Abstract
The endogenous circadian timekeeping system modulates human physiology and behavior with a near 24 h periodicity conferring adaptation to the ~24 h solar light-dark cycle. Thus, the circadian timekeeping system times physiology and behavior so that it is prepared for environmental changes. The term circadian implies an endogenous “clock-driven” process. However, not all observed daily patterns in physiology and behavior are clock driven and instead may be due to environmental or behavioral factors. For example, the barren rock on the top of a mountain shows a daily temperature oscillation that is not endogenous to the rock but instead is caused by the sun heating the rock during the day and radiative heat loss after sunset. Other factors such as wind, rain, and cloud cover impact the observed daily temperature oscillation of the rock. Similarly, some of the daily patterns observed in physiology and behavior are driven by external factors, while others arise from the interaction between circadian and behavioral processes (e.g., sleep-wake, fasting-feeding). To improve understanding of the mechanisms underlying observed daily patterns in physiology and behavior in humans, a variety of circadian protocols have been implemented (Tables 13.1 and 13.2). These protocols will be reviewed in the following pages, and the strengths and limitations of each will be discussed. First, we review markers of the endogenous clock in humans.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Constant Routine
- Forced Desynchrony
- Ultra-short Sleep-Wake Schedule
- Shift of Sleep to the Daytime
- Circadian Phase
- Circadian Period
- Circadian Amplitude
- Melatonin
- Temperature
1 Markers of Circadian Rhythms in Humans In Vivo
The master clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus [1, 2]. Peripheral cell-autonomous clocks have also been observed in tissues outside the brain, such as fibroblasts, red blood and mononuclear cells, adipose tissue, pancreatic islet cells, skeletal myotubes, hepatocytes, and cardiomyocytes, and in immortalized cancer cell lines [3–14]. Unlike nonhuman models, scientists do not have direct access to the SCN in humans and instead use marker rhythms driven by the SCN to indicate phase, amplitude, and period of the circadian clock. Estimations of circadian timing of the internal biological clock (i.e., phase) and the strength or robustness of the observed oscillation (i.e., amplitude) are informative when determining changes in response to environmental or physiological perturbations. Phase represents the time within the circadian cycle at which a particular event occurs (e.g., minimum, maximum, onset, offset, midpoint), whereas amplitude is commonly defined as the magnitude between the mesor and the maximum of a rhythm. The mesor is defined as the value midway between the maximum and minimum of a fitted rhythm or time series, and thus amplitude is approximately half of the minimum to maximum range of the rhythm.
1.1 Melatonin
The most commonly used circadian marker rhythm in humans is the melatonin rhythm. Melatonin is easily measured in saliva, blood, and urine and is the most precise marker of circadian phase and period [15–19] when exposure to light is controlled. High melatonin levels are considered to be a marker of the biological night (Fig. 13.1). During entrainment when the circadian clock is synchronized to the 24 h day, melatonin levels rise on average ~2 h prior to habitual bedtime [20], peak during the nighttime, and return to low levels shortly after habitual wake time [21]. Note that there are large individual differences in the timing of the melatonin rhythm, which are larger in the modern environment versus after exposure to the natural light-dark cycle [21]. Further, there are large individual differences in peak melatonin levels [22]. A common misperception is that exposure to darkness increases melatonin levels. Rather, the SCN controls the melatonin circadian rhythm via a multisynaptic pathway. This pathway includes an efferent projection to the paraventricular nucleus of the hypothalamus (PVN) and a descending projection to sympathetic preganglionic neurons in the upper thoracic spinal cord which, in turn, project to the superior cervical ganglion (SCG). Postganglionic nerve fibers from the SCG then release norepinephrine to stimulate beta- and alpha-adrenergic receptors on the pineal gland [23]. Beta-adrenergic receptor activation signals the pineal gland to synthesize melatonin from tryptophan via several enzymatic steps. If maintained in constant conditions, the melatonin rhythm continues to rise and fall independent of light exposure. If exposure to light occurs during the biological night, melatonin levels will be acutely reduced as photic input from the retina to the SCN results in inhibition of the multisynaptic SCN-pineal circuit, removing the rate-limiting step of melatonin synthesis. Thus, to accurately assess melatonin levels, samples must be collected every 30–60 min under dim light conditions (e.g., <8 lx maximum).
Phase relationships between the primary circadian phase marker rhythms driven by the SCN in humans. High melatonin and low core temperature levels represent the biological night, whereas low melatonin and high core temperature levels represent the biological day. Cortisol levels are lowest in the first part of the biological night, peak near the end of the biological night, and decrease across the biological day
A variety of markers have been developed to quantify the timing of melatonin rhythm. Changes over and under arbitrarily defined thresholds are often used. For example, melatonin onset is most commonly defined as when melatonin levels rise above 10 pg/ml in plasma, or 3–4 pg/ml in saliva, as salivary levels are 30–40 % of plasma levels [19, 24]. Other thresholds for melatonin onset are when melatonin levels rise two standard deviations above a stable low daytime baseline [19] and the time of a threshold change is calculated by linear interpolation. If the entire melatonin rhythm is assessed, individualized thresholds can be computed to determine the thresholds of when melatonin levels reach 25 % of the three-harmonic fitted peak-to-trough melatonin amplitude [18] and the 50 % mean crossing [15], for example. Melatonin offset is considered the time at which melatonin levels return to low daytime levels, falling below these thresholds, and the melatonin midpoint is the time midway between the onset and offset. The fitted melatonin peak has also been used as a phase marker, and comparisons of the various markers show similar variability in their estimates of circadian phase [15].
1.2 Body Temperature and Cortisol
Two other commonly used circadian marker rhythms in humans are body temperature and cortisol (Fig. 13.1). The SCN controls rhythms in body temperature via multisynaptic projections to the preoptic temperature control center of the hypothalamus, and through direct effects of melatonin on peripheral vasodilation. The SCN also controls rhythms in cortisol via input into the endocrine hypothalamic pituitary adrenal (HPA) axis, as well as through a multisynaptic neural pathway to the adrenal glands that bypasses the HPA axis [25]. Core and distal skin (e.g., hands and feet) body temperatures show approximate inverse rhythms with high core and low distal skin temperatures during the daytime and low core and high distal skin temperatures at night. The fitted minimum of the core body temperature rhythm is the most common circadian temperature phase marker. Accurate assessment of the circadian rhythms in body temperatures requires control of posture, activity, and sleep, as changes in these factors alter the observed temperature rhythm (e.g., physical activity acutely increases core temperature and sleep reduces core temperature). Cortisol levels peak in the morning, decrease across the day, are low near habitual bedtime, and rise throughout the night (Fig. 13.1). Given the pulsatile nature of cortisol, accurate assessment of the cortisol rhythm requires frequent sampling (e.g., every 20–30 min).
2 Protocols to Evaluate Circadian Phase and Amplitude in Humans
2.1 Constant Routines
The constant routine protocol, a modification by Czeisler and colleagues (discussed in [26]) of the Mills test [27], can be used to assess the phase and amplitude of the clock immediately upon release from entrainment into “constant conditions” (Table 13.2). The constant routine protocol controls for factors that influence circadian variables of interest by making constant or equally distributing factors such as ambient light and temperature, physical activity and posture, nutrition intake, and sleep-wake state across the circadian cycle (Fig. 13.2, Table 13.1). Constant environmental conditions include dim-ambient light commonly maintained at ~1.5 lx in the angle of gaze (~0.6 W/m2), ambient temperature in the thermoneutral range (e.g., 22–24 °C), and a light bed sheet pulled up to the waist to maintain a constant temperature microclimate. Constant behavioral conditions include continuous wakefulness to control for sleep-induced changes in physiology and constant posture (i.e., bed rest with the head raised to a 35–45° angle) to control for posture or activity-induced changes (e.g., participants remain in bed and use bedpans/urinals). Continuous monitoring by research staff is required to ensure constant wakefulness and consistent posture. Concurrent brain wave recording is also recommended to maximize continuous wakefulness, as microsleeps will occur and unattended subjects will fall asleep. Nutrition intake is often in the form of miniature snacks distributed equally across the circadian cycle (e.g., hourly) or via less commonly used continuous enteral feeding or venous glucose infusion (Table 13.1). Meals are typically identical across the constant routine (e.g., one-fourth of a ham sandwich, room-temperature juice and water), isocaloric, and total daily calories consumed are increased by ~5 % to account for the higher energy needs associated with extended wakefulness [28]. At a minimum, ~24 h is required to assess a full circadian cycle if melatonin is used as a circadian phase marker, and more time (i.e., 1.5–2 days) is needed if body temperature is used, given residual effects of prior sleep on temperature. Constant routines are often ~40 h in duration and thus recovery sleep occurs at habitual bedtime.
The constant routine protocol is typically preceded by subjects maintaining their habitual and consistent wake-sleep schedule in the home environment. The first one to two nights in the laboratory may consist of the habitual wake and sleep schedules with typical meals. The constant routine protocol on days 3–4 is composed of miniature hourly snacks (s), constant posture, wakefulness, dim light, and ambient temperature. Participants are continuously monitored during the constant routine to ensure wakefulness and compliance with procedures. A 40 h constant routine permits assessment of multiple measure of circadian phase such as body temperature, melatonin, and cortisol and recovery sleep at the habitual sleep time. Constant routines also commonly precede and follow exposure to a phase-shifting stimulus to determine the change in circadian phase. B breakfast, L lunch, D dinner, S snack, orange boxes habitual meals, black boxes scheduled sleep, underline scheduled wakefulness
Comparisons between the constant routine and typical sleep-wake conditions demonstrate that the circadian rhythm in melatonin is minimally influenced by sleep-wake state [29], assuming that ambient light is maintained at dim levels. Circadian rhythms in body temperature and cortisol are, however, influenced by sleep-wake state [21, 29, 30]. The sleep-induced decrease in core temperature [31] (increase in distal skin temperature) and posture-/activity-induced increase in core temperature (decrease in distal skin temperature) can mask the circadian temperature rhythms, resulting in imprecise estimates of circadian phase and amplitude. Similarly, there is a sleep-induced decrease in cortisol levels shortly after the beginning of the sleep episode and a wake-induced increase [32, 33].
Limitations of the constant routine procedure include constant wakefulness, which requires a degree of sleep deprivation that may influence outcomes of interest (i.e., circadian time is not the only factor changing); time (i.e., minimum of 24 h); protocol costs; and a tightly controlled environment which cannot be easily performed outside of the laboratory. Additionally, the constant routine cannot be used to assess circadian period nor interactions between circadian phase and wake-sleep influences on physiology and behavior (Table 13.2). Furthermore, like most laboratory protocols, findings may not translate directly to real-world conditions.
Variations on the constant routine include constant posture protocols that either permit sleep and typical meals [34] or modified constant routines that permit sleep and some changes in posture (e.g., bathroom breaks). Constant posture and modified constant routine protocols still include control for dim-ambient light and thermoneutral ambient temperature and can therefore still be used to determine circadian timing of the melatonin rhythm. The constant posture protocol has been used to measure the melatonin rhythm during wakefulness and sleep from blood samples taken via an indwelling catheter with an extension tubing that exits a room porthole to allow blood to be assessed with minimal disruption of sleep [34]. Individuals can also be awakened from sleep to obtain saliva samples from which to assess melatonin levels. When assessing the melatonin rhythm using saliva instead of blood sampling, food and fluid intake are typically proscribed ~30 min prior to collecting saliva samples, and the mouth is rinsed 30 min prior to sample collection to reduce the risk of food contamination of saliva samples. Furthermore, if posture is not constant, seated posture is typically maintained for ~15 min prior to sample collection. If sleep or changes in posture are permitted, circadian body temperature rhythm phase cannot be precisely determined [35, 36].
Limitations of constant posture and modified constant routines include time (i.e., minimum of 24 h) and protocol costs, and require a tightly controlled laboratory environment. These routines cannot be easily performed outside of the laboratory. Additionally, when daytime sleep opportunities are allowed, total sleep time is reduced relative to nighttime sleep opportunities [37], and such differences in total sleep time could influence outcomes of interest.
2.2 Assessment of the Dim-Light Melatonin Onset
The phase of the melatonin rhythm can also be assessed without performing a constant routine. As noted, melatonin, as opposed to temperature and cortisol, is less impacted by posture and meals. As long as light is maintained at dim levels and food and posture are controlled prior to sample collection (e.g., food proscribed 30 min and posture consistent 15 min immediately prior to the sample), saliva and blood samples can be used to accurately assess melatonin levels. Generally, the dim-light melatonin onset (DLMO) can be determined from samples obtained starting ~7 h prior to and ending ~1 to 2 h after habitual bedtime, assuming that the subject is stably entrained. Saliva sampling can also be easily performed outside of the laboratory when measuring and controlling light levels [38–40].
Limitations of the DLMO assessment include the inability to assess melatonin amplitude or other melatonin markers such as the midpoint and offset and melatonin duration. If the subject is not entrained, 24 h sampling may be required to obtain the melatonin onset, which would permit assessment of melatonin offset, midpoint, and amplitude.
3 Protocols to Evaluate Circadian Period and Amplitude in Humans
Accurate assessment of circadian period in sighted humans requires assessment in the absence of external synchronizers, or under tightly controlled exposure to synchronizers, which ensures their even distribution with respect to circadian phase.
3.1 “Free-Running” Temporal Isolation Protocol
In the 1960s Aschoff and Wever began performing their classic bunker studies in an isolation unit free from natural time cues [41]. Subjects lived in isolation, typically self-selecting their own meal, sleep-wake and light-dark schedules. These and related studies provided information on fundamental concepts of circadian physiology. For example, findings showed circadian rhythms in body temperature and associations between body temperature and sleep, e.g., subjects most commonly chose to initiate sleep on the downward curve of the body temperature rhythm [42, 43] and slept the longest when sleep was initiated near the body temperature minimum. Observed periods for urine corticosteroids and rectal temperature were on average ~25 h [44], and shorter periods were observed in subjects who napped versus those who maintained one primary sleep episode per “day” [45]. Limitations of these “free-running” temporal isolation protocols include subjects self-selected environmental (e.g., light) and behavioral (sleep and meals) events at a limited number of circadian phases. Thus, the nonuniform distribution of zeitgebers, or synchronizers, with respect to circadian phase likely had an impact on the outcomes observed [17, 46]. Experiments by Middleton et al. [47, 48] performed in constant dim light with self-selected sleep-wake schedules, but with knowledge of clock time, revealed circadian periods closer to 24 h (e.g., 24.3 h).
3.2 Forced Desynchrony in Laboratory Conditions
In 1938, Nathaniel Kleitman and Bruce Richardson performed a month-long study ~30 m underground in Mammoth Cave, Kentucky. During their study, they pioneered the forced desynchrony protocol. Kleitman and Richardson lived on a 28 h day and compared circadian temperature rhythms on 28 h versus 24 h day for 1 week each [49]. Regardless of the day length, ~24 h body temperature rhythms were observed, indicating that the ~24 h circadian rhythm in humans is not dependent upon the environmental light-dark cycle. Modern versions of the forced desynchrony protocol established by Czeisler and colleagues [17, 18, 31, 34, 44, 50–55] scheduled individuals to live in the laboratory in dim light-dark, wake-sleep cycles that are outside the range of entrainment of the human circadian clock (e.g., 20 h, 28 h, or 42.85 h days; Fig. 13.3, Tables 13.1 and 13.2). These laboratory studies last for at least 1 week on the non-24 h day length and often 2–4 weeks in order to obtain precise estimates of circadian period upon release from entrainment and revealed circadian period estimates close to 24 h (i.e., ~24.15 h) [17, 18, 53]. In these studies, participants live in an environment free of time cues (i.e., no access to timepieces, sunlight, or electronic devices), and light-dark exposure, physical activity, and food intake are all controlled. The specific day lengths noted above were chosen as their harmonics do not overlap with 24 h (i.e., the sought-after period) nor do their harmonics share any harmonics with a ~24 h period [17].
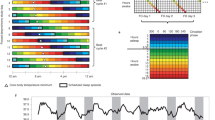
Double raster plot of the 28 h forced desynchrony protocol during which subjects initially start off on a 24 h day (T = 24 h). Typically, participants first undergo a 40 h constant routine (not shown here; but see Fig. 13.2) and then transition to a non-24 h day length that is outside the range of entrainment of the near 24 h period of the human circadian clock under controlled dim light conditions. Common forced desynchrony day lengths include T = 28 h (shown), 20 h, and 42.85 h. The example 28 h day length shown includes a 2:1 wake to sleep ratio of scheduled 18 h and 40 min wake and 9 h and 20 min sleep. The forced desynchrony is also often followed by a second constant routine protocol to assess circadian phase. The blue line illustrates the trajectory of a core body temperature minimum with a period longer than 24 h
The traditional forced desynchrony protocol maintains the habitual 2:1 wake to sleep ratio (e.g., 18 h and 40 min scheduled wake and 9 h and 20 min scheduled sleep for the 28 h day), although modifications have been made to examine interactions between sleep homeostasis and circadian phase [56–62]. When scheduling wake-sleep for a 2:1 ratio of the imposed day length, sleep duration is not equal at all circadian phases and will be shorter during the biological day [50, 63]. Furthermore, the forced desynchrony protocol assumes a symmetrical phase response curve for any photic and non-photic phase shifts that may persist despite the minimization of these influences. Relative coordination between observed rhythms—for example, between the melatonin rhythm and wake-sleep cycle—may result and thus influence the observed melatonin rhythm. However, this does not appear to affect the estimation of period, providing that the data train is sufficiently long [17]. The traditional forced desynchrony protocol requires a facility with maximal control over environmental conditions and has a high cost in both subject and experimenter time (Table 13.1). Related recruitment of participants for such longer term in-laboratory studies is laborious, and large demands are placed on laboratory resources for such studies.
Variations on the forced desynchrony protocol include ultrashort sleep-wakefulness cycles. Ultrashort sleep cycles schedule sleep and wake in brief segments across one or more circadian cycles. Versions include the 90 min day with scheduled 60 min wake and 30 min sleep opportunities [64] and the 20 min day with scheduled 13 min wake and 7 min sleep opportunities [65] (Fig. 13.4). The ultrashort versions of the forced desynchrony protocol are more cost-effective and provide a similar overall circadian period estimate as the traditional forced desynchrony protocol [66–68]. However, the precision and the error in the estimate for individual participants have not been reported but are likely larger than the traditional forced desynchrony estimates. Limitations of the ultrashort forced desynchrony protocol include an inability to assess circadian phase and amplitude (with the exception of melatonin phase if light is dim during scheduled wake) (Table 13.2). Further, this approach results in an inability to assess interactions between circadian phase and changes in the duration of wakefulness or sleep on outcomes. Like the traditional forced desynchrony protocol, sleep duration is not equal at all circadian phases.
3.3 Forced Desynchrony in Free-Living Conditions
Another approach to examine circadian period has been to study visually impaired individuals living outside of the laboratory. Studies of blind individuals in the real world provide circadian period estimates that are similar, but longer, to those found in forced desynchrony protocols, but with less precision due in large part to the infrequent sampling of urine (e.g., every 4–8 h) for circadian phase markers in these studies [69]. Other limitations include the long duration of such field studies and uneven distribution of non-photic time cues in the blind (e.g., activity, sleep-wake cycle, meal times, working hours, social interactions, alcohol, caffeine, medication) that may induce daily advances or delays of the circadian system, effectively shortening or lengthening the measured period. Some blind individuals also maintain photic input into the SCN. Another difference from most laboratory studies is that the observed period in the blind is likely assessed long after the “release” from entrainment and therefore may have different properties.
3.4 Analysis of Data from Forced Desynchrony Protocols to Estimate Circadian Period
Frequently sampled melatonin, temperature, and cortisol data from forced desynchrony protocols are often analyzed with harmonic regression models using an exact maximum likelihood fitting procedure [17]. Data can be fitted with periodic components of the imposed sleep-wake cycle and the sought-for circadian period, together with their harmonics. Such techniques utilize the frequently sampled data available in the dataset and are thus robust and provide the most precise estimates of circadian period and amplitude. Alternatives include fitting linear regression through daily circadian phase estimates, which are less precise as they include error in the phase estimate as well as show higher variance in the period estimate. As with all circadian protocols, the derived estimates of phase, amplitude, and period are only as accurate as the robustness of the analytic techniques and degree of experimental control over factors impinging on the outcome of interest. Masking effects may influence the observed rhythms without impinging upon, and thus not representing, the phase, amplitude, and period of the master clock in the SCN.
4 Protocols to Evaluate Circadian Rhythms in Physiology and Behavior
Beyond using the above circadian protocols for assessment of phase, amplitude, and period of the master clock in humans, other physiological and behavioral data can also be collected and aligned to the known circadian phase makers (e.g., melatonin or core body temperature) to provide evidence for circadian variation in such parameters (Table 13.2). For example, constant routine protocols have shown circadian variation in blood pressure and thyroid-stimulating hormone (TSH) [29, 70], whereas daily patterns in prolactin, human growth hormone (hGH), and parathyroid hormone (PTH) are sleep-wake dependent [29]. In addition, many aspects of cognitive function, including reaction time, cognitive processing speed, math processing speed and accuracy, visual search abilities, executive function/decision-making skills, as well as alertness, sleepiness, mood, hunger, and appetite are influenced by time awake and/or circadian time of day [50, 55, 71–75]. When considering sleep-wake versus circadian-dependent variables, it is important to consider whether altered sleep duration is playing a role in the observed changes. For example, it is known that hunger and appetite hormones leptin and ghrelin, as well as free fatty acids, are altered during sleep restriction [76, 77]. Therefore, sleep duration must be taken into account when considering the effects of these circadian protocols on outcome variables.
Another circadian protocol used to examine circadian versus wakefulness-sleep patterns in physiology is to shift sleep to the daytime (Fig. 13.5) [37, 78], reflective of patterns found in night work, to see whether daily patterns remain synchronous with the melatonin circadian rhythm, move with the wake-sleep cycle, or are abolished. Using such protocols, findings provide evidence that hGH and peptide-YY (PYY) are wake-sleep/feeding-fasting driven, whereas diet-induced thermogenesis is primarily influenced by the circadian clock [79, 80]. Other manipulations may then be necessary to determine the specific non-circadian factors driving such patterns that are altered by a shift in wake-sleep (e.g., wake-sleep, feeding-fasting, physical activity-inactivity).
Forced desynchrony protocols with a reasonable amount of “daily” wake time have been use to describe interactions between circadian and wake-sleep-driven processes. Findings from forced desynchrony protocols have shown many physiological and behavioral variables which are under circadian control and/or interact with wake-sleep factors. For example, findings from forced desynchrony protocols have shown circadian rhythms in physiology such as blood pressure [70], epinephrine, norepinephrine, heart rate, platelet aggregability [81], glucose tolerance in response to meals [82–84], EEG activity during wakefulness and sleep, susceptibility to presyncope [85], and periodic limb movements [86]. Some of these outcomes, including EEG and performance, show changes that are dependent upon the levels of sleep homeostasis and circadian phase and their interaction. For example, in many of the papers cited above, the amplitude of the circadian variation in performance builds with time awake.
Building on this, recent research has focused on the effects of sleep and circadian manipulations on human metabolomics and transcriptomics [87–91] in an effort to elucidate altered mechanisms and biochemical pathways by which these manipulations confer increased risk for disease states and to identify potential health and disease biomarkers.
5 Summary and Conclusions
The circadian protocols reviewed above have been used to elucidate much information about physiology and behavioral outcomes that are controlled and/or modulated by circadian timing. In the end, when interested in determining mechanisms that contribute to daily patterns in physiology and behavior, it is important to recognize that the integrated timing of circadian clock, wake-sleep, feeding-fasting, physical activity-inactivity, and light-dark cycles likely serve to enhance daily oscillations and promote robustness. A well-tuned system that has robust and coordinated oscillations in these parameters is likely to promote maximal cognitive and physiological health outcomes. Understanding the mechanisms underlying daily patterns in physiology and behavior also permits the development of countermeasure strategies for when these daily patterns are unavoidably disrupted, such as during shift work, jet lag and circadian rhythm, and/or sleep-wake disorders. Additional research is needed to understand the interacting factors that produce robust daily patterns in physiology and behavior, some of which will be circadian and others that will be driven by other factors. For example, manipulation of meal timing, physical activity, and environmental conditions in the above circadian protocols will add to our understanding of biological mechanisms controlling daily patterns in physiology.
Notes
- 1.
Suggested readings in the reference section are denoted with an asterisk (*).
References
Suggested readings in the reference section are denoted with an asterisk (*).
Stopa EG, King JC, Lydic R, Schoene WC (1984) Human brain contains vasopressin and vasoactive intestinal polypeptide neuronal subpopulations in the suprachiasmatic region. Brain Res 297:159–163
Lydic R, Schoene WC, Czeisler CA, Moore-Ede MC (1980) Suprachiasmatic region of the human hypothalamus: homolog to the primate circadian pacemaker? Sleep 2:355–361
Brown SA et al (2005) The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol 3, e338. doi:10.1371/journal.pbio.0030338
Boivin DB et al (2003) Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102:4143–4145. doi:10.1182/blood-2003-03-0779
Gomez-Santos C et al (2009) Circadian rhythm of clock genes in human adipose explants. Obesity 17:1481–1485. doi:10.1038/oby.2009.164
Saini C et al (2016) A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes Metab 18:355–365. doi:10.1111/dom.12616
Perrin L et al (2015) Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab 4:834–845. doi:10.1016/j.molmet.2015.07.009
Gaspar L, Brown SA (2015) Measuring circadian clock function in human cells. Methods Enzymol 552:231–256. doi:10.1016/bs.mie.2014.10.023
Burke TM et al (2015) Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med 7:305ra146. doi:10.1126/scitranslmed.aac5125
Davidson AJ, Castanon-Cervantes O, Stephan FK (2004) Daily oscillations in liver function: diurnal vs circadian rhythmicity. Liver Int 24:179–186. doi:10.1111/j.1478-3231.2004.0917.x
Davidson AJ, London B, Block GD, Menaker M (2005) Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens 27:307–311
Storch KF et al (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417:78–83. doi:10.1038/nature744
Zvonic S et al (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55:962–970
O’Neill JS, Reddy AB (2011) Circadian clocks in human red blood cells. Nature 469:498–503. doi:10.1038/nature09702
*Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE (2002) Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 17:181–193
Klerman H et al (2012) Analysis method and experimental conditions affect computed circadian phase from melatonin data. PLoS ONE 7, e33836. doi:10.1371/journal.pone.0033836
*Czeisler CA, et al (1999) Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284:2177–2181
*Wright KP Jr., Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA (2001) Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci U S A 98:14027–14032. doi:10.1073/pnas.201530198
Voultsios A, Kennaway DJ, Dawson D (1997) Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythm 12:457–466
Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA (2005) Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythm 20:168–177. doi:10.1177/0748730404274265
Wright KP Jr et al (2013) Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol 23:1554–1558. doi:10.1016/j.cub.2013.06.039
Burgess HJ, Fogg LF (2008) Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE 3, e3055. doi:10.1371/journal.pone.0003055
Moore RY (1996) Neural control of the pineal gland. Behav Brain Res 73:125–130
*Lewy AJ, Cutler NL, Sack RL (1999) The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms 14:227–236
Buijs RM et al (1999) Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11:1535–1544
Czeisler CA, Wright KP (1999) In: Zee PC, Turek FW (eds) Regulation of sleep and circadian rhythms. Marcel Dekker, New York, pp 147–180
Mills JN, Minors DS, Waterhouse JM (1978) Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. J Physiol 285:455–470
Jung CM et al (2011) Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 589:235–244. doi:10.1113/jphysiol.2010.197517
*Czeisler CA, Klerman EB (1999) Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res 54:97–130. discussion 130–132
Waterhouse J et al (1999) The effect of activity on the waking temperature rhythm in humans. Chronobiol Int 16:343–357
*Dijk DJ, Czeisler CA (1995) Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci 15:3526–3538
Wright KP Jr et al (2015) Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun 47:24–34. doi:10.1016/j.bbi.2015.01.004
Gribbin CE, Watamura SE, Cairns A, Harsh JR, Lebourgeois MK (2012) The cortisol awakening response (CAR) in 2- to 4-year-old children: effects of acute nighttime sleep restriction, wake time, and daytime napping. Dev Psychobiol 54:412–422. doi:10.1002/dev.20599
Scheer FA, Wright KP Jr, Kronauer RE, Czeisler CA (2007) Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE 2, e721. doi:10.1371/journal.pone.0000721
Klerman EB, Lee Y, Czeisler CA, Kronauer RE (1999) Linear demasking techniques are unreliable for estimating the circadian phase of ambulatory temperature data. J Biol Rhythm 14:260–274
Moul DE, Ombao H, Monk TH, Chen Q, Buysse DJ (2002) Masking effects of posture and sleep onset on core body temperature have distinct circadian rhythms: results from a 90-min/day protocol. J Biol Rhythm 17:447–462
McHill AW et al (2014) Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A 111:17302–17307. doi:10.1073/pnas.1412021111
LeBourgeois MK et al (2013) Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythm 28:322–331. doi:10.1177/0748730413506543
Burgess HJ, Wyatt JK, Park M, Fogg LF (2015) Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep 38:889–897. doi:10.5665/sleep.4734
Pullman RE, Roepke SE, Duffy JF (2012) Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO). Sleep Med 13:703–706. doi:10.1016/j.sleep.2011.11.008
Aschoff J, Gerecke U, Wever R (1967) Desynchronization of human circadian rhythms. Jpn J Physiol 17:450–457
Zulley J, Wever R, Aschoff J (1981) The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflugers Arch - Eur J Physiol 391:314–318
Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS (1980) Human sleep: its duration and organization depend on its circadian phase. Science 210:1264–1267
Aschoff J, Wever R (1976) Human circadian rhythms: a multioscillatory system. Fed Proc 35:236–232
Campbell SS, Dawson D, Zulley J (1993) When the human circadian system is caught napping: evidence for endogenous rhythms close to 24 hours. Sleep 16:638–640
Phillips AJ, Czeisler CA, Klerman EB (2011) Revisiting spontaneous internal desynchrony using a quantitative model of sleep physiology. J Biol Rhythm 26:441–453. doi:10.1177/0748730411414163
Middleton B, Arendt J, Stone BM (1997) Complex effects of melatonin on human circadian rhythms in constant dim light. J Biol Rhythm 12:467–477
Middleton B, Arendt J, Stone BM (1996) Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res 5:69–76
*Kleitman N (1939) Sleep and wakefulness. The University of Chicago Press, Chicago
Darwent D et al (2010) Contribution of core body temperature, prior wake time, and sleep stages to cognitive throughput performance during forced desynchrony. Chronobiol Int 27:898–910. doi:10.3109/07420528.2010.488621
Dijk DJ, Duffy JF, Czeisler CA (1992) Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res 1:112–117
Dijk DJ, Czeisler CA (1994) Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett 166:63–68
Duffy JF et al (2011) Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A 108(Suppl 3):15602–15608. doi:10.1073/pnas.1010666108
Gronfier C, Wright KP Jr, Kronauer RE, Czeisler CA (2007) Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A 104:9081–9086. doi:10.1073/pnas.0702835104
Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ (1999) Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Phys 277:R1152–R1163
*Zhou X et al (2012) Mismatch between subjective alertness and objective performance under sleep restriction is greatest during the biological night. J Sleep Res 21:40–49. doi:10.1111/j.1365-2869.2011.00924.x
Ferguson SA et al (2012) The influence of circadian time and sleep dose on subjective fatigue ratings. Accid Anal Prev 45(Suppl):50–54. doi:10.1016/j.aap.2011.09.026
Zhou X et al (2011) Sleep, wake and phase dependent changes in neurobehavioral function under forced desynchrony. Sleep 34:931–941. doi:10.5665/SLEEP.1130
Matthews RW et al (2012) Simulated driving under the influence of extended wake, time of day and sleep restriction. Accid Anal Prev 45(Suppl):55–61. doi:10.1016/j.aap.2011.09.027
Sargent C, Darwent D, Ferguson SA, Kennaway DJ, Roach GD (2012) Sleep restriction masks the influence of the circadian process on sleep propensity. Chronobiol Int 29:565–571. doi:10.3109/07420528.2012.675256
Paech GM, Ferguson SA, Sargent C, Kennaway DJ, Roach GD (2012) The relative contributions of the homeostatic and circadian processes to sleep regulation under conditions of severe sleep restriction. Sleep 35:941–948. doi:10.5665/sleep.1956
Kosmadopoulos A et al (2015) The efficacy of objective and subjective predictors of driving performance during sleep restriction and circadian misalignment. Accid Anal Prev. doi:10.1016/j.aap.2015.10.014
Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA (1997) Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol 505(Pt 3):851–858
Carskadon MA, Dement WC (1975) Sleep studies on a 90-minute day. Electroencephalogr Clin Neurophysiol 39:145–155
Tzischinsky O, Shlitner A, Lavie P (1993) The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J Biol Rhythm 8:199–209
Eastman CI, Molina TA, Dziepak ME, Smith MR (2012) Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans). Chronobiol Int 29:1072–1077. doi:10.3109/07420528.2012.700670
Eastman CI, Suh C, Tomaka VA, Crowley SJ (2015) Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep 5:8381. doi:10.1038/srep08381
Micic G et al (2013) The endogenous circadian temperature period length (tau) in delayed sleep phase disorder compared to good sleepers. J Sleep Res 22:617–624. doi:10.1111/jsr.12072
Lockley SW et al (2000) Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol 164:R1–R6
Shea SA, Hilton MF, Hu K, Scheer FA (2011) Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res 108:980–984. doi:10.1161/CIRCRESAHA.110.233668
Zhou X et al (2011) Dynamics of neurobehavioral performance variability under forced desynchrony: evidence of state instability. Sleep 34:57–63
Sargent C, Zhou X, Matthews RW, Darwent D, Roach GD (2016) Daily rhythms of hunger and satiety in healthy men during one week of sleep restriction and circadian misalignment. Int J Environ Res Public Health 13:170. doi:10.3390/ijerph13020170
Burke TM, Scheer FA, Ronda JM, Czeisler CA, Wright KP Jr (2015) Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res 24:364–371. doi:10.1111/jsr.12291
Scheer FA, Morris CJ, Shea SA (2013) The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 21:421–423. doi:10.1002/oby.20351
Lo JC et al (2012) Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS ONE 7, e45987. doi:10.1371/journal.pone.0045987
Broussard JL et al (2015) Sleep restriction increases free fatty acids in healthy men. Diabetologia 58:791–798. doi:10.1007/s00125-015-3500-4
Broussard JL et al (2016) Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity 24:132–138. doi:10.1002/oby.21321
Goichot B et al (1998) Effect of the shift of the sleep-wake cycle on three robust endocrine markers of the circadian clock. Am J Phys 275:E243–E248
Morris CJ et al (2015) The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity 23:2053–2058. doi:10.1002/oby.21189
Mohr U, Hondius Boldingh W, Althoff J (1972) Identification of contaminating Clostridium spores as the oncolytic agent in some chalone preparations. Cancer Res 32:1117–1121
Scheer FA et al (2010) Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 107:20541–20546. doi:10.1073/pnas.1006749107
Morris CJ et al (2015) Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A 112:E2225–E2234. doi:10.1073/pnas.1418955112
Scheer FA, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106:4453–4458. doi:10.1073/pnas.0808180106
Buxton OM et al (2012) Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4:129ra143. doi:10.1126/scitranslmed.3003200
Hu K, Scheer FA, Laker M, Smales C, Shea SA (2011) Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 123:961–970. doi:10.1161/CIRCULATIONAHA.110.943019
Duffy JF, Lowe AS, Silva EJ, Winkelman JW (2011) Periodic limb movements in sleep exhibit a circadian rhythm that is maximal in the late evening/early night. Sleep Med 12:83–88. doi:10.1016/j.sleep.2010.06.007
Davies SK et al (2014) Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A 111:10761–10766. doi:10.1073/pnas.1402663111
Ang JE et al (2012) Identification of human plasma metabolites exhibiting time-of-day variation using an untargeted liquid chromatography-mass spectrometry metabolomic approach. Chronobiol Int 29:868–881. doi:10.3109/07420528.2012.699122
Archer SN, Oster H (2015) How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res 24:476–493. doi:10.1111/jsr.12307
Moller-Levet CS et al (2013) Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A 110:E1132–E1141. doi:10.1073/pnas.1217154110
Archer SN et al (2014) Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A 111:E682–E691. doi:10.1073/pnas.1316335111
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer (India) Pvt. Ltd.
About this chapter
Cite this chapter
Broussard, J.L., Reynolds, A.C., Depner, C.M., Ferguson, S.A., Dawson, D., Wright, K.P. (2017). Circadian Rhythms Versus Daily Patterns in Human Physiology and Behavior. In: Kumar, V. (eds) Biological Timekeeping: Clocks, Rhythms and Behaviour. Springer, New Delhi. https://doi.org/10.1007/978-81-322-3688-7_13
Download citation
DOI: https://doi.org/10.1007/978-81-322-3688-7_13
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-3686-3
Online ISBN: 978-81-322-3688-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)