Abstract
In this chapter, we show how to assess local bioavailable 87Sr/86Sr isotopic ratios of archaeological strata by analysis of water, leached soil samples and vegetation from selected archaeological sites. We present a detailed description and instruction of how to calculate the data. In this pilot study, calculation of cut-off values between local and non-local stable strontium isotopic ratios was performed for five locations in the Austrian Inn Valley. These cut-off values, determined as 87Sr/86Sr(fauna) ± 0.001, agree within acceptable limits with the measured isotopic signature of excavated animal bone material from the chosen archaeological sites. This way, the vertebrate consumer can clearly be classified as a local or non-local individual. Our model calculation, which leads to a reliable geo-dependent bioavailable 87Sr/86Sr isotopic ratio, reveals a high dependence on the isotopic signal from groundwater rather than from soil.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Numerous attempts have been made for an assessment of local bioavailable 87Sr/86Sr isotopic ratios, the knowledge of which is essential for the determination of local or primarily non-local bio-archaeological finds at a site. The majority of these investigations focus on the comparison of measured stable strontium isotopic ratios in archaeological bones and teeth with those of the regional geology (e.g. Grupe et al. 1997; Müller et al. 2003; Tütken 2010). It has only occasionally been attempted to introduce other factors such as water and vegetation into the discussion on local bioavailable 87Sr/86Sr isotopic ratios (see Drouet et al. 2005; Xin and Hanson 1994). Our study tries to integrate all relevant factors which mix in the consumer’s tissues, be it man or animal. Vertebrates ingest strontium with their food and drinking water , whereby the strontium isotopic ratio of plants is influenced to a major degree by the respective ratio of the atmosphere and the soluble mineral soil components.
We undertook detailed re-investigations of several archaeological sites to establish a firm basis for model calculations of local bioavailable strontium isotopic signals, which are in turn based on mixing models integrating strontium isotopic ratios and strontium concentrations of the relevant parameters water, soil , and vegetation (Faure and Mensing 2005; Grupe et al. 2011). The first data sets, the results of which are presented in this chapter, concentrate on the Inn Valley , part of the archaeologically highly relevant transalpine Inn-Eisack-Etsch-Brenner passage (see www.for1670-transalpine.uni-muenchen.de). It was expected that the model calculation should lead to more reliable results with regard to the determination of place of origin of individual finds, which are hitherto frequently fraught with ambiguity.
It should be stressed, however, that model calculations alone do not permit the assessment of the reliability and correctness of this method. It is necessary to test and control the calculated data by analysis of 87Sr/86Sr isotopic ratios in archaeological vertebrate bone finds from the respective sites. Here, three species were chosen: domestic cattle (Bos taurus), domestic pig (Sus domestica), and red deer (Cervus elaphus). Taken all data together, a reliable image of the local, geo-dependent bioavailable 87Sr/86Sr isotopic ratio emerges. This is in turn indispensable for any attempt to quantify immigrant people or imported animals (or animal parts/raw material) at a site to firmly distinguish mobility from migration and trade.
Material: Samples and Site Description
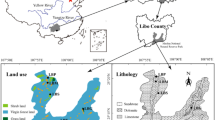
Between 2013 and 2014, groundwater (naturally composed of atmospheric and soil conditioned components), “weathered soil ” (comparable to acid leached soil), and vegetation from selected archaeological sites throughout the Inn Valley were sampled during several field excursions (Fig. 1). Soil samples were taken from the archaeological strata of the chosen sites to establish a baseline soil composition in order to correctly assess the growth of past and wild cultivated plants as closely as possible. Care was taken to exclude possible influences of modern fertilisers. Soil samples were subjected to a leaching step prior to analysis (see below; see also Drouet et al. 2005). Groundwater was taken from neighbouring springs or wells and therefore consists of both precipitation (rain water) and surface water , enriched with soil components. Unfortunately, no archaeological plant material was available for analysis. Modern vegetation samples were therefore taken directly from the location of the soil sample. To avoid species-specific peculiarities, hazelnut branches (Corylus avellana) were sampled because of their availability at nearly every site (Göhring 2014).
87Sr/86Sr isotopic map of the Inn Valley between the cities of Telfs and Kufstein. The investigated archaeological sites are marked with numbers. The Inn Valley is one of the largest East–west extending valleys of the Alps filled with glacial and fluviatile sediments (yellow), occasionally cut through by tertiary sandy outcrops. Moraine and fluviatile sediments of the Inn Valley prevalently enriched with crystalline rocks have mixed 87Sr/86Sr < 0.709–0.7135. The Inn Valley forms the boundary between the Northern Calcareous Alps (blue, various types of calcareous rocks, 87Sr/86Sr = 0.707–0.709) and the Central Alps which are made primarily of crust-dominated crystalline rocks (pink—of acid magmatic origin, and beige—of sedimentary and acid volcanic origin, 87Sr/86Sr > 0.71). Lacustrine sediments, partly of tertiary age and often positioned on high Inn terraces underlain by crystalline rocks, have 87Sr/86Sr ratios > 0.7135. Mantle-dominated rocks are shown in green (87Sr/86Sr < 0.707)
The following archaeological sites were chosen:
Fritzens Pirchboden (Code Number 206)
The Pirchboden is a hill site located above the town of Fritzens, the type locality of the Fritzens-Sanzeno culture (sixth century bc; Lang 1998). The archaeological soil consists of argillaceous sand, light brown with crystalline components of boulder pavement of varying size, definitely of moraine origin ( 87Sr/86Sr = 0.714, profile depth = 100–110 cm). Branches of hazelnut were sampled, and spring water from a nearby well.
Innsbruck-Mühlau, Kalvarienberg (Code Number 215)
The soil was taken from the sidecut of a road. It consists of brown rendzina with calcareous fragments and some crystalline pebbles ( 87Sr/86Sr = 0.70876, profile depth = 25–30 cm). It was covered by landslide material interspersed with carbonate fragments only. Vegetation (hazelnut branches) and water from a well were sampled next to church at the Kalvarienberg.
Pfaffenhofen/Ilm, Hörtenberg (Code Number 230)
The Hörtenberg is a conspicuous hill near the town of Pfaffenhofen and is composed of quartz -phyllite. The Iron Age archaeological site (Fassbinder 2010) is located at the northern hill slope near the “Maierhof” farm. The soil sample was taken from a road sidecut, 100 cm below surface. It consists of grey soft clay ( 87Sr/86Sr = 0.72533, profile depth 100–130 cm), whereby the whole fine-grained loamy profile intercalated with chalk bands suggests a lacustrine sequence. The vegetation sample again consists of hazelnut branches; spring water originates from a well inside the “Maierhof” farm.
Wiesberg Buchberg (Code Number 236)
The Buchberg is a conspicuous hill built by Triassic carbonate rocks overlain by glacial sediments loaded with rounded crystalline components, and was once a Bronze Age settlement site (Pöll 2014). The archaeological soil sample consists of sandy silt ( 87Sr/86Sr = 0.71081, profile depth = 50–60 cm). The vegetation sample is hazelnut ; spring water originates from a fountain in the nearby village of Jenbach.
Ampass Widumfeld (Code Number 237)
The Widumfeld is located east of the town of Ampass and is of archaeological relevance from the Iron Age until Roman times (Tomedi et al. 2001; Castellan and Tomedi 2006). The soil sample ( 87Sr/86Sr = 0.71736, profile depth 50–60 cm) contains small pebbles of quartz -phyllite and probably relates to an argillaceous sandy valley or lake filling from post-Roman times. We assume that the sediment source has remained similar during post-glacial times and that no fundamental change in strontium isotopic ratios of the reservoir has taken place. Again, vegetation samples consist of hazelnut branches; spring water originates from the nearby forest.
Analytical Methods
Bone
The surfaces of the bone samples were manually removed by grinding, and the remaining sample was washed ultrasonically in distilled water until the water remained clear. The air-dried sample was then defatted with diethylether for 5 h in a Soxhlet, air-dried and etched ultrasonically with HCOOH (98 %) for 5 min. Finally, the sample was washed in distilled water until the wash solution reached a pH of 5–6. The air-dried sample was then ashed for 12 h at 800 °C in a muffle furnace and homogenised to a fine powder. This bone meal was dissolved in 1 mL concentrated HNO3 (65 %) on a hot plate at 100 °C and dried. For the column separation, the sample was dissolved in 1 mL 6 N HNO3. This solution was used for the separation of the strontium fraction for the isotopic analysis by mass spectrometry, after a chromatographic column separation by use of Sr SPEC resin. All acids were of ultrapure quality. Distilled water was always of doubly distilled quality.
Water
1.5 L water was filtered through an MN 615 filter (if necessary). Ten millilitre of the sample was then evaporated to dryness and dissolved for 14 h in 65 % HNO3 in a Teflon beaker on a hot plate at 100 °C, followed by a chromatographic column separation (see above).
Soil
The soil samples were dried and sieved with a 500 μm sieve. Three hundred milligram of this sample was dissolved in a Teflon beaker in 2 mL 35 % HCl (suprapure) for 12 h at 120 °C, cooled to room temperature and centrifuged at 11,000 rpm for 10 min. The clear solution was separated and evaporated to dryness, followed by a chromatographic column separation (see above).
Wood
From 150 mg of hazelnut wood each, only the annual rings were analysed; all other parts were removed mechanically. The wood was dried at 80 °C in an oven to constant weight, and then ashed for 12 h at 800 °C. Four hundred milligram of the ash was processed by chromatographic column separation (see above).
Mass Spectrometry
87Sr/86Sr was analysed with a Thermal Ionisation Mass Spectrometer Finnigan MAT 261.5 on single tungsten filaments. Significant extant Rb was evaporated from the loaded filament by controlled preheating before the isotopic Sr composition was measured. For quality control and to check for the proper operation of the mass spectrometer, a certified reference material was measured under the same conditions as the samples (SrCO3, NIST SRM 987, 87Sr/86Sr: 0.710210 ± 0.000056 STD, n = 110). Isotope mass fractionation during analysis was corrected by referencing to an invariant 88Sr/86Sr value of 8.37521. Total analytical uncertainty (precision + accuracy) for 87Sr/86Sr on natural samples is assumed to be <50 ppm. Standard Reference Material SRM 1400 “Bone ash” (NIBS, Washington DC) was used with regard to the wet ashing and Rb–Sr separation. Measurement precision was ± 0.00001.
Strontium Mixing Model for the Assessment of Local Geo-dependent Bioavailable 87Sr/86Sr Isotopic Ratios in Archaeological Fauna
Humans and animals ingest strontium with food and drinking water hence strontium from these sources with their source-specific isotopic ratio mixes in the consumer’s body. The 87Sr/86Sr isotopic ratio in a consumer’s tissue is therefore always a mixed isotopic ratio. In plants , 87Sr/86Sr is largely due to the atmospheric water and the solubilised mineral component in soil . It became obvious in the course of our research that local faunae and florae are not supplied with a uniform water composition, but that the mix of groundwater [groundwater X(wat) and ocean or rainwater X(atm)] could exhibit completely different values (see Fig. 2).
Chosen end members for the water composition are those of the North Atlantic ocean water ( 87Sr/86Sr (atm) = 0.709024, Sr (atm) = 0.0013 ppm; Veizer 1989) and of the locally sampled groundwater X(wat), respectively. Groundwater can be described as rainwater, the composition of which has been altered by solubilised mineral components in the soil . It is necessary to stress that no surface water has been sampled for this study, but rather water from shallow fountains (5–6 m maximum) or from springs near their bottom. Due to the residence time of the water in the soil, a measurable uptake of mineralogical components which are clearly related to the parent rock is evidenced (Table 1).
For an assessment of the other components contributing to the mixed isotopic ratio measured in the consumer’s skeleton , soil and vegetation samples were taken and measured. Soil was sampled from the archaeological sites at a level equivalent to the surface at the time of the prehistoric settlement whenever possible. This way, the strontium isotopic ratio of such soil was measured that had once driven the prehistoric plant growth and was introduced into the prehistoric food chain. The soil samples were processed and leached to access a 87Sr/86Sr ratio comparable to the former bioavailable one. Since no archaeological plant material was available, modern hazelnut was chosen not only because it was abundant at most of the former archaeological localities, but especially because the main roots reach into a depth of 30–40 cm and thus below the agricultural horizon.
For this pilot study, five archaeological sites from the Inn Valley were chosen, whereby the local geology is characterised by lacustrine-fluviatile sediments (sites nos 230 and 237), or by glacial sediments of the last glaciation period (sites nos 206 and 236). In contrast, the Innsbruck/Mühlau Kalvarienberg site (no 215) exhibits a soil composition which is best described as weathered soil on carbonate rock (rendzina ). Archaeozoological remains of the three vertebrate species mentioned above were available from all five sites.
For the calculation of the local bioavailable 87Sr/86Sr isotopic ratio which should be reflected in the skeleton of residential animals (and also in humans; see Table 2 in the appendix), the following published data were used:
For the atmospheric water component X(atm), we took the 87Sr/86Sr ratio and the strontium content of modern ocean water (0.709024 ± 0.000032; Veizer 1989). Other values published by e.g. Graustein and Armstrong (1983) or Dupré et al. (1994) are influenced by aerosol and dust particle burdens (Xin and Hanson 1994). Strontium concentrations in precipitation can be highly variable but usually do not exceed 1 ppb (Xin and Hanson 1994). For Central Belgium, Drouet et al. (2005) measured a mean concentration of 1.1 ± 0.3 ppb (n = 9). For our model calculation, we took an atmospheric strontium concentration Sr (atm) of 0.0013 ppm. This value correlates with that of sample BP1 determined from a post-glacial environment in Central Belgium (Drouet et al. 2005).
87Sr/86Sr of groundwater X(wat) was measured; the strontium content was estimated. Both the stable strontium isotopic ratio and the strontium content of groundwater adjust to the respective local geological settings dependent on the residence time in the soil . Xin and Hanson (1994) published measurements of both soil and groundwater in the Peconic river watershed (New York) and found 87Sr/86Sr isotopic ratios between 0.71 and 0.7113 and strontium contents between 25 and 34 ppb. It is obvious that both the strontium concentration and the strontium isotopic ratio adjust to the geological conditions according to the residence time below ground (Voerkelius et al. 2010). For Denmark, Frei and Frei (2011) published decreasing mean strontium concentrations between 1 and 0.125 ppm for surface water with a 87Sr/86Sr variability between 0.708 and 0.711. Accordingly, we chose a strontium concentration of 0.06 ppm for carbonatic water with a 87Sr/86Sr isotopic ratio <0.709, 0.03 ppm Sr for soils with a 87Sr/86Sr isotopic ratio between 0.709 and 0.7135, and 0.02 ppm Sr for soils with a 87Sr/86Sr ratio >0.7135.
With these isotopic and concentration data, the stable strontium isotopic ratio of the water consumed by the fauna [drinking water X(aq-f)] can be calculated. We suggest that the proportion of atmospheric water f(atm,aq-f) in the drinking water averages 30 %. This percentage correlates with the one calculated from data of Xin and Hanson (1994) as a mixture of soil water (10–50 cm depth) and atmospheric (rain) water component.
With regard to the strontium concentrations in soil [Sr (wea)] and the frequency distribution of the water components in the atmosphere [f(atm, aq-f)], and the fauna [(aq-f, fauna)], variations of 50 % were assumed. Standard deviations (STDev) were taken into account with regard to the measurement error. To present an example, the complete model calculation is shown in Table 2 (see appendix) for sample no. 230 and visualised by the resulting mixing diagram (Fig. 3).
The mixing diagram illustrates the mixing of three components with different strontium contents and different stable strontium isotopic ratios: groundwater X(wat), leached soil X(wea), and vegetation X(veg). Straight lines represent the non-linear mixing of the respective end members. X(aq-v) = vegetation water is a mixture of groundwater X(wat) and rain/atmospheric water X(atm) with a proportion f(atm, aq-v) of 94 %. X(aq-f) = drinking water is a mixture of the same components with a proportion f(atm, aq-f) of only 30 %. Strontium in vegetation [X(veg)] is made up of 99.9 % strontium from vegetation water X(aq-v) and only 0.1 % of strontium from soil water X(wea). The geo-dependent bioavailable strontium composition in faunal bone [X(fauna)] is a 1:1 mixture of strontium from drinking water X(aq-f) and from vegetation X(veg). All investigated samples reflect a far less abundance of strontium from soil than from the surrounding water component for the creation of the local bioavailable87Sr/86Sr isotopic ratio than expected. Acronyms: see appendix
It is depicted from Fig. 3 that the data points for drinking water X(aq-f) and the sampled groundwater X(wat) plot closely together (X stands for both the isotopic ratio and the strontium concentration of the respective components). With regard to this position in the mixing diagram , drinking water cannot combine with the measured soil components X(wea) to the strontium mixing in the vegetation X(veg). Consequently, the water taken up by the vegetation must have a composition different from that of the drinking water of the fauna. The vegetation water X(aq-v) should rather plot at the intersection of the mixing lines groundwater X(wat)—atmospheric water X(atm) and soil X(wea)—vegetation X(veg) (Fig. 3). The calculated data for the vegetation water in Fig. 3 are 87Sr/86Sr (aq-v) = 0.712189 and Sr (aq-v) = 0.00242 ppm, whereby the atmospheric contribution to the vegetation water f(atm, aq-v) = 94 %. Note the nonlinear variation of f(atm, aq-v) along the straight mixing line in the diagram (see Faure and Mensing 2005). With a proportion f(aq-v, veg) of more than 99 %, the influence of the strontium component in vegetation water to the whole vegetation is remarkably high.
We would like to stress the dependencies between the proportion of rainwater in vegetation water [f(atm, aq-v)] and the proportion of vegetation water in the whole vegetation [f(aq-v, veg) = factor K (see Table 2). K is calculated by use of the strontium concentrations from vegetation, vegetation water, and leached soil . In the case of K = 1, data points X(veg) and X(aq-v) would plot in the same place, and a 100 % dependency of 87Sr/86Sr of the whole vegetation from 87Sr/86Sr in vegetation water would be the case. Since this is rather improbable, we chose an f(atm, aq-v) which renders K < 1 automatically. This way, f(atm, aq-v) declines to 0.94 (sample 230). But still, only 6 % of the 87Sr/86Sr ratio in the whole vegetation would be due to the soil component; 94 % would still stem from the water component. While such a value seems to be very high, it is not unusual in nature (see e.g. Green et al. 2004; Grupe et al. 2011).
The strontium concentration of the leached soil Sr (wea), that of the vegetation water Sr(aq-v) and its abundance in the vegetation f(aq-v, veg) = 0.9994, permits the calculation of the strontium concentration in the vegetation Sr(veg) and results in 0.0026 ppm. This value, however, is far lower than some other published strontium concentrations in wood . Stem wood of different tree species had a mean concentration of about 5 ppm (Dijksta et al. 2003). Drouet et al. (2005) measured a mean concentration of 2.6 until 2.8 ppm for the genus Fagus, and Lambertz and Welling (2010) report a range of 1–10 ppm Sr in dry wood of various species. Just as in other tissues, Sr binds at Ca sites in plants which are mainly components of the cell wall (such as pectin acids, cellulose, and lignine; Torre et al. 1992), whereby the cellulose has a particularly low affinity to Ca and therefore also for Sr. Calculation of our model is performed under the assumption that in the course of mixing the components vegetation X(veg) and water X(aq-f) to form the Sr concentration in fauna X(fauna), the total Sr content of the vegetation is of no relevance but rather the bioavailable proportion of strontium only. We assume that it is only the strontium component which solubilised in the vegetation water is available to the consumer. This would in turn correspond to 0.05–0.1 % of the total strontium content of the plant .
Surprisingly, the calculated strontium concentration in animal bone [Sr (fauna)] is at first glance also very low (0.0085 ppm Sr according to the mixing diagram ) and no longer comparable with the average total Sr content in bone (130–140 ppm; see Grupe et al. 1997). While the total Sr content in a bulk bone sample is the result of a long accumulation process lasting many years due to the long biological half-life of the apatite , the mixing diagram rather reflects the actual, time-dependent uptake in the course of the remodelling processes. Therefore, the resulting Sr content of 0.0085 ppm only is a function of the known slow remodelling of bone. Likewise, the low Sr content in vegetation which results from the mixing diagram is due to the time-dependent mineral uptake and precipitation into the plant tissue.
For an assessment of the local bioavailable and geo-dependent stable strontium isotopic ratio in vertebrate bone, possible strontium components provided by the diet are vegetation X(veg) and drinking water X(aq-f). Assuming that both components mix with the same proportion [f(aq-f, fauna) = 0.5], then the local 87Sr/86Sr isotopic ratio for the find with the code number 230 is 0.714913 (Table 2, appendix).
Table 2 also presents an error estimation for the data. For the measured 87Sr/86Sr ratios, the standard deviation of the measurement value was used, but no error estimation was carried out with regard to the Sr contents. Even a 100 % deviation only led to marginal changes. For the calculation of the proportion of the mixing components, a variation of 50 % was assumed with the exception of f(atm, aq-v), which varies between 0.1 and 2 %. A higher variation would be incompatible with the fact that K = f(aq-v, veg) must not exceed 1. For the sample no. 230, error estimation resulted in 87Sr/86Sr = 0.714913 ± 0.000604 (0.084 %, STDev, mean of errors).
Results
Table 1 presents the results obtained at all five sites chosen for this pilot study.
While it is highly plausible that the 87Sr/86Sr of the vegetation is largely due to the water that has been taken up by the plant , the low influence (1–6 %) of the bioavailable soil strontium component is surprising at first glance. This phenomenon is however particularly conspicuous when the 87Sr/86Sr isotopic ratios of groundwater and soil are significantly different from each other (see samples with the code numbers 230, 236). To relate the expected 87Sr/86Sr ratio in residential fauna to the bioavailable strontium isotopic ratio of the soil alone may therefore be totally misleading. Groundwater and vegetation indispensably need to be considered in addition.
The variability of local 87Sr/86Sr ratios in fauna defines the cut-off value which differentiates between local and non-local individuals. One possibility of defining this cut-off value is the traditional statistical way by use of the threefold error (=3 × STDev). Other authors decided for a fixed value such as ±0.001 (highly significant in terms of the measurement precision; see Grupe et al. 1997) which is also used in our study because on average, this value agrees well with the 3 × STDev of the calculated 87Sr/86Sr ratios in fauna (Table 1; 3 × STDevmean = 0.00101). By defining the cut-off values as 87Sr/86Sr(fauna) ± 0.001 this way, the variation of calculated 87Sr/86Sr of local vertebrates with the code number 230 would range from 0.713913 to 0.715913. The measured 87Sr/86Sr ratios in the three cattle bones from the site were 0.714405 ± 0.000135, 0.715243 ± 0.000264, and 0.716443 ± 0.000141 (±2*STDev). The first two individuals should therefore have been of local origin; the third one can no longer be considered local.
Discussion
In sum, the analysis of archaeological animal bone finds and the modelled local, bioavailable 87Sr/86Sr isotopic ratios lead to the definition of three domains which correspond with the regional soil types (Fig. 4):
Test of local or non-local origin of recovered animal bones from all five sites. Calculated local geo-dependent 87Sr/86Sr isotopic ratios are indicated by site code numbers with the corresponding cut-off error bar (±0.001). Marked domains of different isotopic ratios(87Sr/86Sr <0.709; 0.709–0.7135; >0.7135) correlate with local geological sediment /soil types (see text). Non-local animals according to the model calculation are numbered and marked in white and local animals in black
-
Domain 1: 87Sr/86Sr < 0.709; brown calcareous soil , rendzina .
-
Domain 2: 0.709 ≤ 87Sr/86Sr ≤ 0.7135; moraine soil , often in hillside location, coarse detrital and mostly crystalline material.
-
Domain 3: 87Sr/86Sr ≥ 0.7135, fluvio-lacustrine sediment s (silty alluvial deposits, fine sand and silt, lacustrine clay partly of tertiary origin).
The majority of the investigated animals exhibit 87Sr/86Sr isotopic ratios between 0.709 and 0.7135 and must have spent their lives on moraine soil . This was expected since the Inn Valley glacier had once been one of the largest glaciers in the whole alpine area, filling the Inn Valley to a depth of several hundred metres. At other locations such as Pirchboden (code no. 206) and Buchberg (code no. 236), animals had been slaughtered which had obviously been raised elsewhere. A complete agreement of all data (calculated local 87Sr/86Sr isotopic ratio and measured ratios of cattle , pig , and red deer ) was found at only one site (Widumfeld, Ampass, code no. 237), and no agreement at all was found between calculated and measured data for Innsbruck-Mühlau, code no. 215).
In sum, the model calculations permit for a much finer-scaled solution of isoscapes in the Inn Valley as the available geological map (Fig. 1). Furthermore, an association of single non-local bone finds to defined regions is possible beyond the mere detection of non-local finds by way of the exclusion principle.
References
Castellan SN, Tomedi G (2006) Archäologische Untersuchungen im Zuge der Renovierung des Ampasser Widums. Archaeo Tirol Kleine Schriften 5:221–223
Drouet T, Herbauats J, Gruber W, Demaiffe D (2005) Strontium isotope composition as a tracer of calcium sources in two forest ecosystems in Belgium. Geoderma 126:203–223
Dupré B, Négrel P, Seimbille F, Allegre CJ (1994) 87Sr/86Sr ratio variation during a rain event. Atmos Environ 28:617–620
Fassbinder JWE (2010) The Iron age settlement of Pfaffenhofen-Hörtenberg, Tirol (Austria): Akademie und BLfD zu Prospektion in Tirol. Denkmalpf Info 146:73–74
Faure G, Mensing TM (2005) Isotopes. Principles and applications. Wiley, Hoboken, NJ
Frei KM, Frei R (2011) The geographic distribution of strontium isotopes in Danish surface waters – a base for provenance studies in archaeology, hydrology and agriculture. Appl Geochem 26:326–340
Göhring AB (2014) Sauerstoff-Isotopiekartierung einer Alpenpassage anhand rezenter botanischer Proben. MSc Thesis, LMU München
Graustein WC, Armstrong RL (1983) The use of 87Sr/86Sr ratios to measure atmosphere transport into forest watersheds. Science 219:298–392
Green GP, Bestland EA, Walker GS (2004) Distinguishing sources of base cations in irrigated and natural soils: evidence from strontium isotopes. Biogeochemistry 68:199–225
Grupe G, Price TD, Schröter P, Söllner F, Johnson CM, Beard BL (1997) Mobility of Bell Beaker people revealed by strontium isotope ratios of tooth and bone: a study of southern Bavarian skeletal remains. Appl Geochem 12:517–525
Grupe G, von Carnap-Bornheim C, Söllner F (2011) Stable strontium isotope mapping for provenance studies in archaeology – different material, different signals? Bull Soc Suisse d’Anthrop 17:67–76
Lambertz G, Welling J (2010) Die chemische Zusammensetzung von naturbelassenem Holz. Expertise im Auftraf des Bundesverbandes Holzpackmittel, Paletten, Exportverpacing (HPE) e.V. Johann Heinrich von Thünen-Institut, Bundesforschungsinstitut für Ländliche Räume, Wald und Fischerei, Institut für Holztechnologie und Holzbiologie, Hamburg
Lang A (1998) Das Gräberfeld von Kundl im Tiroler Inntal. Studien zur vorrömischen Eisenzeit in den zentralen Alpen. Frühgeschichtliche und Provinzialrömische Archäologie. Materialien und Forschungen 2. Marie Leidorf, Rhaden/Westf
Müller W, Fricke H, Halliday AN, McCulloch MT, Wartho J-A (2003) Origin and migration of the alpine iceman. Science 302:862–866
Pöll J (2014) Der frühbronzezeitliche Vollgriffdolch vom Buchberg bei Wiesing. Conservatum est Schlern-Schriften 363:317–344
Tomedi G, Appler H, Castallen SN (2001) Vorbericht zu den Grabungen von ArchaeoTirol am Ampasser Widenfeld im Jahr 2000. ArchaeoTirol Kleine Schriften 3:91–98
Torre M, Rodriguez AR, Saura-Calixto F (1992) Study of the interactions of calcium ions with lignin, cellulose and pectine. J Agric Food Chem 40:1762–1766
Tütken T (2010) Die Isotopenanalyse fossiler Skelettreste – Bestimmung der Herkunft und Mobilität von Menschen und Tieren. Tagungen des Landesmuseums für Vorgeschichte Halle 3:33–51
Van Dijksta AD, Breemen N, Jongmans AG, Davies GR, Likens GE (2003) Calcium weathering in forested soils and the effect of different tree species. Biochemistry 62:253–275
Veizer J (1989) Strontium isotopes in seawater through time. Ann Rev Earth Planet Sci 17:141–167
Voerkelius S, Lorenz GD, Rummel S, Quétel CR, Heiss G, Baxter M, Brach-Papa C, Deters-Itzelberger P, Hölzl S, Hoogewerff J, Ponzevera E, VanBocxstaele M, Ueckermann H (2010) Strontium isotopic signatures of natural mineral waters, the reference to a simple geological map and its potential for authentication of food. Food Chem 118:933–940
Xin G, Hanson N (1994) Strontium isotope study of the Peconic river watershed, Long Island, New York. MSc thesis, State University of New York at Stony Brook
Acknowledgements
This study was financially supported by the Deutsche Forschungsgemeinschaft. Sample processing was performed by Anita Toncala and mass spectrometry was performed by Stefan Hölzl.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
- f(atm, aq-f):
-
Abundance of the atmospheric water (=rainwater) component in the drinking water
- f(atm, aq-v):
-
Abundance of the atmospheric water (=rainwater) component in the vegetation water
- f(aq-v, veg):
-
Abundance of the vegetation water component in the vegetation
- f(aq-f, fauna):
-
Abundance of the drinking water component in the fauna
- 87Sr/86Sr (atm):
-
Sr isotopic ratio in the atmosphere (ocean water, rainwater, taken from the literature)
- 87Sr/86Sr (wat):
-
Sr isotopic ratio in the groundwater (sampled water from wells or groundwater horizon)
- 87Sr/86Sr (aq-f):
-
Sr isotopic composition of drinking water (mixture of rain and groundwater ) incorporated into the fauna
- 87Sr/86Sr (aq-v):
-
Sr isotopic composition of vegetable water (mixture of rain and groundwater )
- 87Sr/86Sr (veg):
-
Sr isotopic ratio in the vegetation (wood , measured)
- 87Sr/86Sr (fauna):
-
Sr isotopic composition of the fauna (localised Sr isotopic ratio, human and animal, calculated)
- 87Sr/86Sr (wea):
-
Sr isotopic composition of weathered soil , bioavailable component (gained by leaching of sampled soil with 1 N HCl; for details see methods section)
- Sr (atm) (ppm):
-
Sr content of the atmospheric or rainwater (taken from the literature)
- Sr (wat) (ppm):
-
Sr content of the groundwater (taken from the literature)
- Sr (aq-v) (ppm):
-
Sr content in vegetation water, mixture of ground and rainwater
- Sr (aq-f) (ppm):
-
Sr content in drinking water , mixture of ground and rain water
- Sr (veg) (ppm):
-
Sr content of the vegetation (wood , time-dependent assimilated, calculated)
- Sr (fauna) (ppm):
-
Sr content incorporated into the fauna, time-dependent assimilated (human and animal, calculated)
- Sr (wea) (ppm):
-
Sr content of the weathered soil (taken from literature)
- X(…):
-
correlation of 87Sr/86Sr and Sr content for the special case
Equation of the mixing hyperbola in coordinates of the 87Sr/86Sr ratio and the Sr concentration (Sr) (Faure and Mensing 2005)
( 87Sr/86Sr ) M = a/(Sr ) M + b, with
The numerical values of a and b entirely depend on the 87Sr/86Sr ratios and Sr concentrations of the components A and B in the mixture M.
f A is the abundance of A in the mixture M
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Söllner, F., Toncala, A., Hölzl, S., Grupe, G. (2016). Determination of Geo-dependent Bioavailable 87Sr/86Sr Isotopic Ratios for Archaeological Sites from the Inn Valley (Austria): A Model Calculation. In: Grupe, G., McGlynn, G. (eds) Isotopic Landscapes in Bioarchaeology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-48339-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-48339-8_7
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-48338-1
Online ISBN: 978-3-662-48339-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)