Abstract
Present-day cultivars of crop species have become susceptible to a range of biotic and abiotic stresses because of their narrow genetic base. The genes imparting resistance to these stresses are no more available within the cultivated species but are present in many wild and weedy relatives. To transfer desirable genes to the cultivars, the production of wide hybrids is an important pre-breeding requirement. One of the major limitations of wide hybridization is the presence of strong crossability barriers that operate before as well as after fertilization. During the last several decades, many biotechnological methods have become available, and their integration into the traditional methods of wide hybridization greatly increases the efficacy and reduces the time and efforts needed. Some of these include pollen storage, application of growth substances, stump pollination, placental pollination, in vitro fertilization and embryo rescue in the form of ovary, ovule and embryo culture. This review highlights the efficacy of these techniques in realizing wide hybrids and emphasizes the use of combination of techniques to make these approaches more successful. The interest on production and use of sexual wide hybrids has greatly reduced after the development of the techniques of somatic hybridization and genetic transformation. Although they provide powerful technologies to achieve some specific objectives of the breeding program, they cannot replace sexual hybridization for gene transfer. It is important for the plant breeders to refine and exploit fully some of the underexploited techniques described in this review in raising wide sexual hybrids.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brassica hybrids
- Crossability barriers
- Embryo rescue
- In vitro fertilization
- Ovary and ovule cultures
- Placental pollination
- Pollen storage
- Wide hybrids
17.1 Introduction
The transfer of desirable genes to crop species from other accessions or species through sexual hybridization has been one of the most effective crop improvement programs (Goodman et al. 1987). Until recently, the major objectives of crop improvement programs have been on increasing the yield and improving the quality and genetic uniformity of the cultivars. Breeders have been able to achieve remarkable success on these lines in almost all crop species. Intensive breeding and selection for economic traits over the years have greatly reduced genetic variability in crop species. Because of their narrow genetic base, present-day cultivars have become susceptible to a range of biotic and abiotic stresses. Although most of the cultivars have the genetic potential for high yield under optimal agronomic conditions, their increased susceptibility to the stresses brings down the yield substantially (Boyer 1982). Broadening the genetic base of cultivated species, therefore, has become an important pre-breeding requirement to safeguard crop yield in the coming years.
In recent years, the objectives of the breeding program, apart from improvement in yield and quality, have become more diverse. Some of these additional breeding objectives are:
-
1.
To breed varieties tolerant/resistant to biotic and abiotic stresses.
-
2.
To breed varieties that have lower requirement for environmentally unfriendly chemicals particularly pesticides and herbicides. Excessive use of agrochemicals has been a major environmental problem of modern agriculture. The trend is to grow crops ‘organically’ without the use of environmentally unfriendly agrochemicals.
-
3.
To breed varieties that can be grown on marginal lands. A large area of agricultural land is being degraded, particularly in developing countries, due to water logging, soil erosion, salinity, alkalinity and contamination with industrial chemicals. The recovery of such vast areas of land is not feasible because of the lack of suitable technologies and/or the high cost involved. Breeding crops that can be grown on marginal lands has become an alternate strategy.
Because of the constant erosion of genetic diversity in our crop species, genes imparting the required traits are no longer available within the cultivated species. A large number of wild and weedy relatives of crop species, however, form a good repository of such desirable genes (Harlan 1976). The plant breeders, therefore, often have to extend the breeding program across the species limits to tap genes of the wild and weedy species (wide hybridization). A good number of examples of successful gene transfer through wide hybridization are already available (Hawkes 1977; Hadley and Openshaw 1980; Stalker 1980; Goodman et al. 1987; Hermsen 1992; Kalloo 1992; Chopra et al. 1996; Sareen et al. 1992; Wang et al. 2005; Prakash et al. 2011). The primary requirement for transferring genes from wild species to the cultivars is the production of wide hybrids (hybrids from distantly related species).
In addition to the transfer of desirable genes from wild species to the cultivars, wide hybridization is also one of the standard approaches to develop new cytoplasmic male sterile lines (through cytoplasmic substitution), which are important for crop species (Labana et al. 1992; Shivanna and Sawhney 1997; Shivanna 2003; Prakash et al. 2011). Another application of wide hybrids is to develop new alloploid crops such as triticale (Triticum × Secale) and Raphanobrassica (Raphanus × Brassica) (Sareen et al. 1992). Wide hybrids are also useful to study a range of problems of traditional and molecular cytogenetics, particularly for elucidating homology (Prakash et al. 2011).
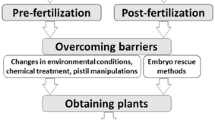
The presence of strong crossability barriers between the species is the major constraint in any wide hybridization program. Early plant breeders used to resort to mass pollination, generally in thousands, to realize a few wide hybrids. Now, a number of biotechnological approaches have become available to overcome crossability barriers. Integration of these techniques with conventional breeding program greatly improves the efficiency of wide hybridization and reduces markedly the time and cost of breeding. This review briefly discusses some of the important biotechnological approaches that have been used to produce wide hybrids through sexual pathway and highlights their importance in the coming decades.
The following are a few general guidelines to be followed to achieve success in any wide hybridization program:
-
1.
It is desirable to try different accessions of both the parents in crossing experiments since genetic variability within the species may affect the intensity of the barriers. Some of the accessions/genotypes may turn out to be more compatible when compared to others.
-
2.
It is also necessary to check the viability of the pollen sample used for crosses and the receptivity of the stigma at the time of pollination (Shivanna and Rangaswamy 1992). If possible, it is better to use pollen grains collected from freshly dehisced anthers.
-
3.
Many of these crosses show unilateral incompatibility (de Nettancourt 2001); it is, therefore, better to make crosses in both the directions.
-
4.
Recent studies have shown that knowledge on the phylogenetic relationships of the cultivar with its wild relatives would help in selecting the wild parent which is likely to be more successful (Kubota et al. 2011). For example, in Hydrangea, interspecific crosses were found to be successful up to an average genetic distance of 0.01065. The crosses are likely to fail when the average genetic distance is 0.01385 and higher (Mendoza et al. 2013a, b). Similarly, in wheat-rye hybrids, significant differences in the frequency of normal embryo development were found when different lines of rye were used in rye × wheat crosses (Taira et al. 2011). It would therefore be helpful to check the available information on phylogenetic relationship of the selected species and use closely related parents for making crosses.
17.2 Crossability Barriers
Crossability barriers operate at different levels before and after fertilization (Sastri 1985; Shivanna 1997; de Nettancourt 2001). One of the common barriers particularly in distantly related species is the physical barrier imposed by geographical and/or temporal isolation of the parent species. The male and female parents do not grow at the same place and/or their flowering may not be synchronous, thus preventing effective pollination. Pre-fertilization barriers inhibit pollen germination or pollen tube entry into the stigma or subsequent growth of the pollen tubes before reaching the ovule. More often these barriers operate in a cumulative way; the proportion of pollen grains that complete germination or post-germination growth of pollen tubes at different levels may get reduced at each step, resulting in the failure of fertilization. In multiovulate systems, fertilization may occur in a few ovules which may not be sufficient to activate the development of the fruit and thus no seeds are realized.
Post-fertilization barriers operate at various levels: abortion of hybrid embryos at preglobular/globular/later stages, failure of hybrid seeds to germinate or the seedling to grow up to the flowering, hybrid sterility and lack of recombination. Post-fertilization barriers also operate in a cumulative way with the result that no usable hybrid is realized. A number of biotechnological approaches are now available to overcome such barriers. Identification of the barrier(s) would help in the selection of effective method(s) to circumvent the barrier(s).
17.3 Methods to Overcome Physical Barriers
17.3.1 Pollen Storage
Pollen storage is one of the simple and effective methods to overcome physical barriers imposed by temporal and spatial isolation of the parent species. A number of techniques are now available to store pollen grains for extended periods in viable condition. When once a suitable technique is standardized for a given species, pollen grains of the male parent can be stored and used routinely for pollination when the female parent flowers. Apart from its application to overcome physical barriers, successful pollen storage eliminates the need to grow pollen parent continuously in the breeding program. Another important application of pollen storage relevant to the breeding program is that it provides a convenient and simple means of pollen exchange amongst breeders within and between countries. Many of the horticultural societies such as the American Rhododendron Society maintain their own pollen banks which are accessible to their members (Mayer 1983). Pollen grains are generally free from pathogens even when the parent plant is infected. Except for some viral diseases, there are no authentic reports of systemic transmission of fungal and bacterial diseases through pollen (Mink 1993). Therefore, the quarantine restrictions for the exchange of germplasm through pollen are much less when compared to seeds and vegetative parts of the plant. The utility of ‘pollen banks’, which would ensure the availability of pollen of the desired species/variety at any time of the year and at any place, has been emphasized since long. Pollen banks would greatly facilitate the breeding program, particularly of tree species which have to complete their juvenile phase, often lasting for several years, before flowering. Extensive literature, available on pollen storage, has been reviewed regularly (Johri and Vasil 1961; King 1965; Stanley and Linskens 1974; Shivanna and Johri 1985; Towill 1991; Hanna and Towill 1995; Barnabas and Kovacs 1997; Ganeshan and Rajasekaran 2000; Shivanna 2003). Many of these reviews list the pollen species stored for various periods under different storage conditions. The following is a brief description of the important methods used to store pollen grains of various species:
-
Storage of pollen under low temperature (+4 to −20 °C) and humidity (<10 % RH) conditions is one of the simple and commonly used methods particularly for horticultural species. Pollen grains are kept in small unsealed vials and stored in a desiccator or a suitable airtight vial containing an appropriate dehydrating agent such as dry silica to maintain low RH (Shivanna and Johri 1985; Shivanna and Rangaswamy 1992). The sealed desiccators are then kept in a refrigerator or a deep freeze. This method is very convenient and effective for short-term storage (for a few weeks/months) and has been used extensively by amateur horticulturists.
-
Storage under subfreezing temperatures (ca −20 °C) is effective for storing pollen grains of several species for more than a year. Frozen pollen of Rhododendron in home freezer has been reported to be viable for 3 years (Mayer 1983). Pollen grains of cereals in general cannot withstand desiccation and need to be stored under high RH in the refrigerator. Even under these conditions, viability of cereal pollen lasts only for a few days (Shivanna and Heslop-Harrison 1981).
-
Storage of freeze-dried/vacuum-dried pollen is an effective method for long-term storage. Freeze-drying involves rapid freezing of pollen (−60 to −80 °C) and gradual removal of water under sublimation. In vacuum-drying, the pollen is subjected to simultaneous cooling and vacuum-drying. Freeze-dried and vacuum-dried pollen grains generally do not show any differences in their responses to storage. The freeze-dried pollen is usually stored at sub-zero temperatures. For effective use of this method, optimum pollen water content, duration of drying and subsequent rehydration have to be optimized (Barnabas and Kovacs 1997). Freeze-drying method has been effective for long-term storage of pollen grains of a number of species (King 1965; Towill 1991). However, in recent years, this method has not been very popular probably because of the success achieved by using a simpler technique of cryopreservation.
-
Cryopreservation of pollen is another effective method for long-term pollen storage and has become more popular than freeze-drying method in recent years. In this method, pollen grains are dried to bring their water content below a threshold level and stored in liquid nitrogen (Towill 1991; Barnabas and Kovacs 1997). Initial attempts to cryopreserve pollen grains of cereals were not successful largely because of their susceptibility to desiccation (which is critical for cryopreservation). Unlike the earlier methods used for drying pollen grains over a desiccant, Barnabas and her associates used a ‘pollen drier’ in which the air of 20 °C and 20–40 % humidity is blown through pollen, facilitating a rapid but gentle and uniform drying (Barnabas and Kovacs 1997). Through this drying method, it was possible to successfully cryopreserve the pollen grains of many cereals for several years.
17.4 Methods to Overcome Pre-fertilization Barriers
A number of techniques have been developed over the years to overcome pre-fertilization barriers that operate after pollination but before fertilization. Some of these methods along with a few examples are presented in Table 17.1. Additional examples are found in Van Tuyl and De Jeu (2003) and Shivanna (2003). According to Bates and Deyoe (1973), the inhibition reaction of pollen in the pistil is analogous to immunochemical reaction found in animals. On the basis of this hypothesis, the flowers were treated with some immunosuppressors such as E-amino caproic acid, salicylic acid or acriflavin (using similar methods used for treatment with growth substances), and a few wide hybrids were realized in some cereals and legumes (Van Tuyl and De Jeu 2003; Shivanna 2003). However, so far, there are no evidences to indicate the involvement of immunochemical reactions in crossability barriers, and there have been no additional reports on the success of immunosuppressors in realizing wide hybrids.
17.4.1 In Vitro Pollination of Cultured Ovules
In this method, instead of carrying out pollination on the stigma, pollen grains are deposited directly on the ovules. In one of these methods, a group of ovules are excised from the ovary and cultured on a nutrient medium (Kanta et al. 1962; Kameya and Hinata 1970); the ovule mass is dusted with pollen grains. In another method termed placental pollination, the entire mass of ovules intact on the placenta(e) together with a short length of pedicel is excised and cultured by inserting only the pedicel in the medium (Rangaswamy and Shivanna 1967). Pollen grains are dusted on cultured ovule mass (Shivanna and Rangaswamy 1992). As this technique eliminates pollen-pistil interaction altogether and brings pollen grains in direct contact with the ovules, it is likely to be more effective than other methods to overcome pre-fertilization barriers. In successful pollinations, pollen grains germinated on the ovule mass; pollen tubes entered the ovules and effected fertilization. The fertilized ovules developed into seeds. Ovule pollination has been used successfully to produce interspecific as well as intergeneric hybrids particularly by Zenkteler and his associates (Zenkteler 1980, 1990; Zenkteler and Bagniewska-Zadworna 2001). Some of the successful crosses include Melandrium album × M. rubrum, M. album × Silene schafta (Zenkteler 1980, 1990) and several interspecific crosses of Nicotiana (Reed and Collins 1978; DeVerna et al. 1987; Zenkteler 1990). Attempts were also made to obtain interspecific/intergeneric hybrids in Brassicaceae (Kameya and Hinata 1970; Zenkteler 1990; Zenkteler et al. 1987). Hybrids were realized in a few combinations, but in some others, although there was normal fertilization, hybrid embryos degenerated. Isolation and culture of embryos on a suitable nutrient medium would probably enable the production of hybrids in such crosses also. In an interesting treatment, placental cultures of some flowering plants were pollinated with pollen grains of a few gymnosperms; pollen grains of Pinus and Ephedra could germinate on placental cultures of several flowering plants (Zenkteler and Bagniewska-Zadworna 2001). Although some pollen tubes were occasionally seen entering the ovules, no hybrid embryos were realized.
17.4.2 In Vitro Fertilization
Fertilization in flowering plants takes place deep inside the ovule, and this is one of the limitations to conduct experimental studies on fertilization. Following the dramatic progress of protoplast technology of somatic cells, a number of investigators started work on isolation of male and female gametes of flowering plants in the 1980s. Success was soon achieved in isolation of protoplasts of embryo sacs and sperm cells of a number of species and to keep them in viable condition for considerable length of time (Cass 1997; Mathys-Rochon et al. 1997). In three-celled pollen, sperm cells were isolated by incubating pollen grains in hypotonic medium that results in bursting of pollen grains or mechanically rupturing the pollen grains by gentle grinding in an isolation medium. The released sperm cells were purified and washed suitably. In two-celled pollen, a semi-vitro method (Shivanna and Rangaswamy 1992) was used to allow pollen tubes to grow partly inside the style and then emerge into a nutrient medium. This was followed by the treatment of pollen tubes with cell wall-degrading enzymes or subjecting pollen tubes to osmotic shock to the release of sperm cells from the tip of pollen tubes (Shivanna et al. 1987). Subsequently it was possible to isolate the components of the embryo sac (egg, synergids, and central cell). By 1990s, all the basic requirements needed to try in vitro fertilization in flowering plants were available.
Kranz and his associates were the first to achieve in vitro fertilization in maize by bringing isolated egg and sperm cells together under the microscope in microdroplets of the fusion medium (Kranz and Lorz 1993; Kranz 1997; Okamoto and Kranz 2005). In vitro formed zygotes were grown successfully by using nurse culture method (by culturing zygotes on a semipermeable membrane placed on fast-growing non-morphogenetic cell suspension cultures derived from maize embryos or microspores) into plantlets and eventually into adult fertile plants. Subsequently the sperm cell was also fused with the central cell; the fusion product did not give rise to the embryo but gave rise to an unorganized tissue comparable to the endosperm in vivo. Subsequently several modifications in the protocols to improve the efficacy of fusion and embryo development have been reported (Kranz 1997; Wang et al. 2006; Kranz et al. 2008).
Apart from its application in tackling a range of problems fundamental to fertilization (see Okamoto and Kranz 2005; Wang et al. 2006), one of the most obvious practical applications of in vitro fertilization is in realizing wide hybrids. Although the success has so far been confined in achieving fertilization and embryo development between gametes of compatible species, studies of Kranz and his associates (Kranz 1997, 2008; Scholten and Kranz 2001) have shown that there is no technical difficulty in achieving in vitro fertilization between isolated egg and sperm cells in interspecific and intergeneric combinations. However, the development of zygotes is restricted to crosses between closely related species (Kranz and Dresselhaus 1996). Hybrid zygotes between maize (egg donor) and sperm cell of many other members of Poaceae gave rise to multicellular structures, while those resulting from the egg of maize and sperm of a distant species, Brassica, failed to divide. Further studies are needed to extend this technology to other species and to realize useful interspecific and intergeneric hybrids from in vitro fused zygotes.
17.5 Methods to Overcome Post-fertilization Barriers
Post-fertilization barriers operate after fertilization. Depending on the extent of reproductive isolation between the parent species, the embryo abortion may initiate at a very early stage of development or after the growth of embryo to different stages.
17.5.1 Embryo Rescue
Embryo rescue has become the most effective and routinely used technique to overcome post-fertilization barriers. When the abortion occurs at a very early stage, it is difficult to excise the embryo and also to culture it successfully as its nutrient requirements are more complex and precise. Embryo excision is a problem even at later stages when the developing seeds are very small. In such instances, it is more convenient to culture the whole ovule or even the ovary. Ovule and ovary culture facilitates embryo growth in situ without exposure to in vitro disturbances. In crosses where the embryo aborts at later stages, embryo culture is the most ideal. There are a large number of successful hybrids realized through embryo rescue (see also Chap. 18 of this volume). For details of the technique and comprehensive examples, the reader may refer to Maheshwari and Rangaswamy (1965), Raghavan (1977, 1986, 1999), Rangan (1982), Williams et al. (1987), Sharma et al. (1996) and Van Tuyl and De Jeu (2003). A modified technique of embryo rescue termed ‘sequential culture’ has been reported to be more effective than either ovary or ovule culture alone in realizing many wide hybrids (Nanda Kumar et al. 1988; Agnihotri 1993; Shivanna 2000). In sequential culture, ovaries are cultured 4–8 days after pollination; cultured ovaries are taken out 7–10 days after culture, dissected under aseptic conditions, and enlarged ovules (young seeds) are then re-cultured on a fresh medium. In successful crosses, cultured ovules grow further and germinate in vitro. In some crosses, it was necessary to dissect the embryo from cultured ovules and re-culture the embryo. Some examples of wide hybrids realized through ovary, ovule, embryo and sequential cultures are presented in Table 17.2. In a few crosses of legumes, hybrid embryos have been transplanted into the developing endosperm of compatible seeds (after removing compatible embryo), and such transplanted embryo-endosperm complex has been successfully cultured (Williams and Latour 1980).
17.5.2 Wide Hybrids in Brassica
Brassica is an important oilseed crop in several countries. All the three major cultivated species, B. rapa, B. juncea and B. napus, are susceptible to a number of diseases, pests and abiotic stresses and result in low productivity. The genes imparting resistance to these stresses are not available in the cultivated species, but many of their wild relatives possess such genes (see Prakash et al. 2011). A large number of wide hybrids have been produced in crop brassicas with many of their wild relatives through embryo rescue, especially from the University of Delhi in collaboration with the Indian Agricultural Research Institute, New Delhi (Shivanna 1995, 2003; Prakash et al. 2011). The details of the crossability barriers between all the cultivated species and 12–20 wild relatives have been documented by Singh et al. (2007). Of the 100 cross combinations tested, 73 crosses showed pre-fertilization barriers and 27 crosses showed post-fertilization barriers. All the wide hybrids produced by this group through embryo rescue are listed in Table 17.3.
17.6 Bridge Cross Hybrids
As pointed out earlier, the strength of the crossability barriers of the cultivar may vary with different wild relatives. The barriers may not be so strong with some of the wild relatives, but such species may not have desirable traits; those that have desirable traits may show strong barriers with the cultivar. Another simple method, referred to as bridge cross method, has been used by the breeders to circumvent such barriers. In this method, the hybrid is produced between the cultivar and one of the wild species (which does not show strong barriers with the cultivar), and this hybrid or its amphidiploid is used as the bridge species to raise hybrids with another wild species (which shows strong barriers with the cultivar but has desirable trait). This approach has been very effective in a number of crop plants – wheat, tobacco, potato, lettuce, sugarcane and Brassica (see Shivanna 2003). In some combinations, it was possible to realize bridge cross hybrids through field pollinations without resorting to application of any special technique. Bridge cross technique is simple and very useful particularly to transfer the cytoplasm from one species to the other (Shivanna 1995, 2000), although the transfer of nuclear genes through this method involves more elaborate breeding program (Hadley and Openshaw 1980). The bridge cross hybrids raised in Brassica by Delhi group are listed in Table 17.4.
17.7 The Development of New Cytoplasmic Male Sterile (CMS) Lines in Brassica
Male sterile lines are very important to exploit hybrid vigor in crop plants. By using male sterile line as the female parent, intra-line pollinations are prevented completely in hybrid seed production plots. Male sterile lines developed through different approaches (genic male sterile lines, cytoplasmic male sterile lines, male sterility induced through application of chemicals and also through recombinant DNA technology) are available. Of these, cytoplasmic male sterile (CMS) lines have several advantages over others in producing hybrid seeds (see Shivanna and Sawhney 1997; Shivanna 2003). One of the standard approaches to produce CMS lines is through the development of alloplasmic lines (containing the cytoplasm of one species and the nuclear genes of another species) as many of them result in cytoplasmic male sterility. This involves production of wide hybrids (wild species as the female and cultivar as the male parent). As the cytoplasm is inherited only through the egg in a majority of species, the hybrids possess the cytoplasm of the wild species and the nuclear genome of both the parents. The hybrid or its amphiploid is repeatedly backcrossed with the cultivar (as male parent) to eliminate the nuclear genome of the wild species. Although many CMS lines were available in crop Brassica (Banga 1992; Shivanna 2000; Prakash et al. 2011), they show many limitations such as chlorophyll deficiency, thermosensitivity of male sterility and presence of female sterility. Also, many of them do not have suitable restorers needed for hybrid seed production. There has been a need to produce more CMS and restorer lines. The Delhi University group has been able to produce several new CMS lines in the background of the cytoplasm of different wild species. These CMS lines are listed in Table 17.5.
Restorer lines have been developed subsequently for some of these alloplasmic lines: B. juncea carrying Diplotaxis catholica cytoplasm (Pathania et al. 2003); B. rapa (Deol et al. 2003), B. juncea (Banga et al. 2003) and B. napus (Janeja et al. 2003) carrying Enarthrocarpus lyratus cytoplasm; and B. juncea carrying D. erucoides cytoplasm (Bhat et al. 2005).
17.8 Multiplication of Hybrids
The number of hybrids realized in wide crosses even after using biotechnological methods is rather limited. A large number of hybrids are needed for morphological and cytological studies, induction of amphiploidy to restore fertility and to raise backcross progeny. Hybrids can easily be multiplied through the use of in vitro culture technique (Nanda Kumar and Shivanna 1991; Agnihotri et al. 1990a, b; Shivanna 2000). Culture of shoot tips or single node segments on a medium containing one of the cytokinins is effective in inducing multiple shoots. The shoots are isolated and cultured on an auxin-containing medium to induce rooting. The plantlets are then hardened and transferred to the soil. Hybrids can also be multiplied through the induction of callus from hybrid embryos or hypocotyl segments and subsequent regeneration of plantlets through shoot and root regeneration pathway or somatic embryogenesis pathway (Nanda Kumar and Shivanna 1991; Agnihotri et al. 1990a, b).
17.9 Concluding Remarks
The production of wide hybrids is an important pre-breeding step in transferring genes across species limits. Early breeders, in spite of low rate of success, were able to realize a number of wide hybrids in many crop species and to transfer important traits from the wild species to the cultivars. Integration of some of the tools of biotechnology to the breeding program has made the production of wide hybrids more effective, and a large number of wide hybrids have been produced through this approach. Pollen storage is probably the simplest and effective technique to overcome nonsynchronous flowering and geographical isolation of the parent species. Although several pollen banks of many horticultural species have come up to supply pollen for the breeding programs, pollen banks for crop species are yet to be established as a routine facility at the international level. Establishment of effective pollen banks, similar to seed banks, at global level would greatly facilitate the breeding programs of crop species.
There is a scope for further refinement of many of the available techniques to overcome pre-fertilization barriers. For example, by carrying out stump pollination or stylar grafting on cultured pistils (instead of flowers retained on plants), the operations can be performed and controlled more efficiently. Cultured pistils would provide a more convenient system to conduct additional treatments such as irradiation or high-temperature treatment to the pistil, which have not yet been tried seriously to raise wide hybrids. Cultured pistils are also ideal to perform intra-stylar pollinations (in hollow-styled systems) and mixed/mentor pollinations. These treatments can be combined with manipulation of the medium and other conditions to improve the growth of cultured pistils. Also, the full potentials of other in vitro techniques particularly in vitro pollination of ovules and in vitro fertilization are yet to be fully exploited.
More importantly, a combination of techniques integrating the methods used to overcome pre- and post-fertilization barriers is likely to give better results. In the absence of such integration, even when a particular method is effective in circumventing pre-fertilization barriers, it may not yield hybrids because of the operation of post-fertilization barriers. For example, in crosses between B. rapa and B. juncea, showing pre-fertilization barriers, bud pollination and stump pollination increased the frequency of ovules that were fertilized. However, hybrids could be recovered only when bud pollination and stump pollination were combined with ovule culture (Bhat and Sarla 2004). Combination of placental pollination with embryo rescue would be particularly effective in providing suitable conditions for hybrid embryos to continue their growth. Thus, plant breeders in the coming decades should try combination of techniques rather than a single technique to raise wide hybrids.
Although a large number of wide hybrids have been realized in a number of crop species, introgression of desirable traits from wild species to the cultivars is limited due to lack of intergenomic chromosome homoeology. This is one of the major problems to achieve alien gene transfer even after getting wide hybrids. Interspecific hybridization and polyploidization in ornamental plants are more simple and straightforward, as it aims to produce novel cultivars combining the traits of both the parents rather than to transfer specific traits from one parent to the other (Kato and Mii 2012). Even the fertility of the hybrid may not be a serious problem in ornamental plants as many of them can be propagated through vegetative multiplication or through micropropagation.
The interest on wide hybridization through sexual pathway has greatly reduced after the development of the techniques of somatic hybridization and genetic transformation. There are not many reports on the production of wide sexual hybrids in recent years. Although both somatic hybridization and genetic transformation have great potential in specific areas of crop improvement program, they cannot replace sexual hybrids; they can only supplement them. Thus, there is a continuous need for the production of sexual wide hybrids in the coming years. It is necessary for the breeders to re-establish interest in sexual hybrids, particularly wide hybrids for transferring desirable traits from wild species/accessions to the cultivars.
References
Agnihotri A (1993) Hybrid embryo rescue. In: Lindsey K (ed) Plant tissue culture manual: Fundamentals and applications. Kluwer Academic Publishers, Dordrecht, pp E4:1–8
Agnihotri A, Gupta V, Lakshmikumaran M, Shivanna KR et al (1990a) Production of Eruca-Brassica hybrid by embryo rescue. Plant Breed 104:281–289
Agnihotri A, Shivanna KR, Raina SN et al (1990b) Production of Brassica napus x Raphanobrassica hybrids by embryo rescue: an attempt to induce shattering resistance in B. napus. Plant Breed 105:292–299
Alonso LC, Kimber G (1980) A haploid between Agropyron junceum and Triticum aestivum. Cereal Res Commun 8:355–358
Banga SS (1992) Heterosis and its utilization. In: Labana KS, Banga SS, Banga SK (eds) Breeding oilseed Brassicas. Narosa Publishing House, New Delhi, pp 21–43
Banga SS, Deol JS, Banga SK (2003) Alloplasmic male sterile Brassica juncea with Enarthrocarpus lyratus cytoplasm and the introgression of gene(s) for fertility restoration from cytoplasmic donor species. Theor Appl Genet 106:1390–1395
Barnabas B, Kovacs M (1997) Storage of pollen. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 293–314
Bates LS, Deyoe CW (1973) Wide hybridization and cereal improvement. Econ Bot 27:401–512
Batra VS (1991) Hybridization between the cultivated species of Brassicas and their two wild relatives Diplotaxis and Erucastrum. PhD thesis. University of Delhi
Batra V, Shivanna KR, Prakash S (1989) Hybrids of wild species Erucastrum gallicum and crop Brassicas. In: Proceedings of the 6th International Congress. SABRAO. Tsukuba, pp 443–446
Batra V, Prakash S, Shivanna KR (1990) Intergeneric hybridization between Diplotaxis siifolia, a wild species, and crop brassicas. Theor Appl Genet 80:537–541
Bhat S, Sarla N (2004) Identification and overcoming barriers between Brassica rapa L. em. Metzg. and B. nigra (L.) Koch crosses for the resynthesis of B. juncea (L.) Czern. Genet Resour Crop Evol 51:455–469
Bhat SR, Prakash S, Kirti PB, Dineshkumar V, Chopra VL (2005) A unique introgression from Moricandia arvensis confers male fertility to two different cytoplasmic male sterile lines of Brassica juncea. Plant Breed 124:117–120
Boyer JS (1982) Plant productivity and environment. Science 218:443–466
Bridges MP, Langhans R, Graig R (1989) Biotechnological breeding techniques for Alstroemeria. Herbertia 45:93–96
Cass DD (1997) Isolation and manipulation of sperm cells. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 352–362
Chen NC, Parrot JF, Jacobs T et al (1978) Intergeneric hybridization of food grain legumes by unconventional methods of breeding. In: International Mungbean symposium AVRDC. Taiwan, pp 247–252
Chopra VL, Kirti PB, Prakash S (1996) Assessing and exploiting genes of breeding value of distant relatives of crop brassicas. Genetica 97:305–312
Chrungu B, Mohanty A, Verma N, Shivanna KR (1999) Production and characterization of interspecific hybrids between Brassica murorum and crop brassicas. Theor Appl Genet 98:608–613
Cisneros A, Tel-Zur N (2010) Embryo rescue and plant regeneration following interspecific crosses in the genus Hylocereus (Cactaceae). Euphytica 174:73–82
Das S (1993) Intergeneric hybridization between Erucastrum varium, a wild species and cultivated Brassicas. MPhil thesis. University of Delhi
De Jeu MJ, Sasbrink H, Garriga Caldere F, Piker J (1992) Sexual reproduction biology of Alstroemeria. Acta Horticult 325:571–575
de Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants. Springer, Berlin
Deol JS, Shivanna KR, Prakash S, Banga SS (2003) Enarthrocarpus lyratus–based cytoplasmic male sterility and fertility restorer system in Brassica rapa. Plant Breed 122:438–440
DeVerna JW, Myers JR, Collins GB (1987) Bypassing prefertilization barriers to hybridization in Nicotiana using in vitro pollination and fertilization. Theor Appl Genet 73:665–671
Ganeshan S, Rajasekaran PE (2000) Current status of pollen cryopreservation research: relevance to tropical horticulture. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm: current research progress and application. JIRCAS/IPGRI, Taukuba/Rome, pp 360–365
Goodman RM, Hauptli H, Crossway A, Knauf VC (1987) Gene transfer in crop improvement. Science 236:48–54
Gundimeda HR, Prakash S, Shivanna KR (1992) Intergeneric hybrids between Enarthrocarpus lyratus, a wild species and crop brassicas. Theor Appl Genet 83:655–662
Hadley HH, Openshaw SJ (1980) Interspecific and intergeneric hybridization. In: Fehr RW, Hadley HH (eds) Hybridization in crop plants. American Society of Agronomy: Crop Science Society of America, Madison, pp 133–159
Hanna WW, Towill LE (1995) Long-term pollen storage. Plant Breed Rev 13:179–207
Harlan JR (1976) Genetic resources in wild relatives of crops. Crop Sci 16:329–333
Hawkes JG (1977) The importance of wild germplasm in plant breeding. Euphytica 26:615–621
Hermsen JG (1992) Introductory considerations on distant hybridization. In: Kalloo G, Chowdhury JB (eds) Distant hybridization in crop plants. Springer, Berlin, pp 1–14
Herrick JF, Murray BG, Hemmett KRW (1993) Barriers preventing hybridization of Lathyrus odoratus and L. chrysanthus. N Z J Hort Sci 21:115–121
Heslop-Harrison Y, Reger BJ, Heslop-Harrison J (1985) Wide hybridization: pollination of Zea mays L. by Sorghum bicolor (L.) Moench. Theor Appl Genet 70:252–258
Ikeda N, Niimi Y, Han D (2003) Production of seedling from ovules excised at the zygote stage in Lilium spp. Plant Cell Tissue Organ Cult 73:159–166
Ishizaka H, Uematsu J (1992) Production of interspecific hybrid in Cyclamen persicum Mill. and C. hederifolium Aiton. by ovule culture. Jpn J Breed 42:353–366
Iwai S, Kishi C, Nakata K, Kawashima N (1986) Production of Nicotiana tabacum x Nicotiana acuminata hybrid by ovule culture. Plant Cell Rep 5:403–404
Janeja HS, Banga SK, Bhasker PB, Banga SS (2003) Alloplasmic male sterile Brassica napus with Enarthrocarpus lyratus cytoplasm: introgression and molecular mapping of an E. lyratus chromosome segment carrying a fertility restoring gene. Genome 46:792–797
Janson J, Reinders MC, Van Tuyl JM, Keijzer CJ (1993) Pollen tube growth in Lilium longiflorum following different pollination techniques and flower manipulation. Acta Bot Neerl 4:461–472
Johri BM, Vasil IK (1961) Physiology of pollen. Bot Rev 27:325–381
Kalloo G (1992) Utilization of wild species. In: Kallo G, Chowdhuy JB (eds) Distant hybridization in crop plants. Springer, Berlin, pp 149–167
Kameya T, Hinata K (1970) Test tube fertilization of excised ovules in Brassica. Japan J Breed 20:253–260
Kanoh K, Hayashi M, Serizawa Y, Konishi T (1988) Production of interspecific hybrids between Lilium longiflorum and L. elegans by ovary slice culture. Japan J Breed 38:278–282
Kanta K, Rangaswamy NS, Maheshwari P (1962) Test tube fertilization in a flowering plant. Nature 194:1214–1217
Kato J, Mii M (2012) Production of interspecific hybrids in ornamental plants. Methods Mol Biol 877:233–245
King JR (1965) The storage of pollen particularly by the freeze drying method. Bull Torrey Bot Club 92:270–287
Knox RB, Willing RR, Ashford E (1972) Pollen wall proteins: role as recognition substances in interspecific incompatibility in poplars. Nature 237:381–383
Kranz E (1997) In vitro fertilization with single isolated gametes. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 377–391
Kranz E, Dresselhaus T (1996) In vitro fertilization with isolated higher plant gametes. Trends Plant Sci 1:82–89
Kranz E, Lorz H (1993) In vitro fertilization with single isolated gametes results in zygotic embryogenesis. Plant Cell 5:739–746
Kranz E, Hoshino Y, Okamoto T (2008) In vitro fertilization with isolated higher plant gametes. Methods Mol Biol 427:51–69
Kubota S, Konno I, Kanno A (2011) Molecular phylogeny of the genus Asparagus (Asparagaceae) explains interspecific crossability between the garden asparagus (A. officinalis) and other Asparagus species. Theor Appl Genet 124:345–354
Kuboyama U, Chung CS, Takeda G (1994) The diversity of interspecific pollen-pistil incongruity in Nicotiana. Sex Plant Reprod 7:250–258
Labana KS, Banga SS, Banga SK (eds) (1992) Breeding oilseed Brassicas. Narosa Publishing House, New Delhi
Maheshwari P, Kanta K (1961) Intraovarian pollination in Eschscholzia californica Cham., Argemone mexicana L. and Argemone ochroleuca Sweet. Nature 191:304
Maheshwari P, Rangaswamy NS (1965) Embryology in relation to physiology and genetics. In: Preston RD (ed) Advances in botanical research, vol 2. Academic, New York, pp 219–321
Malik M, Vyas P, Rangaswamy NS, Shivanna KR (1999) Development of two new cytoplasmic male sterile lines in Brassica juncea through wide hybridization. Plant Breed 118:75–78
Mathys-Rochon S, Mol R, Faure C et al (1997) Isolation and micromanipulation of the embryo sac and egg cell in maize. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 363–376
Mayer M (1983) A pollen bank update. J Am Rhododendron Soc 37(4):635–644
McCoy TJ, Echt CS (1993) Potential of trispecies bridge crosses and random amplified polymorphic DNA markers for introgression of Medicago daghestanica and M. pironae germplasm into alfalfa (M. sativa). Genome 36:594–601
Mehetre SS, Aher AK (2004) Embryo rescue: a tool to overcome incompatible interspecific hybridization in Gossypium Linn. – a review. Indian J Biotech 3:29–36
Mendoza CG, Wanke S, Salomo K et al (2013a) Application of the phylogenetic informativeness method to chloroplast markers: a test case of closely related species in tribe Hydrangeae (Hydrangeaceae). Mol Phylo Evol 66:233–242
Mendoza CG, Wanke S, Goetghebeur P, Samain P (2013b) Facilitating wide hybridization in Hydrangeas cultivars: a phylogenetic and marker-assisted breeding approach. Mol Breed 32:233–239
Mink GI (1993) Pollen and seed transmitted viruses and viroids. Annu Rev Phytopathol 31:991–995
Mohanty A (1996) Hybridization between crop brassicas and some of their wild allies. PhD thesis. University of Delhi
Mohanty A, Chrungu B, Verma N, Shivanna KR (2009) Broadening the genetic base of crop brassicas by production of a new intergeneric hybrid. Czech J Genet Plant Breed 45:117–122
Morgan ER (2004) Use of in ovulo embryo culture to produce interspecific hybrids between Gentiana triflora and Gentiana lutea. N Z Crop Hort Sci 32:343–347
Mujeeb-Kazi A (1981) Triticum timopheevii x Secale cereale crossability. J Hered 72:227–228
Nanda Kumar PBN, Shivanna KR (1991) In vitro multiplication of a sterile interspecific hybrid, Brassica fruticulosa x B. campestris. Plant Cell Tiss Organ Cult 26:17–22
Nanda Kumar PBA, Shivanna KR (1993) Intergeneric hybridization between Diplotaxis siettiana and crop brassicas for the production of alloplasmic lines. Theor Appl Genet 85:770–776
Nanda Kumar PBA, Shivanna KR, Prakash S (1988) Wide hybridization in Brassica: crossability barriers and studies on the hybrid and synthetic amphidiploid of B. fruticulosa x B. campestris. Sex Plant Reprod 1:234–239
Nanda Kumar PBA, Prakash S, Shivanna KR (1989) Wide hybridization in Brassica: studies on interspecific hybrids between cultivated species (B. napus, B. juncea) and a wild species (B. gravinae). In: Proceedings of the 6th International Congress, Society for the Advancement of Breeding Researches in Asia and Oceania (SABRAO). Tsukuba, pp 435–438
Nomura Y, Oosawa K (1990) Production of interspecific hybrids between Allium chinense and A. thunbergii by in ovulo embryo culture. Japan J breed 40:531–535
Obata Y, Niimi Y, Nakano M et al (2000) Interspecific hybrids between Lilium nobilissimum and L. regale produced via ovules-with-placental-tissue culture. Sci Hortic 84:191–204
Okamoto T, Kranz E (2005) In vitro fertilization – a tool to dissect cell specification from a higher plant zygote. Curr Sci 89:1861–1869
Park CH, Walton PD (1990) Intergeneric hybrids and an amphiploid between Elymus canadensis and Psathyrostachys juncea. Euphytica 45:217–222
Pathania A, Bhat SR, Dinesh Kumar V et al (2003) Cytoplasmic male sterility in alloplasmic Brassica juncea carrying Diplotaxis catholica cytoplasm: molecular characterization and genetics of fertility restoration. Theor Appl Genet 107:455–461
Prakash S, Bhat SR, Quiros CF et al (2011) Brassica and its close allies: cytogenetics and evolution. Plant Breed Rev 31:21–187
Raghavan V (1977) Applied aspects of embryo culture. In: Reinert J, Bajaj YPS (eds) Applied and fundamental aspects of plant cell, tissue and organ cultures. Springer, Berlin, pp 357–397
Raghavan V (1986) Variability through wide crosses and embryo rescue. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants. Academic, New York, pp 613–663
Raghavan V (1999) Molecular embryology of flowering plants. Cambridge University Press, New York
Rangan TS (1982) Ovary, ovule and nucellar cultures. In: Johri BM (ed) Experimental embryology of vascular plants. Springer, Berlin, pp 105–129
Rangaswamy NS, Shivanna KR (1967) Induction of gamete compatibility and seed formation in axenic cultures of a diploid self-incompatible species of Petunia. Nature 216:937–939
Rao GU (1995) Development and characterization of new alloplasmics in crop brassicas. PhD thesis. University of Delhi
Rao GU, Shivanna KR (1996) Development of a new alloplasmic CMS Brassica napus in the cytoplasmic background of Diplotaxis siifolia. Cruciferae Newsl 18:68–69
Rao GU, Batra VS, Prakash S, Shivanna KR (1994) Development of a new cytoplasmic male sterile system in Brassica juncea through wide hybridization. Plant Breed 112:171–174
Rao GU, Lakshmikumaran M, Shivanna KR (1996) Production of hybrids, amphiploids and backcross progenies between a cold tolerant wild species E. abyssinicum and crop brassicas. Theor Appl Genet 92:786–790
Reed SM, Collins GB (1978) Interspecific hybrids in Nicotiana through in vitro culture of fertilized ovules. J Hered 69:311–315
Sabja AM, Mok DWS, Mok MC (1990) Seed and embryo growth in pod cultures of Phaseolus vulgaris and P. acutifolia. Hort Sci 25:1288–1291
Sage TL, Strumas F, Cole WW, Barret S (2010) Embryo rescue and plant regeneration following interspecific crosses in the genus Hylocereus (Cactaceae). Euphytica 174:73–82
Sareen PK, Chowdhury JB, Chowdhury K (1992) Amphiploids/synthetic crop species. In: Kalloo G, Chowdhury JB (eds) Distant hybridization in crop plants. Springer, Berlin, pp 62–81
Sastri DC (1985) Incompatibility in angiosperms: significance in crop improvement. Adv Appl Biol 10:1–111
Sastri DC, Moss JP (1982) Effects of growth regulators on incompatible crosses in the genus Arachis. J Exp Bot 33:1293–1301
Sastri DC, Shivanna KR (1976) Attempts to overcome interspecific incompatibility in Sesamum by using recognition pollen. Ann Bot 40:891–893
Sastri DC, Moss JP, Nalini MS (1983) The use of in vitro methods in groundnut improvement. In: Sen SK, Giles KL (eds) Proceedings of the international symposium on plant cell culture in crop improvement. Plenum Press, New York, pp 365–370
Scholten S, Kranz E (2001) In vitro fertilization and expression of transgenes in gametes and zygotes. Sex Plant Reprod 14:35–40
Sharma DR, Kaur R, Kumar K (1996) Embryo rescue in plants—a review. Euphytica 89:325–337
Shivanna KR (1995) Incompatibility and wide hybridization. In: Chopra VL, Prakash S (eds) Oilseed and vegetable Brassicas: an Indian perspective. Oxford-IBH, New Delhi, pp 77–102
Shivanna KR (1997) Crossability barriers. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 261–272
Shivanna KR (2000) Wide hybridization in Brassica. In: Jaiswal V, Rai AK, Uma J, Singh JS (eds) The changing scenario in plant sciences (Professor Mohan Ram’s Commemoration Vol). Allied Publishers Limited, New Delhi, pp 197–212
Shivanna KR (2003) Pollen biology and biotechnology. Science Publishers, Enfield
Shivanna KR, Heslop-Harrison J (1981) Membrane state and pollen viability. Ann Bot 47:759–770
Shivanna KR, Johri BM (1985) The angiosperm pollen: structure and function. Wiley Eastern, New Delhi
Shivanna KR, Rangaswamy NS (1992) Pollen biology: a laboratory manual. Springer, Berlin
Shivanna KR, Sawhney VK (eds) (1997) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York
Shivanna KR, Xu H, Taylor P, Knox RB (1987) Isolation of sperms from pollen tubes of flowering plants during fertilization. Plant Physiol 87:647–650
Singh R, Shivanna KR, Prakash S (2007) Studies on crossability barriers between cultivated species and wild allies of crop Brassicas. In: Proceedings of the 12th international rapeseed congress, vol 1. Science Press USA, Wuhan, pp 272–276
Singsit C, Hannaeman RE Jr (1991) Rescuing abortive inter-EBN potato hybrids through double pollination and embryo rescue. Plant Cell Rep 9:475–478
Stalker ST (1980) Utilization of wild species for crop improvement. Adv Agron 33:111–147
Stanley RG, Linskens HF (1974) Pollen: biology, biochemistry and management. Springer, Berlin
Stettler RF (1968) Irradiated mentor pollen: its use in remote hybridization in black cotton wood. Nature 219:746–747
Subramanyam NC (1999) Hybrids from wide hybridization in grass genera: progress and prospects. In: Kishore R (ed) Plant tissue culture and biotechnology: emerging trends. University Press, Hyderabad, pp 11–21
Taira T, Lelley E, Larter EN (2011) Influence of parental rye on the development of embryos and endosperm of wheat–rye hybrids. Can J Bot 56:386–390
Towill LE (1991) Cryopreservation. In: Dodds JH (ed) In vitro method for conservation of plant genetic resources. Chapman & Hall, London, pp 41–70
Van Tuyl JM, De Jeu MJ (2003) Methods for overcoming interspecific crossability barriers. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 273–292
Van Tuyl JM, van Dien MP, van Creij MGM et al (1991) Application of in vitro pollination, ovary culture, ovule culture and embryo rescue for overcoming incongruity barriers in interspecific Lilium crosses. Plant Sci 74:115–126
Verma N (1993) Production of intergeneric hybrids between Brassica cossoniana and crop brassicas through embryo rescue. MPhil thesis. University of Delhi
Villar M, Gaget-Faurobert M (1997) Mentor effects in pistil-mediated pollen-pollen interactions. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge University Press, New York, pp 315–332
Vyas P (1993) Studies on wide hybridization between crop brassicas and species of Diplotaxis. PhD thesis. University of Delhi
Vyas P, Prakash S, Shivanna KR (1995) Production of wide hybrids and backcross progenies between Diplotaxis erucoides and crop brassicas. Theor Appl Genet 90:549–553
Wang A-Y, Chen D-L, Cai DT (2005) Application of wide hybridization and allopolyploidization in rice breeding. Plant Sci J 23:491–495
Wang YY, Kuang A, Russell SC, Tian HQ (2006) In vitro fertilization as a tool for investigating sexual reproduction of angiosperms. Sex Plant Reprod 19:103–115
Wang H, Jiang J, Chen S, Qi X et al (2014) Rapid genetic and epigenetic alterations under intergeneric genomic shock in newly synthesized Chrysanthemum morifolium × Leucanthemum paludosum hybrids (Asteraceae). Genome Biol Evol 6:247–259
Wietsma WA, De Jong KY, Van Tuyl WA (1994) Overcoming prefertilization barriers in interspecific crosses of Fritillaria imperialis and F. raddeana. Plant Cell Incomp Newslett 26:89–92
Williams EG, de Latour G (1980) The use of embryo culture with transplanted nurse endosperm for the production of interspecific hybrids in pasture legumes. Bot Gaz 141:252–257
Williams EG, Maheshwaran G, Hutchinson JF (1987) Embryo and ovule culture and crop improvement. Plant Breed Rev 5:181–236
Zenkteler M (1980) Introvarian and in vitro pollination. In: Vasil IK (ed) Perspective in plant cell and tissue culture. Intl Rev Cytol (Suppl) 11B:137–156
Zenkteler M (1990) In vitro fertilization and wide hybridization in higher plants. Crit Rev Plant Sci 9:267–279
Zenkteler M, Maheshwaran G, Williams EG (1987) In vitro placental pollination in Brassica campestris and B. napus. J Plant Physiol 128:245–250
Zenktler M, Bagniewska-Zadworna A (2001) Distant in vitro pollination of ovules. In: Rangaswamy NS (ed) Phytomorphology golden jubilee issue: trends in plant sciences. International Society of Plant Morphologists, New Delhi, pp 225–235
Acknowledgement
KRS thanks the Indian National Science Academy for the award of INSA Senior Research Fellow (2003–2008) and INSA Honorary Scientist (2009 to date).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Shivanna, K.R., Bahadur, B. (2015). Efficacy of Biotechnological Approaches to Raise Wide Sexual Hybrids. In: Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K. (eds) Plant Biology and Biotechnology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2283-5_17
Download citation
DOI: https://doi.org/10.1007/978-81-322-2283-5_17
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2282-8
Online ISBN: 978-81-322-2283-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)