Abstract
Objectives: To describe early perihemorrhagic changes after lobar intracerebral hemorrhage (ICH) using multiparametric neuromonitoring [intracranial pressure (ICP), cerebral blood flow (CBF), tissue oxygenation (PbrO2), microdialysis (MD)].
Methods: Seven anaesthetized male swine were examined over 12 h. Four cerebral probes were inserted around the ICH (ICP, MD, CBF and PbrO2). A right frontal autologous arterial ICH (1.5 mL) was induced in all animals.
Results: Initial ICH creation was hampered by using a soft 22-G cannula. A modified injection technique with a 90° bent steel cannula (20 G) allowed for an 87.5% success rate in ICH formation. After induction of ICH, ICP significantly increased from 2 mmHg to 9 mmHg. No significant PbrO2 or CBF reduction occurred during the monitoring period. Consequently, microdialysis did not indicate overall mean deterioration in the hematoma group over time. The indicator of ischemia (extracellular lactate) did not increase significantly during the monitoring period. Individual monitoring episodes demonstrated hypoxic episodes with consecutive metabolic derangement. These effects were reversible by optimizing CPP and FiO2.
Conclusion: We established a reproducible cortical ICH model using multiparametric neuromonitoring. Subtle changes in ICP were observed. No evidence for the existence of a perihemorrhagic ischemic area was found, hypothetically because of the small hematoma size. Individual animals underwent critical PbrO2 and CBF decreases with consecutive metabolic derangement. The effect of larger hematoma volumes should be evaluated with this setup in future studies to study volume-dependent deterioration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The acute changes in the perihemorrhagic zone (PHZ) of spontaneous ICH remain unclear. In theory, depression of perfusion may lead to metabolic changes because of lack of tissue oxygenation. Some previous studies using PbrO2 measurement and microdialysis have shown evidence of perihemorrhagic tissue hypooxygenation or of an increase in extracellular concentrations of glutamate after ICH [1, 2]. Currently no experimental model exists that simultaneously measures intracranial pressure, oxygenation, perfusion and metabolism in the PHZ of ICH. Therefore, we utilized human cerebral multimodal monitoring probes (ICP, PbrO2, CBF and microdialysis) to study the PHZ in a porcine model of lobar ICH.
Methods
Animal Preparation
Animal protocols for the experiments were approved by the institutional animal care and use committee (protocol no.: 35-9185.81/G-60/06). A standardized operative setup was followed as described elsewhere [3]. Seven male swine with an average mean weight of 30.1 ± 1.62 (SD) kg were anesthetized with ketamine (10 mg/kg), midazolam (5 mg/kg) and azaperone (40 mg/kg) administered by intramuscular injection. Animals were orally intubated and mechanically ventilated (FiO2 = 0.3). Anesthesia was maintained using 1.5% isoflurane inhalation. Rectal and brain temperatures (Tr, Tbr) were continuously monitored. Rectal temperature was maintained between 35.5°C and 37°C. After surgical exposure of the right femoral artery, a 4-F catheter was placed for permanent monitoring of mean arterial blood pressure (MAP). A venous line was placed in the right ear vein, and capillary oxygen saturation (SO2) was monitored from the left ear.
Operative Procedure
Five burr holes (diameter: 5 mm) were placed over the right hemisphere. One burr hole was placed parietally through which the ICP probe (Neurovent-P®, Raumedic AG, Helmbrechts, Germany) was placed. Immediately along the coronal suture three burr holes were placed 0.8, 1.2 and 1.6 cm off midline, respectively. Through these burr holes the monitoring probes were inserted next to each other. Medially the thermodiffusion regional CBF probe (Hemedex td-rCBF, Codman®, USA) was followed by the PbrO2 probe (Neurovent®-TO, Raumedic AG, Helmbrechts, Germany) and microdialysis probe (CMA 70, Solna, Sweden) most laterally (Fig. 1). All intraparenchymal devices were introduced and secured transcutaneously prior to insertion into the brain. An equilibration period of 2 h was allowed for the probes to run in and the animals to stabilize. Further frontally, a soft 22-G cannula for induction of the ICH was introduced via the fifth burr hole, which was located 1.5 cm in front of the coronal suture and 0.8 cm lateral to the sagittal suture. In seven subjects (data not shown completely), an autologous arterial hematoma was created following the description of Quereshi et al. and Hemphil et al. [1, 4]. Polyvinyl chloride tubing was attached to a stopcock at the distal end of the spinal needle and to the femoral artery. At the initiation of hematoma formation, autologous blood was introduced into the brain under arterial pressure via this tubing. However, we aimed to target the cortico-subcortical area in order to avoid deep-seated ICH location. The injected hematoma volume was 1.5 mL. Neither the catheters nor the syringes were flushed with heparin. Following first experiences with the described injection method, we adapted the technique and used a 90° bent steel 20-G cannula. After catheter placements and ICH injection, the burr holes were sealed with bone wax and monitoring was conducted for 10 h to complete the monitoring period of 12 h. Postmortem cranial vaults were opened completely, brains were removed, and intraparenchymal catheter locations and ICH size and position were macroscopically inspected (Fig. 2).
Photograph illustrating the catheter positioning in relation to the injection site of the hematoma. Most parietally the ICP probe is introduced. Just in front of the coronal suture three monitoring probes are placed next to each other. Most medial is the CBF probe followed by the PbrO2 probe and microdialysis probe. Frontally, the burr hole site for the hematoma is marked (white arrow)
Monitoring
All relevant physiological parameters, such as mean arterial pressure (MAP), intracranial pressure (ICP), cerebral perfusion pressure (CPP), brain and rectal temperature (Tb, TR), heart rate (HR), oxygen saturation (SO2), PbrO2 and rCBF, were continuously recorded. Microdialysates were perfused with a rate of 0.3 μL/min, and samples were taken every 30 min. The microdialysis vials were collected and analyzed after the experiment was finished. All other parameters were recorded online. Blood-gas analyses were achieved every 2 h. The experiments did not test any therapeutic measure in all but one animal in which an increase of FiO2 and regulation of CPP were allowed.
Statistical Analysis
For statistical analysis we used a standard software package (SPSS 14.0). All data were normally distributed. A paired Student’s t-test was used. The 95% confidence interval was calculated for the mean difference of all parameters. The p < 0.05 level was used to assess significance.
Results
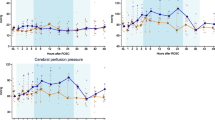
Using the ICH injection technique with the soft cannula, an ICH of intended volume was formed in 7/11 (64%) of animals. The improved technique resulted in an 87.5% success rate. The monitoring results were as follows: ICP increased shortly after hematoma formation and rose until it reached a plateau at 8 mmHg after 6 h of monitoring. This increase was statistically significant (p < 0.001). The early ICP increase was adjoined by an increase in CBF from 27 mL/100 mg/min to 34 mL/100 mg/min after 3 h. After 10 h CBF decreased to baseline values. PbrO2 stayed unchanged for 6 h after the hematoma placement. However, towards the end of the experiment PbrO2 reached 30 mmHg. Metabolic mean values did not show any evidence of parenchymal ischemia as neither extracellular glutamate (not displayed) nor lactate increased significantly compared to baseline measurements. These results are displayed in Table 1. Individual monitoring episodes demonstrated immediate dramatic oxygen decrease and subsequent increase of metabolic indicators of cerebral ischemia. These effects were reversible by increasing the fraction of inhaled oxygen (FiO2) from 30% to 80% and modulation of CPP (Fig. 3).
Original recording episode of an individual animal illustrating the PbrO2, lactate/pyruvate ratio, glutamate and ICP. At 12.00 h the ICH was created. One can appreciate that ICP increased from 8 mmHg to 11 mmHg, and tissue oxygenation dramatically decreased subsequently under the hypoxic threshold (below grey dashed line) of 10 mmHg. As an early indicator of pending ischemia lactate/pyruvate ratio and extracellular glutamate increased up to clearly pathological values. Regulation of CPP from 55 mmHg to 60 mmHg and increase of FiO2 from 30% to 80% (initiation 15.45 h, double lined box) led to reversal of the PbrO2 decrease and the metabolic deterioration. The usefulness of additional monitoring is evident as ICP did not indicate any critical circumstance in this subject
Discussion
The theory that cerebral ischemia contributes to secondary neuronal injury after ICH has been discussed in several publications, but the results of different groups are inconsistent, and there is an ongoing debate about the existence of a “perihemorrhagic penumbra” [1, 2, 5–7]. Experimental projects are often subject to the criticism that results cannot be transferred into human pathologies. For example, assessing CBF with laser Doppler flowmetry in rats is not comparable to online CBF recording using a td-CBF probe in patients. However, multimodal monitoring tools are progressing as useful tools in the setting of various types of stroke [8–11]. Therefore, this study was performed on the basis of a multimodal monitoring approach with cerebral probes used in the clinical setting (ICP, CBF, PbrO2 and microdialysis).
As superficial lobar hematomas are more amenable to effective therapy such as surgical evacuation, we at first investigated experimental ICH in pigs as described. The injection method that we used has been previously described by Qureshi et al. and Hemphil et al. [1, 4]. We adapted this method to the porcine anatomy. The injection technique was modified using a 90° angled 20-G steel cannula, which reliably led to the formation of consistent ICH of intended volume. Using this model, hypoxic episodes were found, but metabolic or histological correlates were not assessed. Others found that glutamate, as an indicator of pending ischemia, significantly increased in the early phase after ICH formation [2]. The authors concluded that the exact role of these amino acids in the pathogenesis of neuronal injury observed in ICH needs to be defined.
From previous publications it was reported that cerebral perfusion may be reduced in tissues surrounding the hematoma. In this context, Belayev et al. performed a combined study of perfusion monitoring by laser Doppler flow and neurobehavioral tests in mice after ICH. A significantly reduced perfusion (35–50%) was sustained over 90 min and went along with substantial neurological deficits in these animals [12]. An MRI study at 7 T in rats additionally showed that cerebral perfusion may be diminished around the hematoma, which correlated to histological cell loss [13]. Similarly, relevant perfusion reductions measured by MRI were seen in 18 patients [14]. However, it remains difficult to establish perihemorrhagic perfusion and structural patterns using artifact-prone tools like MRI. In our previous results we found that there is no relevant ischemic tissue in the immediate surrounding of the hematoma as measured by DWI, although we were able to show that perfusion is reduced on the affected side (5–27%) in comparison to the healthy side [6].
There are limitations to our study. We do not present histological correlates to our findings. However, once more significant perihemorrhagic changes are evident, histopathological studies seem mandatory. Effects on functional recovery cannot be proved as neurological assessments were not performed as the animals were sacrificed at the end of the study.
In conclusion, we examined continuous multiparametric characteristics of small volume ICH in swine in the acute phase. We improved the injection technique of ICH that resulted in an acceptable success rate of hematoma creation of approximately 90%. In the current study only subtle changes in ICP but no changes in CBF, PbrO2 and microdialysis were observed. With the use of larger hematoma volumes, one might be able to elicit perfusion and tissue oxygen changes with subsequent impact on metabolic indices. Future studies should therefore focus on increasing hematoma size and elucidate the effects of contralateral hematomas (to differentiate purely volume-/ICP-dependent effects from ischemic/neurotoxic effects). Our model could also allow studying en vogue but yet unclear phenomena like cortical spreading depolarizations in their role in the pathophysiology of the PHZ in ICH.
Conflict of interest statement We declare that we have no conflict of interest.
References
Hemphill JC 3rd, Morabito D, Farrant M, Manley GT (2005) Brain tissue oxygen monitoring in intracerebral hemorrhage. Neurocrit Care 3:260–270
Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, Guterman LR, Hopkins LN (2003) Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med 31:1482–1489
Orakcioglu B, Sakowitz OW, Neumann J-O, Kentar MM, Unterberg AW, Kiening KL (2010) Evaluation of a novel brain tissue oxygenation probe in an experimental swine model. Neurosurgery 67(6):1716–1722
Qureshi AI, Hanel RA, Kirmani JF, Yahia AM, Hopkins LN (2002) Cerebral blood flow changes associated with intracerebral hemorrhage. Neurosurg Clin N Am 13:355–370
Carhuapoma JR, Wang PY, Beauchamp NJ, Keyl PM, Hanley DF, Barker PB (2000) Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral hemorrhage. Stroke 31:726–732
Orakcioglu B, Becker K, Sakowitz OW, Herweh C, Kohrmann M, Huttner HB, Steiner T, Unterberg A, Schellinger PD (2008) MRI of the perihemorrhagic zone in a rat ICH model: effect of hematoma evacuation. Neurocrit Care 8(3):448–455
Schellinger PD, Fiebach JB, Hoffmann K, Becker K, Orakcioglu B, Kollmar R, Juttler E, Schramm P, Schwab S, Sartor K, Hacke W (2003) Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke 34:1674–1679
Meixensberger J, Jaeger M, Vath A, Dings J, Kunze E, Roosen K (2003) Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry 74:760–764
Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR, Unterberg AW (2001) On-line microdialysis following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 77:141–144
Unterberg AW, Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR (2001) Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 94:740–749
Vajkoczy P, Roth H, Horn P, Lucke T, Thome C, Hubner U, Martin GT, Zappletal C, Klar E, Schilling L, Schmiedek P (2000) Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg 93:265–274
Belayev L, Saul I, Curbelo K, Busto R, Belayev A, Zhang Y, Riyamongkol P, Zhao W, Ginsberg MD (2003) Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke 34:2221–2227
Knight RA, Han Y, Nagaraja TN, Whitton P, Ding J, Chopp M, Seyfried DM (2008) Temporal MRI assessment of intracerebral hemorrhage in rats. Stroke 39:2596–2602
Pascual AM, Lopez-Mut JV, Benlloch V, Chamarro R, Soler J, Lainez MJ (2007) Perfusion-weighted magnetic resonance imaging in acute intracerebral hemorrhage at baseline and during the 1st and 2nd week: a longitudinal study. Cerebrovasc Dis 23:6–13
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag/Wien
About this chapter
Cite this chapter
Orakcioglu, B. et al. (2011). Multiparametric Characterisation of the Perihemorrhagic Zone in a Porcine Model of Lobar ICH. In: Zhang, J., Colohan, A. (eds) Intracerebral Hemorrhage Research. Acta Neurochirurgica Supplementum, vol 111. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0693-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0693-8_4
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0692-1
Online ISBN: 978-3-7091-0693-8
eBook Packages: MedicineMedicine (R0)