Abstract
Schizophrenia alters basic brain processes of perception, emotion, and judgment to cause hallucinations, delusions, thought disorder, and cognitive deficits. Unlike neurodegeneration diseases that have irreversible neuronal degeneration and death, schizophrenia lacks agreeable pathological hallmarks, which makes it one of the least understood psychiatric disorders. With identification of schizophrenia susceptibility genes, recent studies have begun to shed light on underlying pathological mechanisms. Schizophrenia is believed to result from problems during neural development that lead to improper function of synaptic transmission and plasticity, and in agreement, many of the susceptibility genes encode proteins critical for neural development. Some, however, are also expressed at high levels in adult brain. Here, we will review evidence for altered neurotransmission at glutamatergic, GABAergic, dopaminergic, and cholinergic synapses in schizophrenia and discuss roles of susceptibility genes in neural development as well as in synaptic plasticity and how their malfunction may contribute to pathogenic mechanisms of schizophrenia. We propose that mouse models with precise temporal and spatial control of mutation or overexpression would be useful to delineate schizophrenia pathogenic mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Excitatory synaptic transmission
- Inhibitory synaptic transmission
- Neuromodulators
- Schizophrenia
- Schizophrenia susceptibility genes
1 Introduction
Schizophrenia alters basic brain processes of perception, emotion, and judgment to cause hallucinations, delusions, thought disorder, and cognitive deficits. It is a mental disorder that affects 0.5–1% of the population worldwide with devastating consequences for affected individuals and their families and is the seventh most costly illness in the USA. Unlike neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) that have irreversible neuronal degeneration and death, nerve cells in schizophrenia generally do not degenerate or die. Because of the lack of pathological hallmarks, schizophrenia remains to be one of the least understood psychiatric disorders. With identification of schizophrenia susceptibility genes, recent studies have begun to shed light on underlying pathological mechanisms. All brain functions depend on the function of synapses, connections between neurons. It is now widely believed that schizophrenia results from problems during neural development that lead to improper function of synaptic transmission and plasticity (Eastwood 2004; McCullumsmith et al. 2004; Mirnics et al. 2001; Nikolaus et al. 2009; Stephan et al. 2006). Intriguingly, many of the schizophrenia susceptibility genes encode proteins that have been implicated in synapse formation and/or function. This chapter focuses on the relationship between synaptic transmission and schizophrenia. We will first review evidence for altered neurotransmission at glutamatergic, GABAergic, dopaminergic, and cholinergic synapses in schizophrenia and discuss the roles of susceptibility genes in neural development and synaptic plasticity and how their malfunction may contribute to the pathogenic mechanisms of schizophrenia.
2 Altered Synaptic Transmission in Schizophrenia
2.1 The Glutamatergic Pathway
The interest in alterations of glutamatergic neurotransmission as potential pathological mechanisms in schizophrenia was raised when phencyclidine (PCP) was found to reduce noncompetitively excitation of neurons by NMDA (Anis et al. 1983). Earlier, PCP had been shown to produce transient psychotic symptoms in healthy individuals including thought disorder, blunted affect, and cognitive impairments that resemble those in schizophrenic patients (Fauman et al. 1976; Luby et al. 1959). Ketamine, a PCP derivative and a dissociative anesthetic drug, was also able to generate in healthy individuals transient schizophrenia-like (positive and negative) symptoms and impair cognitive functions that depend on the prefrontal cortex (PFC) (Adler et al. 1999; Krystal et al. 1994; Lahti et al. 2001; Malhotra et al. 1997). In schizophrenic patients, ketamine exacerbates preexisting symptoms (Lahti et al. 1995; Malhotra et al. 1997). Taken together, these results suggest a role of reduced glutamatergic function in schizophrenic pathology.
In agreement with this hypothesis were findings that glutamate levels, which inversely correlate with the severity of positive symptoms (Faustman et al. 1999), are significantly lower in the cerebrospinal fluid (CSF) and in brain tissues of schizophrenic patients (Kim et al. 1980; Tsai et al. 1995). Glutamate release from synaptosomes prepared from frozen brain samples of schizophrenics was reduced in response to NMDA or kainic acid (Sherman et al. 1991b). In addition, postmortem analysis shows reduced mRNA and enzymatic activity of glutamate carboxypeptidase II (GCP II), the enzyme that degrades the neuropeptide N-acetylaspartylglutamate (NAAG), which is a reversible antagonist of NMDA receptors (Hakak et al. 2001; Tsai et al. 1995). It is controversial whether levels of NMDA or AMPA receptors are reduced in schizophrenics. Increased mRNA levels were reported in some studies (Akbarian et al. 1996; Dracheva et al. 2001; Kristiansen et al. 2006) while other studies showed a decrease (Akbarian et al. 1995, 1996; Dracheva et al. 2001; Kristiansen et al. 2006; Mirnics et al. 2000). Morphologically, dendritic length and dendritic spine density are reduced in the cerebral cortex of schizophrenic patients (Garey et al. 1998; Glantz and Lewis 2000) although the density of pyramidal neurons was shown to be increased in the dorsal lateral PFC (DLPFC) in schizophrenics (Selemon and Goldman-Rakic 1999).
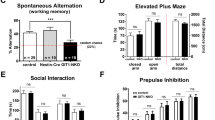
Adult rodents, when treated with NMDA antagonists, become hyperactive (Nabeshima et al. 1983; Sturgeon et al. 1979) and are impaired in prepulse inhibition (Bakshi and Geyer 1995; Bakshi et al. 1994), a behavioral deficit thought to model psychotic symptoms. They are also deficient in social interactions, a negative symptom (Sams-Dodd 1995, 1996) and cognition functions such as working memory (Jentsch et al. 1997). Mutant mice which expressed 5% of normal level of NR1 showed behavioral deficits relevant to schizophrenia including hyperactivity, impaired social interaction, and cognitive dysfunction, which can be ameliorated by antipsychotic treatments (Mohn et al. 1999).
Glutamatergic synapses are present on projection cells as well as interneurons. Both could be the target of “glutamatergic hypofunction.” Interestingly, in acutely prepared hippocampal slices, GABAergic interneurons were tenfold more sensitive to NMDA receptor inhibitors than were pyramidal neurons (Grunze et al. 1996). Therefore, GABAergic interneurons should be more vulnerable than pyramidal cells to glutamatergic hypofunction. Hypoactivity of GABAergic neurons would result in impaired inhibition of projection cells and thus cognitive deficits. When the essential subunit of NMDA receptor NR1 was selectively eliminated in parvalbumin (PV)-positive interneurons, mutant mice are impaired in spatial working memory, but their spatial open field exploratory activity and their social activity are normal (Korotkova et al. 2010). Interestingly, when NR1 is ablated in about 50% of cortical interneurons during postnatal development, mutant mice exhibit novelty-induced hyperlocomotion and are impaired in mating and nest building (Belforte et al. 2010). These observations suggest that NMDA receptors in different types of interneurons could have distinct functions. Metabotropic glutamate receptors have also been implicated in schizophrenia. Pretreatment with LY354740, a selective agonist for metabotropicglutamate 2/3 (mGlu2/3) receptors, attenuated the disruptive effects of PCP on locomotion, stereotypy, working memory, and cortical glutamate efflux (Moghaddam and Adams 1998). These results suggest that mGlu2/3 receptor agonists have antipsychotic properties and may provide a new alternative for the treatment of schizophrenia.
2.2 The GABAergic Pathway
Dysfunctions of GABA transmission have also been implicated in the processes leading to psychosis (Keverne 1999; Lacroix et al. 2000). Psychotic symptoms in schizophrenia have been found to be correlated with reduced GABAergic inhibition in the medial temporal region (Busatto et al. 1997). GABAergic interneurons, representing about 20–30% of neocortical neurons, are a population that is extremely heterogeneous, varying in morphology, expression of markers, laminar distribution, and electrophysiological properties (Ascoli et al. 2008; Markram et al. 2004). Embedded in the network of principal cells, they innervate different domains of these neurons. For example, basket cells target the somata and proximal dendrites, chandelier cells form axoaxonic synapses on the axon initial segments. Somatostatin (SOM)-positive or Martinotti interneurons innervate distal dendrites and presumably regulate other inputs of principle cells. Thus, it is generally believed that GABAergic interneurons play a critical role in controlling cell excitability, spike timing, synchrony, and oscillatory activity in the mammalian central nervous system (McBain and Kauer 2009). Albeit fewer in number than principal cells, a single GABAergic neuron can innervate multiple principle cells and thus could potentially alter the activity of thousands of downstream neurons.
In situ hybridization studies demonstrated overall reduced levels of the 67-kDa isoform of glutamic acid decarboxylase (GAD67), the primary enzyme of GABA synthesis, in the PFC area 9 of the left hemisphere of schizophrenic brains (Akbarian et al. 1995). Similar results were obtained in a better controlled study of PFC area 9 of the right hemisphere (Volk et al. 2000). The reduction in GAD67 expression may not be due to antipsychotic medications because long-term treatment with haloperidol did not affect GAD67 mRNA expression in the PFC of monkeys (Volk et al. 2000). Moreover, the activity of GAD was significantly reduced in nucleus accumbens, amygdala, hippocampus, and putamen from schizophrenic postmortem brains (Bird et al. 1977). In agreement, GABA release from synaptosomes of schizophrenic brains was decreased (Sherman et al. 1991a, b). These results suggest that decreased GAD67 mRNA expression in the association regions of the neocortex may be a frequent feature of schizophrenia. Moreover, the binding of [3H]nipecotic acid, a ligand for labeling GABA uptake sites, was reported to be reduced in schizophrenic brains (Reynolds et al. 1990; Simpson et al. 1989). In addition, also the mRNA and protein levels of GAT1 (GABA membrane transporter 1), a protein responsible for reuptake of released GABA into nerve terminals, are reduced in the DLPFC of subjects with schizophrenia (Lewis et al. 1999; Volk et al. 2001).
Early studies reported a loss of small neurons in cortical layer II (Benes et al. 1991). However, subsequent studies failed to see a significant reduction of GAD67-positive neurons (Akbarian et al. 1995; Volk et al. 2000). Similarly, parvalbumin (PV)-positive interneurons were found to be reduced (Beasley and Reynolds 1997) or unchanged (Woo et al. 1997) in DLPFC in schizophrenia. Nevertheless, evidence appeared to be compelling that GABAergic function is reduced in the DLPFC of schizophrenic patients. Maybe as a compensatory mechanism, expression of GABAA receptor in superficial layers of the cortex of schizophrenic brains was increased (Benes et al. 1992; Hanada et al. 1987).
Intriguingly, GABAergic alternation in schizophrenia appears to be interneuron type specific. GAD67 expression is normal in 70% of GABAergic interneurons in the DLPFC but reduced or undetectable in the remaining 30% GABAergic neurons (Akbarian et al. 1995; Volk et al. 2000). The affected interneurons express PV, whereas those expressing calretinin appeared to be normal (Hashimoto et al. 2003). PV-positive neurons include basket cells that form perisomatic synapses onto pyramidal neurons and chandelier cells that form characteristic linear arrays of terminals (termed cartridges) on the axon initial segments of pyramidal neurons. GAT1 levels appear to be selectively reduced in chandelier axon cartridges in the DLFC of schizophrenic patients (Woo et al. 1998). On the other hand, GABAA receptors are upregulated on the postsynaptic membranes facing the axon initial segments, probably to compensate deficient GABAergic transmission (Volk et al. 2002).
Reduced GABA signaling from chandelier cells to pyramidal neurons could contribute to the pathophysiology of working memory dysfunction. Networks of PV-positive GABA neurons, formed by both chemical and electrical synapses, give rise to oscillatory activity in the gamma band range, the synchronized firing of a neuronal population at 30–80 Hz (Whittington et al. 2011). Thus, decreased inhibitory GABA transmission in schizophrenic patients might contribute to psychotic symptoms in schizophrenia. Consistent with this hypothesis, disinhibition of the ventral hippocampus by the GABAA antagonist picrotoxin would result in similar psychosis-related behavioral disturbances such as hyperactivity and decreased PPI (Bast et al. 2001).
2.3 The Cholinergic Pathway
The association of cholinergic pathways with schizophrenia was as ancient as the illness was diagnosed. Schizophrenic patients are often heavy smokers (Lohr and Flynn 1992), and acetylcholine-induced convulsion and atropine-induced coma were used to treat schizophrenia (Forrer and Miller 1958). Substantial evidence has accumulated over the years that suggests the involvement of dysfunction, mostly hypofunction, of cholinergic transmission in schizophrenia (Neubauer et al. 1975; Tandon et al. 1989). Acetylcholine modulates transmission of various neurotransmitters including glutamate, GABA, dopamine, and serotonin. Postmortem studies of brains of schizophrenic patients were ambiguous about protein levels and activity of choline acetyltransferase (ChAT), the enzyme crucially involved in the synthesis of acetylcholine, and AChE, the enzyme that degrades acetylcholine. Protein or activity levels were reported as increased, decreased, or unchanged. A more recent study suggested decreased levels of ChAT mRNA and a decreased number of ChAT-positive cells in striatum, particularly in the ventral striatum (Holt et al. 1999, 2005).
Acetylcholine acts by stimulating two types of receptors in the brain: nicotinic and muscarinic receptors. For neuronal nicotinic receptors, there are nine α and three β subunits; the predominant subtypes are the homomeric α7 and heteromeric α4 β2 subtypes (Paterson and Nordberg 2000). There are five types of muscarinic receptors (M1–5), each encoded by an individual gene. A region of chromosome 15, 15q13-14, that contains the α7 AChR subunit gene has been associated with schizophrenia, and SNPs have been described in the promoter region of the α7 subunit gene (Freedman et al. 1997). Studies using postmortem tissue suggest a decreased density of the α7 nicotinic subtype in the brains of schizophrenics (Freedman et al. 1995; Kucinski et al. 2010; Marutle et al. 2001). However, α7 AChR null mutant mice are normal in prepulse inhibition, water maze test, and fear conditioning except for increased anxiety in the open field test (Paylor et al. 1998). Animal studies demonstrate that α7-specific agonists can ameliorate positive and negative symptoms, improve learning and memory (water maze and Y maze), and attentional deficits (auditory gating) (Thomsen et al. 2010; Tregellas et al. 2011). In patients with schizophrenia, α7 agonists appeared to have procognitive effects (Thomsen et al. 2010). These observations suggest that this receptor subtype may be responsible for the inheritance of a pathophysiological aspect of the illness.
As mentioned above, many schizophrenic patients are extremely heavy nicotine users, even in comparison with other psychiatric patients (de Leon et al. 1995; Hamera et al. 1995). α7 subunit mRNA and protein levels are lower in schizophrenic nonsmokers compared to control nonsmokers and are brought to control levels in schizophrenic smokers (Mexal et al. 2010). Intriguingly, several types of sensory processing deficits, including auditory sensory processing and eye-tracking abnormalities, could be normalized by nicotine, delivered as gum, or by smoking (Adler et al. 1993; Olincy et al. 1998). These observations suggest that schizophrenic patients may smoke to self-medicate endogenous behavioral deficits (Goff et al. 1992).
Initial investigations with quinuclidinyl benzilate (QNB), an antagonist that binds to all five subtypes of muscarinic receptors, were inconsistent on levels of muscarinic receptors in brains of schizophrenic patients. Ligand-binding studies with pirenzepine, an M1-specific antagonist, revealed consistently decreased levels in the DLPFC tissues from subjects with schizophrenia (Scarr et al. 2009). A reduction of pirenzepine binding may be schizophrenia-specific because it was not observed in patients with bipolar disorder or major depression (Zavitsanou et al. 2004). In primates, M1 muscarinic receptors are located postsynaptically in noncholinergic asymmetric and cholinergic symmetric synapses in cortical layers III and V/VI (Mrzljak et al. 1993). They may modulate the cholinergic input from the basal forebrain and intrinsic cortical cholinergic activity (Zhang et al. 2006). M1 mutant mice were normal in hippocampal learning and memory (Miyakawa et al. 2001; Shinoe et al. 2005) but were impaired in behavioral tasks requiring interactions between the hippocampus and cortex (Anagnostaras et al. 2003).
2.4 The Dopaminergic Pathway
The original dopamine hypothesis of schizophrenia, proposed over 40 years ago, associates hyperactivity of dopamine transmission with schizophrenia. It was based on effective antipsychotic drugs that appear to act by blocking dopamine D2 receptors and their antipsychotic potency as usually positively correlated with their D2 antagonistic activity (van Rossum 1966). Drugs which inhibit the reuptake of dopamine such as amphetamine can induce schizophrenia-like psychosis in nonpsychotic subjects (Angrist and Gershon 1970; Bell 1973; Gardner and Connell 1972) and exacerbate psychotic symptoms in schizophrenic patients (Laruelle et al. 1999; Lieberman et al. 1987). It was then believed that schizophrenia is associated with hyperactivity of subcortical mesolimbic D2 pathways in the brain. In support of this notion, positron emission tomography studies indicate that schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations (Breier et al. 1997; Laruelle et al. 1996). Striatal dopamine overactivity was observed in patients with “at risk mental states” (ARMS) that might eventually lead to the outbreak of psychosis (Howes et al. 2006).
D2-dependent antipsychotics are effective for positive symptoms but not negative symptoms and cognitive deficits in schizophrenic patients. These functions are mainly controlled by the neocortex where the density of D2 receptors is several times lower than that of D1 receptors (De Keyser et al. 1988; Hall et al. 1994). D1 receptor–mediated signaling regulates the critical patterns of sustained neuronal firing in the DLPFC during working memory tasks (Sawaguchi 2001; Williams and Goldman-Rakic 1995) and has been shown to be critical for cognitive functions subserved by the DLPFC, such as executive cognition and working memory (Sawaguchi and Goldman-Rakic 1991, 1994). Recent postmortem and imaging studies have suggested that the mesocortical dopaminergic projection to the PFC may be hypoactive (Toda and Abi-Dargham 2007). Dopaminergic axons from mesocortical regions were reduced in the DLPFC of schizophrenic patients (Akil et al. 1999). Probably to compensate for the reduced dopaminergic input, D1 receptor binding in the DLPFC was increased in in vivo imaging studies of drug-free and drug-naive schizophrenia subjects (Abi-Dargham et al. 2002). In some case, the D1 receptor binding was decreased in schizophrenic patients (Okubo et al. 1997). In summary, the D1 upregulation does not actually contribute to the impairment of working memory as D1 receptor antagonist worsen cognitive deficits in schizophrenia (Abi-Dargham and Moore 2003).
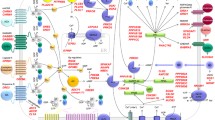
3 Functions of Schizophrenia Susceptibility Genes in Synapse Formation and Transmission
Many of the schizophrenia susceptibility genes have been implicated in neural development. In addition, recent evidence suggests that they may also regulate neurotransmission and synaptic plasticity. A comprehensive overview about the synaptic function of various schizophrenia susceptibility genes is given below.
3.1 D2 DR
Brain imaging studies have found an increase in the density and occupancy of D2 receptors in the striatum of schizophrenic patients (Abi-Dargham et al. 1998; Abi-Dargham et al. 2000; Wong et al. 1986). Also, several studies suggest that at least in a subpopulation of patients the observed increase in D2 receptor binding may be genetically determined (Hirvonen et al. 2004, 2005; Lawford et al. 2005; Zvara et al. 2005). D2 receptors are localized at the postsynaptic membrane of medium spiny neurons in the striatum (Gerfen 1992). In the PFC, where the expression levels of dopamine transporters are low (Sesack et al. 1998), the D2 receptor is localized at dopaminergic terminals to control the reuptake and the release of dopamine (Usiello et al. 2000) and at GABAergic terminals to control the release of GABA (Tseng and O’Donnell 2004). These D2 receptors are thought to fine-tune the firing of pyramidal neurons. Consistent with a major function of D2R as autoreceptors, the ability of dopamine to inhibit the firing of neurons in the midbrain or to inhibit the dopamine release in striatal projection areas is lost in D2R KO mice (Mercuri et al. 1997; Rouge-Pont et al. 2002). However, no in vivo genetic studies clarified the functions of D2 receptor in GABAergic interneurons. Overexpression of D2 receptor in medium spiny neurons in the striatum causes impairments in cognitive processes in the transgenic mice (Kellendonk et al. 2006). The transgenic mice are also impaired in incentive motivation that relates to negative symptoms. Interestingly, the cognitive, but not motivational, deficits persisted long after D2 receptor expression was switched off, suggesting that transient expression during prenatal development was sufficient to cause cognitive deficits in adulthood.
3.2 DISC1
The disrupted in schizophrenia (DISC) gene locus was first identified as a risk factor for major mental illness through study of a large Scottish family in which a balanced translocation between chromosomes 1 and 11 cosegregates with schizophrenia, bipolar disorder, and recurrent major depression (Millar et al. 2000; St Clair et al. 1990). This translocation directly disrupts the DISC1 protein and leads to a C-terminal truncated mutation of DISC1 (Millar et al. 2000). In addition to the translocation, several putative pathogenic mutations have been identified through sequencing DISC1 exons in patients (Song et al. 2008). DISC1 seems to serve as a scaffolding protein interacting with many proteins ranging from transcription factors, phosphodiesterases, and proteins implicated in cytoskeletal and centrosomal organization (Kamiya et al. 2008; Millar et al. 2003, 2005; Miyoshi et al. 2003; Morris et al. 2003; Ozeki et al. 2003). Consistent with this idea, studies in cell culture as well as in Drosophila and mice suggest that DISC1 may be involved in neuronal migration, positioning, differentiation, and neurite extension (Duan et al. 2007; Kamiya et al. 2005). DISC1 is expressed at the postsynaptic membrane of asymmetric synapses in human neocortex (Kirkpatrick et al. 2006). Mutant mice were generated to carry a 25-bp deletion in exon 6 of the Disc1 gene, which express a truncated DISC1 protein mimicking the mutant DISC1 found in the Scottish family (Kvajo et al. 2008). These mice exhibit fewer synaptic spines in the dentate gyrus, deficits in short-term plasticity at CA3/CA1 synapses, and impaired working memory (Kvajo et al. 2008). Depletion of DISC1 in newborn neurons in adult mice causes their mispositioning and accelerated formation of dendritic spines and synapses. DISC1-deficient newborn neurons also exhibit enhanced excitability (Duan et al. 2007).
3.3 DTNBP1/Dysbindin
Both linkage and association studies have implicated dystrobrevin-binding protein 1 (Dysbindin or DTNBP1) as a promising susceptibility gene for schizophrenia (Kirov et al. 2004; Schwab et al. 2003; Straub et al. 1995, 2002; Tang et al. 2003). mRNA or protein levels of dysbindin were decreased in prefrontal cortex (PFC) and hippocampus (Talbot et al. 2004; Tang et al. 2009; Weickert et al. 2004, 2008) from schizophrenic patients. Dysbindin is a member of a protein complex, known as biogenesis of lysosome-related organelle complex 1 (BLOC-1). This complex is involved in vesicle trafficking and dendritic branching (Ghiani et al. 2010). In cultured neurons, increase and suppression of dysbindin expression can promote and inhibit glutamate release, respectively (Numakawa et al. 2004). The Sandy mice, which lack dysbindin protein owing to a deletion in the gene Dtnbp1 (encoding dysbindin) (Li et al. 2003), have a decreased rate of vesicle release, a correlated decrease in vesicle pool size, and an increased thickness of the postsynaptic density (Chen et al. 2008). In Sandy mice, deep-layer pyramidal neurons in the PFC showed reduced miniature and evoked EPSCs, and impaired paired-pulse facilitation, suggesting that dysbindin may regulate excitatory transmission in the PFC possibly by a presynaptic mechanism (Jentsch et al. 2009). Decreased levels of dysbindin are associated with reduction in NMDA-evoked currents in PFC pyramidal neurons and in NR1 expression (Karlsgodt et al. 2011). The Sandy mice showed mild deficit in spatial working memory (Jentsch et al. 2009), which appears to correlate with levels of NR1 expression (Karlsgodt et al. 2011).
3.4 NRG1 and ErbB4
Several linkage studies in independent populations have identified neuregulin 1 (NRG1) and its receptor ErbB4 as susceptibility genes of schizophrenia (Nicodemus et al. 2006; Norton et al. 2006; Stefansson et al. 2002, 2003; Yang et al. 2003). NRG1 isoforms (types I and IV) and the ErbB4 isoform (JMa, CYT1) are expressed at higher levels in the PFC and hippocampus of schizophrenic patients (Hashimoto et al. 2004; Law et al. 2007; Law et al. 2006; Silberberg et al. 2006). Another group reported a marked increase in NRG1-induced ErbB4 activation in the prefrontal cortex in schizophrenia, while the total level of NRG1 and ErbB4 did not alter (Hahn et al. 2006). NRG1 is a family of EGF domain–containing trophic factors that acts by activating ErbB tyrosine kinases (Mei and Xiong 2008). In vitro studies suggest that NRG1-ErbB4 signaling may regulate neuronal migration and gene expression of NMDA and GABA receptors (Mei and Xiong 2008). However, these notions were challenged by studies of mutant mice (Barros et al. 2009; Brinkmann et al. 2008; Chen et al. 2010a; Gajendran et al. 2009).
ErbB4 in rodents is enriched in GABAergic interneurons (Fazzari et al. 2010; Huang et al. 2000; Lai and Lemke 1991; Vullhorst et al. 2009; Yau et al. 2003). During development, NRG1-ErbB4 appears to play a role in the formation of excitatory synapses on GABAergic interneurons and inhibitory synapses on projection cells (Fazzari et al. 2010; Ting et al. 2011). Both NRG1 and ErbB4 are expressed in adult brain. Acute treatment of hippocampal slices with soluble NRG1 suppresses the induction of long-term potentiation (LTP) (Huang et al. 2000). Evidence suggests that this effect is mediated by enhanced GABAergic transmission. We have recently demonstrated that NRG1 acts to promote GABA release and thus control the firing of pyramidal neurons and suppresses long-term potentiation (LTP) (Chen et al. 2010b; Huang et al. 2000; Wen et al. 2010; Woo et al. 2007). Ablation of ErbB4 in parvalbumin-positive interneurons causes schizophrenia-relevant phenotypes in mutant mice including hyperactivity, impaired prepulse inhibition, and working memory deficits (Wen et al. 2010).
In addition to inhibitory neurons, ErbB4 is highly expressed in midbrain dopaminergic neurons in rodents, monkeys, and humans (Abe et al. 2009; Steiner et al. 1999; Zheng et al. 2009). NRG1 has been shown to promote dopamine release in the striatum, hippocampus, and medial prefrontal cortex (Kato et al. 2010; Kwon et al. 2008; Yurek et al. 2004). In vitro studies suggest that NRG1 enhances the survival of dopaminergic neurons (Zhang et al. 2004). However, mutant mice where ErbB4 is ablated in the entire brain showed normal structure of the substantial nigra pars compacta and no deficits in motor performance, suggesting that ErbB4 is not required for the development or survival of dopaminergic neurons (Thuret et al. 2004). It will be interesting to generate dopaminergic neuron–specific ErbB4 mutant mice to determine whether NRG1-ErbB4 signaling is important for neurotransmission at dopaminergic synapses.
It is controversial whether NRG1 regulates excitatory synapse formation in pyramidal neurons and glutamatergic transmission. Overexpression of ErbB4 and suppression of its expression by ErbB4 shRNA promoted or inhibited the formation of glutamatergic synapses in pyramidal neurons of neonatal hippocampal slices (Li et al. 2007), suggesting a potential role in excitatory synapse formation. However, when ErbB4 is ablated specifically in CaMKII-positive neurons, it had no effect on basal glutamatergic transmission (Chen et al. 2010b). Acute treatment of soluble NRG1 did not alter paired-pulse facilitation (PPF) (Huang et al. 2000; Iyengar and Mott 2008), suggesting no effects of NRG1 on glutamate release. However, NRG1 mutant mice showed altered PPF and short-term plasticity (Bjarnadottir et al. 2007). Treatment with NRG1 decreased NMDAR-mediated excitatory postsynaptic currents in PFC slices and reduced whole-cell NMDAR currents in acutely isolated PFC pyramidal neurons by elevating intracellular Ca2+ and stimulating ERK activity (Gu et al. 2005). In hippocampal slices, however, NRG1 appeared to have little effect on NMDAR- or AMPAR-mediated basic transmission (Chen et al. 2010b). In human postmortem hippocampal tissues, NRG1 could attenuate ligand-induced phosphorylation of NMDA receptors and its association with signaling partners (Hahn et al. 2006).
NRG1 regulates the expression of the α7 nicotinic acetylcholine receptors (nAChRs) (Liu et al. 2001; Sandrock et al. 1997; Usdin and Fischbach 1986; Yang et al. 1998). Consistent with these reports, decreased α7 nAChR mRNA and protein in schizophrenic patients is associated with the genetic variation of NRG1 (Mathew et al. 2007). Recent studies of NRG1 mutant mice indicate that type III NRG1 regulates the axonal targeting of α7 nAChR and is required for the enhancement of hippocampal transmission by nicotine (Hancock et al. 2008; Zhong et al. 2008).
3.5 Future Directions
It is clear that synaptic transmission and plasticity are disrupted in schizophrenia. The disruption could be caused by problems that occurred during neural development and/or after brain wiring is complete. Interestingly, Rett syndrome–like neurological deficits of MeCP2 mutant mice can be reversed in adult stage (Guy et al. 2007). It would be important to determine whether this occurs to mutant mice of schizophrenia candidate genes, which would require the reversible transgenic or knockout strategies. Tet-Off system is commonly used to overexpress individual genes which can be reversed by doxycycline (Mayford et al. 1996). Tamoxifen-inducible Cre mice were generated to reactivate the genes by removing the loxP-STOP-loxP cassette (Guy et al. 2007; Hayashi and McMahon 2002). Another important question is to demonstrate the deficit in neural circuitry in schizophrenia. For example, recent studies showed impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia which has the microdeletion on the human chromosome 22 (Sigurdsson et al. 2010). More recent paper reported that the efficacy of ventral hippocampus input to the nucleus accumbens is reduced in the type III NRG1 heterozygotes mutant mice (Nason et al. 2011). The third question to be addressed is how the dysfunction of different types of GABAergic interneurons contributes to the schizophrenia. Optogenetics, a new emerging technique which enables the activation or inactivation of different types of neurons with spatial and temporal control (Boyden et al. 2005; Gradinaru et al. 2009; Petreanu et al. 2009), is obviously of great advantage to address this question. Recent study demonstrated the critical roles of parvalbumin-positive interneurons in gamma-frequency synchronization in vivo using optogenetics (Sohal et al. 2009). Finally, how can we test the hypothesis that a synaptic defect is responsible for schizophrenia in humans? A direct way would be to study synaptic behavior in the brains of affected individuals, but this can not yet be done in the intact human brain. A possible alternative route involves the production of induced pluripotent stem cells (Takahashi et al. 2007; Yu et al. 2007) from adult cells derived from schizophrenic patients and then inducing these iPS cells to form neurons and synapses. The neuronal culture is also potentially useful in screening the individual antischizophrenia drugs.
References
Abe, Y., Namba, H., Zheng, Y., & Nawa, H. (2009). In situ hybridization reveals developmental regulation of ErbB1-4 mRNA expression in mouse midbrain: Implication of ErbB receptors for dopaminergic neurons. Neuroscience, 161, 95–110.
Abi-Dargham, A., Gil, R., Krystal, J., Baldwin, R. M., Seibyl, J. P., Bowers, M., van Dyck, C. H., Charney, D. S., Innis, R. B., & Laruelle, M. (1998). Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. The American Journal of Psychiatry, 155, 761–767.
Abi-Dargham, A., Mawlawi, O., Lombardo, I., Gil, R., Martinez, D., Huang, Y., Hwang, D. R., Keilp, J., Kochan, L., Van Heertum, R., et al. (2002). Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience, 22, 3708–3719.
Abi-Dargham, A., & Moore, H. (2003). Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. The Neuroscientist, 9, 404–416.
Abi-Dargham, A., Rodenhiser, J., Printz, D., Zea-Ponce, Y., Gil, R., Kegeles, L. S., Weiss, R., Cooper, T. B., Mann, J. J., Van Heertum, R. L., et al. (2000). Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 97, 8104–8109.
Adler, L. E., Hoffer, L. D., Wiser, A., & Freedman, R. (1993). Normalization of auditory physiology by cigarette smoking in schizophrenic patients. The American Journal of Psychiatry, 150, 1856–1861.
Adler, C. M., Malhotra, A. K., Elman, I., Goldberg, T., Egan, M., Pickar, D., & Breier, A. (1999). Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. The American Journal of Psychiatry, 156, 1646–1649.
Akbarian, S., Kim, J. J., Potkin, S. G., Hagman, J. O., Tafazzoli, A., Bunney, W. E., Jr., & Jones, E. G. (1995). Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry, 52, 258–266.
Akbarian, S., Sucher, N. J., Bradley, D., Tafazzoli, A., Trinh, D., Hetrick, W. P., Potkin, S. G., Sandman, C. A., Bunney, W. E., Jr., & Jones, E. G. (1996). Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. Journal of Neuroscience, 16, 19–30.
Akil, M., Pierri, J. N., Whitehead, R. E., Edgar, C. L., Mohila, C., Sampson, A. R., & Lewis, D. A. (1999). Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. The American Journal of Psychiatry, 156, 1580–1589.
Anagnostaras, S. G., Murphy, G. G., Hamilton, S. E., Mitchell, S. L., Rahnama, N. P., Nathanson, N. M., & Silva, A. J. (2003). Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nature Neuroscience, 6, 51–58.
Angrist, B. M., & Gershon, S. (1970). The phenomenology of experimentally induced amphetamine psychosis–preliminary observations. Biological Psychiatry, 2, 95–107.
Anis, N. A., Berry, S. C., Burton, N. R., & Lodge, D. (1983). The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. British Journal of Pharmacology, 79, 565–575.
Ascoli, G. A., Alonso-Nanclares, L., Anderson, S. A., Barrionuevo, G., Benavides-Piccione, R., Burkhalter, A., Buzsaki, G., Cauli, B., Defelipe, J., Fairen, A., et al. (2008). Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Reviews Neuroscience, 9, 557–568.
Bakshi, V. P., & Geyer, M. A. (1995). Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology, 122, 198–201.
Bakshi, V. P., Swerdlow, N. R., & Geyer, M. A. (1994). Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. Journal of Pharmacology and Experimental Therapeutics, 271, 787–794.
Barros, C. S., Calabrese, B., Chamero, P., Roberts, A. J., Korzus, E., Lloyd, K., Stowers, L., Mayford, M., Halpain, S., & Muller, U. (2009). Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 106, 4507–4512.
Bast, T., Zhang, W. N., & Feldon, J. (2001). Hyperactivity, decreased startle reactivity, and disrupted prepulse inhibition following disinhibition of the rat ventral hippocampus by the GABA(A) receptor antagonist picrotoxin. Psychopharmacology, 156, 225–233.
Beasley, C. L., & Reynolds, G. P. (1997). Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophrenia Research, 24, 349–355.
Belforte, J. E., Zsiros, V., Sklar, E. R., Jiang, Z., Yu, G., Li, Y., Quinlan, E. M., & Nakazawa, K. (2010). Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature Neuroscience, 13, 76–83.
Bell, D. S. (1973). The experimental reproduction of amphetamine psychosis. Archives of General Psychiatry, 29, 35–40.
Benes, F. M., McSparren, J., Bird, E. D., SanGiovanni, J. P., & Vincent, S. L. (1991). Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Archives of General Psychiatry, 48, 996–1001.
Benes, F. M., Vincent, S. L., Alsterberg, G., Bird, E. D., & SanGiovanni, J. P. (1992). Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. Journal of Neuroscience, 12, 924–929.
Bird, E. D., Spokes, E. G., Barnes, J., MacKay, A. V., Iversen, L. L., & Shepherd, M. (1977). Increased brain dopamine and reduced glutamic acid decarboxylase and choline acetyl transferase activity in schizophrenia and related psychoses. Lancet, 2, 1157–1158.
Bjarnadottir, M., Misner, D. L., Haverfield-Gross, S., Bruun, S., Helgason, V. G., Stefansson, H., Sigmundsson, A., Firth, D. R., Nielsen, B., Stefansdottir, R., et al. (2007). Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: Differential synaptic function in NRG1+/– knock-outs compared with wild-type mice. Journal of Neuroscience, 27, 4519–4529.
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., & Deisseroth, K. (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience, 8, 1263–1268.
Breier, A., Su, T. P., Saunders, R., Carson, R. E., Kolachana, B. S., de Bartolomeis, A., Weinberger, D. R., Weisenfeld, N., Malhotra, A. K., Eckelman, W. C., et al. (1997). Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America, 94, 2569–2574.
Brinkmann, B. G., Agarwal, A., Sereda, M. W., Garratt, A. N., Muller, T., Wende, H., Stassart, R. M., Nawaz, S., Humml, C., Velanac, V., et al. (2008). Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron, 59, 581–595.
Busatto, G. F., Pilowsky, L. S., Costa, D. C., Ell, P. J., David, A. S., Lucey, J. V., & Kerwin, R. W. (1997). Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. The American Journal of Psychiatry, 154, 56–63.
Chen, X. W., Feng, Y. Q., Hao, C. J., Guo, X. L., He, X., Zhou, Z. Y., Guo, N., Huang, H. P., Xiong, W., Zheng, H., et al. (2008). DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. The Journal of Cell Biology, 181, 791–801.
Chen, Y., Hancock, M. L., Role, L. W., & Talmage, D. A. (2010a). Intramembranous valine linked to schizophrenia is required for neuregulin 1 regulation of the morphological development of cortical neurons. Journal of Neuroscience, 30, 9199–9208.
Chen, Y. J., Zhang, M., Yin, D. M., Wen, L., Ting, A., Wang, P., Lu, Y. S., Zhu, X. H., Li, S. J., Wu, C. Y., et al. (2010b). ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America, 107, 21818–21823.
De Keyser, J., Claeys, A., De Backer, J. P., Ebinger, G., Roels, F., & Vauquelin, G. (1988). Autoradiographic localization of D1 and D2 dopamine receptors in the human brain. Neuroscience Letters, 91, 142–147.
de Leon, J., Dadvand, M., Canuso, C., White, A. O., Stanilla, J. K., & Simpson, G. M. (1995). Schizophrenia and smoking: An epidemiological survey in a state hospital. The American Journal of Psychiatry, 152, 453–455.
Dracheva, S., Marras, S. A., Elhakem, S. L., Kramer, F. R., Davis, K. L., & Haroutunian, V. (2001). N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. The American Journal of Psychiatry, 158, 1400–1410.
Duan, X., Chang, J. H., Ge, S., Faulkner, R. L., Kim, J. Y., Kitabatake, Y., Liu, X. B., Yang, C. H., Jordan, J. D., Ma, D. K., et al. (2007). Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell, 130, 1146–1158.
Eastwood, S. L. (2004). The synaptic pathology of schizophrenia: Is aberrant neurodevelopment and plasticity to blame? International Review of Neurobiology, 59, 47–72.
Fauman, B., Aldinger, G., Fauman, M., & Rosen, P. (1976). Psychiatric sequelae of phencyclidine abuse. Clinical Toxicology, 9, 529–538.
Faustman, W. O., Bardgett, M., Faull, K. F., Pfefferbaum, A., & Csernansky, J. G. (1999). Cerebrospinal fluid glutamate inversely correlates with positive symptom severity in unmedicated male schizophrenic/schizoaffective patients. Biological Psychiatry, 45, 68–75.
Fazzari, P., Paternain, A. V., Valiente, M., Pla, R., Lujan, R., Lloyd, K., Lerma, J., Marin, O., & Rico, B. (2010). Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature, 464, 1376–1380.
Forrer, G. R., & Miller, J. J. (1958). Atropine coma: A somatic therapy in psychiatry. The American Journal of Psychiatry, 115, 455–458.
Freedman, R., Coon, H., Myles-Worsley, M., Orr-Urtreger, A., Olincy, A., Davis, A., Polymeropoulos, M., Holik, J., Hopkins, J., Hoff, M., et al. (1997). Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences of the United States of America, 94, 587–592.
Freedman, R., Hall, M., Adler, L. E., & Leonard, S. (1995). Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biological Psychiatry, 38, 22–33.
Gajendran, N., Kapfhammer, J. P., Lain, E., Canepari, M., Vogt, K., Wisden, W., & Brenner, H. R. (2009). Neuregulin signaling is dispensable for NMDA- and GABA(A)-receptor expression in the cerebellum in vivo. Journal of Neuroscience, 29, 2404–2413.
Gardner, R., & Connell, P. H. (1972). Amphetamine and other non-opioid drug users attending a special drug dependence clinic. British Medical Journal, 2, 322–325.
Garey, L. J., Ong, W. Y., Patel, T. S., Kanani, M., Davis, A., Mortimer, A. M., Barnes, T. R., & Hirsch, S. R. (1998). Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry, 65, 446–453.
Gerfen, C. R. (1992). The neostriatal mosaic: Multiple levels of compartmental organization. Trends in Neurosciences, 15, 133–139.
Ghiani, C. A., Starcevic, M., Rodriguez-Fernandez, I. A., Nazarian, R., Cheli, V. T., Chan, L. N., Malvar, J. S., de Vellis, J., Sabatti, C., & Dell’Angelica, E. C. (2010). The dysbindin-containing complex (BLOC-1) in brain: Developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Molecular Psychiatry, 15, 204–215.
Glantz, L. A., & Lewis, D. A. (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry, 57, 65–73.
Goff, D. C., Henderson, D. C., & Amico, E. (1992). Cigarette smoking in schizophrenia: Relationship to psychopathology and medication side effects. The American Journal of Psychiatry, 149, 1189–1194.
Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M., & Deisseroth, K. (2009). Optical deconstruction of parkinsonian neural circuitry. Science, 324, 354–359.
Grunze, H. C., Rainnie, D. G., Hasselmo, M. E., Barkai, E., Hearn, E. F., McCarley, R. W., & Greene, R. W. (1996). NMDA-dependent modulation of CA1 local circuit inhibition. Journal of Neuroscience, 16, 2034–2043.
Gu, Z., Jiang, Q., Fu, A. K., Ip, N. Y., & Yan, Z. (2005). Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. Journal of Neuroscience, 25, 4974–4984.
Guy, J., Gan, J., Selfridge, J., Cobb, S., & Bird, A. (2007). Reversal of neurological defects in a mouse model of Rett syndrome. Science, 315, 1143–1147.
Hahn, C. G., Wang, H. Y., Cho, D. S., Talbot, K., Gur, R. E., Berrettini, W. H., Bakshi, K., Kamins, J., Borgmann-Winter, K. E., Siegel, S. J., et al. (2006). Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature Medicine, 12, 824–828.
Hakak, Y., Walker, J. R., Li, C., Wong, W. H., Davis, K. L., Buxbaum, J. D., Haroutunian, V., & Fienberg, A. A. (2001). Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 98, 4746–4751.
Hall, H., Sedvall, G., Magnusson, O., Kopp, J., Halldin, C., & Farde, L. (1994). Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacol, 11, 245–256.
Hamera, E., Schneider, J. K., & Deviney, S. (1995). Alcohol, cannabis, nicotine, and caffeine use and symptom distress in schizophrenia. The Journal of Nervous and Mental Disease, 183, 559–565.
Hanada, S., Mita, T., Nishino, N., & Tanaka, C. (1987). [3H]muscimol binding sites increased in autopsied brains of chronic schizophrenics. Life Sciences, 40, 259–266.
Hancock, M. L., Canetta, S. E., Role, L. W., & Talmage, D. A. (2008). Presynaptic type III neuregulin1-ErbB signaling targets {alpha}7 nicotinic acetylcholine receptors to axons. The Journal of Cell Biology, 181, 511–521.
Hashimoto, R., Straub, R. E., Weickert, C. S., Hyde, T. M., Kleinman, J. E., & Weinberger, D. R. (2004). Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Molecular Psychiatry, 9, 299–307.
Hashimoto, T., Volk, D. W., Eggan, S. M., Mirnics, K., Pierri, J. N., Sun, Z., Sampson, A. R., & Lewis, D. A. (2003). Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience, 23, 6315–6326.
Hayashi, S., & McMahon, A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Developmental Biology, 244, 305–318.
Hirvonen, M., Laakso, A., Nagren, K., Rinne, J. O., Pohjalainen, T., & Hietala, J. (2004). C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Molecular Psychiatry, 9, 1060–1061.
Hirvonen, J., van Erp, T. G., Huttunen, J., Aalto, S., Nagren, K., Huttunen, M., Lonnqvist, J., Kaprio, J., Hietala, J., & Cannon, T. D. (2005). Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Archives of General Psychiatry, 62, 371–378.
Holt, D. J., Bachus, S. E., Hyde, T. M., Wittie, M., Herman, M. M., Vangel, M., Saper, C. B., & Kleinman, J. E. (2005). Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: An in situ hybridization study. Biological Psychiatry, 58, 408–416.
Holt, D. J., Herman, M. M., Hyde, T. M., Kleinman, J. E., Sinton, C. M., German, D. C., Hersh, L. B., Graybiel, A. M., & Saper, C. B. (1999). Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience, 94, 21–31.
Howes, O. D., Smith, S., Gaughran, F. P., Amiel, S. A., Murray, R. M., & Pilowsky, L. S. (2006). The relationship between prolactin levels and glucose homeostasis in antipsychotic-treated schizophrenic patients. Journal of Clinical Psychopharmacology, 26, 629–631.
Huang, Y. Z., Won, S., Ali, D. W., Wang, Q., Tanowitz, M., Du, Q. S., Pelkey, K. A., Yang, D. J., Xiong, W. C., Salter, M. W., et al. (2000). Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron, 26, 443–455.
Iyengar, S. S., & Mott, D. D. (2008). Neuregulin blocks synaptic strengthening after epileptiform activity in the rat hippocampus. Brain Research, 1208, 67–73.
Jentsch, J. D., Tran, A., Le, D., Youngren, K. D., & Roth, R. H. (1997). Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacol, 17, 92–99.
Jentsch, J. D., Trantham-Davidson, H., Jairl, C., Tinsley, M., Cannon, T. D., & Lavin, A. (2009). Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacol, 34, 2601–2608.
Kamiya, A., Kubo, K., Tomoda, T., Takaki, M., Youn, R., Ozeki, Y., Sawamura, N., Park, U., Kudo, C., Okawa, M., et al. (2005). A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nature Cell Biology, 7, 1167–1178.
Kamiya, A., Tan, P. L., Kubo, K., Engelhard, C., Ishizuka, K., Kubo, A., Tsukita, S., Pulver, A. E., Nakajima, K., Cascella, N. G., et al. (2008). Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: A candidate for psychiatric illnesses. Archives of General Psychiatry, 65, 996–1006.
Karlsgodt, K. H., Robleto, K., Trantham-Davidson, H., Jairl, C., Cannon, T. D., Lavin, A., & Jentsch, J. D. (2011). Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biological Psychiatry, 69, 28–34.
Kato, T., Abe, Y., Sotoyama, H., Kakita, A., Kominami, R., Hirokawa, S., Ozaki, M., Takahashi, H., & Nawa, H. (2010). Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: Implication in neurodevelopmental hypothesis for schizophrenia. Molecular Psychiatry, 16, 307–320.
Kellendonk, C., Simpson, E. H., Polan, H. J., Malleret, G., Vronskaya, S., Winiger, V., Moore, H., & Kandel, E. R. (2006). Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron, 49, 603–615.
Keverne, E. B. (1999). GABA-ergic neurons and the neurobiology of schizophrenia and other psychoses. Brain Research Bulletin, 48, 467–473.
Kim, J. S., Kornhuber, H. H., Schmid-Burgk, W., & Holzmuller, B. (1980). Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neuroscience Letters, 20, 379–382.
Kirkpatrick, B., Xu, L., Cascella, N., Ozeki, Y., Sawa, A., & Roberts, R. C. (2006). DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. The Journal of Comparative Neurology, 497, 436–450.
Kirov, G., Ivanov, D., Williams, N. M., Preece, A., Nikolov, I., Milev, R., Koleva, S., Dimitrova, A., Toncheva, D., O’Donovan, M. C., et al. (2004). Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biological Psychiatry, 55, 971–975.
Korotkova, T., Fuchs, E. C., Ponomarenko, A., von Engelhardt, J., & Monyer, H. (2010). NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron, 68, 557–569.
Kristiansen, L. V., Beneyto, M., Haroutunian, V., & Meador-Woodruff, J. H. (2006). Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Molecular Psychiatry, 11, 705.
Krystal, J. H., Karper, L. P., Seibyl, J. P., Freeman, G. K., Delaney, R., Bremner, J. D., Heninger, G. R., Bowers, M. B., Jr., & Charney, D. S. (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry, 51, 199–214.
Kucinski, A. J., Stachowiak, M. K., Wersinger, S. R., Lippiello, P. M., & Bencherif, M. (2010). Alpha7 neuronal nicotinic receptors as targets for novel therapies to treat multiple domains of schizophrenia. Current Pharmaceutical Biotechnology, 12, 437–448.
Kvajo, M., McKellar, H., Arguello, P. A., Drew, L. J., Moore, H., MacDermott, A. B., Karayiorgou, M., & Gogos, J. A. (2008). A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proceedings of the National Academy of Sciences of the United States of America, 105, 7076–7081.
Kwon, O. B., Paredes, D., Gonzalez, C. M., Neddens, J., Hernandez, L., Vullhorst, D., & Buonanno, A. (2008). Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proceedings of the National Academy of Sciences of the United States of America, 105, 15587–15592.
Lacroix, L., Spinelli, S., Broersen, L. M., & Feldon, J. (2000). Blockade of latent inhibition following pharmacological increase or decrease of GABA(A) transmission. Pharmacology, Biochemistry, and Behavior, 66, 893–901.
Lahti, A. C., Koffel, B., LaPorte, D., & Tamminga, C. A. (1995). Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacol, 13, 9–19.
Lahti, A. C., Weiler, M. A., Tamara Michaelidis, B. A., Parwani, A., & Tamminga, C. A. (2001). Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacol, 25, 455–467.
Lai, C., & Lemke, G. (1991). An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron, 6, 691–704.
Laruelle, M., Abi-Dargham, A., Gil, R., Kegeles, L., & Innis, R. (1999). Increased dopamine transmission in schizophrenia: Relationship to illness phases. Biological Psychiatry, 46, 56–72.
Laruelle, M., Abi-Dargham, A., van Dyck, C. H., Gil, R., D’Souza, C. D., Erdos, J., McCance, E., Rosenblatt, W., Fingado, C., Zoghbi, S. S., et al. (1996). Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America, 93, 9235–9240.
Law, A. J., Kleinman, J. E., Weinberger, D. R., & Weickert, C. S. (2007). Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Human Molecular Genetics, 16, 129–141.
Law, A. J., Lipska, B. K., Weickert, C. S., Hyde, T. M., Straub, R. E., Hashimoto, R., Harrison, P. J., Kleinman, J. E., & Weinberger, D. R. (2006). Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proceedings of the National Academy of Sciences of the United States of America, 103, 6747–6752.
Lawford, B. R., Young, R. M., Swagell, C. D., Barnes, M., Burton, S. C., Ward, W. K., Heslop, K. R., Shadforth, S., van Daal, A., & Morris, C. P. (2005). The C/C genotype of the C957T polymorphism of the dopamine D2 receptor is associated with schizophrenia. Schizophrenia Research, 73, 31–37.
Lewis, D. A., Pierri, J. N., Volk, D. W., Melchitzky, D. S., & Woo, T. U. (1999). Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biological Psychiatry, 46, 616–626.
Li, B., Woo, R. S., Mei, L., & Malinow, R. (2007). The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron, 54, 583–597.
Li, W., Zhang, Q., Oiso, N., Novak, E. K., Gautam, R., O’Brien, E. P., Tinsley, C. L., Blake, D. J., Spritz, R. A., Copeland, N. G., et al. (2003). Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nature Genetics, 35, 84–89.
Lieberman, J. A., Kane, J. M., & Alvir, J. (1987). Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology, 91, 415–433.
Liu, Y., Ford, B., Mann, M. A., & Fischbach, G. D. (2001). Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. Journal of Neuroscience, 21, 5660–5669.
Lohr, J. B., & Flynn, K. (1992). Smoking and schizophrenia. Schizophrenia Research, 8, 93–102.
Luby, E. D., Cohen, B. D., Rosenbaum, G., Gottlieb, J. S., & Kelley, R. (1959). Study of a new schizophrenomimetic drug: Sernyl. American Medical Association: Archives of Neurological Psychiatry, 81, 363–369.
Malhotra, A. K., Pinals, D. A., Adler, C. M., Elman, I., Clifton, A., Pickar, D., & Breier, A. (1997). Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology, 17, 141–150.
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., & Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience, 5, 793–807.
Marutle, A., Zhang, X., Court, J., Piggott, M., Johnson, M., Perry, R., Perry, E., & Nordberg, A. (2001). Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. Journal of Chemical Neuroanatomy, 22, 115–126.
Mathew, S. V., Law, A. J., Lipska, B. K., Davila-Garcia, M. I., Zamora, E. D., Mitkus, S. N., Vakkalanka, R., Straub, R. E., Weinberger, D. R., Kleinman, J. E., et al. (2007). Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Human Molecular Genetics, 16, 2921–2932.
Mayford, M., Bach, M. E., Huang, Y. Y., Wang, L., Hawkins, R. D., & Kandel, E. R. (1996). Control of memory formation through regulated expression of a CaMKII transgene. Science, 274, 1678–1683.
McBain, C. J., & Kauer, J. A. (2009). Presynaptic plasticity: Targeted control of inhibitory networks. Current Opinion in Neurobiology, 19, 254–262.
McCullumsmith, R. E., Clinton, S. M., & Meador-Woodruff, J. H. (2004). Schizophrenia as a disorder of neuroplasticity. International Review of Neurobiology, 59, 19–45.
Mei, L., & Xiong, W. C. (2008). Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nature Reviews Neuroscience, 9, 437–452.
Mercuri, N. B., Saiardi, A., Bonci, A., Picetti, R., Calabresi, P., Bernardi, G., & Borrelli, E. (1997). Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience, 79, 323–327.
Mexal, S., Berger, R., Logel, J., Ross, R. G., Freedman, R., & Leonard, S. (2010). Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. Journal of Molecular Neuroscience, 40, 185–195.
Millar, J. K., Christie, S., & Porteous, D. J. (2003). Yeast two-hybrid screens implicate DISC1 in brain development and function. Biochemical and Biophysical Research Communications, 311, 1019–1025.
Millar, J. K., Pickard, B. S., Mackie, S., James, R., Christie, S., Buchanan, S. R., Malloy, M. P., Chubb, J. E., Huston, E., Baillie, G. S., et al. (2005). DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science, 310, 1187–1191.
Millar, J. K., Wilson-Annan, J. C., Anderson, S., Christie, S., Taylor, M. S., Semple, C. A., Devon, R. S., St Clair, D. M., Muir, W. J., Blackwood, D. H., et al. (2000). Disruption of two novel genes by a translocation co-segregating with schizophrenia. Human Molecular Genetics, 9, 1415–1423.
Mirnics, K., Middleton, F. A., Lewis, D. A., & Levitt, P. (2001). Analysis of complex brain disorders with gene expression microarrays: Schizophrenia as a disease of the synapse. Trends in Neurosciences, 24, 479–486.
Mirnics, K., Middleton, F. A., Marquez, A., Lewis, D. A., & Levitt, P. (2000). Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron, 28, 53–67.
Miyakawa, T., Yamada, M., Duttaroy, A., & Wess, J. (2001). Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. Journal of Neuroscience, 21, 5239–5250.
Miyoshi, K., Honda, A., Baba, K., Taniguchi, M., Oono, K., Fujita, T., Kuroda, S., Katayama, T., & Tohyama, M. (2003). Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Molecular Psychiatry, 8, 685–694.
Moghaddam, B., & Adams, B. W. (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science, 281, 1349–1352.
Mohn, A. R., Gainetdinov, R. R., Caron, M. G., & Koller, B. H. (1999). Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell, 98, 427–436.
Morris, J. A., Kandpal, G., Ma, L., & Austin, C. P. (2003). DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: Regulation and loss of interaction with mutation. Human Molecular Genetics, 12, 1591–1608.
Mrzljak, L., Levey, A. I., & Goldman-Rakic, P. S. (1993). Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: Morphological evidence for cholinergic modulation of excitatory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America, 90, 5194–5198.
Nabeshima, T., Yamada, K., Yamaguchi, K., Hiramatsu, M., Furukawa, H., & Kameyama, T. (1983). Effect of lesions in the striatum, nucleus accumbens and medial raphe on phencyclidine-induced stereotyped behaviors and hyperactivity in rats. European Journal of Pharmacology, 91, 455–462.
Nason, M. W., Jr., Adhikari, A., Bozinoski, M., Gordon, J. A., & Role, L. W. (2011). Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology, 36, 488–496.
Neubauer, H., Adams, M., & Redfern, P. (1975). The role of central cholinergic mechanisms in schizophrenia. Medical Hypotheses, 1, 32–34.
Nicodemus, K. K., Luna, A., Vakkalanka, R., Goldberg, T., Egan, M., Straub, R. E., & Weinberger, D. R. (2006). Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Molecular Psychiatry, 11, 1062–1065.
Nikolaus, S., Antke, C., & Muller, H. W. (2009). In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behavioural Brain Research, 204, 1–31.
Norton, N., Moskvina, V., Morris, D. W., Bray, N. J., Zammit, S., Williams, N. M., Williams, H. J., Preece, A. C., Dwyer, S., Wilkinson, J. C., et al. (2006). Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 141B, 96–101.
Numakawa, T., Yagasaki, Y., Ishimoto, T., Okada, T., Suzuki, T., Iwata, N., Ozaki, N., Taguchi, T., Tatsumi, M., Kamijima, K., et al. (2004). Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Human Molecular Genetics, 13, 2699–2708.
Okubo, Y., Suhara, T., Suzuki, K., Kobayashi, K., Inoue, O., Terasaki, O., Someya, Y., Sassa, T., Sudo, Y., Matsushima, E., et al. (1997). Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature, 385, 634–636.
Olincy, A., Ross, R. G., Young, D. A., Roath, M., & Freedman, R. (1998). Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacol, 18, 175–185.
Ozeki, Y., Tomoda, T., Kleiderlein, J., Kamiya, A., Bord, L., Fujii, K., Okawa, M., Yamada, N., Hatten, M. E., Snyder, S. H., et al. (2003). Disrupted-in-Schizophrenia-1 (DISC-1): Mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proceedings of the National Academy of Sciences of the United States of America, 100, 289–294.
Paterson, D., & Nordberg, A. (2000). Neuronal nicotinic receptors in the human brain. Progress in Neurobiology, 61, 75–111.
Paylor, R., Nguyen, M., Crawley, J. N., Patrick, J., Beaudet, A., & Orr-Urtreger, A. (1998). Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: A behavioral characterization of Acra7-deficient mice. Learning and Memory, 5, 302–316.
Petreanu, L., Mao, T., Sternson, S. M., & Svoboda, K. (2009). The subcellular organization of neocortical excitatory connections. Nature, 457, 1142–1145.
Reynolds, G. P., Czudek, C., & Andrews, H. B. (1990). Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biological Psychiatry, 27, 1038–1044.
Rouge-Pont, F., Usiello, A., Benoit-Marand, M., Gonon, F., Piazza, P. V., & Borrelli, E. (2002). Changes in extracellular dopamine induced by morphine and cocaine: Crucial control by D2 receptors. Journal of Neuroscience, 22, 3293–3301.
Sams-Dodd, F. (1995). Automation of the social interaction test by a video-tracking system: Behavioural effects of repeated phencyclidine treatment. Journal of Neuroscience Methods, 59, 157–167.
Sams-Dodd, F. (1996). Phencyclidine-induced stereotyped behaviour and social isolation in rats: A possible animal model of schizophrenia. Behavioural Pharmacology, 7, 3–23.
Sandrock, A. W., Jr., Dryer, S. E., Rosen, K. M., Gozani, S. N., Kramer, R., Theill, L. E., & Fischbach, G. D. (1997). Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science, 276, 599–603.
Sawaguchi, T. (2001). The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neuroscience Research, 41, 115–128.
Sawaguchi, T., & Goldman-Rakic, P. S. (1991). D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science, 251, 947–950.
Sawaguchi, T., & Goldman-Rakic, P. S. (1994). The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology, 71, 515–528.
Scarr, E., Cowie, T. F., Kanellakis, S., Sundram, S., Pantelis, C., & Dean, B. (2009). Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Molecular Psychiatry, 14, 1017–1023.
Schwab, S. G., Knapp, M., Mondabon, S., Hallmayer, J., Borrmann-Hassenbach, M., Albus, M., Lerer, B., Rietschel, M., Trixler, M., Maier, W., et al. (2003). Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. American Journal of Human Genetics, 72, 185–190.
Selemon, L. D., & Goldman-Rakic, P. S. (1999). The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biological Psychiatry, 45, 17–25.
Sesack, S. R., Hawrylak, V. A., Matus, C., Guido, M. A., & Levey, A. I. (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. Journal of Neuroscience, 18, 2697–2708.
Sherman, A. D., Davidson, A. T., Baruah, S., Hegwood, T. S., & Waziri, R. (1991a). Evidence of glutamatergic deficiency in schizophrenia. Neuroscience Letters, 121, 77–80.
Sherman, A. D., Hegwood, T. S., Baruah, S., & Waziri, R. (1991b). Deficient NMDA-mediated glutamate release from synaptosomes of schizophrenics. Biological Psychiatry, 30, 1191–1198.
Shinoe, T., Matsui, M., Taketo, M. M., & Manabe, T. (2005). Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. Journal of Neuroscience, 25, 11194–11200.
Sigurdsson, T., Stark, K. L., Karayiorgou, M., Gogos, J. A., & Gordon, J. A. (2010). Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature, 464, 763–767.
Silberberg, G., Darvasi, A., Pinkas-Kramarski, R., & Navon, R. (2006). The involvement of ErbB4 with schizophrenia: Association and expression studies. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 141B, 142–148.
Simpson, M. D., Slater, P., Deakin, J. F., Royston, M. C., & Skan, W. J. (1989). Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neuroscience Letters, 107, 211–215.
Sohal, V. S., Zhang, F., Yizhar, O., & Deisseroth, K. (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature, 459, 698–702.
Song, W., Li, W., Feng, J., Heston, L. L., Scaringe, W. A., & Sommer, S. S. (2008). Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochemical and Biophysical Research Communications, 367, 700–706.
St Clair, D., Blackwood, D., Muir, W., Carothers, A., Walker, M., Spowart, G., Gosden, C., & Evans, H. J. (1990). Association within a family of a balanced autosomal translocation with major mental illness. Lancet, 336, 13–16.
Stefansson, H., Sarginson, J., Kong, A., Yates, P., Steinthorsdottir, V., Gudfinnsson, E., Gunnarsdottir, S., Walker, N., Petursson, H., Crombie, C., et al. (2003). Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. American Journal of Human Genetics, 72, 83–87.
Stefansson, H., Sigurdsson, E., Steinthorsdottir, V., Bjornsdottir, S., Sigmundsson, T., Ghosh, S., Brynjolfsson, J., Gunnarsdottir, S., Ivarsson, O., Chou, T. T., et al. (2002). Neuregulin 1 and susceptibility to schizophrenia. American Journal of Human Genetics, 71, 877–892.
Steiner, H., Blum, M., Kitai, S. T., & Fedi, P. (1999). Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Experimental Neurology, 159, 494–503.
Stephan, K. E., Baldeweg, T., & Friston, K. J. (2006). Synaptic plasticity and dysconnection in schizophrenia. Biological Psychiatry, 59, 929–939.
Straub, R. E., MacLean, C. J., Ma, Y., Webb, B. T., Myakishev, M. V., Harris-Kerr, C., Wormley, B., Sadek, H., Kadambi, B., O’Neill, F. A., et al. (2002). Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Molecular Psychiatry, 7, 542–559.
Straub, R. E., MacLean, C. J., O’Neill, F. A., Burke, J., Murphy, B., Duke, F., Shinkwin, R., Webb, B. T., Zhang, J., Walsh, D., et al. (1995). A potential vulnerability locus for schizophrenia on chromosome 6p24-22: Evidence for genetic heterogeneity. Nature Genetics, 11, 287–293.
Sturgeon, R. D., Fessler, R. G., & Meltzer, H. Y. (1979). Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. European Journal of Pharmacology, 59, 169–179.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872.
Talbot, K., Eidem, W. L., Tinsley, C. L., Benson, M. A., Thompson, E. W., Smith, R. J., Hahn, C. G., Siegel, S. J., Trojanowski, J. Q., Gur, R. E., et al. (2004). Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. The Journal of Clinical Investigation, 113, 1353–1363.
Tandon, R., Dutchak, D., & Greden, J. F. (1989). Cholinergic syndrome following anticholinergic withdrawal in a schizophrenic patient abusing marijuana. The British Journal of Psychiatry, 154, 712–714.
Tang, J., LeGros, R. P., Louneva, N., Yeh, L., Cohen, J. W., Hahn, C. G., Blake, D. J., Arnold, S. E., & Talbot, K. (2009). Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Human Molecular Genetics, 18, 3851–3863.
Tang, J. X., Zhou, J., Fan, J. B., Li, X. W., Shi, Y. Y., Gu, N. F., Feng, G. Y., Xing, Y. L., Shi, J. G., & He, L. (2003). Family-based association study of DTNBP1 in 6p22.3 and schizophrenia. Molecular Psychiatry, 8, 717–718.
Thomsen, M. S., Hansen, H. H., Timmerman, D. B., & Mikkelsen, J. D. (2010). Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: From animal models to human pathophysiology. Current Pharmaceutical Design, 16, 323–343.
Thuret, S., Alavian, K. N., Gassmann, M., Lloyd, C. K., Smits, S. M., Smidt, M. P., Klein, R., Dyck, R. H., & Simon, H. H. (2004). The neuregulin receptor, ErbB4, is not required for normal development and adult maintenance of the substantia nigra pars compacta. Journal of Neurochemistry, 91, 1302–1311.
Ting, A. K., Chen, Y., Wen, L., Yin, D. M., Shen, C., Tao, Y., Liu, X., Xiong, W. C., & Mei, L. (2011). Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. Journal of Neuroscience, 31, 15–25.
Toda, M., & Abi-Dargham, A. (2007). Dopamine hypothesis of schizophrenia: Making sense of it all. Current Psychiatry Reports, 9, 329–336.
Tregellas, J. R., Tanabe, J., Rojas, D. C., Shatti, S., Olincy, A., Johnson, L., Martin, L. F., Soti, F., Kem, W. R., Leonard, S., et al. (2011). Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biological Psychiatry, 69, 7–11.
Tsai, G., Passani, L. A., Slusher, B. S., Carter, R., Baer, L., Kleinman, J. E., & Coyle, J. T. (1995). Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Archives of General Psychiatry, 52, 829–836.
Tseng, K. Y., & O’Donnell, P. (2004). Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. Journal of Neuroscience, 24, 5131–5139.
Usdin, T. B., & Fischbach, G. D. (1986). Purification and characterization of a polypeptide from chick brain that promotes the accumulation of acetylcholine receptors in chick myotubes. The Journal of Cell Biology, 103, 493–507.
Usiello, A., Baik, J. H., Rouge-Pont, F., Picetti, R., Dierich, A., LeMeur, M., Piazza, P. V., & Borrelli, E. (2000). Distinct functions of the two isoforms of dopamine D2 receptors. Nature, 408, 199–203.
van Rossum, J. M. (1966). The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Archives Internationales de Pharmacodynamie et de Thérapie, 160, 492–494.
Volk, D. W., Austin, M. C., Pierri, J. N., Sampson, A. R., & Lewis, D. A. (2000). Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry, 57, 237–245.
Volk, D., Austin, M., Pierri, J., Sampson, A., & Lewis, D. (2001). GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: Decreased expression in a subset of neurons. The American Journal of Psychiatry, 158, 256–265.
Volk, D. W., Pierri, J. N., Fritschy, J. M., Auh, S., Sampson, A. R., & Lewis, D. A. (2002). Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cerebral Cortex, 12, 1063–1070.
Vullhorst, D., Neddens, J., Karavanova, I., Tricoire, L., Petralia, R. S., McBain, C. J., & Buonanno, A. (2009). Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. Journal of Neuroscience, 29, 12255–12264.
Weickert, C. S., Rothmond, D. A., Hyde, T. M., Kleinman, J. E., & Straub, R. E. (2008). Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophrenia Research, 98, 105–110.
Weickert, C. S., Straub, R. E., McClintock, B. W., Matsumoto, M., Hashimoto, R., Hyde, T. M., Herman, M. M., Weinberger, D. R., & Kleinman, J. E. (2004). Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Archives of General Psychiatry, 61, 544–555.
Wen, L., Lu, Y. S., Zhu, X. H., Li, X. M., Woo, R. S., Chen, Y. J., Yin, D. M., Lai, C., Terry, A. V., Jr., Vazdarjanova, A., et al. (2010). Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proceedings of the National Academy of Sciences of the United States of America, 107, 1211–1216.
Whittington, M. A., Cunningham, M. O., LeBeau, F. E., Racca, C., & Traub, R. D. (2011). Multiple origins of the cortical gamma rhythm. Developmental Neurobiology, 71, 92–106.
Williams, G. V., & Goldman-Rakic, P. S. (1995). Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature, 376, 572–575.
Wong, D. F., Wagner, H. N., Jr., Tune, L. E., Dannals, R. F., Pearlson, G. D., Links, J. M., Tamminga, C. A., Broussolle, E. P., Ravert, H. T., Wilson, A. A., et al. (1986). Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science, 234, 1558–1563.
Woo, R. S., Li, X. M., Tao, Y., Carpenter-Hyland, E., Huang, Y. Z., Weber, J., Neiswender, H., Dong, X. P., Wu, J., Gassmann, M., et al. (2007). Neuregulin-1 enhances depolarization-induced GABA release. Neuron, 54, 599–610.
Woo, T. U., Miller, J. L., & Lewis, D. A. (1997). Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. The American Journal of Psychiatry, 154, 1013–1015.
Woo, T. U., Whitehead, R. E., Melchitzky, D. S., & Lewis, D. A. (1998). A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 95, 5341–5346.
Yang, X., Kuo, Y., Devay, P., Yu, C., & Role, L. (1998). A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron, 20, 255–270.
Yang, J. Z., Si, T. M., Ruan, Y., Ling, Y. S., Han, Y. H., Wang, X. L., Zhou, M., Zhang, H. Y., Kong, Q. M., Liu, C., et al. (2003). Association study of neuregulin 1 gene with schizophrenia. Molecular Psychiatry, 8, 706–709.
Yau, H. J., Wang, H. F., Lai, C., & Liu, F. C. (2003). Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: Preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cerebral Cortex, 13, 252–264.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920.
Yurek, D. M., Zhang, L., Fletcher-Turner, A., & Seroogy, K. B. (2004). Supranigral injection of neuregulin1-beta induces striatal dopamine overflow. Brain Research, 1028, 116–119.
Zavitsanou, K., Katsifis, A., Mattner, F., & Huang, X. F. (2004). Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacol, 29, 619–625.
Zhang, L., Fletcher-Turner, A., Marchionni, M. A., Apparsundaram, S., Lundgren, K. H., Yurek, D. M., & Seroogy, K. B. (2004). Neurotrophic and neuroprotective effects of the neuregulin glial growth factor-2 on dopaminergic neurons in rat primary midbrain cultures. Journal of Neurochemistry, 91, 1358–1368.
Zhang, Y., Hamilton, S. E., Nathanson, N. M., & Yan, J. (2006). Decreased input-specific plasticity of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptors. Cerebral Cortex, 16, 1258–1265.
Zheng, Y., Watakabe, A., Takada, M., Kakita, A., Namba, H., Takahashi, H., Yamamori, T., & Nawa, H. (2009). Expression of ErbB4 in substantia nigra dopamine neurons of monkeys and humans. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33, 701–706.
Zhong, C., Du, C., Hancock, M., Mertz, M., Talmage, D. A., & Role, L. W. (2008). Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. Journal of Neuroscience, 28, 9111–9116.
Zvara, A., Szekeres, G., Janka, Z., Kelemen, J. Z., Cimmer, C., Santha, M., & Puskas, L. G. (2005). Over-expression of dopamine D2 receptor and inwardly rectifying potassium channel genes in drug-naive schizophrenic peripheral blood lymphocytes as potential diagnostic markers. Disease Markers, 21, 61–69.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag/WIen
About this chapter
Cite this chapter
Yin, DM., Chen, YJ., Sathyamurthy, A., Xiong, WC., Mei, L. (2012). Synaptic Dysfunction in Schizophrenia. In: Kreutz, M., Sala, C. (eds) Synaptic Plasticity. Advances in Experimental Medicine and Biology, vol 970. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0932-8_22
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0932-8_22
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0931-1
Online ISBN: 978-3-7091-0932-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)