Abstract
After intracerebral hemorrhage (ICH), hemoglobin (Hb) that is released from erythrocytes within the brain hematoma is highly cytotoxic and leads to severe brain edema and direct neuronal damage. Therefore, neutralization of Hb could represent an important target for reducing the secondary injury after ICH. Haptoglobin (Hp), an endogenous Hb-binding protein in blood plasma, is found in this study to be upregulated in the hematoma-affected brain after ICH. Both in vivo and in vitro studies indicate that Hp upregulation is primarily mediated by oligodendrocytes. Hp acts as a secretory protein capable of neutralizing the cell-free Hb. We also found in an “ICH-like” injury that Hp-KO mice show the most severe brain injury and neurological deficits, whereas Hp-Tg mice are the most resistant to ICH injury, suggesting that a higher Hp level is associated with the increased resistance of animals to hemolytic product-mediated brain injury after ICH. We conclude that brain-derived Hp plays a cytoprotective role after ICH, and Hp may represent a new potential therapeutic target for management of ICH.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Haptoglobin (Hp) is an endogenous hemoglobin (Hb)-binding protein present in blood plasma. In blood, Hp binds cell-free Hb (derived from damaged or senescent erythrocytes) to form highly stable Hb-Hp complexes [1]. This process is capable of shielding and stabilizing the heme iron within the Hb hydrophobic pocket and thereby blocking the pro-oxidative property of Hb [2, 3]. Normally, Hp is produced by hepatocytes and then released to the blood [4, 5] where it binds the cell-free Hb and mediates the fast removal of Hb from circulation [6, 7]. The clearance of the Hp-Hb complexes occurs via circulating blood monocytes and tissue macrophages that express the Hb scavenger receptor CD163 [8–10]. In addition, the uptake of Hp-Hb complexes by hepatocytes plays an important role in iron recycling. Hp indirectly exerts a broad range of anti-inflammatory activities and plays an anti-oxidant role most notably by virtue of its ability to bind free Hb, accelerating the rapid clearance of Hb by monocytes and macrophages.
The Hp reservoir in blood plasma is relatively high, allowing for efficient buffering of the Hb in case of acute hemolytic events. Although the role of Hp in intravascular hemolysis is well accepted, the role of Hp in the brain is essentially unknown [11]. Limited studies suggest the presence of Hp in the neural retina [12] and subarachnoid blood clot [13]; however, its expression profile and role in the brain is not clear.
Following ICH, a large amount of Hb is released into the brain parenchyma after erythrocytes undergo hemolysis within the hematoma [14, 15]. The cell-free Hb is a potent neurotoxin capable of initiating deleterious reactions by causing oxidative damage to lipids, DNA and proteins [16, 17]; caspase activation [18, 19]; blood-brain-barrier (BBB) disruption [14, 15, 20]; and irreversible neuronal damage [21–23]. We postulate that Hp may benefit the brain after ICH assuming Hp can either penetrate to the brain through the disrupted BBB or being locally produced by the brain cells. Here, we review our finding [24] regarding the expression, localization and biological function of Hp in the brain after ICH.
Results
Hp Is Increased in the Brain After ICH: The Role of Oligodendrocytes
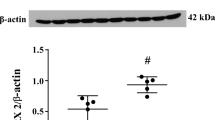
To explore the role of Hp in the brain after ICH, we first tested the expression of Hp protein in the brain using Western blot analysis. In naïve Sprague-Dawley (SD) rats, Hp expression in the brain is very low. However, Hp protein in the brain increases robustly after ICH, which is seen in as soon as 3 h and subsequently increases over time in the first 3 days and remains at higher levels for at least 7 days in the hematoma-affected brain tissues [24]. To verify the origin of brain Hp protein, we measured the Hp mRNA changes in the brain tissue using RT-PCR. We found that Hp mRNA levels share a similar temporal pattern with Hp protein changes, suggesting that Hp is produced locally in the brain. However, we cannot rule out that additional Hp protein may enter the brain through a disrupted BBB.
Next, we studied the spatial distribution of Hp in the brain using immunofluorescence [24]. We found that the Hp signal is strong in the hematoma-affected cortex, corpus callosum and striatum (Fig. 1A, a–c). Using double immunolabeling to identify the specific cell types that express Hp, we found that Hp is primarily co-localized within the MBP-positive oligodendrocytes [24]. Specifically, Hp immunopositive signals localize in the oligodendrocytes’ soma within the grey matter, as well in the fine processes around the myelinated nerve tract in the white matter at the corpus callosum and striatum.
(A) The representative Hp immunofluorescence to show Hp localization in the ICH-affected brain. The SD rats were subjected to M-ICH and analyzed for presence of Hp at 24 h using immunofluorescence (now converted to black and white and inverted). Hp immunopositive cells were detected in cerebral cortex (a), corpus callosum (b) and basal ganglia (c) adjacent to the hematoma. Scale bar = 50 μm. ICH: location of ICH. (B) Photograph of Hp localization in the rat primary neuron-glial co-cultures. The oligodendrocytes are visualized with anti-myelin basic protein antibody (MBP, d). The rat Hp is visualized with sheep anti-Hp (e). A merged image of Hp and MBP is shown in (f). The scale bar = 100 μm. Note: Among all of the brain cell types in the field, only MBP-positive cells are immunopositive for Hp. Hp proteins localize in the soma and the fine processing of MBP-positive oligodendrocytes
To further validate the in vivo findings, we studied Hp expression using a primary neuron-glial co-culture system generated from E-18 rat embryos. We found that Hp protein and mRNA are uniquely expressed by MBP-positive oligodendrocytes (Fig. 1B, d–f). Interestingly, the Hp immunopositive signal is detected throughout the soma and fine processes, which is similar to the Hp localizations detected in the rat brains [24].
Using Hp ELISA, we found that Hp is a secretary protein as it can be released by oligodendrocytes into the culture media [24].
Hp Produced by Oligodendrocytes Is Protective to Neurons and Oligodendrocytes
To establish if the Hp produced and secreted by oligodendrocytes is biologically functional, we employed oligodendrocytes cultured from Hp-KO [25] and Hp-Tg [26] mice brains. As expected, at 20 days in culture, Hp-KO oligodendrocytes have practically no Hp presence in the culture media, whereas Hp-Tg oligodendrocytes release high levels of Hp into the culture media (Fig. 2a, b). We then collected the oligodendrocyte-conditioned media and transferred it to the neuron cultures. Compatibility of the media transfer was also confirmed. Next, we measured the injury to the neurons caused by hemolytic products by subjecting the neurons to an “ICH-like” injury (lysed RBC + hypoxia) and assessing the LDH released into the culture media by the injured neurons. At 24 h after adding lysed RBCs, the neurons in the medium from Hp-overexpressing oligodendrocytes demonstrated significantly less injury as compared to the neurons in the medium from Hp-deficient oligodendrocytes (Fig. 2c, d). This suggests that Hp produced by oligodendrocytes can neutralize the hemolytic product-mediated neurotoxicity. In parallel studies, we found that the Hp-Tg oligodendrocytes, similar to neurons, are much more resistant than the Hp-KO oligodendrocytes to the RBC lysate-mediated damage, suggesting that Hp is cytoprotective not only to neurons, but also to oligodendrocytes, the cells that produce Hp [24].
Hp produced by oligodendrocytes is cytoprotective. (a) A representative photograph of oligodendrocytes-enriched culture (OEC) prepared from mouse brain at 20 days in vitro. The oligodendrocytes are visualized with MBP immunofluorescence. (b) Bar graph of quantifying Hp protein in the OEC culture media prepared from Hp-KO and Hp-Tg mice, as determined using Hp ELISA. *p ≤ 0.05. (c) A representative photograph of mouse primary cortical neuron cultured for 15 days. The neurons are visualized with MAP2 immunofluorescence. (d) Bar graph of LDH showing the injury to neurons in culture in response to “ICH-like” injury (lysed RBC + hypoxia) in presence of media conditioned by OEC from Hp-KO or Hp-Tg mice. The OEC conditioned media was directly transferred into the neuronal culture by replacing 2/3 volume of the neuronal culture medium and incubated for 15 min before “ICH-like” injury. The LDH in culture media was determined at 24 h. The data are displayed as mean ± SEM (n = 3). *p ≤ 0.05
The Levels of Hp Expression Are Associated with Resistance to ICH-Mediated Brain Damage
To further define the relationship between Hp and ICH, we subjected the Hp-KO, Hp-Tg and WT control mice to a modified ICH injury (M-ICH). In this M-ICH injury model, we injected lysed RBCs (not whole blood) into the basal ganglia to better mimic the events associated with blood toxicity following hemolysis in the hematoma. At 7 days after M-ICH, we assessed brain damage by determining the neurological deficits (NDS; a composite score from a battery of behavioral tests, including foot fault, cylinder, postural reflex and corner tests) [27]. As predicted, Hp-KO mice suffer the most severe neurological deficits, whereas Hp-Tg mice are the least susceptible to M-ICH injury (Fig. 3), demonstrating that Hp in the brain is in fact important in protecting the animals from hemolytic product-mediated brain damage after ICH.
Neurological deficit score (NDS) after M-ICH. The Hp-KO, Hp-Tg and WT control mice were subjected to M-ICH, and the neurological deficit was measured at day 7 by a battery of behavioral tests. The data are expressed as mean ± SEM (n = 9) and displayed as percent changes of the NDS in WT mice. *p ≤ 0.05, from all other groups
Conclusion
Hp expression after ICH is increased in the brain, where it is primarily confined to oligodendrocytes, and can be secreted into the extracellular space to play a cytoprotective role against the toxicity of hemolysis products. The temporal and spatial profile of Hp synthesis in the brain appears to be highly strategic for optimal control of hemolysis-mediated cytotoxicity [28, 29]. We propose that Hp could be a potential therapeutic target for ICH.
References
Wada T et al (1970) Autoradiographic study on the site of uptake of the haptoglobin-hemoglobin complex. J Reticuloendothel Soc 8(2):185–193
Zuwala-Jagiello J (2006) Haemoglobin scavenger receptor: function in relation to disease. Acta Biochim Pol 53(2):257–268
Yang F et al (2003) Haptoglobin reduces lung injury associated with exposure to blood. Am J Physiol Lung Cell Mol Physiol 284(2):L402–L409
Hoj L et al (1984) Secretion rates of immunoglobulins, albumin, haptoglobin and complement factors C3 and C4 in the perfused jejunum and ileum of human Salmonella carriers. Acta Pathol Microbiol Immunol Scand C 92(2):129–132
Bowman BH, Kurosky A (1982) Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet 12:189, 453–454
Schaer DJ et al (2006) CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107(1):373–380
Philippidis P et al (2004) Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 94(1):119–126
Ascenzi P et al (2005) Hemoglobin and heme scavenging. IUBMB Life 57(11):749–759
Schaer DJ et al (2005) Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol 74(1):6–10
Schaer DJ, Alayash AI, Buehler PW (2007) Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid Redox Signal 9(7):991–999
D’Armiento J, Dalal SS, Chada K (1997) Tissue, temporal and inducible expression pattern of haptoglobin in mice. Gene 195(1):19–27
Chen W et al (1998) Expression of the protective proteins hemopexin and haptoglobin by cells of the neural retina. Exp Eye Res 67(1):83–93
Borsody M et al (2006) Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology 66(5):634–640
Bhasin RR et al (2002) Experimental intracerebral hemorrhage: effect of lysed erythrocytes on brain edema and blood-brain barrier permeability. Acta Neurochir Suppl 81:249–251
Xi G, Keep RF, Hoff JT (1998) Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg 89(6):991–996
Nakamura T et al (2006) Iron-induced oxidative brain injury after experimental intracerebral hemorrhage. Acta Neurochir Suppl 96:194–198
Nakamura T et al (2005) Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res 1039(1–2):30–36
Regan RF, Panter SS (1993) Neurotoxicity of hemoglobin in cortical cell culture. Neurosci Lett 153(2):219–222
Wang X et al (2002) Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke 33(7):1882–1888
Keep RF et al (2008) Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl 105:73–77
Aronowski J, Hall CE (2005) New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res 27(3):268–279
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5(1):53–63
Koeppen AH, Dickson AC, McEvoy JA (1995) The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci 134(Suppl):102–112
Zhao X et al (2009) Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci 29(50):15819–15827
Lim SK et al (1998) Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92(6):1870–1877
Yang F et al (1993) Characterization of the mouse haptoglobin gene. Genomics 18(2):374–380
Zhao X et al (2007) Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 38(12):3280–3286
Wagner KR, Dwyer BE (2004) Hematoma removal, heme, and heme oxygenase following hemorrhagic stroke. Ann NY Acad Sci 1012:237–251
Wagner KR et al (2003) Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab 23(6):629–652
Acknowledgements
This study was supported in part by NIH/NINDS projects RO1NS060768, R21NS057284 and RO1NS064109. We thank Drs. Franklin G. Berger and Karen Barbour (University of South Carolina, Columbia, SC), who kindly provided us the Hp-Tg and Hp-KO mice.
Conflict of interest statement We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag/Wien
About this chapter
Cite this chapter
Zhao, X. et al. (2011). Cytoprotective Role of Haptoglobin in Brain After Experimental Intracerebral Hemorrhage. In: Zhang, J., Colohan, A. (eds) Intracerebral Hemorrhage Research. Acta Neurochirurgica Supplementum, vol 111. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0693-8_17
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0693-8_17
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0692-1
Online ISBN: 978-3-7091-0693-8
eBook Packages: MedicineMedicine (R0)