Abstract
Studies of social ecology can benefit from long-term observations, as these provide researchers with opportunities to distinguish between the relative contributions of life history, demographics, and ecological pressures to the development of social patterns. Long-term study can provide the means of interpreting changes in stable social patterns relative to changes in environmental factors or availability of members of specific age-sex classes. The strength of social patterns can be measured by their persistence from one generation to the next, and as individuals pass life history milestones.
The Sarasota Dolphin Research Program has been engaged in studies of bottlenose dolphins along the central west coast of Florida, including Sarasota Bay, since 1970. The research includes focal animal behavioral observations, photographic identification surveys, biopsy darting for genetic and contaminant samples, and occasional capture–release efforts to examine the animals’ behavior, ecology, life history, population biology, health, and concentrations and effects of environmental contaminants. More than 4,800 individuals have been identified in the bays and coastal Gulf of Mexico waters of the region, including the approximately 160 dolphins using Sarasota Bay on a regular basis. A mosaic of adjacent, often slightly overlapping dolphin communities has been identified based on sighting locations and social associations, and genetic findings support these designations. These communities are genetically distinguishable but not isolated.
The communities of dolphins residing in and around Sarasota Bay, the most intensively studied animals, are characterized by a high level of multigenerational site fidelity and low levels of emigration and immigration. The social structure includes three basic components: nursery groups built around females with young of similar age, juvenile groups, and adult males, mostly in strongly bonded, long-term male pairs or sometimes as single individuals. This overall structure has remained relatively stable through five generations; however, core area use, group size, and some social association patterns show variability over time. Paternity testing suggests that male pair-bonding may improve reproductive success. Female reproductive success appears to be related to mother’s age, experience, and environmental contaminant residues. Older, more experienced mothers are more successful in rearing young over the typical 3- to 6-year period of association; these females have also previously depurated organochlorine contaminants that otherwise might have influenced reproduction and health.

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Studies of social ecology can benefit from long-term observations, as these provide researchers with opportunities to distinguish between the relative contributions of life history, demographics, and ecological pressures to the development of social patterns. Long-term study can provide the means of interpreting changes in stable social patterns relative to changes in environmental factors or availability of members of specific age-sex classes. The strength of social patterns can be measured by their persistence from one generation to the next, and as individuals pass life history milestones.
Many delphinid cetaceans live in complex societies (Norris and Dohl 1980a; Wells et al. 1999; Mann et al. 2000). Long lifespans, in some species exceeding 50 years, likely contribute to the observed social complexity. To fully understand the social structure of these animals and the ecological influences on social patterns, it is beneficial to observe individuals throughout the course of their lives. Several field studies of dolphins have begun to approach this goal. Hawaiian spinner dolphin (Stenella longirostris) studies initiated by Ken Norris and Tom Dohl in the late 1960s have been continued at intervals over several decades, providing insights into the structure and dynamics of the fluid societies of these small delphinids (Norris and Dohl 1980b; Norris et al. 1994). Studies of killer whales (Orcinus orca) initiated by Michael Bigg near Vancouver Island in the early 1970s and continued by others to the present (Bigg 1982; Ford and Fisher 1983; Parsons et al. 2009; Foster et al. 2012) have led to detailed descriptions of the workings of these highly stable societies. The social behavior of dusky dolphins (Lagenorhynchus obscurus) has been studied in depth in Argentina and later in New Zealand by Bernd and Melany Würsig and colleagues since the mid-1970s (Würsig and Würsig 1980; Benoit-Bird et al. 2004; Weir et al. 2008).
One of the first of the long-term studies of delphinid social ecology began in Sarasota Bay, Florida, in 1970, with common bottlenose dolphins (Tursiops truncatus) (Scott et al. 1990a; Wells 1991, 2003, 2009a). The study reported in this chapter has followed individually identified resident dolphins for more than 42 years, in some cases, and across at least five maternally related generations. Long-term observations combined with information on life history and ecology have begun to provide an understanding of the social structure of this dolphin community and the environmental and demographic factors influencing this structure.
2 Background
2.1 Study Area
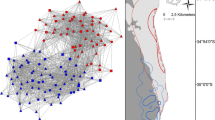
The study area includes the inshore and coastal waters along the central west coast of Florida, from Tampa Bay southward through Charlotte Harbor and Pine Island Sound, and the Gulf of Mexico to about 5–10 km offshore (Fig. 8.1). Most of the research effort has been concentrated in Sarasota Bay and adjacent bays, sounds, and Gulf waters within 1 km of the shore because of initial findings of dolphin residency to these waters (Irvine and Wells 1972) and their proximity to our base of operations at Mote Marine Laboratory in Sarasota. The shallow (<4 m deep), sheltered bay and estuarine waters are separated from the Gulf of Mexico by a series of barrier islands, communicating with the Gulf through narrow, deeper (up to ~10-m-deep) passes. The bays contain areas of shallow seagrass meadows and are fringed by mangroves, along with manmade features such as bridges, piers, and seawalls. Natural or dredged channels 3–4 m deep run through seagrass meadows and sand or mud flats. A gently sloping, shallow sandy bottom extends offshore from the beaches on the Gulf sides of the barrier islands. Sarasota and Manatee Counties, which encompass the Sarasota Bay study area, are heavily populated, with more than 687,000 people (as of 2008), and more than 45,000 registered vessels (as of 2008). Beach- and boat-based tourism is of major importance to the region.
2.2 History of the Sarasota Dolphin Research Program
A pilot tagging study conducted through Mote Marine Laboratory during 1970–1971 to investigate movements and activities of common bottlenose dolphins (Tursiops truncatus) along the central west coast of Florida, and to test tag designs, initially identified patterns of residency for dolphins in this region, setting the stage for continuing research (Irvine and Wells 1972). Additional research, including radio-tagging and radio-tracking, along with observational studies and some photographic identification efforts through the University of Florida during 1975–1978, confirmed previous residency findings. During this work and subsequent opportunistic surveys in 1979, re-identifications of 92 % of the dolphins tagged during 1970–1971 suggested that residency might be long term (Irvine et al. 1981). The high frequency of resightings of identifiable individuals led to an initial description of home range and social patterns (Wells et al. 1980).
Seasonal, systematic photographic identification surveys were initiated in 1980, and continued through 1989 through the University of California, Santa Cruz, and Dolphin Biology Research Institute. Since 1989, the program has been coordinated through the Chicago Zoological Society. Seasonal surveys continued until 1993, when year-round, monthly surveys were implemented and continued through the present (Scott et al. 1990a; Wells 1991, 2003). These surveys are conducted from small (<8-m-long) outboard-powered vessels following standard routes through the study area, selected daily depending on previous coverage and conditions. When dolphin groups are encountered, data are collected on location, time, environmental parameters, dolphin activities, numbers of dolphins, calves, and young-of-the-year (YOY). Photographs are taken of dolphin dorsal fins to identify individuals from distinctive markings (Scott et al. 1990b; Würsig and Jefferson 1990); the resulting identification catalog included more than 4,800 dolphins as of the end of 2013. Through 2013, the sighting database included more than 113,000 individual identifications from more than 41,000 dolphin group records collected since 1970, with some individuals having been resighted more than 1,400 times each.
Life history and health data are obtained through occasional capture–release sessions, in which small groups of selected dolphins are encircled with a 500-m-long, 4-m-deep seine net in shallow water (<2 m deep). Each individual is brought aboard a specialized veterinary examination vessel, where sex is determined; it is weighed, measured for a standard suite of lengths and girths (Read et al. 1993; Tolley et al. 1995), measured ultrasonically for blubber thickness (Wells 1993; Noren and Wells 2009), examined by a veterinarian externally and through ultrasonography, sampled, marked if necessary (Wells 2009b), photographed, and released (Wells et al. 2004). Samples are collected for basic health profiles (Wells et al. 2004; Hall et al. 2007; Schwacke et al. 2009), which are used as reference values for comparison with other populations experiencing unusual mortality events, abnormal environmental conditions, or anthropogenic stressors (St. Aubin et al. 2013; Schwacke et al. 2010, 2011). Samples are also collected for microbiology and disease processes (Buck et al. 2006; Burdett Hart et al. 2010, 2011; Nollens et al. 2009; Rowles et al. 2011; Hart et al. 2012), immune system function (Lahvis et al. 1995; Ruiz et al. 2009), serology (Duignan et al. 1996; Venn-Watson et al. 2008), reproductive hormones (Wells et al. 1987), biotoxins (Fire et al. 2008; Twiner et al. 2011), kidney health (Venn-Watson et al. 2010), and environmental contaminant concentrations (Schwacke et al. 2002; Wells et al. 2005; Houde et al. 2005, 2006; Hall et al. 2006; Bryan et al. 2007; Woshner et al. 2008; Yordy et al. 2010a,b,c,d; Kucklick et al. 2011; Miller et al. 2011). When ages are not known from long-term observations of mothers with calves, age is determined from examination of growth layer groups in a sectioned tooth (Hohn et al. 1989) and, experimentally, through telomere analyses (Dunshea et al. 2011). Genetic samples are collected for evaluating relationships, including paternity (Duffield and Wells 1991, 2002; Sellas et al. 2005). Since 1984, more than 700 sets of measurements have been collected from more than 225 individuals, with some measured up to 15 times. Since 1988, more than 700 blood samples have been collected from more than 230 individuals, some sampled as many as 15 times.
Capture–release sessions also provide opportunities to measure hearing abilities (Mann et al. 2010) and obtain acoustic recordings for studies of communication, including signature whistle characteristics and development (Fripp et al. 2005; Sayigh et al. 1990, 1995, 2007; Watwood et al. 2004, 2005; Esch et al. 2009), as well as to perform acoustic playback experiments to examine whistle function (Sayigh et al. 1999; Janik et al. 2006, 2013). Individual dolphins have been recorded during capture–release sessions since 1975, mostly via a suction cup-mounted hydrophone, and during focal animal behavioral follows, resulting in recordings from 225 individuals, some recorded up to 16 times and/or spanning more than 30 years.
In combination, the observational and capture–release datasets have led to the compilation of reproductive histories of more than 100 mothers with 300 of their calves. Some females have been observed with as many as 10 calves during the course of our research. Data include birthdates of calves, calf sex, mother’s age at time of birth (including age at first birth in some cases), duration of the mother–calf association, and circumstances leading to separation. These datasets also provide crucial background data in support of focal animal behavioral observations. Since 1992, more than 2,073 focal follows have been conducted on more than 143 different individuals followed up to 61 times each.
Ecological studies involve collaborative efforts. Carcasses recovered by Mote Marine Laboratory’s Stranding Investigations Program are examined and necropsied for determination of cause of death and for collection of standardized measurements and biological samples, including stomach contents (Barros and Wells 1998; Wells et al. 2008; Fauquier et al. 2009; DeLynn et al. 2011). Since 1985, more than 65 dolphins with sighting histories in our database have been recovered, and stomach contents have been collected from 33 Sarasota Bay residents. Stomach content data are examined relative to data from quantitative purse seine survey operations conducted during winter and summer field seasons to determine the abundance, distributions, length frequencies, body conditions, and species assemblages of fish using Sarasota Bay (Gannon et al. 2009; Berens McCabe et al. 2010). During more than 1,189 sets of the purse seine since 2004, more than 480,790 fish of 132 species have been caught, examined, measured, and released. Data on injuries from shark bites and stingray barbs are collected during necropsies and health assessments.
2.3 Study Population
Strong site fidelity has been demonstrated by dolphins using Sarasota Bay. Dolphins were considered to be residents of Sarasota Bay if they were seen at least ten times and more than half of their sighting records occurred within the core study area, the region bounded by Tampa Bay to the north and Venice Inlet to the south, and the barrier island chain to the west (Fig. 8.1). For 2007, the most recent year for which population analyses have been completed, 155 identifiable dolphins (and their dependent calves) met these criteria. On average, 89 % (±12 % SD) of the sightings of these animals occurred within Sarasota Bay. Of the 67 dolphins present in 2007 known to be at least 15 years old, 96 % had been observed in the area over a span of at least 15 years, with some observed for as many as 37 years.
The 155 identifiable dolphins comprised 96 % of the dolphins seen in Sarasota Bay, indicating that the total number of dolphins using the bay in 2007, including unmarked animals, was about 163. In 2007, about 84 % of the resident dolphins were of known sex (52 % F and 48 % F), and about 90 % were of known age class (42 % subadult and 58 % adult). As of 2013, the oldest resident male recorded to date was 50 years old, and the oldest female was 63 years old, based on long-term observations and growth layer groups in teeth (Hohn et al. 1989). The number of identifiable dolphins using Sarasota Bay on a regular basis has varied over time, ranging between 111 and 166 during the 15-year period of consistent survey effort from 1993 through 2007. Variations in abundance appear to have occurred at least partially in response to changes in commercial fishing regulations, resulting in increased prey abundance, and the periodic occurrence of severe harmful algal blooms (Karenia brevis red tides). Annual fecundity rate averaged about 0.14, recruitment rate to age 1 year averaged about 0.05, loss rate including known mortalities plus disappearances averaged about 0.04–0.06, and annual immigration rates were about 0.03 and emigration rates about 0.03–0.05 during 1980–1987 (Wells and Scott 1990) and during 1993–2007 (unpublished data).
3 Life History and Social Relationships in the Study Population
3.1 Reproduction and Development
The resident female bottlenose dolphins of Sarasota Bay tend to remain associated with the Bay throughout their lives, facilitating monitoring of reproductive success. Most calves are born during late spring or early summer and remain with their mothers for the next 3–6 years. Typically, separation from the mother occurs before the birth of her next calf. Males reach sexual maturity at 10–13 years of age, whereas females mature at 5–12 years of age (Wells and Scott 1999; Wells 2003). Many females give birth to their first calf at 8 to 10 years of age, following a 12-month gestation period. The female reproductive lifespan is prolonged, with a few females as old as 48 years successfully producing and rearing calves. Paternity tests have demonstrated that males in the age range of 13–40 years of age at least sire calves (Duffield and Wells 2002). Physical maturity is reached by females by about 12 years of age, and males by about 20 years of age, leading to significant sexual dimorphism in body length, mass, and other features (Read et al. 1993; Tolley et al. 1995).
3.2 Basic Societal Components
The bottlenose dolphins of Sarasota Bay are distributed at any given time in units referred to as groups (= school or sighting; Wells et al. 1987), defined operationally as “cohesive collections of conspecifics in a limited area (typically within several hundred meters), often engaged in similar activities and moving in the same general direction, maintained by social factors as a unit; groups may be stable over long periods of time or may change composition over periods ranging from minutes to weeks” (Wells et al. 1999). For Sarasota Bay bottlenose dolphins, these units are generally more similar to the small, changeable “parties” of chimpanzees (Goodall 1983) than to the permanent “pods” of killer whales (Bigg 1982). This working definition is a useful and replicable classification tool for the biologist in the field, and long-term observations of repeated patterns suggest that the observed groupings have biological meaning as well. However, our definition likely does not accurately reflect the dolphins’ full perspective on what constitutes an interacting social unit. In the murky estuarine waters of Sarasota Bay and vicinity, acoustic communication plays an important role in dolphin interactions. Signature whistles are believed to be used as contact calls in these environments (Watwood et al. 2005), and the active space for these whistles has been estimated to range from hundreds of meters to kilometers, depending on the sound attenuation characteristics of the habitat (Quintana-Rizzo et al. 2006). Thus, dolphins beyond the researcher’s sight may be interacting with the dolphins under observation. For example, on occasions when a member of a strongly bonded adult male pair is observed alone, the pair is often seen together again a short time later, and it is suspected that the males were in acoustic contact while “separated” (Watwood et al. 2005). Consideration of bottlenose dolphin groups should specify the kinds of interactions of interest.
The Sarasota Bay dolphins live in a fission–fusion society exhibiting the full spectrum of the term “group” as already defined (Wells et al. 1987; Connor et al. 2000; Wells 2003). They swim in small groups composed typically of 5 to 7 dolphins, ranging on rare, brief occasions to as many as 30 individuals (Wells et al. 1980, 1987). Variations in group size likely reflect demographic conditions as well as the ways in which the dolphins balance taking advantage of the benefits of group formation, such as protection from predation, while minimizing the costs, for example, from feeding competition (Wells et al. 1980). With a few exceptions, observed group composition changes frequently, over minutes to hours. Several basic group categories based on age, sex, and reproductive state can be described for Sarasota Bay dolphins, including (1) nursery groups, (2) juvenile groups of young dolphins independent of their mothers, and (3) adult males, typically as strongly bonded pairs.
Nursery groups, consisting of females with their most recent offspring, are the largest groups in the area. The reproductive state of the female and the age of her calf appear to be more important determinants of group composition than other factors such as relatedness (Wells et al. 1987). Group size decreases with calf age (Wells et al. 1987). Females with calves at similar levels of dependency tend to swim together, presumably because they must contend with similar needs for increased feeding to support lactation and because they must adjust their swimming patterns to facilitate frequent bouts of nursing. Female associates tend to be drawn from a pool of other mothers who inhabit significantly overlapping ranges. Wells (1991) referred to these female groups involving recurring associations as “bands,” reflecting the long-term social and geographic relationships among the females. Because of the extended reproductive lifespan of the Sarasota Bay dolphins, the pool of potential associates may include multiple generations of related and unrelated females who may swim together if they are in reproductive synchrony. The band structure that was well defined in the 1980s has changed during the past 20 years. Wells (2003) described the initial stages of these changes as involving the reduction of the more northerly Anna Maria band through mortality and lack of recruitment, and fissioning of the more southerly Palma Sola Band following growth from successful recruitment of female offspring during the 1980s and the loss of two of the oldest members in the early 1990s. The Anna Maria Band has ceased to exist, the previous members of the Palma Sola band continue to swim in smaller groups of only a few females, and a band of Tampa Bay females regularly summers in the northern portion of the Sarasota community’s range.
The most stable components of the nursery groups are the individual mother–calf pairs, with associations typically lasting 3–6 years. The period of mother–calf association typically extends well beyond the time of nutritional weaning and appears to provide opportunities for calves to learn important skills, such as feeding techniques (Wells 2003). Nutritional weaning is believed to occur during the second year of life, even though lactation and nursing may continue at a reduced level. Some calves orphaned in their second year of life have survived without being adopted by other resident females, even when related adult females are in the area. Older calves, especially females, will sometimes maintain close associations with their mothers and new siblings for months or more, presumably learning about calf rearing, and perhaps providing relief to the mother if she can share some rearing responsibilities with her older daughter.
Females without calves are often found together. In some cases, these associations involve females in their fifties and are believed to be post reproductive as they have not given birth for 13–20 years (Fig. 8.2). These presumed post-reproductive females associate with younger mothers and their calves, including kin, from time to time, but the relationships and presumed functions are not nearly so consistent or clear as those reported for killer whales (Foster et al. 2012).
The separation of calves from their mothers can occur abruptly, as is typically the case with male offspring, or it can be gradual, involving an incremental reduction in frequency and duration of associations over a number of months, as is observed more commonly for female calves (Wells et al. 1987; Wells 1991, 2003; McHugh et al. 2011a). Most newly independent calves join others in juvenile groups, while others may remain mostly alone within a very limited range for months before assimilating into groups with other juveniles. Juvenile groups are fluid in composition from day to day, include both sexes, and may include a broad range of ages up to early or mid-teens, in some cases reflecting delayed social maturity after individuals become sexually mature. These appear to be important formative years for the social development of young dolphins, as juveniles often engaged in social interactions that will take on greater importance later in life, such as copulations, affiliative behaviors, and agonistic behaviors. Juveniles interact with a large number of individuals of all age and sex classes, suggesting that this is a period of social exploration (McHugh et al. 2011a). Associations occurring during this phase often are maintained or recur throughout the individual’s life.
The duration of involvement in juvenile groups varies by sex. Females tend to leave juvenile groups before males. Female association patterns begin to change in association with the birth of a female’s first calf, but stronger associations with experienced mothers typically occur with subsequent calves (Owen 2001). Males mature later than females, and often associate with juveniles until they develop a strong pair bond with another male of similar age (Wells et al. 1987).
Alliances between adult males, in the form of long-term stable pair bonds, are among the strongest features of the Sarasota Bay bottlenose dolphin social structure, with average half-weight association coefficients of 0.753 (Wells 1991, 2003; Owen et al. 2002). Pair-bond formation is the norm for males in Sarasota Bay, with more than 93 % forming an alliance by age 20 (Owen et al. 2002). At any given time, about 57 % of adult or potentially adult males are paired, and 72 % of males 20 years or more of age are paired (Owen et al. 2002). The remaining males appear to be in transition, developing alliances or having lost an alliance partner. The average minimum age for first-time pair-bond formation is 11 years (Owen et al. 2002). Alliances are usually formed by individuals within less than 4 years of age of one another and who have been among the top five associates of each other within the 5 years preceding pair formation (Owen 2003). As has been noted by Goldberg and Wrangham (1997) for chimpanzees, males do not preferentially form alliances with close relatives; alliance partners are no more related to one another than they are to non-alliance males (Owen 2003). Some alliances have been observed over more than two decades, and about half of alliances end because of the loss of a partner (Owen 2003). Alliances may provide enhanced predator protection, which will grant pairs access to habitats where prey and predators may be more abundant (Owen 2003). Adult male pairs rarely associate with other males. They commonly move between groups of adult females, and may spend days to a week or more engaged in mate guarding with reproductively receptive females, engaging in sequential female defense polygyny (Moors 1997; Owen et al. 2002). Males play no role in calf rearing.
3.3 Social Matrix
Geography is the key defining feature of the Sarasota Bay bottlenose dolphin society. The inshore region from southern Tampa Bay to Venice Inlet and within several kilometers of the Gulf shore (Fig. 8.1) is the stage upon which the lives and social interactions of the resident dolphins are played out over decades and across generations. Sarasota Bay dolphins are constantly on the move through this region, in small groups that encounter other groups, and often change composition through joinings and separations as a result of these encounters. The resident dolphins interact with, and occasionally interbreed with, dolphins in adjacent ranges, but to a lesser extent than with the dolphins who share a Sarasota Bay home range (Wells 1986; Duffield and Wells 1991, 2002). The nature of these encounters and associations within a long-established range is reminiscent of the communities of chimpanzees (Goodall 1983), leading to adoption of this term as a descriptor of the kind of bottlenose dolphin social unit evident through much of the species inshore range in the southeastern United States (Wells 1986, Wells et al. 1987). As applied to dolphins, the term is defined as a regional society of animals sharing ranges and social associates, but exhibiting genetic exchange with other social units (Wells et al. 1999). A community is distinguished from the similar concept of a “population” by the fact that the latter is typically defined as a closed reproductive unit.
Geographic and physiographic features help to define the community range. More than 89 % of the resident sightings occur inshore of the barrier island chain bounding Sarasota Bay to the west and south, and south of an extensive shallow sandbank that delineates Tampa Bay from Sarasota Bay. Sighting frequencies for Sarasota Bay residents in the Gulf of Mexico decrease with distance from passes leading into Sarasota Bay, in contrast to the pattern of even distribution along the coast as exhibited by dolphins who rarely enter Sarasota Bay (Fazioli et al. 2006). Most Sarasota Bay residents have been recorded from all parts of the community range at some time in their lives, but individuals tend to frequent specific core areas, often characterized by habitat type. For example, some individuals spend most of their time in the vicinity of shallow seagrass meadows, while others emphasize the deeper, more open waters of Sarasota Bay proper in their daily movements. Upon reaching independence, calves often occupy all or part of their mother’s core area.
Our understanding of Sarasota dolphin community parameters has evolved over time, with expanded regional survey coverage beginning in the 1980s and with the accumulation of long-term individual sighting records. The size of the community range is currently estimated at about 125 km2 (Wells 2003; Urian et al. 2009), up from the 85 km2 reported from the much more limited dataset from the early years of the research program in the 1970s (Wells et al. 1980). Adult males tend to range farther than females (including up to 150 km2 or more), occasionally leaving the community range for months or more before returning, presumably in search of breeding opportunities (Wells 1991; Urian et al. 2009). At least in part as a result of increased survey coverage (spatial and temporal) and incorporation of improvements in photographic identification techniques, estimates of the numbers of dolphins using Sarasota Bay have increased from about 100 for the early years (Wells et al. 1980; Wells and Scott 1990) to about 160 residents in recent years (Wells 2009a, b).
Bottlenose dolphins are distributed continuously along the central west coast of Florida, including waters adjacent to Sarasota Bay. Consideration of genetics, ranging patterns, social associations, and stable isotope analyses has demonstrated the existence of a mosaic of communities in this region (Wells 1986; Wells et al. 1987; Duffield and Wells 1991, 2002; Sellas et al. 2005; Urian et al. 2009; Barros et al. 2010; Bassos-Hull et al., 2013). These communities are not isolated, behaviorally or genetically. About 15 % of calves born to Sarasota Bay resident mothers were sired by nonresident males (Duffield and Wells 2002). Community ranges sometimes overlap, and mixing occurs where community ranges are in close proximity. About 14 % to 17 % of groups including Sarasota dolphins also include dolphins from other communities (Wells et al. 1987; Fazioli et al. 2006). Agonistic interactions between dolphins from different communities occur, but are not frequently observed.
4 Factors Associated with Long-Term Social Stability and Variability
4.1 Site Fidelity
Strong long-term site fidelity ensures the possibility of frequent encounters with other community members. Social relationships, once developed, can be maintained through repeated contact. Dolphins born to community members tend to remain in the community, leading to the concurrent existence within the community of matrilineally related individuals spanning as many as five generations. Dolphins originally marked during the initial 1970–1971 tagging project were observed in the Sarasota Bay area for 27 years, on average, before they died or disappeared, and two of the original individuals were still seen in 2013, 42 years after their initial marking.
Natal site philopatry of both sexes appears to be the rule, but it is not absolute. Dispersal outside the community is not common (Wells 2003; Sellas et al. 2005), but can involve either males or females. Individuals may also leave the community range temporarily for periods of months, or in rare cases years, and some of these have been observed in nearby communities. Within the Sarasota community range, core areas may shift for some individuals over time. For example, a summer influx of females from southern Tampa Bay into the northern waters of the Sarasota community range beginning in the 1990s coincided with a southward shift in the core areas of several lifelong Sarasota Bay resident females (Wells 2003).
Overall, the Sarasota community range has exhibited great stability over four decades of observations, across multiple generations of residents. Similarly, dolphins seen primarily in Gulf coastal waters, Tampa Bay, or Charlotte Harbor/Pine Island Sound have been observed in those same waters over several decades (Urian et al. 2009; Wells 2009a, b; Bassos-Hull et al. 2013). Sellas et al. (2005) found strong genetic subdivision between dolphins inhabiting the coastal Gulf of Mexico and inshore waters including Sarasota Bay, in spite of the lack of isolation. The observed genetic distinctions support the idea that inshore communities may have existed for more than a few generations, perhaps as far back as the geological formation of the bays themselves (Sellas et al. 2005). The communities along the central west coast of Florida have continued to exist in spite of catastrophic environmental perturbations, including harmful algal blooms and hurricanes. Red tides from the toxic dinoflagellate Karenia brevis occur every few years along the central west coast of Florida, killing large numbers of marine vertebrates, including fish and sometimes dolphins (Fire et al. 2007, 2008). A severe red tide lasting 11 months in 2005 resulted in the loss of more than 70 % of primary dolphin prey fish in the Sarasota Bay area (Barros and Wells 1998; Gannon et al. 2009). The red tide resulted in temporary shifts in group size and habitat use (McHugh et al. 2011b), but the long-term residents remained within the long-established community range. In 2004 Category 4 Hurricane Charley struck Charlotte Harbor, immediately south of Sarasota Bay. In spite of tremendous coastal devastation and extensive pollution, the long-term resident dolphins remained in the area, and overall dolphin abundance appeared unchanged (Bassos-Hull et al. 2013). The concept of a stable, long-term, geographically based bottlenose dolphin community appears to be sufficiently robust to allow differentiation of units through consideration of a variety of parameters, including genetics (Duffield and Wells 1991, 2002; Sellas et al. 2005), ranging and social association patterns (Wells 1986; Wells et al. 1987; Urian et al. 2009), and stable isotopes (Barros et al. 2010). Strong attachment to a long-term community range facilitates development and maintenance of social relationships with other residents.
4.2 Life History
Protracted maternal investment and long lifespans also contribute to the development and maintenance of social relationships. The continued association of mothers and calves well beyond nutritional weaning suggests the importance of this relationship for calf learning. Mothers interact with a large number of associates (Wells et al. 1987), exposing their calves, in a protected context, to individuals with whom they may interact for decades to come. Associations in juvenile groups lead to male pair bonds that can last for decades (Wells et al. 1987; Owen et al. 2002). Repeated associations of adult females over the course of rearing as many as ten calves through a reproductive lifespan of four decades can improve the females’ probabilities for successful calf rearing (Owen 2001; Wells 2003).
5 Factors Associated with Reproductive Success
5.1 Female Reproductive Success
Female reproductive success is related to the mother’s age and level of experience (Wells 2000, 2003). Fewer than half of mothers less than 10 years of age successfully rear their calves through the first year (Wells 2003). Considering calf parity regardless of age, 58 % of first-time mothers, including some presumably primiparous females in their early teens, are successful through their first year of calf-rearing (Fig. 8.3). This percentage increases over the next two calves before declining somewhat with subsequent calves.
Age-related female reproductive success likely results from a combination of factors. Primiparous mothers are significantly smaller on average (length and mass) than multiparous mothers, suggesting that some of the younger mothers may not be fully developed as they attempt to rear their first calf. First-time mothers also have higher concentrations of lipohilic organochlorine pollutants such as PCBs and pesticides in their tissues, which they transfer to their calves via their fat-rich milk (Wells et al. 2005; Yordy et al. 2010b). Estimates suggest that a mother may transfer 80 % of her body burden of these organochlorine contaminants to her calf in the first few months of lactation (Cockcroft et al. 1989). Concentrations in Sarasota Bay mothers before lactation, accumulated over the first 6–13 years of life, exceed hypothesized thresholds for health and reproductive impacts, placing the first-born calves at higher risk for survival (Schwacke et al. 2002; Hall et al. 2006). First-born calves in Sarasota Bay exhibit higher concentrations than subsequent calves as a legacy of their mother’s original contaminant load (Wells et al. 2005). This process of depuration reduces the available contaminants for subsequent calves, reducing risks. However, as calving intervals and associated intervals between lactation periods lengthen later in a female’s life (Wells 2000), tissue concentrations of contaminants increase (Wells et al. 2005), perhaps explaining at least in part the decline in first-year survival of calves after the third birth (Fig. 8.3).
Social factors also influence female reproductive success and may be related to maternal experience. Calves raised in larger and more stable groups demonstrated the highest survival (Wells 2000). These kinds of groups likely provide enhanced protection from threats such as predation, aggressive conspecifics, or boat collisions (Wells and Scott 1997), and would provide opportunities for learning through observation, allomaternal care, and socialization. Owen (2001) found that experienced mothers tended to include other mothers with calves as close associates more frequently than did first-time mothers. Multiparous mothers also demonstrated increased control over their calf’s environment as compared to first-time mothers, by maintaining greater synchrony and keeping them closer (Owen 2001).
5.2 Male Reproductive Success
Social factors appear to play a stronger role in male reproductive success than do biological factors such as age or size for Sarasota Bay dolphins. Males associate preferentially with breeding females well before the beginning of the breeding season, perhaps to influence female choice later (Owen et al. 2002). Genetic paternity tests have shown that the Sarasota dolphins do not engage in monogamy, although some males may sire more than one calf through a particular female (Duffield and Wells 2002). Sires may be young or old, ranging in age from 13 to 40 years, and they may be larger or smaller individuals as compared to other adult males, including their alliance partners (Duffield and Wells 2002; Wells 2003). However, paired males sire disproportionately more calves than do unpaired males, suggesting an evolutionary basis for the development of these cooperative alliances. Potentially receptive females are the nearest neighbors of male alliances significantly more often, and for longer periods of time, than they are with unpaired males, providing paired males with greater access to mating opportunities (Owen 2003). Aggressive interactions between males and females in reproductive contexts appear to be much less common in Sarasota Bay than at other sites where male alliances have been observed, such as Shark Bay, Western Australia, suggesting either a greater role for female choice, or that control of females may be more subtle, perhaps influenced by the significant sexual dimorphism observed in Sarasota Bay (Wells et al. 1987; Tolley et al. 1995; Moors 1997; Connor et al. 2000; Owen 2003; Wells 2003).
6 Conclusions
Bottlenose dolphin social systems are the result of at least 10 to 12 million years (Myr) of delphinid evolution (Barnes 2002). The species has faced a wide range of environmental changes during its evolutionary history, and has adapted to these changes in part through the development of a high degree of behavioral plasticity, including variability in social structure (Wells et al. 1999; Mann et al. 2000; Reynolds et al. 2000). The occurrence of bottlenose dolphins in Sarasota Bay is a relatively recent phenomenon, because it has only been a few thousand years since the barrier islands and shallow bays of Florida’s west coast appeared in their current configuration. Disentangling the basic, core features of bottlenose dolphin societies from the range of variability that provides the species with crucial evolutionary resiliency in the face of environmental change is challenging. Long lifespans and social changes associated with life history milestones provide additional complications, but through long-term study they also offer opportunities for developing an understanding of the factors influencing social structure. Research carried out over much of the lifespan of an individual allows observations through changing environmental conditions, leading to identification of persistent patterns. Similarly, repeated observations across multiple generations allow the identification of age-related social patterns. Variations on these general themes provide indications of the potential range of responses to environmental changes.
Strong site philopatry over multiple decades, in combination with long lifespans and the co-occurrence of as many as five generations of related individuals, provide a solid basis for repeated interactions with familiar individuals, leading to long-term social relationships and contributing to a relatively stable social system. Patterns of social associations relative to age, sex, and reproductive status have been repeated consistently across generations and through dramatic environmental changes, allowing a description of the fundamental social structure. A stable society facilitates cultural transmission of knowledge, as has been noted for Sarasota Bay dolphins relative to feeding behaviors, for example (Wells 2003).
Although strong site fidelity establishes conditions supporting the development of a stable, long-term social system, it can also create problems for the animals because it exposes them to localized threats. Coastal bottlenose dolphins are facing increasing threats of human origin from such sources as environmental contaminants (Schwacke et al. 2002; Wells et al. 2005; Woshner et al. 2008; Yordy et al. 2010a), recreational and commercial fishing gear ingestion and entanglement (Wells and Scott 1994; Wells et al. 1998, 2008; Powell and Wells 2011), boat traffic and collisions (Wells and Scott 1997; Nowacek et al. 2001; Buckstaff 2004; Wells et al. 2008), and provisioning by humans (Cunningham-Smith et al. 2006; Powell and Wells 2011). The cumulative effects of anthropogenic and natural threats can place the continued survival of the long-term resident community at risk. For example, in 2006 about 2 % of the Sarasota Bay community died from ingestion of recreational fishing gear following the severe red tide of 2005 that depleted available prey, a level of additional mortality that was unsustainable (and fortunately did not continue). To date, the dolphins of Sarasota Bay have not demonstrated a capacity for shifting their community range in response to dramatic environmental changes such as severe red tides. If these dolphins occupy an ecological “cul-de-sac” where range shifts are precluded, then this raises important concerns for the future, when global climate disruption will likely alter the local environment significantly (Wells 2010). How much capacity will these animals have to respond to environmental changes?
From an applied perspective, a stable, geographically based community can serve as a biologically meaningful unit for wildlife management purposes (Wells 1986; Urian et al. 2009; Bassos-Hull et al. 2013). The ability to relate community exposure to specific local anthropogenic threats facilitates development and implementation of mitigation measures. Mitigation of known anthropogenic threats will become increasingly important as the animals face new threats such as global climate disruption. The behavioral plasticity and long reproductive lifespan of the species may provide a high degree of resiliency, but the capacity of the animals to respond to existing and emerging threats is likely not without bounds. Successful human mitigation of anthropogenic threats will provide the animals with increased capacity to respond to changes in their environment and will help to provide opportunity for the continued long-term stability of the community.
References
Barnes LG (2002) Dephinoids, evolution of the modern families. In: Perrin WF, Würsig B, Thewissen JGM (eds) Encyclopedia of marine mammals. Academic, San Diego, pp 314–316
Barros NB, Wells RS (1998) Prey and feeding patterns of resident bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. J Mammal 79(3):1045–1059
Barros NB, Ostrom P, Stricker C, Wells RS (2010) Stable isotopes differentiate bottlenose dolphins off west central Florida. Mar Mamm Sci 26:324–336
Bassos-Hull K, Perrtree R, Shepard C, Schilling S, Barleycorn A, Allen J, Balmer B, Pine W, Wells R (2013) Long-term site fidelity and seasonal abundance estimates of common bottlenose dolphins (Tursiops truncatus) along the southwest coast of Florida and responses to natural perturbations. J Cetacean Res Manag 13:19–30
Benoit-Bird KJ, Würsig B, McFadden CJ (2004) Dusky dolphin (Lagenorhynchus obscurus) foraging in two different habitats: active acoustic detection of dolphins and their prey. Mar Mamm Sci 20:215–231
Berens McCabe E, Gannon DP, Barros NB, Wells RS (2010) Prey selection in a resident common bottlenose dolphin (Tursiops truncatus) community in Sarasota Bay, Florida. Mar Biol 157(5):931–942
Bigg M (1982) An assessment of killer whale (Orcinus orca) stocks off Vancouver Island, British Columbia. Rep Int Whaling Comm 32:655–666
Bryan CE, Christopher SJ, Balmer BC, Wells RS (2007) Establishing baseline levels of trace elements in blood and skin of bottlenose dolphins in Sarasota Bay, Florida: implications for non-invasive monitoring. Sci Total Environ 388:325–342
Buck JD, Wells RS, Rhinehart HL, Hansen LJ (2006) Aerobic microorganisms associated with free-ranging bottlenose dolphins in coastal Gulf of Mexico and Atlantic Ocean waters. J Wildl Dis 42:536–544
Buckstaff KC (2004) Effects of watercraft noise on the acoustic behavior of bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar Mamm Sci 20:709–725
Burdett Hart L, Wells RS, Adams JD, Rotstein DS, Schwacke LH (2010) Modeling lacaziosis lesion progression in common bottlenose dolphins Tursiops truncatus using long-term photographic records. Dis Aquat Org 90:105–112
Burdett Hart L, Rotstein DS, Wells RS, Schwacke LH (2011) Lacaziosis and lacaziois-like prevalence among common bottlenose dolphins (Tursiops truncatus) from the west coast of Florida, USA. Dis Aquat Org 95:49–56
Cockcroft V, DeKock A, Lord D, Ross G (1989) Organochlorines in bottlenose dolphins Tursiops truncatus from the east coast of South Africa. S Afr J Mar Sci 8:207–217
Connor RC, Wells RS, Mann J, Read AJ (2000) The bottlenose dolphin, Tursiops spp.: social relationships in a fission–fusion society. In: Mann J, Connor RC, Tyack PL, Whitehead H (eds) Cetacean societies: field studies of dolphins and whales. University of Chicago Press, Chicago, pp 91–126
Cunningham-Smith P, Colbert DE, Wells RS, Speakman T (2006) Evaluation of human interactions with a wild bottlenose dolphin (Tursiops truncatus) near Sarasota Bay, Florida, and efforts to curtail the interactions. Aquat Mammal 32:346–356
DeLynn RE, Lovewell G, Wells RS, Early G (2011) Congenital scoliosis of a bottlenose dolphin. J Wildl Dis 47(4):979–983
Duffield DA, Wells RS (1991) The combined application of chromosome, protein and molecular data for the investigation of social unit structure and dynamics in Tursiops truncatus. In: Hoelzel AR (ed) Genetic ecology of whales and dolphins. Report of the International Whaling Commission, Special Issue 13. Cambridge, pp 155–169
Duffield DA, Wells RS (2002) The molecular profile of a resident community of bottlenose dolphins, Tursiops truncatus. In: Pfeiffer CJ (ed) Molecular and cell biology of marine mammals. Krieger, Melbourne, pp 3–11
Duignan PJ, House C, Odell DK, Wells RS, Hansen LJ, Walsh MT, St. Aubin DJ, Rima BK, Geraci JR (1996) Morbillivirus infection in bottlenose dolphins: Evidence for recurrent epizootics in the western Atlantic and Gulf of Mexico. Marine Mammal Science 12:499-515
Dunshea G, Duffield D, Gales N, Hindell M, Wells RS, Jarman SN (2011) Telomeres as age markers in animal molecular ecology. Mol Ecol Resour 11:225–235
Esch C, Sayigh L, Wells R (2009) Quantification of parameters of signature whistles of bottlenose dolphins. Mar Mamm Sci 25:976–986
Fauquier DA, Kinsel MJ, Dailey MD, Sutton GE, Stolen MK, Wells RS, Gulland FMD (2009) Prevalence and pathology of lungworm infection in bottlenose dolphins (Tursiops truncatus) from southwest Florida. Dis Aquat Org 88:85–90
Fazioli KL, Hofmann S, Wells RS (2006) Use of coastal Gulf of Mexico waters by distinct assemblages of bottlenose dolphins, Tursiops truncatus. Aquatic Mammals 32:212–222
Fire SE, Fauquier D, Flewelling LJ, Henry M, Naar J, Pierce R, Wells RS (2007) Brevetoxin exposure in bottlenose dolphins (Tursiops truncatus) associated with Karenia brevis blooms in Sarasota Bay, Florida. Mar Biol 152:827–834
Fire SE, Flewelling LJ, Wang Z, Naar J, Henry MS, Pierce RH, Wells RS (2008) Florida red tide and brevetoxins: association and exposure in live resident bottlenose dolphins (Tursiops truncatus) in the eastern Gulf of Mexico, USA. Mar Mamm Sci 24:831–844
Ford JKB, Fisher HD (1983) Group-specific dialects of killer whales (Orcinus orca) in British Columbia. In: Payne RS (ed) Communication and behavior of whales. Westview, Boulder, pp 129–161
Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, Croft DP (2012) Adaptive prolonged postreproductive life span in killer whales. Science 337:1313
Fripp D, Owen C, Quintana-Rizzo, Shapiro A, Buckstaff K, Jankowski K, Wells RS, Tyack P (2005) Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Anim Cognit 8:17–26
Gannon DP, Berens EJ, Camilleri SA, Gannon JG, Brueggen MK, Barleycorn A, Palubok V, Kirkpatrick GJ, Wells RS (2009) Effects of Karenia brevis harmful algal blooms on nearshore fish communities in southwest Florida. Mar Ecol Prog Ser 378:171–186
Goldberg TL, Wrangham RW (1997) Genetic correlates of social behaviour in wild chimpanzees: evidence from mitochondrial DNA. Anim Behav 54:559–570
Goodall J (1983) The chimpanzees of Gombe: patterns of behavior. Belknap Press of Harvard University Press, Cambridge
Hall AJ, McConnell BJ, Rowles TK, Aguilar A, Borrell A, Schwacke L, Reijnders PJH, Wells RS (2006) An individual-based model framework to assess the population consequences of polychlorinated biphenyl exposure in bottlenose dolphins. Environ Health Perspect 114(suppl 1):60–64
Hall AJ, Wells RS, Sweeney JC, Townsend FI, Balmer BC, Hohn AA, Rhinehart HL (2007) Annual, seasonal and individual variation in hematology and clinical blood chemistry profiles in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida. Comp Biochem Physiol A 148:266–277
Hart LB, Rotstein DS, Wells RS, Allen J, Barleycorn A, Balmer BC, Lane SM, Speakman T, Zolman ES, Stolen M, McFee W, Goldstein T, Rowles TK, Schwacke LH (2012) Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS One 7(3):e33081. doi:10.1371/journal.pone.0033081
Hohn AA, Scott MD, Wells RS, Sweeney JC, Irvine AB (1989) Growth layers in teeth from known-age, free-ranging bottlenose dolphins. Mar Mamm Sci 5(4):315–342
Houde M, Wells RS, Fair PA, Bossart GD, Hohn AA, Rowles TK, Sweeney JC, Solomon KR, Muir DCG (2005) Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ Sci Technol 39:6591–6598
Houde M, Balmer BC, Brandsma S, Wells RS, Rowles TK, Solomon KR, Muir DCG (2006) Perfluorinated alkyl compounds in relation with life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA. Environ Toxicol Chem 25:2405–2412
Irvine B, Wells RS (1972) Results of attempts to tag Atlantic bottlenose dolphins (Tursiops truncatus). Cetology 13:1–5
Irvine AB, Scott MD, Wells RS, Kaufmann JH (1981) Movements and activities of the Atlantic bottlenose dolphin, Tursiops truncatus, near Sarasota, Florida. Fish Bull US 79:671–688
Janik V, Sayigh LS, Wells RS (2006) Signature whistle shape conveys identity information to bottlenose dolphins. Proc Natl Acad Sci USA 103:8293–8297
Janik VM, King SL, Sayigh LS, Wells RS (2013) Identifying signature whistles from recordings of groups of unrestrained bottlenose dolphins (Tursiops truncatus). Mar Mamm Sci 29:109–122
Kucklick J, Schwacke L, Wells R, Hohn A, Guichard A, Yordy J, Hansen L, Zolman E, Wilson R, Litz J, Nowacek D, Rowles T, Pugh R, Balmer B, Sinclair C, Rosel P (2011) Bottlenose dolphins as indicators of persistent organic pollutants in waters along the US East and Gulf of Mexico coasts. Environ Sci Technol 45:4270–4277
Lahvis GP, Wells RS, Kuehl DW, Stewart JL, Rhinehart HL, Via CS (1995) Decreased lymphocyte responses in free-ranging bottlenose dolphins (Tursiops truncatus) are associated with increased concentrations of PCBs and DDT in peripheral blood. Environ Health Perspect 103:67–72
Mann J, Connor RC, Tyack PL, Whitehead H (eds) (2000) Cetacean societies: field studies of dolphins and whales. University of Chicago Press, Chicago
Mann D, Hill-Cook M, Manire CA, Greenhow D, Montie E, Powell J, Wells RS, Bauer G, Cunningham-Smith P, Lingenfelser R, DiGiovanni R, Stone A, Brodsky M, Stevens R, Kieffer G, Hoetjes P (2010) Hearing loss in stranded odontocete dolphins and whales. PLoS One 5(11):e13824. doi:10.1371/journal.pone.0013824
McHugh KA, Allen JB, Barleycorn A, Wells RS (2011a) Natal philopatry, ranging behavior, and habitat selection of juvenile bottlenose dolphins in Sarasota Bay, FL. J Mammal 92:1298–1313
McHugh KA, Allen JB, Barleycorn AA, Wells RS (2011b) Severe harmful algal bloom events influence juvenile common bottlenose dolphin behavior and sociality in Sarasota Bay, Florida. Mar Mamm Sci 27:622–643
Miller DL, Woshner V, Styer EL, Ferguson S, Knott KK, Gray MJ, Wells RS, O’Hara TM (2011) Histological findings in free-ranging Sarasota Bay bottlenose dolphin (Tursiops truncatus) skin: mercury, selenium and seasonal factors. J Wildl Dis 47(4):1012–1018
Moors TL (1997) Is ‘menage a trois’ important in dolphin mating systems? Behavioral patterns of breeding female bottlenose dolphins. M.Sc. thesis, University of California, Santa Cruz
Nollens HH, Rivera R, Palacios G, Wellehan JFX, Saliki JT, Caseltine SL, Smith CR, Jensen ED, Hui J, Lipkin WI, Yochem PK, Wells RS, St. Leger J, Venn-Watson S (2009) New recognition of Enterovirus infections in bottlenose dolphins (Tursiops truncatus). Vet Microbiol 139:170–175
Noren SR, Wells RS (2009) Postnatal blubber deposition in free-ranging common bottlenose dolphins (Tursiops truncatus) with considerations to buoyancy and cost of transport. J Mammal 90:629–637
Norris KS, Dohl TP (1980a) The structure and function of cetacean schools. In: Herman LM (ed) Cetacean behavior: mechanisms and functions. Wiley, New York, pp 211–261
Norris KS, Dohl TP (1980b) The behavior of the Hawaiian spinner porpoise, Stenella longirostris. Fish Bull US 77:821–849
Norris KS, Würsig B, Wells RS, Würsig M (1994) The Hawaiian spinner dolphin. University of California Press, Los Angeles
Nowacek SM, Wells RS, Solow AR (2001) Short-term effects of boat traffic on bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar Mamm Sci 17:673–688
Owen CFW (2001) A comparison of maternal care by primiparous and multiparous bottlenose dolphins, Tursiops truncatus: does parenting improve with experience? M.Sc. thesis, University of California, Santa Cruz, CA
Owen ECG (2003) The reproductive and ecological functions of the pair-bond between allied adult male bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Ph.D. dissertation, University of California, Santa Cruz
Owen ECG, Hofmann S, Wells RS (2002) Ranging and social association patterns of paired and unpaired adult male bottlenose dolphins, Tursiops truncatus, in Sarasota, Florida, provide no evidence for alternative male strategies. Can J Zool 80:2072–2089
Parsons KM, Balcomb KC III, Ford JKB, Durban JW (2009) The social dynamics of southern resident killer whales and conservation implications for this endangered population (Orcinus orca). Anim Behav 77:963–971
Powell JR, Wells RS (2011) Recreational fishing depredation and associated behaviors involving common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar Mamm Sci 27:111–129
Quintana-Rizzo E, Mann DA, Wells RS (2006) Estimated communication range of social sounds used by bottlenose dolphins (Tursiops truncatus). J Acoust Soc Am 120:1671–1683
Read AJ, Wells RS, Hohn AA, Scott MD (1993) Patterns of growth in wild bottlenose dolphins, Tursiops truncatus. J Zool Lond 231:107–123
Reynolds JE III, Wells RS, Eide SD (2000) The bottlenose dolphin: biology and conservation. University Press of Florida, Gainesville, p 289
Rowles TK, Schwacke LS, Wells RS, Saliki JT, Hansen L, Hohn A, Townsend F, Sayre RA, Hall AJ (2011) Evidence of susceptibility to morbillivirus infection in cetaceans from the United States. Mar Mamm Sci 27:1–19
Ruiz C, Nollens HH, Venn-Watson S, Green LG, Wells RS, Walsh MT, Chittick E, McBain JF, Jacobson ER (2009) Baseline circulating immunoglobulin G levels in managed collection and free-ranging bottlenose dolphins (Tursiops truncatus). Dev Comp Immunol 33:449–455
Sayigh LS, Tyack PT, Wells RS, Scott MD (1990) Signature whistles of free-ranging bottlenose dolphins Tursiops truncatus: stability and mother-offspring comparisons. Behav Ecol Sociobiol 26:247–260
Sayigh LS, Tyack PL, Wells RS, Scott MD, Irvine AB (1995) Sex difference in signature whistle production of free-ranging bottlenose dolphins, Tursiops truncatus. Behav Ecol Sociobiol 36:171–177
Sayigh LS, Tyack PL, Wells RS, Solow AR, Scott MD, Irvine AB (1999) Individual recognition in wild bottlenose dolphins: a field test using playback experiments. Anim Behav 57:41–50
Sayigh LS, Esch HC, Wells RS, Janik VM (2007) Facts about signature whistles of bottlenose dolphins (Tursiops truncatus). Anim Behav 74:1631–1642
Schwacke LH, Voit EO, Hansen LJ, Wells RS, Mitchum GB, Hohn AA, Fair PA (2002) Probabilistic risk assessment of reproductive effects of polychlorinated biphenyls on bottlenose dolphins (Tursiops truncatus) from the southeast United States coast. Environmental Toxicology and Chemistry 21:2752–2764.
Schwacke LH, Hall AJ, Townsend FI, Wells RS, Hansen LJ, Hohn AA, Bossart GD, Fair PA, Rowles TK (2009) Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am J Vet Res 70:973–985
Schwacke LH, Twiner MJ, De Guise S, Balmer BC, Wells RS, Townsend FI, Rotstein DC, Varela RA, Hansen LJ, Zolman ES, Spradlin TR, Levin M, Leibrecht H, Wang Z, Rowles TK (2010) Eosinophilia and biotoxin exposure in bottlenose dolphins (Tursiops truncatus) from a coastal area impacted by repeated mortality events. Environ Res 110:548–555
Schwacke LH, Zolman ES, Balmer BC, De Guise S, George RC, Hoguet J, Hohn AA, Kucklick JR, Lamb S, Levin M, Litz JA, McFee WE, Place NJ, Townsend FI, Wells RS, Rowles TK (2011) Anemia, hypothyroidism, and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proc R Soc B Biol Sci 279:48–57
Scott MD, Wells RS, Irvine AB (1990a) A long-term study of bottlenose dolphins on the west coast of Florida. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic, San Diego, pp 235–244
Scott MD, Wells RS, Irvine AB, Mate BR (1990b) Tagging and marking studies on small cetaceans. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic, San Diego, pp 489–514, 653 pp
Sellas AB, Wells RS, Rosel PE (2005) Mitochondrial and nuclear DNA analyses reveal fine scale geographic structure in bottlenose dolphins (Tursiops truncatus) in the Gulf of Mexico. Conserv Genet 6:715–728
St. Aubin DJ, Forney KA, Chivers SJ, Scott MD, Danil K, Romano T, Wells RS, Gulland FMD (2013) Hematological, serum and plasma chemical constituents in pantropical spotted dolphins (Stenella attenuata) following chase, encirclement and tagging. Mar Mamm Sci 29:14–35
Tolley KA, Read AJ, Wells RS, Urian KW, Scott MD, Irvine AB, Hohn AA (1995) Sexual dimorphism in wild bottlenose dolphins (Tursiops truncatus) from Sarasota, Florida. J Mammal 76(4):1190–1198
Twiner MJ, Fire S, Schwacke L, Davidson L, Wang Z, Morton S, Roth S, Balmer B, Rowles T, Wells R (2011) Concurrent exposure of bottlenose dolphins (Tursiops truncatus) to multiple toxins in Sarasota Bay, Florida, USA. PLoS One 6(3):e17394. doi:10.1371/journal.pone.0017394
Urian KW, Hofmann S, Wells RS, Read AJ (2009) Fine-scale population structure of bottlenose dolphins, Tursiops truncatus, in Tampa Bay, Florida. Mar Mamm Sci 25:619–638
Venn-Watson S, Rivera R, Smith CR, Saliki JT, Casteline S, St. Leger J, Yochem P, Wells RS, Nollens H (2008) Exposure to novel parainfluenza virus and clinical relevance in two bottlenose dolphin (Tursiops truncatus) populations. Emerg Infect Dis 14:397–405
Venn-Watson S, Townsend FI, Daniels RL, Sweeney JC, McBain JW, Klatsky LJ, Hicks CL, Staggs LA, Rowles TK, Schwacke LH, Wells RS, Smith CR (2010) Hypocitraturia in common bottlenose dolphins (Tursiops truncatus): assessing a potential risk factor for urate nephrolithiasis. Comp Med 60:1–5
Watwood SL, Tyack PL, Wells RS (2004) Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav Ecol Sociobiol 55:531–543
Watwood SL, Owen ECG, Tyack PL, Wells RS (2005) Signature whistle use by free-swimming and temporarily restrained bottlenose dolphins, Tursiops truncatus. Anim Behav 69:1373–1386
Weir JS, Duprey NMT, Würsig B (2008) Dusky dolphin (Lagenorhynchus obscurus) subgroup distribution: are shallow waters a refuge for nursery groups? Can J Zool 86:1225–1234
Wells RS (1986) Structural aspects of dolphin societies. Ph.D. dissertation, University of California, Santa Cruz
Wells RS (1991) The role of long-term study in understanding the social structure of a bottlenose dolphin community. In: Pryor K, Norris KS (eds) Dolphin societies: discoveries and puzzles. University of California Press, Berkeley, pp 199–225
Wells RS (1993) Why all the blubbering? BISON Brookfield Zoo 7(2):12–17
Wells RS (2000) Reproduction in wild bottlenose dolphins: Overview of patterns observed during a long-term study. In: Duffield D, Robeck T (eds) Bottlenose dolphin reproduction workshop report. AZA Marine Mammal Taxon Advisory Group, Silver Spring, pp 57–74
Wells RS (2003) Dolphin social complexity: lessons from long-term study and life history. In: de Waal FBM, Tyack PL (eds) Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press, Cambridge, pp 32–56
Wells RS (2009a) Learning from nature: bottlenose dolphin care and husbandry. Zoo Biol 28:1–17
Wells RS (2009b) Identification methods. In: Perrin WF, Würsig B, Thewissen JGM (eds) Encyclopedia of marine mammals, 2nd edn. Elsevier, San Diego, pp 593–599
Wells RS (2010) Feeling the heat: potential climate change impacts on bottlenose dolphins. Whalewatcher J Am Cetacean Soc 39(2):12–17
Wells RS, Scott MD (1990) Estimating bottlenose dolphin population parameters from individual identification and capture-release techniques. In: Hammond PS, Mizroch SA, Donovan GP (eds) Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Report of the International Whaling Commission, Special Issue 12, Cambridge, pp 407–415
Wells RS, Scott MD (1994) Incidence of gear entanglement for resident inshore bottlenose dolphins near Sarasota, Florida. In: Perrin WF, Donovan GP, Barlow J (eds) Gillnets and cetaceans. Report of the International Whaling Commission, Special Issue 15, p 629
Wells RS, Scott MD (1997) Seasonal incidence of boat strikes on bottlenose dolphins near Sarasota, Florida. Mar Mamm Sci 13:475–480
Wells RS, Scott MD (1999) Bottlenose dolphin Tursiops truncatus (Montagu, 1821). In: Ridgway SH, Harrison R (eds) Handbook of marine mammals: the second book of dolphins and porpoises, vol 6. Academic, San Diego, pp 137–182
Wells RS, Boness DJ, Rathbun GB (1999) Behavior. Pp. 324-422 In: Reynolds JE III, Rommel SA (eds) Biology of marine mammals. Smithsonian Institution Press, Washington, DC. Pp 324–422
Wells RS, Irvine AB, Scott MD (1980) The social ecology of inshore odontocetes. In: Herman LM (ed) Cetacean behavior: mechanisms and functions. Wiley, New York, pp 263–317
Wells RS, Scott MD, Irvine AB (1987) The social structure of free-ranging bottlenose dolphins. In: Genoways H (ed) Current mammalogy, vol 1. Plenum Press, New York, pp 247–305
Wells RS, Hofmann S, Moors TL (1998) Entanglement and mortality of bottlenose dolphins (Tursiops truncatus) in recreational fishing gear in Florida. Fish Bull 96:647–650
Wells RS, Rhinehart HL, Hansen LJ, Sweeney JC, Townsend FI, Stone R, Casper D, Scott MD, Hohn AA, Rowles TK (2004) Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth 1:246–254
Wells RS, Tornero V, Borrell A, Aguilar A, Rowles TK, Rhinehart HL, Hofmann S, Jarman WM, Hohn AA, Sweeney JC (2005) Integrating life history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Sci Total Environ 349:106–119
Wells RS, Allen JB, Hofmann S, Bassos-Hull K, Fauquier DA, Barros NB, DeLynn RE, Sutton G, Socha V, Scott MD (2008) Consequences of injuries on survival and reproduction of common bottlenose dolphins (Tursiops truncatus) along the west coast of Florida. Mar Mamm Sci 24:774–794
Woshner V, Knott K, Wells R, Willetto C, Swor R, O’Hara T (2008) Mercury and selenium in blood and epidermis of bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida (USA): interaction and relevance to life history and hematologic parameters. EcoHealth 5(1):1–11. doi:10.1007/s10393-008-0164-2
Würsig B, Jefferson TA (1990) Methods of photo-identification for small cetaceans. In: Hammond PS, Mizroch SA, Donovan GP (eds) Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. Report of the International Whaling Commission, Special Issue 12, Cambridge, pp 43–55
Würsig B, Würsig M (1980) Behavior and ecology of the dusky dolphin, Lagenorhynchus obscurus, in the South Atlantic. Fish Bull 77:871–890
Yordy JE, Mollenhauer MAM, Wilson RM, Wells RS, Hohn A, Sweeney J, Schwacke LH, Rowles TK, Kucklick JR, Peden-Adams MM (2010a) Complex contaminant exposure in cetaceans: a comparative E-SCREEN analysis of bottlenose dolphin blubber and mixtures of four persistent organic pollutants. Environ Toxicol Chem 29:2143–2153
Yordy J, Wells RS, Balmer BC, Schwacke L, Rowles T, Kucklick JR (2010b) Life history as a source of variation for persistent organic pollutant (POP) patterns in a community of common bottlenose dolphins (Tursiops truncatus) resident to Sarasota Bay, FL. Sci Total Environ 408:2163–2172
Yordy JE, Pabst DA, McLellan WA, Wells RS, Rowles TK, Kucklick JR (2010c) Tissue-specific distribution and whole body burden estimates of persistent organic pollutants in the bottlenose dolphin (Tursiops truncatus). Environ Toxicol Chem 29:1–11
Yordy JE, Wells RS, Balmer BC, Schwacke LH, Rowles TK, Kucklick JR (2010d) Partitioning of persistent organic pollutants (POPs) between blubber and blood of wild bottlenose dolphins: implications for biomonitoring and health. Environ Sci Technol 44:4789–4795
Acknowledgments
Many people over the past 43 years have contributed to the information presented in this chapter. Without the initial efforts of Blair Irvine and Michael Scott in the 1970s (and continuing today), there would have been no long-term study to report. Over the years the program has benefited greatly from the dedicated services of our laboratory managers and field coordinators, including Kim Urian, Sue Hofmann, Kim Bassos-Hull, Stephanie Nowacek, and Jason Allen, along with myriad staff, students, colleagues, and volunteers. Crucial information on ages has been provided by Aleta Hohn, and on genetic relationships by Debbie Duffield. Major support for ongoing operations has been provided by the Chicago Zoological Society, the Batchelor Foundation, NOAA’s Fisheries Service, Disney, Earthwatch Institute, the U.S. Marine Mammal Commission, Dolphin Quest, and Mote Marine Laboratory. Many thanks to Katherine McHugh for her review of an early draft.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Wells, R.S. (2014). Social Structure and Life History of Bottlenose Dolphins Near Sarasota Bay, Florida: Insights from Four Decades and Five Generations. In: Yamagiwa, J., Karczmarski, L. (eds) Primates and Cetaceans. Primatology Monographs. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54523-1_8
Download citation
DOI: https://doi.org/10.1007/978-4-431-54523-1_8
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54522-4
Online ISBN: 978-4-431-54523-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)