Abstract
In this chapter, we review the latest developments concerning the role of sphingosine 1-phosphate (S1P) in cancer. Particular focus is paid to the role of sphingosine kinases 1 and 2, S1P lyase and S1P-dependent signalling networks in both solid tumours and haematological cancer. The potential of this S1P-dependent pathophysiology as a therapeutic target for the treatment of cancer is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
There are six major hallmarks of cancer. These are (1) enhanced proliferation, (2) evasion of growth suppression, (3) enhanced cell survival/reduced apoptosis, (4) acquisition of replicative immortality/reduced senescence, (5) angiogenesis and creation of a tumour microenvironment and (6) increased invasiveness and metastasis. These hallmarks can be initiated by genetic instability including activating or inactivating mutations of oncogenes and tumour suppressors, respectively. Sphingosine 1-phosphate (S1P) is a bioactive lipid that has been implicated in cancer. S1P is formed by the phosphorylation of sphingosine, catalysed by sphingosine kinase (two isoforms termed SK1 and SK2) and is degraded by S1P lyase or dephosphorylated by S1P phosphatase. Extracellular S1P activates a family of G-protein-coupled S1P receptors that couple to various cellular signalling pathways (Pyne and Pyne 2000), whereas intracellular S1P directly binds to intracellular protein targets such as the E3 ligase, TRAF2. The role of S1P in cancer has recently been extensively reviewed (Pyne and Pyne 2010), and therefore, the purpose of this chapter is to update progress in this area in the last 2 years. We describe recent advances which demonstrate that S1P, S1P receptors, sphingosine kinase and S1P lyase are involved in aberrant cellular processes that contribute to the hallmarks of cancer. Strategies targeting SK1 are described which provide impetus for the development of novel SK1 inhibitors that might be used for therapeutic treatment of cancer in the future and that might offer better disease management options.
2 Sphingosine Kinase and Cancer
There is substantial evidence of a role for SK1 in numerous cancers (Pyne and Pyne 2010). For instance, there is increased SK1 expression in stomach, lung, brain, colon, kidney and breast cancers and non-Hodgkins lymphoma (Pyne and Pyne 2010). Many factors influence SK1 expression, such as hypoxia, growth factors and cytokines, and this has a clear indication concerning prognosis. Thus, high expression of SK1 in the tumours of ER+ breast cancer patients is associated with reduced patient survival and increased disease recurrence (Long et al. 2010a; Watson et al. 2010). One of the reasons for poor prognosis might be because SK1 is a sensor for S1P binding to S1P receptors that enables tuning of the migratory response to S1P in ER+ breast cancer cells (Long et al. 2010a). In this regard, the binding of S1P to S1P3 receptors promotes the translocation of SK1 from the cytoplasm to the plasma membrane of ER+ MCF-7 breast cancer cells, and this is a key step in promoting migration. This is exemplified by the finding that siRNA knockdown of SK1 reduces the ability of S1P to induce activation of ERK-1/2, which involves EGF receptor transactivation (Sukocheva et al. 2006; Long et al. 2010a, b) and is required for migration (Long et al. 2010a). Moreover, siRNA knockdown of SK1 reduces the expression of S1P3 in these cells (Long et al. 2010a). Thus, SK1 appears to modulate responsiveness of these cancer cells to S1P by regulating the expression of the S1P3 receptor (Fig. 1). A poor prognosis of grade 4 astrocytoma patients also correlates with high expression of SK1 in tumours (Van Brocklyn et al. 2005). Additional evidence highlighting a key role for SK1 in cancer progression is the finding that the genetic deletion of SK1 in mice significantly reduced 4-nitroquinoline-1-oxide (4-NQO)-induced head and neck squamous cell carcinogenesis, associated with decreased cell proliferation, activation of caspase-3 and ablated AKT pro-survival signalling (Shirai et al. 2011).
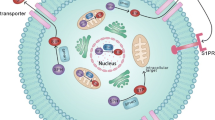
Schematic showing how SK1 acts as a sensor that governs responsiveness of oestrogen receptor-positive breast cancer cells to S1P in terms of regulating the formation of a migratory phenotype. SK1 regulates the expression of S1P3, which binds S1P to activate the ERK-1/2 pathway. Phosphorylated ERK-1/2 accumulates in actin-enriched lamellipodia and the nucleus where it can regulate metalloproteinase expression, both of which are required for allowing these cells to migrate in response to S1P
One of the most significant recent advances relating to SK1 and cancer has been made by Heffernan-Stroud et al. who have demonstrated a functional interaction between SK1 and the tumour suppressor p53. Mice lacking both p53 tumour-suppressor alleles develop thymic lymphoma, while mice lacking only one p53 allele develop osteosarcomas and soft tissue sarcoma (Donehower et al. 1992; Jacks et al. 1994; Lozano 2010). Genotoxic stress has been shown to increase ceramide levels via a p53-dependent mechanism leading to apoptosis. Indeed, the treatment of Molt-4 leukaemia cells with actinomycin D induces the degradation of SK1, while over-expression of the papilloma virus E6 protein, which targets p53 itself for degradation, prevents the removal of SK1 (Taha et al. 2004). Heffernan-Stroud et al. have now demonstrated that genotoxic stress (actinomycin, etoposide, UV) induces the degradation of SK1 in MCF-7 breast cancer cells (Heffernan-Stroud et al. 2011). This appears to be via a p53-dependent activation of caspase-2. Significantly, deletion of SK1 in p53 deficient mice completely abrogates thymic lymphomas and prolongs survival by 30 %. The tumour suppression by p53 is linked with the elevation of sphingosine and ceramide levels and increased expression of cell cycle inhibitors p21 and p16 resulting in tumour cell senescence (Fig. 2). Therefore, certain cancers might be driven by p53-inactivating mutations that increase SK1 stability and expression. The role of SK1 in cancer is also exemplified by its regulation by other tumour suppressors. In this regard, various extracellular stimuli can activate SK1 via its phosphorylation by ERK-1/2 at Ser225 which increases its activity and promotes its translocation to the plasma membrane (Pitson et al. 2003). Deactivation of SK1 occurs via its dephosphorylation by protein phosphatase 2A (PP2A) (Barr et al. 2008), facilitated by the B’α (B56α/PR61α/PPP2R5A) regulatory subunit of PP2A (Pitman et al. 2011), a well-defined tumour-suppressor protein (Arnold and Sears 2008).
3 Interaction of Sphingosine 1-Phosphate Receptors and Sphingosine Kinase 1 with Oncogenes
There is now evidence for several functional partnerships between SK1, S1P receptors and oncogenes. For instance, H-RAS has been shown to increase SK1 activity in NIH3T3 fibroblasts (Xia et al. 2000). Indeed, transformation of these cells into fibrosarcoma is inhibited by SK1 inhibitors and by over-expression of the G82D dominant negative SK1 mutant (Xia et al. 2000). SK1 is also activated by the GDP bound form of eukaryotic elongation factor 1A (eEF1A) (Leclercq et al. 2011). Translationally controlled tumour protein (TCTP) is an eEF1A guanine nucleotide dissociation inhibitor which when over-expressed in cells also activates SK1. Intriguingly, an oncogenic form of eEF1A1 called prostate tumour inducer-1 (PTI-1) which lacks the GDP–GTP-binding domain activates SK1, and this is required for PTI-1-induced neoplastic transformation (Leclercq et al. 2011). HER2/ErbB2 also functionally interacts with S1P4 in ER− MDA-MB-453 breast cancer cells to promote enhanced ERK-1/2 signalling (Long et al. 2010b), and S1P stimulates the tyrosine phosphorylation of HER2 in MKN28 and MKN74 gastric cancer cells (Shida et al. 2005). Paradoxically, SK1 can negate the function of HER2 in ER+ breast cancer cells (Long et al. 2010a). This involves a process in which HER2 increases SK1 expression, which results in a negative feedback reduction in HER2 expression and ablated migration of these cells in response to S1P. High levels of SK1 in a HER2/ER+ background induce a deregulation of p21-activated protein kinase 1 which normally functions to promote migration of these cells in response to S1P. Therefore, SK1 can induce tolerance to HER2 in ER+ breast cancer cells, and this might have clinical significance as high cytoplasmic SK1 expression in HER2+/ER+ breast tumours is associated with increased patient survival and reduced disease recurrence in patients treated with tamoxifen (Long et al. 2010a).
4 Advances in Sphingosine Kinase Inhibitors
In the past 10 years, a number of SK1 inhibitors have been synthesised. SK1-I (BML-258; (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol) is a water-soluble sphingosine analogue that is specific for SK1 and enhances survival of mice in an orthotopic intracranial tumour model (Kapitonov et al. 2009). SKi (or SKI-II, 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole) inhibits both SK1 and SK2 and reduces S1P levels, inhibits proliferation and induces apoptosis in various cancer cell lines (French et al. 2003). Several natural products, B-5354c (Kono et al. 2000a), F-12509A (Kono et al. 2000b) and S-15183a,b (Kono et al. 2001), have been isolated, which inhibit SK activity in vitro. More recently, FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride) has been identified as a SK1 inhibitor (Tonelli et al. 2010; Lim et al. 2011a). Indeed, the inhibition of SK1 activity by FTY720 induces prostate cancer cell apoptosis in a manner independent of S1P receptors (Pchejetski et al. 2010). Moreover, γ irradiation, which does not affect SK1 activity alone, synergised with FTY720 in inhibiting SK1 activity and inducing apoptosis. This can be reverted by over-expression of SK1, which protects the cancer cells from FTY720-induced apoptosis. These findings have translational significance because FTY720 also increases the sensitivity of prostate tumours to radiation in vivo without toxic side effects (Pchejetski et al. 2010).
There are also other examples expanding the utility of FTY720 as an anticancer agent. For example, FTY720 has been shown to reduce gastric cancer cell proliferation, and this is associated with G1 phase cell cycle arrest and apoptosis (Zheng et al. 2010). This involves an increase in the expression of PTEN, which subsequently ablates the phosphorylation of AKT and MDM2 (Zheng et al. 2010). MDM2 is an E3 ubiquitin ligase that catalyses the ubiquitination of p53, which enables its proteasomal degradation and which maintains low levels of p53 in cells. Therefore, FTY720 induces a post-translational increase in p53 stability and expression, which is associated with the induction of p53-regulated genes involved in growth arrest and apoptosis, such as Cip1/p21, p27 and BH3-only proteins. FTY720 is also cytotoxic in human ovarian cancer cells (Zhang et al. 2010). This occurs via a mechanism that is independent of caspase-3 activity and involves cellular swelling and cytoplasmic vacuolisation, typical of necrotic cell death (Zhang et al. 2010). FTY720 also promotes autophagy in these cells as evidenced by formation of autophagosomes and the accumulation of LC3-II. Interestingly, the siRNA knockdown of Beclin 1 or LC3 leads to enhanced necrotic cell death in response to FTY720, suggesting that stimulation of autophagy is a rebound survival response that impedes FTY720-induced necrotic death. Thus, targeting autophagy might augment the necrotic death promoting activity of FTY720 in ovarian cancer.
FTY720 also has marked inhibitory effects on hepatocellular carcinoma (Omar et al. 2011). In this case, FTY720 induces apoptosis by activating reactive oxygen species and PKCδ. In addition, the compound OSU-2S has been synthesised from FTY720. This compound lacks S1P1 receptor activity, thereby excluding the potential side effect of lymphopenia (as seen with FTY720, once phosphorylated) that might compromise anticancer activity. OSU-2S exhibits higher potency than FTY720 and is also active in vivo, reducing tumour growth in both ectopic and orthotopic models of hepatocellular carcinoma without significant toxicity (Omar et al. 2011).
Structural modification of the FTY720 scaffold has produced (S)-FTY720 vinylphosphonate, which is also an SK1 inhibitor (Tonelli et al. 2010). The treatment of MCF-7 breast cancer cells with FTY720, (S)-FTY720 vinylphosphonate or SKi prevents the formation of actin-enriched lamellipodia in response to S1P (Lim et al. 2011a), suggesting that these inhibitors block formation of the migratory phenotype, and which might have application for preventing metastasis. Indeed, treatment of MCF-7 cells with SKi reduces their migration to S1P (Long et al. 2010a). Furthermore, siRNA knockdown of SK1 similarly prevents actin rearrangement into lamellipodia (Long et al. 2010a) (Fig. 1). This is reminiscent of the action of FTY720 in vivo where it decreases metastasis in a mouse model of breast cancer and which is associated with impaired formation of filopodia (Azuma et al. 2002). Remarkably, the treatment of MCF-7 breast cancer and LNCaP prostate cancer cells with FTY720, (S)-FTY720 vinylphosphonate or SKi also induces a novel ubiquitin-proteasomal degradation of SK1 (Tonelli et al. 2010; Loveridge et al. 2010; Lim et al. 2011a), and this results in apoptosis, thereby offering additional enhanced efficacy options for the treatment of cancer. Recently, Macdonald et al. have synthesised amidine-based SK1 inhibitors which exhibit nanomolar potency and reduce intracellular S1P levels in human leukaemia U937 cells (Kennedy et al. 2011), thereby providing evidence for the first time that it is possible to achieve high, clinically relevant potency.
There is also new emerging evidence for an important role of SK2 in cancer. This is exemplified by the finding that the siRNA knockdown of SK2 enhances doxorubicin-induced apoptosis of breast or colon cancer cells (Sankala et al. 2007). Surprisingly, siRNA knockdown of SK2 elevates SK1 expression and increases intracellular S1P in A498, Caki-1 or MDA-MB-231 cells (Gao and Smith 2011). However, knockdown of SK2 in these cells reduces cell proliferation and migration/invasion, and this is actually more effective than knockdown of SK1, which results in reduced intracellular S1P and has no effect on SK2 expression. The knockdown of SK1 or SK2 also have differential effects on p53, p21, ERK-1, ERK-2, FAK and VCAM1 indicating that SK1 and SK2 have non-overlapping functions. These studies provide evidence that loss of SK2 has a stronger anticancer effect in these cell lines compared with loss of SK1 (Gao and Smith 2011) although this is unexpected given the widely documented pro-cancerous role of SK1-derived S1P. The increase in intracellular S1P upon loss of SK2 together with reduced proliferation suggests that SK2 regulates a discrete intracellular pool of S1P that is functionally compartmentalised from S1P formed by SK1 in the cancer cell types studied (see Pyne et al. 2009 for a review of spatial aspects of S1P signalling). The reduction in SK2-derived S1P upon knockdown of SK2 might then be masked by the net increase in intracellular S1P levels caused by the compensatory elevation in SK1 expression. Alternatively, it is possible that unidentified non-catalytic functions of SK2 regulate some of the effectors. This is not without foundation as SK2 interacts with Bcl2 via its BH3 domain, although this interaction promotes apoptosis (Liu et al. 2003). Nevertheless, these studies strongly support the idea that the synthesis of SK2 inhibitors might be a strategy for the development of anticancer agents. In this regard, two new SK2-selective inhibitors, namely (R)-FTY720 methyl ether ((R)-FTY720-OMe) and ABC294640, have been synthesised and characterised (Lim et al. 2011b; French et al. 2010).
(R)-FTY720-OMe has one of the prochiral hydroxyl groups of FTY720 replaced by a methoxy group, thereby blocking the site of phosphorylation by SK2. (R)-FTY720-OMe is a selective, competitive (with sphingosine) inhibitor of SK2. The treatment of MCF-7 cells with (R)-FTY720-OMe prevents actin enrichment into lamellipodia in response to S1P suggesting application to inhibit metastasis (Lim et al. 2011b). The aryladamantane compound, ABC294640 [3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide] is a competitive inhibitor (with sphingosine) of SK2 activity and reduces intracellular S1P formation in cancer cells (French et al. 2010). The Ki values for inhibition of SK2 by both (R)-FTY720-OMe and ABC294640 are very similar (16.5 vs. 10 μM, respectively). ABC294640 suppresses the proliferation of several tumour cell lines and inhibits tumour cell migration associated with the loss of actin microfilaments. In addition, ABC294640 has significant in vivo activity and inhibits tumour progression in mice with mammary adenocarcinoma xenografts (French et al. 2010).
More recent studies have demonstrated that ABC294640 induces autophagy in A-498 kidney carcinoma, PC-3 prostate and MDA-MB-231 breast cancer cells (Beljanski et al. 2010). Interestingly, combined treatment of A-498 cells with ABC294640 and an autophagy inhibitor (3-methyladenine) induces apoptosis, demonstrating a close balance between autophagic and apoptotic processes. In this regard, Smith et al. proposed a model for the action of ABC294640 (Beljanski et al. 2010): in normal conditions, tumour cells operate under conditions of a low autophagic flux. This enables balancing the energy demand for growth, which is high and is driven by the ERK pathway. In contrast, the apoptotic rate is low due to high AKT pro-survival signalling. When the cells are treated with ABC294640, there is a marked reduction in ERK and AKT signalling leading to the inhibition of proliferation and pro-survival signalling. However, the inhibition of SK2 activity by ABC294640 results in the accumulation of ceramide which, in turn, stimulates autophagic death and which predominates over apoptotic death. However, autophagy induced by ABC294640 is blocked in the presence of 3-methyladenine, and therefore, ABC294640 induces apoptosis as the predominant mechanism. In this respect, it is interesting to note that (R)-FTY720-OMe induces apoptosis in HEK 293 cells (Lim et al. 2011b). Smith et al. have also found that ABC294640 delays tumour growth and increases autophagy markers in severe combined immunodeficient mice with A-498 xenografts, albeit the number of apoptotic cells did not increase (Beljanski et al. 2010).
ABC294640 has also been shown to decrease ER-regulated gene expression, and this is accounted for by the ability of ABC294640 to also bind to the antagonist ligand-binding domain of the oestrogen receptor, ERα. In this case, ABC294640 functions as a partial antagonist similar to tamoxifen (Antoon et al. 2010). This property of ABC294640 might be physiologically relevant as ABC294640 inhibits ER+ breast cancer tumour formation in vivo. Similarly, SKi has also been reported to function as an ER antagonist (Antoon et al. 2011). These additional activities of ABC294640 and SKi enable a multipronged attack on breast cancer. In this regard, (S)-FTY720 vinylphosphonate might also be exploited to launch a multipronged attack on cancer as it can affect multiple targets. With regard to S1P-related targets, (S)-FTY720 vinylphosphonate inhibits both SK1 and SK2 activities (Lim et al. 2011a). It is also a full antagonist of S1P1,3,4 (K i 208, 15 and 1,190 nM, respectively) and a partial antagonist of S1P2 and S1P5 (Valentine et al. 2010). Additionally, (S)-FTY720 vinylphosphonate reduces the expression of the androgen receptor in androgen-independent LNCaP-AI cells (Tonelli et al. 2010), which might provide additional anti-proliferative activity.
The importance of S1P receptors in cancer is highlighted by several studies. For instance, S1P stimulates the migration of fibrosarcoma cells through S1P1 (Fisher et al. 2006) and gastric cancer cells through S1P3 (Yamashita et al. 2006). High membrane S1P1 expression in the ER+ breast cancer tumours is associated with shorter time to disease recurrence on tamoxifen, and high cytoplasmic S1P1 and S1P3 tumour expression is linked with reduced disease specific survival times (Watson et al. 2010). In addition, IL-6/Jak2 signalling is linked with S1P1, and this is required for the sustained activation of Stat3, which promotes cancer progression (Lee et al. 2010). A positive feedback loop exists, whereby Stat3 increases the expression of S1P1 resulting in further activation of Stat3 and up-regulation of IL-6 expression, which enhances tumour growth and metastasis.
Taking all these findings together, it is logical to propose that the combined inhibition of SK1 and/or SK2 and/or the antagonism of S1P receptors in cancer might provide better efficacy options compared with agents that inhibit each of these targets alone.
5 Advances on the Role of S1P Lyase in Cancer
The human SGPL1 gene, which encodes S1P lyase (SPL), maps to chromosomal regions that are commonly mutated in cancer, and one can consider SPL as having tumour-suppressor activity. Expression of SPL is regulated principally by Sp1 and a Sp1/GATA-4 complex which bind to the SGPL1 gene promoter (Ito et al. 2011). However, SPL expression is not directly correlated with cellular S1P levels in lung cancer cell lines (Ito et al. 2011). Nevertheless, deficiency in SPL induces resistance to etoposide and doxorubicin. Moreover, the reduction in doxorubicin-induced apoptosis in Sgpl-/- cells is associated with up-regulation of the anti-apoptotic proteins, Bcl2 and Bcl-xl. The deficiency of SPL is also linked with increased proliferation, anchorage-independent growth and formation of tumours in mice. Importantly, SPL expression is decreased in human melanoma cell lines, thereby suggesting reduced tumour-suppressor activity that might contribute to the progression of this cancer (Colié et al. 2009).
6 Advances in Other Specific Cancers
Several recent significant advances have been made concerning the role of S1P in haematological cancers:
6.1 Myeloid Leukaemia
The BCR/ABL1 oncogene is produced by the Philadelphia chromosome translocation t(9;22)(q34;q11) and is a linked to poor prognosis in chronic myelogenous leukaemia (CML). It is also present in 20 % of adult acute lymphoblastic leukaemias (ALL). BCR/ABL1 encodes a 210 kDa constitutively active tyrosine kinase that is present in CML patients, where it enhances survival of myeloid progenitors leading to myeloproliferative disease. The p190-encoded Bcr/Abl has similar transforming ability but stimulates proliferation of lymphoid progenitors. The Bcr/Abl oncoprotein activates signalling pathways that result in increased survival and ablated differentiation of hematopoietic progenitors.
The Bcr/Abl tyrosine kinase has been shown to increase SK1 expression, and this appears necessary for regulating the level of Mcl-1 and other anti-apoptotic Bcl-2 family members that enhance cell survival (Li et al. 2007; Bonhoure et al. 2008). In CML cells, Bcr/Abl increases SK1 expression through its activation of the ERK-1/2, PI3K and Janus kinase 2 pathways (Li et al. 2007) and AKT2/mTOR in imatinib-resistant CML cells (Marfe et al. 2011). The major treatment option for CML involves the use of the Bcr/Abl tyrosine kinase inhibitor, imatinib, which reduces SK1 expression, and this appears to contribute to the therapeutic efficacy of imatinib (Bonhoure et al. 2008). However, CML and Ph1 ALL patients develop resistance dependent on the over-expression or mutation of Bcr/Abl. Moreover, SK1 confers resistance of CML cells to imatinib (Baran et al. 2007). Indeed, the knockdown of SK1 expression by small interference RNA increases the sensitivity of resistant cells to imatinib in terms of the induction of apoptosis (Marfe et al. 2011) and enforced expression of SK1 in K562 cells increases the S1P/C18-ceramide ratio and prevents apoptosis to imatinib (Baran et al. 2007). These findings have been replicated in other CML cells. For instance, the ceramide:S1P ratio is increased in response to imatinib in imatinib-sensitive LAMA84 cells, while the ratio is unaltered in imatinib-resistant cells. Additionally, over-expression of SK1 in imatinib-sensitive cells impairs apoptosis by inhibiting caspase-3 activation and cytochrome C/Smac release. This involves the SK1-dependent modulation of Bim, Bcl-xL and Mcl-1 expression (Bonhoure et al. 2008). Furthermore, daunorubicin-sensitive but not insensitive leukaemia cells (CML, AML and ALL) exhibit an elevated ceramide:S1P ratio when treated with daunorubicin and sensitivity to daunorubicin is restored by inhibiting SK1 activity (Sobue et al. 2008).
Taken together, these findings identify SK1 as having a very important role in regulating the sensitivity of leukaemic cells to chemotherapeutic agents. Indeed, Ogretman et al. have recently provided a molecular explanation for the role of SK1 in the acquisition of resistance to imatinib in CML (Salas et al. 2011). They demonstrated that increased SK1/S1P enhances Bcr/Abl1 protein stability. This involves S1P formed by SK1 being released to act on S1P2 receptor which inactivates PP2A and thereby prevents Bcr/Abl1 degradation (Fig. 3). This is consistent with the fact that the proteasomal degradation of Bcr/Abl1 requires its dephosphorylation by PP2A/SHP-1. Moreover, pharmacological inhibition or siRNA knockdown of SK1 restores dephosphorylation and proteasomal degradation of Bcr/Abl1 and enhances the inhibition of growth by imatinib or nilotinib in primary CD34+ mononuclear cells obtained from chronic phase and blast crisis CML patients, imatinib-insensitive CML cells and murine progenitor cells expressing the wild-type or mutant (Y253H or T315I) Bcr/Abl1. SK1 inhibition also enhances the sensitivity of 32D/T315I-Bcr/Abl1-derived mouse allografts to growth inhibition by nilotinib (Salas et al. 2011).
Interestingly, Bcr/Abl1 inactivates the tumour suppressor PP2A by enhancing the expression of the PP2A inhibitor SET (Neviani et al. 2005). The effect of the functional loss of PP2A in chronic phase and blast crisis CML patient cells has been assessed using FTY720, which is an activator of PP2A (Neviani et al. 2007). The recent findings made by Salas et al. (2011) and Tonelli et al. (2010) can now be used to elaborate the mechanism of action of FTY720. Neviani et al. (2007) showed that whilst normal CD34+ or CD34+/CD19+ bone marrow cells were unaffected, FTY720 induced apoptosis of CML-BCCD34+ and Ph1 ALLCD34+/CD19+ progenitors and impaired clonogenicity of imatinib/dasatinib-sensitive and -resistant Bcr/Abl myeloid and lymphoid cell lines. These workers proposed that the restoration of PP2A activity by FTY720 reduces Bcr/Abl expression by inducing its proteasomal degradation. In addition, exogenous S1P counteracts the effect of FTY720 on Bcr/Abl stability, which is consistent with a role for extracellular S1P in preventing Bcr/Abl degradation, as reported by Salas et al. (2011). Moreover, SK inhibitors reduce the phosphorylation and stability of Bcr/Abl suggesting that inhibition of SK1 is sufficient to prevent the SK1-S1P2 autocrine loop that functions to inactivate PP2A. As mentioned previously, we have shown that FTY720 is a competitive inhibitor of SK1 (Lim et al. 2011a). In addition, FTY720 promotes the ubiquitin-proteasomal degradation of SK1 to create SK1 null cancer cells (Tonelli et al. 2010). It is therefore possible that the effect of FTY720 on Bcr/Abl stability is mediated not only by the direct activation of PP2A but also by inhibition/downregulation of SK1, which would theoretically restore PP2A activity by interrupting the S1P/S1P2 functional loop (Fig. 4). Alternatively, the activation of PP2A activity by FTY720 might function in a negative feedback loop to inactivate SK1 (Barr et al. 2008) that has been phosphorylated by ERK-1/2 on S225 (Pitson et al. 2003), thereby providing an additional route for reducing SK1 activity. FTY720 also suppresses Bcr/Abl-dependent (including mutated p210/p190Bcr/Abl (T315I)) leukaemogenesis without exerting any toxicity. Therefore, the action of FTY720 on SK1 inhibition/downregulation and restoration of PP2A activity might represent a clear example of where modulation of ceramide-sphingosine-S1P rheostat has a well-defined clinical translational potential.
Schematic showing how FTY720-dependent inhibition/degradation of SK1 might perturb the ceramide-sphingosine-S1P rheostat to prevent the inhibitory effect of S1P/S1P2 on PP2A/SHP-1, thereby enhancing Bcr/Abl degradation and improving sensitivity of CML to imatinib. This might represent an additional mechanism to direct activation of PP2A by FTY720
ALL is the most common form of childhood cancer. Notably, FTY720 reduces survival of Ph(+) and Ph(−) ALL cell lines. However, this occurs by a mechanism that is independent of PP2A and caspase-3 (Wallington-Beddoe et al. 2011). FTY720 also induces autophagy as evidenced by increased LC3II expression and autophagic flux. Interestingly, FTY720 stimulates reactive oxygen species (ROS), and therefore, the antioxidant N-acetyl-cysteine (NAC) partially reverses the cytotoxic effects of this compound. In this respect, it is noteworthy that shRNA knockdown of SK1 or an SK1 inhibitor induces ROS formation in carcinoma (Huwlier et al. 2011), suggesting close coupling between SK1 and ROS formation.
6.2 Multiple Myeloma
Multiple myeloma cell lines and cells isolated from patients that are unresponsive to conventional therapeutics undergo apoptosis in response to FTY720. This is mediated by activation of caspase-8, -9 and -2 and altered BAX cleavage and mitochondrial potential (Yasui et al. 2005). However, FTY720 also reduced IL-6-stimulated Akt phosphorylation, Stat3 and ERK-1/2 activation, IGF-I-stimulated Akt activation and TNFα-stimulated IKB and NFκB phosphorylation in these cells. A Mcl-1-dependent pathway is involved in the FTY720-induced apoptosis of B cell malignancies and primary B cells from chronic lymphocytic leukaemia (CLL) patients (Liu et al. 2008). FTY720 can also induce prolonged survival of mice with disseminated B cell lymphoma/leukaemia (Liu et al. 2008).
Lymphoma: The oncogenic activity of Runx (Runx1, 2 and 3) genes has been demonstrated in transgenic mice that develop lymphoma and which display marked synergism with over-expressed Myc. Interestingly, three genes involved in sphingolipid metabolism have been identified as direct targets for Runx-dependent transcriptional regulation (Kilbey et al. 2010). Sgpp1 (which encodes S1P phosphatase-1) is decreased, whereas Ugcg (which encodes UDP–glucose ceramide galactosyltransferase) and St3gal5/Siat9 (which encodes GM3 synthase) are both increased. This is in line with the finding that ectopic over-expression of Runx reduces intracellular long-chain ceramides and elevates extracellular S1P in NIH3T3 fibroblasts. Runx expression also reduces the activation JNK and p38 MAPK, which are essential proteins involved in ceramide-induced death.
7 Summary and Future Directions
There is now ample evidence that sphingosine kinase has tumour-promoting activity via direct or functional interaction with oncogenes. Cancer progression might occur because positive selection and clonal expansion are consequences of SK1 conferring a significant survival and growth advantage to the cancer cells. S1P lyase is pro-apoptotic and therefore exhibits tumour-suppressing activity. This highlights the key role for regulating the position of the ceramide-sphingosine-S1P rheostat in cancer. The release of S1P from cells or its partitioning into the plasma membrane and close proximity association with S1P receptors in addition to its binding to intracellular targets such as HDAC1/2 (Hait et al. 2009) and TRAF2 (Alvarez et al. 2010) might influence ubiquitination, phosphorylation, methylation, glycosylation and redox pathways conducive to enhanced survival and invasiveness of cancer cells. The functional interaction between S1P receptors and oncogenes is an additional level of complexity that might contribute to cancer and is worthy of further investigation. These processes are therefore viable targets for therapeutic intervention as exemplified by the effectiveness of SK inhibitors in in vivo cancer models (Pyne and Pyne 2010). Indeed, the SK inhibitor L- threo-dihydrosphingosine (safingol) in combination with the DNA cross-linking agent, cisplatin is effective in phase I clinical trials for the treatment of advanced solid phase tumours (Dickson et al. 2011). More successful translation to the clinic requires the development of compounds with nanomolar potency, enhanced efficacy (e.g. exemplified by inhibitor-induced proteasomal degradation of SK1) and improved bioavailability. In addition, we believe that a major focus of future research will be in the identification and characterisation of new intracellular targets that bind S1P. Indeed, it is possible that the activation/inhibition of subsets of intracellular targets might, in concert with oncogenes, confer a particular phenotype to the cancer. Identification of these S1P-dependent signalling pathways will open up new translational possibilities for therapeutically targeting cancer using personalised medicines so that this disease can be treated more effectively than is currently the case.
References
Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S (2010) Sphingosine 1-phosphate is a missing co-factor for the E3 ligase TRAF2. Nature 465:1084–1088
Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, Rhodes LV, Ashe HB, Wiese TE, Smith CD, Burow ME, Beckman BS (2010) Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology 151:5124–5135
Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, Wiese TE, Burow ME, Beckman BS (2011) Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signalling in human breast cancer. J Mol Endocrinol 46:205–216
Arnold HK, Sears RC (2008) A tumor suppressor role for PP2A–alpha through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev 27:147–158
Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T, Nonomura N, Suzuki S, Okuyama A, Katsuoka Y (2002) Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res 62:1410–1419
Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B (2007) Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib induced apoptosis in K562 human chronic myeloid lekaemia cells. J Biol Chem 282:10922–10934
Barr RK, Lynn HE, Moretti PA, Khew-Goodall Y, Pitson SM (2008) Deactivation of sphingosine kinase 1 by protein phosphatase 2A. J Biol Chem 283:34994–35002
Beljanski V, Knaak C, Smith CD (2010) A novel sphingosine kinase inhibitor induces autophagy in tumour cells. J Pharmacol Exp Ther 333:454–464
Bonhoure E, Lauret A, Barnes DJ, Martin C, Malavaud B, Kohama T, Melo JB, Cuvillier O (2008) Sphingosine kinase-1 is a downstream regulator of imatinib-induced apoptosis in chronic myeloid leukaemia cells. Leukemia 22:971–979
Colié S, Van Veldhoven PP, Kedjouar B, Bedia C, Albinet V, Sorli SC, Garcia V, Djavaheri-Mergny M, Bauvy C, Codogno P, Levade T, Andrieu-Abadie N (2009) Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res 69:9346–9353
Dickson MA, Carvajal RD, Merrill AH Jr, Gonen M, Cane LM, Schwartz GK (2011) A phase I clinical trial of safingol in combination with Cisplatin in advanced solid tumours. Clin Cancer Res 17:2484–2492
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215–221
Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE (2006) Tumour cell invasion of collagen matrices requires coordinate lipid agonist-induced G protein and membrane-type matrix metalloproteinase-1-dependent signalling. Mol Cancer 5:69
French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JLM, Yun JK, Smith CD (2003) Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res 63:5962–5969
French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD (2010) Pharmacology and antitumour activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther 333:129–139
Gao P, Smith CD (2011) Ablation of sphingosine kinase-2 inhibits tumour cell proliferation and migration. Mol Cancer Res 9(11):1509–19
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S (2009) Regulation of histone acetylation in the nucleus by sphingosine 1-phosphate. Science 325:1254–1257
Heffernan-Stroud LA, Helke KL, Jenkins RW, De Costa AM, Hannum YA, Obeid LM (2011) Defining a role for sphingosine kinase 1 in p53-dependent tumours. Oncogene 31(9):1166–75
Huwlier A, Kotelevets N, Xin C, Pastukhov O, Pfeilschiffter J, Zangemeister-Wittke U (2011) Loss of sphingosine kinase 1 in carcinoma cells increases formation of reactive oxygen species and sensitivity to doxorubicin-induced DNA damage. Br J Pharmacol 162:532–543
Ito H, Yoshida K, Murakami M, Hagiwara K, Sasaki N, Kobayashi M, Takagi A, Kojima T, Sobue S, Suzuki M, Tamiya-Koizumi K, Nakamura M, Banno Y, Nozawa Y, Murate T (2011) Heterogeneous sphingosine-1-phosphate lyase gene expression and its regulatory mechanism in human lung cancer cell lines. Biochim Biophys Acta 1811:119–128
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA (1994) Tumour spectrum analysis in p53-mutant mice. Curr Biol 4:1–7
Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S (2009) Targeting sphingosine kinase 1 inhibits AKT signalling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res 69:6915–6923
Kennedy AJ, Mathews TP, Kharel Y, Field SD, Moyer ML, East JE, Houck JD, Lynch KR, Macdonlad TL (2011) Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J Med Chem 54:3524–3548
Kilbey A, Terry A, Jenkins A, Borland G, Zhang Q, Wakelam MJ, Cameron ER, Neil JC (2010) Runx regulation of sphingolipid metabolism and survival signalling. Cancer Res 70:5860–5869
Kono K, Tanaka M, Ogita T, Kohama T (2000a) Characterisation of B-5354c, a new sphingosine kinase inhibitor, produced by a marine bacterium. J Antibiot (Tokyo) 53:759–764
Kono K, Tanaka M, Ogita T, Hosoya T, Koyama T (2000b) F-12509A, a new sphingosine kinase inhibitor, produced by a discomycete. J Antibiot (Tokyo) 53:459–466
Kono K, Tanaka M, Ono Y, Hosoya T, Ogita T, Kohama T (2001) S-15183a and b, new sphingosine kinase inhibitors, produced by a fungus. J Antibiot (Tokyo) 54:415–420
Leclercq TM, Moretti PA, Pitson SM (2011) Guanine nucleotides regulate sphingosine kinase 1 activation by eukaryotic elongation factor 1A and provide a mechanism for eEF1A-associated oncogenesis. Oncogene 30:372–378
Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H (2010) STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 16:1421–1428
Li QF, Huang WR, Duan HF, Wang H, Wu CT, Wang LS (2007) Sphingosine kinase-1 mediates BCR/ABL-induced upregulation of Mcl-1 in chronic myeloid leukemia cells. Oncogene 26:7904–7908
Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, Pyne NJ (2011a) FTY720 analogues as sphingosine kinase 1 inhibitors: Enzyme inhibition kinetics, allosterism, proteasomal degradation and actin rearrangement in MCF-7 breast cancer cells. J Biol Chem 286:18633–18640
Lim KG, Sun C, Bittman R, Pyne NJ, Pyne S (2011b) (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell Signal 23:1590–1595
Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S (2003) Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278:40330–40336
Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, Lehman A, Perrotti D, Chen CS, Dalton JT, Muthusamy N, Byrd JC (2008) FTY720 demonstrates promising pre-clinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood 111:275–284
Long JS, Edwards J, Watson C, Tovey S, Mair K, Schiff R, Natarajan V, Pyne NJ, Pyne S (2010a) Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor positive breast cancer cells. Mol Cell Biol 30:3827–3841
Long JS, Fujiwara Y, Edwards J, Tannahill C, Tigyi G, Pyne S, Pyne NJ (2010b) Sphingosine 1-phosphate 4 uses HER2 (ErbB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J Biol Chem 285:35957–35966
Loveridge C, Tonelli F, Leclecq T, Lim KG, Long S, Berdyshev E, Tate RJ, Natarajan V, Pitson SM, Pyne NJ, Pyne S (2010) The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem 285:38841–38852
Lozano G (2010) Mouse models of p53 functions. Cold Spring Harb Perspect Biol 2(4):a001115
Marfe G, Di Stefano C, Gambacurta A, Ottone T, Martini V, Abruzzese E, Mologni L, Sinibaldi-Salimei P, de Fabritis P, Gambacorti-Passerini C, Amadori S, Birge RB (2011) Sphingosine kinase 1 overexpression is regulated by signalling through PI3K, AKT2, and mTOR in imatinib-resistant chronic myeloid leukemia cells. Exp Hematol 39:653–665.e6
Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, Roy DC, Valtieri M, Bruner-Klisovic R, Caligiuri MA, Bloomfield CD, Marcucci G, Perrotti D (2005) The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 8:355–368
Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen C-S, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D (2007) FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome–positive acute lymphocytic leukemia. J Clin Invest 117:2408–2421
Omar HA, Chou CC, Berman-Booty LD, Ma Y, Hung JH, Wang D, Kogure T, Patel T, Terracciano L, Muthusamy N, Byrd JC, Kulp SK, Chen CS (2011) Antitumor effects of OSU-2S, a nonimmunosuppressive analogue of FTY720, in hepatocellular carcinoma. Hepatology 53:1943–1958
Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M, Salunkhe V, Teissié J, Malavaud B, Waxman J, Cuvillier O (2010) FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res 70:8651–8661
Pitman MR, Barr RK, Gliddon BL, Magarey AM, Moretti PA, Pitson SM (2011) A critical role for the protein phosphatase 2A B’alpha regulatory subunit in dephosphorylation of sphingosine kinase 1. Int J Biochem Cell Biol 43:342–347
Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW (2003) Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J 22:5491–5500
Pyne S, Pyne NJ (2000) Sphingosine 1-phosphate signalling in mammalian cells. Biochem J 349:385–402
Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10:489–503
Pyne S, Lee SC, Long J, Pyne NJ (2009) Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signal 21:14–21
Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, Apohan E, Sentelle RD, Smith C, Gault CR, Obeid LM, El-Shewy HM, Oaks J, Santhanam R, Marcucci G, Baran Y, Mahajan S, Fernandes D, Stuart R, Perrotti D, Ogretmen B (2011) Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr/Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 117:5941–5952
Sankala HM, Hait NC, Paugh SW, Shida D, Lépine S, Elmore LW, Dent P, Milstien S, Spiegel S (2007) Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res 67:10466–10474
Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H (2005) Lysophospholipids transactivate HER2/neu (erbB-2) in human gastric cancer cells. Biochem Biophys Res Commun 327:907–914
Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, Obeid LM, Ogretmen B, Kawamori T (2011) A role of sphingosine kinase 1 in head and neck carcinogenesis. Cancer Prev Res 4:454–462
Sobue S, Nemoto S, Murakami M, Ito H, Kimura A, Gao S, Furuhata A, Takagi A, Kojima T, Nakamura M, Ito Y, Suzuki M, Banno Y, Nozawa Y, Murati T (2008) Implications of sphingosine kinase 1 expression level for the cellular sphingolipid rheostat: relevance as a marker for daunorubicin sensitivity of leukaemia cells. Int J Haematol 87:266–275
Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P (2006) Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol 173:301–310
Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, Dbaibo GS, Hannun YA, Obeid LM (2004) Downregulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53. J Biol Chem 279:20546–20554
Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ (2010) FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal 22:1536–1542
Valentine WJ, Kiss GN, Liu J, Gotoh M, Murakami-Murofushi K, Pham TC, Baker DL, Parrill AL, Lu X, Sun C, Bittman R, Pyne NJ, Tigyi G (2010) (S)-FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, signals as a pan-antagonist of sphingosine 1-phosphate GPCR and inhibits autotaxin activity. Cell Signal 22:1543–1553
Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW (2005) Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: role of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol 64:695–705
Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ (2011) FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy 7:707–715
Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, Pyne S, Pyne NJ, Edwards J (2010) High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol 177:2205–2215
Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA (2000) An oncogenic role of sphingosine kinase. Curr Biol 10:1527–1530
Yamashita H, Kitayama J, Shida D, Yamaguchi H, Mori K, Osada M, Aoki S, Yatomi Y, Takuwa Y, Nagawa H (2006) Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation. J Surg Res 130:80–87
Yasui H, Hideshima T, Raje N, Roccaro AM, Shiraishi N, Kumar S, Hamasaki M, Ishitsuka K, Tai YT, Podar K, Catley L, Mitsiades CS, Richardson PG, Albert R, Brinkmann V, Chauhan D, Anderson KC (2005) FTY720 induces apoptosis in multiple myeloma cells and overcomes drug resistance. Cancer Res 65:7478–7484
Zhang N, Qi Y, Wadham C, Wang L, Warren A, Di W, Xia P (2010) FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: a protective role of autophagy. Autophagy 6:1157–1167
Zheng T, Meng X, Wang JB, Chen X, Yin D, Liang Y, Song X, Pan S, Jiang H, Liu L (2010) PTEN- and p53-mediated apoptosis and cell cycle arrest by FTY720 in gastric cancer cells and nude mice. J Cell Biochem 111:218–228
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Wien
About this chapter
Cite this chapter
Pyne, S., Pyne, N.J. (2013). New Perspectives on the Role of Sphingosine 1-Phosphate in Cancer. In: Gulbins, E., Petrache, I. (eds) Sphingolipids in Disease. Handbook of Experimental Pharmacology, vol 216. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1511-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1511-4_3
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1510-7
Online ISBN: 978-3-7091-1511-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)