Abstract
“Environmental nanotechnology” is considered to play a key role in the shaping of current environmental engineering and science. The conventional environmental remedial techniques seem to be relatively ineffectual in the face of currently extensively expanding load of pollutants that permeate the air, water, and soil environment. Nanotechnology can provide a way to purify the air and water resources by utilizing nanoparticles as a catalyst and/or sensing systems. In the present research chapter, the potential of nanotechnological products and processes and their application to clean up the environment contaminants have been discussed. Water treatment and purification techniques based on nanotechnology have been highlighted. These also include the environmental and energy application of nanotechnology which focuses on clean technology, reducing global warming, eco-friendly and efficient energy-generating techniques, eco-friendly surface coating, remediation techniques, and environmental monitoring. Environmental nanoscience products, devices, and processes have an impact on socioeconomic aspects for maintaining a clean environment for sustainable development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Environmental nanotechnology
- Nanoproducts
- Clean technology
- Eco-friendly coating
- Environmental monitoring
- Remediation
Introduction
Nanotechnology is a broad and interdisciplinary area of research and development activity that has been growing exponentially worldwide. Nanotechnology will provide the capacity to create affordable products with dramatically improved performance. This will come through basic understanding of works to control and manipulate matter at the nanometer scale through the incorporation of nanostructures and nanoprocesses into technological innovations. A revolution has been occurring in science and technology, based on the recently developed ability to measure, manipulate, and organize matter on this scale. Nanotechnology is fundamentally changing the way materials and devices will be produced in the future. The ability to synthesize nanoscale building blocks with precisely controlled size and composition and then to assemble them into large structure with unique properties and functions will revolutionize materials, manufacturing, and their applications.
Nanoscience and engineering could significantly affect molecular understanding of nanoscale processes that take place in the environment: the generation and remediation of environmental problems through control of emissions, the development of new “green” technology that minimize the production of undesirable by-products, and the remediation of existing waste sites and streams. Nanotechnology will also afford the removal of the smallest contaminants from water supplies and air and the continuous measurement and mitigation/prevention of pollution to clean up the environment.
New technologies offer interesting opportunities for sustainable solutions, depending on the area of application and the framework conditions given. The research results show that there are varying implications at different life-cycle stages and application levels concerning the resource consumption. Therefore, NT applications and their implications need to be carefully studied, taking a life-cycle-wide perspective as well as looking at different levels (e.g., the production of NT products, the use phase including systemic effects). Using the experience gained in investigating the sustainability impacts of emerging technologies, nano tech offer great promise for delivering new and improved Environment technology (Carvajal 2004). Nanotechnology can make a considerable contribution to sustainable development through its combination of economic, ecological, and social benefits. Sustainable development means balancing economic, ecological, and social requirements in order to meet present needs without restricting the opportunities available to future generation.
Potential of Nanotechnology
Nanoscience is associated with the intimate understanding and control of matter at dimensions of roughly 1–100 nm and is intrinsically important because of the genuine expectation that the properties of materials at this scale will differ significantly from those of their bulk counterparts. Nanotechnology involves the imaging, measuring, modeling, and manipulation of materials at this length scale. The synthesis and fabrication of functional nanomaterials with predictive, rational strategies is a major focal point of research for many groups worldwide (Xia et al. 2008; Xu et al. 2009).
In practice, this effort has entailed the design, production, and characterization of a myriad of nanostructures including nanoparticles, nanocubes, nanorods, nanowires, and nanotubes, which maintain fundamentally interesting size-dependent electronic, optical, thermal, mechanical, magnetic, chemical, and other physical properties. From the perspective of applications, these structures have wide-ranging utility in areas as diverse as catalysis, energy storage, fiber engineering, fuel cells, biomedicine, computation, power generation, photonics, pollution remediation, and sensing. Tables 7.1 and 7.2 demonstrate nanomaterials and its properties and applications.
Nanotechnology: Multidisciplinary Aspects
Nanotechnology is the design, characterization, production, and application of structures, devices, and systems by controlling the shape and size at the nanometer scale; environmental nanotechnology (E-nano) products can be developed for a wide array of urgently needed environmental remediation. The nanoparticles/nanostructures made by mechanical and/or microbial action with fundamental building blocks are among the smallest human-made objects and exhibit novel physical, chemical, and biological properties, which has wider application for pollution prevention, detection, monitoring, and remediation of pollutants. Environmental nanotechnology would be the new innovation to remediate and treat the contaminants to acceptable levels. Environmental scientists and engineers are already working with nanoscale structure to manipulate matter of the atomic or molecular scale that has cut across disciplines of chemistry, physics, biology, and even engineering (Fig. 7.1).
In research communities, there are statements that NT is still not well defined. Some defines it on the basis of physical dimension of structures ranging from 0.1 to 100 nm; it means different for different disciplines of people. Definitions of NT are as the applications of it. The following table outlines the set of definitions related to its discipline (Table 7.3).
Nanotechnology is a result of convergence of traditional fields of physics, biology, and chemistry. It depends on the contribution from among these and other disciplines. The convergence of these traditional fields in the environment and energy is well illustrated in Fig. 7.2 which also shows the scales of the areas of interaction. There are considerable debates in the scientific community about the boundaries of the new disciplines emerging from this convergence, e.g., between microtechnology and nanotechnology, but it is becoming clear that in practice no clear division can be made. Thus, for example, sensors and biochips at nanoscale need to be packaged for commercial application using microtechnology.
Nanotechnology and Energy
Nanotechnology has been shown to directly contribute to the long-term energy sustainability through the development of energy-saving and renewable energy technologies. Nanoscience accounts for the development of a vast variety of high-performance materials for thermal insulation as well as lighter and stronger materials for vehicle production and LEDs for application in energy-efficient lighting devices and displays. In the field of renewable energy, nanotechnology has contributed to the development of a variety of solar photovoltaic (PV) technologies for electricity production. PV nanotechnologies have lower production costs than the expensive, conventional silicon crystalline technology. The thin-film copper–indium–gallium diselenide (CIGS) technology is considered as the most promising PV nanotechnology. CIGS cells show very high conversion efficiency compared to the rest of the nano-PV technologies, and their manufacturing costs are relatively low. One of the promising areas of NT is under increased usage of renewable energies like hydrogen, solar, and cleaner fuels. Currently, conventional technologies provide hydrogen as energy carrier in fuel cells, but there are drawbacks in it, because unfortunately, a convenient hydrogen storage system does not exist today. The current technologies include compressed gases (like natural gas, propane), which occupy larger volumes and metal hydrides, which are very heavy and therefore result in a reduced driving range for automobiles (National Renewable Energy Laboratory, n.d.). CNTs are essentially tiny, lightweight cylinders with diameters the size of several hydrogen molecules, and they can provide a solution to the storage problems.
Research carried showed that these CNTs are capable of adsorbing hydrogen (Dillon et al. 1999), and this indicates that CNTs are the ideal building blocks for constructing safe, efficient, and high-density adsorbents for hydrogen storage application like fuel cells and batteries in electronic and automobile applications. For example, these nanotubes have been reported to store up to 20 l of hydrogen per gram. This has significant implication for reducing costs – transporting and storing hydrogen (Khan et al. 2003). CNTs absorb trace quantities of CO2 present in natural gas, so researchers are currently developing CNT membranes that could revolutionize natural gas purification technology. And also there are ongoing research to make use of CNTs for desalination and treatment of wastewater. These nanotubes can enhance water treatment and also out complete reverse osmosis or distillation processes using at least 10 times less energy than reverse osmosis and at least 1,000 times less energy than distillation (Bruns 2000). It is envisaged that NT will show potentially significant impacts in environmental and energy applications. These include developing new green processing technologies that minimize the generation of undesirable by-products. It can be used to monitor and remediate existing waste sites and polluted wastewater resources. Nanotechnology, in particular nanofabrication, offers a variety of tools to contribute for solving the energy crisis, since creating materials and devices smaller than 100 nanometers (nm) offers new ways to capture, store, and transfer energy. The level of control that nanofabrication provides could help to solve many problems that the world is facing related to current energy generation technologies, including the array of alternative, renewable energy approaches. The conversion of primary energy sources, i.e., the sun into electricity, heat, and kinetic energy, can be made more efficient and environmentally friendly using nanotechnology. Producing electricity through the conversion of sunlight, known as solar photovoltaics (or solar cells), is a field where nanostructured materials and nanotechnology are contributing greatly. Successful research could result in a significant reduction of the manufacturing cost of these solar cells and also improve efficiency. Cell types being investigated include thin-layer solar cells, dye solar cells, or polymer solar cells. The use of a layer of quantum dots – tiny blobs of one semiconductor grown on the surface of another, added behind the conventional multilayer compound – is also being investigated. It is anticipated that nanotechnology will help to develop the ideal solar cell, incorporating optimum structure and design.
Nanofabrication of materials is also being used in other energy conversion processes where specific, extreme conditions need to be withstood, such as heat-resistant turbine materials. Coal-fired power stations can also be made more environmentally friendly using nano-optimized membranes, which separate out and store the carbon dioxide. Thermoelectric energy conversion using nanostructured semiconductors promises increases in efficiency that could pave the way for a broad application in the utilization of waste heat, e.g., from car or human body heat for portable electronics in textiles. Hydrogen fuel cell technology is another area where nanotechnology can be applied to improve efficiency. Other renewable energy sources are also being “improved” using nanotechnology including wind energy, using lighter, more durable nano-based materials for rotor blades; geothermal, using nano-coatings and composites for wear-resistant drilling equipment; hydro-/tidal power, using nano-coatings for corrosion protection; and biomass energy (“biofuels”), using nano-based precision farming to optimize yields.
Benefits of Nano-Energy
-
Reduced energy consumption – By optimizing/increasing efficiency in energy storage, generation, and conservation, energy consumption will decrease through nanotechnology applications.
-
Environmentally friendly – Nanotechnology can contribute to “cleaning” up and reducing the environmental impact throughout the value chain of the energy sector.
-
Cheaper – The use of nanotechnology can reduce the cost of energy production, distribution, and storage, since it has the advantage of reducing the amount of power outputs. Through miniaturization, nanotechnology also provides an opportunity to tailor-make solutions.
-
Independent power sources – The application of nanotechnology in the energy sector could contribute to providing alternative sources of energy to the national grid.
-
Facilitate transition to renewable – The application of nanotechnology in the energy sector could facilitate the transition from fossil fuels to renewable energy.
Clean Technology
Nanotechnology involves atom-by-atom construction; it will be able to create substances and finished objects without producing the dangerous and messy by-products that most current manufacturing processes produce. Nanodevices will operate in a liquid containing the necessary raw materials usually carbon or silicon, with trace amounts of other elements as needed, and will simply plug the appropriate atoms in the appropriate places to produce the desired end product. Such processes should produce few by-products, and those by-products can be purified readily by other nanodevices and recycled back into the feedstock (Reynolds 2001). Toxic waste generated during the various industrial processes consists of harmless atoms arranged into noxious molecule. With inexpensive energy and equipment able to work at the molecular level, these wastes can be converted into harmless forms (Drexler et al.1991).
In addition, most products of NT and abundant elements like carbon in diamond or diamondoid form will be the basis of most nanomanufacturing. Products made of such materials will be strong, which uses smaller amounts of materials, and carbon is an abundant material, meaning that little in the way, exploration and extraction will be needed. As a greenhouse remediation measure, nanodevices could extract carbon dioxide (CO2) form in the air, if desired (Reynolds 2001). Presently, the atom-by-atom-based manufacturing in the industries is achieving efficient, environment-friendly processes by using some nanomaterials like nanocatalysts. The nanocatalysts are improving the efficiency and specificity of chemical reactions, which results in the design of light and strong materials that can lead to saving energy and raw materials (ION 2001). Apart from this, NT could contribute to cleaner industrial production process or products, mainly through the use of raw materials and energy. These trends includes miniaturization of devices design and new catalyst at the manoscale level.
Nanomaterial could also help to develop greener technologies, hence reducing CO2 emissions and energy consumption for buildings and transport. For example, US buildings account for about 40 % of the country’s total energy consumption and are responsible for 39 % of CO2 emissions. Transport’s contribution amounts to another 33 %. By combining the use of light-emitting diodes (LEDs) and super-insulating and self-cleaning windows, together with the implementation of more powerful rooftop photovoltaic (PV) panels and nanosensors, monitoring energy usage, energy efficiency, and environmental sustainability of buildings would be enhanced. In particular, the improvement of solar photovoltaic (PV) is linked to the development of new materials with higher efficiency in solar energy conversion. Nanostructured organic photovoltaics can now reach a power efficiency of 8 %. Although still lower than traditional silicon-based PV, given their high flexibility and relatively cheap industrial production, these organic PV panels have found applications in many sectors, ranging from buildings to transport and electronic equipment. Improved recycling in industrial processes would enable the recovery of precious transition metal ions from aqueous solutions and mixtures, in parallel to a decrease in solid waste and greenhouse gas (GHG) emissions. Such recycling could be achieved through recovery and reuse of magnetic nanoparticles via magnetic separation.
Furthermore, highly porous nanomaterials encompassing metal organic and zeolitic imidazole frameworks capture large amounts of CO2, in parallel to metallic iron nanoparticles which degrade organic contaminants like chlorinated hydrocarbons. The development of new catalytic nanoparticles for transport technology, at lower cost than the currently used platinum-based catalytic converters, would guarantee cleaner emissions. New nanotechnologies based on carbon nanotubes (CNTs), leading to new materials between 10 and 56 times stronger than steel but significantly lighter, would reduce fuel consumption (PNNL 2002).
Nano-Enhanced Green Industry Technologies
Environmentally friendly catalysis is possible using cooperative catalytic systems incorporated in mesoporous silica nanoparticles (MSNs). Nano Scientists have described how catalyst work inside the pore of MSNs and tailored to perform a series of reactions – for instance, to synthesize biodiesel from free fatty acids in vegetable oils. These catalytic MSNs are environmentally benign and easy to recycle. It is possible to tailor the activity and stability of nanoparticle catalysts by modifying their port structure. Researcher’s goal is to make stable catalysts for use in removing poisonous carbon monoxide from the air in places such as mining shafts. Scientists have described a promising method for synthesizing nanogold and nanopalladium catalysis in an ordered mesoporous support structure with controllable pore sizes. The synthesis can be done at low temperatures using microwaves. A novel nonporous sorbent effectively removes mercury and other toxic heavy metals from wastewater generated during offshore oil and gas platform drilling. The material, which is called thiol-SAMs, removes 99 % of mercury from gas-condensate liquid containing 800 ppm of mercury.
Polymer nanospheres offer a new way to selectively detect hazardous materials in aquatic environment. Scientists have described how to prepare polymer nanospheres that change their shape and optical properties whenever a certain chemical is present. The change can be measured by surface plasma on resonance, which enables the detection of pollutants at the level of parts per billion (ppb). The nanospheres can be tailored to sense a specific chemical by molecular imprinting of the polymers. The method could prove particularly useful for detecting pharmaceuticals and other emerging pollutants in waterways that are currently difficult to detect.
Nano-Enhanced Cleanup Technologies
Magnetic nanoparticles could become an important green tool for removing arsenic from drinking water, particularly for point-of-use treatment in developing countries. Nanoparticles of iron oxide bind strongly and specifically to arsenic and can be separated out of solutions using magnets. Scientists determined that particles of 12 nm size removed 99.2 % of the arsenic in solution. Zerovalent nanoparticles of iron and magnesium more effectively degrade heavy metals and organic solvents in water sediments when they are combined with emulsion liquid membranes. The membranes increased the contact between these catalytic nanoparticles and the targeted pollutants. This is a promising remediation technology.
Eco-Friendly Coating: Pollution Prevention
Pollution prevention is reducing or eliminating waste at the source by modifying production processes, promoting the use of nontoxic or less-toxic substances, implementing conservation techniques, and reusing materials rather than putting them into the waste stream. The unique and potentially useful properties of nanomaterials have dramatically increased surface areas and reactivity that improved strength and weight ratios and increased electrical conductivity and changes in color and opacity. Materials designed to take advantage of these properties are finding application in a variety of areas, such as electronics, medicine, and environmental protection.
Nanoproducts
Examples of products with potential for preventing pollution include coatings that are free of volatile organic compounds and diisocyanates, safer surfactants, and self-cleaning surfaces. Nanotechnology and nanomaterials can help to create alternatives to light-emitting or absorbing applications that previously relied upon heavy metal-based semiconductors. Nanocomposites may be used in a variety of products, resulting in reduced need for addition of flame-retardant chemicals. In addition, products including a variety of tools, automobile and airplane components, and coatings can be made harder and more wear, erosion, and fatigue resistant than conventional counterparts. Examples are as follows:
-
(a)
Alternatives to Chrome VI Coatings: Chrome and cadmium plating provides very effective surface treatments for wear and corrosion reduction. Electrolytic hard chrome (EHC) is applied using the reduction of hexavalent chrome in a solution of chromic acid. The EHC plating process is a less efficient process which results in vigorous gas evolution at the electrode. This results in airborne mist which contains hexavalent chrome, a known carcinogen. Several alternative technologies exist to coat a substrate with metal without using electrolytic solutions or plating baths. These technologies do not eliminate the use of metal coatings, but they do eliminate the use of nonmetal toxic components such as cyanide from the plating process. They can also reduce the amount of metal-contaminated wastewater and sludge that is generated from plating. These alternative technologies include thermal spray coating, vapor deposition, and chemical vapor deposition and can be used to apply alternate materials.
-
Powdermet, Inc., has developed a combined top-down/bottom-up approach to this problem, utilizing a novel process for producing nano-featured feedstock materials for the alternative technologies mentioned above. This approach reduces both the amount of waste produced and the amount of post-plating processing required resulting in a hard, corrosion-resistant coating.
-
-
(b)
Energetics, Including Safer Non-lead Primers to Prevent Pollution: Researchers are currently developing nanoengineered thermites (nanothermite) with tailored properties that are expected to replace lead azide and lead styphnate primers in the near future. Nanothermites are comprised of a mixture of fuel and oxidizer nanostructures. Typically Al nanoparticles are used as fuel and metal oxides like CuO, MoO3, and Bi2O3. Environmentally safe chemicals are utilized for the preparation of these materials. Nanothermites are self-assembled and coated with polymers or explosives in nanoscale for applications such as primers, reactive materials, and propellant initiators. These materials are also integrated with microdevices for various applications such as microthrusters, power generators, micro-shock generators for drug delivery, and microinitiators.
-
(c)
Nanoscale Thermoelectric Materials and Devices: Increasingly, the ability to provide power and cooling is a major factor in how large-scale computer systems, as in Internet data centers, are designed and managed. This stems from the fact that advanced high-performance electronics in many situations are currently limited by the ability to remove heat from the operating chip. This heat is typically concentrated in hot spots in the chip, experiencing very high heat fluxes. Such thermal problems are also relevant to small-scale computationally intense, wireless, and communication devices that rely on advanced algorithms for voice, video, and error-free data operations. Component- and system-level heat flux densities are projected to increase as computers and other digital devices add increased functionality. Strategies to manage these problems will have a profound impact on future technology and economic growth. Concomitant with these technology issues are the emerging problems of rising energy costs that are demanding higher efficiency in automobiles to cooling systems, as well as in other energy-intensive industries. It is worth noting that nearly 60 % of the world’s useful energy is wasted as heat. Thus, it is worth considering the recovery of even a fraction of this heat by converting to useful electric power. Complicating this mix is the urgent need for limiting CO2 levels in the atmosphere to contain global warming and minimizing ozone-depleting refrigerants. This shows how nanoscale thermoelectric materials and device technology can have an impact on such a broad spectrum of emerging technological, energy, and environmental issues.
Nanoprocesses
Processes that could prevent pollution include more efficient industrial chemical production through the use of nanoscale catalysts and the bottom-up self-assembly of materials, resulting in processing efficiency, reduction of waste in manufacturing, and stronger materials with fewer defects. In addition, the ability to enhance and tune chemical activity can result in catalysts that improve the efficiency of chemical reactions in automobile catalytic converters, power generation plants, and manufacturing facilities. Examples are as follows:
-
(a)
Nanoengineering Materials for Pollution Prevention: Concrete and Reduced CO 2 Emissions. Protecting the built environment from the forces of the natural world with dams and seawalls is an important work, as is protecting the natural environment from the engineered world. But the twenty-first-century engineer should also look to the natural world as a powerful design partner and a source of sustainable solutions. A good place to begin is by studying the way natural materials are constructed at the nanoscale and drawing inspiration from them as we engineer our own materials. For example, the civil engineer’s construction material of choice includes concrete, the oldest engineered building material and one of the most widely consumed materials on earth. Each year, 2.1 billion tons of cement, the primary component of concrete, is manufactured, enough to produce one cubic meter of concrete for every person alive. Unfortunately, cement is a major source of atmospheric carbon dioxide largely because it is made by burning fossil fuel to heat a limestone and clay powder to 1,500°C, which changes its molecular structure. When the cement powder is later mixed with water and gravel, the invested energy is released into chemical bonds that form calcium silicate hydrates, the glue that binds the gravel to make concrete. The production of cement accounts for 7–8 % of all human-generated carbon dioxide emissions. If novel cement can be engineered, whose manufacture produces only half as much CO2, a significant reduction in total CO2 emissions will be achieved. The application of nanotechnology may make it possible for all this to happen.
-
(b)
Stereoselective Green Chemistry Strategies Using Crystal-to-Crystal Reactions: The Advantages of Molecular Nanocrystals. It has recently been shown that photochemical excitation of crystalline ketones bearing radical stabilizing substituents on their two α-positions leads to efficient photodecarbonylation reactions. Experiments with crystals of carefully designed carbonyl compounds can be used to accomplish the formation of sigma bonds between adjacent quaternary stereogenic centers in good chemical yields and with remarkable chemical control. The exceptional simplicity, chemoselectivity, and stereospecificity of the solid-state reactions have been combined with well-developed ketone chemistry for the preparation of several natural products, including the sesquiterpenes cuparenone, herbertenolide, and touchuinyl acetate. Simple scale-up strategies based on nanocrystals and the use of sunlight suggest a remarkable potential for specialty chemical applications for these crystal-to-crystal reactions.
-
(c)
Nanotechnology as a Tool to Advance Pollution Prevention in the Semiconductor/IT Industry. Nanotechnology is not new to the semiconductor/IT (information technology) industry. Many features in semiconductors and integrated circuits have been at the nanoscale for decades. The International Technology Roadmap for Semiconductors identifies nanotechnology, both traditional semiconductor nanotechnology and novel nanomaterials, as a critical key to the future development of the industry, both in extending the current CMOS (complementary metal oxide semiconductor) scaling and in positioning beyond the current CMOS platform. The expanded use of nanotechnology and the incorporation of nanomaterials into semiconductor/IT processes and products not only provide potential solutions to many technical and business challenges facing the industry, but they also offer a tremendous opportunity to advance pollution prevention. Examples of nano-applications in semiconductor/IT processes and products that may result in energy and resource conservation, waste minimization, and energy-efficient products include bottom-up self-assembly, nanoarchitecture, and nanophotonics.
Environmental Monitoring
Environmental monitoring plays an important role in natural and resource conservation. It is crucial for environmental policy and research, because it provides policymakers, scientists, and the public with the data needed to understand and improve the environment.
-
(a)
Air Monitoring: Conventional air pollution monitoring employs large, fixed systems, which often fail to detect “hot spot” pollution peaks (Court et al. 2004). Solid-state gas sensors (SGSs), based on nanocrystalline metal oxide thin films, provide faster response with real-time analysis capability, higher resolution, simplified operation, and lower running costs compared to conventional methods, such as chemiluminescence and infrared spectrometry (Rickyerby and Morrison 2006).
-
(b)
Water Monitoring: When the EU Water Framework Directive was implemented in 2000, new regulations for water monitoring of organic substances were imposed and measurements had to be done down to microgram-per-liter (μgL−1) levels. Such accuracy, however, was difficult to achieve with conventional water monitoring technologies, and a necessity for faster, more sensitive systems appeared. This is how the automated water analyzer computer-supported system (AWACSS) was created.
-
(c)
Microbial Monitoring and Detection: The use of QDs as a fluorescence labeling system in microbial detection has been successfully demonstrated. Thiolated CdSe-core QDs could be conjugated with wheat germ agglutinin (WGA), a lectin that is commonly found in gram-positive bacteria (Kloepfer et al. 2003). By reacting with bacterial cells, this QD-conjugated WGA can bind to sialic acid and N-acetylglucosaminyl residues on bacterial cell walls. QDs can also be bioconjugated with a substrate such as iron, which is essential for the growth of pathogenic organisms inside a human host. Pathogenic bacteria normally contain receptors for a human host’s own shuttle protein transferrin and can harvest iron from transferrin (Modun et al. 2000). Using this metabolism-specific approach, transferrin-conjugated QDs can be transported through the membrane into metabolically active cells of iron-deprived Staphylococcus aureus and detected under fluorescence excitation. In contrast, no QD signal is observed in nonpathogenic bacteria. QDs can be further conjugated with specific antibodies to detect pathogenic microorganisms such as Cryptosporidium parvum and Giardia lamblia (Zhu et al. 2004).
The detection and monitoring of microorganisms can be further accelerated using nanoparticles in a fluorescence labeling system in microfluidic devices. Nanoparticle-based fluorescence reporting systems can be further developed to achieve rapid bacterial detection at the single-cell level (Zhao et al. 2004). To do so, the fluorescence intensity of individual nanoparticles was greatly increased through the encapsulation of thousands of fluorescent dye molecules in a protective silica matrix. After modifying the surface of these silica nanoparticles, they were conjugated with antigens specific to Escherichia coli strain O157 and used in immunological assays. With the improvement in the fluorescence reporting system, the fluorescence intensity emitted by one E. coli O157 cell was sufficient to be detected using a normal spectrofluorometer in a conventional plate-based immunological assay or to be accurately enumerated using a laboratory-made flow cytometer within 60 s of sample preparation.
Nanoparticles can be further used to immobilize microbial cells that can degrade or biorecover specific chemicals (Shan et al. 2005). Unlike conventional cell immobilization on micron-sized media or a fixed surface, magnetic nanoparticles (i.e., Fe3O4) were functionalized with ammonium oleate and coated on the surface of Pseudomonas delafieldii. By applying an external magnetic field to these microbial cells, these magnetic nanoparticle-coated cells were concentrated at a specific location on the reactor wall, separated from the bulk solution, and recycled for the treatment of the same substrate. These microbial cells were added into a bioreactor at a high biomass concentration and were demonstrated to desulfurize organic sulfur from fossil fuel (i.e., dibenzothiophene) as effectively as non-nanoparticle-coated cells (Shan et al. 2005). In environmental research, these nanoparticles further enhance the detection sensitivity of microbial monitoring and the degradation and recovery efficiency of chemicals.
Water Treatment–Based Nanotechnologies
Water Treatment Technologies Involves the Following
-
(a)
Filtration: Filtration involves removing the pollutants through a straining mechanism. Nanoparticles enhance traditional filtration methods because they are more strongly attracting the pollutants as they pass through the filter.
-
(b)
Desalination: Desalination is a very specific water treatment that focuses on creating freshwater from saltwater. Given that most of the Earth is covered with saltwater, desalination could be an important process to ensure that the world has enough drinking water. Desalination is often conducted using reverse osmosis.
-
(c)
Disinfection: Waterborne pathogens are the major cause of diseases and infection. A common disinfection technique uses powders or chlorine-based chemicals to kill bacteria in water. Nanotechnology can improve the disinfection capabilities of these chemicals, and new nanotechnology applications can kill organisms that are developing resistance to traditional disinfection techniques.
-
(d)
Photocatalysis: This application uses light to clean the water. As opposed to other techniques that simply collect pollutes from a dirty source, photocatalysis actually degrades the pollutants. In this process, a catalyst, often titanium dioxide, is placed into the dirty water. When UV light hits the particle, it begins a chemical reaction that degrades the pollutants in the system.
-
(e)
Remediation: In general remediation refers to removing heavy metals and other pollutants from water that was contaminated due to industrial processes. Remediation processes have to be quick and cheap in order to clean the effluent as it leaves industrial plants.
-
(f)
Sensors: It is important that pollutants be detected in water sources so that they can be treated. Nanosensors promise to be a cheap and easy method to detect whether a water source has been contaminated.
Water Purification
Water must be purified in order to remove harmful materials and make it suitable for human uses. Contaminants can include metals like cadmium, copper, mercury, lead, nickel, zinc, chromium, and aluminum; nutrients include phosphate, ammonium, nitrate, nitrite, phosphorus, and nitrogen, and biological elements such as bacteria, viruses, parasites, and biological agents from weapons. UV light is an effective purifier, but it is energy intensive, and application in large-scale systems is sometimes considered cost prohibitive. Chlorine, also commonly used in water purification, is undesirable because its production is one of the world’s most energy-intensive industrial processes, consuming about 1 % of the world’s total electricity output. Nanotechnology is opening new doors to water decontamination, purification, and desalinization and providing improved detection of waterborne harmful substances.
For example, iron nanoparticles have high surface area and reactivity and can be used to detoxify carcinogenic chlorinated hydrocarbons in groundwater. They can also render heavy metals like lead and mercury insoluble, reducing their contaminations. Dendrimers, with their spongelike molecular structure, can clean up heavy metals by trapping ions in their pores. Nanoscale filters have a charge membrane enabling them to treat both metallic and organic contaminant ions via both steric filtration based on the size of openings and Donnan filtration based on electrical charge. They can also be self-cleaning.
Gold nanoparticles coated with palladium have proven to be 2,200 times better than palladium for removing trichloroethylene from groundwater. In addition, photocatalytic nanomaterials enable ultraviolet light to destroy pesticides, industrial solvents, and germs. Titanium dioxide, for example, can be used to decontaminate bacteria-ridden water. When exposed to light, it breaks down bacterial cell membranes killing bacteria like E. coli. Purification and filtration of water can also be achieved through nanoscale membrane or using nanoscale polymer “brushes” coated with molecules that can capture and remove poisonous metals, proteins, and germs (Elvin 2007).
A new sterilizer, the RVK-NI, mixes ozone nanobubbles with oxygen microbubbles to produce, according to manufacturer Royal Electric, almost completely bacteria-free water for food processing. Ozone gas is a naturally occurring type of oxygen that is formed as sunlight passes through the atmosphere; it can be generated artificially by passing high-voltage electricity through oxygenated air. Because ozone is an unstable highly reactive form of oxygen, it is 51 times more powerful than chlorine, the oxidizer used by most food processers. With it, manufacturers can forego the use of environmentally harmful chlorine or other chemicals used in conventional water disinfection process. The ozone process is also said to kill bacteria and other microbes 3,000 times faster than chlorine. Desalination is another critical area of water purification. Dais Analytic Corporation, for example, is currently preparing its nanoclear desalinization process for commercialization. Low-cost techniques for water purification, self-cleaning, evaporation, reduction, and desalination could have tremendous impact by providing adequate supplies of clean water.
Water Treatment
Conventional water treatment technologies include filtration, ultraviolet radiation, chemical treatment, and desalination, whereas the nano-enabled technologies include a variety of different types of membranes and filters based on carbon nanotubes, nanoporous ceramics, magnetic nanoparticles, and other nanomaterials.
In a recent study, several polymeric nanofiltration and reverse osmosis membranes were tested for the treatment of brackish groundwater (water that is salty, but less so than seawater). The tests showed that nanofiltration membranes can produce potable water from the brackish groundwater. As expected, the reverse osmosis membranes removed about 99 % of all the solutes, but the concentrations of essential nutrients, such as calcium and magnesium ions, were reduced to levels that were below the specifications of the World Health Organization standard for drinking water. The product water therefore had to be spiked with these nutrients to provide drinking water of the required quality.
These studies also underline the importance of making communities aware of the actual quality of their drinking water because it is not possible to detect contaminants, for example, by simply observing the physical properties of the water (i.e., smell, taste, and color).
However, it is not enough to develop technical solutions to these problems; the technology must also be transferred to the country that needs it. To be effective, technology transfer must be accompanied by technology adaptation and technology adoption to take account of the technical capability, infrastructure, and market potential of the developing country that needs the technology.
Nanofiltration
Nanofiltration is a relatively recent membrane filtration process, which holds promise to deliver cost-effective water and air treatment solutions. Nanomembranes (NMs) are used not only to remove contaminants from polluted water and air but also for desalination of salty water. There are two types of NMs currently available on the market: nanofilters, using either carbon nanotubes (CNTs) or nanocapillary arrays to mechanically remove impurities, and reactive NMs, where functionalized NPs chemically convert the contaminants into safe by-products.
Ordered arrays of densely packed, vertically aligned CNTs are used as membranes to filter out water impurities while letting the water flow freely through the filter. Carbon nanotube membranes (CNMs) are able to remove almost all kinds of contaminants including bacteria, viruses, and organic pollutants. CNMs are also effective in desalinating salty water.
Until now, several NM types, which effectively remove CO2 from industrial flue gases, were developed from both polymeric and inorganic materials (i.e., carbon-based membranes, mesoporous oxide membranes, and zeolite membranes). These membranes show better selectivity and higher removal capacity than their conventional alternatives, and they are often more cost-efficient (Fujiokya et al. 2007). The large-scale industrial application of the CO2-removing NMs would contribute to the reduction of the anthropogenic CO2 emissions in the atmosphere and impede climate change.
Recently, enzymatic quorum quenching (in the form of a free enzyme or an immobilized form on a bead) was successfully applied to a submerged membrane bioreactor with a microfiltration membrane for wastewater treatment as a novel approach to control membrane biofouling. In this study, a quorum-quenching enzyme (acylase) was directly immobilized onto a nanofiltration membrane to mitigate biofouling in a nanofiltration process. In a flow cell experiment, the acylase-immobilized membrane with quorum-quenching activity prohibited the formation of mushroom-shaped mature biofilm due to reduced secretion of extracellular polymeric substances (EPS). The acylase-immobilized membrane maintained more than 90 % of its initial enzyme activity for more than 20 iterative cycles of reaction and washing procedure. In the lab-scale continuous crossflow nanofiltration system operated at a constant pressure of two bar, the flux with the acylase-immobilized nanofiltration (NF) membrane was maintained at more than 90 % of its initial flux after a 38 h operation, whereas that with the raw NF membrane decreased to 60 % accompanied with severe biofouling. The quorum-quenching activity of the acylase-immobilized membrane was also confirmed by visualizing the spatial distribution of cells and polysaccharides on the surface of each membrane using confocal laser scanning microscope (CLSM) image analysis technique.
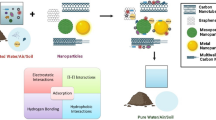
Metal Ion Recovery from Solutions by Dendrimer Filtration
Diallo et al. (2005) have developed a dendrimer-enhanced ultrafiltration (DEF) system that can remove dissolved cations from aqueous solution ions using low-pressure membrane filtration (Fig. 7.3). DEF works by combining dendrimers with ultrafiltration or microfiltration membranes. Functionalized and water-soluble dendrimers with large molar mass are added to an incoming aqueous solution and bind with the target ions. For most metal ions, a change in solution acidity and/or salinity causes the dendrimers to bind or release the target metal ions. Thus, a two-stage filtration process can be used to recover and concentrate a variety of dissolved ions in water including Cu(II), Ag(I), and U(VI). A key feature of the DEF process is the combination of dendritic polymers with multiple chemical functionalities with the well-established separation technologies of ultrafiltration (UF) and microfiltration (MF). This allows a new generation of metal ion separation processes to be developed that are flexible, reconfigurable, and scalable. The flexibility of DEF is illustrated by its modular design approach. DEF systems will be designed to be “hardware invariant” and thus reconfigurable in most cases by simply changing the “dendrimer formulation” and “dendrimer recovery system” for the targeted metal ions of interest. The DEF process has many applications including the recovery of valuable metal ions such as platinum group metals, rare-earth metals, and actinides from mineral/hydrometallurgical processing solutions, in situ leach mining solutions, and industrial wastewater solutions.
Recovery of metal ions from aqueous solutions by dendrimer filtration (Adapted from Diallo 2008)
Research is under way to use advance nanotechnology in water purification for safe drinking. Nanotechnology, the deliberate manipulation of matter at size scales of less than 100 nm, holds the promise of creating new materials and devices which take advantage of unique phenomena realized at those length scales, because of their high reactivity due to the large surface to volume ratio (Ichinose et al. 1992). Nanoparticles are expected to play a crucial role in water purification (Stoimenov et al. 2002). The environmental fate and toxicity of a material are critical issues in material selection and design for water purification. No doubt that nanotechnology is better than other techniques used in water treatment, but today the knowledge about the environmental fate, transport, and toxicity of nanomaterials (Colvin 2003) is still in infancy. Advances in nano science and engineering suggest that many of the current problems involving water quality could be resolved or greatly diminished by using nonabsorbent, nanocatalysts, bioactive nanoparticles, nanostructured catalytic membranes, submicron, nanopowder, nanotubes, magnetic nanoparticles, granules, flake, high surface area metal particle supramolecular assemblies with characteristic length scales of 9–10 nm including clusters, micromolecules, nanoparticles, and colloids have a significant impact on water quality in natural environment (Mamadou and Savage 2005). Nano sensors are used for detection of pesticides (Nair and Pradeep 2004) and biological agents including metals (e.g., cadmium, copper, lead, mercury, nickel, zinc), nutrients (e.g., phosphate, ammonia, nitrate, nitrite), cyanide organics, algae (e.g., cyanobacterial toxins) viruses, bacteria, parasites, antibiotics, and biological agents are used for terrorism. Innovations in the development of novel technologies to desalinate water are among the most exciting and seem to have promise (Diallo et al. 2005). Opportunities and challenges of using nanomaterials in the purification of surface water, groundwater, and industrial wastewater streams are a matter of continuing concern. A misconception and one of the many impressions that people have about the future of nanotechnology is the expectation that nanoparticles can be used to kill harmful organisms, to repair body tissue, to improve water quality, and to cure disease. Recent applications of nanoparticulate silver have included open wound and burn treatment, and preliminary studies have shown that a 20 ppm silver colloidal suspension (∽30 nm diameter) in purified water has a 100 % cure rate for malaria. Titanium dioxide, especially as nanoparticulate anatase, is also an interesting antibacterial, with notable photocatalytic behavior. But ultrafine anatase has also been identified as cytotoxic and in vivo studies have shown that it can be severely toxic in the respiratory system (Oberdörste 2001; Ishibashi 2000). Nanocapsules and nanodevices may present new possibilities for drug delivery, gene therapy, medical diagnostics, antimicrobial activity, etc. The effect of particle size on the adsorption of dissolved heavy metals to iron oxide and titanium dioxide nanoparticles is a matter of laboratory-scale experiments. Iron oxide and titanium dioxide are good sorbents for metal contaminants. Spherical nanoparticle have a similar size and shape like resin beads that are used in water purification. Ligands, fulvic acids, humic acids, and their aggregates have a significant impact on contaminant mobility, reactivity, and bioavailability. Nanoparticles can also be designed and synthesized to act as either separation or reaction media for pollutants. The high surface area to mass ratios of nanoparticles can greatly enhance the adsorption capacities of sorbent materials. In addition to having high specific surface areas, nanoparticles also have unique adsorption properties due to different distributions of reactive surface sites and disordered surface regions. Their extremely small feature size is of the same scale as the critical size for physical phenomena, for example, the radius of the tip of a crack in a material may be in the range 1–100 nm. The way a crack grows in a larger-scale, bulk material is likely to be different from crack propagation in a nanomaterial where crack and particle size are comparable. Fundamental electronic, magnetic, optical, chemical, and biological processes are also different at this level.
Environmental Nanotechnology: Perspectives
Biogenic Synthesis of Nanoparticles Using Microorganisms
Numerous microorganisms are reported to biosynthesize gold and silver nanoparticles by NADPH-dependent reductase enzymes that reduce metal salts to nanoparticles through electron shuttle enzymatic metal reduction process (Table 7.4). The pollution detection nanostructures and/or devices are being used for a variety of environmental applications.
Sensors
The characterization of environmental sensors is based primarily on the physics involved and their operating mechanisms. For example, chromatography relies on the separation of complex mixtures by percolation through a selectively adsorbing medium with subsequent detection of compounds of interest. Electrochemical sensors include sensors that detect signal changes (e.g., resistance) caused by an electric current being passed through electrodes that interact with chemicals. Mass sensors rely on disturbances and changes to the mass of the surface of the sensors during interaction with chemicals. Optical sensors detect changes in visible light or other electromagnetic waves during interactions with chemicals. Within each of these categories, some sensors may exhibit characteristics that overlap with those of other categories. For example, some mass sensors may rely on electrical excitation or optical settings. The nanostructure material developed for detection of pollution monitoring and remediation is highlighted in Table 7.5.
Sensors: Biosensors, Electrochemical Sensors, Mass Sensors, Optical Sensors, Gas Sensors
Nanotechnology and Pollution Control
Pollution results from resource production and consumption, which in their current state are wasteful. Nanofabrication holds much potential for effective pollution control, but it currently faces many problems that prevent it from mass commercialization, particularly its high cost. Nanotechnology plays a vital role in air and water pollution control. Air pollution can be remediated using nanotechnology in several ways. One is through the use of nanocatalysts with increased surface area for gaseous reactions. Catalysts work by speeding up chemical reactions that transform harmful vapors from cars and industrial plants into harmless gases. Catalysts currently in use include a nanofiber catalyst made of manganese oxide that removes volatile organic compounds from industrial smokestacks. Other methods are still in development. Another approach uses nanostructured membranes that have pores small enough to separate methane or carbon dioxide from exhaust. John Zhu of the University of Queensland is researching carbon nanotubes (CNTs) for trapping greenhouse gas emissions caused by coal mining and power generation. CNT can trap gases up to a hundred times faster than other methods, allowing integration into large-scale industrial plants and power stations. This new technology both processes and separates large volumes of gas effectively, unlike conventional membranes that can only do one or the other effectively.
As with air pollution, harmful pollutants in water can be converted into harmless chemicals through chemical reactions. Trichloroethene, a dangerous pollutant commonly found in industrial wastewater, can be catalyzed and treated by nanoparticles. Studies have shown that these materials should be highly suitable as hydrodehalogenation and reduction catalysts for the remediation of various organic and inorganic groundwater contaminants. Nanotechnology eases the water-cleansing process because inserting nanoparticles into underground water sources is cheaper and more efficient than pumping water for treatment. The deionization method using nanosized fibers as an electrode is not only cheaper but also more energy efficient. Traditional water filtering systems use semipermeable membranes for electrodialysis or reverse osmosis. Decreasing the pore size of the membrane to the nanometer range would increase the selectivity of the molecules allowed to pass through. Membranes that can even filter out viruses are now available. Also widely used in separation, purification, and decontamination processes are ion exchange resins, which are organic polymer substrate with nanosized pores on the surface where ions are trapped and exchanged for other ions (Alchin 2008). Ion exchange resins are mostly used for water softening and water purification. In water, poisonous elements like heavy metals are replaced by sodium or potassium. However, ion exchange resins are easily damaged or contaminated by iron, organic matter, bacteria, and chlorine.
Recent developments of nanowires made of potassium manganese oxide can clean up oil and other organic pollutants while making oil recovery possible (Yuan et al. 2008). These nanowires form a mesh that absorbs up to 20 times its weight in hydrophobic liquids while rejecting water with its water-repelling coating. Since the potassium manganese oxide is very stable even at high temperatures, the oil can be boiled off the nanowires and both the oil and the nanowires can then be reused (Yuan et al. 2008).
Removal of Pollutant by Adsorbent
The nanoparticles have been investigated as adsorbent for the removal of organic and inorganic contaminants. The nanosized metal oxides and natural nanosized clays have been investigated for the removal of metals and inorganic ions. Besides, oxidized and hydroxylated CNTs are good absorbers for metals such as Cu, Ni, Cd, and Pb. Pristine multiwalled CNTs have been found to be stronger adsorber materials for organometallic compounds. CNTs have also been found as a powerful adsorbent for a wide variety of organic compounds from aquatic environment, which include dioxin, polynuclear aromatic hydrocarbons (PAHs), DDT and its metabolites, PBDEs, chlorobenzenes and chlorophenols, trihalomethanes, bisphenol A and nonylphenol, phthalate esters, dyes, pesticides, and herbicides such as sulfuron derivatives, atrazine, and dicamba. Nanoporous polymers which have cross-linked and copolymerized with functionalized CNTs have been demonstrated for a high sorption capacity for a variety of organic compounds such as p-nitrophenol and trichloroethylene.
Nanoremediation Technologies
Environmental protection and pollution issues are frequently discussed worldwide as topics that need to be addressed sooner rather than later. Nanotechnology can strive to provide and fundamentally restructure the technologies currently used in environmental detection, sensing, and remediation for pollution control. Some nanotechnology applications that are near commercialization include nanosensors and nanoscale coatings to replace thicker, more wasteful polymer coating that prevent corrosion, nanosensors for detection of aquatic toxins, nanoscale biopolymers for improved decontamination and recycling of heavy metals, nanostructured metals that break down hazardous organics at room temperature, smart particles for environmental monitoring and purification, and nanoparticles as novel photocatalyst for environmental cleanup.
Environmental cleanup has promoted the development of highly efficient photocatalysts that can participate in detoxification reactions. Environmental remediation by photocatalysts comes with several advantages: direct conversion of pollutants to nontoxic by-products without the necessity for any other associated disposal steps, use of oxygen as oxidant and elimination of expensive oxidizing chemicals, potential for using free and abundant solar energy, self-regeneration and recycling of photocatalyst, etc. A significant amount of research on semiconductor-catalyzed photooxidation of organic chemicals has been carried out during the past 15 years. The ability to catalyze the destruction of a wide variety of organic chemicals and complete oxidation of organics to CO2 and dilute mineral acids in many cases, the lack of inherent toxicity, and the resistance to photodegradation at low cost render this process highly suitable for environmental remediation.
Degradation of Pollutants Using Semiconductors: TiO2 and ZnO
The semiconductor TiO2 nanoparticles have been extensively studied for oxidative transformation of organic and inorganic contaminants (Obare and Meyer 2004; Hoffmann et al. 1995). These are now used in a variety of products such as self-cleaning glass, disinfectant tiles, and filters for air purification (Fujishima et al. 1999). TiO2 electrodes have the capacity to determine the chemical oxygen demand of water and are used as sensors for monitoring contaminated water (Kim et al. 2001). TiO2 nanoparticles can be immobilized on different supports which are used for the solar detoxification of water and air. These engineered nanoparticles are known for their interaction with organic, inorganic, and biological contaminants such as heavy metals, organochlorine pesticide, arsenic, and phosphates in water, induced by ultraviolet light. TiO2 leads to pollutant degradation through two everyday chemical reactions: reduction and oxidation. Once excited by UV and TiO2 electron–hole pairs develop, these electrons have sufficient oxidizing potential to oxidize pollutants in wastewater (Bahnemann 2004). Interestingly, the combination of UV and TiO2 generates bactericidal activity, which attacks several types of bacteria. This approach thus provides a comprehensive treatment procedure since chemical species and pathogens can be removed from wastewater simultaneously. Other nanoparticles with semiconducting properties, such as ZnO, ZnS, F2O3, and CdS, can be used for photocatalysis oxidation. TiO2 is biologically and chemically inert and has demonstrated great resistance to corrosion along with the capacity to be used repetitively without substantial loss of catalytic activity, and it is therefore inexpensive to use. In light of these properties, TiO2 is potentially more attractive for environmental applications than other oxidative nanoparticles (Pirkanniemi and Sillanpaa 2002). However, TiO2 requires ultraviolet light and, consequently, is effective only for treatment of transparent wastewater. Several research groups have attempted to overcome this limitation by extending the nanoparticle excitation into visible light with the doping of TiO2 with transition metal ions or sensitizing dye such as Ru (II) polypyridyl complex, or investigated an approach involving a tube reactor production based on hollow glass tubes (Kamat and Meisel 2003). The tubes are extremely coated with TiO2 and UV light passes through the hollow tubes. Preliminary results suggested that combining ultrasound processes such as sonolysis and photocatalysis improves pollutant oxidation and could be an effective approach. Several pilot projects are under way to evaluate the potential of solar reactors to generate the energy required for TiO2 excitation and detoxification of polluted water. If these attempts are successful, sunlight could play an economic and eco-friendly role in the treatment of wastewater.
Nanoparticulate titanium dioxide (TiO2) has been traditionally used in environmental remediation because of its low toxicity, high photoconductivity, high photostability, availability, and low cost (Wallington 2005). Novel technologies and improved processes, however, enabled the development of a variety of Tio2 photocatalytic derivatives. Metals such as copper (Cu), silver (Ag), gold (Au), and platinum (Pt) have been tested for their ability to improve the decontamination activity of TiO2 with Cu, for example, accelerates the reduction of hexavalent chromium (Cr+6)ions. The coupling of TiO2 with Au or Ag results in similar reductive capabilities. The TiO2-based p–n junction nanotubes (NTs) represent the most recent innovation in the field of nanophotocatalysts. The NTs contain platinum (Pt) (inside) and TiO2 (on the outside). The TiO2 coating of the tubes acts as an oxidizing surface, while the inside of the tube is reductive (Cheng and Cheng 2005). The ability of p–n junction on NTs to destroy toluene was tested by Chen et al., and the results showed that they exhibit much higher decontamination rates than non-nanotube materials (Cheng and Cheng 2005).
Unlike TiO2, semiconductor nanoparticles such as ZnO are able to emit strongly in the visible region, and the visible emissions of ZnO are usually very sensitive to whole scavengers such as phenols or iodide ions. ZnO particles thus seem good candidates for use as sensors for chemical compounds. It has been claimed that sensor system based on ZnO could reach a detection sensitivity of 1 ppm. Furthermore, ZnO has the ability to induce contaminant degradation under ultraviolet light (Kamat and Meisel 2003). Consequently, the use of ZnO can be considered as a promising way to simultaneously sense and destroy toxic chemicals. It has been reported that nanostructured ZnO films could simultaneously detect and degrade organic compounds in water. Such a catalyst system is useful to induce contaminant degradation where the system senses a targeted molecule, thus avoiding destruction of molecules present in the environment.
Degradation of Pollutants Using Iron Nanoparticles
Laboratory research has established that nanoscale metallic iron is very effective in destroying a wide variety of common contaminants such as chlorinated methanes, brominated methanes, trihalomethanes, chlorinated ethane, chlorinated benzenes, other polychlorinated hydrocarbons, pesticides, and dyes (Zhang 2003). The basis for the reaction is the corrosion of zerovalent iron in the environment. Contaminants such as tetrachloroethene can readily accept the electrons from iron oxidation and be reduced to ethane. However, nanoscale zerovalent iron (nZVI) can reduce not only organic contaminants but also the inorganic anions nitrate, which is reduced to ammonia (Sohn et al. 2006; Liou et al. 2006) perchlorate (plus chlorate or chlorite) and then reduced to chloride (Cao et al. 2005), selenate, arsenate (Kanel et al. 2006), arsenite (Jegadeesan et al. 2005), and chromate (Ponder et al. 2000; Manning et al. 2007). nZVI is also efficient in removing dissolved metals from solution, e.g., Pb and Ni (Li and Zhang 2006). The reaction rates for nZVI are at least 25–30 times faster and also sorption capacity is much higher compared with granular iron (Li et al. 2006). The metals are reduced to either zerovalent metals or lower oxidation states, e.g., Cr (III), and are surface complexed with the iron oxides that are formed during the reaction. Some metal can increase the dechlorination rate of organics and also lead to more benign products, whereas other metals decrease the reactivity (Lien et al. 2007). Most of the research using nZVI has been devoted to groundwater and soil remediation. Nanoremediation methods entail the application of reactive nanomaterials for transformation and detoxification of pollutants. These nanomaterials have properties that enable both chemical reduction and catalysis to mitigate the pollutants of concern. For nanoremediation in situ, no groundwater is pumped out for aboveground treatment, and no soil is transported to other places for treatment and disposal (Otto et al. 2008).
Nanomaterials have highly desired properties for in situ applications. Because of their minute size and innovative surface coatings, nanoparticles may be able to pervade very small spaces in the subsurface and remain suspended in groundwater, allowing the particles to travel farther than larger, macro-sized particles and achieve wider distribution. However, in practice, current nanomaterials used for remediation do not move very far from their injection point (Tratnyek and Johnson 2006). Many different nanoscale materials have been explored for remediation, such as nanoscale zeolites, metal oxides, carbon nanotubes and fibers, enzymes, various noble metals [mainly as bimetallic nanoparticles (BNPs)], and titanium dioxide. Of these, nanoscale zerovalent iron (nZVI) is currently the most widely used. The different nanomaterials, along with the pollutants they could potentially remediate, are listed in Supplemental Material for a comprehensive overview of the chemistry and engineering of various nanotechnology applications addressed in Supplemental Material used for remediation (Theron et al. (2008) and Zhang (2003)). nZVI particles range from 10 to 100 nm in diameter, although some vendors sell micrometer-scale iron powders as “nanoparticles.” Typically, a noble metal (e.g., palladium, silver, copper) can be added as a catalyst. The second metal creates a catalytic synergy between itself and Fe and also aids in the nanoparticles’ distribution and mobility once injected into the ground (Saleh et al. 2007; Tratnyek and Johnson 2006; U.S. EPA 2008). These BNPs may contain more than two different metals. The second metal is usually less reactive and is believed to promote Fe oxidation or electron transfer (U.S. EPA 2008). Some noble metals, particularly palladium, catalyze dechlorination and hydrogenation and can make the remediation more efficient (U.S. EPA 2008; Zhang and Elliott 2006).
In the 1990s, Fe at the nanoscale was synthesized from Fe(II) and Fe(III) to produce particles ranging from 10 to 100 nm, initially using borohydride as the reductant, and examined in laboratory studies. Zhang (2003) tested nZVI for the transformation of a large number of pollutants, most notably halogenated organic compounds commonly detected in contaminated soil and groundwater. The author reported that nanoscale Fe particles are very effective for the transformation and detoxification of a variety of common environmental pollutants, including chlorinated organic solvents, organochlorine pesticides, and polychlorinated biphenyls (PCBs). According to Zhang (2003), Fe-mediated reactions should produce an increase in pH and a decrease in the solution redox potential created by the rapid consumption of oxygen, other potential oxidants, and the production of hydrogen. Although batch reactors produce pH increases of 2–3 and an oxidation–reduction potential (ORP) range of −500 to −900 mV, it is expected that the pH and ORP would be less dramatic in field applications where other mechanisms reduce the chemical changes (Zhang 2003). Previous work showing an increase of pH by 1 and an ORP in the range of −300 to −500 mV supports this assessment (Elliott and Zhang 2001; Glazier et al. 2003). Zhang (2003) also showed that modifying Fe nanoparticles could enhance the speed and efficiency of the remediation process.
The first field application was reported in 2000. Nanoparticles have been shown to remain reactive in soil and water for up to 8 weeks and can flow with the groundwater for >20 m. In one study, Zhang (2003) produced a 99 % reduction of TCE within a few days of injection.
Because nanoscale particles are so small, Brownian movement or random motion, rather than wall effects, dominates their physical movement or transport in water. The movement of micrometer-scale particles, especially microscale metal particles, is largely controlled by gravity-induced sedimentation because of their size and high density. In the absence of significant surface electrostatic forces, nanosized particles can be easily suspended in water during the design and manufacturing stages, thus providing a versatile remediation tool that allows direct injection as a liquid into the subsurface where contaminants are present. Coating the Fe particles to improve mobility and catalytic reaction rates is important. Some of the particles flow with the groundwater and remain in suspension for various amounts of time, whereas others are filtered out and bind to soil particles, providing an in situ treatment zone that could hold back emanating plumes (Henn and Waddill 2006).
The high reactivity of nZVI particles is in part a direct result of their high specific surface area. For example, nZVI produced by the borohydride method has surface areas in the range of 20–40 m2/g, which can yield 10–1,000 times greater reactivity compared with granular Fe, which has a surface area <1 m2/g (Wang and Zhang 1997). nZVI’s small particle size also allows more of the material to penetrate into soil pores, and it can be more easily injected into shallow and deep aquifers.
Initially, Fe nanoparticles have a core of ZVI and an outer shell of Fe oxides, which suggest the following redox reactions:
where s is solid, aq is aqueous, g is gas, and l is liquid (Matheson and Tratnyek 1994).
Although Fe nanoparticles have been shown to have a strong tendency to form microscale aggregates, possibly because of their weak surface charges, coatings can be applied to change the surface properties. These different forms of Fe could be useful for the separation and transformation of a variety of contaminants, such as chlorinated organic solvents, organochlorine pesticides, PCBs, organic dyes, various inorganic compounds, and the metals As(III) (trivalent arsenic), Pb(II) (bivalent lead), copper [Cu(II) (bivalent copper)], Ni(II) (bivalent nickel), and Cr(VI) (hexavalent chromium) (Sun et al. 2006).
Nanoremediation, particularly the use of nZVI, has site-specific requirements that must be met in order for it to be effective. Adequate site characterization is essential, including information about site location, geologic conditions, and the concentration and types of contaminants. Geologic, hydrogeologic, and subsurface conditions include composition of the soil matrix, porosity, hydraulic conductivity, groundwater gradient and flow velocity, depth to water table, and geochemical properties (pH, ionic strength, dissolved oxygen, ORP, and concentrations of nitrate, nitrite, and sulfate). All of these variables need to be evaluated before nanoparticles are injected to determine whether the particles can infiltrate the remediation source zone and whether the conditions are favorable for reductive transformation of contaminants. The sorption or attachment of nanoparticles to soil and aquifer materials depends on the surface chemistry (i.e., electrical charge) of soil and nanoparticles, groundwater chemistry (e.g., ionic strength, pH, and presence of natural organic matter), and hydrodynamic conditions (pore size, porosity, flow velocity, and degree of mixing or turbulence). The reactions between the contaminants and the nZVI depend on contact or probability of contact between the pollutant and nanoparticles (U.S. EPA 2007, 2008).
Degradation of Pollutants Using Bismuth Vanadate (BiVO4) Photocatalyst
One of the promising non-titania-based visible-light-driven semiconductor photocatalyst is bismuth vanadate (BiVO4). It was applied as catalyst in oxidative dehydrogenation of ethyl benzene to styrene (Kudo et al. 1999) and as photocatalyst where O2 was successfully evolved from aqueous silver nitrate solution under visible-light radiation (Kudo et al. 2001). There are exactly three main crystal forms of BiVO4, known as tetragonal zircon-type structure, tetragonal scheelite structure, and monoclinic distorted scheelite structure (Gotic et al. 2005). The color of BiVO4 varies from inhomogeneously yellow brown to homogeneously lemon yellow and depends on many factors including phase composition, stoichiometry, particle size, and morphology. The photocatalytic activity of BiVO4 has also been reported to be strongly influenced by the crystal structure; monoclinic BiVO4 showed better photoactivity than tetragonal (Kudo et al. 1999).
Several synthesis methods have been used to prepare monoclinic BiVO4 such as sonochemical method (Zhou et al. 2006), solid-state reaction (Gotic et al. 2005), reflux (Zhou et al. 2007), solgel (Hirota et al. 1992), and hydrothermal method (Liu et al. 2003; Zhang and Zhang 2009; Zhang et al. 2006, 2009). The photocatalytic activities of BiVO4 nanocrystals prepared by sonochemical methods, evaluated by decolorization of methyl orange under visible-light irradiation, showed better photodegradation rate (95 % in 30 min) than that of sample prepared by solid-state reaction (8 %).
The photocatalytic activities of BiVO4 were evaluated by degrading methylene blue (MB) dye solution under visible-light irradiation. A 23 W light bulb was used as a visible-light source and the experiments were carried out at room temperature for 4 h. Prior to photocatalysis experiment, the amount of MB dye removed via photolysis and adsorption process was determined. Eleven percent of the dye was removed via photolysis after 4 h of irradiation time. However, under visible-light irradiation, significant increase in the percentage removal of MB was observed. Even though higher percentage of MB photodegraded by BiVO4 has been reported, the authors have used a much higher intensity of light (200 W of Xe arc lamp) (Yu et al. 2009) compared to these studies (23 W). This indicates the potential of the prepared BiVO4 to be used as photocatalyst in photodegrading MB dye at low light intensity. The removal of MB dye by BiVO4 is influenced by the surface area and the crystal structure of the catalyst. The higher the surface area of the catalyst, the higher is the removal by adsorption process. Removal of MB via photocatalytic processes is more dominant than adsorption process for a more distorted monoclinic scheelite BiVO4 (Abdullah et al. 2009).
Degradation of Pollutants Using CdS
As an important II–IV compound semiconductor, CdS, with direct bandgap energy of 2.4 eV at room temperature, has attracted much attention because of its unique properties and potential application in light-emitting diodes, flat-panel displays, solar cells, photocatalysis, and thin-film transistors. Since a material’s properties depend greatly on its morphological features, nanostructured CdS with different sizes, shapes, and dimensionalities has been fabricated and characterized. Extensive studies have prepared CdS nanostructures with various shapes, such as nanowires, nanobelts, and self-organized spheres on the scale of micro-/nanometers. The size, shape, and crystalline structure of semiconductor nanocrystallites, which lead to considerable changes in the recombination of electrons and holes trapped at spatially separated donors and acceptors, are important in determining their optical properties. For instance, Xu et al. reported the synthesis of nanostructured flower-like CdS by a solvothermal method using ethylenediamine as a structure-directing template. Qing Xia et al. successfully synthesized a wide range of cadmium sulfide (CdS) three-dimensional (3D) polycrystalline walnut-like nanocrystals by a solvothermal method with polyvinylpyrrolidone (PVP) as stabilizer. Lin et al. synthesized uniform-sized CdS hollow nanospheres via hydrothermal treatment of aqueous solutions of cadmium acetate and thiourea and reported that the spheres could be made solid or hollow by manipulating the precursor Cd/S molar ratio in the synthesis system. Li et al. synthesized CdS microspheres assembled from high-quality nanorods by a hydrothermal method using PVP as a surfactant. Danjun Wang et al. synthesized novel 3D dendritic CdS nanoarchitectures via a facile template-free hydrothermal process using CdSO4 and thiourea as precursors and cetylpyridinium chloride (CPC) as a capping reagent. Zhang et al. prepared CdS submicro- and microspheres by a convenient hydrothermal process through the reactions of CdCl2 and Na2S2O3 in aqueous solution at a relatively low temperature. The solution phase or hydrothermal or solvothermal technique has been demonstrated as an effective method for preparing low-dimensional nanomaterials from sulfides. Liu et al. (2012) have synthesized TiO2 film coated on fiber glass cloth by solgel method and further sensitized by CdS nanoparticles using a sequential chemical bath deposition technique. Gaseous benzene was adopted as a model pollutant to evaluate the photocatalytic performances of the supported TiO2 films sensitized with variable content of CdS nanoparticles, which are obtained by adjusting the concentration of Cd2+ or S2− precursor solution. Along with the increase of the CdS amount, the photocatalytic activity of these samples initially increases and then shows a downward trend. When the concentration of Cd2+ or S2− precursor solution achieved 0.005 M, the sample performs the best photocatalytic property and the degradation efficiency reaches 92.8 % and 32.7 % under uv–vis and visible-light irradiation, respectively. The enhancement of the photocatalytic activity could be attributed to the formation of micro-heterojunction. However, a thick CdS sheath-covered catalyst surface will form when a high concentration of Cd2+ or S2− solution is used. The low photogenerated electron–hole pairs’ separation efficiency and the photo corrosion process of CdS decrease the photocatalytic activity gradually.
Single-crystalline CdS nanoparticles were synthesized for the first time by the composite-molten-salt (CMS) method, which had numerous advantages including one step, ambient pressure, low temperature, template free, and low cost. The influence of temperature, growth time, and amount of salts on the morphology of CdS nanoparticles was systematically investigated. It shows that a smaller size of CdS nanoparticles can be obtained under lower temperature, less growth time, and more composite salts. uv–vis reflection spectrum of the nanoparticles reveals that the nanoparticles have a bandgap of 2.34 eV. Photoluminescence spectrum was also carried out to explore its optical property. Photocatalytic degradation of rhodamine B (RhB) and methylene blue (MB) in the presence of the CdS nanoparticles was compared with that in the presence of the commercial TiO2 nanoparticles under the simulated sunlight (Li et al. 2012).
Doping of Photocatalysts for Effective Degradation
Doping of TiO2 has been an important approach in bandgap engineering to change the optical response of semiconductor photocatalysts. The main objective of doping is to induce a bathochromic shift, i.e., a decrease of the bandgap or introduction of intra-bandgap states, which results in the absorption of more visible light. Doping may lead to photocatalytic systems that exhibit enhanced efficiency (Carp et al. 2004). There are three different main opinions regarding modification mechanism of TiO2 doped with nonmetals:
-
1.
Bandgap narrowing: Asahi et al. (2001) found N 2p state hybrids with O 2p states in anatase TiO2 doped with nitrogen because their energies are very close, and thus, the bandgap of N-TiO2 is narrowed and able to absorb visible light.
-
2.
Impurity energy level: Hiroshi et al. (2003) stated that TiO2 oxygen sites substituted by the nitrogen atom form isolated impurity energy levels above the valence band. Irradiation with UV light excites electrons in both the VB and the impurity energy levels, but illumination with visible light only excites electrons in the impurity energy level.
-
3.
Oxygen vacancies: Ihara et al. (2003) concluded that oxygen-deficient sites formed in the grain boundaries are important to emerge vis-activity, and nitrogen doped in part of oxygen-deficient sites is important as a blocker for reoxidation.
Noble metals including Pt, Ag, Au, Pd, Ni, Rh, and Cu have been reported to be very effective at enhancing photocatalysis by TiO2 (Rupa et al. 2009; Adachi et al. 1994; Wu and Lee 2004). Because the Fermi levels of these noble metals are lower than that of TiO2, photoexcited electrons can be transferred from the conduction band of TiO2 to metal particles deposited on the surface of TiO2, while photogenerated holes in the valence band remain on TiO2. This greatly reduces the possibility of electron–hole recombination, resulting in efficient separation and higher photocatalytic activity.
Sustaining a Clean Environment
Mitigating Ultimate Climate Change Impact
Nanoscale metal organic frameworks (MOFs) and zeolite imidazole frameworks (ZIFs) are promising CO2 sorbents with high adsorption capacity, selectivity, and reversibility. However, the first generation of nanoscale MOFs and ZIFs can only perform a single function, i.e., CO2 separation. Thus, their use alone might not lead to the revolutionary advances needed to significantly decrease the atmospheric release of greenhouse gases by capturing CO2 and converting it to useable products (e.g., fuels and chemicals). The vision for the 5–10 year time frame is that convergence between nanotechnology, chemical separations, catalysis, and systems engineering will lead to revolutionary advances in CO2 capture and conversion technologies, including:
-
Nanoscale sorbents containing functionalized size- and shape-selective molecular cages that can capture CO2 and convert it to useable products
-
Nanoporous fibers and/or membranes containing functionalized size- and shape-selective molecular cages that can capture CO2 and convert it to useable products
In addition to CO2 capture, transformation, and storage, geoengineering is being considered as a potential climate mitigation technology. The ultimate goal of geoengineering is to reduce global warming by developing and deploying large-scale “cooling” systems in the stratosphere.
Reducing Global Warming
As explained, clean nanotechnologies have the potential to produce plentiful consumer goods with much lower throughput of materials and much less production of waste and to reduce sources of chemical pollution. According to the vision of Drexler, the natural feedstock will be the input materials; using them will emit the same materials, which can be reused again. With NT, energy through solar cells and clean fuels from solar energy, air, and water can be made. With cheap solar and fuel energy, coal and petroleum can be replaced, ignored, and left in the ground. Like this many sources, pollution can be step by step eliminated.
The release of other gases, such as chlorofluorocarbons (CFCs) used in foaming plastics, is often a side of primitive manufacturing processes. Foaming plastics will hardly be used during molecular manufacturing. These materials can be replaced or controlled, and they include the gases most responsible for ozone depletion. Nanotechnology based devices/systems also absorb CO2 that could help mitigation of global warning and bring the planet’s ecosystem back into balance.
Sustaining Biodiversity
In the next 10+ years, it is expected that nanotechnology will contribute significantly to the preservation of biodiversity through the development and implementation of:
-
Advanced sensors and devices for monitoring ecosystem health (e.g., soil/water composition, nutrient/pollutant loads, microbial metabolism, and plant health)
-
Advanced sensors and devices for monitoring and tracking animal migration in terrestrial and marine ecosystems
-
Cost-effective and environmentally acceptable solutions to the global sustainability challenges, including energy, water, environment, and climate change
Socioeconomic Aspects for Sustainable Development
Nanoscience is at the unexplored frontiers of science and engineering, and it offers one of the most exciting opportunities for innovation in technology. It is important to have social scientists study the processes by which nanoscience is conducted and nanotechnology is developed even at this early stage. The knowledge gained will help policymakers and the public understand how nanoscience and nanotechnology are advancing, how those advances are being diffused, and how to make necessary course corrections. Insight into innovation process will also grow. Social scientists and scholars possess many effective ways of studying the development of new technology and its implications for society. Applications of a scientific idea to a technical problem, technology transfer, and an introduction of products into the marketplace can be tracked through statistics on research and development investments, patent applications, and new products and services. The societal impacts of nanotechnology may be of great scope and variety. The domains and measures of potential social impacts include economic growth, employment statistics, social transformations, and medical statistics.
Nanoscience may also enable new materials and technologies that reduce economic dependence on other kinds of natural resources. As the global economy continues to be transformed by new technology, a keen competition will develop for talent, intellectual property, capital, and technical expertise. Technical innovations will increasingly shape economies and market robustness. Technology will continue to drive global and domestic GDP. In the economic environment, nanotechnology comprehensively integrated into the economy due to high readiness, effective strategic planning, and widespread investments by business, education, labor, and government. The study of socioeconomic aspects of nanoscale science and technology (NST) will provide green nanoproducts, nanoprocesses, and their applications in science, engineering, and technology for sustainable development.
Priorities for Promoting Nanotechnology
Educate and train a new generation of scientists and workers skilled in nanoscience and nanotechnology at all levels. Develop scientific curricula and programs designed to:
-
(a)
Introduce nanoscale concepts into mathematics, science, engineering, and technological education
-
(b)
Include societal implications and ethical sensitivity in the training of nanotechnologist
-
(c)
Produce sufficient number and variety of well-trained social and economic scientists prepared to work in the nanotechnology area
-
(d)
Develop effective means for giving nanotechnology students an interdisciplinary perspective while strengthening the disciplinary expertise they will need to make maximum professional contributions
-
(e)
Establish fruitful partnerships between industry educational institutions to provide nanotechnology students adequate experience with nanoscale fabrication, manipulation, and characterization techniques
References
Abdullah AH, Ali NM, Tahir MIM (2009) Synthesis of bismuth vanadate as visible-light photocatalyst. Malays J Anal Sci 13:151–157
Adachi K, Ohta K, Mizuno T (1994) Photocatalytic reduction of carbon dioxide to hydrocarbon using copper-loaded titanium dioxide. Sol Energ 53:187–190
Allchin D (2008) Nobel ideals and noble errors. Am Biol Teacher 70(8):489–492 (BioOne)
Asahi R, Morikawa T, Ohwaki T, Aoki K, Tag Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293(5528):269–227. doi:10.1126/science.1061051
Bahnemann D (2004) Photocatalytic water treatment: solar energy applications. Sol Energ 77:445–459
Bruns B (2000) Nanotechnology and the commons: implications of open source abundance in millennial quasi-commons. http://www.cm.ksc.co.th/~bruns/opennan2.htm. Accessed 26 Nov 2002
Cao J, Elliott D, Zhang W-X (2005) Perchlorate reduction by nanoscale iron particles. J Nanoparticle Res 7(4–5):499–506
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–117
Carvajal AR (2004) Methodological approach for the assessment of technology from a sustainability perspective. M.Sc. thesis at the Technical University of Freiberg, Germany in cooperation with the Wuppertal Institute
Cheng SH, Cheng J (2005) Carbon nanotubes delay slightly the hatching time of zebrafish embryos. 229th American Chemical Society meeting, San Diego
Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 10:1166–1170
Court E, Daar AS, Martin E, Acharya T, Singer PA (2004) Will Prince Charles et al diminish the opportunities of developing countries in nanotechnology? http://nanotechweb.org/cws/article/indepth/18909
Diallo MS (2008) Water treatment by dendrimer enhanced filtration. US Patent, 7,470,369
Diallo MS, Christie S, Swaminathan P, Johnson JH Jr, Goddard WA III (2005) Dendrimer-enhanced ultrafiltration. 1. Recovery of Cu(II) from aqueous solutions using Gx-NH2 PAMAM dendrimers with ethylene diamine core. Environ Sci Technol 39(5):1366–1377
Dillon AC, Gennett T, Jones KM, Alleman JL, Parilla PA, Heben MJ (1999) A simple and complete purification of single-walled carbon nanotube materials. Adv Mater 11:1354
Drexler EK, Peterson C, Pergamit G (1991) (Online) http://www.foresight.org/UTF/Unbound_LBW/chapt_9.html. Accessed 26 Nov 2002
Elliott D, Zhang W (2001) Field assessment of nanoparticles for groundwater treatment. Environ Sci Technol 35:4922–4926
Elvin G (2007) Nanotechnology for green buildings. Green Technology forum US, Indianapolis. http://www.nsti.org/BioNano2007/showabstract.html?absno=3026
Fujiokya Y, Yamada K, Kazama S, Yogo K, Kai T et al (2007) In development of innovative gas separation membranes through sub-nano scale materials control. Research symposium presentation. Research Institute of Innovative Technology for the Earth (RITE)
Fujishima A, Hashimoto K, Watanabe T (1999) Tio2 photocatalysis. Fundamentals and applications. BKC, Tokyo
Glazier R, Venkatakrishnan R, Gheorghiu F, Walata L, Nash R, Zhang W (2003) Nanotechnology takes root. Civil Eng 73(5):64–69
Gotic M, Music S, Ivanda M et al (2005) Synthesis and characterisation of bismuth(III) vanadate. J Mol Struct 744:535–540
Henn KW, Waddill DW (2006) Utilization of nanoscale zero-valent iron for source remediation – a case study. Remediation 16(2):57–77
Hiroshi I, Watanabe Y, Hashimoto K (2003) Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J Phys Chem B 107(23):5483–5486. doi:10.1021/jp030133h
Hirota K, Komatsu G, Yamashita M et al (1992) Formation, characterization and sintering of alkoxy-derived bismuth vanadate. Mat Res Bull 27(7):823–830
Hoffmann MR, Martin ST, Choi W, Bahenmann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69
Ichinose N, Ozaki Y, Kashu S (1992) Superfine particle technology. Springer, London
Ihara T, Miyoshi M, Triyama Y, Marsumato O, Sugihara S (2003) Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl Catal B 42:403–409
Institute of Nanotechnology (2001) Featured report (Online). http://www.nano.org.uk/esant4.htm. Accessed 26 Nov 2002
Ishibashi KI (2000) Generation and deactivation processes of super oxide formed on TiO2 film illuminated by very weak UV light in air or water. J Phys Chem B 104:4934–4938
Jegadeesan G, Mondal K, Lalvani SB (2005) Arsenate remediation using nanosized modified zerovalent iron particles. Environ Prog 24(3):289–296
Kamat PV, Meisel D (2003) Nanoscience opportunities in environmental remediation. CR Chim 6:999–1007
Kanel SR, Greneche JM, Choi H (2006) Arsenic (V) removal from groundwater using nanoscale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol 40:2045–2050
Khan EU, Kane RL, Patrinos AA, Kripowicz RS, Krebs MA (2003) U.S. and global carbon reductions: the role of advanced fossil and non-fossil technologies, sequestration and research (Online). http://www.worldenergy.org/wecgeis/publications/default/tech_papers/17th_congress/3_4_07.asp. Accessed 2 May 2003
Kim YC, Sasaki S, Yano K, Ikebukuro K, Haphimoto K, Karube I (2001) Photocatalytic sensor for the determination of chemical oxygen demand using flow injection analysis. Anal Chim Acta 432(59)
Kloepfer JA, Mielke RE, Wong MS, Nealson KH, Stucky G, Nadeau JL (2003) Quantum dots as strain- and metabolism-specific microbiological labels. Appl Environ Microbiol 69:4205–4213
Kudo A, Omori K, Kato H (1999) A novel process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J Amer Chem Soc 121:11459–11467
Kudo A, Kato H, Tokunaga S (2001) Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. J Chem Mater 12:4624–4628
Li XQ, Zhang WX (2006) Iron nanoparticles: the core-shell structure and unique properties for Ni(II) sequestration. Langmuir 22:4638–4642
Li XQ, Elliott DW, Zhang WX (2006) Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State Mater Sci 31:111–122
Li X, Hu C, Wang X et al (2012) Photocatalytic activity of CdS nanoparticles synthesized by a facile composite molten salt method. Appl Surf Sci 258:4370–4376
Lien HL, Jhao YS, Chen LH (2007) Effect of heavy metals on dechlorination of carbon tetrachloride by iron nano particles. Environ Eng Sci 24(1):21–30
Liou YH, Lo SL, Kuan WH, Lin CJ, Weng SC (2006) Effect of precursor concentration on the characteristics of nanoscale zerovalent iron and its reactivity of nitrate. Water Res 40:2485–2492
Liu JB, Hao W, Shu W et al (2003) Hydrothermal preparation of BiVO4 powders. Mater Sci Eng B 104: 36–39
Liu Z, Fang P, Wang S et al (2012) Photocatalytic degradation of gaseous benzene with CdS-sensitized TiO2 film coated on fiberglass cloth. J Mol Catal A Chem 363–364:159–165
Mamadou SD, Savage N (2005) Nanoparticles and water quality. J Nano Res 7:325–330
Manning BA, Kiser JR, Kwon H, Kanel SR (2007) Spectroscopic investigation of Cr(III)- and Cr(VI)-treated nanoscale zerovalent iron. Environ Sci Technol 41:586–592
Matheson LJ, Tratnyek PG (1994) Reductive dehalogenation of chlorinated methanes by iron metal. Environ Sci Technol 28(12):2045–2053
Modun B, Morrissey J, Williams P (2000) The staphylococcal transferrin receptor: a glycolytic enzyme with novel functions. Trends Microbiol 8:231–237
Nair AS, Pradeep T (2004) Reactivity of Au and Ag nanoparticles with halocarbons. Appl Nanosci 59–63
Obare SO, Meyer GJJ (2004) Nanostructured materials for environmental remediation of organic contaminants in water. Environ Sci Health A 39:2549
Oberdörste G (2001) Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health 74: 1–8
Otto M, Floyd M, Bajpai S (2008) Nanotechnology for site remediation. Remediation 19(1):99–108
Pacific Northwest National Laboratory (2002) PNNL nanotechnology: grand challenges. (Online). http://www.pnl.gov/nano/grand/. Accessed 26 Nov 2002
Pirkanniemi K, Sillanpaa M (2002) Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere 48:1047–1060
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569
Reynolds GH (2001) ELR news & analysis, environmental regulation of nanotechnology: some preliminary observations (Online). http://www.foresight.org/impact/31.10681.pdf. Accessed 6 Nov 2002
Rickyerby D, Morrison M (2006) In nanotechnology and the environment: a European perspective. J Sci Technol Adv Mat 8:19–24
Rupa AV, Divakar D, Sivakumar T (2009) Titania and noble metals deposited titania catalysts in the photodegradation of tartrazine. Catal Lett 132:259–267
Saleh N, Sirk K, Liu YQ et al (2007) Surface modifications enhance nanoiron transport and NAPL targeting in saturated porous media. Environ Eng Sci 24(1):45–57
Shan GB, Xing JM, Zhang HY et al (2005) Biodesulfurization of dibenzothiophene by microbial cells coated with magnetite nanoparticles. Appl Environ Microbiol 71:4497–4502
Sohn K, Kang SW, Ahn S et al (2006) Fe(0) nanoparticles for nitrate reduction: stability, reactivity, and transformation. Environ Sci Technol 40(17):5514–5519
Stoimenov PK, Klinger RL, Marchin GL et al (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18:6679–6686
Sun YP, Li X, Cao J, Zhang W, Wang HP (2006) Characterization of zero-valent iron particles. Adv Colloid Interface Sci 120:47–56
Theron J, Walker JA, Cloete TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34:43–69
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today 1(2):44–48
United States Environment Protection Agency (2007) Nanotechnology white paper. EPA 100/B-07/001. U.S. Environmental Protection Agency, Washington, DC
United States Environment Protection Agency (2008) Nanotechnology for site remediation: fact sheet. EPA 542-F-08-009. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/tio/download/remed/542-f-08-009.pdf. Accessed 20 Oct 2009
Wallington K (2005) In emerging nanotechnologies for site remediation and waste treatment. Report, North Carolina State University
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
Wu NL, Lee MS (2004) Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int J Hydro Energ 29:1601–1605
Xia Q, Chena X, Zhaoa K, Liua J (2008) Synthesis and characterizations of polycrystalline walnut-like CdS nanoparticle by solvothermal method with PVP as stabilizer. Mater Chem Phys 111(1):98–105
Xu D, Sushil KK, Wenli D (2009) Preparation, characterization and photocatalytic activity of flowerlike cadmium sulfide nanostructure. Sep Purif Technol 68(1):61–64
Yu J, Zhang Y, Kudo A (2009) Synthesis and photocatalytic performances of BiVO4 by ammonia coprecipitation process. J Solid State Chem 182:223–228
Yuan J, Liu X, Akbulut O et al (2008) Superwetting nanowire membranes for selective absorption. Nat Nanotechnol 3(6):332–336
Zhang W-X (2003) Nanoscale iron particles for environmental remediation: an overview. J Nano Res 5:323–332
Zhang W-X, Elliott DW (2006) Applications of iron nanoparticles for groundwater remediation. Remediation 16(2):7–21
Zhang A, Zhang J (2009) Characterization of visible-light-driven BiVO4 photocatalysts synthesized via a surfactant-assisted hydrothermal method. Spectrochim Acta Part A 73:336–341
Zhang L, Chen D, Jiao X (2006) Monoclinic structured BiVO4 nanosheets: hydrothermal preparation, formation mechanism, coloristic and photocatalytic properties. J Phys Chem B 110:2668–2673
Zhang A, Zhang J, Cui N et al (2009) Effects of pH on hydrothermal synthesis and characterization of visible-light-driven BiVO4 photocatalyst. J Mol Catal A Chemical 304:28–32
Zhao XJ, Hilliard LR, Mechery SJ et al (2004) A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Nat Acad Sci USA 101:15027–15032
Zhou L, Wang W, Liu S et al (2006) A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. J Mol Catal A Chem 252:120–124
Zhou L, Wang W, Zhang L et al (2007) Single-crystalline BiVO4 microtubes with square cross-sections: microstructure, growth mechanism and photocatalytic property. J Phys Chem 111:13659–13664
Zhu L, Ang S, Liu W-T (2004) Quantum dots as a novel immunofluorescent detection system for Cryptosporidium parvum and Giardia lamblia. Appl Environ Microbiol 70:597–598
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Fulekar, M.H., Pathak, B., Kale, R.K. (2014). Nanotechnology: Perspective for Environmental Sustainability. In: Fulekar, M., Pathak, B., Kale, R. (eds) Environment and Sustainable Development. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1166-2_7
Download citation
DOI: https://doi.org/10.1007/978-81-322-1166-2_7
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1165-5
Online ISBN: 978-81-322-1166-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)