Abstract
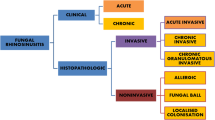
Histopathological examination forms the basis of diagnosis, classification, and management of invasive fungal rhinosinusitis [1, 2]. In cases where fungal infection is suspected on clinical grounds, demonstration of fungal elements should be sufficient to start treatment, but histopathological examination is necessary to confirm the diagnosis of “invasive” fungal infection and to properly classify the fungal disease for further management.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Histopathological examination forms the basis of diagnosis, classification, and management of invasive fungal rhinosinusitis [1, 2]. In cases where fungal infection is suspected on clinical grounds, demonstration of fungal elements should be sufficient to start treatment, but histopathological examination is necessary to confirm the diagnosis of “invasive” fungal infection and to properly classify the fungal disease for further management.
Histopathological diagnosis of invasive fungal diseases has three components, namely, (a) demonstration of fungal elements (b) identifying tissue invasiveness, and (c) identifying type of inflammatory reaction – on the basis of which further classification is made.
In majority of the cases, fungal elements can be identified on hematoxylin and eosin (H&E)-stained sections. However, identification becomes easy with special staining techniques like periodic acid-Schiff (PAS) stain and Gomori’s (Grocott–Gomori’s) silver methenamine (GMS) preparation. Gomori methenamine has been described to be the most sensitive of the commonly used stains, and it has been recommended that a negative diagnosis should not be given unless a silver stain has been performed [3].
In both, PAS and GMS techniques, the principle is to demonstrate polysaccharide-rich fungal walls. In PAS staining, periodic acid oxidizes polysaccharides in to aldehydes which on reaction with Schiff’s reagent produces purple or magenta color. Walls of fungal hyphae are stained magenta color highlighting the fungal structure.
In Grocott–Gomori’s (or Gomori’s) methenamine silver technique, the polysaccharides are oxidized in to pair of aldehydes. The aldehydes react with silver nitrate and reduce it in to metallic silver that deposits on the fungal wall making it stand out. In fact, GMS is not a stain in the strictest sense but causes silver deposition that makes the fungal elements more easily demonstrable.
Both the PAS stain and GMS techniques are not specific for fungi. They only make the polysaccharide-rich fungal walls easily identifiable. There are many other structures in tissues – including collagen bands and vessel walls. To the uninitiated, these may look like fungal elements. Fungi are identified by their structural characteristics and not on positive or negative staining.
Invasiveness of the disease is determined by the presence of the fungal elements in the submucosa, invasion into the vessel walls and the lumen with consequent vascular thrombosis, and infarction of the tissue. While in the chronic disease, there is predominantly chronic inflammatory cell component or there is granulomatous reaction.
Based on the type of inflammatory reaction and temporal profile of the pathologic process, invasive fungal rhinosinusitis is divided in to three groups:
-
(a)
Acute fulminant invasive rhinosinusitis
-
(b)
Chronic invasive fungal rhinosinusitis
-
(c)
Chronic granulomatous rhinosinusitis
Acute Fulminant Invasive Fungal Rhinosinusitis
This occurs mainly in patients with immunosuppression, or ketoacidosis of diabetes mellitus, or uremia of chronic renal disease but is also being reported in immunocompetent patients. The fungi most often involved are Mucor species or Aspergillus. There is usually little or no appreciable inflammatory cell reaction. Fungal hyphae invade the tissue especially the vessel walls, resulting in thrombosis of the vessels and consequent infarction of the tissue. Both invasive mucormycosis and aspergillosis have an affinity for invading blood vessels [4–7] (Fig. 1). In particular, invasive Mucor has a strong affinity for arteries. Histopathological features include growth along the internal elastic lamina that results in dissection away from the media, as well as growth into the blood vessel lumen, producing endothelial damage and initiating thrombosis [8] (Fig. 2).
These fungi, having once invaded the vessels, disseminate rapidly in fulminant fashion, extending to intracranial spaces, carotid artery, etc. and prove fatal in a very short time. Rapid diagnosis and prompt treatment may save at least some of these patients.
Histopathologists involved in diagnosis of such cases should be alerted to the possibility of acute invasive fungal infection. Intraoperative frozen section examination may save considerable time in diagnosis. If intraoperative frozen section examination facility is not available and if the primary physician/surgeon communicates to the pathologist of the clinical suspicion of invasive fungal sinusitis, then routine processing may be done in rapid fashion and the result promptly communicated to the treating physician without delay.
Once the possibility of fungal infection is considered, frozen section diagnosis should not be difficult. Vascular invasion, infarction of the tissue, and presence of fungi are not difficult to identify. Fungi can be identified even on Toluidine blue stain or H&E staining. Mucor species which is most often involved in acute fulminant processes can be identified by broad (10–15 μm wide) ribbon-like aseptate fungal hyphae which fold on themselves and branch at right angles (Figs. 3, 4, and 5), while Aspergillus species which also may cause acute invasive fulminant process (Figs. 8 and 9) appears as narrow (2–5 μm wide) septate acutely branching fungal hyphae (Figs. 7 and 8).
Chronic Invasive Fungal Rhinosinusitis (Fig. 6)
In these cases, there is usually destruction of the tissue with chronic abscess-like inflammatory reaction with presence of fungal elements within the exudate. There is usually nothing – either clinically or histologically to alert the pathologist about the possibility of fungal etiology and hence the diagnosis may be missed. Constant alertness and a high level of suspicion of fungal etiology in all such cases irrespective of the underlying disorders helps in identifying such cases. Unlike the neutrophil-rich, highly necrotic, and angiotrophic process seen in acute invasive fungal sinusitis, there is a low-grade mixed cellular infiltrate in affected tissues [9]. Usually this occurs in diabetics, is slowly progressive, and elicits limited inflammation.
Chronic Granulomatous Fungal Rhinosinusitis
Other terms for this condition include “indolent fungal sinusitis” and primary paranasal granuloma. Published cases include those from Sudan (due to Aspergillus flavus) and St Louis, MO [10, 11]. Patients invariably have no predisposing factors, and the lesion usually presents as a destructive process raising the suspicion of malignancy. There is often destructive growth eroding in to the orbit. Histologically this presents as chronic granulomatous process with non-necrotizing granuloma composed of clusters or sheets of foreign-body-type multinucleated giant cells with fair number of eosinophils in the background. The combination of sheets/clusters of foreign-body-type multinucleated giant cells with eosinophils in the background should be enough to alert the pathologist about possibility of fungal (mainly Aspergillus flavus) disease. Close look at the giant cells even in frozen sections or paraffin sections stained with hematoxylin and eosin stain should reveal short well-stained or shadow-like unstained fungal hyphae in the cytoplasm of the giant cells (Fig. 7). GMS stain (Fig. 8) and PAS stain (Fig. 9) also reveal the characteristic features of the fungus within the giant cells. Tuberculosis does not commonly involve the paranasal sinuses. Also concomitant stains for mycobacteria are negative. Hence, any granulomatous disease causing destruction of bone that initially clinically presents as a malignancy in young immunocompetent patients should alert the pathologist to the possibility of chronic invasive granulomatous sinusitis.
In all these cases, fungal etiology is missed by the inexperienced pathologist not because of the difficulty in identifying the fungi histologically but because of the lack of awareness of this possibility. Clinicians who suspect fungal etiology should make it a point to discuss the case with the concerned pathologist personally. Acute invasive fungal rhinosinusitis is one of the most critical emergency situations for the pathologists. Diagnosis in such cases should be rapid with prompt communication to the treating physician of the diagnosis.
The pathologist should also be able to identify noninvasive fungal sinusitis and differentiate it from the invasive variety. Noninvasive fungal rhinosinusitis includes “fungal ball” and allergic fungal sinusitis (AFS). These have characteristic histopathological appearance with absence of fungal invasion of the tissues.
Fungal Ball
In these cases, histopathology shows multitude of pale fungal hyphae compressed in the center, with appearance of morphology at the periphery (Fig. 10). The surrounding mucosa usually shows dense mixed inflammatory infiltrate.
Allergic Fungal Sinusitis (Fig. 11a, b)
The diagnosis of AFS is primarily histopathological although there are clinical and radiological features to distinguish it from invasive fungal sinusitis. Characteristic allergic mucin is seen on H&E stains as strongly staining laminated concretions of pyknotic and degranulated eosinophils surrounded by areas of lightly staining mucin sprinkled with Charcot–Leyden crystals [12–14]. Staining of allergic mucin with fungal stains like Gomori’s (GMS) may show scattered fungal hyphae within the allergic mucin [12–16]. There is no evidence of mucosal fungal invasion, including features of tissue invasion like mucosal necrosis, granuloma formation, or giant cells. An important fact to remember is that allergic mucin without fungal involvement can be seen in patients with the non-AFS “eosinophilic mucin rhinosinusitis” [17, 18].
References
deShazo RD, O’Brien M, Chapin K, et al. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181–8.
Das A, Bal A, Chakarabarti A, Panda N, Joshi K. Spectrum of fungal rhinosinusitis; a histopathologist’s perspective. Histopathology. 2009;54:854–9.
Schell WA. Histopathology of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:251–76.
Barnes L, Peel RL. Head and neck pathology: a text/atlas of differential diagnosis. New York: Igahu-Shoin; 1990. p. 170.
Michaels L. Ear, nose and throat histopathology. New York: Springer; 1987. p. 146–8.
Johnson JT. Infections. In: Cummings CW, Krause CJ, editors. Otolaryngology head neck surgery, vol. 2. 2nd ed. St. Louis: Mosby Year Book; 1993. p. 931–3.
Myerowitz RL, Guggenheimer J, Barnes L. Infectious diseases of the head and neck. New York: Marcel Dekker; 1985. p. 1784–6.
Straatsma BR, Zimmerman LE, Gass JDM. Phycomycosis: a clinicopathologic study of fifty one cases. Lab Invest. 1962;11:963–85.
Milro CM, Blanshard JD, Lucas S, Michaels L. Aspergillosis of the nose and paranasal sinuses. J Clin Pathol. 1989;42:123–7.
Veress B, Malik OA, el-Tayeb AA, et al. Further observations on the primary paranasal Aspergillosis granuloma in the Sudan: a morphological study of 46 cases. Am J Trop Med Hyg. 1973;22:765–72.
Currens J, Hutcheson PS, Slavin RG, Citardi MJ. Primary paranasal Aspergillus granuloma: a case report and review of literature. Am J Rhinol. 2002;16:165–8.
Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol. 1998;102:387–94.
Katzenstein AA, Sale SR, Greenburger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983;72:89–93.
Gourley DS, Whisman BA, Jorgensen NL, et al. Allergic Bipolaris sinusitis. Clinical and histopathological characteristics. J Allergy Clin Immunol. 1990;85:583–91.
Bent III JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–8.
de Shazo RD, Swain RE. Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol. 1995;95:24–35.
Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000;110:799–813.
Ramadan HH, Quraishi HA. Allergic mucin sinusitis without fungus. Am J Rhinol. 1997;11:145–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Deshpande, R.B. (2014). Histopathology of Invasive Fungal Rhinosinusitis. In: Mankekar, G. (eds) Invasive Fungal Rhinosinusitis. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1530-1_5
Download citation
DOI: https://doi.org/10.1007/978-81-322-1530-1_5
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1529-5
Online ISBN: 978-81-322-1530-1
eBook Packages: MedicineMedicine (R0)