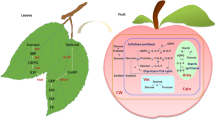
Abstract
Fruit sweetness is one of the most important properties for both fresh and processed tomatoes (Solanum lycopersicum L.). Previously, development of varieties with high sugar content has primarily depended on organoleptic assessments by breeders because of insufficient information regarding the biological mechanism(s) controlling fruit sweetness. However, during the last two decades, research on fruit metabolic physiology and functional genomics has produced substantial progress in tomato. Among sugar-metabolic enzymes, acid invertase rather than sucrose synthase has been known to be involved in regulation of fruit sugar level and its composition. On the other hand, recent studies have revealed that proteins which are not directly related to sugar metabolism such as sucrose transporter, starch biosynthetic enzyme, and vacuolar processing enzyme affect on sugar level and its composition in tomato fruit. In this chapter, those new findings reported by recent studies are introduced, and then the possibility of molecular breeding for the modification of sugar content and composition in tomato fruit will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
Fruit sweetness is one of the most important properties for determining the market value of both fresh and processed tomatoes (Solanum lycopersicum L.). Fruit sweetness is generally represented as the total soluble solids content (TSS, measured as °Brix). In fact, fruit sweetness primarily depends on the soluble sugar content and its composition. The soluble sugar content and composition largely influence not only the organoleptic quality of fruit but also its processing efficiency; fruits with higher soluble sugar content (i.e., less water content) provide economic advantages such as fewer concentration steps and saving transportation and raw material costs (Stark et al. 1996). Therefore, high fruit sugar content is profitable from a commercial point of view and has been one of the major targets of breeding programs, together with yield and disease resistance, in most berry fruit crops, including tomato. However, the development of new fruit varieties with high sugar content has depended primarily on organoleptic assessments by breeders because of insufficient information regarding the biological mechanism(s) controlling fruit sweetness. Over the last two decades, research on fruit metabolic physiology and functional genomics has produced substantial progress for quality breeding in tomato. In this chapter, recent research progress on the regulation mechanisms of sugar accumulation is introduced from a physiological point of view, and the applications of this research to organoleptic quality breeding in tomato are discussed.
Fruit development in tomato is generally classified into three stages (Ho and Hewitt 1986; Ho 1996). In the first stage, the cell number increases as a result of active cell division, which affects the potential mature fruit size. In the second stage, the fruit size enlarges due to rapid cell expansion, and in the third stage, fruit ripening occurs and is associated with ethylene production and an increase in cell respiration. Generally, sugar accumulation in the fruit is thought to proceed by the following steps: (1) active assimilation (mainly sucrose) and water influx into the fruit via the vascular system, (2) sugar metabolism and the biosynthesis of starch as a transient storage form of carbohydrate, and (3) the breakdown of starch and intensive increases in the levels of hexose sugars, such as glucose and fructose (Dinar and Stevens 1981; Schaffer and Petreikov 1997). Steps (1) and (2) occur in the late first and second stages of tomato growth. Rapid sucrose hydrolysis and starch accumulation cause intensive sink strength in the immature fruit (Ho 1996). Step (3) occurs during ripening, which is accompanied by rapid fruit softening and drastic metabolic shifts in the major fruit components, such as organic acids, carotenoids, hexose sugars, and cell wall components (Carrari et al. 2006). Of course, these processes are affected by cultivation management and environmental conditions, such as temperature, rainfall level, humidity, and insolation conditions (Prudent et al. 2011). Our understanding of fruit carbohydrate status, including sugar content and composition, has generally been based on the concept that the futile cycles of sucrose/hexose interchange, which are governed by sucrose synthase, sucrose phosphatase synthase, and invertase, regulate the sink strength and sugar level and composition (Nguyen-Quoc and Foyer 2001). These enzymes are thought to be involved in the intra- and intercellular transport of sugars among vacuoles and the cytosol and apoplasts involved in futile cycles in cooperation with sucrose/hexose transporters. In this chapter, the basic mechanisms regulating sugar level and composition are first described, and several new findings reported by recent studies are introduced. Finally, the possibility of molecular breeding for the modification of sugar content and composition in tomato fruit is discussed.
9.2 Invertase Plays a Key Role in Determining Fruit Sugar Content and Composition
It is now well known that sugars function as signal molecules as well as carbon energy sources in the various stages of the plant life cycle. The amount and balance of sucrose and its cleavage products are particularly important in regulating both metabolism and development. There are two notable enzymes involved in the cleavage of disaccharide sucrose to monosaccharides: sucrose synthase and invertase. Because sugars are important regulators of gene expression in plants, these enzymes are thought to participate in the control of various developmental processes. Sucrose synthase (EC2.4.1.13) converts sucrose into fructose and UDP-glucose. By contrast, invertase (EC 3.2.1.26) irreversibly catalyzes the hydrolytic cleavage of sucrose into glucose and fructose. In tomato, sucrose synthase was considered to be a major factor determining the fruit sink strength because of the following reasons: (1) there is a strong correlation among sucrose synthase activity, ADP-glucose pyrophosphorylase, and starch accumulation in early developing fruit (Robinson et al. 1988; Yelle et al. 1988); (2) sucrose synthase activity increases in parallel with fruit growth, and there is a linear correlation between its activity and the final fruit size in both wild species and commercial varieties (Sun et al. 1992); and (3) antisense transgenic plants in which the sucrose synthase activity was inhibited displayed reduced fruit setting and sucrose import capacity in young fruit (D’Aoust et al. 1999). Meanwhile, the suppression of the sucrose synthase gene in tomato did not lead to remarkable alterations in starch and sugar accumulation in the fruit (Chengappa et al. 1999). Although a clear correlation between sink strength and sucrose synthase activity has been observed, there is poor evidence that sucrose synthase is directly involved in the control of fruit sugar content and composition in tomato.
On the other hand, increasing evidence in the last two decades has indicated that invertase is an essential factor for regulating sugar content in tomato fruit. In plants, invertases are classified into three isozyme types—cell wall invertase (CWIN) (also often referred to as apoplastic or extracellular invertase), vacuolar invertase (VIN), and cytoplasmic invertase (CIN) (also referred to as neutral invertase)—according to their solubility, subcellular localization, isoelectric point (pI), and optimal pH (Sturm 1999). Among these isozyme types, CWIN and VIN are characterized as acid invertases because of their acidic optimal pH, whereas CIN is characterized as a neutral invertase due to its neutral optimal pH. Several studies have revealed the diverse roles of invertase in the plant life cycle, including its participation in various responses to abiotic and biotic stresses such as drought, hypoxia, high temperature, wounding, and pathogen infection. In addition, invertase regulates seed and pollen development, sugar composition in fruit, and sugar storage in sink organs, among other effects (see Roitsch and González 2004, for a review). Most of these processes are considered to result from modified gene expression and changes in carbohydrate partitioning, which are regulated by sucrose and its cleavage products as signal molecules. In plants, CWIN and VIN have similar properties except for their cellular localization and pI; that is, both enzymes are β-fructofuranosidases with acidic optimum pH values, are glycoproteins, and share high sequence homology (Unger et al. 1994). In contrast to CWIN and VIN, little information is available on CIN in tomato, and its physiological function has yet to be elucidated.
In tomato, genetic and biochemical analyses investigating differences in sugar composition between wild species and S. lycopersicum, from which most cultivars are derived, have proven that the two acid invertases CWIN and VIN are involved in the determination of fruit sugar content and composition in different stages (Yelle et al. 1988; Klann et al. 1993; Fridman et al. 2000; Husain et al. 2001; Miron et al. 2002). Utilizing progeny lines derived from interspecific crossing between S. lycopersicum and a wild species, S. chmielewskii, which mainly accumulates sucrose instead of reducing hexoses in its fruit, Klann et al. (1993) revealed a lack of VIN activity during fruit maturation, which resulted in the sucrose-accumulation property of S. chmielewskii. In addition, S. pimpinellifolium showed higher VIN activity and hexose contents in red fruit compared with S. lycopersicum (Husain et al. 2001). These results indicate that VIN changes the fruit sugar balance to high hexose and low sucrose during maturation in tomato. This observation was also supported by a transgenic approach utilizing antisense transgenic tomato plants in which VIN gene (TIV1) expression was suppressed, resulting in a marked increase in sucrose and a decrease in hexose content in the fruit (Ohyama et al. 1995). Interestingly, although the invertase activity was largely suppressed in the transgenic tomato, remarkable differences in fruit development and total soluble solids were not observed compared with a non-transgenic plant. These results suggest that vacuole-localizing acid invertase expression in the later fruit developmental stage is not essential for determining the total sugar level and fruit sink strength.
During the last decade, a cell wall invertase has attracted attention for its influence on the total soluble solids in tomato. CWIN is thought to be an essential enzyme for supplying carbohydrates into sink organs through the hydrolytic cleavage of phloem-unloaded sucrose to hexose. To date, four genes encoding CWIN—LIN5, LIN6, LIN7, and LIN8—have been identified in tomato, and tissue-specific expression patterns have been reported (Godt and Roitsch 1997; Ohyama et al. 1998; Fridman and Zamir 2003). Transcriptional analyses have indicated that LIN5, LIN6, and LIN7 are mainly expressed in the floral reproductive organs, whereas LIN8 is expressed in the vegetative organs, such as the roots and leaves. Among the floral-expressing LINs, whereas LIN7 showed very limited expression in stamens and pollen, LIN5 was strongly expressed in ovaries and immature green fruit in addition to in the floral organs. By contrast, LIN6 showed a broader expression pattern, including expression in the roots, stems, leaves, and young fruit (Fridman and Zamir 2003; Ohyama et al. 2006). From a physiological point of view, the most interesting isozyme is most likely LIN6, as this gene responds to various biotic and abiotic stimuli, including wounding, pathogen infection, and sugars (Godt and Roitsch 1997; Ohyama et al. 1998; Sinha et al. 2002). LIN6 expression is also induced in response to cytokines and brassinosteroids, a group of phytohormones known to promote cell division and active growth in plants (Godt and Roitsch 1997; Goetz et al. 2000). The responses of LIN6 are considered essential in the process of supplying carbohydrates to damaged tissues and to growing organs to accommodate the increasing demand for metabolic energy. Among the other CWINs, LIN7, which is expressed specifically in stamens and pollen, was suggested to be involved in heat stress tolerance because the expression level of LIN7 was significantly higher in a heat stress-tolerant variety than in a sensitive variety, and its expression was specifically promoted by heat stress in the heat-tolerant variety (Li et al. 2012). Li et al. (2012) also suggested that the high import ability of sucrose into young fruits contributes to the heat tolerance of the variety and should be provided by LIN7 expression.
For fruit sugar content, the most important CWIN should be LIN5 because the encoding gene is the most abundantly expressed in the fruit in the early development stages (Godt and Roitsch 1997; Ohyama et al. 1998; Fridman and Zamir 2003). This assumption is further supported by a QTL mapping study utilizing an introgression line developed from an interspecific cross between S. pennellii and S. lycopersicum (Eshed and Zamir 1995). A field trial revealed that 23 QTLs were related to a high total soluble solid content, and one of those was mapped on chromosome 9 as Brix9-2-5 (Eshed and Zamir 1995, 1996). By fine mapping utilizing an F2 hybrid population generated from near isogenic lines (NILs), Brix9-2-5 was finally mapped to a 484 bp region ranging from exon 3 to exon 4 of the LIN5 gene originating from S. pennellii (Fridman et al. 2000). Comparing the specific region of LIN5, there are several differences between the two species. For instance, three SNPs with amino acid substitutions, 18 bp and 7 bp repeat sequences, and a hypothetical ORF of 30 amino acids were found in the S. pennellii-derived sequence. Subsequent analyses revealed that one of the SNPs near the catalytic site facilitates the enzyme kinetics of LIN5, facilitates the uptake of assimilate, and results in increased TSS in the fruit of plants bearing the Brix9-2-5 allele (Fridman et al. 2002, 2004). An RNAi-based approach confirmed the role of LIN5 in the regulation of the fruit Brix content. In addition, the suppression of LIN5 revealed its multiple functions in normal floral and fruit development, including functions related to morphology, size, number, and pollen development. Furthermore, reductions in phytohormone levels, such as ABA, JA, and GA, and the expression of genes associated with biosynthesis and/or with the response of those phytohormones were also observed in the LIN5 RNAi plant, suggesting its role in hormone metabolism (Zanor et al. 2009).
These studies have led to substantial progress in understanding the genetic and transcriptional regulation processes governing the roles of invertase in plants. Additionally, during the last decade, increasing evidence has indicated that invertase activity is regulated by posttranscriptional suppression through its inhibitory protein (Hothorn et al. 2004; Jin et al. 2009). In tomato, the suppression of the invertase inhibitor (INVINH1) leads to posttranscriptional increases in CWIN activity, seed weight, and hexose levels in fruit without any alteration in VIN and CIN activities (Jin et al. 2009). The cellular and subcellular localization of INVINH1 occur in the apoplast vasculature (phloem parenchyma) of young fruit; this expression pattern is consistent with that of LIN5. Other types of invertase inhibitors have also been reported in tomato, and one of these, SolyCIF, showed specific localization to the cell wall compartment (Reca et al. 2008). Invertase inhibitors localizing to both apoplasts and vacuoles have been identified in other crops (Rausch and Greiner 2004), indicating that INVINH comprises a gene family and functions as a modulator for invertase activity in an intracellular-specific manner.
As described above, regarding the physiological function of cytoplasmic invertase (CIN), there is little information available for plants because of its low and unstable enzymatic activity, and few genes have been isolated. In tomato continuous sucrose import during fruit ripening is important for a wild relative Lycopersicum species, cheesmanii, and there is a positive correlation between CIN activity and hexose levels in the fruit of plants exposed to salinity stress (Balibrea et al. 1996, 2006). These results indicate that CIN functions in a specific genetic background, such as wild Lycopersicum germplasm, or under specific environmental conditions, such as salinity stress.
9.3 Sucrose Unloading and Starch Accumulation in Immature Fruit Partially Determines the Fruit Sugar Level
Alongside invertase, early studies have related the starch level in the immature and mature green fruit stages to the level of soluble solids in ripe tomato fruit (Davies and Cocking 1965; Dinar and Stevens 1981; Robinson et al. 1988; Schaffer and Petreikov 1997). ADP-glucose pyrophosphorylase (AGPase, EC 2.7.7.27) is proposed to regulate starch biosynthesis during the early stages of fruit development (Schaffer and Petreikov 1997; Schaffer et al. 2000). AGPase catalyzes the synthesis of ADP-glucose from glucose-1-phosphate and ATP (Preiss 1988), which is the first regulatory step in starch biosynthesis in plants (Tsai and Nelson 1966; Lin et al. 1988; Müller-Röber et al. 1992; Stark et al. 1992). Plant AGPase is a hetero-tetrameric enzyme composed of two small and two large subunits (Morell et al. 1987). The former subunits function as the catalytic molecule, and the latter subunits function as allosteric modulators (Okita et al. 1990). In tomato AGPase, there are two isoforms of the small subunit and three isoforms of the large subunit (Chen and Janes 1997). One gene encoding the small subunit (AgpS1) and three genes encoding the large subunit (AgpL1, AgL2, and AgL3) have thus far been isolated as cDNAs (Chen et al. 1998; Park and Chung 1998). The predominant transcripts in developing fruit are AgpL1 and AgpS1, and the expression of these two genes peaks during the early development stages, which are responsible for starch accumulation (Park and Chung 1998; Petreikov et al. 2006; Yin et al. 2010). Plant AGPase genes are regulated at the transcriptional level by phosphate, nitrate, and sugars (Müller-Röber et al. 1990; Scheible et al. 1997; Nielsen et al. 1998; Sokolov et al. 1998; Li et al. 2002). Additionally, our previous work revealed that AgpS1 and AgpL1 were specifically upregulated at the transcriptional level by salinity stress in early developing fruits in an ABA- and osmotic-stress-independent manner (Yin et al. 2010). In fact, the AgpL1 response to salinity was found to be a sugar-mediated response, as evidenced by the elevated carbohydrate influx into the fruit under salinity stress (Yin et al. 2010). The observation that starch synthesis in fruit is dependent on the sugar supply is consistent with the results of N’tchobo et al. (1999). Auxin has also been demonstrated to be involved in starch biosynthesis and sugar accumulation. Amyloplast development and the gene expression of starch biosynthetic enzymes, including AGPase, were suppressed by auxin in tobacco-cultured cells (Miyazawa et al. 1999). In tomato, the downregulation of auxin response factor 4 (SlARF4), a member of the transcription factor family regulating auxin-responsive genes, led to increased chlorophyll content with excessive numbers of chloroplasts in the fruit (Jones et al. 2002). Further analyses revealed that the SlARF4-suppressed line showed enhanced starch accumulation in early developing fruit and increased sugar contents in mature fruit, suggesting a negative role of SlARF4 and auxin on starch and sugar accumulation in tomato fruit (Sagar et al. 2013).
Enhanced starch accumulation in young fruit was also observed in different germplasms with higher invertase activity and the total soluble solids content than those of normal tomato cultivars, such as in the S. pennellii-derived introgression line possessing the Brix9-2-5 allele (Robinson et al. 1988; Baxter et al. 2005). Expression analyses with the promoter-GUS transgenic plants driven by the AgpL1 and AgpS1 promoters revealed a high expression of both genes in the vascular tissue of young fruit, stems, and roots at the transcriptional level (Xing et al. 2005; Goto et al. 2013). Vasculature-specific expression was also observed for CWIN and for the invertase inhibitor gene in tomato (Jin et al. 2009). A CWIN has been thought to play a role in supplying carbohydrate energy to sink organs in the hexose form as well as in the apoplastic hydrolysis of sucrose. The associated expression pattern of these genes suggests the functional collaboration of CWIN and AGPase in vascular tissue. In contrast to invertase, sucrose synthase seems less likely to be related to starch synthesis in tomato fruit because a transgenic plant with suppressed sucrose synthase expression exhibited unaltered starch accumulation and sugar content in its fruits (Chengappa et al. 1999). However, Baroja-Fernández et al. (2012) reported the involvement of sucrose synthase in starch biosynthesis through cytoplasmic ADP-glucose production in Arabidopsis. The contribution of sucrose synthase to starch synthesis is still unclear in tomato.
9.4 Inhibition of the Sucrose Transporter Affects Fruit Sugar Content as well as Seed Development and Yield in Fruit
In plants, photoassimilates are mainly translocated as nonreducing disaccharides, such as sucrose, from the source leaves to the sink tissues/organs. The sucrose transporter (SUT, also known as SUC) is an essential membrane protein for the long-distance transport of sucrose, in particular at phloem loading/unloading in source/sink organs in higher plants. In tomato, three SUT genes—LeSUT1, LeSUT2, and LeSUT4—were isolated, and it was demonstrated that all proteins localize in the phloem sieve element (Barker et al. 2000; Weise et al. 2000). LeSUT1 is specifically expressed in phloem companion cells in source leaves and is suggested to play a crucial role in phloem loading. LeSUT2 is mainly expressed in sink organs, such as stems and fruits, and in anthers, whereas LeSUT4 is expressed in ovaries and immature fruit (Barker et al. 2000; Hackel et al. 2006; Weise et al. 2000). Among these SUTs, the antisense inhibition of LeSUT2 led to significant reductions in the soluble sugar (glucose, fructose, and sucrose) and starch contents in young fruits, which was accompanied by reductions in yield due to decreased fertility and fruit size (Hackel et al. 2006). Because there were no significant changes in the carbohydrate composition in the leaves of the antisense SUT transgenic lines, the results observed in the fruit were not due to a reduction in the sucrose supply from the source leaves. Although LeSUT2 has been suggested to act as a sucrose sensor (Barker et al. 2000), it has bilateral functions as a physiologically functional transporter of fruit sugar content. These results also showed that the mode of phloem unloading is mediated by an apoplastic step in young fruit. Although early studies in the 1980s suggested that a symplastic pathway functions in young fruit, it is not likely to be associated with phloem unloading in sink organs. However, the function of LeSUT4 has still not been elucidated, although phylogenetic analyses have indicated that this transporter can be categorized as a type III SUT, which are associated with vacuolar membranes (Endler et al. 2006; Reinders et al. 2012). Therefore, LeSUT4 may function in the control of sugar composition in cooperation with vacuolar acid invertase. Taken together, these findings show that apoplastic invertase, AGPase, and sucrose transporters play essential roles in vascular apoplasts in a coordinated manner and the carbohydrate dynamism in early developing fruit is very important for determining the sugar level in red ripe fruit.
9.5 Novel Vacuolar Processing Enzyme Is Involved in the Control of Fruit Sugar Composition in Mature Fruit
Recently, newly isolated vacuolar processing enzymes (VPE) were demonstrated to participate in the modification of sugar content and composition in tomato fruit. VPE proteins are members of the cysteine proteinase family, which is well conserved among various organisms, including plants. VPE was originally identified as a cysteine proteinase involved in the processing of seed storage proteins (Hara-Nishimura et al. 1991). In tomato, five genes (SlVPE1–SlVPE5) coding a VPE protein were isolated and characterized: SlVPE1 and SlVPE2 as seed coat types, SlVPE4 as a seed type, and SlVPE3 and SlVPE5 as vegetative types (Ariizumi et al. 2011). Based on histochemical analyses of the promoter-GUS plants, both SlVPE3 and SlVPE5 exhibited specific expression in the vascular bundles from the seeds to the placenta and around the endocarp tissue in the fruit during all developmental stages. A transgenic approach revealed increased sugar accumulation in mature fruits of the RNAi tomato lines with decreased VPE expression. Among the SlVPEs, the suppression of SlVPE5 had the greatest effect on the fruit sugar content. These results indicate that SlVPEs participate in the regulation of fruit sugar content as a negative regulator in tomato. The target proteins of SlVPEs have yet to be identified. In Arabidopsis, γVPE, an ortholog of SlVPE, targets various types of hydrolases, such β-glycosidase, α-galactosidase, and α-mannosidase (Rojo et al. 2003). Because γVPE is also involved in the proteolysis of a vacuolar invertase, β-FRUCTOSIDASE4, it is likely to display a similar mechanism to that of SlSVPs; that is, reduced VPE activity caused by the suppression of SlVPE5 produces increased invertase activity in vacuoles, resulting in enhanced sugar accumulation in transgenic RNAi fruits (Ariizumi et al. 2011).
9.6 Molecular Breeding for the Modification of Fruit Sugar Content and Composition in Tomato
As described above, several experimental trials have successfully modified fruit sugar contents or composition in tomato by modifying the expression of target genes using transgenic approaches. Stark et al. (1996) developed transgenic tomato lines overexpressing a small subunit gene of a bacterial ADP-glucose pyrophosphorylase (glgC16) that displayed higher starch accumulation in young fruit and total soluble solids increased by approximately 20 % in mature fruit. No remarkable changes in the phenotype or growth of the plant or in the size and yield of fruits were observed. Indeed, an overexpression approach for sugar/starch biosynthetic enzyme genes may be a simple and effective way to generate new tomato varieties with high sugar contents. However, considering the recent consumer attitude against genetically modified crops (GMO), transgenic approaches would be undesirable for the development of a commercial variety. From this point of view, a knockdown strategy, such as mutant screening based on a reverse genetic approach, would be a valid option. In tomato, ethyl methanesulfonate (EMS)-mutagenized populations assisted by TILLING (Targeting Induced Local Lesions in Genomes; Till et al. 2004) have been developed (Okabe et al. 2011; Just et al. 2013). For a new variety with high sugar contents, negative regulators, such as invertase inhibitor (INVINH1), auxin response factor (SlARF4), and vacuolar processing protein (SlVPE5), are potential candidate genes for mutant screening.
During the last decade, the availability of genomic resources has rapidly expanded in tomato, including high-resolution linkage maps, DNA markers, ESTs, and the tomato genomic sequence (Tomato Genome Consortium 2012). In combination with these genomic resources, the availability of existing genetic resources, including wild species-derived introgression/recombinant inbred lines (Eshed and Zamir 1995; Fulton et al. 2002; Causse et al. 2004; Prudent et al. 2009), has also improved. With the accumulation of QTLs and/or mutation alleles for high sugar contents into an appropriate germplasm (so-called pyramiding), a more efficient and rapid breeding system will be established for a new cultivar with high organoleptic quality in the near future.
References
Ariizumi T, Higuchi K, Arakaki S, Sano T, Asamizu E, Ezura H (2011) Genetic suppression analysis in novel vacuolar processing enzymes reveals their roles in controlling sugar accumulation in tomato fruits. J Exp Bot 62:2773–2786
Balibrea ME, Santa Cruz AM, Bolarín M, Pérez-Alfocea F (1996) Sucrolytic activities in relation to sink strength and carbohydrate composition in tomato fruit growing under salinity. Plant Sci 118:47–55
Balibrea ME, Martinez-Andújar C, Cuartero J, Bolarín M, Pérez-Alfocea F (2006) The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Funct Plant Biol 33:279–288
Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12:1153–1164
Baroja-Fernández E, Muñoza FJ, Lia J, Bahajia A, Almagroa G, Monteroa M, Etxeberriac E, Hidalgoa M, Sesmaa MT, Pozueta-Romero J (2012) Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proc Natl Acad Sci USA 109:321–326
Baxter CJ, Carrari F, Bauke A, Overy S, Hill SA, Quick PW, Fernie AR, Sweetlove LJ (2005) Fruit carbohydrate metabolism in an introgression line of tomato with increased fruit soluble solids. Plant Cell Physiol 46:425–437
Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor MI, Nunes-Nesi A, Nikiforova V, Centero D, Ratzka A, Pauly M, Sweetlove LJ, Fernie AR (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol 142:1380–1396
Causse M, Duffe P, Gomez MC, Buret M, Damidaux R, Zamir D, Gur A, Chevalier C, Lemaire-Chamley M, Rothan C (2004) A genetic map of candidate genes and QTLs involved in tomato fruit size and composition. J Exp Bot 55:1671–1685
Chen BY, Janes HW (1997) Multiple forms of ADP-glucose pyrophosphorylase from tomato fruit. Plant Physiol 113:235–241
Chen BY, Janes HW, Gianfagna T (1998) PCR cloning and characterization of multiple ADP-glucose pyrophosphorylase cDNA from tomato. Plant Sci 6:59–67
Chengappa S, Guilleroux M, Wendy P, Shields R (1999) Transgenic tomato plants with decreased sucrose synthase are unaltered in starch and sugar accumulation in the fruit. Plant Mol Biol 40:213–221
D’Aoust MA, Yelle S, Nguyen-Quoc B (1999) Antisense inhibition of tomato fruit sucrose synthase decrease fruit setting and the sucrose unloading capacity of young fruit. Plant Cell 11:2407–2418
Davies JN, Cocking EC (1965) Changes in carbohydrates, proteins and nucleic acids during cellular development in tomato fruit locule tissue. Planta 67:242–253
Dinar M, Stevens MA (1981) The relationship between starch accumulation and soluble solids content of tomato fruits. J Am Soc Hortic Sci 106:415–418
Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG (2006) Identification of a vacuolar sucrose transporter in barley and arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141:196–207
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141:1147–1162
Eshed Y, Zamir D (1996) Less-than-additive epistatic interactions of quantitative trait loci in tomato. Genetics 143:1807–1817
Fridman E, Zamir D (2003) Functional divergence of a syntenic invertase gene family in tomato, potato and Arabidopsis. Plant Physiol 131:603–609
Fridman E, Pleban T, Zamir D (2000) A recombination hotspot delimits a wild-species quantitative trait locus for tomato sugar content to 484 bp within an invertase gene. Proc Natl Acad Sci USA 97:4718–4723
Fridman E, Liu YS, Carmel-Goren L, Shoresh AGM, Pleban T, Eshed Y, Zamir D (2002) Two tightly linked QTLs modify tomato sugar content via different physiological pathways. Mol Gen Genomics 266:821–826
Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D (2004) Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305:1786–1789
Fulton TM, Bucheli P, Voirol E, López J, Pétiard V, Tanksley SD (2002) Quantitative trait loci (QTL) affecting sugars, organic acids, and other biochemical properties possibly contributing to flavor, identified in four advanced backcross populations of tomato. Euphytica 127:163–177
Godt D, Roitsch T (1997) Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 115:273–282
Goetz M, Godt D, Roitsch T (2000) Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J 22:515–522
Goto Y, Nonaka S, Yin YG, Koiwa T, Asamizu E, EzuraH MC (2013) Isolation and characterisation of the ADP-glucose pyrophosphorylase small subunit gene (AgpS1) promoter in tomato (Solanum lycopersicum L.). Plant Biotechnol 30:279–286
Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C (2006) Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 45:180–192
Hara-Nishimura I, Inoue K, Nishimura M (1991) A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett 294:89–93
Ho LC (1996) The mechanism of assimilate partitioning and carbohydrate compartmentation in fruit in relation to the quality and yield of tomato. J Exp Bot 47:1239–1243
Ho LC, Hewitt JD (1986) Fruit development. In: Atherton JG, Rudich J (eds) The tomato crop. Chapman and Hall, London, pp 201–240
Hothorn M, Wolf S, Aloy P, Greiner S, Scheffzek K (2004) Structural insights into the target specificity of plant invertase and pectin methylesterase inhibitory proteins. Plant Cell 16:3437–3447
Husain SE, James C, Shields R, Foyer CH (2001) Manipulation of fruit sugar composition but not content in Lycopersicon esculentum fruit by introgression of an acid invertase gene from Lycopersicon pimpinellifolium. New Phytol 150:65–72
Jin Y, Ni DA, Ruan YL (2009) Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 21:2072–2089
Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latché A, Pech JC, Bouzayen M (2002) Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J 32:603–613
Just D, Garcia V, Fernandez L, Bres C, Mauxion JP, Petit J, Jorly J, Assali J, Bournonville C, Ferrand C, Baldet P, Lemaire-Chamley M, Mori K, Okabe Y, Ariizumi T, Asamizu E, Ezura H, Rothan C (2013) Micro-Tom mutants for functional analysis of target genes and discovery of new alleles in tomato. Plant Biotechnol 30:225–231
Klann EM, Chetelat RT, Bennett AB (1993) Expression of acid invertase gene controls sugar composition in tomato (Lycopersicon) fruit. Plant Physiol 103:863–870
Li XY, Xing JP, Thomas JG, Harry JW (2002) Sucrose regulation of ADP-glucose pyrophosphorylase subunit genes transcript levels in leaves and fruits. Plant Sci 162:239–244
Li Z, Palmer WM, Martin AP, Wang R, Rainsford F, Jin Y, Patrick JW, Yang Y, Ruan YL (2012) High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J Exp Bot 63:1155–1166
Lin TP, Caspar T, Somerville C, Preiss J (1988) A starch-deficient mutant of Arabidopsis thaliana with low ADP-glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol 88:1175–1181
Miron D, Petreikov M, Carmi N, Shen S, Levin I, Granot D, Zamski E, Schaffer AA (2002) Sucrose uptake, invertase localization and gene expression in developing fruit of Lycopersicon esculentum and the sucrose-accumulating Lycopersicon hirsutum. Physiol Plant 115:35–47
Miyazawa Y, Sakai A, Miyagishima S, Takano H, Kawano S, Kuroiwa T (1999) Auxin and cytokinin have opposite effects on amyloplast development and the expression of starch synthesis genes in cultured bright yellow-2 tobacco cells. Plant Physiol 121:461–469
Morell MK, Bloom M, Knowles V, Preiss J (1987) Subunit structure of Spinach leaf ADP glucose pyrophosphorylase. Plant Physiol 85:182–187
Müller-Röber B, Kossamann J, Hannah LC, Willmitzer L, Sonnewald U (1990) One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genomics 224:136–146
Müller-Röber B, Sonnewald U, Willmitzer L (1992) Inhibition of ADP-glucose pyrophosphorylase leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11:1229–1238
N’tchobo H, Dali N, Nguyen-Quoc B, Foyer CH, Yelle S (1999) Starch synthesis in tomato remains constant throughout fruit development and is dependent on sucrose supply and sucrose synthase activity. J Exp Bot 50:1457–1463
Nguyen-Quoc B, Foyer CH (2001) A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J Exp Bot 52:881–889
Nielsen TH, Krapp A, Röper-Schwarz U, Stitt M (1998) The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP-glucose pyrophosphorylase is modified by nitrogen and phosphate. Plant Cell Environ 21:443–455
Ohyama A, Ito H, Sato T, Nishimura S, Imai T, Hirai M (1995) Suppression of acid invertase activity by antisense RNA modifies the sugar composition of tomato fruit. Plant Cell Physiol 36:369–376
Ohyama A, Nishimura S, Hirai M (1998) Cloning of cDNA for a cell wall-bound acid invertase from tomato (Lycopersicon esculentum) and expression of soluble and cell wall-bound invertases in plants and wounded leaves of L. esculentum and L. peruvianum. Genes Genet Syst 73:149–157
Ohyama A, Suwabe K, Nunome T, Fukuoka H (2006) Characterization of the promoter of the Wiv-1(Lin6) gene encoding a wound-inducible cell wall-bound acid invertase in tomato. Plant Biotechnol 23:365–371
Okabe Y, Asamizu E, Saito T, Matsukura C, Ariizumi T, Bres C, Rothan C, Mizoguchi T, Ezura H (2011) Tomato TILLING technology: development of a reverse genetics tool for the efficient isolation of mutants from Micro-Tom mutant libraries. Plant Cell Physiol 52:1994–2005
Okita TW, Nakata PA, Anderson JM, Sowokinos J, Morell M, Preiss J (1990) The subunit structure of potato tuber ADP-glucose pyrophosphorylase. Plant Physiol 93:785–790
Park SW, Chung WI (1998) Molecular cloning and organ-specific expression of three isoforms of tomato ADP-glucose pyrophosphorylase gene. Gene 206:215–221
Petreikov M, Shen S, Yeselson Y, Levin I, Bar M, Schaffer AA (2006) Temporally extended gene expression of the ADP-Glc pyrophosphorylase large subunit (AgpL1) leads to increased enzyme activity in developing tomato fruit. Planta 224:1465–1479
Preiss J (1988) Biosynthesis of starch and its regulation. In: Preiss J (ed) The biochemistry of plants, vol 14. Academic, San Diego, CA, pp 181–254
Prudent M, Causse M, Génard M, Tripodi P, Grandillo S, Bertin N (2009) Genetic and physiological analysis of tomato fruit weight and composition: influence of carbon availability on QTL detection. J Exp Bot 60:923–937
Prudent M, Lecomte A, Bouchet JP, Bertin N, Causse M, Genard M (2011) Combining ecophysiological modelling and quantitative trait locus analysis to identify key elementary processes underlying tomato fruit sugar concentration. J Exp Bot 62:907–919
Rausch T, Greiner S (2004) Plant protein inhibitors of invertases. Biochim Biophys Acta 16962:253–261
Reca IB, Brutus A, D’Avino R, Villard C, Bellincampi D, Giardina T (2008) Molecular cloning, expression and characterization of a novel apoplastic invertase inhibitor from tomato (Solanum lycopersicum) and its use to purify a vacuolar invertase. Biochimie 90:1611–1623
Reinders A, Sivitz AB, Ward JM (2012) Evolution of plant sucrose uptake transporters. Front Plant Sci 3:2–12
Robinson NL, Hewitt JD, Bennett AB (1988) Sink metabolism in tomato fruit. I. Developmental changes in carbohydrate metabolizing enzymes. Plant Physiol 87:727–730
Roitsch T, González MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV (2003) A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA 100:7389–7394
Sagar M, Chervin C, Mila I, Hao Y, Roustan JP, Benichou M, Gibon Y, Biais B, Maury P, Latché A, Pech JC, Bouzayen M, Zouine M (2013) SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol 161:1362–1374
Schaffer AA, Petreikov M (1997) Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113:739–746
Schaffer AA, Levin I, Ogus I, Petreikov M, Cincarevsky F, Yeselson E, Shen S, Gilboa N, Bar M (2000) ADP-glucose pyrophosphorylase activity and starch accumulation in immature tomato fruit: the effect of a Lycopersicon hirsutum-derived introgression encoding for the large subunit. Plant Sci 152:135–144
Scheible WR, Gonzàlez-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9:783–798
Sinha AK, Hofmann MG, Römer U, Köckenberger W, Elling L, Roitsch T (2002) Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol 128:1480–1489
Sokolov LN, Dejardin A, Kleczkowski LA (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem J 336:681–687
Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM (1992) Regulation of the amount of starch in plant tissues by ADP-glucose pyrophosphorylase. Science 258:287–292
Stark DM, Barry GF, Kishore GM (1996) Improvement of fruit quality traits through enhancement of starch biosynthesis. Ann N Y Acad Sci 792:26–36
Sturm A (1999) Invertases. primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7
Sun J, Loboda T, Sung S-JS, Black CC (1992) Sucrose synthase in wild tomato, Lycopersicon chmielewskii, and tomato fruit sink strength. Plant Physiol 98:1163–1169
The Tomato Genome Consortium (TGC) (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485:635–641
Till BJ, Reynolds SH, Weil C, Springer N, Burtner C, Kim Y, Bowers E, Codomo CA, Enns LC, Odden AR, Greene EA, Comai L, Henikoff S (2004) Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biol 4:12
Tsai CY, Nelson OE (1966) Starch-deficient maize mutant lacking adenosine diphosphate glucose pyrophosphorylase activity. Science 151:341–343
Unger C, Hardegger M, Lienhard S, Sturm A (1994) cDNA cloning of carrot (Daucus carota) soluble acid, 6-fructofuranosidases and comparison with the cell wall isoenzyme. Plant Physiol 104:1351–1357
Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12:1345–1355
Xing J, Li X, Luo Y, Gianfagna TJ, Janes HW (2005) Isolation and expression analysis of two tomato ADP-glucose pyrophosphorylase S(large)subunit gene promoters. Plant Sci 169:882–893
Yelle S, Hewitt JD, Nieder M, Robinson NL, Damon S, Bennett AB (1988) Sink metabolism in tomato fruit. III. Analysis of carbohydrate assimilation in a wild species. Plant Physiol 87:731–736
Yin YG, Kobayashi Y, Sanuki A, Kondo S, Fukuda N, Ezura H, Sugaya S, Matsukura C (2010) Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J Exp Bot 61:563–574
Zanor MI, Osorio S, Nunes-Nesi A, Carrari F, Lohse M, Usadel B, Kühn C, Bleiss W, Giavalisco P, Willmitzer L, Sulpice R, Zhou YH, Fernie AR (2009) RNA interference of LIN5 in tomato confirms its role in controlling brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol 150:1204–1218
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Matsukura, C. (2016). Sugar Accumulation in Tomato Fruit and Its Modification Using Molecular Breeding Techniques. In: Ezura, H., Ariizumi, T., Garcia-Mas, J., Rose, J. (eds) Functional Genomics and Biotechnology in Solanaceae and Cucurbitaceae Crops. Biotechnology in Agriculture and Forestry, vol 70. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-48535-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-48535-4_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-48533-0
Online ISBN: 978-3-662-48535-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)