Abstract
Chronic itch is a multidimensional physical state strongly associated with emotional and cognitive aspects of suffering that causes the urge to scratch. Pathophysiology, psychological stress, and social milieu can influence itch. Here, we review brain neuroimaging research in humans that detects functional and anatomic changes in health and disease states. New data are emerging that are shaping our understanding of itch mechanisms and scratching—the behavioral response as well as the effect of treatments and brain dynamics during itch. Future developments will continue to expand our knowledge of itch mechanisms, allowing translation to clinical assessment and novel therapies focused on the brain, the final relay of itch transmission.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and magnetoencephalography (MEG) have been commonly used during itch episodes to objectively document pruritus (Hsieh et al. 1994; Darsow et al. 2000; Drzezga et al. 2001; Mochizuki et al. 2003, 2007, 2009; Herde et al. 2007; Leknes et al. 2007; Papoiu et al. 2012). The majority of the studies focused on healthy subjects. Only a few studies have assessed brain processing of itchy stimuli in patients with chronic pruritus and demonstrated that it differs from that of healthy controls (Schneider et al. 2008; Ishiuji et al. 2009). As yet there is no clear itch neuromatrix similar to the pain neuromatrix. However, it is clear that there are unique areas that differentiate pain from itch. Here, we review progress in neuroimaging research that is contributing to the development of tools that are clinically relevant to future management of chronic itch patients.
2 Itch Transmission to the Superior Centers of the Central Nervous System
7Two main neuronal pathways have been described for itch transmission: one mediated by histamine and the other by protease-activated receptors PAR2(4) that can be exogenously stimulated by spicules of cowhage (Mucuna pruriens). The continuing spinothalamic pathways ascend via the lateral spinothalamic tract and (mostly) maintain their specialization up to their thalamic (third neuron) stations (Davidson et al. 2009, 2012). Only recently with development of higher-resolution fMRI has the thalamus been shown as the first relay in the brain for itch stimuli associated with both histamine and cowhage. It is known that the spinothalamic neurons project in the ventral posterior lateral (VPL), ventral posterior inferior (VPI), and posterior nuclei, while cowhage-sensitive neurons additionally end in the suprageniculate and medial geniculate nuclei (Davidson et al. 2012). Functional MRI data demonstrated that cowhage itch activated more strongly than histamine, in particular a thalamic area consistent with the location of the mediodorsal nucleus (Leknes et al. 2007; Papoiu et al. 2012) which is connected with the orbitofrontal area and the limbic system. Of note, a dysfunction of this circuit was suggested to occur in processing itch in atopic patients (Leknes et al. 2007).
3 Itch Transmission from the Thalamus to the Cortex
As yet the exact projections of the thalamic neurons conveying itch information from the thalamus to the cerebral cortex have not been identified. Future studies assessing brain connectivity during itch stimuli will provide us with better insights on these projections.
4 Cortical Regions that Are Important During Itch Processing
Numerous brain imaging studies, irrespective of the investigative modalities, demonstrated that there are several cortical regions in humans that are considered to be important for the perception of itch. The primary and secondary somatosensory cortices (S1, S2), the insula and Anterior cingulate cortex (ACC), and the prefrontal cortex (PFC) are commonly activated, often bilaterally during itch stimuli. These areas are not exclusively involved in itch and are known to be highly activated by pain and other sensory stimuli. The activity of these areas reflects the multidimensional character of the itch experience and the complexity of itch processing in the brain. Areas such as the ACC and insula are associated with an emotional–affective response recruiting deep-seated areas of the limbic system, areas connected to craving and unpleasantness as well as pleasure and addiction. Itch is a bothersome, intrusive, acute sensation, requiring immediate action; thus, major arms of the cerebral itch response are involved in refocusing attention, planning the motor action, and seeking itch relief. Other areas that have shown high activity during itch are the premotor, motor, and supplementary motor areas as well as the cerebellum which may control the urge to scratch. The precuneus (medial parietal cortex) is prominently activated by the sensation of itch (Mochizuki et al. 2009; Papoiu et al. 2012). This area is related to memory retrieval, visuospatial processing, and self-awareness and has rarely been reported in imaging of pain processing. It is considered part of the default network. The insula has a paramount role in processing itch information; it is a cortical region linked to salience, self-awareness/interoception, and addiction (among others). The insula is considered a major hub for processing visceroceptive and interoceptive inputs and is significantly involved in the processing of pain and, especially, in assessing stimulus intensity. The bilateral insula is activated in patients with end-stage renal disease (ESRD) pruritus at rest.
The claustrum is a discrete gray matter area whose role has recently been emphasized in itch processing. The functional specialization and connectivity of the claustrum seem very fitting for a region involved with itch sensing since it has the capability to analyze, compare, and integrate sensory information from various inputs; it is connected to almost all areas of the cortex, but (especially) with the somatosensory cortex, thalamus, and limbic structures (cingulate cortex, hippocampus, amygdala). The claustrum is closely linked, functionally and anatomically, with the insula. It is activated more extensively by a complex, transient, fluctuating itch stimulus, such as cowhage, than by a rather constant stimulus like histamine (Papoiu et al. 2012). Activation of the claustrum (as well as of the insula) is largely correlated with the perceived itch intensity, although some discrete areas are activated irrespective of itch stimulus intensity (Papoiu et al. 2012). The insula and claustrum (in particular) are activated continuously, while itch intensity varied and is fully activated bilaterally when histamine and cowhage stimuli are administered at the same time (Papoiu et al. 2012). These features suggest a principal role in itch processing for these regions.
5 Prefrontal and Limbic Control
Numerous imaging studies have demonstrated activation of the PFC by applying itch stimuli, suggesting that this region can regulate the perception and behavioral expression of itch in humans in a manner very similar to pain. The PFC is connected to limbic regions that regulate motivation and emotion. Coactivation of these regions during itch stimuli implies that the motivational and emotional aspects of itch are also regulated by this network. A recent imaging study of the cerebral processing of scratching suggested that the dorsolateral prefrontal cortex (DLPFC) controls the itch response via possible connections with the amygdala to suppress the itch (Yosipovitch et al. 2008). These areas could be targets for drug therapies and psychological biobehavioral treatments for chronic itch.
Frontal limbic regions reciprocally connected to the brain stem via periaqueductal gray matter (PAG), nucleus accumbens (NAc), and tegmentum can inhibit or exacerbate the itch experience via inhibition or activation of neurons in the spinal cord (Carstens 1997; Millan 2002; Davidson and Giesler 2010).
6 Differential Cerebral Processing of Histamine and Cowhage Itches
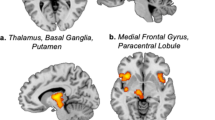
In healthy individuals, the cerebral representation of cowhage itch displays a common core of activation with that of histamine itch while showing certain features that are clearly unique (Fig. 1). These activations provide a comprehensive detail of itch processing in the brain of the two major itch pathways. Of note, cowhage evokes a more extensive activation of the insula, claustrum, globus pallidus, caudate body, putamen, and thalamic nuclei on the contralateral side of the stimuli (Fig. 1). These differences may be related to not only an intrinsic specificity in cortical projection, but also to the fluctuating quality and associated nociceptive signaling (e.g., stinging, burning) elicited by cowhage. These sensations are frequently reported in many cases of chronic itch (Yosipovitch et al. 2002).
The overlap of brain activations induced by histamine itch (in green) and by cowhage itch (in blue) illustrates the regions coactivated (in red) and distinct areas activated separately by the two itch pathways. Standard Talairach space coordinates. The color tones displayed correspond to Z score values as shown in the color bar. ACC anterior cingulate cortex, PCC posterior cingulate cortex, SPL superior parietal lobule, M1 primary motor cortex, S1 primary somatosensory area, SMG supramarginal gyrus, MTG middle temporal gyrus, IPL inferior parietal lobule, S2 secondary somatosensory area, VPL ventral posterior lateral nucleus (of thalamus) (can be appended separately; from NeuroImage 59(4) 2012)
7 Brain Processing of Chronic Itch
A few neuroimaging studies have been performed in patients with chronic itch. These studies have revealed altered function (Schneider et al. 2008; Ishiuji et al. 2009; Papoiu et al. 2014); one study also demonstrated altered structure in the frontolimbic regions compared to healthy controls.
In atopic dermatitis, which is the most common skin disease causing chronic itch, activation of the ACC and DLPFC is directly correlated with disease severity (as measured by the Eczema Area and Severity Index (EASI) score, a standardized validated clinical tool). Intensity of histamine-induced itch correlates with activations in the ACC and insula. Overall, the pattern of association between activation and perceived itch intensity is different in healthy volunteers (Schneider et al. 2008; Ishiuji et al. 2009; Yosipovitch group, unpublished). The distinction between the patterns evoked by histamine and cowhage itches, clearly identified in healthy individuals, appears to be blurred in chronic itch diseases (ESRD and AD). Recently, an investigation of structural and functional perfusion differences between ESRD patients with chronic pruritus and healthy individuals found a significant thinning of the gray matter in the thalamus, insula, ACC, precuneus, and caudate body (areas involved in itch processing) in the ESRD patients. Assessment was by voxel-based morphometry (VBM), a technique that quantitates gray matter densities. These MR-based changes were not demonstrated in two chronic itch states: atopic eczema and psoriasis (unpublished data). In pruritic patients with ESRD, persistent perfusion increases at baseline in the insula, ACC, claustrum, amygdala, hippocampus, and NAc—areas that are known to be highly activated by itch (Fig. 2). Interestingly, baseline brain perfusion in chronic itch of atopics and psoriatics does not differ from that in healthy subjects. These results further suggest that chronic itch states differ in brain imaging patterns. Moreover, the processing of cowhage itch appeared altered in ESRD, while no significant differences could be demonstrated in the processing of histamine-induced itch. In ESRD pruritus, multiple brain activations appear to work either directly or are inversely correlated with perceived itch intensity, suggesting a dual modulation of itch perception. These unique features can be facilitated by the reduced gray matter thickness in ESRD affecting critical areas involved in itch processing, thus revealing a form of neocortical plasticity. As with chronic pain, central neuronal remodeling occurs (Parise et al. 2014). In ESRD, it appears that the PAR2-mediated itch pathway is already overstimulated, in association with an overexpression of PAR2 in the skin. This may lead to a tonic inhibition of cortical processing of acute cowhage itch when induced in the preexistent context of ESRD.
Baseline state (resting) perfusion increases or brain activations identified in ESRD patients with chronic pruritus in significant contrast with healthy volunteers. Brain perfusion was higher at baseline in ESRD patients with chronic pruritus compared to healthy individuals in the insula, claustrum, ACC, amygdala, entorhinal cortex, and subcallosal gray matter—nucleus accumbens. Arterial spin labeling fMRI; p < 0.05. STG superior temporal gyrus, S2 secondary somatosensory area, ACC anterior cingulate cortex
A central “top-to-bottom” inhibition of itch is drawn from the parallel descending pathway for suppression of pain and proposes that the PAG modulates the activity of spinal interneurons. According to this model, descending inputs directed toward itch receptive neurons in the dorsal horn exert an inhibitory action, effectively silencing them (Carstens 1997; Davidson and Giesler 2010). Another possibility is that the cortical projection of itch information into S1/S2 may be inhibited via cortico-cortical inhibitory loops, in a similar fashion to mechanisms that have been known to operate in chronic pain (Henry et al. 2011). Our recent findings in ESRD patients possibly suggest that a tonic inhibition may be exerted at the neocortical level to selectively limit the receptive fields for PAR2-mediated itch processing in S1, precuneus, and insula. These findings are of significant interest because they offer insight into mechanisms the brain may employ to process and modulate itch sensation. Contrary to a widely accepted paradigm in the neuroimaging literature, it is thus possible that a higher intensity itch does not necessarily translate into a higher or more extensive activation of the cerebral cortex. Moreover, longitudinal studies from patients with chronic pain, which shares many similarities with chronic itch, suggest that abnormalities in gray matter densities within brain regions such as the ACC and insula can resolve after treatments that reduce the symptom (Rodriguez-Raecke et al. 2009).
8 Craving for Itch Relief and Its Cerebral Mechanisms
The sensation of itch and the immediate craving for itch relief manifested as the urge to scratch are inseparable. Four recent fMRI studies have investigated the cerebral processing of scratching. In three of them, experimenters scratched the subjects’ skins using brushes and copper plates (Yosipovitch et al. 2007, 2008; Vierow et al. 2009; Mochizuki et al. 2014). In the fourth one, visualized active scratching provided a more robust scratch response (Papoiu et al. 2014). The observed brain regions common to these studies were the PFC, ACC, insula, secondary S2, and cerebellum (Fig. 3). The significant activations of the PFC and insula during scratching are interesting since these regions, in particular the PFC, are less sensitive to other tactile stimuli such as a vibrotactile stimulus (Gelnar et al. 1999; Seitz and Roland 1992; Coghill et al. 1994; Golaszewski et al. 2002), Hagen and Pardo 2002; Burton et al. 2004). Several clinical studies on patients with addictive behaviors have also demonstrated the importance of the PFC in motivation and reward. Reward circuits have been linked with the pleasurability of scratching and may play an important role in itch inhibition, as well as in the formation of “vicious” itch–scratch cycles. Recent findings suggest that the reward system of the midbrain, more specifically the ventral tegmentum (VTA), and substantia nigra, as well as the NAc, may play a role in the urge to scratch and the subsequent satisfaction (pleasure) derived from scratching, via connections with the insula, ACC, and striatum (Papoiu et al. 2014; Mochizuki et al. 2013, 2014). The involvement of VTA and NAc underscores the addictive nature of the itch–scratch cycle and also suggests a role for the dopaminergic system in itch relief (Papoiu et al. 2013, 2014).
9 Role of the PAG in Itch Inhibition
The PAG has been considered the major descending inhibitory control of pain (Millan 2002). A PET study showed increased PAG activity, while itch sensation was reduced, by applying pain stimuli (Mochizuki et al. 2003). Thus, it is likely that descending inhibitory control has the potential to inhibit itch sensation. However, our recent fMRI studies of scratching did not show activation of the PAG; in fact, deactivation of the PAG was noted with high Z scores. Additionally, there was significant activation of the ventral tegmentum (which is closely related to the PAG) during scratching an itch. Thus, more precise investigations using electrophysiological techniques with animals, and fMRI with higher spatial resolution (e.g., 7 T), will be necessary to clarify whether descending PAG inhibitory control is associated with itch inhibition by scratching.
10 Modulation of Itch Targeting the Brain
Currently, there are no published studies on the effect of antipruritic drugs against itch-related changes in brain wiring. We recently demonstrated that butorphanol, a kappa opioid agonist and mu opioid antagonist, known to exert antipruritic effects in the spinal cord (Dawn and Yosipovitch 2006) completely suppressed the itch induced experimentally with histamine (Papoiu et al. 2015). The functional MRI data showed that, in comparison with the placebo, butorphanol produced a bilateral deactivation of the claustrum, insula, and putamen, areas described to be activated during itch processing. The inhibition of histamine itch by butorphanol was paralleled by well-defined, significant activations which mapped to nucleus accumbens bilaterally and to a subcallosal gray matter area on the midline consistent with the location of septal nuclei. Our results indicate that the antipruritic action of butorphanol is mediated by these two formations, known to express a high density of κ opioid receptors (Peckys and Landwehrmeyer 1999; Peckys and Hurd 2001) on which it is likely the κ opioid agonist, butorphanol, acts. This is the first clear identification of discrete structures within the human brain capable of exerting itch suppressions upon opioid activation.
Another study assessed the effect of acupuncture on itch-evoked activation and demonstrated reduced activation in the insula, putamen, and premotor and prefrontal cortical areas (Napadow et al. 2012). An overactive limbic system (anterior cingulate cortex–amygdala–nucleus accumbens) may reflect a more intense, unbalanced craving for itch relief, accompanied by activations in the insula (as seen in ESRD pruritus at baseline) as well as an increase in acute itch induction in atopic eczema itch and psoriatic itch. This leads to the amplification of compulsive scratching behavior and more distress. Therefore, addressing the emotional and psychological suffering by targeting these areas is a cornerstone for building a successful therapy for pruritus. Brain areas involved in self-awareness (precuneus) and self-perception (insula) are involved, confirming the observation that itch is a very intrusive and disturbing sensation, perturbing the well-being of the person.
Neuroimaging studies using quantitative arterial spin labeling (ASL) enable us to measure regional changes in cerebral blood flow in itch states associated with slow (tonic) activities and provide insight not only on the physiological responses (or their dysfunction), but also serve as a window into the supramodal functions of the mind and psyche. Cognitive and emotional aspects of the itch experience influence the higher-level integration of physical stimuli. A successful treatment would need to target and interrupt the vicious itch–scratch cycle and offer a solution for the intense, “amplified” craving for relief. This raises the possibility that cognitive behavioral techniques could be helpful in limiting the emotional and affective impact of this bothersome symptom. Refocusing attention on tasks unrelated to itch could be one avenue worth exploring, since these approaches have been shown to be effective in diminishing the perception of pain (Zeidan et al. 2011). These “mindfulness”-reframing techniques may prove to be even more effective for the subjective relief of itch, since itch (contrary to pain) can be easily generated via a central induction mechanism (the “contagious itch” phenomenology). Therefore, if there is a central “source” that is capable of producing an itch sensation (in the absence of external pruritogenic stimuli), there must be a way to reverse the mechanism, turning it into a cure.
11 Contagious Itch
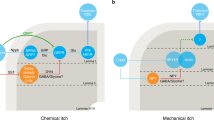
Contagious itch is an intriguing phenomenon in daily life. Thus, observers, while looking at others scratching an itch, themselves feel itchy. It has been studied in humans and primates (Niemeier et al. 2000; Papoiu et al. 2011; Feneran et al. 2012; Holle et al. 2012; Lloyd et al. 2012). The elucidation of central mechanisms underlying contagious itch is of high interest since it could provide invaluable clues for the treatment of itch. A recent brain imaging study pointed toward BA44 and the premotor cortex (BA6) as significant areas activated during the process of “itch induction.” These findings can be seen as a first step in attempting to elucidate the phenomenon (Holle et al. 2012). Another brain imaging study of contagious itch reported that a functional coupling of the insula and basal ganglia likely plays an important role in triggering scratching while viewing and imagining situations associated with itch (Mochizuki et al. 2013) (Fig. 4). Stated bluntly, the existence of a single, specific itch (processing) center remains elusive. It is becoming increasingly clear that the complex neuronal processes involved in processing itch cannot be reduced to a single cortical or subcortical area.
Increased functional connectivity while imaging the itch sensation. Red regions: brain regions in that activity significantly increased functional connection with activity in the insula. Green regions: the functional connectivity while imaging the itch sensation was significantly stronger than that while imaging the pain sensation. TA the tegmentum, BG the basal ganglia, ACC the anterior cingulate cortex, Gp the globus pallidus. The figure has been reproduced with permission of the International Association for the Study of Pain® (IASP)
Contagious itch is significantly easier to induce in atopic dermatitis sufferers than in healthy volunteers. Interestingly, the itch induced by visual cues had a scattered, wide body distribution. We suspect that there are several brain centers that can support the generation of a somatic sensation in the absence of peripheral stimulation. Further elucidation of brain mechanisms behind this phenomenon can provide insight on specific central areas that can be targeted and used in the therapeutic approach to relieve itch. A functional coupling between the insula and basal ganglia could be one of the targets to control scratching behavior.
12 Future Directions
Linking functional human brain imaging, such as brain perfusion measurements with quantitative arterial spin labeling, to anatomical studies, such as voxel-based morphometry, is a powerful way to gain insight into brain regions associated with the itch–scratch cycle. Arterial spin labeling provides us with techniques that measure tonic events, such as itch, and to identify the neural correlates that underpin unrelenting spontaneous itch that occurs in chronic itch patients. As imaging techniques are refined, we will be able to explore in depth the mechanisms of temporal activation of structures that appear unrelated. Combining techniques such as magnetoencephalography to fMRI will further add to our understanding of the itch–scratch cycle. Techniques assessing functional connectivity using diffusion tensor imaging, that enables the study of white matter and neuronal pathways, will increase our understanding of visualizing the connections between the different areas involved in itch. Spinal cord fMRI is evolving as a new tool and will hopefully enable us to simultaneously assess neural activities from dorsal horn, brain stem, to cortex. Magnetic resonance spectroscopy will enable us to quantify changes in brain metabolites (e.g., dopamine glutamate) that are involved in itch.
The challenge now is to improve the sensitivity and specificity of these techniques so we can use them as tools in the assessment of chronic itch and the effectiveness of treatments.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- AD:
-
Atopic dermatitis
- ASL:
-
Arterial spin labeling
- BA6:
-
Brodmann area 6
- BA44:
-
Brodmann area 44
- DLPFC:
-
Dorsolateral prefrontal cortex
- DTI:
-
Diffusion tensor imaging
- EASI:
-
Eczema Area and Severity Index
- ESRD:
-
End-stage renal disease
- fMRI:
-
Functional magnetic resonance imaging
- MEG:
-
Magnetoencephalography
- NAc:
-
Nucleus accumbens
- PAG:
-
Periaqueductal gray matter
- PAR2:
-
Protease-activated receptor 2
- PFC:
-
Prefrontal cortex
- PET:
-
Positron emission tomography
- S1:
-
Primary somatosensory cortex
- S2:
-
Secondary somatosensory cortex
- VBM:
-
Voxel-based morphometry
- VPI:
-
Ventral posterior inferior
- VPL:
-
Ventral posterior lateral
- VTA:
-
Ventral tegmentum
References
Burton H, Sinclair RJ, McLaren DG (2004) Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum Brain Mapp 23:210–228
Carstens E (1997) Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol 77:2499–2514
Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH (1994) Distributed processing of pain and vibration by the human brain. J Neurosci 14:4095–4108
Darsow U, Drzezga A, Frisch M, Munz F, Weilke F, Bartenstein P, Schwaiger M, Ring J (2000) Processing of histamine-induced itch in the human cerebral cortex: a correlation analysis with dermal reactions. J Invest Dermatol 115:1029–1033
Davidson S, Giesler GJ (2010) The multiple pathways for itch and their interactions with pain. Trends Neurosci 33:550–558
Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ Jr (2009) Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci 12:544–546
Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ Jr (2012) Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol 108:1711–1723
Dawn AG, Yosipovitch G (2006) Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol 54:527–531
Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P (2001) Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain 92:295–305
Feneran AN, O’Donnell R, Press A, Yosipovitch G, Cline M, Dugan G, Papoiu AD, Nattkemper LA, Chan YH, Shively CA (2012) Monkey see, monkey do: contagious itch in nonhuman primates. Acta Derm Venereol 93:27–29
Gelnar PA, Krauss BR, Sheehe PR, Szeverenyi NM, Apkarian AV (1999) A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10:460–482
Golaszewski SM, Siedentopf CM, Baldauf E, Koppelstaetter F, Eisner W, Unterrainer J, Guendisch GM, Mottaghy FM, Felber SR (2002) Functional magnetic resonance imaging of the human sensorimotor cortex using a novel vibrotactile stimulator. Neuroimage 17:421–430
Hagen MC, Pardo JV (2002) PET studies of somatosensory processing of light touch. Behav Brain Res 135:133–140
Henry DE, Chiodo AE, Yang W (2011) Central nervous system reorganization in a variety of chronic pain states: a review. PMR 3:1116–1125
Herde L, Forster C, Strupf M, Handwerker HO (2007) Itch induced by a novel method leads to limbic deactivations: a functional MRI study. J Neurophysiol 98:2347–2356
Holle H, Warne K, Seth AK, Critchley HD, Ward J (2012) Neural basis of contagious itch and why some people are more prone to it. Proc Natl Acad Sci U S A 109:19816–1921
Hsieh JC, Hägermark O, Stahle-Bäckdahl M (1994) Urge to scratch represented in the human cerebral cortex during itch. J Neurophysiol 72:3004–3008
Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G (2009) Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol 161:1072–1080
Leknes SG, Bantick S, Willis CM, Wilkinson JD, Wise RG, Tracey I (2007) Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J Neurophysiol 97:415–422
Lloyd DM, Hall E, Hall S, McGlone FP (2012) Can itch-related visual stimuli alone provoke a scratch response in healthy individuals? Br J Dermatol 168:106–111
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K (2003) Imaging of central itch modulation in the human brain using positron emission tomography. Pain 105:339–346
Mochizuki H, Sadato N, Saito DN, Toyoda H, Tashiro M, Okamura N, Yanai K (2007) Neural correlates of perceptual difference between itching and pain: a human fMRI study. Neuroimage 36:706–717. Erratum in: 2008. Neuroimage 39:911–912
Mochizuki H, Inui K, Tanabe HC, Akiyama LF, Otsuru N, Yamashiro K, Sasaki A, Nakata H, Sadato N, Kakigi R (2009) Time course of activity in itch-related brain regions: a combined MEG-fMRI study. J Neurophysiol 102:2657–2666
Mochizuki H, Baumgärtner U, Kamping S, Ruttorf M, Schad LR, Flor H, Kakigi R, Treede RD (2013) Cortico-subcortical activation patterns for itch and pain imagery. Pain 154:1989–1998
Mochizuki H, Tanaka S, Morita T, Wasaka T, Sadato N, Kakigi R (2014) The cerebral representation of scratching-induced pleasantness. J Neurophysiol 111:488–498
Napadow V, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, Pfab F (2012) The brain circuitry mediating antipruritic effects of acupuncture. Cereb Cortex 24:873–882
Niemeier V, Kupfer J, Gieler U (2000) Observations during an itch inducing lecture. Dermatol Psychosom 1(Suppl 1):15–18
Papoiu ADP, Wang H, Coghill RC, Chan YH, Yosipovitch G (2011) Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol 164:1299–1303
Papoiu ADP, Coghill RC, Kraft RA, Wang H, Yosipovitch G (2012) A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage 59:3611–3623
Papoiu AD, Nattkemper LA, Sanders KM, Kraft RA, Chan YH, Coghill RC, Yosipovitch G (2013) Brain's reward circuits mediate itch relief. a functional MRI study of active scratching. PLoS One 8:e82389 (erratum in PLoS One. 2014;9(1))
Papoiu AD, Emerson NM, Patel TS, Kraft RA, Valdes-Rodriguez R, Nattkemper LA, Coghill RC, Yosipovitch G (2014) Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol 112:1729–1738
Papoiu AD, Kraft RA, Coghill RC, Yosipovitch G (2015) Butorphanol suppression of histamine itch is mediated by nucleus accumbens and septal nuclei: a pharmacological FMRI study. J Invest Dermatol 135:560–568
Parise M, Kubo TT, Doring TM, Tukamoto G, Vincent M, Gasparetto EL (2014) Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia. J Headache Pain 15:17
Peckys D, Hurd YL (2001) Prodynorphin and kappa opioid receptor mRNA expression in the cingulate and prefrontal cortices of subjects diagnosed with schizophrenia or affective disorders. Brain Res Bull 55:619–624
Peckys D, Landwehrmeyer GB (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88:1093–1135
Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A (2009) Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 29:13746–13750
Schneider G, Ständer S, Burgmer M, Driesch G, Heuft G, Weckesser M (2008) Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur J Pain 12:834–841
Seitz RJ, Roland PE (1992) Vibratory stimulation increases and decreases the regional cerebral blood flow and oxidative metabolism: a positron emission tomography (PET) study. Acta Neurol Scand 86:60–67
Vierow V, Fukuoka M, Ikoma A, Dörfler A, Handwerker HO, Forster C (2009) Cerebral representation of the relief of itch by scratching. J Neurophysiol 102:3216–3224
Yosipovitch G, Goon AT, Wee J, Chan YH, Zucker I, Goh CL (2002) Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int J Dermatol 41:212–216
Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC (2007) Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol 156:629–634
Yosipovitch G, Ishiuji Y, Patel TS, Hicks MI, Oshiro Y, Kraft RA, Winnicki E, Coghill RC (2008) The brain processing of scratching. J Invest Dermatol 128:1806–18011
Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC (2011) Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 31:5540–5548
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Yosipovitch, G., Mochizuki, H. (2015). Neuroimaging of Itch as a Tool of Assessment of Chronic Itch and Its Management. In: Cowan, A., Yosipovitch, G. (eds) Pharmacology of Itch. Handbook of Experimental Pharmacology, vol 226. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44605-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-44605-8_4
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44604-1
Online ISBN: 978-3-662-44605-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)