Abstract
The goal of this introductory chapter is to provide an overview of the design, evolution, and basic characteristics of the disc and the vertebrae that comprise the human spine. As with any survey, the state of current knowledge reflects the work of earlier cohorts of individuals whose insightful observations relied almost entirely on observation, argument, and inductive reasoning. Over the centuries, sequential observations by men like Aristotle, Vesalius, Hunter, and Winslow have all contributed to understanding how the oversized human head can restrictively swivel on the multiple bones of the vertebrate spine and in doing so provide our species with its huge biological advantage.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Evolutionary Considerations
The goal of this introductory chapter is to provide an overview of the design, evolution, and basic characteristics of the disc and the vertebrae that comprise the human spine. As with any survey, the state of current knowledge reflects the work of earlier cohorts of individuals whose insightful observations relied almost entirely on observation, argument, and inductive reasoning. Over the centuries, sequential observations by men like Aristotle, Vesalius, Hunter, and Winslow have all contributed to understanding how the oversized human head can restrictively swivel on the multiple bones of the vertebrate spine and in doing so provide our species with its huge biological advantage.
It needs to be acknowledged that the spine as we know it with the intervening intervertebral discs is a relatively late phylogenetic development in the animal kingdom. It was preceded by the appearance of a stiff rodlike structure, the notochord. In animals that lack backbones, the notochord provides rigidity and some resilience to the organism, promotes formation of an extended shape, and protects the overlying spinal cord. The defining characteristic of vertebrates, the backbone, first appeared in the fossil record about 500 million years ago, during the Ordovician period. While details of the transition (notochord to spine) are missing, the 500-million-year-old tiny Middle Cambrian fossil chordate, Pikaia, possessed a notochord that separated the distinct head and tail regions; Haikouichthys—a small early Cambrian fish-like fossil—exhibited well-developed eyes as well as muscle blocks typical of early vertebrates (Shu et al. 2003). Box 1.1 shows the metameric structure of Pikaia.
The appearance of the spine probably signaled the most critical event in evolution of higher organisms. The stimulus for invertebrate chordates to develop the complex mineralized metameric structure that characterizes the vertebrate subphylum is still unknown; even less understood is how evolutionary pressures prompted the development of the intervertebral disc, an event that permitted rapid locomotion and flexion. Remarkably, evidence is mounting that this evolutionary jump might be the result of the development and expression of microRNAs (Iwama et al. 2013). Rather than viewing this type of transformation as a slow evolutionary process, Garstang (1928) has proposed that both cephalochordates and vertebrates evolved along separate pathways in prehistory. This worker proposed that as a result of neotenicFootnote 1 evolution, our ancestor may have been a sessile, ascidian wormlike organism.
As organisms evolved a mineralized vertebrate axial skeleton, the biological advantages offered by the spine motion segment provided functions that profoundly influenced the activities of other organ systems. Not surprisingly, aside from allowing extension of the body with some flexibility, the vertebral bone protects the spinal cord. Other advantages of the vertebral bones are that they provide sites for attachment of the axial skeleton to the appendicular bones via the pectoral and pelvic girdles; additionally, the attachment of muscles and ribs to vertebrae facilitates functional changes required for locomotion and respiration. With respect to the discs that separate each of the vertebrae, specific functions are to allow movement of the individual vertebrae, transmit forces between vertebrae, and serve as hydrodynamic shock absorbers.
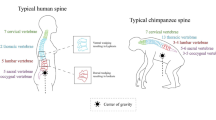
In humans and other primates, the spine permitted adoption of a vertical posture facilitating the transition from arboreal to terrestrial locomotion. The upright bipedal stance afforded evolutionary advantages including extended three-dimensional vision: enhanced depth perception would be expected to enhance manual dexterity, which in turn would promote skills linked to tool creation. That these same influences also promoted weaponization added to the uniqueness of the human race and its determination to limit its own growth and development. Away from the appendicular skeleton, the stable, strong, flexible, and vertical spine permitted evolutionary changes in the bones of the skull, allowing marked cranial growth and development. Thus, over time, the head, albeit balanced precariously at the tip of the vertical spine, together with the bones of the arms, ribs, and legs, would undergo phenotypic alterations that characterize primates and humans. Moreover, the change in the biomechanical status of the appendicular skeleton would impact the size, shape, and depth of the pelvis. These evolutionary changes provided animals with an enormous biological advantage, moving the organism away from the wormlike characteristics of our distant ancestors to the frenetic and often random activities of modern day bipedal hominids.
Other chapters of this book will ask the following questions: Why did these transitions take place, and what or how are the biomechanical forces accommodated by the skeleton and the musculature? What gene clusters are altered to support this critical evolutionary change, and what is the fate of the notochord itself—can notochordal remnants influence the functional and developmental biology of the spine? Hopefully, insights generated by these developmental, molecular, mechanical, physiological, and biochemical studies of the spine will provide answers to questions concerning the health and function of the intervertebral disc—answers that could not be derived through extant anatomical and pathological analysis.
1.2 Development of the Vertebrae and Intervertebral Disc
The vertebrae develop from individual ossification centers which are well documented historically (Kerkring 1717; Albinus 1737; Rambaud and Renault 1864). Probably the most detailed report in the twentieth century was by Peacock (1951). The reader is urged to review the latter report for more details; the developmental biology of the intervertebral disc and the vertebrae is discussed in great detail in Chap. 3.
The vertebral bodies are formed by fusion of sclerotome from two adjacent somites: thus, tissue from the caudal portion of one sclerotome fuses with cranial sclerotome of the succeeding somite. The dense connective tissue of the two halves of each sclerotome becomes two centers of chondrogenesis. At each putative vertebrae, two more chondrogenic centers appear laterally and grow backwards to form the cartilage precursor to the neural arch. During this phase of development, the notochord becomes compressed by the pressure exerted by the cartilage and may persist for a while as a “mucoid streak.” However, between the developing vertebrae, notochordal tissue is retained and subsequently forms the intervertebral disc. At these sites, notochordal cells become enclosed in a dense ring of connective tissue, the putative annulus fibrosus. Noteworthy, some notochordal cells may remain in the cartilage; at a later time, cells buried in the bone of the centrum may serve as a site for tumor formation (see Chaps. 3 and 17). The nucleus pulposus is thus formed early in fetal life from notochordal elements and grows rapidly in late fetal life and early infancy. By birth, it occupies half of the intervertebral space in the lumbar region, and by 1 year it occupies almost three quarters of the space. It is thought that there is some remodeling of the intervertebral space early in life (Taylor 1975).
By the seventh week of life, the cartilage undergoes endochondral ossification. Dorsal and ventral blood vessels invade the two cartilage anlagen and trigger their replacement with bone. Subsequently, the anterior and posterior portions of the calcified centrum fuse. Along the anterior and lateral periphery of the vertebrae, cartilage plates appear to form the apophysis. This is the site for insertion of the fibers of the annulus fibrosus. As the centrum ossifies, the cartilage anlage of the neural arch is replaced by bone. The two sides of the arch fuse and then join together with the centrum. The process begins in the upper cervical region and extends caudally. The laminae are also formed in cartilage—they join together after birth and then fuse with the rest of the vertebrae between the third and seventh years of life. Vertebrae growth is mediated by chondrogenic activity at the growth plate. Actually, as the centrum has two centers of growth, it should be labeled as a synchondrosis. Histologically, prior to closure in the 17th–25th year, a well-defined zone of hypertrophic chondrocytes is visible. Once growth has ceased, the only remaining cartilage is the endplate.
2 Anatomical and Molecular Structure of the Intervertebral Discs
Medieval anatomists were the first to recognize that the vertebrae were separated by soft “gristle”-like structures, the intervertebral discs. In his intricate drawings of the spine, Winslow (1776) provided a detailed description of the disc, which considering the limitations posed by the distortions of hand lenses was remarkably accurate. Another analysis of spinal anatomy and the intervertebral disc in health and disease was performed by one of the most prolific anatomists of the nineteenth century, Hubert von Luschka. In his monograph Die Halbgelenke des menschlichen Körpers (1868), von Luschka described the gross and microscopic structure of the intervertebral discs from birth to death. Almost at the same time, Humphrey (1858) in his book A Treatise on the Human Skeleton provided a detailed description of each of the discs. He reported the looping fibrils of the annulus fibrosus and noted the absence of blood vessels in the nucleus and inner annulus fibrosus. Studies of age changes in the disc were subsequently noted by Henle (1872), Poirier and Charpy (1899), Fick (1904) and Petersen (1930), and Bohmig (1930). As far as we can tell, the earliest comment on the relationship of the disc to the notochord was reported by the Austrian anatomist Schaffer early in the twentieth century (1910).
2.1 Form and Function of the Intervertebral Discs
Depending on age, time of day, occupation, and disease state, the discs make up approximately 15–20 % of the length of the spinal column. Aside from absorbing biomechanical forces, each disc permits movement of the spinal column. Undoubtedly, flexibility decreases with age, while spinal movements at all stages of life can be severely limited by disease. Since vertebrae themselves are relatively inelastic, movement in the spine is mediated notably by the tissues of the intervertebral disc. Although the mobility of contiguous vertebrae (motion segments) can be viewed as limited, the integrated motion of the 33 intervertebral discs together with movement at the zygapophyseal joints permits all of the critical movements of the spine without compromising nerve or muscle function.
The famous English anatomist Henry Gray (1827–1861) classified articulations between vertebrae as “amphiarthroses in which the contiguous bony surfaces are either connected by broad flattened discs of fibrocartilage, of a more or less complex structure.” By definition, these joints permit very little motion. Shapiro et al. (2012) compared the structure-function relationships of both the intervertebral disc and synovial joints. On first consideration, the intervertebral disc could be seen as being very different from the generic synovial joint. However, on reflection, the separate tissues of the intervertebral disc are very similar to that of the diarthrodial joint: both types of joints are lined by cartilage, they are limited by an external ligament, and the joint space contains molecules that promote lubrication (lubricin and hyaluronan) and elevate the osmotic pressure (aggrecan). Indeed, even the presence of a band of nucleus pulposus tissue across the joint is not out of line with what is known of complex diarthrodial joints that contain cartilage and fibrocartilage discs and menisci. Related to the function of the nucleus pulposus and the inner annulus, it is not yet clear whether a distinct synovial-like membrane exists in the intervertebral disc. Whether inner annulus is derived from the notochordal sheath has not been determined. Nevertheless, like the cells of the synovium, the resident disc cells do have the ability to mount a robust defense against bacterial attack (Nerlich et al. 2002; Jones et al. 2008).
In terms of movement, the current classification of the disc as an amphiarthrosis would indicate very limited mobility. However, biomechanical studies of the motion segment with or without contributions from the zygapophyseal joints indicate that there is wide range of motion between vertebrae. Moreover, the actual movement of the cervical, thoracic, and lumbar vertebrae includes flexion-extension, axial rotation, and lateral bending, as well as translatory motions. These three-dimensional movements are more in line with those of a diarthrodial joint rather than an amphiarthrosis where movements are slow and motion is limited. Probably the major difference between appendicular diarthrodial joints and the axial intervertebral joints lies in their development. Although the joints originate from different mesenchymal elements, the nucleus pulposus is derived from a unique embryonic tissue, the notochord; deletion studies indicate here too there are considerable similarities in the expression of genes that govern organ development and maturation. Recent investigations indicate that joint formation and even function are dependent on the expression of a number of genes including those of the Hox family, BMPs, and GDF5 (Brunet et al. 1998; Archer et al. 2003; Pacifici et al. 2005). Indeed, deletion of Ext1 influences not just the development of limb joints but also the formation of the intervertebral disc (Mundy et al. 2011). This topic is considered further in Chap. 3.
Based on the overt structural and functional similarities between the intervertebral disc and the synovial joint and recognizing that while some differences exist between these articulations, it would seem logical to place the disc in the same grouping as the diarthrodial joint. Further, since the intervertebral motion segment displays movement in three dimensions and the spine itself provides further rotatory movements, Shapiro et al. (2012) were of the opinion that it should be classified not as an amphiarthrosis, “a slightly moveable joint,” but as a complex polyaxial joint.
2.2 Spinal Curvature
While the intervertebral discs and the zygapophyseal joints provide sites for vertebral motion, the overall shape of the spine as well as curvatures in specific regions of the spine is dependent on intrinsic genetic factors as well as biomechanical forces mediated through the pull of muscles, ligaments, and gravity. Encoded curvatures are seen in the cervical, lumbar, and sacral regions of the spine. On adoption of a vertical stance, and with maturation, these curvatures become more distinct (Fig. 1.1c). However, about 2–3 % of the population exhibit deficits in axial curvature, which vary from simple bending with little functional implications to excessive bending which impacts not just locomotor activities, but other critical functions associated with the spinal nerves. The “hunchback” spine, kyphosis, is due to excessive posterior bending of the thoracic motion segments (Fig. 1.2a); when the cervical and lumbar anterior spinal curvatures become excessive, this condition is termed lordosis (Fig. 1.2b). While these latter conditions are deviation in the anterior-posterior (cephalic-caudal) axis of the spine, abnormal bending is also seen in the lateral (side-to-side) dimensions. Scoliosis can affect any part of the spine; the most common regions are in the thoracic and the lower lumbar spine. These exaggerated musculoskeletal warps in spinal architecture are evident almost entirely in human populations even in royalty (Richard III); their occurrence in rodents is infrequent. Thus, from an experimental viewpoint, rodents and lagomorphs make useful models to investigate the molecular control of spinal curvature.
Spinal curvature: kyphosis and lordosis. Anterior-posterior radiographs of the spine showing (a) kyphosis (excessive posterior bending of the thoracic motion segments) and (b) lordosis (extreme anterior bending of the lumbar and often the cervical spine). (c) The complete spine showing the natural curvature in the cervical, lumbar, and sacral regions (From Bougery and Jacob (1833). Plate 6)
Human vertebrae. (a–c) show vertebrae from the cervical spine (below C2); (d–f) show vertebrae from the thoracic region of the spine; (g–i) show lumbar vertebrae. a, d, and g anterior-posterior aspects of the vertebrae; b, e, and h are superior views; c, f, and i are lateral views of the spine. VB vertebral body or centrum, P pedicle, L lamina, TP transverse process, VF vertebral foramen, SP spinous process, TF transverse foramen, SAS superior articular surface, SCF superior costal facet, TCF transverse costal facet, AP accessory process, SAF superior articular facet (From Bougery and Jacob (1833). Plates 8 and 9)
Clinical analysis of the types of abnormal spinal bending indicates that the most common form of this condition is idiopathic, i.e., of unknown origin. Nevertheless, the etiology of this condition is likely to be multifactorial as both environmental and genetic factors have been implicated. A second form of scoliosis is neuromuscular which is secondary to other conditions, such as cerebral palsy or a myopathy. In the elderly, abnormalities in axial bending are often due to degenerative disc disease and spondylolisthesis. Possibly, the most intriguing form of scoliosis is congenital in origin, a rare condition that is evident early in childhood (usually within the first 6–8 weeks). Radiographically, the spine exhibits fused vertebrae, single or multiple hemivertebrae, a vertebral bar, block vertebrae, and wedge-shaped or butterfly vertebrae. If left untreated, almost all of these congenital anomalies result in deformities and loss of normal function. Since the anomalies occur early in development, this form of scoliosis has been linked to patterning, particularly during the period of somitogenesis (Chal and Pourquie’ 2009).
As will be discussed in considerable detail in Chap. 3, somitogenesis occurs at a very early stage in development and is the process whereby the mesoderm of the developing embryo undergoes a carefully timed segmentation process; somites are generated that specify skeletal muscles, dermis, vertebrae, ribs, and annulus fibrosus. Very recent work by Pourquie’ (2011) has shown that the trigger for the rhythmic production of somites involves three major signaling pathways: Notch (Jiang et al. 2000), Wnt/β-catenin (Dequeant et al. 2006), and fibroblast growth factor (Benazeraf et al. 2010) which are integrated into a molecular circuit. The oscillatory activities of this circuit generate a highly coordinated developmental event that serves as a traveling wave of gene expression along the anterior-posterior axis of the developing embryo. Pourquie’ (2011) refers to this synchronized change in gene expression in the pre-mesodermal cells as the “segmentation clock.” Clearly any activity that interferes with coordinated gene expression and the development of the waves of gene oscillations will impact somitogenesis which in turn will influence the subsequent formation of the vertebrae and the intrinsic curvature of the axial skeleton. Although this system was developed from studies in mice, it is most likely that these new understandings will impact our understanding and ultimately the treatment of congenital scoliosis.
2.3 Gross Morphology and Dimensions of the Disc
The sizes of the discs in the human skeleton have been assessed by a number of investigators, especially in relationship with age, underlying conditions, and responses to surgery. Disc thickness can be assessed by radiography and other forms of imaging analysis. Frobin et al. (1997) made a determined effort to measure the disc and vertebrae height using archived radiographic measurements of the spine. This approach was complicated by a number of factors that included artifacts due to image distortion, axial rotation and lateral tilt, and even magnification. To account for these problems, algorithms were developed that generated dimensionless parameters. The study showed that lumbar vertebrae and discs were larger in males and females and in males there appeared to be no or little impact of age. More recently, magnetic resonance imaging (MRI) was used to provide direct information on the discs of seven healthy males aged 22–30 (Belavý et al. 2011).
Some general comments about disc dimensions are as follows. Disc height (cephalic-rostral dimensions) varies according to the spinal region. In the cervical spine, the disc height is about 3 mm, whereas in the lumbar spine, it is 9–17 mm; in the thoracic spine, the thickness is about 5 mm. In the cervical spine, the discs are thicker in the anterior region than posterior, thus helping to provide the curvature that is characteristic of the neck. In the thoracic spine, the discs are of constant thickness, whereas in the lumbar spine, they are again thickest anteriorly. Radiographs have been used to assess disc parameters in animals most commonly used in spine research (O’Connell et al. 2007).
2.4 Tissues of the Intervertebral Disc
The major functional role of the disc is mechanical: it allows movements between the axial and appendicular skeleton and the head; it accommodates applied loads; and to some extent the disc protects the spinal cord and nerve roots. The discs themselves are complex tissues comprising an outer circumferential ring of fibrocartilage, the annulus fibrosus which encloses a central proteoglycan-rich core, the nucleus pulposus. The nucleus is sandwiched caudally and cephalically by the cartilage endplates of the contiguous vertebrae. Since details of the biochemical, developmental, and biomechanical aspects of each of the disc tissues are provided in considerable detail in other chapters of this book, the sections below merely highlight the major characteristics of the endplate cartilage, nucleus pulposus, and the annulus fibrosus.
2.4.1 Annulus Fibrosus
As an introduction to these topics, it is worth noting that the annulus can be divided into an inner fibrocartilagenous region and an outer or peripheral fibrous zone (Souter and Taylor 1970). It was reported that the outer annulus fibrosus is composed of very well-defined collagen I fibers that bundle to form long parallel concentric lamellae. Marchand and Ahmad (1990) showed that the number of fiber bundles varies from 20 to 62. The thickness of lamellae varies both circumferentially and radially and increases markedly with age, location, and vertebral type. The central annulus fibers are inserted into the endplate cartilage, while those at the periphery are anchored to the vertebral bone. In terms of collagen organization and cell content, this region is not unlike tendon or ligament.
The inner annulus fibrosus represents roughly 50 % of the total radial thickness. Designated by some workers as the transitional zone, it differs substantially from the outer region. Compared with the outer annulus where the cells are elongated and fusiform and extend in the long axis of the fibrils, the cells of the inner annulus are spherical in shape and many resemble chondrocytes. These cells are few in number with short processes. A further difference between the inner and outer annulus is their chemical composition. The inner annulus contains collagens I and II. While aggrecan is present in both regions of the annulus, decorin and biglycan are found mainly in the outer annulus. The other protein of significance is elastin which accounts for 2 % of the dry tissue weight.
2.4.2 Nucleus Pulposus
The nucleus pulposus is derived from the notochord and notochordal cells remain in the tissue after birth and into adult life. During development, the nucleus is highly cellular: after birth, the number of cells is reduced; in the adult, the cell density is very low. The histology of the nucleus pulposus cells is unique and complex: large cells arranged mainly in clusters and separated by an abundant extracellular matrix. Among the large notochordal cells, much smaller cells possibly derived from the notochordal sheath can also be seen. The large cells appear to have numerous vacuoles, which has prompted some authorities to describe them as “physaliphorous.” Probably the most complete TEM analysis of the nucleus of the adult rabbits was described by Gan et al. (2003). These workers showed that the nucleus pulposus contained cell clusters embedded in a proteoglycan-collagen matrix. The cells exhibited a well-defined Golgi system, an extensive endoplasmic reticulum, and a complex vesicular system filled with beaded structures (proteoglycans). Neither necrotic nor apoptotic cells were evident. A remarkable finding was that the cells contain few if any mitochondria. A defining characteristic of the cells was the presence of numerous cytoplasmic processes.
With respect to the extracellular matrix, nucleus pulposus cells secrete aggrecan, as well as collagens I and II. The matrix also contains collagens IX and XI, and collagen X has also been reported to be present during degeneration. Because of the presence of aggrecan, the disc exhibits a high osmotic pressure; moreover, since it has no blood supply, the oxygen tension within the disc is very low. These limitations have prompted the Risbud group to note that nucleus pulposus cells “tune” their metabolism to the available oxygen supply (see Chap. 6 for details). In this case, nucleus pulposus cells evidence almost complete reliance on the glycolytic pathway to generate metabolic energy (Agrawal et al. 2007).
2.4.3 Endplate Cartilage
The caudal and cephalic ends of the disc are covered by a layer of cartilage, the endplate. This thin layer of hyaline cartilage is maximally thick in the newborn and thins with age; in the adult, the actual width is about 0.5–1 mm. It serves not just as an interface between the soft nucleus pulposus and the dense bone of the vertebrae, but as a biomechanical barrier that prevents the disc from applying pressure directly to the bone. It is the presence of the cartilage layer that provides the motion segment with its joint-like characteristics. Some authorities believe that the cartilage also plays a role in maintaining the viability of cells of the nucleus pulposus (Dahia et al. 2009). Structurally, the endplate resembles articular cartilage. Thus, it contains chondrocytes embedded in an aggrecan-rich and collagen II extracellular matrix. Although the cells do not undergo terminal differentiation, collagen X may be present in the central region of the endplate perhaps in relationship to focal areas of endochondral bone formation. The endplate transitions into bone through a region of calcified cartilage.
In his review of the cartilage, Moore noted that vascular channels penetrate the cartilage, but at maturity the vessels become narrow, constricted, or even obliterated. It is likely that this change impacts the nutrient supply to both the cartilage and the disc (Moore 2000). Crock and Yoshizawa (1976) reported that the central region of the endplate where there is a high concentration of channels is freely permeable to small molecules. On the other hand, Nachemson et al. (1970) noted that at the tissue periphery, the cartilage is much less permeable to low molecular weight dyes. Clinically, it is not uncommon to note that the central region undergoes sclerosis or mineralization with alterations in the mechanical properties of the cartilage. When this occurs, nucleus pulposus tissue can be forced through the endplate into the underlying bone of the vertebrae. This phenomenon is known as Schmorl’s nodes which Schmorl himself considered to be linked to degenerative changes at the cartilage bone interface (see Box 1.2).
3 Vertebral Structure
Since the book is devoted to the intervertebral disc, there is little need to review the detailed anatomy of each of the vertebrae. Instead, we herein provide broad brush strokes that delineate the general features of human vertebrae; this is followed by a few comments about individual vertebrae and the sacrum. Detailed images of each of the vertebrae are shown in Figs. 1.2 and 1.3.
Human cervical vertebrae: atlas and axis. (a–c) show the atlas (C1) and (d–f) indicate the axis (C2) vertebrae. (a, d) are superior views; (c, f) show inferior views; (b, e) are lateral aspects of the vertebrae. VF vertebral foramen, AT anterior tubercle, PT posterior tubercle, AA anterior arch, PA posterior arch, TF transverse foramen, TP posterior tubercle, SAF superior articulating facet, SAS superior articulating surface, PAS posterior articulating surface, L lamina, SP spinous process, D dens or odontoid process (From Bougery and Jacob (1833). Plate 7)
At first sight, the architecture of the vertebrae appears to be very complex, each bone being riddled with numerous nooks, crannies, protrusions, and extrusions. However, the basic organization of the 24 articulating bones of the spine is quite simple: the vertebral structure reflects its two primary functions, articulation with contiguous vertebrae and protection of the spinal cord. From an anatomical viewpoint, a canal is formed as bone is deposited around the cord. This canal, the vertebral foramen, houses and protects the spinal cord. The remaining structure of the vertebrae forms in the caudal-cephalic direction a boat-like shape (albeit designed by a drunken engineer), while the anterior-posterior axis exhibits a very inexact pyramidal-like structure (albeit designed by a heat-affected Pharaonic architect). The base of the pyramid is comprised of a robust bone, the centrum or body, while the sides of the pyramid form a bone arch or lamella (the hull) that surrounds the spinal cord. The apex of the arch extends backwards to form the spinous process (the keel). This process is very well developed in the thoracic spine where it serves as a site for attachment of the powerful muscles of the back. Projecting upwards and forwards from the base of the lamellae are transverse processes (retractable stabilizers) which are sites of origin of the pedicles that form a base for the articulating zygapophyseal (facet) joints: the superior (cephalic) articulating process articulates with the zygapophyseal joints of the contiguous cephalic vertebrae; projecting downwards and backwards from the laminae is the inferior articulating process from which a facet joint is formed with the contiguous caudal vertebrae. “Portholes” at the junction of the “fin” and the “lateral stabilizers” provide openings, “intervertebral foramina,” for the nerves that flow from and into the spinal cord. In terms of general anatomy, other than C1 and C2, the largest portion of a typical vertebra is the bony centrum, the weight-bearing region of the vertebrae. With increasing distance from C3, there is a significant increase in the robustness of the centrum and the vertebrae, thus the lumbar vertebrae and its centrum are larger than vertebrae of either the thoracic or cervical spine. In cervical and even upper thoracic vertebrae, on the cephalic bone surface, a ring-like protuberance, the uncus, may be present. This ossified structure, the uncinate process, serves to limit movement at the intervertebral disc and forms the so-called uncovertebral joints (joints of von Luschka, see Box 1.2).
3.1 Cervical Vertebrae (Figs. 1.2 and 1.3)
In line with the generalized numbering system of the individual regions of the spine, the cervical vertebrae are sequentially numbered from rostral to caudal (C1 to C7); C1 and C2, the atlas and axis vertebrae, respectively, form the joint complex that permits the spinal column to articulate with the head via the occipital condyles. Neither of these vertebrae have a well-defined body; indeed, the atlas can be viewed as a ring of dense membrane bone. Bound to the skull by very strong ligaments, these vertebrae allow a range of motion that permits up and down as well as rotational movements of the skull. Thus, the joint between the atlas (named after the God who balanced the world on his shoulders) and the occiput (“hole in the head”), the atlanto-occipital joint, permits flexion and extension (basically nodding), while the atlanto-axial joint (C1 and C2) allows nodding, gliding, and rotation. Rotation of the head and with it the atlas is mediated by the odontoid process or dens, a bony peg-like extension of C2 into C1. The actual interaction between C1 and C2 is complex with a number of centers of movement: the pivoting odontoid process of the axis and the gliding facet joints between the axis and atlas vertebrae. Noteworthy there is no disc between the occiput and the atlas or between the axis and the atlas; the first intervertebral disc is between the axis C2 and C3. The detailed anatomy of the axis and atlas are shown in Fig. 1.3; the anatomy of C4–C7 is shown in Fig. 1.2.
3.2 Thoracic Vertebrae
In general, the twelve thoracic vertebrae have the same functional role as the other axial vertebrae. They are larger in size than in the cervical spine, but smaller than those of the lumbar region. Common architectural features of the thoracic vertebrae are that the body (centrum) and the spinous processes are large and unlike vertebrae of the lumbar region, the spinous processes point downwards (see Fig. 1.2f, i). A major function of the thoracic spine is stability and through articulations with the ribs provides protection for the lungs and the heart. Of the bones that comprise the rib cage, the seven cephalic thoracic vertebrae are attached to the sternum via 12 pairs of ribs. As such, each sternal rib articulates with two vertebrae: sites of attachment are through joints on the inferior and superior aspects of the centrum and a third facet located at the end of the transverse process (costal facets). The remaining thoracic vertebrae are attached to the unanchored ribs (also known as floating ribs) by similar types of articulations.
3.3 Lumbar Spine (Fig. 1.2)
Like the thoracic spine, the robustness of the lumbar vertebrae increases from L1 to L5. When compared with the vertebrae of the other regions of the spine, the individual lumbar vertebrae are the most massive of all: in most cases being wider and longer. However, unlike the thoracic spine, the lumbar spine curves inwards to form the concavity in the lower back. The direction of the curve is probably due to the pull of the viscera of the lower region of the body. Motion around the lumbar spine is considerably greater than the thoracic spine, the facet, and disc joints, permitting a significant degree of flexion and extension. The lumbar body (centrum) is wide in all directions and exhibits concavities on both cephalic and caudal surfaces as well as being slightly constricted at the sides. Like the thoracic vertebrae, the spinous process projects backwards while the large pedicles display deep inferior vertebral notches. The L2 segment is the level at which the spinal nerves come together to form the cauda equina.
3.4 The Sacrum and Coccygeal Bones (Fig. 1.4)
Human sacrum and coccygeal bones. The ala (A) of the sacrum articulates with the ileum (I) of the pelvis at the sacroiliac joints (SIJ). The sacrum consists of five fused vertebrae (S1–S5). The superior portion of the sacrum articulates with L5 (lumbar sacrum articulation, LSA) while the inferior aspect fuses with the bones of the coccyx (C1–C5). Running through the sacrum is a continuation of the vertebral canal from which the sacral nerves emerge through both anterior (ASF) and posterior foramina. SAP superior articulating process, SP sacral promontory, AS apex of sacrum (From Lizars (1857). Plate III, Bones of the pelvis)
The sacrum is a very strong robust multibone triangular complex (S1–S5) which is joined at its upper end to the lumbar vertebrae at L5 while its lower portion associates with the coccyx. The five fused bones of the sacrum integrate the two halves of the pelvis. The sacrum is united to the ileum by fibrocartilage which accommodates and transmits the weight of the upper body mass. The inferior end of the sacrum articulates with the five fused bones of the coccyx. Intervertebral discs are not present in the bones of the sacrum or the coccyx (Box 1.3).
4 Vertebrae and Intervertebral Discs of Animals
4.1 Anatomical Considerations
While considerable space is devoted to the sand rat (see Chap. 20) as well as other quadrupeds (see Chap. 18), it is worthwhile summarizing some key features of small animals that are used extensively in studies of the intervertebral disc. In contrast to the vertically orientated human vertebral column, the almost horizontal spine of quadrupeds is subjected to a different series of biomechanical forces. Discussing the cat spine, Macpherson and Ye note, “Not surprisingly, the force vectors on all of the vertebrae differ substantially from the human. The axial skeleton may be considered as a segmented beam with the legs as pillar supports and two overhanging regions, the head-neck segments and the tail” (Macpherson and Ye 1998). At the rostral end of the spine, the animal’s head is supported through the muscles and ligaments of the cervical spine. The first two vertebrae are ring shaped and are organized to allow for controlled movements of the head. Like the human, these vertebrae do not have the robust body, but exhibit all of the articulations for spinal movement. Macpherson and Ye (1998) propose that the support for the head is provided by muscles that join the spine with the scapula. These muscles include the levator scapulae and serratus ventralis, which are inserted into the transverse processes of C3 to T9/10, and the rhomboids which join the scapula to the spinous process of C4 to T4. Together these muscles “suspend the trunk from the scapulae much like the wires on a suspension bridge.”
In the rat, the 12 thoracic vertebrae form an S-shaped curve (see Fig. 1.5). These vertebrae display well-developed long spinous processes that are intermediate in size between cervical and lumbar, and they exhibit facets for articulation with the ribs. Like the human spine, the lumbar vertebrae are the most massive in the rat with very well-defined intervertebral discs. The last lumbar vertebra articulates with the sacrum. The body of these composite vertebrae forms a slab of bone in which there is loss of intervertebral discs and the zygapophyses and lateral processes are fused (Fig. 1.5). The sacrum articulates with the pelvis through the ilium, thereby transferring the weight of the posterior region of the body to the femurs. Thus, forces applied to the animal’s body are transmitted across the almost horizontal sacrum (usually at S1 and often S2) to the vertical legs.
Axial skeleton of the rat. Micro-CT analysis of the rat. Note the 12 thoracic vertebrae form an S-shaped curve with facets for articulation with the ribs. The last lumbar vertebra articulates with the sacrum which articulates with the pelvis through the ilium, thereby transferring the weight of the posterior region of the body to the femurs and the almost vertical legs. The tail is composed of 28–30 vertebrae which, with increasing distance from the sacrum, exhibits a progressive loss of centrum mass and decrease in the identity of articulating surfaces, processes, and foramina. Eventually, the neural arch becomes fused with the centrum (Figure provided with kind permission by Dr. Rasesh Kapadia, Scanco Medical, Switzerland)
Composed of a variable number of vertebrae (about 28–30), the tail represents the final region of the spine. While the first few vertebrae are anatomically complete, with increasing distance from the sacrum, there is a change in vertebra size and complexity. There is a progressive loss of centrum mass and decrease in the identity of articulating surfaces and processes and foramina. Eventually, the neural arch becomes fused with the centrum, while the diameter of the intervertebral foramen becomes narrowed and indistinct. Since some studies of the intervertebral disc are performed in the caudal region of the spine, these anatomical limitations need to be taken into account when devising studies of the caudal intervertebral discs.
4.2 Conservation of Vertebral Number
The vertebral formula for humans is surprisingly constant: 7 cervical, 12 thoracic, 5 lumbar, 5 fused vertebrae that make up the sacrum, and 4 or 5 coccygeal bones. Details of the vertebral formula for a number of common mammals are shown in Box 1.2. In nonmammalian species, considerable differences exist in the vertebral formula. Snakes have a large number of thoracic (between 100 and 200) and caudal (between 15 and 140) vertebrae; the extinct marine Plesiosaurus had more than 70 cervical vertebrae (Narita and Kuratani 2005).
For both humans and many mammals, the number of vertebrae in the cervical region of mammals appears to be constant. Galis (1999) analyzed the vertebral formula data for mammals from the Descriptive Catalogue of the Osteological Series Contained in the Museum of the Royal College of Surgeons of England compiled by Richard Owen in 1853. This catalogue contains data of 133 species from 15 orders of mammals and showed that a very high percentage of animals, possibly with the exception of carnivores, expressed seven cervical vertebrae (Table 1.1).
Galis (1999) reported that occasionally, there is a loss of a single cervical vertebra (C7) with a concomitant increase in the number of thoracic vertebrae and the appearance of a cervical rib. Associated with this change, in the space between the clavicle and the rib (the thoracic outlet), there is often nerve and blood vessel compression, a condition described as thoracic outlet syndrome (TOS) (Makhoul and Machleder 1992). Correlated with cervical rib formation, Schumacher et al. (1992) reported that there was an increase in childhood cancer including neuroblastoma, Wilms tumor, Ewing sarcoma, and lymphoblastic and myeloid leukemia. It is likely that this developmental anomaly is a result of aberrant Hox gene expression. Thus, a higher incidence of cervical rib is seen in the phenotype of Hoxa-4, Hoxd-4, Hoxa-5, and Hoxa-6 knockouts or overexpression of Hoxb-7 or Hoxb-8 D (Aubin et al. 1998). The relationship between Hox expression and development of the axial skeleton in mammals is developed in more detail in Chap. 3.
As an aside, while a vertebrae-dependent increase in rib number is correlated with disease, loss of a rib has biblical implications.
But for Adam, no suitable helper was found. So the LORD God caused the man to fall into a deep sleep; and while he was sleeping, he took one of the man’s ribs and closed up the place with flesh. Then the LORD God made a woman from the rib he had taken out of the man, and brought her to the man.
Whether Adam had TOS or suffered from headaches due to cervical tension or loss of a rib is not known. For a discussion of this and other biblical possibilities including the emergence of the baculum (ossified penis bone), please read Gilbert and Zevit (2001).
5 Summary of Critical Concepts Discussed in the Chapter
-
The intervertebral disc/vertebrae were preceded phylogenetically by the notochord which provided rigidity and some resilience to the organism, promoted formation of an extended shape, and protected the overlying spinal cord.
-
The vertebral bodies are formed by fusion of sclerotome from two adjacent somites: following formation of the neural arch, remnants of the notochord subsequently form the nucleus pulposus of the intervertebral disc.
-
Synchronized change in gene expression in the pre-mesodermal cells activates the “segmentation clock.” The coordinated expression of a limited number of genes provides waves of gene oscillations which control somitogenesis. Disturbances in this system influence the subsequent formation of the vertebrae and the intrinsic curvatures of the axial skeleton.
-
The discs comprise an outer circumferential ring of fibrocartilage, the annulus fibrosus. The annulus encloses a central proteoglycan-rich core known as the nucleus pulposus and bounded by the cartilage endplates of the contiguous vertebrae.
-
As a joint, the disc is classified as an amphiarthrosis with very limited mobility. Biomechanical studies indicate that there is wide range of motion between vertebrae that are more in line with those of a diarthrodial joint rather than an amphiarthrosis.
-
The vertebrae protect the spinal cord and serve as sites for connection of the pectoral and pelvic girdles and as bone for attachment of muscle and rib for functional changes that enhanced locomotion and respiration. Specific functions of the discs include acting as hydrodynamic shock absorbers as well as providing flexibility to the whole spine.
-
The vertebral formula for primates is well conserved: in humans 7 cervical, 12 thoracic, 5 lumbar, 5 fused vertebrae that make up the sacrum, and 4 or 5 coccygeal bones. Occasionally, there is a loss of a single cervical vertebra (C7) with a concomitant increase in the number of thoracic vertebrae and the appearance of a cervical rib.
Notes
- 1.
Term used to describe the retention in the adult of traits or phenotype expressed in the immature state.
References
Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV (2007) Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol 293:C621–C631
Archer CW, Dowthwaite GP, Francis-West P (2003) Development of synovial joints. Birth Defects Res C Embryo Today 69:144–155
Aubin J, Lemieux M, Tremblay M, Behringer RR, Jeannotte L (1998) Transcriptional interferences at the Hoxa4/Hoxa5 locus: importance of correct Hoxa5 expression for the proper specification of the axial skeleton. Dev Dyn 212:141–156
Belavý DL, Bansmann PM, Böhme G, Frings-Meuthen P, Heer M, Rittweger J, Zange J, Felsenberg D (2011) Changes in intervertebral disc morphology persist 5 mo after 21-day bed rest. J Appl Physiol 111:1304–1314
Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie’ O (2010) A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466:248–252
Bougery JM, Jacob NH (1833) Atlas of complete treatise on human anatomy comprising operative medicine. C.A. Delaunay, Paris
Brunet LJ, McMahon JA, McMahon AP, Harland RM (1998) Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280:1455–1457
Chal J, Pourquie’ O (2009) Patterning and differentiation of the vertebrate spine. In: Pourquie O (ed) The skeletal system. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 41–116
Crock HV, Yoshizawa H (1976) The blood supply of the lumbar vertebral column. Clin Orthop Relat Res 115:6–21
Dahia CL, Mahoney EJ, Durrani AA, Wylie C (2009) Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976) 34:456–462
Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, Pourquie’ O (2006) A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science 314:1595–1598
Frobin W, Brinckmann P, Biggemann M, Tillotson M, Burton K (1997) Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin Biomech (Bristol, Avon) 12(Suppl 1):S1–S63
Galis F (1999) Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool 285:19–26
Gan JC, Ducheyne P, Vresilovic EJ, Swaim W, Shapiro IM (2003) Intervertebral disc tissue engineering I: characterization of the nucleus pulposus. Clin Orthop Relat Res 411:305–314
Garstang W (1928) The morphology of the tunicata, and its bearings on the phylogeny of the chordata. Q J Microsc Sci 72:51–187
Gilbert SF, Zevit Z (2001) Congenital human baculum deficiency: the generative bone of Genesis 2:21–23. Am J Med Genet 101:284–285
Iwama H, Kato K, Imachi H, Murao K, Masaki T (2013) Human microRNAs originated from two periods at accelerated rates in mammalian evolution. Mol Biol Evol 30(3):613–626
Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowicz D, Lewis J (2000) Notch signalling and the synchronization of the somite segmentation clock. Nature 408(6811):475–9
Jones P, Gardner L, Menage J, Williams GT, Roberts S (2008) Intervertebral disc cells as competent phagocytes in vitro: implications for cell death in disc degeneration. Arthritis Res Ther 10:R86
Lizars J (1857) A system of anatomical plates of the human body; accompanied with descriptions, and physiological, pathological, and surgical observations. Lizars, Edinburgh
Macpherson JM, Ye Y (1998) The cat vertebral column: stance configuration and range of motion. Exp Brain Res 119:324–332, RESEARCH ARTICLE
Makhoul RG, Machleder HI (1992) Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg 16:534–542
Marchand F, Ahmed AM (1990) Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine (Phila Pa 1976) 15:402–410
Moore RJ (2000) The vertebral end-plate: what do we know? Eur Spine J 9:92–96
Mundy C, Yasuda T, Kinumatsu T, Yamaguchi Y, Iwamoto M, Enomoto-Iwamoto M et al (2011) Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol 351:70–81
Nachemson A, Lewin T, Maroudas A, Freeman MA (1970) In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand 41:589–607
Narita Y, Kuratani S (2005) Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J Exp Zool B Mol Dev Evol 304:91–106
Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N (2002) Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine 27:2484–2490
O’Connell GD, Vresilovic EJ, Elliott DM (2007) Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 32:328–333
Pacifici M, Koyama E, Iwamoto M (2005) Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today 75:237–248
Peacock A (1951) Observations on the pre-natal development of the intervertebral disc in man. J Anat 85(Pt 3):260–274
Pourquie’ O (2011) Vertebrate segmentation from cyclic gene networks to scoliosis. Cell 145:651–663
Schumacher R, Mai A, Gutjahr P (1992) Association of rib anomalies and malignancy in childhood. Eur J Pediatr 151:432–434
Shapiro IM, Vresilovic EJ, Risbud MV (2012) Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone 50:771–776
Shu DG, Morris SC, Han J, Zhang ZF, Yasui K, Janvier P, Chen L, Zhang XL, Liu JN, Li Y, Liu HQ (2003) Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature 421(6922):526–529
Souter WA, Taylor TK (1970) Sulphated acid mucopolysaccharide metabolism in the rabbit intervertebral disc. J Bone Joint Surg Br 52:371–384
Taylor JR (1975) Growth of human intervertebral discs and vertebral bodies. J Anat 120(Pt 1):49–68
Tubbs RS, Vahedi P, Loukas M, Shoja MM, Cohen-Gadol AA (2011) Hubert von Luschka (1820–1875): his life, discoveries, and contributions to our understanding of the nervous system: Historical vignette. J Neurosurg 114:268–272
Acknowledgments
The authors would like to thank Dr. Chris Keppler for the radiographs shown in Fig. 1.2, the Smithsonian Institution for the permission to reproduce the image of Pikaia (Box 1.1), Scanco Medical for the use of the microCT image shown in Fig. 1.5, and F. Michael Angelo, MA, for use of the plates shown in Figs. 1.1, 1.2, 1.3, and 1.4. Lastly, the authors wish to thank the NIH and NIAMS for the ongoing support through grants AR050087 and AR055655.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Shapiro, I.M., Risbud, M.V. (2014). Introduction to the Structure, Function, and Comparative Anatomy of the Vertebrae and the Intervertebral Disc. In: Shapiro, I., Risbud, M. (eds) The Intervertebral Disc. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1535-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1535-0_1
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1534-3
Online ISBN: 978-3-7091-1535-0
eBook Packages: MedicineMedicine (R0)