Abstract
Itch is a complex sensory modality that can be evoked by an extremely diverse set of stimuli and has multiple components of disease etiology. Thus, determining the basic molecular and cellular players is essential before we can tackle the more complex aspects of itch. The identification of novel itch receptors has been extremely fruitful and has uncovered novel signaling pathways and pruritogens. Mrgprs encode a family of G protein-coupled receptors, many of which are expressed specifically in sensory nerves and function as itch receptors in mediating histamine-independent itch. In this chapter, we will review the discovery of the receptor family, their specific expression, their roles as itch receptors, and the itch-inducing agonists. Furthermore, we will summarize the results indicating that Mrgpr-expressing sensory neurons are itch-sensing neurons. In the end we will discuss the role of Mrgprs and Mrgpr-positive neurons in chronic itch.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Itch
- Mrgprs
- GPCR
- G protein-coupled receptor
- DRG neurons
- Chloroquine
- BAM8-22
- β-alanine
- SLIGRL
- Mouse
- Knockout
- Skin
- Labeled line
1 Introduction
Organisms use their sensory systems—including vision, audition, olfaction, gustation, and somatosensation—to detect stimuli in the environment. At the cell’s surface, receptors and ion channels play an essential role in every sensory system by acting as the molecular sensors of their respective stimuli. The most famous example is the family of olfactory receptors expressed in olfactory receptor neurons (Buck and Axel 1991; Gogos et al. 2000; Lomvardas et al. 2006).
Itch sensation, a subtype of somatosensation, can be evoked by a wide range of compounds with a variety of chemical structures, including small molecule compounds, peptides, proteases, cytokines, and lipid components (Paus et al. 2006; Patel and Dong 2010). Presumably, these itch-inducing substances—or pruritogens—activate the membrane receptors and/or ion channels that are expressed in sensory nerve endings in the skin, and the activation of these receptors triggers the electrical impulses that are delivered through a series of connected nerves to the brain, where the itch sensation is generated. The best-characterized pruritogen is histamine, which is released from mast cells and activates histamine receptors located in sensory nerves in the skin. In addition to histamine-dependent itch, a wealth of pharmacological, clinical, electrophysiological, and molecular data support the existence of histamine-independent itch (Shelley and Arthur 1957; Schmelz et al. 1997; Ikoma et al. 2005; Namer et al. 2008). Indeed, most chronic itch conditions are not alleviated by antihistamines, suggesting the involvement of other itch mediators and receptors. Therefore, understanding the basic molecular and cellular mechanisms that underlie histamine-independent itch will improve methods for treating chronic itch. In this chapter, we will summarize the recent findings regarding a family of G protein-coupled receptors called Mrgprs, many of which are expressed specifically in sensory nerves and function as itch receptors in mediating histamine-independent itch.
2 Identification of the Mrgpr Family of Receptors
In 1997, Michael Caterina and David Julius cloned the TRPV1 gene, which encodes a noxious heat sensor and capsaicin receptor (Caterina et al. 1997, 2000; Tominaga et al. 1998). This seminal discovery prompted us to search for additional molecules that are expressed specifically in small-diameter nociceptors in the mouse DRG. Using a cDNA subtractive screening approach, we isolated a cDNA clone that is enriched in small-diameter DRG neurons and encodes a G protein-coupled receptor (Dong et al. 2001). Because this receptor shares sequence homology with the proto-oncogene Mas1, we named this new gene MrgA1 for “Mas1-related gene”; the name was later changed to MrgprA1 for “Mas1-related G protein-coupled receptor.” Subsequent database searches and genomic DNA screening using MrgprA1 as a probe led to the identification of additional Mrgpr member genes. Ultimately, approximately 50 Mrgpr genes have been identified in the mouse genome. Based on their sequence homology, we grouped these genes into the MrgprA, MrgprB, and MrgprC subfamilies (Dong et al. 2001). Genomic and bioinformatics analyses suggest that these Mrgpr subfamilies arose from gene duplication and expansion, likely via retroviral LINE elements present in the mouse Mrgpr loci (Zylka et al. 2003). Like the olfactory receptor family (which was also expanded in the mouse genome), approximately half of all MrgprA, MrgprB, and MrgprC genes are pseudogenes, with shortened open-reading frames caused by premature stop codons due to mutations. The human genome contains at least four Mrgpr genes, which are highly homologous with the murine MrgprA through MrgprC genes (Dong et al. 2001). However, because the human proteins do not form orthologous pairs with their rodent counterparts, we named them human MrgprX1 through MrgprX4. In the following section, we will discuss why the human genome contains fewer Mrgprs than the mouse genome. In addition to these multimember subfamilies, the mouse genome also contains several single-member Mrgpr subfamilies called MrgprD through MrgprH, which have clear human orthologs.
A striking feature of Mrgprs is their specific expression patterns. Using in situ hybridization and genetic labeling approaches, we found that many Mrgpr genes are expressed exclusively in subsets of small-diameter DRG neurons (see below for a detailed description; Fig. 1) (Dong et al. 2001). The Mrgpr gene family was also discovered independently by a research group at AstraZeneca, and they introduced the term “sensory neuron-specific receptors” (SNSRs) (Lembo et al. 2002). Consistent with our results, they used RT-PCR and found that several human MrgprXs are expressed selectively in subsets of human DRG neurons.
Venn diagram summarizing the expression of Mrgprs in DRG neurons. The small- to medium-diameter neurons represent the major primary sensory neuron population in the DRG and can be grouped into two partially overlapping subpopulations known as the peptidergic (CGRP positive; blue) and non-peptidergic (IB4 positive; green) nociceptors. Most—if not all—CGRP-positive nociceptors also express TRPV1 channels (red). MrgprA3-positive and MrgprC11-positive neurons largely overlap (purple) and are positive for both CGRP and IB4. Finally, MrgprD-positive cells (orange) are IB4 positive and CGRP negative. MrgprD-positive and β-alanine sensitive neurons represent a nonoverlapping subpopulation of itch-sensing neurons
3 Ligands and Agonists
In contrast to olfactory receptors that are poorly expressed and trafficked in heterologous cells, expression of Mrgprs in most heterologous cells (e.g., HEK293 and CHO cells) is rather straightforward. By adding a C-terminal GFP tag to Mrgprs, we can easily follow the trafficking of the receptors to the plasma membrane (Dong et al. 2001; Han et al. 2002). Because Mrgprs have the highest sequence homology with G protein-coupled receptors that have peptide ligands (e.g., the formyl peptide receptors), we specifically searched for peptides as the likely ligands for Mrgprs. We used a conventional Ca2+ imaging assay to monitor Mrgpr activation in HEK cells expressing Gα15, a promiscuous G protein that couples G protein-coupled receptors to a signal transduction pathway that ultimately triggers the release of intracellular Ca2+ (Offermanns and Simon 1995). Using this heterologous system (i.e., Mrgprs expressed in Gα15-expressing HEK293 cells), we screened a panel of peptides from the Sigma catalog and identified several RFamide peptides—including the molluscan peptide FMRFamide and the mammalian peptides NPFF, NPAF, and γ2-MSH—that function as agonists for the mouse MrgprA1, MrgprA4, and MrgprC11 receptors (Dong et al. 2001; Han et al. 2002). Although these agonists share a common C-terminal RF(Y)G or RF(Y) amide motif, they activate MrgprA1, MrgprA4, and MrgprC11 receptors with different sensitivities (Dong et al. 2001; Han et al. 2002).
Interestingly, the AstraZeneca group reported that BAM8-22 (a 15-amino acid C-terminal fragment of pro-enkephalin that ends with the amino acid RYG) and γ2-MSH activate human MrgprX1 and rat MrgprC receptors, respectively (Lembo et al. 2002). We also found that BAM8-22 is a potent activator of mouse MrgprC11 and triggers internalization of the receptor from the plasma membrane to the cytosol (Han et al. 2002). The initial identification of Mrgpr ligands provided us with essential tools to probe the function of Mrgprs. For example, we found that all of the Mrgprs with an identified ligand (e.g., the MrgprC11 and MrgprX1 receptors) signal through the Gαq subunit (Han et al. 2002) and some receptors also couple to the Gαi subunit (Chen and Ikeda 2004; Crozier et al. 2007). Therefore, regular heterologous cells (i.e., without Gα15) can be used to express Mrgprs and monitor the receptor’s activation using Ca2+ imaging. This approach can also be used to study the activation of native Mrgprs in DRG neurons. In addition, we also identified TRP channels as the downstream effector targets of Mrgpr activation (Wilson et al. 2011).
More recently, we discovered that mouse MrgprA3 and human MrgprX1 are receptors for the antimalarial drug chloroquine (Liu et al. 2009). A Japanese group reported that β-alanine binds to and activates both human and mouse MrgprD receptors (Shinohara et al. 2004). Thus, BAM8-22, chloroquine, and β-alanine are agonists of both mouse and human Mrgprs, and they have been used as probes to study histamine-independent itch (see below). As mentioned above, there are far fewer human Mrgprs than mouse Mrgprs. Furthermore, human MrgprXs do not form orthologous pairs with their mouse counterparts, despite their sequence homology. However, the ligand specificity of human and mouse Mrgprs suggests that the human MrgprX1 receptor is a functional homolog of both mouse MrgprA3 and MrgprC11 receptors. It is therefore tempting to speculate that during the evolution of the mouse genome, multiple copies of Mrgprs arose through duplication, and each copy has its own specific agonist (e.g., chloroquine for MrgprA3, BAM8-22 for MrgprC11), whereas the human genome contains only a few Mrgpr receptors, each of which serves as a receptor for multiple agonists (e.g., MrgprX1 for both chloroquine and BAM8-22). This broadly tuned mechanism at the receptor level may preserve a functional diversity in a relatively small family of human Mrgprs during evolution.
4 Mrgprs as Itch Receptors
Despite their specific expression patterns, analyzing the in vivo function of Mrgpr receptors has proven to be quite challenging. Because the mouse genome contains so many Mrgpr genes, the conventional single-gene knockout strategy was not feasible due to possible compensation by other genes. In addition, we lacked sufficient information to decide which Mrgpr to study first. Finally, given the lack of orthologs between mouse MrgprA–Cs and human MrgprXs, generating and studying mouse Mrgpr single-knockout mice seemed to have little clinical relevance. Therefore, we chose to genetically delete a group containing several Mrgpr genes from the mouse genome, a viable strategy given that the MrgprA, MrgprB, and MrgprC genes are clustered on mouse chromosome 7. Using the Cre recombinase-loxP strategy, we deleted an 845-kb stretch of genomic DNA containing 12 full Mrgpr genes (including MrgprA3 and MrgprC11) but no additional genes (Liu et al. 2009). Importantly, homozygous mice carrying this large chromosomal deletion (called Mrgpr-cluster Δ −/− mice) survive to adulthood with no detectable developmental or motor function defects. Therefore, Mrgpr-cluster Δ −/− mice are a valuable animal model for studying the role of Mrgprs in itch. Indeed, results obtained from behavioral analyses of these mutant mice have provided key evidence that led us to identify MrgprA3 and MrgprC11 as itch receptors.
4.1 MrgprA3
From all of the pruritogens identified to date, we chose to test the antimalarial drug chloroquine by injection into our Mrgpr-cluster Δ −/− mice (Liu et al. 2009); we selected this pruritogen first because of its medical relevance and wide clinical usage. Most black Africans have a severe itch reaction to chloroquine, and this reaction can be so severe that many patients even refuse to take chloroquine to treat their malaria (Olatunde 1977; Abila et al. 1989; Mnyika and Kihamia 1991; Ademowo et al. 1998); interestingly, other races do not have such an itch response to chloroquine (Sams and Epstein 1965; Spencer et al. 1982). Because patients experience itch when they first ingest chloroquine and because antihistamines do not effectively prevent this side effect, researchers in Africa concluded that chloroquine-induced itch is not an allergic response, nor is it mediated by histamine receptors (Abila et al. 1994). Interestingly, injecting chloroquine into the skin of mice evokes a robust scratching response in the mice, and various inbred strains of mice exhibit different degrees of scratching responses (Green et al. 2006). Based on this response, we tested whether our Mrgpr-cluster Δ −/− mice respond differently from wild-type following chloroquine injection. The results were immediately obvious—the Mrgpr-cluster Δ −/− mice scratched significantly less than the wild-type mice (Liu et al. 2009). We were quite encouraged by this robust phenotype, as we had been searching for a function for Mrgprs in more than 7 years since they were first identified. This important finding opened a new research path in our laboratory that ultimately led to the functional characterization of Mrgprs and Mrgpr-expressing neurons in mediating itch.
To determine the specific itch-inducing effect of chloroquine, we also tested a synthetic precursor of chloroquine (called quinoline), in which one of the benzene rings lacks a specific side chain. Injecting quinoline did not evoke scratch in either wild-type or Mrgpr-cluster Δ −/− mice (Liu et al. 2009), suggesting that the unique chemical structure of chloroquine is required for inducing itch. Because the majority of Mrgpr genes that are deleted in the cluster knockout mice are expressed specifically in DRG neurons, we hypothesized that the chloroquine itch-resistant phenotype of Mrgpr-cluster Δ −/− mice was likely due to a functional deficit in the DRG neurons in these mice. Using Ca2+ imaging in cultured wild-type DRG neurons, we found that a small subpopulation of DRG neurons (representing approximately 5 % of the total population) is indeed activated by chloroquine (Liu et al. 2009). Although this small subpopulation of chloroquine-responding neurons could be easily overlooked or considered to reflect a nonspecific response, cultured DRG neurons from Mrgpr-cluster Δ −/− mice did not respond at all to chloroquine, indicating that the activation of wild-type DRG neurons by chloroquine is both specific and Mrgpr dependent. Similar results were obtained by performing electrophysiological recordings of DRG neurons to monitor their action potentials in response to chloroquine. Taken together, the behavioral and cellular data pointed to the exciting likelihood that Mrgprs are receptors for chloroquine. To test this hypothesis directly, we cloned the cDNAs of each of the 12 deleted Mrgprs and expressed each receptor individually in HEK293 cells (which do not express endogenous Mrgprs). We found that only the MrgprA3-expressing HEK293 cells responded robustly to chloroquine; the remaining 11 lines of Mrgpr-expressing HEK293 cells were completely insensitive to chloroquine (with the exception of MrgprA1-expressing cells, which responded weakly) (Liu et al. 2009). We were quite surprised by this high specificity of activation, particularly given that MrgprA3 is highly homologous to the other MrgprAs that we tested.
4.2 MrgprC11
Our group and others reported that the preproenkephalin peptide BAM8-22 is a specific ligand for both mouse MrgprC11 and human MrgprX1 receptors (Han et al. 2002; Lembo et al. 2002). The precise physiological function of BAM8-22 is currently unclear. However, because both the MrgprA3 and MrgprC11 receptors are expressed in a single subset of small-diameter DRG neurons, we tested whether BAM8-22 is a pruritogen. Indeed, intradermal injection of BAM8-22 evoked a scratch response in wild-type mice (Liu et al. 2009). In collaboration with Robert LaMotte’s group at Yale University, we used cowhage spicules to deliver BAM8-22 to the skin of healthy human volunteers (Sikand et al. 2011). The participants reported an itch response that was similar in intensity to histamine-induced itch. Importantly, the BAM8-22-loaded spicules did not trigger skin flares or wheals (both of which are hallmarks of histamine-mediated itch). Moreover, pretreating the participants with antihistamine cream completely blocked histamine-evoked itch but had no effect on BAM8-22-induced itch. Finally, BAM8-18, a truncated form of BAM8-22 that lacks the Mrgpr-interacting motif, failed to elicit any sensation in the participants. Taken together, these data support the results of our mouse study and suggest that BAM8-22 is likely an endogenous mediator of histamine-independent itch.
SLIGRL is a 6-amino acid peptide cleaved from the N-terminus of activated PAR2 following protease cleavage. SLIGRL and its human ortholog SLIGKV evoke itch in mice and humans, respectively (Steinhoff et al. 2003; Shimada et al. 2006). Although it is generally accepted that this type of itch is mediated by PAR2, no study has directly addressed this issue (e.g., using PAR2-knockout mice). Because SLIGRL is used widely as a pruritogen for studying histamine-independent itch, we tested the ability of this peptide to induce itch in Mrgpr-cluster Δ −/− mice (Liu et al. 2011). To our surprise, the Mrgpr-cluster Δ −/− mice scratched significantly less in response to SLIGRL injection compared with their wild-type littermates. Perhaps even more surprisingly, the SLIGRL-induced itch response in PAR2-knockout mice was similar to the response induced in wild-type mice, suggesting that this response is not mediated by PAR2. In vitro, SLIGRL activated a small subset of both wild-type and PAR2-knockout DRG neurons (approximately 3 % of the total neuron population), whereas Mrgpr-cluster Δ −/− neurons did not respond at all. Interestingly, most of the SLIGRL-sensitive DRG neurons also responded to BAM8-22, suggesting functional overlap.
Based on these unexpected yet compelling results, we next tested whether SLIGRL can activate Mrgprs directly by expressing each of the 12 Mrgprs in heterologous cells and monitoring activation with Ca2+ imaging. Because HEK293 respond to SLIGRL (presumably because they express endogenous PAR2), we used SLIGRL-insensitive Chinese hamster ovary (CHO) cells for these experiments. Strikingly, among the 12 genes that were tested, only MrgprC11-expressing CHO cells were activated by SLIGRL. In parallel experiments, we also found that SLIGKV (the human version) specifically activated human MrgprX2 but not MrgprX1, MrgprX3, or MrgprX4. Based on a dose-response study, we determined that the EC50 of SLIGRL-induced MrgprC11 activation is on par with the EC50 of SLIGRL-induced PAR2 activation, suggesting that the peptide can activate these two receptors equally well. When we examined the SLIGRL sequence in detail, we hypothesized that the C-terminus of the peptide is required for the activation of MrgprC11, as this region contains a sequence motif that is similar to other MrgprC11-activating peptides such as RYamide and RFamide (Dong et al. 2001; Han et al. 2002). We next generated a truncated SLIGR peptide by removing the C-terminal leucine and found that SLIGR failed to activate MrgprC11 in heterologous cells. On the other hand, the truncated peptide still activated PAR2, as the PAR2-activating motif resides at the N-terminus of SLIGRL (which is intact in the truncated peptide). Therefore, SLIGR provided us with a useful tool to separate MrgprC11 and PAR2 signaling. Unlike SLIGRL, SLIGR cannot directly activate wild-type DRG neurons, nor does it evoke a scratching response in wild-type mice. Taken together, these data strongly suggest that SLIGRL-evoked itch is mediated by MrgprC11 but not PAR2.
Using the truncated peptide SLIGR, we also found that PAR2 plays a role in inflammatory pain by sensitizing TRPV1 channels in the DRG. Moreover, we cannot exclude the possibility that PAR2 mediates itch in other cell types such as keratinocytes. It is also highly conceivable that the function of PAR2 in the DRG switches from pain to itch under chronic itch conditions. For example, human psychophysical studies demonstrated that bradykinin and acetylcholine—two stimuli that are normally painful—evoke an itch response when applied to atopic dermatitis patients (Rukwied and Heyer 1999; Hosogi et al. 2006). Various proteases—including plant-derived proteases such as mucunain and papain and mammalian cathepsin S—evoke an itch response that is likely mediated by PAR2 (or related PARs), as PAR2 can be cleaved and activated by these proteases (Hollenberg and Compton 2002; Ossovskaya and Bunnett 2004). This hypothesis can be tested using PAR2-knockout mice. Our results also raise the interesting possibility that Mrgprs are targets for proteases.
4.3 MrgprD
Both MrgprA3 and MrgprC11 are deleted in our Mrgpr-cluster Δ −/− mice. Thus, is it possible that other Mrgprs that are not within the cluster are also itch receptors? A promising candidate is MrgprD, which is located far from the Mrgpr cluster near the telomere of mouse chromosome 7 and which has a clear human ortholog. Genetic labeling experiments revealed that MrgprD-positive axons only innervate the skin and single-gene MrgprD-knockout (MrgprD −/−) mice have been generated (Zylka et al. 2005). Although MrgprD is expressed in 70 % of IB4-positive DRG neurons (which represent approximately 30 % of all DRG neurons), MrgprD −/− mice do not exhibit any sensory (e.g., thermal or mechanical) deficits. Previous studies revealed that β-alanine, a naturally occurring β-form of alanine, directly binds and activates both the human and mouse MrgprD receptors (Shinohara et al. 2004), although a literature search did not yield any published study demonstrating β-alanine-evoked itch. Nevertheless, we noticed that several weblogs by body builders contained many complaints of severe itch experienced immediately after taking oral β-alanine. Interestingly, β-alanine is a popular supplement used to build muscle; β-alanine is a major component of the dipeptide carnosine, a buffer that prevents muscle cells from becoming acidic, thereby decreasing fatigue during exercise (Crush 1970; Derave et al. 2007; Hill et al. 2007). In order to mimic the effect observed in humans, we gave wild-type and MrgprD −/− mice sweetened water containing β-alanine or sweetened water without β-alanine (Liu et al. 2012). The sweetened water alone (without β-alanine) did not elicit scratching in either wild-type or MrgprD −/− mice. However, several minutes after drinking the β-alanine-containing water, the wild-type mice began scratching (primarily at the back of the neck), and this behavior lasted for at least 1 h. In contrast, the MrgprD −/− mice scratched significantly less after drinking the β-alanine-containing water; the level of scratching in the MrgprD −/− mice was comparable to the scratching response in the mice that were given sweetened water without β-alanine. These data suggest that as in humans, β-alanine induces itch in mice and this effect is solely MrgprD dependent.
We were also excited by the ability to induce itch by delivering a compound orally, as we typically induce itch by injecting a pruritogen into the skin. However, many oral drugs have itch as a side effect. Thus, we can employ this oral delivery model to study the mechanisms of itchy side effects in mice. In addition to the oral delivery assay, we also injected β-alanine into the cheek (Liu et al. 2012). Consistent with oral administration, wild-type mice that were injected with β-alanine exhibited robust scratching with apparent pain response; in contrast, the MrgprD −/− mice did not exhibit an itch response. l-alanine itself (which does not activate MrgprD receptors) did not evoke a significant scratch response in wild-type animals, suggesting that only the β-form of the amino acid (i.e., with the amino group located at the β-position from the carboxylate group) can induce MrgprD-mediated itch. At the cellular level, β-alanine directly activated a subset of wild-type DRG neurons, but this response was absent in MrgprD −/− neurons (see below) (Liu et al. 2012). To quantify and analyze the site-specific effect of β-alanine-induced itch in humans, Dr. LaMotte’s group injected β-alanine into the skin of healthy volunteers (Liu et al. 2012). Upon injection of β-alanine, each participant experienced modest itch that was accompanied by tingling and a mild stinging sensation. Importantly, injecting β-alanine did not generate a typical histamine-mediated itchy skin reaction such as flares or wheals. Finally, as reported in mice, l-alanine did not evoke an itch sensation in humans.
Structurally, β-alanine is similar to the primary inhibitory neurotransmitters GABA and glycine, and β-alanine activates both GABA and glycine receptors (albeit with less potency) (Wu et al. 1993; Rajendra et al. 1997). Furthermore, β-alanine binds to glycine’s co-agonist site on NMDA receptors and suppresses glutamatergic signaling (Ogita et al. 1989; Pullan and Powel 1992). Therefore, in the central nervous system, β-alanine acts as an inhibitory neurotransmitter or modulator. Based on these findings, β-alanine-induced itch has been proposed to involve a central mechanism. However, our analysis of knockout mice strongly suggests that β-alanine-induced itch depends solely on MrgprD receptors. Our skin injection experiments in mice and humans combined with the finding that MrgprD receptors are expressed in DRG neurons—but not in CNS neurons—suggest that β-alanine-induced itch is mediated by a peripheral, cutaneous mechanism.
5 Mrgprs as Molecular Markers of Itch-Mediating Neurons
Both itchy and painful stimuli are detected by small-diameter nociceptors in the DRG. Given the similarities between itch-mediating and pain-mediating neurons and the differences between these sensations and their behavioral responses (e.g., pain elicits a withdrawal response and itch elicits a scratch response), several theories have been proposed to explain itch and pain coding. Here, we discuss the two most prominent theories, namely, the intensity and labeled-line theories. The “intensity theory” holds that itch and pain are perceived by a common group of DRG neurons. Neurons discriminate between the two based on the intensity of stimulation received and encode into different patterns of electrical activities and transmit through the same synaptic pathway to the brain. On the other hand, the “labeled-line theory” posits that itch and pain are detected and encoded by discrete groups of DRG neurons and transmitted to the brain via separate synaptic pathway (Patel and Dong 2010, 2011). Other theories such as population coding and contrast coding will not be discussed here due to space constraints, and the reader is referred to published literature for further information regarding these theories (Davidson and Giesler 2010; Patel and Dong 2010, 2011).
5.1 MrgprA3-Positive Neurons
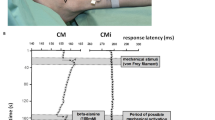
The chloroquine receptor MrgprA3 is expressed in 5–8 % of all DRG neurons (the reported percentage differs depending on the detection method used and the spinal level of the ganglion that is examined) (Liu et al. 2008, 2009; Han et al. 2013). These neurons are positive for both IB4 labeling and CGRP expression and negative for substance P (Han et al. 2013). However, the level of CGRP expression in MrgprA3-positive neurons is lower than in canonical peptidergic neurons. Therefore, the pattern of MrgprA3 expression is not random, but is specific to a subset of DRG neurons. Because MrgprA3-positive neurons can be activated by several pruritogens (including chloroquine, BAM8-22, and histamine) (Liu et al. 2009), they are a promising candidate as the itch-specific neurons. To test this hypothesis, we used the BAC transgenic approach to generate a new mouse line in which a GFP-Cre fusion protein is expressed selectively in MrgprA3-positive neurons (Han et al. 2013). Thanks to recent advances in mouse genetics, we were able to cross this MrgprA3-Cre line with several Rosa26 reporter lines, thus enabling specific expression of various reporter genes in MrgprA3-positive neurons without the need to generate additional mouse lines. With these tools in hand, we first examined the morphology of these DRG neurons using a reporter mouse expressing the red fluorescent protein tdTomato. Strikingly, MrgprA3-positive axons (i.e., red fluorescent fibers) innervate the skin but not any other tissue examined; this finding provides a cellular explanation for why we only feel itch in the skin but not in other, deeper tissues such as muscles and visceral organs (Han et al. 2013). Dr. LaMotte’s group then used their powerful in vivo DRG neuron recording technique to study the cellular properties of MrgprA3-positive neurons (identified clearly by their intrinsic green fluorescence). They recorded soma activation of lumbar DRG neurons in response to various stimuli applied to the receptive fields in the hind limbs and hind paws of live mice. Strikingly, they found that the electrophysiological properties of the MrgprA3-positive neuron population are remarkably homogenous. These neurons are typical polymodal nociceptive neurons, as they have slow conduction velocity (0.5 m/s) and respond to noxious heat, noxious mechanical force, and capsaicin (Han et al. 2013). In addition, MrgprA3-positive neurons can be activated by a variety of pruritogens, including histamine, BAM8-22, chloroquine, and cowhage. On the other hand, MrgprA3-positive neurons do not respond to the MrgprD agonist β-alanine (Han et al. 2013). Interestingly, ablation of MrgprA3-positive neurons specifically affects itch but not pain. Finally, we used a genetic approach to generate mice in which TRPV1 is exclusively expressed in MrgprA3-positive neurons. Strikingly, capsaicin, a normally painful stimulus, caused itch and not a pain response in the MrgprA3-TRPV1 mice. Together, these data strongly suggest that MrgprA3-positive neurons are itch-specific neurons and most fit the labeled-line theory.
5.2 MrgprD-Positive Neurons
Although both MrgprA3-positive neurons and MrgprD-positive neurons stain positive for IB4, these receptors label two discrete subpopulations with both overlapping and nonoverlapping cellular properties (Dong et al. 2001; Zylka et al. 2003). Like MrgprA3-positive neurons, MrgprD-positive neurons also terminate in the most superficial layer of the epidermis (Zylka et al. 2005). However, unlike MrgprA3-positive neurons, MrgprD-positive neurons represent the majority of the IB4-positive neurons (representing approximately 70 % of IB4-positive neurons and 30 % of all DRG neurons). In addition, MrgprD-positive neurons are not CGRP negative (Zylka et al. 2005) (Fig. 1). Based on in vivo DRG recordings in MrgprD-GFP mice, MrgprD-positive neurons are composed of a mixed population of neurons—although all of the neurons responded to noxious mechanical force, only half of the neurons responded to noxious heat (the other half were not sensitive to this stimulus). Furthermore, only the heat-sensitive neurons responded to β-alanine (the heat-insensitive neurons did not respond to β-alanine). Strikingly, none of the MrgprD-positive neurons responded to pruritogens that activate MrgprA3 (Liu et al. 2012). The identification of two distinct itch-responsive neuron populations (MrgprD + and MrgprA3 + neurons, Fig. 2) provides us with a unique opportunity to investigate how itch signals are perceived, encoded, and transmitted in the nervous system.
Two distinct itch-responsive neuron populations (His + /MrgprA3 + and His − /MrgprD + neurons) in the DRG. They detect various exogenous or endogenous itch-inducing molecules via itch receptors on their peripheral axons in the skin and transmit the itch signals to the spinal cord via their central axons
5.3 The Role of Mrgprs-Positive Neurons in Chronic Itch
Chronic pruritus (chronic itch) severely affects both quality of life and productivity. Based on their neuropathophysiological mechanisms and origin, chronic itches can be classified into the following four categories: pruritoceptive itch, which is mediated by the activation of cutaneous afferent fibers; neuropathic itch, which results from diseased or lesioned itch-related neural circuitry; neurogenic itch, which is induced by mediators acting centrally in the absence of neural damage; and psychogenic itch. Of these four types of chronic itch, only pruritoceptive itch arises exclusively from peripheral mechanisms and is most commonly seen in clinical practice. Because Mrgprs are expressed selectively in primary sensory neurons in the peripheral nervous system, here, we will delineate the role of Mrgprs- and Mrgpr-expressing neurons in mediating pruritoceptive chronic itch.
In pruritoceptive chronic itch, the activity of secondary cell types such as keratinocytes or mast cells is believed to trigger the release of substances that act directly on a group of primary sensory neurons in order to elicit the itch sensation (Paus et al. 2006; Davidson and Giesler 2010; Patel and Dong 2011). However, which substances are released from these neurons and which group of sensory neurons is activated are currently unknown, and our lack of this information has greatly impeded our ability to understand chronic itch. As mentioned above, MrgprA3 selectively labels a subpopulation of itch-sensing neurons (Han et al. 2013). Thus, this begs the question of whether this population of neurons is activated by secondary cells to mediate chronic itch. Indeed, our recent studies revealed that ablating MrgprA3-positive neurons dramatically reduced the severity of both allergy-induced and dry skin-induced chronic itch (Han et al. 2013). These results provided the first evidence of a critical group of sensory neurons in chronic itch and opened new avenues for investigating both allergic and nonallergic chronic itch.
Mast cells are the immune effector cells that play a key role in mediating allergic chronic itch. Mast cells originate in the bone marrow and migrate to many peripheral tissues—including the lungs, intestines, peritoneum, and skin—where they mature and then reside (Metcalfe et al. 1997). We previously reported that Kit W-sh /Kit W-sh mice (which lack mast cells due to a chromosomal inversion that affects c-kit gene function) do not scratch in response to allergy, suggesting that mast cells are required for mediating allergy-associated itch (Patel et al. 2011). When mast cells are activated by cross-linking the high-affinity IgE receptor (FcεRI) through an allergen challenge, they release a plethora of preformed mediators—including histamine, serotonin, lipid compounds, proteases, and neuropeptides—to induce an itch sensation (Metcalfe et al. 1997; Metz and Maurer 2009). It is generally accepted that IgE-mediated allergic itch is mediated principally by the action of histamine on its receptor (H1) expressed in skin sensory nerve fibers. However, recent studies revealed that allergic itch is only mildly reduced in H1-deficient mice (Sugimoto et al. 2003), suggesting an involvement of histamine-independent pathways in itch allergy. The identification of MrgprA3-positive neurons as itch-sensing neurons raises an interesting question: Do histamine-independent Mrgprs mediate the communication between mast cells and sensory neurons and induce allergic itch? Future studies will test the function of Mrgprs in allergic itch and will likely provide novel insight into the etiology of allergic itch.
Unlike allergic itch, nonallergic itch lacks antigen specificity. Nonallergic itch can be generated by harmful metabolites, immune factors, or the activity of nonimmune cells such as keratinocytes. Keratinocytes are the most important resident cells in the skin (Metz and Maurer 2009; Raap et al. 2011). In addition to providing an effective barrier against harmful environmental factors, keratinocytes also play an important role in the induction and modulation of cutaneous sensations such as warmth, cold, contact, pain, and itch. Previous studies have shown that keratinocytes can release ATP and prostaglandin E2 (PGE2) to activate sensory fibers in the skin and modulate thermal and pain sensations (Koizumi et al. 2004; Huang et al. 2008; Mandadi et al. 2009). However, the specific signal transduction pathways between keratinocytes and sensory fibers in chronic itch remain poorly understood. Studies have shown that TRPV3 channels are expressed in high levels in keratinocytes and mice with the gain-of-function Gly573Ser substitution in the TRPV3 develop chronic itch (Yoshioka et al. 2009). In addition, overexpressing the stratum corneum chymotryptic enzyme in keratinocytes induces chronic itchy dermatitis in mice (Hansson et al. 2002; Ny and Egelrud 2003). These studies support the notion that keratinocytes play a role in chronic itch. However, which population of sensory neurons detects the substances released from keratinocytes—and thus mediates chronic itch—was unclear. Our recent studies revealed that MrgprA3-positive neurons play a key role in dry skin-induced chronic itch (Han et al. 2013), which has cast a new light on the interaction between keratinocytes and itch-sensing neurons. Furthermore, these results led to the intriguing hypothesis that Mrgprs might be the missing link between keratinocytes and itch-sensing sensory fibers, thus providing a new direction for researchers to explore the signaling cascade between keratinocytes and sensory neurons in nonallergic itch.
6 Future Direction
Itch is a complex sensory modality that can be evoked by an extremely diverse set of stimuli (chemical, mechanical, and thermal) and has multiple components of disease etiology (cutaneous, neurological, and immunological). Thus, determining the basic molecular and cellular players is essential before we can tackle the more complex aspects of itch. Although the identification of novel itch receptors has been extremely fruitful and has uncovered novel signaling pathways and pruritogens, much more research is clearly needed to understand all of the mechanisms that underlie the various forms of itch. For instance, MrgprA3-positive neurons express high levels of histamine receptors and many other Mrgprs and can be activated by many pruritogens (Fig. 2), suggesting a “broadly tuned” cellular mechanism. It is distinct from “finely tuned” chemosensations such as olfaction in which each olfactory receptor neuron expresses only one olfactory receptor. This finding begs the question of how the body discriminates between different types of itch evoked by chemically diverse molecules. In addition, what is the functional relevance of Mrgpr divergence in the mouse genome? What are the endogenous ligands for Mrgprs? Efforts to address these questions will greatly advance our understanding of the molecular and cellular mechanisms underlying itch perception and lead to new frontiers for studying itch.
Abbreviations
- CNS:
-
Central nervous system
- BAM8-22:
-
Bovine adrenal medulla 8-22 peptide
- CGRP:
-
Calcitonin gene-related peptide
- DRG:
-
Dorsal root ganglion
- γ2-MSH:
-
γ2-melanocyte-stimulating hormone
- GABA:
-
Gamma-aminobutyric acid
- GFP:
-
Green fluorescence protein
- IB4:
-
Isolectin B4
- Mrgpr:
-
Mas1-related G protein-coupled receptor
- NPFF:
-
Neuropeptide FF
- NPAF:
-
Neuropeptide AF
- TRPV1:
-
Transient vanilloid receptor 1; the capsaicin receptor
References
Abila BE, Ezeamuzie IC, Jose MMB, Tamunokonbia I (1989) Effects of intradermal chloroquine in healthy black subjects who itch with oral chloroquine and in those who do not itch. Med Sci Res 17:665–667
Abila B, Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, Asomugha L (1994) Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. Afr J Med Med Sci 23:139–142
Ademowo OG, Walker O, Sodeinde O (1998) Prevalence of chloroquine-induced pruritus: evidence for heredofamilial factors. Malaria Infect Dis Afr 8:14–18
Buck L, Axel R (1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65:175–187
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Chen H, Ikeda SR (2004) Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci 24:5044–5053
Crozier RA, Ajit SK, Kaftan EJ, Pausch MH (2007) MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci 27:4492–4496
Crush KG (1970) Carnosine and related substances in animal tissues. Comp Biochem Physiol 34:3–30
Davidson S, Giesler GJ (2010) The multiple pathways for itch and their interactions with pain. Trends Neurosci 33:550–558
Derave W, Ozdemir MS, Harris RC, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E (2007) beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol 103:1736–1743
Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ (2001) A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106:619–632
Gogos JA, Osborne J, Nemes A, Mendelsohn M, Axel R (2000) Genetic ablation and restoration of the olfactory topographic map. Cell 103:609–620
Green AD, Young KK, Lehto SG, Smith SB, Mogil JS (2006) Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 124:50–58
Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI (2002) Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci USA 99:14740–14745
Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X (2013) A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16:174–182
Hansson L, Backman A, Ny A, Edlund M, Ekholm E, Ekstrand Hammarstrom B, Tornell J, Wallbrandt P, Wennbo H, Egelrud T (2002) Epidermal overexpression of stratum corneum chymotryptic enzyme in mice: a model for chronic itchy dermatitis. J Investig Dermatol 118:444–449
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007) Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32:225–233
Hollenberg MD, Compton SJ (2002) International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev 54:203–217
Hosogi M, Schmelz M, Miyachi Y, Ikoma A (2006) Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 126:16–23
Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci 28:13727–13737
Ikoma A, Handwerker H, Miyachi Y, Schmelz M (2005) Electrically evoked itch in humans. Pain 113:148–154
Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M (2004) Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J 380:329–338
Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S (2002) Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci 5:201–209
Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q (2008) Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci 28:125–132
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139:1353–1365
Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X (2011) The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 4:ra45
Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, Lamotte RH, Dong X (2012) Mechanisms of Itch Evoked by beta-Alanine. J Neurosci 32:14532–14537
Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, Axel R (2006) Interchromosomal interactions and olfactory receptor choice. Cell 126:403–413
Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M (2009) TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch 458:1093–1102
Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77:1033–1079
Metz M, Maurer M (2009) Innate immunity and allergy in the skin. Curr Opin Immunol 21:687–693
Mnyika KS, Kihamia CM (1991) Chloroquine-induced pruritus: its impact on chloroquine utilization in malaria control in Dar es Salaam. J Trop Med Hyg 94:27–31
Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M (2008) Separate peripheral pathways for pruritus in man. J Neurophysiol 100:2062–2069
Ny A, Egelrud T (2003) Transgenic mice over-expressing a serine protease in the skin: evidence of interferon gamma-independent MHC II expression by epidermal keratinocytes. Acta Derm Venereol 83:322–327
Offermanns S, Simon MI (1995) G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem 270:15175–15180
Ogita K, Suzuki T, Yoneda Y (1989) Strychnine-insensitive binding of [3H]glycine to synaptic membranes in rat brain, treated with Triton X-100. Neuropharmacology 28:1263–1270
Olatunde A (1977) The practical and therapeutic implications of chloroquine-induced itching in tropical Africa. Afr J Med Med Sci 6:27–32
Ossovskaya VS, Bunnett NW (2004) Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84:579–621
Patel KN, Dong X (2010) An itch to be scratched. Neuron 68:334–339
Patel KN, Dong X (2011) Itch: cells, molecules, and circuits. ACS Chem Neurosci 2:17–25
Patel KN, Liu Q, Meeker S, Undem BJ, Dong X (2011) Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One 6:e20559
Paus R, Schmelz M, Biro T, Steinhoff M (2006) Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 116:1174–1186
Pullan LM, Powel RJ (1992) Comparison of binding at strychnine-sensitive (inhibitory glycine receptor) and strychnine-insensitive (N-methyl-D-aspartate receptor) glycine binding sites. Neurosci Lett 148:199–201
Raap U, Stander S, Metz M (2011) Pathophysiology of itch and new treatments. Curr Opin Allergy Clin Immunol 11:420–427
Rajendra S, Lynch JW, Schofield PR (1997) The glycine receptor. Pharmacol Ther 73:121–146
Rukwied R, Heyer G (1999) Administration of acetylcholine and vasoactive intestinal polypeptide to atopic eczema patients. Exp Dermatol 8:39–45
Sams WM Jr, Epstein JH (1965) The affinity of melanin for chloroquine. J Invest Dermatol 45:482–487
Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE (1997) Specific C-receptors for itch in human skin. J Neurosci 17:8003–8008
Shelley WB, Arthur RP (1957) The neurohistology and neurophysiology of the itch sensation in man. AMA Arch Derm 76:296–323
Shimada SG, Shimada KA, Collins JG (2006) Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530:281–283
Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S (2004) Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem 279:23559–23564
Sikand P, Dong X, LaMotte RH (2011) BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31:7563–7567
Spencer HC, Poulter NR, Lury JD, Poulter CJ (1982) Chloroquine-associated pruritus in a European. Br Med J (Clin Res Ed) 285:1703–1704
Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M (2003) Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 23:6176–6180
Sugimoto Y, Nakamura Y, Hossen MA, Watanabe T, Kamei C (2003) Evaluation of the effects of anti-pruritic drugs on scratch responses using histamine H1 receptor-deficient mice. Eur J Pharmacol 470:113–116
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543
Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14:595–602
Wu FS, Gibbs TT, Farb DH (1993) Dual activation of GABAA and glycine receptors by beta-alanine: inverse modulation by progesterone and 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol 246:239–246
Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, Sakata T, Horikawa T, Arimura A (2009) Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol 129:714–722
Zylka MJ, Dong X, Southwell AL, Anderson DJ (2003) Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA 100:10043–10048
Zylka MJ, Rice FL, Anderson DJ (2005) Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45:17–25
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Liu, Q., Dong, X. (2015). The Role of the Mrgpr Receptor Family in Itch. In: Cowan, A., Yosipovitch, G. (eds) Pharmacology of Itch. Handbook of Experimental Pharmacology, vol 226. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44605-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-662-44605-8_5
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44604-1
Online ISBN: 978-3-662-44605-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)