Abstract
Objective: Oxidative stress contributes significantly to the development of secondary brain injury after intracerebral hemorrhage (ICH). It has been previously demonstrated that hydrogen gas can decrease oxidative stress by scavenging reactive oxygen species. We hypothesized that hydrogen therapy will reduce brain oxidative stress in mice after ICH and thereby will lead to reduced brain edema and improved neurological outcomes.
Materials and Methods: CD1 male mice (weight 30–35 g) were divided into the following groups: sham, ICH + vehicle (room air), ICH + 1-h hydrogen treatment, and ICH + 2-h hydrogen treatment. ICH was induced by injection of bacterial collagenase into the right basal ganglia. The evaluation of outcomes was done at two time points: 24 and 72 h post-ICH. Brain water content was measured for assessment of brain edema (wet/dry weight method), and three neurological tests were performed pre- and postoperatively.
Results: Collagenase injection was found to induce brain edema and impair functional performance of rats. The hydrogen inhalation reduced these effects acutely (24 h); however it exhibited only a tendency to improvement in the delayed study (72 h).
Conclusions: Our results suggest that hydrogen inhalation exerts an acute brain-protective effect in the mouse ICH model. However, the acute hydrogen therapy alone is not sufficient to improve delayed ICH outcomes in this model.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
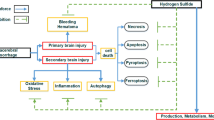
The role of reactive oxygen species (ROS) as mediators of oxidative brain damage after ICH is well accepted. Overproduction of ROS, which cannot be balanced by endogenous antioxidant defenses of brain tissues, results in lipid peroxidation, disruption of the blood-brain barrier (BBB), an increase in brain edema, and neurological deficits. Beneficial effects of antioxidant agents in the ICH model have been recently demonstrated in our laboratory [1]. The inhalation with hydrogen selectively reduced hydroxyl radical and peroxynitrite levels in vitro and exerted an antioxidant effect, reflected by a decreased brain concentration of 4-hydroxynonenal (4-HNE) (the specific marker for lipid peroxidation) and 8OHG (nucleic acid oxidation marker) in a rat MCAO model [2]. To date, no study has assessed the effects of hydrogen gas therapy in the animal model of ICH. In this study, we examined whether hydrogen inhalation provides neuroprotective effects by decreasing brain water content and ameliorating neurological deficits.
Methods
Experimental animals: All procedures and methods for these studies were approved by the Animal Care and Use Committee at Loma Linda University and complied with the Guide for the Care and Use of Laboratory Animals (http://research.llu.edu/forms/appendixb.doc). A total of 30 male CD-1 mice were assigned into the acute (24 h) or delayed (72 h) study. The animals in the acute experiment were divided into sham, ICH treated with vehicle (room air), ICH treated with hydrogen for 1 h, and ICH treated with hydrogen for 2 h. At 24 h after ICH, the animals were tested for neurological deficits and euthanized for brain edema measurements. The animals assigned to the delayed group were partitioned into ICH treated with vehicle and ICH treated with hydrogen for 1 h (since 1-h treatment was effective in the 24-h study). Animals were checked for neurological deficits and euthanized for brain water content measurements at 72 h post-ICH. Since no increase in brain water content was observed after sham surgery, the same group of sham-operated animals was used for the 72-h time point.
Intracerebral hemorrhage induction: We adopted the collagenase-induced intracerebral hemorrhage model in mice, as we described before [3]. Briefly, mice were anesthetized intrapertioneally with a ketamine (100 mg/kg)/xylazine (10 mg/kg) cocktail and positioned prone in a stereotaxic head frame (Stoelting, Wood Dale, IL). An electronic thermostat-controlled warming blanket was used to maintain the core temperature at 37°C. The calvarium was exposed by a midline scalp incision from the nose to the superior nuchal line, and the skin was retracted laterally. With a variable speed drill (Fine Scientific Tools, Foster City, CA), a 1.0-mm burr hole was made 0.9 mm posterior to the bregma and 1.45 mm right-lateral to the midline. A 26-G needle on a Hamilton syringe was inserted with stereotaxic guidance 4.0 mm into the right deep cortex/basal ganglia at a rate 1 mm/min. Collagenase (0.075 units in 0.5 μL saline; Sigma, St Louis, MO) was then infused into the brain at a rate of 0.25 μL/min over 2 min using an automatic infusion pump (Stoelting, Wood Dale, IL). The needle was left in place for an additional 10 min after injection to prevent the possible leakage of collagenase solution. After removal of the needle, the incision was sutured closed, and mice were allowed to recover. Sham operation was performed with needle insertion only.
Brain water content: Mice were euthanized under deep anesthesia. Brains were removed immediately and divided into five parts: ipsilateral and contralateral basal ganglia, ipsilateral and contralateral cortex, and cerebellum. Tissue samples were weighed on an electronic analytical balance. The tissue was then dried at 100°C for 24 h to determine the dry weight. Brain water content (%) was calculated as [(wet weight – dry weight)/wet weight] × 100.
Neurological deficits: Neurological scores were assessed by an independent researcher blinded to the procedure 24 and 72 h after ICH. Three tests were implemented for evaluation of neurological deficits: (1) Modified Garcia test [4]. The Modified Garcia Score is a 21-point sensorimotor assessment system consisting of seven tests with scores of 0–3 for each test (maximum score = 21). These seven tests included: (1) spontaneous activity, (2) side stroking, (3) vibris touch, (4) limb symmetry, (5) climbing, (6) lateral turning, and (7) forelimb walking. Additionally, beam balance and wire hang testing were performed as described before [3]. Both the beam (590 cm in length by 51 cm in width) and wire (550 cm in length by 51 mm in width) were constructed and held in place by two platforms on each side. Mice were put on the center of the beam or wire and allowed to reach the platform. Mice were observed for both their time and behavior until they reached one platform, and scored according to six grades. The test was repeated three times, and an average score was taken [minimum score 0; maximum score (healthy mouse)].
Statistical analysis: Quantitative data are expressed as the mean ± SEM. Statistical significance was verified by analysis of variance (ANOVA) (Dunn’s method) for analysis of acute effect (24-h) and by ANOVA (Tukey test) for analysis of delayed effect (72-h). P < 0.05 was considered statistically significant.
Results
Effect of Inhalation Hydrogen Therapy on Brain Edema
At 24 h after ICH, the water content significantly increased in the ipsilateral basal ganglia of all collagenase-injected animals (Fig. 1a). Hydrogen treatment resulted in a dose-dependent effect where 1-h of inhalation significantly decreased brain water content in hydrogen vs. room-air treated animals, while 2-h of hydrogen inhalation showed no effect. Brain edema progressed to involve bilateral basal ganglia as well as the ipsilateral cortex at 72 h post-ICH (Fig. 1b). At this time point, 1-h of hydrogen inhalation showed only the tendency towards reducing water content in these brain regions (Fig. 1b). Since there was no statistical difference between the brain water content of naïve (non-operated animals) and sham-operated animals at the 24-h time point, we did not expect the formation of brain edema at a later time-point and used the same group of sham-operated animals in both studies.
All animals after collagenase injection had a significant increase of brain water content. One but not 2 h of hydrogen therapy reduced brain edema in the 24-h study group. (a) One hour of hydrogen therapy tended to decrease brain water content in (b) the 72-h group. * Significant difference vs. sham (p < 0.05). #Significant difference vs. sham (p < 0.05). (a) 24 h: sham = 5; (vehicle) = 5; (ICH + Hydrogen 1 h) = 5; (ICH + hydrogen 2 h) = 5. (b) 72 h: sham = 5; (vehicle) = 5; (ICH + hydrogen 1 h)
Effect of Inhalation Hydrogen Therapy on Neurological Outcomes
Neurological deficits were evident in all ICH-animals 24 and 72-h after ICH as tested by the modified Garcia test, wire hanging and beam balance tests. One hour but not 2-h of hydrogen inhalation was able to attenuate these ICH-induced effects in 24-h study (Fig. 2). However, 72-h post-ICH, hydrogen therapy showed only a tendency towards an improvement of neurological deficit. This tendency did not reach statistical significance (Fig. 3).
Discussion
Several studies have shown that antioxidants confer a neuroprotective effect in ICH [5]. Since previous work had demonstrated the antioxidative effects of hydrogen inhalation therapy [2], we postulated that the hydrogen gas treatment will reduce oxidative stress and will protect against brain injury following ICH. In our study we tested the effects of inhalation with hydrogen in the mouse intracerebral hemorrhage model. We used 2.0% of hydrogen since it is known that (1) at such a concentration (less 4% in air), H2 is neither explosive nor harmful; (2) this concentration of hydrogen was most effective in the previous studies [2, 6]. We tested the acute effect (24-h after ICH induction) of hydrogen therapy and compared it to the delayed effect (72-h after ICH). In the acute study, hydrogen inhalation significantly reduced the ICH-induced increase in brain water content and improved the neurological deficit in ICH animals. In the 72-h study, the hydrogen inhalation tended to decrease brain edema; however, the difference between groups did not reach statistical significance. There are several possible explanations for this result. The limited efficacy of hydrogen therapy at 72-h after ICH may relate to the increased severity of inflammatory cell infiltration in the delayed phase after ICH [7]. One of the ROS sources after ICH are peripheral immune cells, which start invading the brain shortly after the hemorrhage and participate in microglial activation. The activated microglia further increase the ROS formation. It has been reported that leukocyte infiltration and microglial activation reached a maximum at 3 days after ICH [8]. Concordantly, Liao et al. observed maximal superoxide production in the peri-hematomal region on day 3 after ICH induction [9]. Another possibility is that hydroxyl radicals, which are the most aggressive reactive oxygen species, overwhelmed the beneficial effect of hydrogen in the delayed phase of hemorrhagic brain injury. Hydroxyl radicals can cause lipid peroxidation, protein oxidation, as well as DNA damage in the cell [10, 12]. These radicals are produced by Fenton chemistry, which requires free iron for the formation of transition metal complexes. Wu et al. demonstrated that ICH upregulated heme oxygenase-1 levels and resulted in iron overload in the brain [11]. The release of iron from the breakdown of hemoglobin occurred during intracerebral hematoma formation. The authors observed a time-depended increase in iron level in the ipsilateral versus contralateral basal ganglia. The difference was insignificant on the first day after ICH induction, however became significant 72 h later [11]. In conclusion, hydrogen therapy can protect the brain against acute injury following ICH. However, further studies are needed to establish the regimen of treatment that would provide lasting protection.
References
Rojas H, Lekic T, Chen W, Jadhav V, Titova E, Martin RD, Tang H, Zhang J (2008) The antioxidant effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir Suppl 105:19–21
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694
Manaenko A, Lekic T, Sozen T, Tsuchiyama R, Zhang JH, Tang J (2009) Effect of gap junction inhibition on intracerebral hemorrhage-induced brain injury in mice. Neurol Res 31:173–178
Garcia JH, Wagner S, Liu KF, Hu XJ (1998) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 29:871–872
Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH (2010) Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma 27(3):627–637
Hayashida K, Sano M, Ohsawa I, Shinmura K Tamaki K, Kimura K, Endo J, Katayama T, Kawamira A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fakuda K (2008) Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 373(1):30–35
Wang J, Rogove AD, Tsirka AE, Tsirka SE (2003) Protective role of tuftsin fragment 1–3 in an animal model of intracerebral hemorrhage. Ann Neurol 54:655–664
Gong C, Hoff JT, Keep RF (2000) Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res 871:57–65
Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, Yang S, Liu P, Xu J, Wang J, Han Z, Han ZC (2009) Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem 24:307–316
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39:44–84
Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G (2003) Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke 34:2964–2969
Chan PH (1996) Role of oxidants in ischemic brain damage. Stroke 27:1124–1129
Acknowledgement
This study is partially supported by NIH NS053407 to J.H. Zhang and NS060936 to J. Tang.
Conflict of interest statement We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag/Wien
About this chapter
Cite this chapter
Manaenko, A., Lekic, T., Ma, Q., Ostrowski, R.P., Zhang, J.H., Tang, J. (2011). Hydrogen Inhalation is Neuroprotective and Improves Functional Outcomes in Mice After Intracerebral Hemorrhage. In: Zhang, J., Colohan, A. (eds) Intracerebral Hemorrhage Research. Acta Neurochirurgica Supplementum, vol 111. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0693-8_30
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0693-8_30
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0692-1
Online ISBN: 978-3-7091-0693-8
eBook Packages: MedicineMedicine (R0)