Abstract
Single-pollen genotyping enables us to track pollen flow that is mediated by animal vectors. By applying this innovative technique to a Camellia japonica and pollinator bird system on Miyake-jima, a volcanic island of the Izu Islands, Japan, we evaluated pollen flow mediated by the pollinator Zosterops japonica under different C. japonica flower densities. These flower densities were affected by a volcanic eruption in 2000 and subsequent large emissions of volcanic gases. The genetic diversity of pollen grains adhering to pollinators in areas with low flower density was greater than in an area with high flower density. This result was consistent with bird pollinator movement elucidated by radio tracking. In areas with low flower density, resulting from volcanic activity, pollinator birds ranged over larger areas to satisfy their energy demands rather than moving to areas with higher flower density. These results indicate that the C. japonica and pollinator bird system can ensure reproductive success even though external environmental factors, such as volcanic activity, have decreased flower density.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.


5.1 Introduction
Ecological observations during and after volcanic activity provide valuable opportunities to study how organisms respond to environmental devastation. Previous studies of volcanic islands have mainly addressed the colonization processes of pioneer plants to examine the primary succession of plant communities (e.g., Kitayama et al. 1995; Tsuyuzaki and del Moral 1995; Thornton et al. 1996; Shanahan et al. 2001; Kamijo et al. 2002; Kamijo and Hashiba 2003). However, no study has focused on the recovery processes of late climax forest communities by secondary succession according to various symbiotic species interactions. Because secondary succession dominates the recovery process, a better understanding of the detailed interactions between organisms during recovery is important.
In an effort to study these community recovery processes, we examined the effects of volcanic activity on a plant–animal system comprising a common broad-leaved evergreen tree species, Camellia japonica, from climax forests, and a pollinating bird, the Japanese white-eye, Zosterops japonica. We examined the hypothesis that Camellia trees are sufficiently robust to serve as a core species in natural forest recovery, even in severely damaged areas. We previously reported the effects of volcanic activity on the maternal reproductive success of C. japonica (Abe and Hasegawa 2008). We found that the net fruit production of C. japonica in a heavily damaged area was nearly the same as that in a less damaged area; this was true because the heavily damaged area had better pollination and seed-set rates compared to the less damaged area, which compensated for the suppressed blossoming and fruit growth in the heavily damaged area where poisonous volcanic gas exposure was intense. Despite the similar maternal reproductive success in terms of the final fruit-set rates in heavily damaged and less damaged sites, the genetic diversity of subsequent generations (seeds) may be different among sites. Knowledge about the genetic diversity of pollen transported by pollinators will deepen our understanding of population maintenance mechanisms and reproductive strategies in C. japonica. These types of studies are currently quite limited for bird-pollinated species (Ward et al. 2005).
In this study, we show how pollinator behavior affects the pollen dispersal of C. japonica at sites having variable flower densities. We do this using a single-pollen genotyping method and radio tracking of pollinator birds. Our previous study (Abe and Hasegawa 2008) suggested that because pollinator birds transport C. japonica pollen grains collected from various areas with low flower density, genetic diversity should increase in C. japonica progeny as a consequence of long-distance pollen dispersal vectors. Recently, direct genotyping of single pollen grains was developed (Matsuki et al. 2007, 2008). In the present study, we reveal characteristics of pollen movement of C. japonica pollinated by Z. japonica by comparing the genetic diversity of pollen grains on the birds between sites having different flower densities. In addition, we also compare the results from radio tracking of pollinator birds with pollen haplotyping. We examined the stability of plant–pollinator systems with regard to volcanic disturbance by combining the single-pollen genotyping method (Matsuki et al. 2007, 2008) with radio tracking, which demonstrated that genetic diversity in pollen grains found on the birds was more diverse in the heavily damaged area that had low flower density. The consequences of these results for plant–pollinator system stability following volcanic disturbances are discussed.
5.2 Materials and Methods
5.2.1 Study Areas
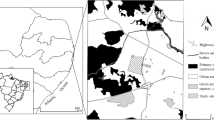
The Izu Islands are a group of volcanic islands located on the western rim of the Pacific Ocean. They are characterized by a humid warm temperate climatic zone with an annual rainfall of more than 2,000 mm and an average air temperature ∼17°C. The volcanically active island Miyake-jima (Fig. 5.1), located about 180 km south of Tokyo, was selected as the study island because the volcano explosively erupted during the summer of 2000. The main vegetation is evergreen broad-leaved dominated by species such as Castanopsis sieboldii, Machilus thunbergii, and Camellia japonica before the 2000 volcanic eruption (Kamijo et al. 2002; Kamijo and Hashiba 2003).
In the winter of 2006, we established three study sites on Miyake-jima [Tubota (TU), Igaya (IG), and Nanto-road 2 (N2); Figs. 5.1 and 5.2] to examine the genetic aspects of reproductive success under various flowering conditions related to volcanic activity.
5.2.2 Study Species
Camellia japonica was selected as the study species. In eastern Asia, C. japonica is a common broad-leaved evergreen tree species in climax forests (Wendel and Parks 1985; Katsuta 1999; Nagamasu 2006). This species is monoecious in its reproductive system. It produces conspicuous red flowers with a large quantity of nectar that attracts birds (Z. japonica and Hypsipetes amaurotis). It flowers during November to March with a peak during January to February on Yakushima Island (Yumoto 1988) and the Izu islands (Abe et al., unpublished). C. japonica is also self-incompatible (Shibata and Ieyumi 1991), and flowers isolated from pollinating birds do not bear fruit (Kunitake et al. 2004). Kunitake et al. (2004) experimentally demonstrated that Z. japonica (Fig. 5.3) is the most effective pollinator of C. japonica on Niijima Island adjacent to Miyake-jima. Although H. amaurotis, Cettia diphone, and Parus major are also potential pollinators at Miyake-jima, Z. japonica was always dominant and represented 78–96% of the flower-visiting birds (Abe and Hasegawa 2008). Z. japonica migrates to warmer places in winter, and some birds fly from mainland Honshu to the Izu islands. Resident birds on the Izu islands are classified as subspecies Z. japonica var. stejnegeri. Kikkawa and Kakizawa (1981) determined that about 9% of Z. japonica caught in winter on Miyake-jima were mainland subspecies. Abe et al. (2006) reported secondary seed dispersal by the Japanese wood mouse (Apodemus speciosus) on Niijima.
5.2.3 Flowering Conditions of C. japonica and Home Ranges of Z. japonica
During winter 2006, the number of flowering trees and flowers per flowering tree were counted, and the flower density (per hectare, ha) was estimated at each study site to analyze the relationship among flowering conditions, pollen genetic diversity, and the home range size of the pollinator bird Z. japonica. Home range sizes were estimated using radiotelemetry and analyzed for birds tracked for more than 1 week.
In the radiotelemetry tracking, birds were captured with 12- to 18-m mist nets (mesh size, 36 mm) that were placed at a central location between the radio-tracking sites (∼100 ha). The capture of birds using mist nets was permitted by the Ministry of the Environment, Government of Japan (permit no. 051118001). Transmitters (model LB-2; Holohil Systems, Canada; http://www.holohil.com) weighing less than 0.5 g were glued to the backs of adult birds heavier than 11 g in body mass, based on the methods of Raim (1978). Any two birds captured at the same time were determined to be a pair. Therefore, we only attached a transmitter to one of them. The life of a transmitter was 12 days (lifespan range, 8–15 days), and its dimensions were 13×6.5×3.5 mm (L×W×H). We radio-tracked birds with transmitters between 5 January and 5 February 2006 at high- and low-flower-density sites at TU, IG, and N2 on Miyake-jima (see Fig. 5.1). The birds were tracked more than three different times per day, between sunrise and sunset (normally at 0700, 1200, and 1600) for active birds and once during the night for inactive birds. The home range of the birds that were continually tracked for more than 1 week was depicted using minimum convex polygons. Radio-tracking equipment comprised a receiver (FT-817; Vertex Standard) that had three handheld Yagi aerial-antenna elements.
5.2.4 Pollen Grain Sampling and Haplotyping
For all birds captured using the methods just described, we first checked for the presence or absence of pollen grains on the beak and then wiped any visible pollen grains off the beak using a cotton-tipped swab (Fig. 5.4). Pollen grains were dropped on a glass slide from the swab, and a single pollen grain was picked up using a needle under a stereomicroscope.
DNA extraction and microsatellite haplotyping of a single pollen grain were conducted according to the method described by Matsuki et al. (2007). Haplotypes were determined using eight pairs of microsatellite polymerase chain reaction (PCR) primers: TMSE-27B03T, TMSE-9E07S, TMSE-25E07T, TMSE-11D02T, TMSE-4B07S, TMSE-10C05T (Taniguchi et al., personal communication), MSCjaH38 (Ueno et al. 1999), and MSCjaR2 (Abe et al. 2006). Microsatellite amplification was then performed using a Multiplex PCR Mix according to the manufacturer’s protocol (Takara Bio). Reactions were incubated in a TaKaRa PCR Thermal Cycler Dice Gradient TP600 (Takara Bio). After an initial denaturation phase of 15 min at 95°C, 30 thermal cycles were performed under the following conditions: denaturation for 30 s at 94°C, annealing for 90 s at 58°C, and extension for 90 s at 72°C, with final extension for 10 min at 72°C. The PCR amplification products were detected using a genetic analyzer (ABI Prism 3100; Applied Biosystems). Individual genotypes were determined using Genotyper software (Applied Biosystems).
5.2.5 Mature Tree Sampling and Genotyping
Because the distribution of C. japonica at the study sites tended to be clumped, we selected a dense area of C. japonica trees as the study plot within each site. From late July to early August 2005, we collected leaves from all mature trees that had floral buds in each 50×60 m area (0.3 ha) at the three study sites. The number of mature trees within each population was 48, 24, and 17 for TU, IG, and the N2 population, respectively. Genomic DNA was extracted from dried or frozen leaves using a modified CTAB method (Murray and Thompson 1980). Genotypes were determined using the same method as in the pollen grain analysis already described.
5.2.6 Analysis of Genetic Diversity
We calculated three genetic parameters: number of alleles (Na), gene diversity (H), and allelic richness (A r). Na and H were estimated using the FSTAT 2.9.3 software (Goudet 2000). The number of gene copies [g] was standardized to 20 for the calculation of A r, according to El Mousadik and Petit (1996). To compare genetic diversity among the study sites, the partitioning of allelic richness among pollen grains (A st[g]) was calculated as A st[g]=1−(A s[g]−1)/(A t[g]−1) (El Mousadik and Petit 1996; Petit et al. 1998; Comps et al. 2001), where A s[g] and A t[g] signify the mean allelic richness within each pollen pool adhering to a bird and the total allelic richness in the pollen pools adhering to birds within each study site, respectively. Values of A st depend on the distribution of alleles among populations. If rare alleles are clustered in some populations (pollen pools, here), high values of A st are expected. On the other hand, even distribution of alleles among pollen pools will give low A st values. From a conservation point of view, more emphasis should be placed on rare alleles, and therefore A st is a more suitable measure than F st (El Mousadik and Petit 1996). Comparisons of genetic diversity among sites were carried out using A st[g] values. Differences in the mean values of the analyzed genetic parameters between bird species and among study sites were analyzed using Mann–Whitney U tests and Kruskal–Wallis tests after a test of equal variances.
5.3 Results
5.3.1 Flowering Conditions and Home Range of Zosterops japonica
The flower and flowering tree densities were highest at TU and lowest at N2 (Table 5.1). For the three sites described above, a total of 18 Zosterops birds were captured (Table 5.2). One bird at TU was determined, by the length of its beak, to be a Z. japonica that had migrated from the Honshu mainland. The others were identified as a nonmigratory resident subspecies from the Izu Islands, Z. japonica var. stejnegeri.
In total, ten birds were tracked for 7–20 days (n=7, 16.6 days at TU; n=3, 7.5 days at IG). The home range sizes were estimated using the minimum convex polygon method (Table 5.3). The mean home range size at the high-flower-density site (TU) was smaller than at the low-flower-density site (IG), although no significant differences were found (Welch’s T test, P=0.13; Table 5.3 and Fig. 5.5).
Home ranges of Zosterops japonica individually tracked using radiotelemetry in winter 2006 on Miyake-jima. Outermost points of locations tracked for more than 1 week were connected by lines to form minimum convex polygons of home ranges. (a) High-flower-density area of Tubota (TU). (b) Low-flower-density area of Igaya (IG). Stars represent points where birds were captured
The mean number of flowers estimated to be within all home ranges was greater at TU than at IG (Table 5.3). In contrast, the mean number of flowering trees estimated to be within all home ranges at TU tended to be smaller than at IG (Table 5.3). However, these differences were not significant (Mann–Whitney U test: Z=1.03, P=0.31).
5.3.2 Microsatellite Haplotyping from Single Pollen Grains
All Zosterops captured using nets (n=22) had pollen grains adhered to their entire beak surfaces. Four Cetta diphone (n=15; 27%) and four Parus major (n=13; 31%) individuals had small amounts of pollen on their beaks. Only one Hypsipetes amaurotis individual was captured with a small quantity of pollen on its beak (see Table 5.2).
The haplotypes of pollen grains found on the 18 captured Z. japonica and 4 recaptured birds were determined (Table 5.2). Additionally, we determined the haplotypes of pollen grains on 1 H. amaurotis, 4 C. diphone, and 4 P. major captured in the low-flower-density areas of IG and N2 (Table 5.2).
In total, 878 pollen grain samples were haplotyped for more than five microsatellite loci and used in the genetic analyses (n=599, mean 27.2, and range 16–48 for Z. japonica; n=279, mean 31.0, and range 22–92 for other birds). All microsatellite loci were amplified in 83% of the pollen grains.
5.3.3 Genetic Diversity
The genotypes of all 89 mature trees were determined at the three study plots (each 0.3 ha: n=48 at TU; n=24 at IG; n=17 at N2). The genetic parameters of the pollen grains found on Z. japonica are summarized in Table 5.4. We summed the data at IG and N2 in the low-flower-density area because of the small number of birds analyzed for pollen. A comparison of the diversity parameters (Na, H, and Ar) for adult trees in the high-flower-density site (TU) to those in the low-flower-density sites (IG and N2) revealed that diversity only slightly differed among different densities and that this difference was not significant (Table 5.4a). On the other hand, the three genetic parameters for the pollen were significantly higher in the low-flower-density sites (IG and N2) than in the high-flower-density site (TU) (Table 5.4b). In addition, A st were significantly lower in the low-flower-density sites (Table 5.4b and Fig. 5.6). Higher genetic diversity parameters in the low-flower-density sites were probably caused by the larger numbers of pollen donors sampled by Z. japonica (see Table 5.3), whereas the lower values of A st in the low-flower-density sites were considered to be the result of more homogeneous distribution of alleles among pollen pools on beaks of Z. japonica in the low-flower-density sites.
Relationship between flower density and the partitioning of allelic richness among pollen grains on each Zosterops individual (A st). Site TU had a high flower density, whereas sites IG and N2 had low flower densities. Circles and bars, each sampled bird and the mean value for each site, respectively
The genetic parameters for the pollen found on H. amaurotis, C. diphone, and P. major are also summarized in Table 5.4. A comparison of the genetic diversity of the pollen found on Z. japonica and the other birds in the low-flower-density sites (IG and N2) showed a significant difference in three parameters (Table 5.4c). However, the mean A st of Z. japonica did not differ from that of the other birds (Table 5.4c).
5.4 Discussion
5.4.1 Resilience of the Camellia japonica–Zosterops japonica System Against Environmental Perturbation
Cascading negative impacts of reduced flower density on pollination, fruit-set, and seed-set rates have been observed in many plant species (Ågren 1996; Mustajärvi et al. 2001; Leimu and Syrjänen 2002). Low fruit-set rates have generally been attributed to pollen or resource limitation (Stephenson 1981; Bawa and Webb 1984; Zimmerman and Pyke 1988; Sperens 1996). Even with efficient seed production that produces a sufficient quantity of pollen, the genetic variation of progeny might decrease because of a decrease in pollen donors. Although long-distance pollen dispersal by insects occurs when there is low tree density as a consequence of sparse distribution or fragmented landscapes (Stacy et al. 1996; Konuma et al. 2000; Dick et al. 2003), the low population numbers or low flowering density increases selfing and inbreeding depression (Aldrich and Hamrick 1998; Obayashi et al. 2002; Latouche-Hallé et al. 2004; Naito et al. 2008). For Shorea leprosula, it was demonstrated that flowering tree density was negatively correlated with the rare allele frequency in the seedling population as well as with pollen dispersal distance, without reference to the biological systems that caused these results (Fukue et al. 2007). In contrast to the conclusions of numerous previous studies, reduced flower density in C. japonica engendered markedly high genetic diversity in pollen grains associated with Zosterops (see Table 5.4) as well as an expansion in the pollen dispersal area (see Fig. 5.5). Moreover, the F st values, or the partitioning of heterozygosity among pollen grains, were lower in the low-flower-density areas (0.267 at IG; 0.169 at N2) compared to the high-flower-density area (0.301 at TU). We propose that specialized interactions between C. japonica and Z. japonica along with the foraging behavior of Z. japonica account for the resilience within the C. japonica–Z. japonica system against environmental perturbation, such as volcanic eruption and anthropogenic habitat destruction, which is discussed next.
Because Z. japonica strongly depends on Camellia flower nectar as a food resource in winter, it is considered a seasonal specialist (Kunitake et al. 2004). During the winter season, invertebrates, fruits, and other flowering plants are scarce, whereas C. japonica flowers that secrete large amounts of nectar are abundant. This phenological concordance between the pollinator’s resource shortage and C. japonica flowering might have stimulated seasonal specialization by omnivorous birds that produced effective pollination services. This specialization may never stop Z. japonica from visiting Camellia flowers, even if volcanic disturbance severely limits the flowering activities of C. japonica; that is, birds without access to high-flower-density areas were forced to seek Camellia flowers in low-flower-density areas. Consequently, the shortage of flower resources increases the home range size of the pollinator (Table 5.3 and Fig. 5.5) and the number of visited flowering trees (Table 5.3) under low flower densities. The corresponding increase in pollen donors not only enhanced genetic diversity of the pollen grains on Zosterops individuals (Table 5.4), but also increased the proportion of flowers visited by pollinators, thereby ensuring higher pollination rates in low-flower-density areas (Abe and Hasegawa 2008).
At the high-flower-density site (TU), 70% of tracked Zosterops moved within the study area, while at the low-flower-density sites (IG and N2) 100% of tracked birds disappeared from the study plot for 9 days or fewer (data not shown). The home ranges in the low-flower-density sites may be more flexible than those in high-flower-density sites. The existence of pollinator birds with a large home range in low-flower-density habitats (Fig. 5.5) must be one of the factors preventing genetic differentiation among pollen grains, because the number of trees visited by Z. japonica in low-flower-density areas was larger than in the high-flower-density area, which would increase the number of pollen donors for each mother tree. High genetic diversity of pollen grains in low-density sites is therefore likely a result of the resilient relationship between C. japonica and Z. japonica. The enlarged area is generated in response to environmental perturbation, leading to an increase in potential pollen donors for recipient Camellia trees in low-flower-density areas.
5.4.2 Conclusions and Future Research
This study examined how reduced flower density affected the pollen movement of Camellia japonica through a combination of single-pollen haplotyping and radio tracking of the specialized pollinator bird, Zosterops japonica. Greater foraging movements of the pollinator in areas with low flower density promote higher genetic diversity of pollen grains transported by Zosterops. These results corresponded to an enhanced efficiency in maternal reproductive success (pollination rate and seed-set rate) of C. japonica in low-flower-density areas (Abe and Hasegawa 2008). A greater dependence on nectar, which results in an increase in the transporting rates of C. japonica pollen grains, makes Z. japonica the most effective pollinator of C. japonica among the potential avian pollinators. The greater genetic diversity within the transported pollen (see Table 5.4) and higher visitation rates by pollinator birds (Kunitake et al. 2004; Abe and Hasegawa 2008) together provide solid evidence that the C. japonica–Z. japonica system, with its innate mechanisms, is robust against environmental perturbation such as volcanic eruption and anthropogenic habitat destruction.
Finally, there are two other issues that should be addressed in future studies. One concern is to determine whether the high genetic diversity found within the pollen adhering to pollinator birds is successfully delivered to the trees by examining the genetic diversity of pollen donors within a fruit or seedling, which would determine the genetic diversity of the next generation in a more direct way. The second issue is to examine how spatial differences in flowering phenology (i.e., flowering time and the number of flowers) among individual trees affect both maternal and paternal reproductive success via pollen flow. Variations in flowering phenology might induce spatial and temporal variation in flower density and affect the degree of spatial restriction of pollen flow. In fact, previous studies have reported that flower density can strongly affect pollen flow (Levin and Kerster 1969). For example, at the TU site with high flower density, the number of flowers per flowering tree varied greatly (see Table 5.1). There may be large variations in genetic diversity among pollen pools that individual trees have received. If Z. japonica were only attracted to Camellia trees that had large amounts of flowers, the number of pollen donors received per tree would decrease, which would lead to genetic differentiation. Therefore, the differences in genetic diversity of pollen pools between the low- and high-flower-density sites might be caused by variations in flowering phenology within each population. To better understand the C. japonica–Z. japonica system, we must determine the consequences of variable pollen flow on genetic differentiation within the populations considering the interactions among flowering time, the number of flowers, and visitation rates of pollinators.
References
Abe H, Hasegawa M (2008) Impact of volcanic activity on a plant–pollinator module in an island ecosystem: the example of the association of Camellia japonica and Zosterops japonica. Ecol Res 23:141–150
Abe H, Matsuki R, Ueno S, Nashimoto M, Hasegawa M (2006) Dispersal of Camellia japonica seeds by Apodemus speciosus revealed by maternity analysis of plants and behavioral observation of animal vectors. Ecol Res 21:732–740
Ågren J (1996) Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria. Ecology 77:1779–1790
Aldrich R, Hamrick JL (1998) Reproductive dominance of pasture trees in a fragmented tropical forest mosaic. Science 281:103–105
Bawa KS, Webb CJ (1984) Flower, fruit, and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Am J Bot 71:736–751
Comps B, Gömöry D, Letouzey J, Thiébaut B, Petit RJ (2001) Diverging trends between heterozygosity and allelic richness during postglacial colonization in the European beech. Genetics 157:389–397
Dick CW, Etchelecu G, Austerlitz F (2003) Pollen dispersal of tropical trees (Dinizia excelsa: Fabaceae) by native insects and African honeybees in pristine and fragmented Amazonian rainforest. Mol Ecol 12:753–764
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Fukue Y, Kado T, Lee SL, Ng KKS, Muhammad N, Tsumura Y (2007) Effects of flowering tree density on the mating system and gene flow in Shorea leprosula (Dipterocarpaceae) in Peninsular Malaysia. J Plant Res 120:413–420
Goudet J (2000) FSTAT: a program to estimate and test gene diversities and fixation indices. Ver. 2.9.1. Available via http://www2.unil.ch/popgen/softwares/fstat.htm
Kamijo T, Hashiba K (2003) Island ecosystem and vegetation dynamics before and after the 2000-year eruption on Miyake-jima Island, Japan, with implications for conservation of the island’s ecosystem. Global Environ Res 7(1):69–78
Kamijo T, Kitayama K, Sugawara A, Urushimichi S, Sakai K (2002) Primary succession of the warm-temperate broad-leaved forest on a volcanic island, Miyake-jima Island, Japan. Folia Geobot 37:71–91
Katsuta M (1999) Camellia Linn. (Camellia). In: Tree seeds in Japan (broad-leaved trees). Japanese Forest Tree Breeding Association, Tokyo, pp 277–282 (in Japanese)
Kikkawa J, Kakizawa R (1981) Agonistic behaviour of Japanese white-eyes Zosterops japonica on Miyake Island. J Yamashina Inst Ornithol 13:60–70
Kitayama K, Mueller-Dombois D, Vitousek PM (1995) Primary succession of Hawaiian montane rain forest on a chronosequence of eight lava flows. J Veg Sci 6:211–222
Konuma A, Tsumura Y, Lee CT, Lee SL, Okuda T (2000) Estimation of gene flow in the tropical-rainforest tree Neobalanocarpus heimii (Dipterocarpaceae), inferred from paternity analysis. Mol Ecol 9:1843–1852
Kunitake Y, Hasegawa M, Miyashita T, Higuchi H (2004) Role of a seasonally specialist bird Zosterops japonica on pollen transfer and reproductive success of Camellia japonica in a temperate area. Plant Species Biol 19:197–201
Latouche-Hallé C, Ramboer A, Bandou E, Caron H, Kremer A (2004) Long-distance pollen flow and tolerance to selfing in a neotropical tree species. Mol Ecol 13:1055–1064
Leimu R, Syrjänen K (2002) Effects of population size, seed predation and plant size on male and female reproductive success in Vincetoxicum hirundinaria (Asclepiadaceae). Oikos 98:229–238
Levin D, Kerster H (1969) The dependence of bee-mediated pollen and gene dispersal upon plant density. Evolution 23:560–571
Matsuki Y, Isagi Y, Suyama Y (2007) The determination of multiple microsatellite genotypes and DNA sequences from a single pollen grain. Mol Ecol Notes 7:194–198
Matsuki Y, Tateno R, Shibata M, Isagi Y (2008) Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. Am J Bot 95:925–930
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Mustajärvi K, Siikamäki P, Rytkönen S, Lammi A (2001) Consequences of plant population size and density for plant–pollinator interactions and plant performance. J Ecol 89:80–87
Nagamasu H (2006) Theaceae. In: Iwatsuki K, Boufford DE, Ohba H (eds) Flora of Japan, vol a. Kodansha, Tokyo, pp 394–411
Naito Y, Kanzaki M, Iwata H, Obayashi K, Lee SL, Muhammad N, Okuda T, Tsumura Y (2008) Density-dependent selfing and its effects on seed performance in a tropical canopy tree species, Shorea acuminata (Dipterocarpaceae). For Ecol Manag 256:375–383
Obayashi K, Tsumura Y, Ihara T, Niiyama K, Tanouchi H, Suyama Y, Washitani I, Lee CT, Lee SL, Muhammad N (2002) Genetic diversity and outcrossing rate between undisturbed and selectively logged forests of Shorea curtisii (Dipterocarpaceae) using microsatellite DNA analysis. Int J Plant Sci 163:151–158
Petit RJ, El Mousadik A, Pons O (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol 12:844–855
Raim A (1978) A radio transmitter attachment for small passerines. Bird-Banding 49:326–332
Shanahan M, Harrison RD, Yamuna R, Boen W, Thornton IWB (2001) Colonization of an island volcano, Long Island, Papua New Guinea, and an emergent island, Motmot, in its caldera lake. V. Colonization by figs (Ficus spp.), their dispersers and pollinators. J Biogeogr 28:1365–1377
Shibata M, Ieyumi S (1991) Self-incompatibility in a wild population of Camellia (in Japanese). In: Kurume Branch Annual Report, vol 4. National Institute of Vegetable and Tea Science, Tsu, Japan, pp 182–183
Sperens U (1996) Is fruit and seed production in Sorbus aucuparia L. (Rosaceae) pollen-limited? Ecoscience 3:325–329
Stacy EA, Hamrick JL, Nason JD, Hubbell SP, Foster RB, Condit R (1996) Pollen dispersal in low-density populations of three neotropical tree species. Am Nat 148:275–298
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Annu Rev Ecol Syst 12:253–279
Thornton IWB, Compton SG, Wilson CN (1996) The role of animals in the colonization of the Krakatau Islands by fig trees (Ficus species). J Biogeogr 23:577–592
Tsuyuzaki S, del Moral R (1995) Species attributes in early primary succession on volcanoes. J Veg Sci 6:517–522
Ueno S, Yoshimaru H, Tomaru N, Yamamoto S (1999) Development and characterization of microsatellite markers in Camellia japonica L. Mol Ecol 8:335–346
Ward M, Dick CW, Gribel R, Lowe AJ (2005) To self, or not to self… A review of outcrossing and pollen-mediated gene flow in neotropical trees. Heredity 19:1–9
Wendel JF, Parks CR (1985) Genetic diversity and population structure in Camellia japonica L. (Theaceae). Am J Bot 72:52–65
Yumoto T (1988) Pollination systems in the cool temperate mixed coniferous and broad-leaved forest zone of Yakushima Island. Ecol Res 3:117–129
Zimmerman M, Pyke GH (1988) Experimental manipulations of Polemonium foliosissimum: effects on subsequent nectar production, seed production and growth. J Ecol 76:777–789
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer
About this chapter
Cite this chapter
Abe, H., Ueno, S., Tsumura, Y., Hasegawa, M. (2011). Expanded Home Range of Pollinator Birds Facilitates Greater Pollen Flow of Camellia japonica in a Forest Heavily Damaged by Volcanic Activity. In: Isagi, Y., Suyama, Y. (eds) Single-Pollen Genotyping. Ecological Research Monographs. Springer, Tokyo. https://doi.org/10.1007/978-4-431-53901-8_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-53901-8_5
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-53900-1
Online ISBN: 978-4-431-53901-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)