Abstract
Mannans are a major constituent of the hemicellulose fraction of lignocelluloses. Mannans perform distinct functions as structural components in cell walls of softwoods and storage functions in seeds. Enzymatic hydrolysis of mannan involves the backbone hydrolyzing endo-β-mannanases and β-mannosidases. Mannans are heteropolymeric and their hydrolysis also requires the action of β-glucosidases and side-chain cleaving α-galactosidases and acetyl mannan esterases. Microorganisms are therefore explored for the production of such repertoire of enzymes so that effective mannan hydrolysis can be achieved. The present chapter discusses the occurrence and structural properties of mannans in plant materials and its hydrolysis using enzymes sourced from various fungi and other microorganisms. The production and properties of mannanolytic enzymes, their cloning and expression in heterologous hosts, and their application have also been discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Hemicelluloses are structural polysaccharides of the plant cell wall. Hemicellulose is associated with cellulose and lignin and forms about 30 % of the lignocellulosic biomass. Lignocellulose is abundant and represents one of the major natural renewable resources and a dominating waste material from agriculture. This renewable resource can be used in several industries, including the pharmaceutical, biofuel, and pulp and paper industries, and many more (Kango et al. 2003; Kango 2007). The generation of feedstock is possible by hydrolysis of lignocellulosic biomass using various microorganisms and their enzymes. The hydrolysis of lignocellulose has become the “hot spot” and a crucial part of lignocellulose biotechnology. According to Chaikumpollert et al. (2004), hemicelluloses form about one third of all the components available in plants and are the second major heteropolymer present in nature. Distribution of hemicellulose in gymnosperms and angiosperms varies. Hemicelluloses consist of different heterogeneous polymers of sugars such as xylose, arabinose, mannose, glucose, galactose, and sugar acids. These hemicelluloses are named according to their main sugar component (80–90 %) which is present in its backbone, e.g., mannose is present in mannan hemicelluloses. Enzyme-based hydrolysis of hemicelluloses, especially mannan and xylan, significantly affects the prospects of biobleaching and saccharification of lignocellulosic biomass (Viikari et al. 1993).
Mannan: Occurrence and Structure
Most of the main-chain sugars in hemicellulose are linked together by β-1,4-glycosidic bonds. Mannans are one of the most important constituents of hemicelluloses in the wall of higher plants. Mannan is composed of repeating units of mannose (a second carbon epimer of glucose) linked by β-1,4-glycosidic linkages. Besides D-mannose, other sugars like glucose, galactose, and acetyl groups can be present in various mannans. Mannans are further classified on the basis of other sugars present in the structure, e.g., glucose-containing mannan is called glucomannan. Similarly, when galactose is present as a side chain linked to the main chain, the polymer is called galactomannan, and when both glucose and galactose are linked to the mannose sugars of the main-chain backbone, it is called galactoglucomannan. Mannan exists in both linear and branched forms with a β-1,4-linked backbone. Mannans are an important part of the hemicellulose family, which are further classified as linear mannan, glucomannan, galactomannan, and galactoglucomannan. Linear mannans are homopolysaccharides which have a main chain composed of 1,4-linked β-d-mannopyranosyl (mannose-mannose) residues. The percentage of galactose in linear mannans is 5 % or less. In glucomannan, the main chain consists of randomly β-1,4-linked D-mannose and D-glucose residues, while in galactomannan, the galactose sugars are present as single side chains substituted on the main-chain sugar, mannose (Fig. 4.1). In galactoglucomannan, galactose sugars are present as single side chains in α-1,6-linkage with the main chain, which consists of both mannose and glucose.
The ratio of sugars present in mannan varies with respect to the different sources from which it is obtained, which indicates the polydiversity of the polymer. In linear mannans, mannose is predominantly present, while in galactomannan the ratio of galactose to mannose is 1:3. True galactomannan is considered to contain more than 5 % of galactose residues in side chains. In galactoglucomannan, the ratio of galactose/glucose/mannose is observed to be 1:1:3. Mannans are found in nature as part of the hemicellulose fraction of hardwood and softwood. It is predominantly found in the endosperm of copra, locust bean, guar beans, seeds of other leguminous plants, coffee beans, roots of the konjac tree, and ivory nuts. Locust bean gum (LBG) is a galactomannan, while mannan from konjac trees is a glucomannan. Linear mannans are the major structural units in woods, in seeds of ivory nut, and in green coffee beans. Petkowicz et al. (2007) separated mannans from ivory nuts into two components, mannan I and mannan II. Mannan I, extracted with alkali, displayed a crystalline structure, while mannan II was not amenable to direct extraction and displayed a less crystalline structure. Molecular size also varied as mannan I was smaller compared to mannan II. Linear mannans are present in the seed coat or exterior section of leguminous plants, while galactomannan occurs in the intermediate section of the seed endosperm. Various gum extracts from plants are conspicuous sources for galactomannan, for example, locust bean gum, tara gum, fenugreek gum, and guar gum. In these sources, the main chain of galactomannan contains 1,4-linked β-d-mannopyranosyl residues with side chains of single 1,6-linked α-d-galactopyranosyl groups attached along the main chain (Fig. 4.1). The distribution of galactose in galactomannan varies between mannans obtained from different sources. It is observed that all types of galactomannans have more than 5 % of galactose residues as side chains. Galactomannan obtained from the endosperm of locust bean or Ceratonia siliqua (carob) has a ratio of 1:4 (galactose/mannose). The galactomannan of the intermediate section of the seed endosperm of Schizolobium amazonicum and Cesalpinia spinosa has a sugar ratio of 1:3 (galactose/mannose) (Table 4.1). The function of galactomannan in seeds, in addition to the retention of water by solvation, is to prevent complete drying of seeds in high atmospheric temperatures so that the enzymes, which are crucial for seed germination, remain active. Liepman et al. (2007) have showed some evidence that mannan also functions as a signaling molecule in plant growth and development. Three-dimensional structure studies of guaran or guar fibers (Cyamopsis tetragonolobus) were done by Chandrasekaran et al. (1998) using x-ray diffraction, and they revealed that the hydrogen of the galactosyl side chain interacted with the mannan backbone and provided structural stability. The structure showed a flat twofold helix with a pitch of 10.38 Å. Glucomannans are the principal components of softwood hemicelluloses and consist of β-1,4-linked D-mannose and D-glucose residues with a 3:1 ratio. Hongshu et al. (2002) obtained glucomannan from ramie (Boehmeria nivea) which contained 95–99 % of D-glucose and D-mannose residues with a ratio of 1.3–1.7:1. The presence of D-galactose residues in glucomannan is very rare, but Puls and Schuseil (1993), working with softwood, observed D-galactose residues attached to the main-chain mannose residues with α-1,6-linked terminal units and observed a ratio of mannose/glucose/galactose as 3:1:0.1. The glucomannan from Amorphophallus (konjac) showed an association with starch-like α-glucan, comprised of 1,4-linked β-d-mannopyranose and D-glucopyranose in 70 % and 30 %, respectively (Aspinall 1959). Kenne et al. (1975) studied distribution of the O-acetyl groups in glucomannan from pine and observed that acetyl groups are irregularly distributed in pine glucomannan. In galactoglucomannans, galactose residues are attached to both D-glucosyl and D-mannosyl units with a α-1,6-linkage, and mannosyl units also have partial substitution by O-acetyl groups. Several reports showed that about 60–65 % of mannose residues in galactoglucomannan from native Norway spruce wood and pulp were acetylated at either the C-2 or C-3 position (Aspinall et al. 1962; Popa and Spiridon 1998; Timell 1967; Willfor et al. 2003). Lundqvist et al. (2002, 2003) extracted galactoglucomannan from spruce (Picea abies) by heat fractionation at different temperatures and characterized it. Galactoglucomannan from spruce contained about one third of D-mannosyl units substituted by O-acetyl groups with an equal distribution between C-2 and C-3 and a molar ratio of 0.1:1:4 (galactose/glucose/mannose). Various types of mannans with their sources and sugar ratios are listed in Table 4.1.
Solubility among mannans towards water varies due to the presence of D-galactose side chains. The solubility of galactomannan and galactoglucomannan is higher in comparison to linear and glucomannan homopolymers. The D-galactose side chains prevent alignment of macromolecules and lead to formation of strong hydrogen bonds (Timell 1965). In addition to aforesaid structures, mannans also display a range of curious structures and configurations. For instance, Ishurd et al. (2004) observed and isolated galactomannan from Retama raetam (Fabaceae). Its backbone consisted mostly of 1-3-linked β-d-mannopyranosyl residues with attachment of galactopyranosyl residues observed at C-6. Nunes et al. (2005) observed arabinosyl and glucosyl residues in galactomannan from green and roasted coffee infusions. The acetyl groups were present in the main chain of mannan at the O-2 position of mannose residues, while arabinose residues were at O-6 of mannose residues as side chains. Omarsdottir et al. (2006) isolated galactomannan from a number of lichen species like foliose lichen (Peltigera canina). The backbone of these mannans displayed odd structures, being composed of α-1,6-linked mannopyranosyl residues with a difference in the side-chain pattern at O-2 and O-4 instead of O-6, which is observed in various galactomannan structures. Singh and Malviya (2006) observed D-glucopyranosyl units in glucomannan from seeds of a medicinal plant, Bryonia laciniosa, which displayed α-1-6-linkages in the main chain with a 1:1.01 ratio of glucose and mannose.
The degree of polymerization (DP) of any macromolecule is a manifestation of the approximate number of monomer units present in polymer. The DP of any polymer influences its various properties such as colligative properties, boiling point, freezing point, solubility, viscosity, toughness, and somatic pressure. The DP also helps in calculating the average molecular weight of the polymer. Petkowicz et al. (2007) isolated mannan from ivory nuts and observed two types, viz., mannan I and II in which mannan I has a lower molecular weight and a DP of ~15, while mannan II had a DP of about ~80 with higher molecular weight. Softwood glucomannans with a 3:1 ratio of mannose:glucose exhibit higher DPs of more than 200. Softwood galactoglucomannans with a 1:1:3 ratio for galactose/glucose/mannose exhibit a DP between 100 and 150. Enzymes play a crucial role in the modification of polymers and its structural analysis. Analysis or sequencing of mannan requires several mannanases from legume seeds and microorganisms to act on the various mannans. Selection of enzymes is important because their differential activity towards substrates reveals the structural difference. A mannanase from Trichoderma reesei was able to hydrolyze fiber-bound galactoglucomannan from pine kraft pulp, while an enzyme from Bacillus subtilis was not effective for its hydrolysis (Ratto et al. 1993). Tenkanen et al. (1997) studied the action of a mannanase from T. reesei on galactoglucomannan in pine kraft pulp and analyzed the hydrolysate by 1H NMR spectroscopy and high-performance anion-exchange chromatography (HPAEC-PAD). The relative amount of sugar residues in the hydrolysate of pine kraft pulp, after extensive hydrolysis by a mannanase from T. reesei, was analyzed. The molar percentage of mannose, glucose, and galactose was 73.4, 20.4, and 5.8, respectively, as determined by 1H NMR spectroscopy, and 71.8, 20.3, and 6.9 by the HPAEC-PAD method. LBG hydrolysis products of the recombinant man5S27 enzyme were analyzed using HPAEC. The approximate percentages of mannose, mannobiose, mannotriose, mannotetraose, mannopentaose, and other sugar oligosaccharides were 3.23, 0.74, 22.14, 2.21, 6.89, and 64.79 (Shi et al. 2011). Analysis of the sequence and percentage of sugars in mannan requires a particular enzyme or enzymes with their homo- and heterosynergistic actions leading to the hydrolysis of hemicelluloses. The analysis of hydrolysate needs a suitable method or a combination of methods like TLC, HPLC, and NMR spectroscopy.
Mannan-Degrading Enzymes and Their Sources
Mannans can be present as linear and branched, homo- as well as heteropolymers. In general, enzymes involved in the hydrolysis of mannan are called as mannanases. Complete biodegradation of mannans necessitates the use of various enzymes. Enzymes that actively participate in mannan hydrolysis include β-mannanase (1,4-β-D-mannan mannohydrolase, EC 3.2.1.78), β-mannosidase (1,4-β-d-mannopyranoside hydrolase, EC 3.2.1.25), β-glucosidase (1,4-β-D-glucoside glucohydrolase, EC 3.2.1.21), α-galactosidase (1,4-α-d-galactoside galactohydrolase, EC 3.2.1.22), and acetyl esterase (EC 3.1.1.6). A number of microorganisms, including fungi, yeast, bacteria, and actinomycetes, produce β-mannanases and other accessory enzymes. Among these, fungi have been investigated by various workers for mannanase production (Dhawan and Kaur 2007; Moreira and Filho 2008; Van Zyl et al. 2010). Some of the prominent mannanase producers are listed in Table 4.2.
β-Mannanase distribution is also observed in plants. Seeds are the most preferred sources for isolation of mannanases. However, other plant organs like fruits also displayed the presence of mannanases (Bourgault et al. 2001). Schroder et al. (2006) obtained endo-β-mannanase from ripe tomato fruit. For mannanase production, microorganisms which are selected from various sources (soil, compost, water, agriculture waste) are grown on basal media containing mannan (LBG) as sole carbon source (Ratto and Poutanen 1988; Puchart et al. 2004; Maijala et al. 2012).
Mode of Action of Mannanases
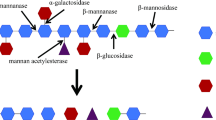
Polysaccharides like mannans can exist in linear, homo, hetero, or branched form. β-Mannanases find application in the extraction of vegetable oil, in the manufacture of instant coffee as a viscosity reducer agent for coffee extract, nutraceuticals such as the production of MOS (mannose oligosaccharides), pharmaceuticals, food and feed, production of second-generation biofuels, paper and pulp, and various other industries (Sachslehner et al. 2000; Van Zyl et al. 2010). At least one main-chain hydrolyzing enzyme, like β-mannanase, and one side-chain hydrolyzing enzyme, like α-galactosidase, are required for the breakdown of branched mannan (LBG). β-Mannanase cleaves internal β-1,4-linked residues of mannose/glucose in the mannan backbone. This enzyme mainly produces oligomannan/oligoglucomannan and is very effective on linear mannan and glucomannan (homopolymer), although the hydrolysis action of this enzyme is affected in galactomannan due to the presence of side chains. β-Mannosidase helps to remove mannose from the nonreducing end of mannan and cleaves β-1,4-linked mannose residues. Similarly, β-glucosidase removes glucose residue from the nonreducing end of the oligoglucomannan and cleaves 1,4-β-d-glucopyranose. Besides these main-chain enzymes, two side-chain cleaving enzymes are very important for complete biodegradation of mannan, viz., α-galactosidase cleaves the α-1,6 glycosidic bonds between galactose and the main-chain sugars (mannose/glucose) and leads to hydrolysis of D-galactopyranosyl side chains of galactomannan and galactoglucomannan. Acetyl mannan esterase removes acetyl groups from galactoglucomannan. The delineation of various mannanases action is shown in Fig. 4.2.
Mannan composition also affects the action of enzymes, and to achieve complete degradation of heteromannan like locust bean gum, fungi and bacteria have to produce three enzymes, namely, β-mannanase, β-mannosidase, and α-galactosidase (Hilge et al. 1998). These hemicellulases also show synergistic action. When β-mannanase and β-mannosidase (main-chain cleaving enzymes) or α-galactosidase and acetyl mannan esterase (side-chain cleaving enzymes) cooperate, it is called homosynergistic action. Heterosynergy refers to the interaction of main- and side-chain cleaving enzymes working together (Fig. 4.3). Homosynergy between β-mannosidase and two β-mannanases obtained from the enzyme extract of Sclerotium rolfsii (Gubitz et al. 1996; Moreira and Filho 2008) and heterosynergy between β-mannanase, β-mannosidase, and α-galactosidase have been observed in enzyme extractions of Thermotoga neapolitana 68 on galactomannan (Duffaud et al. 1997).
Microbial Production of Mannanases
The best and richest sources of enzymes are microorganisms (Kirk et al. 2002). For mannanase production, mainly fungi and some bacteria are used at a commercial level and their enzyme systems are reported to be inducible. Hemicelluloses like xylan are not able to cross cell walls; therefore, small oligosaccharides formed as a result of xylan degradation act as an inducer and also play an important role as a regulation factor for xylanase biosynthesis (Singh et al. 2003). Both submerged and solid-state fermentation (SSF) have been examined for mannanase production. The cost of enzymes remains a bottleneck in realizing their application on a large scale. Use of inexpensive substrates, such as by-products of agro-industries and forestry waste, can effectively subsidize the recurring cost of enzyme production. Ratto and Poutanen (1988) have used wheat bran with locust bean galactomannan for mannanase production by bacteria and fungi. Mannanase activities were found to be 256, 34, and 24 nkat ml−1 with Bacillus subtilis, Aspergillus awamori, and T. reesei, respectively. Abdeshahian et al. (2010) used palm kernel cake (PKC) as a substrate for β-mannanase production by Aspergillus niger FTCC5003 through solid-state fermentation. Production was evaluated by response surface methodology on the basis of a central composite face-centered (CCF) design with three independent variables, namely, incubation, temperature, initial moisture content of substrate, and airflow rate. The highest level of β-mannanase (2,117.89 U/g) was obtained when the incubation temperature, initial moisture level, and aeration rate were 32.5 °C, 60 %, and 0.5 l/min, respectively, during SSF. There are many species of fungi reported to produce significantly high mannanase activity. For instance, Lin and Chen (2004) observed 27.4 U/ml mannanase activity in a submerged culture of Aspergillus niger NCH 189. Similarly, Hossain et al. (1996) obtained about 90 U/ml mannanase activity in submerged conditions using Bacillus sp. KK01. Production of enzymes is affected by temperature, pH, agitation, and aeration. The overall production process gives a good outcome in terms of enzyme activity at optimum temperature, pH, and other factors. The effect of the agitation speed, aeration rate, and temperature on the production of β-mannanase by Bacillus licheniformis NK 27 in a batch fermenter was studied by Feng et al. (2003). They concluded that temperature was the most significant factor in β-mannanase production. Feng et al. (2003) obtained a maximum activity of 212 U/ml in 36 h at an aeration rate of 0.75 vvm, agitation of 600 rpm, and a constant temperature of 30 °C. Mannanase production by microorganisms is influenced by the media composition, mostly carbon and nitrogen (Kataoka and Tokiwa 1998; Dhawan and Kaur 2007). Groβwindhager et al. (1999) used glucose and cellulose for S. rolfsii, while Ademark et al. (1998) and Gomes et al. (2007) used locust bean gum (LBG) for A. niger and a thermophilic fungus, Thermoascus aurantiacus. Ferreira and Filho (2004) have used wheat bran as the carbon source for the production of β-mannanase from mesophilic fungus Trichoderma harzianum strain T4. Besides the carbon source, various organic or inorganic nitrogen sources play an important role in mannanase production. Organic nitrogen sources like peptone, yeast autolysate, corn steep liquor (CSL), and beef extract are preferred (Puchart et al. 2004; Zhang et al. 2006; Cui et al. 1999; Kataoka and Tokiwa 1998), while inorganic nitrogen sources like ammonium sulfate, diammonium hydrogen phosphate, ammonium dihydrogen phosphate, and sodium nitrate have been found to play an effective role (Zakaria et al. 1998; Perret et al. 2004). Gomes et al. (2007) achieved the highest β-mannanase and β-mannosidase activity by the thermophilic fungus (Thermoascus aurantiacus) with soya meal as nitrogen source, supplemented with LBG as carbon source. Recently, Mohamad et al. (2011) performed a comparison study of different carbon and nitrogen sources for their effect on mannan-degrading enzyme production by Aspergillus niger. They revealed in their result that guar gum (GG) and bacteriological peptone supported the highest β-mannanase activity. They achieved β-mannanase activities equivalent to 1,495, 1,148, 10.7, 8.8, and 4.6 nkat ml−1 with guar gum (GG), LBG, α-cellulose, glucose, and carboxymethyl cellulose as carbon sources, respectively. Activity levels equivalent to 1,744, 1,168, 817, 241, 113, and 99 nkat ml−1 were achieved with bacteriological peptone, yeast extract, ammonium sulfate, ammonium nitrate, and ammonium chloride as nitrogen sources, respectively. The above results showed that mannanase production by A. niger can be enhanced with GG and LBG. Inorganic nitrogen sources reduced β-mannanase production greatly, while organic nitrogen sources enhanced β-mannanase production. In contrast, Kalogeris et al. (2003) have obtained better production of cellulases by Thermoascus aurantiacus using inorganic nitrogen sources. Various industries like paper and pulp and detergent industries need enzymes that function well at a high pH. Alkaline β-mannanase was obtained for the first time from alkaliphilic Bacillus sp. AM001 by Akino et al. (1987). This mannanase showed a pH optimum between 7.0 and 9.0. Mudau and Setati (2006) have studied endo-mannanase-producing molds from hypersaline environments and observed the effect of salt (NaCl) on growth and enzyme production. All four isolates, Scopulariopsis brevicaulis LMK002, S. candida LMK004, S. candida LMK008, and Verticillium dahliae LMK006, showed growth on NaCl concentrations of up to 10 %. Endo-mannanase production by Scopulariopsis isolates was found to increase with NaCl concentration. Groβwindhager et al. (1999) have shown efficient β-mannanase production by Sclerotium rolfsii and S. coffeicola under derepressed condition by using cellulose- and glucose-based media. They have concluded that cellulose is the best inducer for both S. rolfsii and S. coffeicola strains for mannanase production with maximum activities of 677 and 461 Uml−1, respectively. In a glucose-based medium, activities were 96.6 and 67.7 Uml−1. Glucose is an easily metabolizable substrate, and in the presence of this substrate, glycosyl hydrolase systems get repressed (Ronne 1995; Ruijter and Visser 1997). However, both the strains S. rolfsii and S. coffeicola were observed to produce mannanase activity when a typical repressing substrate, glucose, was used as sole carbon source in batch cultivation. Mannan-degrading enzyme production started only when the glucose concentration in the medium dropped low. High mannanase activity (240 Uml−1) by S. rolfsii CB5191.62 was achieved in a glucose fed-batch system in which glucose concentration in the media was maintained low (Groβwindhager et al. 1999). Table 4.3 displays an overview of production and properties of mannanases from various microorganisms.
Heterologous Production
Higher yield, ease of operational conditions, simple recovery, and downstream processing have prompted several workers towards cloning and heterologous production of mannanases. The recombinant DNA technique provides enormous opportunity to make genetically modified microbial strains. More than 50 % of mannanase-producing microorganisms, which are being used at industrial level, are genetically engineered (Dhawan and Kaur 2007).
S. cerevisiae is not known for production of mannanase by itself, but the heterologous production of endo-β-1,4-mannanase has been done using S. cerevisiae as a genetically modified host by Setati et al. (2001). Similarly, Qiao et al. (2008) have used Pichia pastoris as a host for expression of MAN gene of Bacillus subtilis. It has been observed that, if the same gene encoding mannanase is expressed in different hosts, the resultant recombinant enzymes show somewhat different properties. For instance, MAN1 gene of Aspergillus aculeatus MRC 11624 was cloned and expressed in S. cerevisiae, A. niger, and Y. lipolytica. Besides higher enzyme activity, the resultant recombinant enzymes showed different temperature and pH optima as compared to the native enzymes. Isolation and cloning of genes encoding mannanases and their expression in a suitable host play an important role in the molecular and structural studies of enzyme proteins and protein engineering thereof. Eight essentially conserved active site residues of β-mannanases, viz., Arg-83, His-119, Asn-157, Glu-158, His-224, Tyr-226, Gly-254, and Trp-283, are reported in Bacillus N16-5 mannanase (Ma et al. 2004). A 1,345 bp gene encoding mannanase (ManN) from Aspergillus sulphureus was expressed in Pichia pastoris (Chen et al. 2007). Alkaline β-mannanase (ManA) was cloned by Ma et al. (2004) from Bacillus sp. N165, and its overproduction and optimization have been studied by Lin et al. (2007). They achieved a maximum yield of 310 U/ml after optimization. Recently, Pan et al. (2011) demonstrated heterologous expression of alkaline β-mannanase by a yeast expression system. Pan et al. (2011) have used Kluyveromyces cicerisporus Y179U and Pichia pastoris GS115 for expression of MAN 330 (truncated β-mannanase) and MAN 493. MAN330 and MAN 493 genes were amplified and alkaline mannanase was successfully expressed using Y179U/pUKD-S-MAN 330 and GS115/pPIC-9 k MAN 493 (vectors), and high yields of 1,378 and 1,114 U/ml in shake flasks were obtained, respectively. Both enzymes had a maximum activity at pH 9.5 and 70 °C. β-Mannanase from Bacillus subtilis has been purified and characterized (Jiang et al. 2006). Recently, a thermostable β-mannanase from Bacillus subtilis BCC41051 was expressed in E. coli and Bacillus megaterium (Summpunn et al. 2011). The open reading frame of the gene coding β-mannanase was amplified by PCR using Man-CHF (5′-GTACGCCATATGTTTAAGAAACATACGATCTCTTTGC-3′) and Man-CHR (5′-GTACGCCTCGAGTTCAACGATTGGCGTTAAAGAATC-3′) primers, and the recombinant vector pEManAhis was transfected into E. coli BL21. The gene was expressed and induced by IPTG (isopropyl-β-d-1thiogalactopyranoside), and the highest activity of 415.18 U/ml was obtained. For expression in B. megaterium, E. coli, Bacillus shuttle, and expression vector, Pxb was used. The gene coding for β-mannanase was amplified with the primer Man-F1 (5′-GTACGCGGATCCGACAAATGTTTAAGAAACATACGATC-3′) and Man-R1 (5′-CTGATTCATTCAACGATTGG-3′) and transformed into B. megaterium with the help of a pXManA plasmid. The expression of the cloned gene was induced by xylose to obtain 359 U/ml enzyme activity. Various examples of heterologous production of mannanases, vectors employed, and the properties of recombinant enzyme are presented in Table 4.4. Heterologous expression allows a simpler and cheaper means of production using desired hosts, while induction of β-mannanases by native organisms needs mostly expensive and complex medium components (Kote et al. 2009; Viniegra et al. 2003; Van Zyl et al. 2010).
Applications of Mannanases
Mannan-degrading enzymes find various uses in different industries. More recently, mannanases have been used for the production of manno-oligosaccharides (MOS), feed upgradation, biobleaching, and detergents. The details of these applications are detailed below.
Production of Manno-Oligosaccharide
Mannanases degrade mannans and produce manno-oligosaccharides (MOS) and mannose. The MOS contribute to human health and are considered to confer prebiotic benefits. Fan et al. (2009a, b) have showed that glucomannan enhanced fecal probiotics. Hydrolyzed glucomannan can be used as a prebiotic to augment growth of fecal probiotics. MOS is demonstrated to confer a similar result as oligofructose prebiotics. Kobayashi et al. (1987) have noticed that oligosaccharides, which are used as prebiotics to enhance growth of human intestinal microflora, including manno-oligosaccharide. MOS also used as functional food ingredients.
Coffee and Coconut Oil Extraction
In coffee extract, mannan is present as the main polysaccharide and this mannan increases the viscosity of the coffee extract, which unfavorably affects instant coffee preparation. β-Mannanase is used for reducing the viscosity of coffee extract, because it hydrolyzes mannan into simple sugars. The hydrolyzed mannan is also beneficial to consumer health as it decreases fat utilization. Endo-mannanase is also applicable in coconut extraction. Mannan is present as the main component in the cell wall of coconuts, so the use of mannanase helps to achieve a higher yield of oil and improves the refining properties of oil. Commercially, endo-β-mannanase from Aspergillus niger is marketed as “GAMANASE” for coconut oil extraction (Novo Nordisk, Denmark).
Feed Upgradation
Mannan is commonly present in feed ingredients such as soybean meal, copra meal, and palm kernel meal. Among these meals, soybean meal is mostly used as a protein source in poultry feed. This meal is the product remaining after complete oil extraction from soybean seeds. Due to high levels of mannan, this meal negatively affects growth performance of animals because of its indigestive nature. This mannan component can be hydrolyzed by addition of endo-β-mannanase in the meal. These additions of β-mannanase improve digestibility by hydrolysis of mannan and thereby enhance the performance of poultry. Mannanase produced from Trichoderma longibrachiatum and B. lentus is marketed as “Hemicell” by ChemGen, a US-based company, and is potentially used as an animal feed supplement.
Biobleaching
The use of xylanase in biobleaching of pulp is well known (Viikari et al. 1993). Mannanase is also used in enzymatic bleaching of softwood pulp. The extraction of lignin from softwood is important. For this purpose, alkaline treatment is performed for hydrolyzing hemicelluloses and removal of lignin. This alkaline treatment of wood pulps creates environment pollution, especially water pollution. Application of mannanases has significantly reduced the use of alkaline chemicals in treatment of wood pulps (Gubitz et al. 1997; Puchart et al. 2004). Mannanases also help in removal of lignin from pulps by biobleaching without affecting the quality of fibers. Here are so many thermophilic microorganisms like Thermomyces lanuginosus, Malbranchea cinnamomea, Myceliophthora fergusii, and Bacillus subtilis which are able to produce thermostable mannanases (Maijala et al. 2012; Summpunn et al. 2011). The thermostable β-mannanases and β-mannosidases offer a significant advantage for biobleaching at elevated temperatures. A thermostable extracellular β-mannanase from A. niger was studied by Naganagouda et al. (2009). They have shown that this β-mannanase was active over a wide pH and temperature range. Due to these properties of this enzyme, it is potentially useful in biobleaching and food processing. Tenkanen et al. (1997) have shown that mannanases can be used as an alternative in place of hydrogen peroxide in biobleaching.
Detergent and Textile Industries
Amylases and cellulases are well-known common enzymes which are used for many decades in detergents. Gums are used as stabilizing and thickening agents worldwide in many foods and in various household products. These gums contain mannans like galactomannan, guar gum, and tara gum. Mannanases can be added in detergents to remove this gummy matter from clothes. Detergents or cleaners, which are used to clean contact lenses, and hard surface cleaners also contain mannanases. It is observed that detergents containing mannanase can improve the whiteness of cellulosic material. But utilization of mannanase has been limited because most of the mannanases have their optima around neutral or somewhat acidic pH (4.0–6.0). Some fungal β-mannanases can tolerate an alkaline pH up to eight. β-Mannanase from alkaliphilic Bacillus sp. N16-5, cloned and expressed in Kluyveromyces cicerisporus and Pichia pastoris, had a pH optimum of 9.5 and was stable over a pH range of 5.0–11.0 (Pan et al. 2011). A mannanase preparation, “MANNAWAY,” used in washing detergents is marketed by Novozymes, a US-based company. Galactomannans such as guar gum and LBG are widely employed as print paste in textile printing. Mannanase, used in enzyme consortia, helps to degrade mannan after the printing of cloth.
Other Applications
Mannanase is also used in oil drilling or gas oil well stimulation. There are many fungal and bacterial β-mannanases available which have high-temperature optima. Heterologous production is providing access to thermostable β-mannanases. To open crevices and cracks for oil and gas flow, mannans like guar gum and tara gum are used as polymer solutions with sand particles. Polymer solutions are hydrolyzed with a suitable thermostable mannanase for better oil and gas recovery. Organisms from hydrothermal vents are isolated and used as a source of endo- and exo-mannanase, and this preparation is marketed by companies, for example, pyrolase 160 and pyrolase 200 products are marketed by Diversa Company with a 37–93 °C recommended temperature. In the production of second-generation fuel (bioethanol, biodiesel), mannanases are crucial with other enzymes. Synergy between mannan-degrading enzymes and cellulases was demonstrated with a fivefold increase in glucose yield from lignocellulose polysaccharides (Jorgensen et al. 2010). Partially hydrolyzed guar gum (PHGG) is used as relief agent for irritable bowel syndrome (IBS).
References
Abdeshahian P, Samat N, Hamid AA, Yusoff WM (2010) Utilization of palm kernel cake for production of beta-mannanase by Aspergillus niger FTCC 5003 in solid substrate fermentation using an aerated column bioreactor. J Ind Microbiol Biotechnol 37(1):103–109
Ademark P, Varga A, Medve J, Harjunpää V, Drakenberg T, Tjerneld F, Stålbrand H (1998) Softwood hemicellulose-degrading enzymes from Aspergillus niger: purification and properties of a β-mannanase. J Biotechnol 63:199–210
Akino T, Nakamura N, Horikoshi K (1987) Production of β-mannosidase and β-mannanase by an alkaliphilic Bacillus sp. Appl Microbiol Biotechnol 26:323–327
Araujo A, Ward OP (1990) Hemicellulases of Bacillus species: preliminary comparative studies on production and properties of mannanases and galactanases. J Appl Bacteriol 68:253–261
Arcand N, Kluepfel D, Paradis FW, Morosoli R, Shareck F (1993) Beta-mannanase of Streptomyces lividans 66: cloning and DNA sequence of the manA gene and characterization of the enzyme. Biochem J 290:857–863
Aspinall GO (1959) Structural chemistry of the hemicelluloses. Adv Carbohydr Chem 14:429–468
Aspinall GO, Begbie R, McKay JE (1962) The hemicelluloses of European larch (Larix decidua). Part II. J Chem Soc 214:219
Benech RO, Li X, Patton D, Powlowski J, Storms R, Bourbonnais R, Paice M, Tsang A (2007) Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzyme Microb Technol 41:740–747
Bourgault R, Bewley JD, Alberici A, Decker D (2001) Endo-β-mannanase activity in tomato and other ripening fruits. Hortscience 36:72–75
Chaikumpollert O, Methacanon P, Suchiva K (2004) Structural elucidation of hemicelluloses from Vetiver grass. Carbohydr Polym 57:191–196
Chandrasekaran R, Radha A, Okuyama K (1998) Morphology of galactomannans: an X-ray structure analysis of guaran. Carbohydr Res 306:243–255
Chen X, Cao Y, Ding Y, LuW LD (2007) Cloning, functional expression and characterization of Aspergillus sulphureus β-mannanase in Pichia pastoris. J Biotechnol 8:452–461
Cui F, Shi J, Lu Z (1999) Production of neutral beta-mannanase by Bacillus subtilis and its properties. Wei Sheng Wu Xue Bao 39(1):60–63
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27(4):197–216
Duffaud GD, McCutchen CM, Leduc P, Parker KL, Kelly RM (1997) Purification and characterization of extremely thermostable β-mannanase, β-mannosidase, and α-galactosidase from the hyperthermophilic Eubacterium Thermotoga neapolitana 5068. Appl Environ Microbiol 63:169–177
Fan Z, Wagschal K, Chen W, Montross MD, Lee CC, Yuan L (2009a) Multimeric hemicellulases facilitate biomass conversion. Appl Environ Microbiol 75:1754–1757
Fan Z, Werkman JR, Yuan L (2009b) Engineering of a multifunctional hemicellulose. Biotechnol Lett 31:751–757
Feng Y, He Z, Ong SL, Hu J, Zhang Z, Ng WJ (2003) Optimization of agitation, aeration and temperature conditions for maximum β-mannanase production. Enzyme Microb Technol 32:282–289
Ferreira HM, Filho EXF (2004) Purification and characterization of a β-mannanase from Trichoderma harzianum strain T4. Carbohydr Polym 57:23–29
Gomes J, Terler K, Kratzer R, Kainz E, Steiner W (2007) Production of thermostable β-mannosidase by a strain of Thermoascus aurantiacus: isolation, partial purification and characterization of the enzyme. Enzyme Microb Technol 40:969–975
Großwindhager C, Sachslehner A, Nidetzky B, Haltrich D (1999) Endo-β-1,4 mannanase is efficiently produced by Sclerotium (Athelia) rolfsii under derepressed conditions. J Biotechnol 67:189–203
Gubitz GM, Hayn M, Sommerauer M, Steiner W (1996) Mannan-degrading enzymes from Sclerotium rolfsii: characterization and synergism of two endo β-mannanases and β-mannosidase. Bioresour Technol 58:127–135
Gubitz GM, Haltrich D, Latal B, Steiner W (1997) Mode of depolymerisation of hemicellulose by various mannanases and xylanases in relation to their ability to bleach softwood pulp. Appl Microbiol Biotechnol 47:658–662
Hilge M, Gloor SM, Rypniewski W, Sauer O, Heightman TD, Zimmerman W et al (1998) High-resolution native and complex structures of thermostable β-mannanase from Thermomonospora fusca-substrate specificity in glycosyl hydrolase family 5. Structure 6:1433–1444
Hongshu Z, Jinggan Y, Yan Z (2002) The glucomannan from ramie. Carbohydr Polym 47:83–86
Hossain MZ, Abe JI, Hizukuri S (1996) Multiple forms of β-mannanase from Bacillus sp. KK01. Enzyme Microb Technol 18:95–98
Ishurd O, Kermagi A, Zgheel F, Flefla M, Elmabruk M, Yalin W, Kennedy JF, Yuanjiang P (2004) Structural aspects of water soluble galactomannan from the seeds of Retama raetam. Carbohydr Polym 58:41–44
Jiang Z, Wei Y, Li D, Chai P, Kusakabe I (2006) High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr Polym 66:88–96
Jorgensen H, Sanadi AR, Felby C, Lange NEK, Fischer M, Ernst S (2010) Production of ethanol and feed by high dry matter hydrolysis and fermentation of palm kernel press cake. Appl Biochem Biotechnol 161:318–332
Kalogeris E, Christakopoulos P, Katapodis P, Alexiou A, Vlachou S, Kekos D, Macris BJ (2003) Production and characterization of cellulolytic enzymes from the thermophilic fungus Thermoascus aurantiacus under solid state cultivation of agricultural wastes. Process Biochem 38:1099–1104
Kango N (2007) Lignocellulose biotechnology: harnessing renewable plant materials for energy and chemicals. Everyman’s Sci XLII(4):194–197
Kango N, Agrawal SC, Jain PC (2003) Production of xylanase by Emericella nidulans NK-62 on low-value lignocellulosic substrates. World J Microbiol Biotechnol 19(7):691–694
Kansoh AL, Nagieb ZA (2004) Xylanase and mannanase enzymes from Streptomyces galbus NR and their use in biobleaching of softwood kraft pulp. Anton van Leeuwonhoek 85:103–114
Kataoka N, Tokiwa Y (1998) Isolation and characterization of an active mannanase-producing anaerobic bacterium, C. tertium KT-5A from lotus soil. J Appl Microbiol 84:357–367
Kenne L, Rossel K-G, Svensson S (1975) Studies on the distribution of the O-acetyl groups in pine glucomannan. Carbohydr Res 44:69–76
Kirk O, Borchet TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351
Kobayashi Y, Echigen R, Mada M, Mutai M (1987) Effect of hydrolyzate of konjac mannan and soybean oligosaccharides on intestinal flora in man and rats. In: Mitsuoka T (ed) Intestinal flora and food factors. Gakkai Shuppan Centre, Tokyo, pp 79–97
Kote NV, Patil AGG, Mulimani VH (2009) Optimization of the production of thermostable endo-β-1,4 mannanases from a newly isolated Aspergillus niger gr and Aspergillus flavus gr. Appl Biochem Biotechnol 152(2):213–223
Kurakake M, Komaki T (2001) Production of β-Mannanase and β-Mannosidase from Aspergillus awamori K and their properties. Curr Microbiol 42:377–380
Kurakake M, Sumida T, Masuda D, Oonishi S, Komaki T (2006) Production of galacto-manno-oligosaccharides from guar gum by β-mannanase from Penicillium oxalicum SO. J Agric Food Chem 54:7885–7889
Liepman AH, Nairn CJ, Willats WGT, Sørensen I, Roberts AW, Keegstra K (2007) Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggest diverse roles of mannans in plants. Plant Physiol 143:1881–1893
Lin TC, Chen C (2004) Enhanced mannanase production by submerged culture of Aspergillus niger NCH-189 using defatted copra based media. Process Biochem 39(9):1103–1109
Lin SS, Dou WF, Xu HY, Li HZ, Xu ZH, Ma YH (2007) Optimization of medium composition for the production of alkaline b-mannanase by alkaliphilic Bacillus sp.N16–5 using response surface methodology. Appl Microbiol Biotechnol 75:1015–1022
Lundqvist J, Teleman A, Junel L, Zacchi G, Dahlman O, Tjerneld F, Stalbrand H (2002) Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr Polym 48:29–39
Lundqvist J, Jacobs A, Palm M, Zacchi G, Dahlman O, Stålbrand H (2003) Characterization of galactoglucomannan extracted from spruce (Picea abies) by heat-fractionation at different conditions. Carbohydr Polym 51:203–211
Ma YH, Xue YF, Dou YT, Xu ZH, Tao WY, Zhou PJ (2004) Characterization and gene cloning of a novel b-mannanase from alkaliphilic Bacillus sp. N16–5. Extremophiles 8:447–454
Maijala P, Kango N, Szijarto N, Viikari L (2012) Characterization of hemicellulases from thermophilic fungi. Anton van Leeuwonhoek 101(4):905–917
Mohamad SN, Nagasundara R, Mohamad RR, Ariff AB (2011) Improved mannan-degrading enzymes production by Aspergillus niger through medium optimization. New Biotechnol 28. doi:10.1016/j.nbt.2010.10.008
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Mudau MM, Setati ME (2006) Screening and identification of endomannanase-producing microfungi from hypersaline environments. Curr Microbiol 52:477–481
Naganagouda K, Salimath PV, Mulimani VH (2009) Purification and characterization of endo-β-1,4 mannanase from Aspergillus niger gr for application in food processing industry. J Microbiol Biotechnol 19(10):1184–1190
Nunes FM, Domingues MR, Comibra MA (2005) Arabinosyl and glucosyl residues as structural features of acetylated galactomannans from green and roasted coffee infusions. Carbohydr Res 340:1689–1698
Oda Y, Komaki T, Tonomura K (1993) Production of β-mannanase and β-mannosidase by Enterococcus casseliflavus FL2121 isolated from decayed konjac. Food Microbiol 10:352–358
Omarsdottir S, Petersen BO, Barsett H, Paulsen BS, Duus JØ, Olafsdottir ES (2006) Structural characterization of a highly branched galactomannan from the lichen Peltigera canina by methylation analysis and NMR-spectroscopy. Carbohydr Polym 63:54–60
Pan X, Zhou J, Tian A, Le K, Yuan H, Xue Y, Ma Y, Lu H (2011) High level expression of a truncated β-mannanase from alkaliphilic Bacillus sp. N16-5 in Kluyveromyces cicerisporus. Biotechnol Lett 33:565–570
Perret S, Belaich A, Fierobe HP, Belaich JP, Tardif C (2004) Towards designer cellulosomes in Clostridia: mannanase enrichment of the cellulosomes produced by Clostridium cellulolyticum. Biotechnol Appl Biochem 40:255–259
Petkowicz CLO, Schaefer S, Reicher F (2007) The mannan from Schizolobium parahybae endosperm is not a reserve polysaccharide. Carbohydr Polym 69:659–664
Popa VI, Spiridon J (1998) Hemicelluloses: structure and properties. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility. Marcel Dekker, New York, pp 297–311
Puchart V, Vrsanska M, Svoboda P, Pohl J, Biely P (2004) Purification and characterization of two forms of endo- β-1,4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly T. lanuginosus IMI 158749). Biochim Biophys Acta 1674:239–250
Puls J, Schuseil J (1993) Chemistry of hemicellulose: relationship between hemicellulose structure and enzyme required for hydrolysis. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland Press, London, pp 1–27
Qiao Y, Chen X, Ding H, Yue M (2008) Heterologous expression and characterization of man gene from Bacillus subtilis in Pichia pastoris. Front Biol China 3:26–31
Ratto M, Poutanen K (1988) Production of mannan-degrading enzymes. Biotechnol Lett 10(9):661–664
Ratto M, Siika-aho M, Buchert J, Valkeajarvi A, Viikari L (1993) Enzymatic hydrolysis of isolated and fibrebound galactoglucomannans from pine-wood and pine raft pulp. Appl Microbiol Biotechnol 40:449–454
Reyna ABV, Noyola PP, Calvo-Méndez C, López-Romero E, Flores-Carreón A (1999) Purification and biochemical characterization of two soluble α-mannosidases from Candida albicans. Glycobiology 9:533–537
Ronne H (1995) Glucose repression in fungi. Trends Genet 11:12–17
Roth R, Moodley V, van Zyl P (2009) Heterologous expression and optimized production of an Aspergillus aculeatus endo-1,4-β-mannanase in Yarrowia lipolytica. Mol Biotechnol 43:112–120
Ruijter GJG, Visser J (1997) Carbon repression in Aspergilli. FEMS Microbiol Lett 151:103–114
Sachslehner A, Foidl G, Foidl N, Gubitz G, Haltrich D (2000) Hydrolysis of isolated coffee mannan and coffee extract by mannanases of Sclerotium rolfsii. J Biotechnol 80:127–134
Schroder R, Wegrzyn TF, Sharma NN, Atkinson RG (2006) LeMAN4 endo-β-mannanase from ripe tomato fruit can act as a mannan transglycosylase or hydrolase. Planta 224:1091–1102
Setati ME, Ademark P, VanZyl WH, Hahn-Hagerdal B, Stalbrand H (2001) Expression of the Aspergillus aculeatus endo-β-1,4-mannanase encoding gene (man1) in Saccharomyces cerevisiae and characterization of the recombinant enzyme. Protein Expr Purif 21:105–114
Shi P, Yuan T, Zhao J, Huang H, Luo H, Meng K, Wang Y, Yao B (2011) Genetic and biochemical characterization of a protease-resistant mesophilic β-mannanase from Streptomyces sp. S27. J Ind Microbiol Biotechnol 38:451–458
Singh V, Malviya T (2006) A non-ionic glucomannan from the seeds of an indigenous medicinal plant: Bryonia laciniosa. Carbohydr Polym 64:481–483
Singh S, Madlala AM, Prior BA (2003) Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev 27:3–16
Stalbrand H, Siika-aho M, Tenkanen M, Viikari L (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29:229–242
Summpunn P, Chaijan S, Isarangkul D, Wiyakrutta S, Meevootisom V (2011) Characterization, gene cloning and heterologous expression of β-mannanase from a thermophilic bacillus subtilis. J Microbiol 49:86–93
Takegawa K, Miki S, Jikibara T, Iwahara S (1989) Purification and characterization of exo-α-d-mannosidase from a Cellulomonas sp. Biochim Biophys Acta 991:431–437
Tenkanen M, Makkonen M, Perttula M, Viikari L, Teleman A (1997) Action of Trichoderma reesei mannanase on galactoglucomannan in pine kraft pulp. J Biotechnol 57:191–204
Timell TE (1965) Wood hemicelluloses: part II. Carbohydr Chem 20:409–483
Timell TE (1967) Recent progress in the chemistry of wood hemicelluloses. Wood Sci Technol 1:45–70
Torrie JP, Senior DJ, Saddler JN (1990) Production of β-mannanases by Trichoderma harzianum E58. Appl Microbiol Biotechnol 34:303–307
Van Zyl PJ, Moodley V, Rose SH, Roth RL, Van Zyl WH (2009) Production of the Aspergillus aculeatus endo-1,4-β-mannanase in A. niger. J Ind Microbiol Biotechnol 36:611–667
Van Zyl WH, Rosea SH, Trollopeb K, Gorgensb JF (2010) Fungal β-mannanases: mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem 45:1203–1213
Viikari L, Tenkanen M, Buchert J, Ratto M, Bailey M, Siika-Aho M, Linko M (1993) Hemicellulases for industrial applications. In: Saddler JN (ed) Bioconversion of forest and agricultural plant residues. CAB International, Wallingford, pp 131–182
Viniegra-Gonzalez G, Favela-Torres E, Aguilar CN, Romero-Gomez SJ, Diaz-Godinez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Willfor S, Sjoholm R, Laine C, Roslund M, Hemming J, Holmbom B (2003) Characterisation of water-soluble galactoglucomannan from Norway spruce wood and thermomechanical pulp. Carbohydr Polym 52:175–187
Xiaoyu F, Huang X, Liu P, Lin L, Wu G, Li C, Feng C, Hong Y (2010) Cloning and characterization of a novel mannanase from Paenibacillus sp. BME-14. J Microbiol Biotechnol 20(3):518–524
Yoshida T, Inoue T, Ichishima E (1993) 1,2-i-d-Mannosidase from Penicillium citrinum: molecular and enzymic properties of two isoenzymes. Biochem J 290:349–354
Zakaria MM, Ashiuchi M, Yamamoto S, Yagi T (1998) Optimization for beta-mannanase production of a psychrophilic bacterium, Flavobacterium sp. Biosci Biotechnol Biochem 62:655–660
Zhang Q, Yan X, Zhang L, Tang W (2006) Cloning, sequence analysis, and heterologous expression of a β-mannanase gene from Bacillus subtilis Z-2. Mol Biol 40:368–374
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer India
About this chapter
Cite this chapter
Soni, H., Kango, N. (2013). Microbial Mannanases: Properties and Applications. In: Shukla, P., Pletschke, B. (eds) Advances in Enzyme Biotechnology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1094-8_4
Download citation
DOI: https://doi.org/10.1007/978-81-322-1094-8_4
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1093-1
Online ISBN: 978-81-322-1094-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)




 Mannose
Mannose  Glucose
Glucose  Galactose
Galactose
 Mannose
Mannose  Glucose
Glucose  Galactose
Galactose
 Mannose
Mannose  Glucose
Glucose  Galactose
Galactose