Abstract
Urea and other nonprotein nitrogen compounds in the ration of ruminants as an economical replacement of vegetable and animal proteins have been investigated for more than 100 years. Initially, the death of animals from urea toxicity owing to insufficient knowledge of urea feeding impeded the widespread use of urea in the diets of ruminants. However, following comprehensive research demonstrating its safety and usefulness in many feeding conditions, urea has commonly become an accepted ingredient for the diets of ruminants. A large body of information and understandings related to the mechanisms of urea and other nonprotein nitrogen compound utilization by ruminal microorganisms has been documented. This chapter discusses urea recycling in the gut, urea and ammonia metabolism by rumen microorganisms, ammonia absorption and mechanisms, and symptoms and treatments of urea/ammonia toxicity in ruminants. The ammonia/urea toxicity problems could easily be prevented through proper employment of scientific knowledge of urea feeding to ruminants discussed in this chapter. Opportunities exist for enhancing anabolic use of urea-N by the microorganisms through modulating urea-N recycling into the rumen by dietary and feeding management, which could decrease N wastage into environment and improve efficiency of N utilization in ruminants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
It has been recognized for more than a century that urea and nonprotein nitrogen compounds may be incorporated in the diets of ruminants. As early as 1891, Ehrenberg et al. (1891) and Zuntz (1891) suggested that nonprotein nitrogen (NPN) compounds could be converted to true protein by ruminal microflora and may replace a portion of protein in the diets of ruminants (cited by Huntington and Archibeque 2000; Kertz 2010). Thereafter, few studies were conducted on the use of NPN in ruminant diets until 1937; it was, however, not widely recognized that urea can be converted to proteins in substantial amounts in ruminants (Reid 1953). In reviewing the earlier research, Krebs (1937) concluded that there remained considerable doubt whether NPN compounds were converted to protein in amounts significant to ruminants.
Research on urea feeding took an impetus after Bartlett and Cotton (1938) reported that satisfactory growth of young cattle resulted when urea supplemented protein in the diet. Hart et al. (1939) showed that replacing vegetable protein with urea or ammonium bicarbonate resulted in normal growth in growing cattle and inclusion of soluble sugars to the diet improved the utilization. A number of studies, as reviewed by Reid (1953), subsequently demonstrated that urea-nitrogen fed to ruminants was definitely retained in the body and that the tissues of the growing animals were of normal composition. Digestion studies showed that urea supplements sometimes increased the digestibility of cellulose and crude fiber of low-protein rations. Balance studies provided evidence of increased nitrogen retention in ruminants that gained body weight with supplemental urea. In vitro fermentation techniques and analyses of rumen ingesta showed that concentrations of urea or ammonia decreased, while true protein content increased in the fermentation medium. Chemical and microbiological analyses and the use of tracers proved that urea-nitrogen was, indeed, converted into true protein in the rumen, which subsequently appeared as tissue and milk proteins.
Most extensive research had been conducted on urea or NPN feeding to ruminants throughout the world in animal nutrition. Initially, the death of animals from urea toxicity owing to insufficient knowledge of urea feeding impeded the widespread use of urea in the diets of ruminants. However, following comprehensive research demonstrating its safety and usefulness in many feeding conditions, urea has commonly become an accepted ingredient for the diets of ruminants. This chapter focuses on urea and ammonia metabolism in the rumen in relation to ammonia toxicity in ruminants.
2 Ruminal Urea/Ammonia Metabolism
2.1 Urea/Ammonia Pool in the Rumen
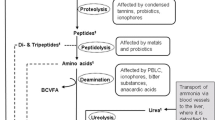
Ammonia and urea arise in the rumen through diet, urea recycling via ruminal wall, and salivary secretion. Transfer of urea into rumen occurs by simple diffusion down the concentration gradient, which is greatly variable depending upon dietary and rumen environmental factors (Owens and Bergen 1983). The transport across the rumen epithelium is also mediated via urea transporters in the luminal and basolateral membrane of the epithelium (Abdoun et al. 2006). Urea transporters in the rumen wall are differentially expressed depending on dietary N concentration (Marini and Amburgh 2003). In ruminants, the amount of urea-N recycled to the gut (as a proportion of total hepatic urea-N output) varies between 29 % and 99 % and N transfer across the gut can be much greater than N intake (Kiran and Mutsvangwa 2007). This mechanism is proposed to have constant supply of nitrogen to preserve rumen microbial population when the nitrogen supply in feed is limited. Urea-N recycling to the gut is influenced by several dietary and ruminal factors. Amount of N intake (Marini et al. 2004), total dry matter intake (Sarraseca et al. 1998), type and frequency of feeding dietary N that is degraded in the rumen (Wickersham et al. 2008; Rémond et al. 2009; Kiran and Mutsvangwa 2010), ruminal fermentable carbohydrate intake, and organic matter digestibility (Kennedy and Milligan 1980) are the major dietary factors regulating the proportion of hepatic urea-N output returning to the gut. Concentrations of ammonia-N and volatile fatty acids, particularly butyrate, urease activity, ruminal CO2 and pH, and plasma urea concentration also exert an important role in trans-epithelial movement of blood urea-N into the rumen (Kennedy and Milligan 1980; Kiran and Mutsvangwa 2010). Ruminal ammonia-N concentration is negatively correlated with urea-N transfer to the rumen (Kennedy and Milligan 1980), while greater urease activity, butyrate, and CO2 increase urea transport through rumen wall (Huntington and Archibeque 2000; Abdoun et al. 2010). Defaunation may enhance the urea cycling as a proportion of endogenous urea synthesis through the rumen wall (Kiran and Mutsvangwa 2010).
As outlined in the review by Lapierre and Lobley (2001), contributions from salivary flow to urea-N entry to the rumen can vary between 15 % and 100 % depending on the type of the diet. Depending on the dietary and ruminal factors, 19–96 % of endogenous urea production may be recycled to the gut, 15–94 % of the recycling may be transferred in saliva, and 25–90 % of urea degraded in the gut may be degraded in the postruminal digestive tract (Huntington and Archibeque 2000). Salivary flow of urea-N into the rumen as a percent of total urea-N entry to the gut was 36 % in forage-fed (Taniguchi et al. 1995) and 16 % in concentrate-fed (Guerino et al. 1991) ruminants. Urea excreted in the urine represents from 25 % to 60 % of endogenous urea production in ruminants (Huntington and Archibeque 2000).
Ammonia is also derived from degradation of dietary protein depending upon source. Amino groups are also split from amino acids and from intact proteins and used by bacteria in the same manner. The solubility of natural proteins varies highly, and thus the rate at which they are hydrolyzed by bacteria differs appreciably. For the less soluble proteins, the process of ammonia liberation is much less rapid and fairly large proportions of the protein may pass through the rumen to the abomasum without being broken down. There are several major known protein-degrading rumen bacteria, i.e., Prevotella ruminicola, Ruminobacter amylophilus, Butyrivibrio fibrisolvens, Selenomonas ruminantium, Prevotella bryantii, and Streptococcus bovis, and amino acid-fermenting bacteria, i.e., Clostridium aminophilum, Clostridium sticklandii, Megasphaera elsdenii, Prevotella ruminicola, Butyrivibrio fibrisolvens, Selenomonas ruminantium, and Streptococcus bovis (Russell 1998; Wallace 1996). Degradation of feed protein and predation of bacteria by protozoa might play a major role in protein degradation in the rumen influencing the ammonia concentration in the rumen (Wallace et al. 1987). The inhibition of the growth of the protein-degrading bacteria and protozoa may decrease protein degradation and metabolism in the rumen, consequently ruminal ammonia concentration (Patra and Saxena 2009; Patra and Yu 2014).
2.2 Hydrolysis of Urea
Upon entering into the rumen, urea is rapidly hydrolyzed to ammonia within 30 min to 2 h (Rekib and Sadhu 1986) by the urease enzyme produced by the ruminal microorganisms.
2.2.1 Bacterial Urease Enzyme
The urease activity depends upon the concentration of urea entering into the rumen. It has been noted that the magnitude of the urease activity was a direct function of the amounts of urea infused. The urease activity may be repressed with high ammonia concentrations and complex organic nitrogen sources in the rumen fluid. Wozny et al. (1977) noted that the activity of the urease in two anaerobes (Selenomonas ruminantium and Peptostreptococcus productus) was depressed two- to six-folds by the inclusion of 50 mM urea as compared to inclusion of none. There is evidence that glutamine synthatase can regulate the production of urease in Selenomonas ruminantium (Smith and Bryant 1979) and the activities of both these enzymes could be increased manyfolds when ammonia was limiting. In general, urease activity increased 2–6 h after feeding and the increases were greater with roughage diets. The purified urease enzyme was inhibited by divalent cations (Mn2+, Mg2+, Ba2+, Hg2+, Cu2+, Zn2+, Cd2+, Ni2+, and Co2+) (Mahadevan et al. 1976), while Spears and Hatfield (1978) using incubated ruminal fluid reported that ruminal urease was stimulated by a number of inorganic ions including Mn2+, Ni2+, and Ba2+, but was inhibited by Cu2+, Zn2+, and Cd2+.
Urease activity is mostly localized on rumen wall epithelium with adherent bacteria, as well as in the rumen fluid (Rybosová et al. 1984). The high ureolytic activity of bacteria adhering to the rumen wall in the presence of a low nitrogen intake is assumed to be one of the partial mechanisms of the hydrolysis of blood urea entering the rumen across the rumen wall and its reutilization in the rumen-liver nitrogen cycle in ruminal mucosa. In in vivo studies with sheep fed with a low-protein diet (3.7 g N/day), urease activity was found to be the highest in the bacteria adhering to the rumen wall, lower in the rumen fluid bacteria, and lowest in the bacteria adhering to feed particles in the rumen (Javorský et al. 1987). However, the urease activities of bacteria adhering to the rumen wall and the rumen fluid bacteria in sheep on a high-protein diet (21 g N/day) were similar, but significantly lower than in sheep with a low N intake; it was lowest in bacteria adhering to feed particles in the rumen (Javorský et al. 1987). In rusitec fermenter study (Czerkawski and Breckenridge 1982), the ureolytic activity per unit volume was usually higher in compartment 2 (space occupied by microorganisms that are loosely associated with the solids) than in compartment 1 (strained rumen contents) or compartment 3 (space occupied by microorganisms that are tightly associated with solid matrix). Specific urease activity (per unit weight of protein or deoxyribonucleic acid) was much higher in compartment 1 than in compartment 2, which decreased markedly with depth of compartment (Czerkawski and Breckenridge 1982).
2.2.2 Ureolytic Bacteria in the Rumen
Following extensive research on the utilization of urea as a replacement of protein and its supplementation in ruminant diet, interests were raised to isolate urea-hydrolyzing microbes for a better understanding of urea metabolism in the rumen. Appleby (1955) and Blackburn and Hobson (1962) reported only a few facultative anaerobes (predominantly staphylococci or micrococci) as being ureolytic. Among the other reported experiments on ureolytic bacteria, Gibbons and Doetsch (1959) isolated an ureolytic bacterium, assigned to the species Lactobacillus bifidus from normally fed cattle. Slyter et al. (1968) reported presumptively identified ureolytic isolates of Propionibacterium sp., Bacteroides sp., Ruminococcus sp., Streptococcus bovis, and an anaerobic Lactobacillus sp. However, these bacteria isolated from cattle fed with semisynthetic, purified diets and the levels of urease activity in the bacteria were not determined. John et al. (1974) isolated an ureolytic strain of Selenomonas ruminantium from the rumen of a steer. Screening of over 1,000 strains of rumen bacteria isolated from sheep rumen on different media, Cook (1976) showed that urease activity was apparently confined to species of Staphylococcus, Lactobacillus casei var. casei, and Klebsiella aerogenes. The ureolytic strain of Streptococcus faecium had a higher urease activity than the other bacteria occurring in higher numbers and possessed urease activity sufficient to account for most of the ureolytic activity of the rumen of roughage-fed sheep. Van Wyk and Steyn (1975) noted that all urease positive isolates were facultative anaerobic, Gram-positive, catalase-positive cocci. Out of ten isolates, nine were identified as Staphylococcus saprophyticus and one as Micrococcus varians. The facultative anaerobic Gram-positive cocci were probably responsible for a large proportion of the urease activity of the rumen fluid.
Ureolytic activity of bacteria differs among the strains. Lauková and Koniarová (1995) tested several strains of bacteria, including Selenomonas ruminantium, Lactobacillus sp., Enterococcus sp., and Staphylococcus sp., from the rumen of domesticated and wild ruminants for their urease activity. It was noted that 56.7 % of Selenomonas ruminantium strains and 18.5 % of lactobacilli manifested medium urease activity. Most of the Enterococcus faecium (62.2 %) and all of the Enterococcus faecalis isolates expressed low urease activity. Streptococcus bovis and Streptococcus uberis did not produce any urease. All the staphylococci screened were urease-producing strains, mostly with medium or low urease activity.
Urease activity was also detected in many strains of nonselectively isolated rumen species, which include Succinivibrio dextrinosolvens, Treponema sp., Ruminococcus bromii, Butyrivibrio sp., Bifidobacterium sp., Bacteroides ruminicola, and Peptostreptococcus productus (Wozny et al. 1977). Most Peptostreptococcus productus strains contain urease; however, the uniformity of this feature in the other species noted above is not known. Propionibacterium, Veillonella, and Megasphaera did not possess urease activity.
2.3 Ammonia Utilization
The ammonia can be utilized by the bacteria for synthesis of amino acids required for their growth. Protein synthesis in the rumen by microorganisms is very closely associated with the activity of these same organisms in breaking down cellulose and other carbohydrate materials and in the formation of organic acids as by-products of this fermentation process. There is evidence, however, that a fairly high proportion of the more soluble proteins such as casein will be utilized by bacteria in about the same way as the ammonia from urea. Through the years many attempts were made to determine the optimum concentrations of ammonia in the rumen and to relate it to synthesis of microbial protein. The in vitro studies suggest that ammonia concentration required for maximal microbial protein synthesis is approximately 50–60 mg/L (Satter and Slyter 1974; Mehrez et al. 1977), while with in vivo studies, Hume et al. (1970) and Pisulewski et al. (1981) obtained best results with 88–133 and 27–100 and mg/L, respectively, which varied depending upon diet. Mehrez et al. (1977) obtained maximal rate of fermentation with 235 mg/L. In all these studies, the concentration of ammonia in the rumen meant the concentration in compartment 1 only. Since the concentration of carbohydrate in this compartment is low, it is unlikely to be the most active metabolic site (Czerkawski and Breckenridge 1982). Utilization of ruminal ammonia-N into the bacterial protein in the rumen is energy dependent, and hence, providing adequate ruminally available energy is associated with lower ruminal ammonia-N concentration and, consequently, increased microbial protein synthesis. Defaunation may also increase urea-N recycling to the GIT and microbial non-ammonia-N supply, thus improving efficiency of N utilization (Kiran and Mutsvangwa 2011).
Utilization of ammonia for synthesis of amino acids by rumen microorganisms involves amination and transamination reactions. When the activities of those involved in ammonia production (urease and NAD and NADP-linked glutamate dehydrogenases) exceed the activities of enzymes those concerned with ammonia utilization (glutamine synthetase, carbamyl phosphokinase, and NADH- and NADPH-linked glutamate dehydrogenases), ammonia concentration in the rumen increases. Ruminal ammonia utilization is also controlled by other factors such as availability of energy and cofactors, substrate levels, and bacterial cell permeability (Allison 1969). Since branched-chain volatile organic acids derive in the rumen mainly from degradation of dietary protein and the deamination of branched-chain amino acids, the feeding of protein free diets can cause depressions in these acids (Oltjen 1969). Concentrations of isobutyrate and isovalerate usually decrease and other volatile fatty acids may also be affected due to urea feeding. Rumen fluid from urea-added treatment contained more total volatile fatty acids, but decreased molar percentages of isobutyrate, isovalerate, and caproate (Czerkawski and Breckenridge 1982). Rumen microorganisms have pathways similar to those of the tricarboxylic acid cycle, which will provide carbon skeletons for amino acid biosynthesis (Allison 1969). However, for synthesis of some amino acids, specific carbon sources are required. For example, isobutyrate, phenylacetate, indole-3-acetate, isovalerate, and 2-methyl-butyrate are precursors of valine, phenylalanine, tryptophan, leucine, and isoleucine, respectively (Allison 1969). When urea is the primary dietary nitrogen source, energy, carbon skeletons, and cofactors required for amino acid biosynthesis may be needed in greater quantities.
2.4 Ammonia Absorption
When ammonia is produced too rapidly in the rumen compared to the utilization by the rumen microorganisms, or if the concentration becomes too high, appreciable amounts are absorbed directly into the bloodstream, reconverted to urea in the liver, excreted through the kidneys in the urine, or reenter through the saliva and the ruminal wall into the rumen. The absorption of ammonia occurs in the lipophilic form as ammonia (NH3) via simple diffusion, the magnitude of which is linearly related to the pH in the ruminal fluid at pH values above 7, while at normal rumen pH of 6–7, ammonia is predominantly absorbed as NH4 + via putative potassium channels in the apical membrane (Abdoun et al. 2006). At normal ruminal pH, NH4 + is converted to ammonia at the entry site in the rumen epithelium before being absorbed into portal blood. In addition, Abdoun et al. (2007) also suggested that the absorption of NH4 + may occur through some transport proteins and the movement of NH4 + across the ruminal epithelium is probably regulated by both chemical and electrical gradients. Absorption of both forms of ammonia across the ruminal wall increases with the increase in ruminal pH and total ammonia-N concentrations. Hence, the relative transport rates of ammonia or NH4 + are determined by the ruminal pH according to the Henderson-Hasselbalch equation (Abdoun et al. 2006). At ruminal pH of 6.5 and low, which is normally observed in most feeding conditions, most of the ammonia is absorbed in the form of NH4 + (Abdoun et al. 2007).
The amount of N absorbed as ammonia ranged from 16 % to 73 % of N intake, which can be several times the amount of N absorbed as amino acids (Parker et al. 1995). Ammonia-N is absorbed across all the sections of the gut and on average 67 % of ammonia-N is absorbed from the reticulo-rumen, while the lower gut, including the small and large intestines, and cecum can account for 33 % (Reynolds and Huntington 1988). However, these proportions vary considerably depending upon dietary chemical composition (Parker et al. 1995). Ammonia-N absorbed across the ruminal wall into the portal blood accounts for up to 50 % of total ammonia-N flow to the liver (Parker et al. 1995). The quantity of ammonia-N absorbed across the ruminal wall is mainly determined by dietary as well as ruminal factors, with the most important factors being dietary protein that is degraded in the rumen, contributions of endogenous urea to the ruminal ammonia-N pool, and dietary ruminally available energy (Reynolds and Kristensen 2008). Under a wide variety of dietary and physiological conditions in growing and lactating cattle, Firkins and Reynolds (2005) concluded that ammonia-N absorption across the gut accounts for about 42 % of dietary N intake. Supply of adequate amount of ruminally available energy is associated with lower ruminal ammonia-N concentration and, consequently, reduced ammonia-N absorption into portal blood.
3 Toxicity of Urea
Urea is not normally toxic, but hydrolysis of urea produces ammonia that is toxic to all mammals. Inability of the liver to convert absorbed ammonia from the rumen to nontoxic urea results in an increased concentration of ammonia in the blood, which is responsible for urea toxicity. Any compounds forming ammonia in the rumen exert toxic effects if the rumen ammonia level is highly increased so that the rate of ammonia absorption from the rumen exceeds the capacity of the liver to remove ammonia from blood and thus leading to a rise in peripheral blood ammonia to a level of more than 10 mg/L (McDonald 1958). Toxicity of ammonia in ruminants may be attributed to a direct toxic effect of ammonium ion in body systems (Lewis 1960). Besides, a disturbance of the acid–base balance and a change in electrolyte balances due to high ammonia concentrations in blood may modify the signs of toxicity (Lewis 1960). Indicators of ammonia toxicity include ruminal ammonia concentration above 1,000 mg/L, rumen pH above 8, and blood ammonia concentration above 20 mg/L (Owens and Bergen 1983). Death of animals may usually occur when blood ammonia concentrations exceed 40 mg/L. Several contributing factors e.g., rumen pH, diets, and adaptation to the animals, modify the degree of ammonia/urea toxicity. Rumen pH, rather than rumen ammonia concentrations, is one of most important contributing factors triggering the symptoms of urea toxicity, since rumen pH determines how quickly and how much ammonia is absorbed into the blood. The classical urea toxicity studies of Bartley et al. (1976) showed that rumen pH had a greater correlation (r = 0.317) with toxicity than rumen ammonia-N concentration (r = 0.039) and blood ammonia-N had the highest correlation (r = 0.707) with toxicity. Kertz et al. (1983) demonstrated this finding in a study in which ammonium chloride was dosed into the rumen of cows at urea-equivalent levels. Because ammonium chloride simply dissociates in the rumen, it would not have the rumen pH-elevating effect of urea hydrolysis. Although ammonium chloride considerably increased rumen ammonia, because rumen pH was not elevated, as occurs with urea hydrolysis, the rumen ammonia was essentially trapped, resulting in no significant increases in blood urea-N and ammonia and no decreases in feed intake.
Urea can be tolerated in greater amounts in the presence of readily available carbohydrate feeds such as cereal grains. Animals fed with balanced or high-carbohydrate feeds and those adjusted to urea-containing feeds can handle larger amounts of urea than those subsisting on low-protein roughage (Kertz 2010). Urea may produce harmful effects under unusual conditions such as for starved or fasted animals due to rapid consumption of urea-containing feeds. However, urea toxicity would not be expected in animals that are fed properly with mixed rations containing urea in the recommended amounts. Coombe and Tribe (1960) reported that 75 g of urea per day was not toxic for sheep when it was carefully mixed with hay. Clark et al. (1951) showed that putting urea into the rumen of sheep (10 g or in aqueous solution) when feed was withheld caused an acute intoxication. Toxicity was shown to be associated with increased alkalinity of the rumen contents. Toxicity of urea was reduced when sheep were fed with poor-quality hay and was nonexistent when lucerne hay or casein plus low-quality hay was fed. It was concluded that the toxicity of urea depends on the activity of the rumen flora in utilizing the ammonia and the presence of available carbohydrate. They noted that sheep fed with poor-quality diets were more susceptible than those well-fed sheep.
4 Signs and Symptoms
Toxicity of urea feeding to ruminants has been investigated since early 1950s. Ammonia exerts toxic effects on the central nervous system, kidney, and heart, producing symptom of urea toxicity. Subclinical toxicity may alter carbohydrate metabolism in the liver and energetic efficiency of lactation (Owens and Bergen 1983). Acute toxicity of ammonia is immediate threat to the life of animals depending upon the dose of urea feeding. Hart et al. (1939) found that cattle fed for a year with a ration containing 4.3 % urea showed hypertrophy of the kidneys upon slaughter, but there was no evidence of toxicity. Those fed with a ration having 2.8 % urea were normal. Harris and Mitchell (1941) could not detect any evidence of injury to withers fed with a ration having 3.2 % urea. When larger doses were given, urea caused toxic effects and even death of ruminants. Dinning et al. (1948) observed that a single dose of 116 g of urea caused ataxia, severe tetany, a retarded respiration rate, and excessive salivation in cattle. However, feeding 200 g properly mixed in the daily feed did not result in unfavorable effects. Clark et al. (1951) showed that putting urea into the rumen of sheep (10 g or more in aqueous solution) after withholding the feed caused an acute intoxication characterized by atony of the rumen, muscular spasms, and sudden death caused by circulatory failure. Toxicity was shown to be associated with increased alkalinity of the rumen contents. Pathological changes may also be found in the liver, lungs, kidneys, and brain (Hart et al. 1939; Antonelli et al. 2004; Srinivasan et al. 2008). There are no characteristic lesions on postmortem examination, but most cases show generalized exfoliation of mucosa, edema and degeneration of rumen mucosa, necrotic enteritis, congestion and hemorrhages in the brain, and lymphocytic infiltrations of the heart, lungs, and kidney (Randhawa et al. 1989).
Davis and Roberts (1959) described the progressive symptoms of urea toxicity. Following administration of a toxic dose of urea, the animals showed uneasiness, muscle and skin tremors, excess salivation, respiratory difficulties, incoordination or ataxia, bloat, tetany, and death. When blood ammonia concentrations exceeded 40 mg/L, all animals died. The toxic dose was about 0.30 g/kg of body weight given as a drench, whereas an 18 g dose was not fatal. In buffaloes, a urea dose of 1.25 g/kg body weight caused similar symptoms and death after 60–150 min (Randhawa et al. 1989). Coombe and Tribe (1958) and Coombe et al. (1960) showed that sheep could ingest very large amounts of urea (100 g/day) provided that the intake was spread over several hours each day; this was achieved by mixing a solution of urea with the roughage; under these circumstances, rumen pH and ammonia concentration remained low. They noted that dosing with urea resulted in a high ammonia concentration and an elevated pH in the rumen, and when the pH value increased to 7.0, rumination time declined, and at pH 7.3, there was complete rumen stasis. This effect was not due to high ammonia concentration as a similar concentration produced by administration of ammonium chloride did not cause stasis at normal rumen pH. It appeared that a rise in rumen pH could enhance any tendency to ammonia intoxication due to increased rate of absorption from the rumen. Coombe and Tribe (1962) noted that feeding of molasses had the effect of reducing rumen pH and ammonia concentration, which could be of significance in reducing the risk of toxicity from urea.
Lewis (1960) reported experiments on ammonia intoxication with different forms of ammonia feeding to sheep. The metabolic observations showed that there were distinct differences following the intraruminal administration of ammonium chloride, ammonium acetate, or urea. Ammonium chloride produced acidosis, with low blood pH, respiratory hyperventilation, and a consequent low blood bicarbonate level; however, it was considered that these changes were not adequate to explain the toxic signs and that toxicity was probably directly related to the blood ammonia level, with a critical concentration about 8 mg/L. Ammonium acetate had no effect on blood pH, but caused a marked fall in blood bicarbonate, which was considered to be due to a respiratory alkalosis produced by the respiratory stimulation from the blood ammonium ion. The critical blood ammonia concentration for toxicity was about 6 mg/L. Addition of urea led to transitory increases in blood pH and bicarbonate, and symptoms of toxicity occurred when blood ammonia attained a level of about 5 mg/L. Lewis (1960) concludes that the toxicity of urea is almost certainly due to a direct effect of the circulating ammonium ion. Clark et al. (1963) confirmed that a 30 g dose of urea, given as a drench, was fatal to the sheep. However, biuret proved nontoxic to sheep in very large doses (250 g) even after the sheep had become adapted by feeding biuret for 9 weeks. Some 20–30 % of a dose of biuret given per rumen fistula was excreted unchanged in the urine (Gray and Clark 1964).
5 Treatments
Acetic acid is an effective antidote, whereas the addition of sodium bicarbonate increased the severity of the symptoms. A 5 % solution of acetic acid or vinegar is an effective cure in many cases if administered orally before severe tetany develops. A case of acute urea toxicity in a buffalo heifer was successfully treated after administering with 2.5 L of 5 % acetic acid followed by 1 L of acetic acid after 30 min (Kulkarni and Kulkarni 2002). Intravenous calcium and magnesium solutions can be effective in decreasing tetany. Using rumen pH and ability to neutralize ammonia as indicators, Oltjen et al. (1964) suggested that acetic acid is several times more effective than glutamic acid in preventing signs of urea toxicity. Though drenching of acetic acid decreases ammonia absorption, it does not reduce ammonia concentration in the blood quickly, and thus, death of animals may occur once tetany develops. However, emptying the rumen contents with surgery may result in rapid decrease of blood ammonia concentration, and possibility of animal survivability increases (Bartley et al. 1976). Animals that exhibit severe toxicity could be treated intravenously with 1 mL/kg body weight of commercial solution of urea-cycle amino acids (improve ammonia metabolism in the liver), 1 mg/kg body weight of furosemide (diuretic), and 20 mL/kg body weight isotonic saline solution (Antonelli et al. 2004). However, acute ammonia toxicity sometimes causes death of animals so rapidly that there is normally not adequate time for many of these treatments.
6 Factors Influencing Urea Utilization and Toxicity
6.1 Readily Available Carbohydrates
Supply of readily available digestible energy is the important factor influencing the efficiency of urea utilization by ruminants. A low level of protein and high level of starch in the ration favor urea utilization. Rations high in digestible energy (high grains) result in better urea utilization; conversely those that are low in digestible energy (high forage) result in a lowered utilization of urea. Utilization of urea by animals fed with high-forage rations may be improved by the addition of grain or molasses. Soluble sugars and cellulose are inferior to starch as sources of energy for ruminal microorganisms because sugars are rapidly fermented by the rumen microorganisms and cellulose is too slowly degradable (Reid 1953). Urea-molasses block and uromol preparations containing urea and readily available carbohydrate and minerals have proved to be good protein supplements for different ruminants (Dass et al. 1996; Mehra et al. 1998; Toppo et al. 1997).
6.2 Frequency of Feeding Urea
A continuous intake of urea will improve its utilization over abrupt or periodic intake (Kertz 2010). Constant supply of urea-N decreases ammonia concentrations in the rumen fluid, which is advantageous for rumen microbial growth and nutrient utilization (Alvarez Almora et al. 2012).
6.3 Level of Urea Fed
Low levels of urea are utilized more efficiently with less problems than high levels. Urea may provide up to 3 % of the concentrate ration or up to 1 % of the total ration for milking cows (Reid 1953). However, urea should not provide more than 25 % of the N in rations containing 12 % protein equivalent for fattening lambs and for pregnant or lactating ewes (Reid 1953).
6.4 Thorough Mixing of Urea-Containing Supplements into the Daily Feed
If urea-containing supplements are mixed with the entire daily ration, the intake of urea at any one time will not likely be great, and the ability of the microbes to synthesize protein will not be exceeded. However, small quantities of urea undiluted by feed (116 g in cattle and 10 g in sheep) and introduced suddenly into the rumen resulted in rapid onset of toxicosis, whereas 180–272 g urea was consumed daily by beef calves or cows without toxicosis when fed along with hay or corn silage (Reid 1953).
6.5 Adequate Supply of Phosphorus, Sulfur, and Trace Minerals
Substitution of urea for natural protein sharply changes the quality and quantity of minerals available for ruminal bacteria and animals. Although needed only in small quantities, these elements are necessary building blocks for microbial protein synthesis. Addition of methionine or S has improved the retention of N by ruminants fed with urea-containing rations (Reid 1953). These often are found in many urea-containing supplements.
6.6 Amount and Solubility of Proteins
Urea is fairly inferior for dairy and beef calves fed with rations containing 12 % or more of protein equivalent, of which three-fourths of the N is supplied by conventional protein sources (Reid 1953). Different vegetable and animal protein sources have different solubilities or rates of hydrolysis in the rumen. Bacteria may prefer highly soluble and readily hydrolyzable protein rather than urea in the ration; thus, dietary proteins may be competitive with urea. Supplementation of tannins or tannin-containing feeds may reduce the degradability of protein (Patra and Saxena 2011), which might be beneficial to decrease ammonia concentrations in the rumen due to urea feeding.
6.7 Urease Inhibitors or Slow-Release Urea Products
Slowing the urea hydrolysis may minimize ammonia wastage and improve utilization. Several inhibitors of ruminal urease activity substantially reduce urease activity; but, inhibitors usually provide a short-term effect because remaining urease capacity is still great enough to hydrolyze ruminal urea and possibly because of microbial adaptation to the inhibitors (Whitelaw et al. 1991; Ludden et al. 2000). Coated urea or slow-release urea products as N supplements have shown equal performance to urea supplements without the potential hazards associated with feed-grade urea (Taylor-Edwards et al. 2009; Kertz 2010). Slow-release urea products provide constant supply of ammonia to rumen microorganism for their growth, which also improves nutrition utilization for low-quality forages and reduces plasma ammonia concentrations (Ribeiro et al. 2011; Huntington et al. 2009).
7 Conclusions
Urea and other NPN sources in the ration of ruminants as an economical replacement of vegetable and animal proteins have been investigated for more than 100 years. A large body of information and understandings related to the mechanisms of urea and other NPN utilization by ruminal microorganisms has been documented. Conventionally, it has been recommended that urea should not contribute more than 25 % of total dietary protein, not more than 3 % of concentrate, and not more than 1 % of the total ration (Reid 1953). On the safer side, Kertz (2010), however, suggests that a more reasonable recommendation for feeding urea to adult cattle is 1 % of the concentrate, 135 g/animal daily, and not more than 20 % of total protein, counting other added NPN sources, which would be appropriate under the most adverse conditions for maintaining normal feed intake. Depending on other dietary situations, the urea source and ration balancing programs, these levels may be safely exceeded. The ammonia/urea toxicity problems could easily be prevented through proper employment of knowledge of urea feeding to ruminants. As most of intake N is excreted through urine as urea, opportunities exist for enhancing anabolic use of urea-N by the microorganisms through modulating urea-N recycling into the rumen, which could decrease N wastage into environment, and improve efficiency of N utilization in ruminants.
References
Abdoun K, Stumpff F, Martens H (2006) Ammonia and urea transport across the rumen epithelium: a review. Anim Health Res Rev 7:43–59
Abdoun K, Stumpff F, Martens H (2007) Ammonia and urea transport across the rumen epithelium: a review. Anim Health Res Rev 7:43–59
Abdoun K, Stumpff F, Rabbani I et al (2010) Modulation of urea transport across sheep rumen epithelium in vitro by SCFA and CO2. Am J Physiol Gastrointest Liver Physiol 298:G190–G202
Allison MJ (1969) Biosynthesis of amino acids by ruminal microorganisms. J Anim Sci 29:797–807
Alvarez Almora EG, Huntington GB, Burns JC (2012) Effects of supplemental urea sources and feeding frequency on ruminal fermentation, fiber digestion, and nitrogen balance in beef steers. Anim Feed Sci Technol 171:136–145
Antonelli AC, Mori CS, Soares PC et al (2004) Experimental ammonia poisoning in cattle fed extruded or prilled urea: clinical findings. Braz J Vet Res Anim Sci 41:67–74
Appleby C (1955) The isolation and classification of proteolytic bacteria from the rumen of the sheep. J Gen Microbiol 12:526–533
Bartlett S, Cotton AG (1938) Urea as a protein substitute in the diet of young cattle. J Dairy Res 9:263–272
Bartley EE, Davidovich AD, Barr GW et al (1976) Ammonia toxicity in cattle. I. Rumen and blood changes associated with toxicity and treatment methods. J Anim Sci 53:835–841
Blackburn TH, Hobson PN (1962) Further studies on the isolation of proteolytic bacteria from the sheep rumen. J Gen Microbiol 29:69–81
Clark R, Oyaert W, Quin JI (1951) Studies on the alimentary tract of the Merino sheep in South Africa. XXI. The toxicity of urea to sheep under different conditions. Onderstepoort J Vet Res 25:73–78
Clark R, Barrett EL, Kellerman JH (1963) A comparison between nitrogen retention from biuret and urea by sheep on a low – protein roughage diet. J S Afr Vet Med Assoc 34:419–423
Cook AR (1976) The elimination of urease activity in Streptococcus faecium as evidence for plasmid coded urease. J Gen Microbiol 9:49–58
Coombe JB, Tribe DE (1958) Toxicity of urea to sheep. Nature (London) 182:116–117
Coombe JB, Tribe DE (1960) The effect of urea on the utilization of low-quality roughage by the ruminant. Proc Aust Soc Anim Prod 3:83–85
Coombe JB, Tribe DE (1962) The feeding of urea supplements to sheep and cattle: the results of penned feeding and grazing experiments. J Agric Sci 59:125–141
Coombe JB, Tribe DE, Morrison JWC (1960) Some experimental observations on the toxicity of urea to sheep. Aust J Agric Res 11:247–256
Czerkawski JW, Breckenridge W (1982) Distribution and changes in urease (EC 3.5.1.5) activity in rumen simulation technique (Rusitec). Br J Nutr 47:331–348
Dass RS, Verma AK, Mehra UR (1996) Effect of feeding urea molasses liquid diet on nutrient utilization, rumen fermentation pattern and blood profile in adult male buffaloes. Buffalo J 12:1–2
Davis GK, Roberts HF (1959) Urea toxicity in cattle, Bulletin No. 611. Florida Agricultural Experiment Station, Gainesville
Dinning JS, Briggs HM, Gallup WD et al (1948) Effect of orally administered urea on the ammonia and urea concentration in the blood of cattle and sheep, with observations on blood ammonia levels associated with symptoms of alkalosis. Am J Physiol 153:41–46
Ehrenberg P, Nitsche H, Muller J (1891) Report on protein substitutions in feeding experiments at Bettlerm (translated title). Z Tierernahr Futtermittlek 1:33–40
Firkins JL, Reynolds CK (2005) Whole animal nitrogen balance in cattle. In: Pfeffer E, Hristov A (eds) Nitrogen and phosphorus nutrition of cattle and environment. CAB International, Cambridge, MA, pp 167–185
Gibbons RJ, Doetsch RN (1959) Physiological study of an obligately anaerobic ureolytic bacterium. J Bacteriol 77:417–428
Gray RS, Clark R (1964) The excretion of biuret in the urine of sheep fed biuret. Onderstepoort J Vet Res 31:91–96
Guerino F, Huntington GB, Erdman RA (1991) The net hepatic flux of metabolites and oxygen consumption in growing beef steers given postruminal casein. J Anim Sci 69:387–395
Harris LE, Mitchell HH (1941) The value of urea in the synthesis of protein in the paunch of the ruminant. 1. In maintenance. J Nutr 22:167–182, 2. In growth J Nutr 22:183–196
Hart EB, Bohstedt G, Deobald HJ et al (1939) The utilization of simple nitrogenous compounds such as urea and ammonium bicarbonate by growing calves. J Dairy Sci 22:785–798
Hume ID, Moir RJ, Somers M (1970) Synthesis of microbial protein in the rumen. I. Influence of the level of nitrogen intake. Aust J Agric Res 21:283–296
Huntington GB, Archibeque SL (2000) Practical aspects of urea and ammonia metabolism in ruminants. J Anim Sci 78:742–749
Huntington GB, Magee K, Matthews A et al (2009) Urea metabolism in beef steers fed tall fescue, orchardgrass, or gamagrass hays. J Anim Sci 87:1346–1353
Javorský P, Rybosová E, Havassy I et al (1987) Urease activity of adherent bacteria and rumen fluid bacteria. Physiol Bohemoslov 36:75–81
John A, Isaacson HR, Bryant MP (1974) Isolation and characteristics of an ureolytic strain of Selenomonas ruminantium. J Dairy Sci 57:1003–1014
Kennedy PM, Milligan LP (1980) The degradation and utilization of endogenous urea in the gastrointestinal tract of ruminants: a review. Can J Anim Sci 60:205–221
Kertz AF (2010) Review: urea feeding to dairy cattle: a historical perspective and review. Prof Anim Sci 26:257–272
Kertz AF, Davidson LE, Cords BR et al (1983) Ruminal infusion of ammonium chloride in lactating cows to determine effect of pH on ammonia trapping. J Dairy Sci 66:2597–2601
Kiran D, Mutsvangwa T (2007) Effect of barley grain processing and dietary ruminally degradable protein on urea nitrogen recycling and nitrogen metabolism in growing lambs. J Anim Sci 85:3391–3399
Kiran D, Mutsvangwa T (2010) Effects of partial ruminal defaunation on urea-nitrogen recycling, nitrogen metabolism, and microbial nitrogen supply in growing lambs fed low or high dietary crude protein concentrations. J Anim Sci 88:1034–1047
Kiran D, Mutsvangwa T (2011) Feeding sunflower oil to partially defaunate the rumen increases nitrogen retention, urea-nitrogen recycling to the gastrointestinal tract and the anabolic use of recycled urea-nitrogen in growing lambs. Br J Nutr 105:1453–1464
Krebs K (1937) Der Wirt der Amide bei der Fütterung des Rindes. Biedermanns Z Tierernähr 9:394–507
Kulkarni S, Kulkarni S (2002) Urea poisoning in a buffalo heifer. Buffalo Bull 2:27–28
Lapierre H, Lobley GE (2001) Nitrogen recycling in the ruminant: a review. J Dairy Sci 84:E223–E236
Lauková A, Koniarová I (1995) Survey of urease activity in ruminal bacteria isolated from domestic and wild ruminants. Microbios 84:7–11
Lewis D (1960) Ammonia toxicity in the ruminant. J Agric Sci 55:111–117
Ludden PA, Harmon DL, Huntington GB et al (2000) Influence of the novel urease inhibitor N-(n-butyl) thiophosphoric triamide on ruminant nitrogen metabolism: II. Ruminal nitrogen metabolism, diet digestibility, and nitrogen balance in lambs. J Anim Sci 78:188–198
Mahadevan S, Sauer F, Erfle JD (1976) Studies on bovine rumen bacterial urease. J Anim Sci 42:745–753
Marini JC, Van Amburgh ME (2003) Nitrogen metabolism and recycling in Holstein heifers. J Anim Sci 81:545–552
Marini JC, Klein JM, Sands JM et al (2004) Effect of nitrogen intake on nitrogen recycling and urea transporter abundance in lambs. J Anim Sci 82:1157–1164
McDonald IW (1958) The utilization of ammonia-nitrogen by the sheep. Proc Aust Soc Anim Prod 2:46–51
Mehra UR, Verma AK, Dass RS (1998) Effect of restricted and ad libitum feeding of urea molasses liquid diet (UMLD) on the performance of adult crossbred cattle. Asian Austral J Anim Sci 1:30–34
Mehrez AZ, Orskov ER, McDonald I (1977) Rates of rumen fermentation in relation to ammonia concentration. Br J Nutr 38:437–443
Oltjen RR (1969) Effects of feeding ruminants non-protein nitrogen as the only nitrogen source. J Anim Sci 28:673–681
Oltjen RR, Robbins JD, Davis RE (1964) Studies involving the use of glutamic acid in ruminant nutrition. J Anim Sci 23:767–770
Owens FN, Bergen WG (1983) Nitrogen metabolism of ruminant animals: historical perspective, current understanding and future implications. J Anim Sci 57:498–518
Parker DS, Lomax MA, Seal CJ et al (1995) Metabolic implications of ammonia production in the ruminant. Proc Nutr Soc 54:549–563
Patra AK, Saxena J (2009) The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev 22:204–219
Patra AK, Saxena J (2011) Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J Sci Food Agric 91:24–37
Patra AK, Yu Z (2014) Effects of vanillin, quillaja saponin, and essential oils on in vitro fermentation and protein-degrading microorganisms of the rumen. Appl Microbiol Biotechnol 98:897–905
Pisulewski PM, Okorie AIU, Buttery PJ et al (1981) Ammonia concentration and protein synthesis in the rumen. J Sci Food Agric 32:759–766
Randhawa SS, Dhaliwal PS, Gupta PP et al (1989) Studies on clinico-biochemical and pathological changes in the urea-induced acute rumen alkalosis in buffalo calves. Acta Vet Brno 58:225–243
Ribeiro SS, Vasconcelos JT, Morais MG et al (2011) Effects of ruminal infusion of a slow-release polymer-coated urea or conventional urea on apparent nutrient digestibility, in situ degradability, and rumen parameters in cattle fed low-quality hay. Anim Feed Sci Technol 164:53–61
Reid JT (1953) Urea as a protein replacement for ruminants: a review. J Dairy Sci 36:955–996
Rekib A, Sadhu DP (1986) Effect of feeding higher doses of urea on the rumen metabolism in goat. Ind Vet J 45:735–739
Rémond D, Bernard L, Savary-Auzeloux I et al (2009) Partitioning of nutrient net fluxes across the portal-drained viscera in sheep fed twice daily: effect of dietary protein degradability. Br J Nutr 102:370–381
Reynolds CK, Huntington GB (1988) Partition of portal-drained visceral net flux in beef steers. 1. Blood flow and net flux of oxygen, glucose and nitrogenous compounds across stomach and post-stomach tissues. Br J Nutr 60:539–551
Reynolds CK, Kristensen NB (2008) Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis. J Anim Sci 86(E Suppl):E293–E305
Russell JB (1998) Strategies that ruminal bacteria use to handle excess carbohydrate. J Anim Sci 76:1955–1963
Rybosová E, Javorský P, Havassy I et al (1984) Urease activity of adherent bacteria in the sheep rumen. Physiol Bohemoslov 33:411–416
Sarraseca A, Milne E, Metcalf MJ et al (1998) Urea recycling in sheep: effects of intake. Br J Nutr 79:79–88
Satter LD, Slyter LL (1974) Effect of ammonia concentration on rumen microbial protein production in vitro. Br J Nutr 32:199–208
Slyter LL, Oltgen RR, Kern DL et al (1968) Microbial species including ureolytic bacteria from the rumen of cattle fed purified diets. J Nutr 94:185–192
Smith CJ, Bryant MP (1979) Introduction to metabolic activities of intestinal bacteria. Am J Clin Nutr 32:149–157
Spears JW, Hatfield EE (1978) Nickel for ruminants i. Influence of dietary nickel on ruminal urease activity. J Anim Sci 47:1345–1350
Srinivasan P, Balasubramaniam GA, Sivaseelan S et al (2008) Accidental acute and sub acute urea poisoning in dairy cows. Indian Vet J 85:243–245
Taniguchi K, Huntington GB, Glenn BP (1995) Net nutrient flux by visceral tissues of beef steers given abomasal and ruminal infusions of casein and starch. J Anim Sci 73:236–249
Taylor-Edwards CC, Hibbard G, Kitts SE et al (2009) Effects of slow-release urea on ruminal digesta characteristics and growth performance in beef steers. J Anim Sci 87:200–208
Toppo S, Verma AK, Dass RS et al (1997) Nutrient utilization and rumen fermentation pattern in crossbred cattle fed different planes of nutrition supplemented with urea molasses mineral block. Anim Feed Sci Technol 64:101–112
Van Wyk L, Steyn PL (1975) Ureolytic bacteria in sheep rumen. J Gen Microbiol 91:225–232
Wallace RJ (1996) Ruminal microbial metabolism of peptides and amino acids. J Nutr 126:1326S–1334S
Wallace RJ, Broderick GA, Brammall ML (1987) Microbial protein and peptide metabolism in rumen fluid from faunated and ciliate-free sheep. Br J Nutr 58:87–93
Whitelaw FG, Milnde JS, Wright SA (1991) Urease (EC 3.5.1.5) inhibition in the sheep rumen and its effect on urea and nitrogen metabolism. Br J Nutr 66:209–225
Wickersham TA, Titgemeyer EC, Cochran RC et al (2008) Effect of rumen degradable intake protein supplementation on urea kinetics and microbial use of recycled urea in steers consuming low-quality forage. J Anim Sci 86:3079–3088
Wozny MA, Bryant MP, Holdeman LV et al (1977) Urease assay and urease-producing species of anaerobes in the bovine rumen and human feces. Appl Microbiol 33:1097–1104
Zuntz N (1891) Observations on the digestion and nutritive value of cellulose (translated title). Pflugers Archiv – Eur J Physiol 49:477–483
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Patra, A.K. (2015). Urea/Ammonia Metabolism in the Rumen and Toxicity in Ruminants. In: Puniya, A., Singh, R., Kamra, D. (eds) Rumen Microbiology: From Evolution to Revolution. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2401-3_22
Download citation
DOI: https://doi.org/10.1007/978-81-322-2401-3_22
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2400-6
Online ISBN: 978-81-322-2401-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)