out in Hydra (David and Campbell 1972; Campbell and David 1974; David and Gierer 1974; Campbell 1976; David and Murphy 1977; David and MacWilliams 1978; David and Plotnick 1980; Herrmann and Berking 1987; Holstein and David 1990a,b; David et al. 1991; Holstein et al. 1991; also reviewed by David et al. 1987; Bode 1996; Bosch 2007a,b). I-cells are characterized by a short G1, an S-phase of 12 h, and a variable G2. The majority of the interstitial stem cells divide with an average cell cycle time of 24 h (Campbell and David 1974). In descendants, commitment to a particular differentiation pathway occurs at the S/G2 boundary. The pathways leading to terminally differentiated nematocytes and nerve cells take 3–5 days. In this time mitotically active epithelial cells undergo one round of division on average.
3.3.3 Ontogeny
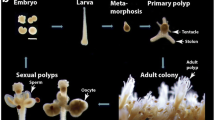
In Hydractinia i-cells first appear in the inner cell mass of the gastrula (Fig. 3.5). Previous reports on other hydroids identified i-cells only later, in the endoderm of planula larvae (van de Vyver 1964, 1967; Weis et al. 1985; Martin and Archer 1986, 1997). The discrepancy probably arose from the densely packaging of cells in the gastrula’s inner cell mass, rendering the identification of single cells in histological preparations difficult. New analyses with specific i-cell markers clearly show these cells as early as the gastrula (Figs. 3.5 and 3.6). During larval development some of these cells cross the mesoglea and emigrate into the ectoderm. Here they give rise to larval-type nematocytes that, in Hydractinia, are used to anchor the larvae onto shells of hermit crabs (Müller and Leitz 2002), and to larval neurons. The latter enable phototactic behavior. A subset of neurosecretory cells confers competence to metamorphose (Schmich et al. 1998; Müller and Leitz 2002; Plickert et al. 2003). Stimulated by external cues the larva will undergo metamorphosis to a primary polyp, the founder of a new colony (Fig. 3.3).
In situ hybridization of genes specifically expressed in i-cells. (A) An Oct4-like POU domain gene, expressed in i-cells in stolons; (B) PL10, expressed in i-cells in stolons (C) GalTSP, a galectin expressed in oocyte precursors; (D) HeGal3, expressed in oocyte precursors; (E) HyGal4, a galectin, expressed in i-cells in stolons; (F) frizzled, the Wnt receptor, expressed in i-cells in stolons. Scale bars approx. 10 μm in (A) and (B), 60 μm in (C) and (D), 100 μm in (E), and 40 μm in (F). Source: Mali, Millane, Frank unpubl
During metamorphosis most i-cells migrate into the ectoderm of the developing stolons. In post-metamorphic development i-cells are predominantly found in the stolonal compartment, enclosed between two ectodermal epithelia (Fig. 3.4). These epithelia provide a meshwork of interstitial spaces through which the cells can migrate to populate new parts of a growing colony. Committed descendants of these stem cells, mainly precursors of nematocytes, nerve cells, germ cells and gland cells, emigrate from the stolonal tissue into newly emerging polyps. The fate of the precursors is sometimes morphologically indicated as follows: nematoblasts by their developing capsules, oogonia by their location in the endodermal germ zone of sexual polyps and their increasing size. Further evidence was provided by gene expression studies and by experimental inferences (see below). The i-cell stem cell niche, at least in Hydractinia, is thought to be at the interstices of stolonal ectodermal epithelia. Polyps, at least in older colonies, may only contain committed cells. These assumptions are partly based on the observation that most i-cells are indeed found in stolons, but they are also supported by some experimental data: While able to regenerate heads, isolated Hydractinia polyps mostly do not regenerate stolons (Müller et al. 1986), whereas stolons bud new polyps regularly. There is an exception to this rule in very young primary polyps (Müller et al. 1986). This could be interpreted as a lack of totipotent stem cells in polyps (young polyps may still contain stem cells).
Sex in Hydractinia is genetically determined (Hauenschild 1954). The occurrence of intersexual polyps harboring both oogonia and spermatogonia, but being functional males results from a particular genetic heritage (Hauenschild 1954) or from the fusion of histocompatible male and female sibling colonies of different sex (Müller 1964). Recent studies suggest that intersexuality may be more widespread than previously thought (unpublished, see below). The evolution of histoincompatibility in Hydractinia (and in colonial invertebrates in general) has been interpreted as a mean to avoid parasitism of an individual colony by allogeneic germ cell precursors immigrating from neighboring colonies (Buss 1982, 1983).
3.3.4 Gene Expression in Stem Cells and Derivatives
Many i-cells in Hydra and Hydractinia express the gene vasa (Mochizuki et al. 2001; Rebscher et al. 2008). In Hydractinia oocytes the protein is found in a perinuclear cloud (nuage). In mature oocytes the mRNA disappears but the protein remains present. In fertilized eggs Vasa is redistributed and located at the polar-body-forming pole where also transcripts of the Wnt pathway (wnt and tcf) are concentrated (Plickert et al. 2006). During cleavage Vasa protein is equally allocated to the first four blastomeres; no early specification and segregation of a germ line is evident, in line with the situation in other clonal invertebrates (Buss 1999). New vasa transcripts and Vasa protein appear in the gastrula and planula, in parallel to the appearance of new i-cells. In primary polyps and colonies derived from them, vasa is expressed by i-cells in stolons and gonozoids. This suggests that in Hydractinia, vasa expression characterizes multipotent or totipotent stem cells with the capability to give rise to somatic and germ cells in mature colonies. This is different from animals that sequester the germ line already during early embryonic development. In the latter, vasa is only expressed in cells restricted to the germ line. In Hydractinia, germ cell restriction of stem cell descendants presumably occurs continuously in the stolon plate of mature colonies or during the migration of i-cells into the gonozoids.
In eggs of the hydrozoan jellyfish Podocoryne carnea transcripts of the germ line and stem cell specific gene Piwi (called Cniwi in cnidarians) were identified (Seipel et al. 2004a). The expression of this gene marks stem cells in many other animals as well. In Podocoryne, Cniwi is upregulated during transdifferentiation of somatic cells into multipotent stem cells. Another interesting gene, expressed in Hydractinia oocytes, is GalTSP. This gene, which encodes a galectin with a number of TSP domains, is expressed in female germ cell precursors (Mali and Frank unpubl). Interestingly, in an in situ hybridization analysis of over 16 male colonies, a few cells expressing the gene were found in all colonies (Fig. 3.6). A related gene in H. symbiolongicarpus, a sibling species to H. echinata, seems to function in immunity and has a very different expression pattern (Schwarz et al. 2007).
In addition to germ line-specific genes, oocytes of Hydractinia contain mRNAs of genes of the canonical Wnt-signaling system (Müller et al. 2007; Plickert et al. 2006; Teo et al. 2006). These genes are also expressed in i-cells in mature animals. In an ongoing study, we (unpubl) have identified many i-cell specific genes. Of particular interest are orthologues of genes known to be stem cell specific in higher animals such as a POU domain gene resembling oct4, a PL10-like gene (unpubl), and more. Studies done on Hydra and Hydractinia identified many genes that mark early nematocytes and nerve cells (see below). The function of these genes is still unclear; however, they are very useful in mapping different, morphologically indistinguishable, sub-populations of i-cell in polyps and colonies.
Cells entering the pathway to neuronal differentiation express proneural genes with sequence similarity to genes of the achaete-scute complex (ac-sc) family of bHLH transcription factors in Hydra and Podocoryne (Grens et al. 1995; Seipel et al. 2004b). Nematocyte precursors, which are considered to represent a particular type of sensory neurons, also express ac-sc (Hayakawa et al. 2004). Many proneuronal genes are under the upstream control of the homeobox gene cnox-2 (Miljkovic-Licina et al. 2007). The actual number of neuronal sub-populations is unknown. Of particular interest are genes that mark only a small fraction of neurons. One example is the CTRN gene (Cnidarian Tachylectin-Related gene in Neurons), expressed in only few neurons around the Hydractinia polyp mouth (Mali et al. 2006) and in their immediate i-cell precursors. It also marks early polyp buds on stolons. In contrast to the above genes, marking only a small neuronal fraction, FMR1, the Hydractinia homolgue of the fragile X mental retardation gene, is a broad neuronal marker (Guduric-Fuchs et al. 2004).
Specific marker genes for nematoblasts are genes that are activated when cells just enter this pathway of differentiation such as a homologue of zic/odd-paired gene (Lindgens et al. 2004) a Dickkopf-3-related gene (Fedders et al. 2004), or genes used to produce the capsule such as spinalin (Hellstern et al. 2006).
3.4 Developmental Potencies of i-cells
Like stem cells in other organisms and many cancer cells, cycling i-cells of hydroids are particular susceptible to agents that interfere with DNA replication and nucleic acid metabolism. This offers an opportunity to study the developmental potency of i-cells by selectively depleting animals of their i-cells and repopulate them with defined donor cells.
3.4.1 Studies on Hydra
Like in other hydroids, the complement of stem cells in Hydra confers potential immortality to the whole individual (genotype). All aged cells are replaced by newly produced cells. The contribution of the various stem cells to this process has been inferred from experimental interferences.
I-cells are lodged in the interstices mainly of the ectodermal epithelium, fewer numbers are found in interstices of the gastrodermis. In Hydra all i-cells can be eliminated by irradiation with x-rays (Strelin 1928; Brien and Reniers-Decoen 1950), or by treatment with nitrogen mustard (Diehl and Burnett 1964; Diehl 1973) or colchicine (Campbell 1976, 1979). The treated animals lose the ability to regenerate, apparently in support of the traditional notion that i-cells being the source of Hydra’s enormous capacity to regenerate (Strelin 1928; Brien and Reniers-Decoen 1950; Diehl and Burnett 1964; Diehl 1973). The evidence these early authors presented was considered invalid by later studies where harsh agents were replaced by the milder acting chemical hydroxy urea (e.g. Sacks and Davis 1979). With time, the treated animals become impoverished of nematocytes and nerve cells – which are derivatives of i-cells. Hence, treated hydras lose the capacity to catch prey and engulf food. Surprisingly these epithelial hydras survive if force-fed, continue to bud new polyps and restore the removed head by regenerating a new one (albeit with inferior quality; Campbell 1976, 1979; Marcum and Campbell 1978; Sacks and Davis 1979; Wanek et al. 1980; Bode et al. 1987; Bosch and David 1987; Holstein and David 1990a). Sf-1, a mutant strain of Hydra magnipapillata that loses its i-cells following a heat shock, displays similar deficiencies but retained regeneration capacity (Terada et al. 1988). Transplanting i-cells from wild type, untreated donors results in reappearance of all lost cell types.
Another experimental set up made use of the ability to dissociate hydras into single viable cell suspensions and the preparation of pellet aggregates by centrifugation (Bode et al. 1973). Aggregates prepared from i-cell-depleted animals were inoculated with few cells from healthy donors (David and Murphy 1977; Bosch and David 1986, 1987, 1990). The i-cells “cloned” in this way gave rise to various cell types or to uni-cultures, e.g. developing a single type of nematocytes or gametes, paralleling the clone-forming units in the mammalian hematopoietic system (see also David and Gierer 1974; Bode et al. 1976, 1987, 1990; Bode and Smith 1977; Bode and David 1978; David and MacWilliams 1978; Berking 1979, 1980; Sugiyama and Fujisawa 1979; Fujisawa and Sugiyama 1980; Bosch and David 1984, 1987; David et al. 1987, 1991; Terada et al. 1988; Fujisawa 1989; Holstein and David 1990a,b; Bosch et al. 1991; Holstein et al. 1991; Zeretzke and Berking 2002). In a recent publication GFP-labeled donor cells were used to trace their migration (Khalturin et al. 2007).
Above results and older studies showing that cellular transitions from gastrodermis to ectodermis or back (reported by Haynes and Burnett 1963; Burnett et al. 1966, 1973; Davis 1970, 1973), were not reproduced unambiguously (Macklin 1968), have led to the conclusion (reviewed by Bode 1996; Bosch 2004, 2007a,b) that three separate compartments of cell lineages exist in the genus Hydra (Fig. 3.7): (1) ectodermal epithelial cells, (2) endodermal epithelial cells, and (3) the interstitial cell lineages. Both epithelial cell lines contain functional epithelio-muscular cells capable of proliferation and self-renewal (sometimes called “epithelial stem cells”) and this enables epithelial hydras to regenerate and bud new polyps. No evidence for “true” epithelial stem cells exists, to the best of our knowledge, in the Hydra literature. By definition, stem cells are undifferentiated whereas Hydra’s “epithelial stem cells” are functional, differentiated cells displaying characteristics of epithelio-muscular cells and/or digestive cells. In well-fed animals the gastric region supplies supernumerary cells that are used to produce new animals by budding.
Fixture of cells in a hydra. There are three compartments with self-renewal capabilities: (1) the ectodermal, (2) the endodermal epithelial cells, and (3) the interstitial cell lineages. Note: each nest of nematoblasts gives rise to only one type of nematocytes. After Müller WA, Developmental Biology, Springer New York 1996
The third Hydra cell lineage comprises the interstitial cells. These cells are “true” stem cells, being undifferentiated. The majority of these cells are lodged in the ectodermal interstices, predominantly in the gastric region. A few are found in the head region; they are never found in the tentacles and peduncle. A type of large i-cells is considered to represent the stem cells proper (while the smaller ones are considered to be committed descendants; reviewed by Bode 1996). The interstitial stem cells are multipotent and give rise to four types of nematocytes, to sensory cells and ganglionic nerve cells, to endodermal gland cells, and to primordial germ cells (Fig. 3.7). The descendants migrate to their destinations in the terminal regions of the polyp, the hypostome with the tentacles (i.e., the head) or the basal disk (foot), and into buds. All hydra cells are displaced and move toward the oral and aboral pole, sloughing off at the tip of tentacles or at the basal disk.
Two features in the production of stem cell descendants are noteworthy: (1) Cells committed to become nematoblasts that undergo up to five rounds of division. The resulting two, four, eight, 16 or 32 nematoblasts adhere to each other by cytoplasmatic bridges, forming nests. Connection by cytoplasmic bridges enables synchronous differentiation. All nematoblasts of a nest form the same type of capsule. The type is determined at the beginning of the pathway (Shimizu and Bode 1995). Only shortly before the nematocytes reach full maturation do the cells separate and quickly migrate to their final sites in the tentacles (Kalthurin et al. 2007, and references therein). (2) Oocytes that engulf sister cells to increase their own size, a feature known from Hydra and some marine hydroids (Cladocoryne, Pennaria, Tubularia; Weismann 1883). In Hydra, oocytes are transitorily multinuclear, but only the original nucleus of the oocyte is thought to survive (Alexandrova et al. 2005).
I-cell descendants with potencies restricted to germ cell pathways were identified in several members of the genus Hydra (Bosch and David 1986; Nishimiya-Fujisawa and Sugiyama 1993). Two i-cell subpopulations, that were isolated from the phenotypic male strain nem-1 of Hydra magnipapillata, were examined for their roles in sex determination. One i-cell subpopulation was restricted to the sperm differentiation pathway, and the other subpopulation restricted to the egg differentiation pathway. Hydras containing only sperm- or egg-restricted stem cells, but no other interstitial stem cell types were maintained by force-feeding for two years. Sex reversals occurred three times during this period. These observations suggest that strain nem-1 (male) contains both sperm and egg-restricted i-cells. Differentiation of eggs, however, is normally suppressed, and only sperm are produced by the sperm-restricted stem cells. Evidence is presented which suggests that similar “phenotypic males”, which normally only produce sperm but contain the stem cell types capable of differentiating into eggs, occur widely in Hydra magnipapillata.
Particular features of Hydra, probably shared with polyps of other hydroid polyps, are the plasticity of the differentiated state of the epithelial and nerve cells. All cells in Hydra are continuously moving from the middle of the body column upward or downward, to the extremities. While migrating, cells have to adopt the phenotypic features of the new region they enter. For example, the last epithelial cell in the tentacle-generating zone is still dividing while its neighbor across the border in the tentacle does no longer divide (Holstein et al. 1991). While crossing this border the cells abruptly express different molecular markers. In the lower body column ectodermal cells that cross the border to the peduncle undergo a conversion into gland cells.
Sensory and ganglionic nerve cells are displaced along the body column together with the epithelial cells. It has been observed that nerve cells in different locations express different neuropeptides. This spatial repertoire appears to be accomplished partly through the replacement of nerve cells by immigrating neuroblasts, and partly by transdifferentiation (Koizumi et al. 1988; Koizumi and Bode 1991).
3.4.2 Studies on Hydractinia
This colonial marine species offers many advantages compared to other cnidarians (Frank et al. 2001). Sexual reproduction, embryonic development from eggs to planula larvae and their metamorphosis into primary polyps, and asexual cloning of colonies can routinely be performed in the laboratory. Mutant strains are available (Müller 2002) and were used as stem cell donors for i-cell-depleted wild-type colonies. I-cells were eliminated using alkylating agents such as mitomycin C (Müller 1966, 1967). Like in Hydra, mild treatments (i.e. low mitomycin C concentration) yield i-cells, nematocytes and germ cells free colonies, that are able to bud new (epithelial) polyps. To unravel the complete potency of migratory stem cells, a harsh treatment (i.e. high mitomycin C concentration) was used to deprive also epithelial cells of their capability of self-renewal, and in the long term (about two to three weeks) causing their death (Müller 1964; Müller et al. 2004a). The dying colonies were used as scaffolding for transplanted i-cells from untreated, BrdU-labeled mutant colonies. The donor colonies differed from the recipient also by sex. The transplanted i-cells migrated into all areas of the dying colonies and differentiated into all cell types of the animal as indicated by the mutant phenotype of the regenerated colonies, BrdU-containing nuclei of epithelial cells, showing that they originated from donor i-cells. The recipient also underwent sex reversal following i-cell transplantation showing again the origin of regeneration (Müller et al. 2004a). The sequence of cell replacement is shown in Fig. 3.8. It is concluded that in Hydractinia i-cells are capable to contribute to all cell types of the animal including epithelia. This does in no way rule out the existence of descendant interstitial cells with more restricted potencies and the immigration of committed descendants with limited abilities to proliferate, namely nematoblasts, neuroblasts and germ cell precursors.
Sequence of cell replacent by immigrating i-cells introduced into stem cell-depleted colonies of Hydractinia. First epithelial cells are replaced, finally new germ cells are produced. From Müller 1967, modified
3.5 Signals Controlling Self-Renewal and Differentiation
The signals controlling self-renewal and differentiation in hydroids have been subjected to speculations and are poorly defined. Key words used to indicate such signals are terms like “positional information” and “feed back signals” (Bode and Smith 1977; Bode and David 1978; Fujisawa et al. 1986, 1990; David et al. 1987, 1991; Fujisawa 1989; Bosch et al. 1991; Teragawa and Bode 1991; cited in Khalturin et al. 2007). An inhibitor suppressing the formation of stenoteles, a type of nematocyte in hydra, is interpreted as a feedback-signaling molecule regulating the density of this cell type (Fujisawa 1988). Oligopeptides have been suggested to control differentiation. For example, a role in regulating neuron differentiation has been attributed to the peptide Hym-355 (Takahashi et al. 2000; further references therein and in Bode 1996). However, in many cultured mammalian neuroblasts or embryonic stem cells, withdrawal of serum with its mitogenic factors stimulates neuronal differentiation (Howard et al. 1993; Wang et al. 2004; Zhang et al. 2005; Shigeta et al. 2006). Unspecific blocking of receptors for mitogenic signals is expected to induce similar effects and there is no easy way to discriminate between stimulation of differentiation by positive signals or by unspecific prevention of further proliferation. Remarkably, i-cells of Hydractinia express a gene for a serum-response factor and a role for this factor in stem cell decision-making has been postulated (Hoffmann and Kroiher 2001).
The Wnt pathway plays a major role in directing i-cells out of self-renewal. Wnt plays a crucial role also in axis formation and patterning of the Hydra polyp (Hobmayer et al. 2000; Broun et al. 2005) and in embryos and polyps of Hydractinia (Müller et al. 2004b, 2007; Plickert et al. 2006). In Hydractinia, a subtype of interstitial cells, probably cells already committed to their final fate (putative nematoblasts, neuroblasts and oogonia) express wnt and the Wnt receptor frizzled (Teo et al. 2006; Müller et al. 2007). Experimental interference was done by treating the animals with pulses of paullones, specific inhibitors of GSK-3β. Blockage of GSK-3β is expected to activate Wnt target genes. The treated colonies produced huge numbers of nematocytes and the polyps developed enlarged heads with high numbers of nerve cells (Fig. 3.9). It should be pointed out that this stimulation of nematocyte and nerve cell production occurs only in a small range of optimal concentrations. With respect to nematocyte production, similar results were recently described for hydra (Kalthurin et al. 2007). Evidence has also been presented for the involvement of the Notch signaling system in the control of the stem cell fate (Kaesbauer et al. 2007).
3.6 Redifferentiation, Transdifferentiation, and Reverse Development in Hydroids
Isolated mononucleated muscle cells of hydroid medusae may transdifferentiate in vitro to new cell types and even form a complex regenerate (Schmid et al. 1982, 1993; Alder and Schmid 1987; Schmid and Plickert 1990; Schmid and Reber-Müller 1995; Yanze et al. 1999). Transdifferentiation events follow a strict pattern, starting with cell type that resembles smooth muscle cells without a preceding DNA replication. This cell type behaves like a stem cell and produces all other cell types of the animal, including new interstitial stem cells and their derivatives, nematocytes, nerve cells and germ cells, and also new epithelial cells. Some preparations developed an inner and an outer layer separated by a basal lamina eventually resulting in a complete manubrium, the feeding organ of a medusa (Fig. 3.10).
Another manifestation of cnidarian developmental plasticity is the ability to “reverse develop” (Schmich et al. 2007, and references therein). Some cnidarians are unique in the animal kingdom because of their unequalled potential to undergo reverse development, such as cases of medusae buds or free swimming medusa that undergo reverse development into the polyp stage (e.g. in Podocoryne carnea) or isolated tentacles in Aurelia that can convert into metamorphosis-competent planula larvae (Lesh-Laurie et al. 1991). These examples and others of reverse development are stress-induced. However, in several cnidarian species reverse development is part of the normal life cycle: Stephanoscyphus planulophorus is a member of the scyphozoan group Coronata. The animals lack germ cells. Instead young medusae, known as ephyrae, transform into planula larvae that metamorphose into polyps (Werner and Hentschel 1983). Likewise, in the scyphozoan Cassiopea andromeda asexually produced buds look and behave like sexually produced planula larvae. Sexual and asexual borne larvae undergo metamorphosis into polyps, induced by the same external cues (Hofmann et al. 1978; Neumann et al. 1980; Thieme and Hofmann 2003). Reverse development exemplifies an unusual high plasticity of the differentiated state of these organisms.
It is reasonable to hypothesize that in cnidarians, which apparently lack morphologically identifiable interstitial stem cells (e.g. Anthozoa), terminally differentiated cells such as nematocytes, nerve cells and germ cells may arise through transdifferentiation of epithelial or other somatic cells having preserved high plasticity (Aochi and Kato 1980; Minasian and Mariscal 1980). Gene expression studies with marker genes such as vasa and nanos for the germ line (Extavour et al. 2005; Torras and Gonzalez-Crespo 2005), and other stem cell markers, may help to trace cell lineages in these animals. The unmatched reversibility of the differentiated state may help to identify genes and factors controlling reversal to stem cell characteristics.
Finally, dedifferentiation and transdifferentiation of fully differentiated epithelial cells, postulated in old literature (Davis 1970, 1973; Haynes and Burnett 1963; Lowell and Burnett 1969), is a topic recently again proposed for Hydra: During head regeneration an early dedifferentiation of digestive cells into blastema-like cells was described (Galliot et al. 2006).
3.7 Stem Cells in Other Cnidarians
Typical i-cells as described here are only known from Hydrozoa. Old textbooks occasionally speak of interstitial or amoeboid cells in Octocorallia (e.g. Kükenthal 1923) but a role as stem cells has not yet been shown or attributed to them. Drawings in old literature point to epithelial cells as source for nematocytes. The dividing precursors of nerve cells and gametes are not identified so far.
With the emergence of the sea anemone, Nematostella vectensis, as a new cnidarian model organism it is to hope that new research will address this issue. Indeed, anti-Vasa antibodies recognize i-cell-like cells in Nematostella (Fig. 3.7 in Extavour et al. 2005). Nematostella is an anthozoan. The class Anthozoa is considered by many Cnidaria researchers to represent the basal group within the Cnidaria. If stem cells and the mechanisms that control their fates are indeed conserved between hydroids and bilaterians, than it would be likely to find i-cells also in sea anemones.
3.8 Perspectives
Stem cell research is at the cutting edge of current research in biology and biomedicine. This is not only due to the basic interest of developmental and cell biologists, but also, and perhaps mainly, because of the great potential of stem cells in regenerative medicine. Mammalian stem cells can be cultured in defined media in vitro for prolonged periods of time, during which they remain in an undifferentiated state. It is also possible to manipulate them, instigating them to differentiate into specialized cell types. Mammalian stem cells can roughly be divided into two main types: embryonic stem cells, and adult (or tissue) stem cells. Embryonic stem cells are derived from the inner cell mass of the blastocyst (the mammalian blastula). They are pluripotent, i.e. able to give rise to any cell type of the embryo proper, but not to all extra-embryonic tissues (they do not contribute to the trophectoderm). Adult stem cells are more restricted in their developmental potencies, generally able to differentiate into a single cell type (= unipotent, e.g. skin stem cell) or several cell types (= multipotent, e.g. the hematopoietic stem cell). The term totipotent in vertebrates refers to the ability to contribute also to all extra-embryonic tissues and this ability is restricted to the fertilized egg and the blastomeres resulting from the first few cleavages. At present these cells are not culturable in vitro. In most invertebrates, in contrast, pluripotency equals totipotency due to the lack of extra-embryonic tissues.
Work on invertebrate stem cells could circumvent several problems associated with working on mammalian (especially human) cells. First, work on invertebrate cells is simpler in technology and, hence, cheaper. Hydroid i-cells, for example, can be observed and manipulated in vivo in the small, translucent animal without the need for costly cell culture technology (the animals grow in seawater). Furthermore, cell behavior in vivo is more likely to reflect “real life” than cells growing in a culture dish. Second, invertebrate stem cell research is interesting from a comparative, evolutionary, perspective (Yanze et al. 1999; Galliot and Schmid 2002; Holstein et al. 2003; Bosch 2004, 2007b). Finally, working on invertebrates minimizes the ethical issues associated with working on vertebrate systems, especially human embryonic stem cells. Clearly, invertebrate stem cell research cannot substitute for work on human cells. It can, however, complement the latter by enabling us to identify universal stem cell genes, both the genes that control differentiation, and those that maintain “stemness” (see Okita et al. 2007; Wernig et al. 2007; Yu et al. 2007). Many of both groups of genes will be shared with mammalian systems, but identifying them in invertebrates would be faster, cheaper, and free of ethical issues.
References
Alder H, Schmid V (1987) Cell cycles and in vitro transdifferentiation and regeneration of isolated, striated muscle of jellyfish. Dev Biol 124:358–369
Alexandrova O, Schade M, Bottger A, David CN (2005) Oogenesis in Hydra: nurse cells transfer cytoplasm directly to the growing oocyte. Dev Biol 28:91–101
Aochi M, Kato KI (1980) The polyp reconstitution in ectoderm and-or endoderm isolated from Arelia aurita. Dev Growth Diff 22:717
Berking S (1979) Control of nerve cell formation from multipotent stem cells in Hydra. J Cell Sci 4:193–205
Berking S (1980) Commitment of stem cells to nerve cells and migration of nerve cell precursors in preparatory bud development in Hydra. J Embryol Exp Morphol 60:373–387
Bode HR (1996) The interstitial cell lineage of hydra: a stem cell system that arose early in evolution. J Cell Sci 109:1155–1164
Bode HR, Berking S, David CN, Gierer A, Schaller H, Trenkner E (1973) Quantitative analysis of cell types during growth and morphogenesis in Hydra. Wilhelm Roux’ Arch 171:269–285
Bode HR, David CN (1978) Regulation of a multipotent stem cell, the interstitial cell of hydra. Progr Biophys Molec Biol 33:189–206
Bode HR, Flick KM, Smith GS (1976) Regulation of interstitial cell differentiation in Hydra attenuata. I. Homeostatic control of interstitial cell population size. J Cell Sci 20:29–46
Bode HR, Gee LW, Cow MA (1990) Neuron differentiation in hydra involves dividing intermediates. Dev Biol 139:231–334
Bode HR, Heimfield S, Chow MA, Huang LW (1987) Gland cells arise by differentiation from interstitial cells in Hydra attenuata. Dev Biol 122:577–585
Bode HR, Smith GS (1977) Regulation of interstitial cell differentiation in Hydra attenuata. II. Correlation of the axial position of interstitial cells with nematocyte differentiation. Wilhelm Roux’ Arch 181:203–213
Bosch TCG (2004) Control of asymmetric cell divisions: will cnidarians provide an answer? Bioessays 26:929–931
Bosch TCG (2007a) Symmetry breaking in stem cells of the basal metazoan Hydra. In: Macieira-Coelho (ed) Asymmetric cell division series, Progr Mol Subcell Biol. Springer, Heidelberg, pp 61–78
Bosch TCG (2007b) Why polyps regenerate and we don’t: towards a cellular and molecular framework for Hydra regeneration. Dev Biol 303:421–433
Bosch TCG, David CN (1984) Growth regulation in Hydra: Relationship between epithelial cell cycle length and growth rate. Dev Biol 104:161–171
Bosch TCG, David CN (1986) Male and female stem cells and sex reversal in Hydrapolyps. Proc Natl Acad Sci USA 83:9478–9482
Bosch TCG, David CN (1987) Stem cells of Hydra magnipapillatacan differentiate somatic and germ cell lines. Dev Biol 121:182–191
Bosch TCG, David CN (1990) Cloned interstitial stem cells grow as contiguous patches in hydra. Dev Biol 138:513–515
Bosch TCG, Rollbühler R, Scheider B, David CN (1991) Role of the cellular environment in interstitial stem cell proliferation in hydra. Dev Genes Evol 200:269–276
Brien P, Reniers-Decoen M (1950) La significance des cellules interstitielles des hydres d’eau douce et le problème de la résèrve embryonaire. Bulletin Biol France Belgique 89:285–325
Broun M, Gee L, Reinhardt B, Bode HR (2005) Formation of the head organizer in hydra involves the canonical wnt pathway. Development 132:2907–2916
Bunting M (1894) The origin of sex cells in Hydractiniaand Podocoryneand the development of Hydractinia. J Morphol 9:203–236
Burnett AL, Davis LE, Ruffin FE (1966) A histological and ultrastructural study of germinal differentiation of interstitial cells arising from gland cells in Hydra viridis. J Morphol 120:1–8
Burnett AL, Lowell R, Cyrlin M (1973) Regeneration of a complete hydra from a single differentiated somatic cell type. In: Burnett AL (ed) Biology of Hydra. Academic Press, New York, pp 225–270
Buss LW (1982) Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc Natl Acad Sci USA 79:5337–5341
Buss LW (1983) Evolution, development, and the units of selection. Proc Natl Acad Sci USA 80:1387–1391
Buss LW (1999) Slime molds, ascidians, and the utility of evolutionary theory. Proc Natl Acad Sci USA 96:8801–8803
Campbell RD (1976) Elimination of hydra interstitial and nerve cells by means of colchicine. J Cell Sci 21:1–13
Campbell RD (1979) Development of hydra lacking interstitial and nerve cells (“epithelial hydra”). In: Subtelny S, Konigsberg IR (eds) Determinants of Spatial Organization. 37th Symp Soc Dev Biol. Academic Press, New York, pp 267–293
Campbell RD, David CN (1974) Cell cycle kinetics and development of Hydra attenuata. II. Interstitial cells. J Cell Sci 16:349–358
David CN, Bosch TCG, Hobmeyer B, Holstein T, Schmidt T (1987) Interstitial stem cells in hydra. In: Loomis WF (ed) Genetic control of development. Alan R Liss, New York, p 389
David CN, Campbell RD (1972) Cell cycle kinetics and development of Hydra attenuata. I. Epithelial cells. J Cell Sci 11:557–568
David CN, Fujisawa T, Bosch TC (1991) Interstitial stem cell proliferation in hydra: evidence for strain-specific regulatory signals. Dev Biol 148:501–507
David CN, Gierer A (1974) Cell cycle kinetics and development of Hydra attenuata. J Cell Sci 16:359–376
David CN, MacWilliams H (1978) Regulation of the self-renewal probability in hydra stem cell clones. Proc Natl Acad Sci USA 75:886–890
David CN, Murphy S (1977) Characterization of interstitial stem cells in hydra by cloning. Dev Biol 58:372–383
David CN, Plotnick I (1980) Distribution of interstitial cells in Hydra attenuata. Dev Biol 76:175–184
Davis LE (1970) Cell division during dedifferentiation and redifferentiation in regenerating isolated gastrodermis of Hydra. Exp Cell Res 60:127–132
Davis LE (1973) Ultrastructural changes during dedifferentiation and redifferentiation in regenerating, isolated gastrodermis. In: Burnett AL (ed) Biology of Hydra. Academic Press, New York, pp 171–219
Diehl FA (1973) The developmental significance of interstitial cells during regeneration and budding. In: Burnett AL (ed) Biology of Hydra. Academic Press, New York, pp 109–141
Diehl FA, Burnett AL (1964) The role of interstitial cells in the maintenance of hydra. I. Specific destruction of interstitial cells in normal, asexual, non-budding animals. J Exp Zool 155:253–260
Extavour CG, Akam M (2003) Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130:5869–5884
Extavour CG, Pang K, Matus DQ, Martindale MQ (2005) Vasa and nanos expression patterns in a sea anemone and the evolution of bilaterian germ cell specification mechanisms. Evol Dev 7:201–215
Fedders H, Augustin R, Bosch TC (2004) A Dickkopf-3-related gene is expressed in differentiating nematocytes in the basal metazoan Hydra. Dev Genes Evol 214:72–80
Frank U, Leitz T, Müller WA (2001) The hydroid Hydractinia: a versatile, informative cnidarian representative. Bioessays 23:963–971
Fujisawa T (1988) Inhibition of stenotele commitment by an endogenous factor in Hydra. J Cell Sci 91, 361–366
Fujisawa T (1989) Role of interstitial cell migration in generating position-dependent patterns of nerve cell differentiation in Hydra. Dev Biol 133:77–82
Fujisawa T, David CN, Bosch TCG (1990) Transplantation stimulates interstitial cell migration in hydra. Dev Biol 138:509–512
Fujisawa T, Nishima C, Sugiyama T (1986) Nematocyte differentiation in hydra. Curr Top Dev Biol 20:281–290
Fujisawa T, Sugiyama T (1980) Nematocyte differentiation from interstitial cells newly introduced into interstitial cell-deficient hydra. In: Tardent P, Tardent R (eds) Developmental and cellular biology of coelenterates. Elsevier/North-Holland Biomedical Press, Amsterdam, pp 319–324
Galliot B, Miljkovic-Licina M, de Rosa R, Chera S (2006) Hydra, a niche for cell and developmental plasticity. Sem Cell Dev Biol 17:492–502
Galliot B, Schmid V (2002) Cnidarians as a model system for understanding evolution and regeneration. Int J Dev Biol 46:39–48
Grens A, Mason E, Marsh JL, Bode HR (1995) Evolutionary conservation of a cell fate specification gene: the Hydra achaete-scute homolog has proneural activity in Drosophila. Development 121(1):4027–4035
Guduric-Fuchs J, Möhrlen F, Frohme M, Frank U (2004) A fragile X mental retardation-like gene in a cnidarian. Gene 343:231–238
Hauenschild C (1954) Genetische und entwicklungsphysiologische Untersuchungen über Intersexualität und Gewebeverträglichkeit bei dem Hydroidpolypen Hydractinia echinata. Wilhelm Roux’ Archiv Entwicklungsmech Org 147:132–138
Hauenschild C (1956) Experimentelle Untersuchungen über die Entstehung asexueller Klone bei der Hydromeduse Eleutheria dichotoma. Zeitschrift Naturforschung 11b:394–402
Hauenschild C (1957) Ergänzende Mitteilung über die asexuellen Medusenklone bei Eleutheria dichotoma. Zeitschrift Naturforschung 12b:412–413
Hayakawa E, Fujisawa C, Fujisawa T (2004) Involvement of Hydra achaete-scutegene CnASHin the differentiation pathway of sensory neurons in the tentacles. Dev Genes Evol 214:486–492
Haynes J, Burnett AL (1963) Dedifferentiation and redifferentiation in Hydra viridis. Science 142:1481–1483
Hellstern S, Stetefeld J, Fauser C, Lustig A, Engel J, Holstein TW, Ozbek S (2006) Structure/function analysis of spinalin, a spine protein of Hydranematocysts. FEBS J 273:3230–3237
Herrmann K, Berking S (1987) The length of S-phase and G2-phase of epithelial cells is regulated during growth and morphgenesis in Hydra attenuata. Development 99:33–39
Hobmayer B, Rentzsch F, Kuhn K, Happel CM, Cramer von Laue C, Snyder P, Rothbächer U, Holstein TW (2000) WNT signaling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407:186–189
Hoffmann U, Kroiher M (2001) A possible role for the cnidarian homologue of serum response factor in decision making by undifferentiated cells. Dev Biol 236:304–315
Hofmann DK, Neumann R, Henne K (1978) Strobilation, budding and initiation of scyphistoma morphogenesis in the rhizostome Cassiopea andromeda(Cnidaria: Scyphozoa). Marine Biol 47:161–176
Holstein TW, David CN (1990a) Cell cycle length, cell size and proliferation rate in Hydrastem cells. Dev Biol 142:392–400
Holstein TW, David CN (1990b) Putative intermediates in the nerve cell differentiation pathway in Hydrahave properties of multipotent stem cells. Dev Biol 142:401–405
Holstein TW, Hobmayer E, David CN (1991) Pattern of epithelial cell cycling in hydra. Dev Biol 148:602–611
Holstein TW, Hobmayer E, Technau U (2003) Cnidarians: an evolutionary conserved model system for regeneration. Dev Dyn 226:257–267
Howard MK, Burke LC, Mailhos C, Pizzey A, Gilbert CS, Lawson WD, Collins MK, Thomas NS, Latchman DS (1993) Cell cycle arrest of proliferating neuronal cells by serum deprivation can result in either apoptosis or differentiation. J Neurochem 60:1783–91
Kalthurin K, Anton-Erxleben F, Milde S, Plötz C, Wittlieb J, Hemmerich G, Bosch TCG (2007) Transgenic stem cells in Hydrareveal an early evolutionary origin for key elements controlling self-renewal and differentiation. Dev Biol 309:32–44
Kaesbauer T, Towb P, Alexandrova O, David CN, Dall’Armi E, Staudigl A, Stiening B, Boettger A (2007) The Notch signaling pathway in the cnidarian Hydra. Dev Biol 303:376–390
Khalturin k, Anton-Erxleben F, Mildel S (2007) Transgenic stem cells in Hydra reveal an early evolutionary origin for key elements controlling self-renewal and differentiation. Dev Biol 309(1):32–44
Koizumi O, Bode HR (1991) Plasticity of the nervous system of adult hydra. III. Conversion of neurons to expression of a vasopressin-like immunoreactivity depends on axial location. J Neurosci 11:2011–2020
Koizumi O, Heimfeld S, Bode HR (1988) Plasticity of the nervous system of adult hydra. II. Conversion of ganglionic cells of the body column into epidermal sensory cells of the hypostome. Dev Biol 129:358–371
Kroiher M, Plickert G, Müller WA (1990) Pattern of cell proliferation in embryogenesis and planula development of Hydractinia echinatapredicts the postmetamorphic body pattern. Roux’s Archiv Dev Biol 199:156–163
Kükenthal W (1923) Octocorallia. In: Krumbach T (ed) Handbuch der Zoologie I. Walter de Gruyter, Leipzig, pp 695, 776
Lentz TL (1965) The fine structure of differentiating interstitial cells in hydra. Zeitschrift Zellforschung mikroskop Anatomie 67:547–560
Lentz TL (1966) The cell biology of hydra. North-Holland Publ, Amsterdam
Lesh-Laurie GE, Hujer A, Suchy P (1991) Polyp regeneration from isolated tentacles of Aurelia scyphistomae: a role for gating mechanisms and cell division. Hydrobiologia 216–217:91–97
Lindgens D, Holstein TW, Technau U (2004) Hyzic, the Hydra homolog of the zic/odd-paired gene, is involved in the early specification of the sensory nematocytes. Development 131:191–201
Littlefield CL (1991) Cell lineages in Hydra: Isolation and characterization of an interstitial stem cell restricted to egg production in Hydra oligactis. Dev Biol 143:378–88
Littlefield CL, Bode HR (1986) Germ cells in Hydra oligactismales. II Evidence for a subpopulation of interstitial stem cells whose differentiation is limited to sperm production. Dev Biol 116:381–386
Lowell RD, Burnett AL (1969) Regeneration of complete hydra from isolated epidermal transplants. Biol Bull 137:312–320
Macklin M (1968) Reversal of cell layers in hydra: a critical re-appraisal. Biol Bull 134:465–472
Mali B, Soza-Ried J, Frohme M, Frank U (2006) Structural but not functional conservation of an immune molecule: a tachylectin-like gene in Hydractinia. Dev Comp Immunol 30:275–281
Marcum BA, Campbell RD (1978) Developmental roles of epithelial and interstitial cell lineages in hydra: analysis of chimeras. J Cell Sci 32:233–247
Martin VJ, Archer WE (1986) Migration of interstitial cells and their derivatives in a hydrozoan planula. Dev Biol 116:486–496
Martin VJ, Archer WE (1997) Stages of larval development and stem cell population changes during metamorphosis of a hydrozoan planula. Biol Bull 192:41–52
Miljkovic M, Mazet F, Galliot B (2002) Cnidarian and bilaterian promoters can direct GFP expression in transfected hydra. Dev Biol 246:377–90
Miljkovic-Licina M, Chera S, Ghila L, Galliot B (2007) Head regeneration in wild-type hydra requires de novo neurogenesis. Development 134:1191–1201
Minasian LL, Mariscal RN (1980) Tissue-specific differentiation of cnidoblasts in a sea anemone (Haliplanella luciae). Am Zool 20:803
Mochizuki K, Nishimiya-Fujisawa C, Fujisawa T (2001) Universal occurrence of the vasa-related genes among metazoans and their germline expression in Hydra. Dev Genes Evol 211:299–308
Müller WA (1964) Experimentelle Untersuchungen über Stockentwicklung und Sexualchimären bei Hydractinia echinata. Roux’ Arch. Entwicklungsmechanik 155:181–268
Müller WA (1966) Elimination der I-Zellen und Hemmung der Planulametamorphose durch alkylierende Cytostatika. Naturwissenschaften 7:184–185
Müller WA (1967) Differenzierungspotenzen und Geschlechtsstabilität der I-Zellen von Hydractinia echinata. Roux Arch Entwicklungsmech 159:412–432
Müller WA (2002) Autoaggressive, multi-headed and other mutant phenotypes in Hydractinia echinata(Cnidaria: Hydrozoa). Int J Dev Biol 46:1023–1033
Müller WA, Frank U, Teo R, Mokady O, Guette C, Plickert G (2007) Wnt signaling in hydroid development: Ectopic heads and giant buds induced by GSK-3beta inhibitors. Int J Dev Biol 51:211–220
Müller WA, Leitz T (2002) Metamorphosis in the Cnidaria. Can J Zool 80:755–1771
Müller WA, Plickert G, Berking S (1986) Regeneration in Hydrozoa: distal versus proximal transformation in Hydractinia. Roux’s Arch Dev Biol 195:513–518
Müller WA, Teo R, Frank U (2004a) Totipotent migratory stem cells in a hydroid. Dev Biol 275:215–224
Müller WA, Teo R, Möhrlen F (2004b) Patterning a multi-headed mutant in Hydractinia: enhancement of head formation and its phenotypic normalization. Int J Dev Biol 48:9–15
Neumann R, Schmahl G, Hofmann DK (1980) Bud formation and control of polyp morphogenesis in Cassiopea andromeda (Scyphozoa). In: Tardent P, Tardent R (eds) Developmental and Cellular Biology of Coelenterates. Elsevier, Amsterdam
Nishimiya-Fujisawa C, Sugiyama T (1993) Genetic analysis of developmental mechanisms in hydra. XX. Cloning of interstitial stem cells restricted to the sperm differentiation pathway in Hydra magnipapillata. Dev Biol 157:1–9
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–318
Plickert G, Jacoby V, Frank U, Müller WA, Mokady O (2006) Wnt signaling in hydroid development: formation of the primary body axis in embryogenesis and its subsequent patterning. Dev Biol 298:368–378
Plickert G, Kroiher M (1988) Proliferation kinetics and cell lineages can be studied in whole mounts and macerates by means of BrdU/anti-BrdU technique. Development 103:791–794
Plickert G, Kroiher M, Munck A (1988) Cell proliferation and early differentiation during embryonic development and metamorphosis of Hydractinia echinata. Development 103:795–803
Plickert G, Schetter E, Verhey van Wijk N, Schlossherr J, Steinbüchl M, Gajewski M (2003) The role of α-amidated neuropeptides in hydroid development – LWamides and metamorphosis in Hydractinia echinata. Int J Dev Biol 47:439–450
Rebscher N, Volk C, Teo R (2008) Vasa protein is a component of the germ plasm in the cnidarian Hydractinia echinata. Dev Dyn 237(6):1736–45
Rebscher N, Zelada-Gonzalez F, Banisch TU, Raible F, Arendt D (2007) Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev Biol 306:599–611
Sacks PG, Davis LE (1979) Production of nerveless Hydra attenuataby hydroxyurea treatments. J Cell Sci 37:189–203
Schmich J, Kraus Y, De Vito D, Graziussi D, Boero F, Piraino S (2007) Induction of reverse development in two marine Hydrozoans. Int J Dev Biol 51(1):45–56
Schmich J, Trepel S, Leitz T (1998) The role of GLWamides in metamorphosis of Hydractinia echinata. Dev Genes Evol 208:267–273
Schmid V, Baader C, Bucciarelli A, Reber-Muller S (1993) Mechanochemical interactions between striated muscle cells of jellyfish and grafted extracellular matrix can induce and inhibit DNA replication and transdifferentiation in vitro. Dev Biol 155:483–496
Schmid V, Wydler M, Alder H (1982) Transdifferentiation and regeneration in vitro. Dev Biol 92:76–488
Schmid V, Plickert G (1990) The proportion altering factor (PAF) and the in vitro transdifferentiation of isolated striated muscle of jellyfish into nerve cells. Differentiation 44:95–102
Schmid V, Reber-Müller S (1995) Transdifferentiation of isolated striated muscle of jellyfish in vitro: the initiation process. Sem Cell Biol 6:109–116
Schwarz RS, Hodes-Villamar L, Fitzpatrick KA, Fain MG, Hughes AL, Cadavid LF (2007) A gene family of putative immune recognition molecules in the hydroid Hydractinia. Immunogenetics 59:233–246
Seipel K, Yanze N, Schmid V (2004a) The germ line and somatic stem cell gene Cniwi in the jellyfish Podocoryne carnea. Int J Dev Biol 48:1–7
Seipel K, Yanze N, Schmid V (2004b) Developmental and evolutionary aspects of the basic helix-loop-helix transcription factors Atonal-like 1 and Achaete-scute homolog 2 in the jellyfish. Dev Biol 269:331–345
Shigeta M, Shibukawa Y, Ihara H, Miyoshi E, Taniguchi N, Gu J (2006) beta1,4-N-Acetylglucosaminyltransferase III potentiates beta1 integrin-mediated neuritogenesis induced by serum deprivation in Neuro2a cells. Glycobiology 16:564–571
Shimizu H, Bode HR (1995) Nematocyte differentiation in hydra: commitment to nematocyte type occurs at the beginning of the pathway. Dev Biol 169:136–150
Shukalyuk AI, Golovnina KA, Baiborodin SI, Gunbin KV, Blinov AG, Isaeva VV (2006) vasa-related genes and their expression in stem cells of colonial parasitic rhizocephalan barnacle Polyascus polygenea(Arthropoda: Crustacea: Cirripedia: Rhizocephala). Cell Biol Int 31:97–108
Strelin GS (1928) Röntgenologische Untersuchungen an Hydren. Wilhelm Roux’ Archiv Entwicklungsmechanik Organismen 115:27–51
Sugiyama T, Fujisawa T (1979) Genetic analysis of developmental mechanisms in hydra. VI. Cellular composition of chimera hydra. J Cell Sci 35:1–15
Takahashi T, Koizumi O, Ariura Y, Romanovitch A, Bosch TC, Kobayakawa Y, Mohri S, Bode HR, Yum S, Hatta M, Fujisawa T (2000) A novel neuropeptide, Hym-355, positively regulates neuron differentiation in Hydra. Development 127:997–1005
Teo R, Möhrlen F, Plickert G, Müller WA, Frank U (2006) An evolutionary conserved role of Wnt signaling in stem cell fate decision. Dev Biol 289:91–99
Terada H, Sugiyama T, Shigenaka Y (1988) Genetic analysis of developmental mechanisms in hydra. XVIII. Mechanism for elimination of the interstitial cell lineage in the mutant strain Sf-1. Dev Biol 126:263–269
Teragawa CK, Bode HR (1991) A head signal influences apical migration of interstitial cells in Hydra vulgaris. Dev Biol 147:293–302
Thieme C, Hofmann DK (2003) Control of head morphogenesis in an invertebrate asexually produced larva-like bud (Cassiopea andromeda; Cnidaria: Scyphozoa). Dev Genes Evol 213:127–133
Torras R, Gonzalez-Crespo S (2005) Posterior expression of nanos orthologs during embryonic and larval development of the anthozoan Nematostella vectensis. Int J Dev Biol 49:895–899
Van de Vyver G (1964) Etude histologique du development d’Hydractinia echinata (Flem) Cahiers de Biologie Marine V:295–310
Van de Vyver G (1967) Etude de development embyryonnaire des hydraires athecates (gymnoblastiques) a gonophores. Archives de Biologie (Liege) 78:451–518
Wanek N, Marcum BA, Campbell RD (1980) Histological structure of epithelial hydra and evidence for the complete absence of interstitial and nerve cells. J Exp Zool 212:1–12
Wang YP, Wang ZF, Zhang YC, Tian Q, Wang JZ (2004) Effect of amyloid peptides on serum withdrawal-induced cell differentiation and cell viability. Cell Res 14:467–72
Weis VM, Keene DR, Buss LW (1985) Biology of hydractiniid hydroids. 4. Ultrastructure of the planula of Hydractinia echinata. Biol Bull 168:403–418
Weismann A (1883) Die Entstehung der Sexualzellen bei Hydromedusen. Gustav Fischer-Verlag, Jena
Weismann A (1885) Die Continuität des Keimplasmas als Grundlage einer Theorie der Vererbung. Gustav Fischer-Verlag, Jena
Weismann A (1892a) Das Keimplasma. Eine Theorie der Vererbung. Gustav Fischer-Verlag, Jena
Weismann A (1892b) Essays on heredity and kinded biological problems (trans. Poulton EB et al). Clarendon Press, Oxford
Weismann A (1904) Keimplasmatheorie, Regeneration. In: Vorträge über Deszendenztheorie, 2nd edn. Gustav Fischer-Verlag, Jena
Werner B, Hentschel J (1983) Apogamous life cycle of Stephanoscyphus planulophorus. Mar Biol 74:301–304
Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448:318–325
Wittlieb J, Khalturin K, Lohmann JU, Anton-Erxleben F, Bosch TC (2006) Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc Natl Acad Sci USA 103:6208–6211
Yanze N, Groger H, Muller P, Schmid V (1999) Reversible inactivation of cell-type-specific regulatory and structural genes in migrating isolated striated muscle cells of jellyfish. Dev Biol 213:194–201
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zeretzke S, Berking S (2002) In the multiheaded strain (mh-1) of Hydra magnipapillatathe ectodermal epithelial cells are responsible for the formation of additional heads and the endodermal epithelial cells for the reduced ability to regenerate a foot. Dev Growth Differ 44:85–93
Zhang E, Li X, Zhang S, Chen L, Zheng X (2005) Cell cycle synchronization of embryonic stem cells: effect of serum deprivation on the differentiation of embryonic bodies in vitro. Biochem Biophys Res Comm 333:1171–1177
Acknowledgements
We thank Peter Schuchert (Muséum d’histoire naturelle, Geneva) and Klaus Sander (University of Freiburg) for providing us with copies of Weissmann’s 1883 work, which had been considered lost for decades. UF is supported by SFI (Science Foundation Ireland), by a Beufort Marine Biodiscovery Grant, and by the Irish Higher Education Authority’s PRTLI Cycle 4 Award.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Frank, U., Plickert, G., Müller, W.A. (2009). Cnidarian Interstitial Cells: The Dawn of Stem Cell Research. In: Rinkevich, B., Matranga, V. (eds) Stem Cells in Marine Organisms. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-2767-2_3
Download citation
DOI: https://doi.org/10.1007/978-90-481-2767-2_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-2766-5
Online ISBN: 978-90-481-2767-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)