Abstract
The lymphatic vasculature is the “sewer system” of our body as it plays an important role in transporting tissue fluids and extravasated plasma proteins back to the blood circulation and absorbs lipids from the intestinal tract. Malfunction of the lymphatic vasculature can result in lymphedema and obesity. The lymphatic system is also important for the immune response and is one of the main routes for the spreading of metastatic tumor cells. The development of the mammalian lymphatic vasculature is a stepwise process that requires the specification of lymphatic endothelial cell (LEC) progenitors in the embryonic veins, and the subsequent budding of those LEC progenitors from the embryonic veins to give rise to the primitive lymph sacs from which the entire lymphatic vasculature will eventually be derived. This process was first proposed by Florence Sabin over a century ago and was recently confirmed by several studies using lineage tracing and gene manipulation. Over the last decade, significant advances have been made in understanding the transcriptional control of lymphatic endothelial cell type differentiation. Here we summarize our current knowledge about the key transcription factors that are necessary to regulate several aspects of lymphatic endothelial specification and differentiation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Overview of the Stepwise Process Leading to the Formation of the Lymphatic Network
Mammals have two interdependent circulatory systems—the blood vasculature and the lymphatic vasculature. Although detailed descriptions of the blood vascular system were available as early as the sixth century bc, those of the lymphatic vasculature were in the seventeenth century ad by Asellius. In contrast to the function of the blood vasculature in transporting blood throughout the body, the lymphatic vasculature is essential for maintaining interstitial fluid homeostasis. The main physiological functions of the lymphatic vasculature include draining and returning fluid from the extracellular tissue spaces back to the blood circulation, absorbing lipids from the intestinal tract and tissues, and transporting immune cells to lymphoid organs.
During the formation of the blood vascular network, the Notch signaling pathway is required to promote arterial cell fate differentiation (De Val and Black 2009; Kokubo et al. 2005; Lawson et al. 2001). On the other hand, the orphan nuclear receptor COUP-TFII promotes venous fate differentiation by inhibiting Notch signaling and other arterial specification genes (You et al. 2005).
Studies on the lymphatic network in the past decade have brought important advances in the understanding of the molecular events that lead to the development of the lymphatic vasculature. At least in the mammalian embryo, the formation of the lymphatic vasculature is a stepwise process that is closely associated with the venous vasculature. The pioneering work by Florence Sabin at the beginning of the last century proposed that the lymphatic vasculature arises from the embryonic veins (Sabin 1902). Detailed lineage tracing analysis performed in mouse embryos almost a century later confirmed Sabin’s prediction that lymphatic endothelial cells (LECs) have a venous origin (Srinivasan et al. 2007). Therefore, a key prerequisite for the genesis of the lymphatic network is the prior formation of the blood vasculature.
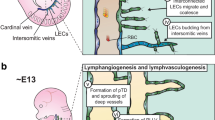
In the mouse embryo, the process leading to the formation of the entire lymphatic vascular network starts inside the cardinal vein (CV) and intersomitic vessels at around embryonic day 9.5 (E9.5) (Srinivasan et al. 2007; Wigle and Oliver 1999; Yang et al. 2012; Hagerling et al. 2013) (Fig. 2.1a). As discussed in detail later, at this stage a subpopulation of venous ECs become specified into the lymphatic lineage by acquiring a very specific molecular footprint. Concomitant with their stepwise loss of venous fate, these ECs will gain lymphatic fate and as such they should be considered LEC progenitors. The formation of the entire lymphatic vascular network is mediated by intermediate structures called lymph sacs. Lymph sacs are formed when most of the LEC progenitors bud off from the veins and migrate into the surrounding mesenchyme. Electron microscopy and immunostaining studies have revealed that adhesion junctions between the venous endothelial cells (VECs) and LEC progenitors are important for maintaining vein integrity during the budding process (Yang et al. 2012; Yao et al. 2012) (Fig. 2.2). As part of the stepwise differentiation process, as they bud off, LEC progenitors start expressing additional genes (e.g., podoplanin) and differentiate into more mature LECs outside the veins (Yang et al. 2012) (Fig. 2.1a, b). Importantly, at the level of the junction of the jugular and subclavian veins, a small fraction of LEC progenitors will remain inside the CV and contribute to the formation of the lymphovenous valves, which are the main valves responsible for the unidirectional return of lymph fluid into the blood circulation (Srinivasan and Oliver 2011) (Fig. 2.1b, d).
Schematic representation of the stepwise process leading to the formation of the mammalian lymphatic vasculature. (a) The cardinal vein (CV) is the source from where LECs are going to be specified. Early during development, the CV expresses the transcription factors COUP-TFII and Sox18. Together, the activity of these factors will be necessary to induce the expression of Prox1 in a subpopulation of venous ECs. The initiation of Prox1 expression at around E9.5 is an indication that LEC specification started and the venous Prox1-expressing ECs should be considered as LEC progenitors. Approximately a day later (E10.5), most of those progenitors will start to bud off from the CV. This process is guided by the graded expression of Vegfc in the surrounding mesenchyme. LECs will bud off from the CV and intersomitic vessels as a chain of interconnected cells. Vegfr3 expression in LECs is maintained by Prox1. As soon as LECs bud from the vein they start to express podoplanin, an indication that lymphatic maturation and differentiation is progressing. As LECs bud, they will start to form the different lymph sacs (starts at around E11.5), intermediate structures from which upon maturation and differentiation, the entire lymphatic network will be formed. (b) Although most Prox1-expressing LEC progenitors will move out from the veins, a small fraction located at the junction of the internal (IJV) or external jugular veins (EJV) with the subclavian vein (SCV) will remain and upon their intercalation with lymph sac (LS) LECs, they will help to form the lymphovenous valves
Prox1-expressing LEC progenitors bud from the CV (a, a′, a″). EM analysis of E10.5 Prox1 +/LacZ embryos showing that Prox1-expressing cells (pink) exit the CV via an active budding mechanism. The budding cell is attached to the venous endothelial cells (blue) by adhesion junctions (arrowheads in (a′) and (a″)). Panels (a′, a″) are high-power magnifications of the black boxed area in (a). (b) Podoplanin expression is only detected once Prox1-expressing LEC progenitors fully exit the CV (dashed line) at around E11.5. (c) Prox1-expressing LEC progenitors that bud off from the CV (dashed line) and ISVs migrate as an interconnected group of cells dorsally and longitudinally into the surrounding mesenchymal tissue at approximately E11.0 in the anterior region of the embryo. (d) A small subpopulation of Prox1-expressing venous ECs remain in the veins and form the lymphovenous valves at the junction of the jugular and subclavian veins at E15.5 (arrowheads in (d)). These valve cells are negative for podoplanin. CV cardinal vein, IJV internal jugular vein, SCV subclavian vein. Scale bars: 10 μm (a), 1 μm (a′, a″), 100 μm (b–d)
Vascular endothelial growth factor C (Vegfc) signaling is indispensable for the budding process. A newly identified player in this pathway, Ccbe1, is also required for LEC budding (Bos et al. 2011; Hogan et al. 2009). In the absence of either molecule, LEC progenitors fail to bud off from the embryonic veins (Karkkainen et al. 2004; Hagerling et al. 2013; Bos et al. 2011). Recent studies have shown that as LECs bud off from the veins, they remain interconnected to each other during migration (Yang et al. 2012; Hagerling et al. 2013) (Fig. 2.2c). These interconnected LECs form strings that gradually organize into the lumenized structures called lymph sacs. Earlier studies argued that by sprouting and remodeling, the lymph sacs and the early lymphatic plexus give rise to the mature lymphatic vasculature, including both superficial lymphatics and the thoracic duct (TD) (Sabin 1902; van der Putte 1975; Oliver 2004). However, digital three-dimensional reconstructions using ultramicroscopy suggest a different model. According to this new model, simultaneous with the formation of primitive lymph sacs, LECs accumulate at the first lateral branch of the intersomitic vessels to form a peripheral longitudinal lymphatic vessel that will give rise to the superficial lymphatic plexus while the primitive lymph sacs will later develop into the TD (Hagerling et al. 2013). After the primitive lymphatic vessels are formed, they will further differentiate into larger collecting vessels and smaller capillaries, which are the two predominant types of vessels of the lymphatic vasculature. Lymphatic capillaries absorb interstitial fluid and carry this lymph toward the larger collecting lymphatic vessels. In the collecting lymphatics, valves form to prevent the backflow of the lymph and separate the lymphatic vessels into functional units called lymphangions (Sacchi et al. 1997; Casley-Smith 1980).
2.2 Transcriptional Control of Lymphatic Specification and Differentiation
2.2.1 Sox18 and COUP-TFII Initiate Lymphatic Endothelial Cell Transcriptional Profiling
So far, only a few transcription factors critical during LEC specification and differentiation have been identified, and how these genes are regulated and how they interact with other critical signaling pathways (e.g., Vegfc–Vegfr3, MAPK/ERK, and Notch signaling) remain to be fully elucidated.
Many transcription factors play an important role in regulating blood endothelial cell (BEC) fate differentiation. Given the common origin and close relationship of venous and lymphatic endothelial cells, it is not surprising that at least some venous endothelial transcription factors are also required for the development of the lymphatic vasculature. For instance, Sox18 encodes an SRY-type HMG box transcription factor and is a member of the SOX gene family. Mutations in SOX18 in humans are associated with hypotrichosis–lymphedema–telangiectasia (Irrthum et al. 2003). Sox18 is expressed in the vascular endothelium and hair follicles in mouse embryos (Pennisi et al. 2000b). Point mutations in Sox18 result in cardiovascular and hair follicle defects in mice; as a consequence, these mice are known as ragged mice (Carter and Phillips 1954; Slee 1957a, b). On the other hand, Sox18 knockout mice display a milder coat defect and no obvious cardiovascular defects in a mixed genetic background (Pennisi et al. 2000a).
Some evidence supports that other Sox transcription factors have redundant functions. For example, Sox18 and Sox7 can compensate for the loss of each other in arteriovenous specification in zebrafish (Herpers et al. 2008; Cermenati et al. 2008; Pendeville et al. 2008). In the mouse, the expression of Sox18 in vascular endothelial cells starts as early as E7.5 in the allantois and yolk-sac blood islands (Pennisi et al. 2000b). Sox18 expression in ECs in the CV is detected at around E9.0, but gets downregulated in LECs at around E14.5 and is not maintained in the adult lymphatic vasculature (Francois et al. 2008). Sox18 is indispensible for generating venous LEC progenitors via its activation of the critical transcription factor Prox1 (prospero homeobox 1, Drosophila prospero-related vertebrate gene) in VECs (Francois et al. 2008). However, this lack of LEC progenitors in Sox18 mutant mice has only been observed in a pure C57BL/6 (B6) background; Sox7 and Sox17 functionally substitute for Sox18 in a mixed background (Hosking et al. 2009). A 4 kb Prox1 promoter region that is sufficient to recapitulate Prox1 expression in vivo contains two conserved SoxF binding sites that in vitro can be bound directly by Sox18 (Francois et al. 2008) (Table 2.1). These results show that Sox18 is a direct upstream activator of Prox1 in ECs in the CV, and as such, also an important early player in the acquisition of LEC progenitor fate. Previously, the MAPK/ERK pathway was shown to regulate lymphatic vessel growth by modulating Vegfr3 expression in mice (Ichise et al. 2010). A recent report revealed that MAPK/ERK signaling might be responsible for activating Sox18 expression in the CV (Deng et al. 2013). Normally, induction of Prox1 expression by Sox18 occurs only in the embryonic veins (i.e., not in the arteries). This specificity could be because arteries fail to express certain specific factors required to activate Prox1 in combination with Sox18 (Francois et al. 2008) or because they express certain genes (repressors) that inhibit Prox1 activation. The finding that gain of function of the MAPK/ERK signaling component RAF1 in ECs induces an abnormal expression level of Sox18 in the veins and dorsal aorta, which in turn activates Prox1 expression in these vessels (Deng et al. 2013), argues that aberrantly activated MAPK/ERK signaling either inhibits arterial-specific repressor(s) or turns on the expression of venous Sox18 coactivators in the arteries.
As mentioned above, in addition to Sox18, other coactivators are necessary to induce Prox1 expression in the veins and initiate the specification of LEC progenitors. The chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) is a venous cofactor that can bind to the Prox1 promoter and activate its expression (Srinivasan et al. 2010). COUP-TFII is a member of the steroid/thyroid hormone receptor superfamily and is highly expressed in the mesenchymal tissue as well as in the blood vascular endothelium during development (Pereira et al. 1995). Within the vasculature, its expression is restricted to the veins, where its activity is required to promote and maintain venous identity by inhibiting Notch activity in VECs, thus blocking the arterial transcriptional program (You et al. 2005). A recent study reported that COUP-TFII activity in the veins can be regulated epigenetically by the chromatin-remodeling enzyme gene Brg1, a member of the SWI/SNF protein family (Davis et al. 2013). Brg1 remodels the chromatin structure of the COUP-TFII promoter region by direct binding, thereby preventing the access of the transcriptional machinery to that region (Davis et al. 2013). Interestingly, COUP-TFII is not only essential for venous cell fate differentiation but is also involved in the specification of LEC progenitors during lymphatic vasculature development. Deletion of COUP-TFII expression from LEC progenitors causes a drastic reduction in the number of LECs (Srinivasan et al. 2007). Moreover, COUP-TFII directly binds conserved binding sites located approximately 9.5 kb upstream of the Prox1 open reading frame (ORF) (Srinivasan et al. 2010) (Table 2.1). These results argue that COUP-TFII is another direct in vivo regulator of Prox1 expression during the early phases of LEC specification in the CV (Fig. 2.1). The maintenance of Prox1 expression in LECs also requires COUP-TFII (see below). In addition, conditional inactivation of COUP-TFII at different embryonic stages revealed that its activity is not only essential for the specification of LEC progenitors but also for the sprouting of dermal lymphatic capillaries prenatally (Lin et al. 2010). In the absence of COUP-TFII, the lymphatic capillaries failed to form filopodial extensions projecting from the vessels. This function of COUP-TFII is via direct transcriptional regulation of Nrp2, a coreceptor for Vegfc in LECs (Lin et al. 2010; Xu et al. 2010) (Table 2.1). However, although COUP-TFII is expressed in LECs throughout life, its activity is not required to maintain quiescent lymphatic vessels in the adult, as normal lymphatic function remains intact when COUP-TFII is inactivated in adult mice (Lin et al. 2010). Thus, Sox18 and COUP-TFII are two transcription factors crucial to initiate Prox1-mediated LEC progenitor specification in the veins. Although both Sox18 and COUP-TFII bind to the Prox1 promoter to induce its expression, either one is sufficient to activate Prox1 expression by itself. However, how these two transcription factors interact with each other in this process remains uncertain.
2.2.2 Prox1 Regulates LEC Progenitor Specification and Differentiation
It is widely accepted that LEC specification begins with Prox1 expression in VECs at around E9.5 (Oliver 2004) (Fig. 2.2). Prox1 (prospero homeobox 1, vertebrate gene related to Drosophila prospero) (Oliver et al. 1993) was the first lymphatic endothelial transcription factor to be identified (Wigle and Oliver 1999). Although Prox1 is expressed in many other tissue types such as the central nervous system, lens, heart, liver, and pancreas (Oliver et al. 1993), its expression in the vascular system is restricted to lymphatic ECs. Prox1 begins to be expressed in a subpopulation of ECs on the anterior CV (LEC progenitors) at around E9.5 and its expression is maintained in LECs through life (Wigle et al. 2002; Wigle and Oliver 1999). Functional inactivation of Prox1 in mice showed that in the absence of Prox1 expression, the embryo was devoid of the entire lymphatic vasculature and died at around E14.5 (Wigle and Oliver 1999). It was later shown that conditional deletion of Prox1 at any developmental or postnatal stage leads to the loss of lymphatic-specific gene expression and the concomitant upregulation of BEC-specific genes in LECs (Johnson et al. 2008). These findings show that Prox1 activity is required for the formation of LEC progenitors and for the maintenance of LEC identity at all developmental stages, including adulthood. Furthermore, it was recently shown that Prox1 activity is also necessary for LECs to bud from the embryonic veins (Yang et al. 2012). Finally, several in vitro gain-of-function studies indicated that ectopic expression of Prox1 in BECs is able to initiate a mature lymphatic-specific gene expression (e.g., Vegfr3 and podoplanin) while suppressing BEC-specific genes (Hong et al. 2002; Petrova et al. 2002). Taken together, these studies indicate that Prox1 is a key transcriptional regulator not only during the specification of LEC progenitors but also during LEC differentiation.
As Prox1 is a central regulator of lymphatic endothelial transcription, its expression is tightly regulated. Several lines of evidence suggest that Prox1 dosage is crucial for its function. In most genetic backgrounds, haploinsufficiency of Prox1 causes perinatal death and pups exhibit characteristics of lymphatic dysfunction (e.g., chylothorax and chylous ascites) (Harvey et al. 2005). In other backgrounds, a small proportion of Prox1 heterozygous animals survive to adulthood; however, their lymphatic vessels are mispatterned and leaky, and mice develop adult-onset obesity (Harvey et al. 2005). All Prox1 heterozygote embryos display edema and occasionally develop blood-filled lymphatics (Srinivasan and Oliver 2011; Harvey et al. 2005). In these embryos, the number of LEC progenitors is significantly decreased and lymphovenous valves are absent (Srinivasan and Oliver 2011). Detailed analyses of Prox1 null embryos in which the ORF of Prox1 was replaced with either LacZ or GFP reporter gene constructs revealed that Prox1 is essential for maintaining its own expression in LEC progenitors; this autoregulation is crucial for LEC identity (Wigle et al. 2002; Srinivasan et al. 2010). At the molecular level, in vitro studies suggest a physical interaction between COUP-TFII and Prox1 in LECs (Lee et al. 2009; Yamazaki et al. 2009). Similarly, a recent study demonstrated that COUP-TFII homodimers induce VEC fate by repressing the Notch target genes HEY1/2, whereas COUP-TFII/Prox1 heterodimers induce or are permissive for the expression of a subgroup of LEC-specific genes (Aranguren et al. 2013). Comprehensive phenotype analyses showed that in COUP-TFII/Prox1 double heterozygotes embryos, the loss of COUP-TFII aggravates the lymphatic defects of Prox1 heterozygotes (Srinivasan et al. 2010). Compared with Prox1 heterozygote embryos, the number of LECs and the expression level of Prox1 are further reduced in COUP-TFII/Prox1 double heterozygotes (Srinivasan et al. 2010). This result argues that the activity of COUP-TFII is required to maintain Prox1 expression in LECs and that the amount of COUP-TFII/Prox1 protein complex is important to regulate Prox1 expression in a dosage-dependent manner (Srinivasan et al. 2010; Srinivasan and Oliver 2011). In addition, embryos with a mutated Prox1 nuclear hormone receptor-binding site in which the interaction between Prox1 and COUP-TFII is abolished displayed similar LEC specification defects (Srinivasan et al. 2010). Taken together, these results support that the COUP-TFII/Prox1 interaction is required to maintain Prox1 expression in LEC progenitors and, therefore, LEC identity during the LEC specification stage. Once LEC progenitors are specified and start to differentiate as they bud off from the CV, COUP-TFII activity is no longer required to maintain Prox1 expression (Srinivasan et al. 2010).
Since COUP-TFII is a crucial regulator in both venous and lymphatic specification (by suppressing Notch activity and triggering Prox1 expression in the veins), it can be speculated that Notch signaling may also be involved in lymphatic vasculature development. It is well known that Notch signaling is critical for the development of the blood vasculature, including arteriovenous specification and angiogenic sprouting (Gridley 2010; Roca and Adams 2007). However, until recently the role of Notch signaling in lymphatic vasculature development remained a matter of debate, mainly because there were no conclusive data demonstrating the presence of either in vivo expression of Notch pathway components in LECs or in vivo functional studies. For example, in vivo deletion of Rbpj (a key mediator of Notch signaling) in ECs did not result in LEC specification defect in the embryos (Srinivasan et al. 2010). On the other hand, in vitro studies showed that ectopic expression of an activated Notch receptor in cultured LECs repressed Prox1, COUP-TFII, and the mature lymphatic marker podoplanin through Hey proteins (downstream effectors of Notch signaling) (Kang et al. 2010). Likewise, addition of soluble Jag1 or Dll4 recombinant protein (Notch ligands) into the culture medium suppressed the expression of Prox1, COUP-TFII, and podoplanin also through Hey proteins (Kang et al. 2010). These data suggest that in vitro Notch signaling inhibits LEC fate. Another study also proposed that blocking Notch promotes LEC sprouting in vitro. This Notch inhibition-induced lymphangiogenesis required Vegfr2 and Vegfr3 signaling (Zheng et al. 2011). Thus, these data indicate that in vitro Notch signaling is involved in both the specification and sprouting of LECs. Contradictory to these results, another study demonstrated that by treating neonatal mouse tail dermis, ears, and retinas with blocking antibodies targeting Notch1 and Dll4, lymphatic vessel sprouting and growth were impaired (Niessen et al. 2011). Recently, some in vivo studies have supported that Notch activity plays a role during embryonic lymphatic development (Murtomaki et al. 2013). These studies showed that Notch signaling components are present in LECs during embryonic development. Also, removal of Notch1 or disturbing Notch transcription in LECs leads to an increase in the number of LEC progenitors and to larger lymph sacs (Murtomaki et al. 2013). These results argue that Notch signaling acts as a negative regulator of LEC specification by repressing Prox1 expression. Taken together, these findings indicate that Notch signaling is a negative regulator of LEC fate decisions during lymphatic vasculature development (Fig. 2.1).
Because of the limited number of LEC progenitors on the embryonic veins and the lack of specific surface markers to sort these cells, the identification of direct in vivo target genes of Prox1 in these cells has been challenging. Several lymphatic genes are regulated by Prox1 expression in vitro (Mishima et al. 2007; Hong et al. 2002; Fritz-Six et al. 2008; Sabine et al. 2012; Harada et al. 2009; Shin et al. 2006). Some studies have suggested that the main receptor of Vegfc signaling, Vegfr3, is a downstream target of Prox1. Vegfr3 is expressed in BECs and is essential for blood vasculature development. Vegfr3 null mouse embryos die at around E10.0 with severe defects in remodeling of the primary vessel networks (Dumont et al. 1998). In wild-type embryos LECs start to express Vegfr3 after E10.5. Vegfc is the most well-characterized Vegfr3 ligand, and in the absence of Vegfc signaling, Prox1 + LEC progenitors fail to bud off from the embryonic veins (Karkkainen et al. 2004). Previous microarray data indicate that ectopic expression of Prox1 in cultured VECs leads to a significant increase in Vegfr3 expression (Hong et al. 2002; Petrova et al. 2002). Furthermore, during inflammation-induced lymphangiogenesis, Prox1 transcriptionally regulates Vegfr3 expression by binding to its promoter together with NF-κB or Ets2 in vitro (Flister et al. 2009; Yoshimatsu et al. 2011). These in vitro data suggest that Vegfr3 is a direct target of Prox1 and that other coactivators such as COUP-TFII, NF-κB, or Ets2 may be involved in this regulatory process (Table 2.1). Our own unpublished data recently confirmed that Vegfr3 is a direct in vivo target of Prox1 in a dosage-dependent manner (Table 2.1). We determined that Prox1 maintains Vegfr3 expression in LEC progenitors and the number of LEC progenitors. Furthermore, the expression level of Prox1 in those cells is further reduced in Vegfr3+/−; Prox1+/− embryos, revealing the existence of a regulatory feedback loop between Prox1 and Vegfr3. Therefore, in addition to COUP-TFII, Vegfr3 also regulates Prox1 expression during the early specification and differentiation of LEC progenitors.
Besides transcriptional regulation, at least in vitro Prox1 expression is also controlled by posttranscriptional regulation. It has been reported that lysine 556 is the major sumoylation site for Prox1 and that sumoylation of Prox1 influences its activity (Pan et al. 2009; Shan et al. 2008). In addition, in vitro data suggest that microRNAs regulate Prox1 levels in LECs, as Prox1 expression is negatively regulated by miR-181 or miR-31 in cultured LECs (Kazenwadel et al. 2010; Pedrioli et al. 2010) (Table 2.1). However, the in vivo function of the posttranscriptional regulation of Prox1 remains unknown. Interestingly, blood flow plays a significant role in modulating lymphatic identity in vivo. In this context, the expression of Prox1 is rapidly lost when lymphatic vessels are exposed to high shear rates from blood flow, leading to the loss of lymphatic identity (Chen et al. 2012). Taken together, these results highlight that as a central player during LEC specification and differentiation, the level of Prox1 is strictly regulated by numerous environmental and genetic factors. More transcription factors and signaling pathways that affect Prox1 expression remain to be discovered.
2.3 Not All Prox1-Expressing LEC Progenitors Will Leave the CV
As discussed above, Prox1 activity is required for the specification of LEC progenitors and for those progenitors to bud off from the CV. However, a recent study has identified a small subpopulation of Prox1-expressing LEC progenitors that will remain in the veins and help to form the lymphovenous valves (Srinivasan and Oliver 2011) (Fig. 2.1b, d). These cells are located at the junction of the jugular and subclavian veins and will not acquire LEC features (e.g., will not express podoplanin). Instead, they express an additional set of markers such as Foxc2 and Itga9 (Fig. 2.1b). Following intercalation with a subpopulation of venous ECs they will form the lymphovenous valves (Figs. 2.1 and 2.2). The formation of Prox1-expressing venous ECs and the derived lymphovenous valves is also dependent on Prox1 activity, as these valves are absent in Prox1 heterozygous mice (Srinivasan and Oliver 2011). This defect is a consequence of defective maintenance of Prox1 expression in LEC progenitors, which is promoted by a reduction in the formation of the COUP-TFII/Prox1 complex (Srinivasan and Oliver 2011). Together, these results support that Prox1-expressing venous ECs are the source of cells that will produce both LECs progenitors and lymphovenous valves. However, what makes some Prox1-expressing ECs remain on the vein remains to be determined.
2.4 Foxc2 Is an Essential Regulator of Lymphatic Maturation and Valve Formation
Once the specified, mature LECs form the primitive lymph sacs and lymphatic plexus, they will differentiate further and give rise to the collecting lymphatic vessels and lymphatic capillaries. The formation of the lymphatic valves is an important step during the maturation of the primitive lymphatic plexus into collecting lymphatics. Foxc2, a member of the forkhead/winged-helix family of transcription factors, is one of the main players in the regulation of this critical step. In humans, point mutations in FOXC2 have been identified as the cause of lymphedema-distichiasis (LD) (Fang et al. 2000; Brice et al. 2002). A similar phenotype was observed in Foxc2 +/− mice (additional row of eyelashes, increased number of lymph nodes, and lymph backflow), suggesting that Foxc2 +/− is a suitable mouse model for LD (Kriederman et al. 2003). Foxc2 is necessary for lymphatic patterning, lymphatic valve formation, and mural cell recruitment during the maturation stage (Petrova et al. 2004). Inactivation of Foxc2 results in dilated lymphatic capillaries that become ectopically covered with smooth muscle actin-positive perivascular cells, whereas normal lymphatic capillaries lack mural cell coverage. It has been suggested that Foxc2 and Vegfr3 cooperate during the patterning of the lymphatic vasculature, and Foxc2 presumably functions downstream of Vegfr3 (Petrova et al. 2004). Although Foxc2 is normally expressed in LECs from E9.5 to adult stages, its activity is not required for the budding and migration of LEC progenitors from the embryonic veins or the formation of lymph sacs (Dagenais et al. 2004). Studies have shown that Foxc2 is also essential for the maturation of collecting lymphatics (Norrmen et al., 2009). Collecting lymphatics start to form around E14.5–15.5, and during their maturation markers for lymphatic capillaries such as Prox1, Vegfr3, and Lyve1 get downregulated and valves start to form. In the absence of Foxc2, the expression of these markers remains high and valves do not form; as a consequence, the primary lymphatic plexus fails to mature into functional collecting lymphatics (Norrmen et al. 2009). Coimmunoprecipitation assays and genome-wide location mapping revealed that Foxc2 physically interacts with NFATc1, a regulator of cardiac valve development (Chang et al. 2004; de la Pompa et al. 1998; Ranger et al. 1998), and functionally cooperates with calcineurin/NFATc1 signaling in transcriptional regulation during the development of collecting lymphatics (Norrmen et al. 2009) (Table 2.1). Importantly, calcineurin/NFATc1 signaling is required for normal lymphatic vascular patterning and LEC-specific gene expression during development. Blocking calcineurin/NFATc1 signaling with the calcineurin inhibitor cyclosporine A in utero results in the loss of podoplanin and Fgfr3 expression in LECs (Kulkarni et al. 2009). Furthermore, Foxc2-calcineurin/NFATc1 signaling is not only important during collecting lymphatic vessel maturation but also indispensible for the formation and maintenance of lymphatic valves. The formation of lymphatic valves starts around E16.0, which is indicated by elevated Prox1 and Foxc2 expression in lymphatic valve-forming cells (Sabine et al. 2012). Foxc2-calcineurin/NFATc1 signaling is activated in developing lymphatic valves, as indicated by the accumulation of nuclear NFATc1 in lymphatic valve-forming cells. Retrograde lymph flow is observed in Foxc2 −/− embryos because of the complete absence of lymphatic valves (Petrova et al. 2004). Removal of calcineurin in ECs is also sufficient to affect the formation of a lymphatic valve territory (Sabine et al. 2012). In addition, the inactivation of calcineurin at any developmental stage results in lymphatic valve defects, indicating that Foxc2–calcineurin/NFATc1 signaling is not only crucial for the initiation of valve formation but also required for the maintenance of lymphatic valves (Sabine et al. 2012). Besides calcineurin/NFATc1 signaling, the gap junction protein Cx37 is also essential for the assembly of the lymphatic valve territory. The clusters of lymphatic valve-forming cells were absent in Cx37-knockout mice, resulting in the absence of lymphatic valves (Kanady et al. 2011; Sabine et al. 2012). In vitro flow analyses revealed that the expression of Cx37 and calcineurin/NFATc1 activation in LECs is regulated by oscillatory fluid shear stress in a Prox1- and Foxc2-dependent manner and that Cx37 depletion significantly decreases calcineurin/NFATc1 activation (Sabine et al. 2012). In vivo, Cx37 was almost completely absent in LECs of Foxc2 −/− embryos (Sabine et al. 2012). Taken together, these results support that Prox1, Foxc2, and shear stress coordinate the expression of Cx37, which in turn activates calcineurin/NFATc1 signaling in the lymphatic valve-forming cells during lymphatic valve morphogenesis.
2.5 Additional Transcription Factors Involved in Lymphatic Development
Tbx1, a member of a conserved family of transcription factors that share a common T-box DNA-binding domain, has been recently identified as a gene whose activity is necessary during lymphatic development (Chen et al. 2010). Tbx1 is associated with the DiGeorge syndrome; however, lymphatic defects are rarely reported in patients with this syndrome (Yagi et al. 2003; Mansir et al. 1999). During mouse development, deletion of Tbx1 from ECs leads to embryonic edema and postnatal lethality between 2 and 4 days after birth because of abdominal chylous ascites (Chen et al. 2010). These mice have severely reduced lymphatic vessel density in the heart, diaphragm, and skin and lack the entire gastrointestinal lymphatic vasculature (Chen et al. 2010). Conditional inactivation of Tbx1 at different developmental stages revealed that Tbx1 activity is required until E14.5 for the formation of the mesenteric lymphatic vasculature (Chen et al. 2010). Mechanistically, chromatin immunoprecipitation analysis has shown that Tbx1 binds to conserved T-box-binding elements in the Vegfr3 promoter to activate its expression (Chen et al. 2010) (Table 2.1).
The Gata binding protein 2 (Gata2) belongs to an evolutionarily conserved family of C4 zinc finger transcription factors. Gata2 was first demonstrated to be essential for hematopoiesis because Gata2 knockout embryos die around E10 due to a failure in primitive hematopoiesis (Tsai et al. 1994). In addition to the hematopoietic lineage, a recent study systematically examined the expression of Gata2 in ECs during embryonic development by using Gata2-GFP knock-in mice. GFP was strongly expressed in arterial and venous BECs (Khandekar et al. 2007). Interestingly, GFP expression was also observed in LECs budding from the veins and in postnatal lymphatic vessels (Khandekar et al. 2007). Gata2 expression was also reported in lymphatic valve cells, suggesting a possible role for the gene in lymphatic valve formation (Kazenwadel et al. 2012). Importantly, conditional inactivation of Gata2 in the endothelial lineage led to edema and hemorrhaging and ultimately embryonic demise at around E16.5. Further analysis revealed that loss of Gata2 caused lymph sac hypoplasia and suggested defective blood–lymphatic separation (Lim et al. 2012). More evidence for Gata2 function in lymphatic vascular development comes from human patients in whom mutations in Gata2 have been characterized as the cause of primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) (Ostergaard et al. 2011). Similarly, in some patients with myelodysplastic syndrome, acute myeloid leukemia, and “MonoMAC” syndrome, mutations in Gata2 are associated with primary lymphedema (Kazenwadel et al. 2012). Little is known about the transcriptional regulation of Gata2. A fragment in intron 4 of Gata2 has been identified as an endothelium-specific enhancer of Gata2. Analysis of this fragment revealed that transcription factors belonging to the Ets family and Scl are activators of Gata2 expression (Khandekar et al. 2007) (Table 2.1). In addition to the upstream regulation of Gata2, in vitro siRNA data suggest that Gata2 regulates the expression of many genes required for valve formation (e.g., Prox1, Foxc2, NFATc1, and Itga9) (Kazenwadel et al. 2012). Taken together, these findings suggest that Gata2 is another newly identified lymphatic-specific transcription factor important for early lymphatic vascular development.
References
Aranguren, X. L., Beerens, M., Coppiello, G., Wiese, C., Vandersmissen, I., Nigro, A. L., et al. (2013). COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or heterodimerisation with PROX1. Journal of Cell Science, 126(Pt 5), 1164–1175.
Bos, F. L., Caunt, M., Peterson-Maduro, J., Planas-Paz, L., Kowalski, J., Karpanen, T., et al. (2011). CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circulation Research, 109, 486–491.
Brice, G., Mansour, S., Bell, R., Collin, J. R., Child, A. H., Brady, A. F., et al. (2002). Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. Journal of Medical Genetics, 39, 478–483.
Carter, T. C., & Phillips, J. S. (1954). RAGGED, a semidominant coat texture mutant in the house mouse. Journal of Heredity, 45, 151–154.
Casley-Smith, J. R. (1980). The fine structure and functioning of tissue channels and lymphatics. Lymphology, 13, 177–183.
Cermenati, S., Moleri, S., Cimbro, S., Corti, P., Del Giacco, L., Amodeo, R., et al. (2008). Sox18 and Sox7 play redundant roles in vascular development. Blood, 111, 2657–2666.
Chang, C. P., Neilson, J. R., Bayle, J. H., Gestwicki, J. E., Kuo, A., Stankunas, K., et al. (2004). A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell, 118, 649–663.
Chen, C. Y., Bertozzi, C., Zou, Z., Yuan, L., Lee, J. S., Lu, M., et al. (2012). Blood flow reprograms lymphatic vessels to blood vessels. Journal of Clinical Investigation, 122, 2006–2017.
Chen, L., Mupo, A., Huynh, T., Cioffi, S., Woods, M., Jin, C., et al. (2010). Tbx1 regulates Vegfr3 and is required for lymphatic vessel development. Journal of Cell Biology, 189, 417–424.
Dagenais, S. L., Hartsough, R. L., Erickson, R. P., Witte, M. H., Butler, M. G., & Glover, T. W. (2004). Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expression Patterns, 4, 611–619.
Davis, R. B., Curtis, C. D., & Griffin, C. T. (2013). BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Development, 140, 1272–1281.
de la Pompa, J. L., Timmerman, L. A., Takimoto, H., Yoshida, H., Elia, A. J., Samper, E., et al. (1998). Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature, 392, 182–186.
De Val, S., & Black, B. L. (2009). Transcriptional control of endothelial cell development. Developmental Cell, 16, 180–195.
Deng, Y., Atri, D., Eichmann, A., & Simons, M. (2013). Endothelial ERK signaling controls lymphatic fate specification. Journal of Clinical Investigation, 123, 1202–1215.
Dumont, D. J., Jussila, L., Taipale, J., Lymboussaki, A., Mustonen, T., Pajusola, K., et al. (1998). Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science, 282, 946–949.
Fang, J., Dagenais, S. L., Erickson, R. P., Arlt, M. F., Glynn, M. W., Gorski, J. L., et al. (2000). Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. American Journal of Human Genetics, 67, 1382–1388.
Flister, M. J., Wilber, A., Hall, K. L., Iwata, C., Miyazono, K., Nisato, R. E., et al. (2009). Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood, 115, 418–429.
Francois, M., Caprini, A., Hosking, B., Orsenigo, F., Wilhelm, D., Browne, C., et al. (2008). Sox18 induces development of the lymphatic vasculature in mice. Nature, 456, 643–647.
Fritz-Six, K. L., Dunworth, W. P., Li, M., & Caron, K. M. (2008). Adrenomedullin signaling is necessary for murine lymphatic vascular development. Journal of Clinical Investigation, 118, 40–50.
Gridley, T. (2010). Notch signaling in the vasculature. Developmental Biology, 92, 277–309.
Hagerling, R., Pollmann, C., Andreas, M., Schmidt, C., Nurmi, H., Adams, R. H., et al. (2013). A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO Journal, 32(5), 629–644.
Harada, K., Yamazaki, T., Iwata, C., Yoshimatsu, Y., Sase, H., Mishima, K., et al. (2009). Identification of targets of Prox1 during in vitro vascular differentiation from embryonic stem cells: Functional roles of HoxD8 in lymphangiogenesis. Journal of Cell Science, 122, 3923–3930.
Harvey, N. L., Srinivasan, R. S., Dillard, M. E., Johnson, N. C., Witte, M. H., Boyd, K., et al. (2005). Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature Genetics, 37, 1072–1081.
Herpers, R., van de Kamp, E., Duckers, H. J., & Schulte-Merker, S. (2008). Redundant roles for Sox7 and Sox18 in arteriovenous specification in zebrafish. Circulation Research, 102, 12–15.
Hogan, B. M., Bos, F. L., Bussmann, J., Witte, M., Chi, N. C., Duckers, H. J., & Schulte-Merker, S. (2009). Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nature Genetics, 41, 396–398.
Hong, Y. K., Harvey, N., Noh, Y. H., Schacht, V., Hirakawa, S., Detmar, M., & Oliver, G. (2002). Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Developmental Dynamics, 225, 351–357.
Hosking, B., Francois, M., Wilhelm, D., Orsenigo, F., Caprini, A., Svingen, T., et al. (2009). Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development, 136, 2385–2391.
Ichise, T., Yoshida, N., & Ichise, H. (2010). H-, N- and Kras cooperatively regulate lymphatic vessel growth by modulating VEGFR3 expression in lymphatic endothelial cells in mice. Development, 137, 1003–1013.
Irrthum, A., Devriendt, K., Chitayat, D., Matthijs, G., Glade, C., Steijlen, P. M., et al. (2003). Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. American Journal of Human Genetics, 72, 1470–1478.
Johnson, N. C., Dillard, M. E., Baluk, P., McDonald, D. M., Harvey, N. L., Frase, S. L., & Oliver, G. (2008). Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes and Development, 22, 3282–3291.
Kanady, J. D., Dellinger, M. T., Munger, S. J., Witte, M. H., & Simon, A. M. (2011). Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Developmental Biology, 354, 253–266.
Kang, J., Yoo, J., Lee, S., Tang, W., Aguilar, B., Ramu, S., et al. (2010). An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood, 116, 140–150.
Karkkainen, M. J., Haiko, P., Sainio, K., Partanen, J., Taipale, J., Petrova, T. V., et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature Immunology, 5, 74–80.
Kazenwadel, J., Michael, M. Z., & Harvey, N. L. (2010). Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood, 116, 2395–2401.
Kazenwadel, J., Secker, G. A., Liu, Y. J., Rosenfeld, J. A., Wildin, R. S., Cuellar-Rodriguez, J., et al. (2012). Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood, 119, 1283–1291.
Khandekar, M., Brandt, W., Zhou, Y., Dagenais, S., Glover, T. W., Suzuki, N., et al. (2007). A Gata2 intronic enhancer confers its pan-endothelia-specific regulation. Development, 134, 1703–1712.
Kokubo, H., Miyagawa-Tomita, S., Nakazawa, M., Saga, Y., & Johnson, R. L. (2005). Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Developmental Biology, 278, 301–309.
Kriederman, B. M., MyLoyde, T. L., Witte, M. H., Dagenais, S. L., Witte, C. L., Rennels, M., et al. (2003). FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Human Molecular Genetics, 12, 1179–1185.
Kulkarni, R. M., Greenberg, J. M., & Akeson, A. L. (2009). NFATc1 regulates lymphatic endothelial development. Mechanisms of Development, 126, 350–365.
Lawson, N. D., Scheer, N., Pham, V. N., Kim, C. H., Chitnis, A. B., Campos-Ortega, J. A., et al. (2001). Notch signaling is required for arterial–venous differentiation during embryonic vascular development. Development, 128, 3675–3683.
Lee, S., Kang, J., Yoo, J., Ganesan, S. K., Cook, S. C., Aguilar, B., et al. (2009). Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood, 113, 1856–1859.
Lim, K. C., Hosoya, T., Brandt, W., Ku, C. J., Hosoya-Ohmura, S., Camper, S. A., et al. (2012). Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. Journal of Clinical Investigation, 122, 3705–3717.
Lin, F. J., Chen, X., Qin, J., Hong, Y. K., Tsai, M. J., & Tsai, S. Y. (2010). Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. Journal of Clinical Investigation, 120, 1694–1707.
Mansir, T., Lacombe, D., Lamireau, T., Taine, L., Chateil, J. F., Le Bail, B., et al. (1999). Abdominal lymphatic dysplasia and 22q11 microdeletion. Genetic Counseling, 10, 67–70.
Mishima, K., Watabe, T., Saito, A., Yoshimatsu, Y., Imaizumi, N., Masui, S., et al. (2007). Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Molecular Biology of the Cell, 18, 1421–1429.
Murtomaki, A., Uh, M. K., Choi, Y. K., Kitajewski, C., Borisenko, V., Kitajewski, J., & Shawber, C. J. (2013). Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development, 140(11), 2365–2376.
Niessen, K., Zhang, G., Ridgway, J. B., Chen, H., Kolumam, G., Siebel, C. W., & Yan, M. (2011). The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood, 118, 1989–1997.
Norrmen, C., Ivanov, K. I., Cheng, J., Zangger, N., Delorenzi, M., Jaquet, M., et al. (2009). FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. Journal of Cell Biology, 185, 439–457.
Oliver, G. (2004). Lymphatic vasculature development. Nature Reviews Immunology, 4, 35–45.
Oliver, G., Sosa-Pineda, B., Geisendorf, S., Spana, E. P., Doe, C. Q., & Gruss, P. (1993). Prox 1, a prospero-related homeobox gene expressed during mouse development. Mechanisms of Development, 44, 3–16.
Ostergaard, P., Simpson, M. A., Connell, F. C., Steward, C. G., Brice, G., Woollard, W. J., et al. (2011). Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nature Genetics, 43, 929–931.
Pan, M. R., Chang, T. M., Chang, H. C., Su, J. L., Wang, H. W., & Hung, W. C. (2009). Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. Journal of Cell Science, 122, 3358–3364.
Pedrioli, D. M., Karpanen, T., Dabouras, V., Jurisic, G., van de Hoek, G., Shin, J. W., et al. (2010). miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Molecular and Cellular Biology, 30, 3620–3634.
Pendeville, H., Winandy, M., Manfroid, I., Nivelles, O., Motte, P., Pasque, V., et al. (2008). Zebrafish Sox7 and Sox18 function together to control arterial-venous identity. Developmental Biology, 317, 405–416.
Pennisi, D., Bowles, J., Nagy, A., Muscat, G., & Koopman, P. (2000a). Mice null for sox18 are viable and display a mild coat defect. Molecular and Cellular Biology, 20, 9331–9336.
Pennisi, D., Gardner, J., Chambers, D., Hosking, B., Peters, J., Muscat, G., et al. (2000b). Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nature Genetics, 24, 434–437.
Pereira, F. A., Qiu, Y., Tsai, M. J., & Tsai, S. Y. (1995). Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. Journal of Steroid Biochemistry and Molecular Biology, 53, 503–508.
Petrova, T. V., Karpanen, T., Norrmen, C., Mellor, R., Tamakoshi, T., Finegold, D., et al. (2004). Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nature Medicine, 10, 974–981.
Petrova, T. V., Makinen, T., Makela, T. P., Saarela, J., Virtanen, I., Ferrell, R. E., et al. (2002). Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO Journal, 21, 4593–4599.
Ranger, A. M., Grusby, M. J., Hodge, M. R., Gravallese, E. M., de la Brousse, F. C., Hoey, T., et al. (1998). The transcription factor NF-ATc is essential for cardiac valve formation. Nature, 392, 186–190.
Roca, C., & Adams, R. H. (2007). Regulation of vascular morphogenesis by Notch signaling. Genes and Development, 21, 2511–2524.
Sabin, F. (1902). On the origin of the lymphatics system from the veins and the development of the lymph hearts and the thoracic duct in the pig. The American Journal of Anatomy, 1, 367–389.
Sabine, A., Agalarov, Y., Maby-El Hajjami, H., Jaquet, M., Hagerling, R., Pollmann, C., et al. (2012). Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Developmental Cell, 22, 430–445.
Sacchi, G., Weber, E., Agliano, M., Raffaelli, N., & Comparini, L. (1997). The structure of superficial lymphatics in the human thigh: Precollectors. Anatomical Record, 247, 53–62.
Shan, S. F., Wang, L. F., Zhai, J. W., Qin, Y., Ouyang, H. F., Kong, Y. Y., et al. (2008). Modulation of transcriptional corepressor activity of prospero-related homeobox protein (Prox1) by SUMO modification. FEBS Letters, 582, 3723–3728.
Shin, J. W., Min, M., Larrieu-Lahargue, F., Canron, X., Kunstfeld, R., Nguyen, L., et al. (2006). Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: A role for FGF signaling in lymphangiogenesis. Molecular Biology of the Cell, 17, 576–584.
Slee, J. (1957a). The morphology and development of ‘ragged’— A mutant affecting the skin and hair of the house mouse I. Adult morphology. Journal of Genetics, 55, 100–121.
Slee, J. (1957b). The morphology and development of ragged—A mutant affecting the skin and hair of the house mouse II. Genetics, Embryology and Gross Juvenile Morphology. Journal of Genetics, 55, 570–584.
Srinivasan, R. S., Dillard, M. E., Lagutin, O. V., Lin, F. J., Tsai, S., Tsai, M. J., et al. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes and Development, 21, 2422–2432.
Srinivasan, R. S., Geng, X., Yang, Y., Wang, Y., Mukatira, S., Studer, M., et al. (2010). The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes and Development, 24, 696–707.
Srinivasan, R. S., & Oliver, G. (2011). Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes and Development, 25, 2187–2197.
Tsai, F. Y., Keller, G., Kuo, F. C., Weiss, M., Chen, J., Rosenblatt, M., et al. (1994). An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature, 371, 221–226.
van der Putte, S. C. (1975). The early development of the lymphatic system in mouse embryos. Acta Morphologica Neerlando-Scandinavica, 13, 245–286.
Wigle, J. T., Harvey, N., Detmar, M., Lagutina, I., Grosveld, G., Gunn, M. D., et al. (2002). An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO Journal, 21, 1505–1513.
Wigle, J. T., & Oliver, G. (1999). Prox1 function is required for the development of the murine lymphatic system. Cell, 98, 769–778.
Xu, Y., Yuan, L., Mak, J., Pardanaud, L., Caunt, M., Kasman, I., et al. (2010). Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. Journal of Cell Biology, 188, 115–130.
Yagi, H., Furutani, Y., Hamada, H., Sasaki, T., Asakawa, S., Minoshima, S., et al. (2003). Role of TBX1 in human del22q11.2 syndrome. Lancet, 362, 1366–1373.
Yamazaki, T., Yoshimatsu, Y., Morishita, Y., Miyazono, K., & Watabe, T. (2009). COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes to Cells, 14, 425–434.
Yang, Y., Garcia-Verdugo, J. M., Soriano-Navarro, M., Srinivasan, R. S., Scallan, J. P., Singh, M. K., et al. (2012). Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood, 120(11), 2340–2348.
Yao, L. C., Baluk, P., Srinivasan, R. S., Oliver, G., & McDonald, D. M. (2012). Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. American Journal of Pathology, 180, 2561–2575.
Yoshimatsu, Y., Yamazaki, T., Mihira, H., Itoh, T., Suehiro, J., Yuki, K., et al. (2011). Ets family members induce lymphangiogenesis through physical and functional interaction with Prox1. Journal of Cell Science, 124, 2753–2762.
You, L. R., Lin, F. J., Lee, C. T., Demayo, F. J., Tsai, M. J., & Tsai, S. Y. (2005). Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature, 435, 98–104.
Zheng, W., Tammela, T., Yamamoto, M., Anisimov, A., Holopainen, T., Kaijalainen, S., et al. (2011). Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood, 118, 1154–1162.
Acknowledgment
We want to thank Josh Stokes (St. Jude) for the generation of Fig. 2.1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Yang, Y., Oliver, G. (2014). Transcriptional Control of Lymphatic Endothelial Cell Type Specification. In: Kiefer, F., Schulte-Merker, S. (eds) Developmental Aspects of the Lymphatic Vascular System. Advances in Anatomy, Embryology and Cell Biology, vol 214. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1646-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1646-3_2
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1645-6
Online ISBN: 978-3-7091-1646-3
eBook Packages: MedicineMedicine (R0)