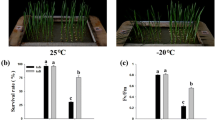
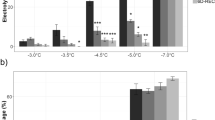
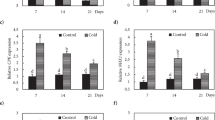
Abstract
In nature several factors including ice nucleation, temperature, freezing (cooling) rate, duration of exposure to ice, thawing rate and post-thaw conditions contribute to the degree of injury caused by frost episodes. Our results show that an increase in cooling rate from 1 to 4 °C h-1 made the difference between survival and death. We have also found that in the critical temperature range where injury occurs, the thaw rate influenced the degree and type of injury. The plasma membrane is a key site of alteration by freeze-thaw stress and cold acclimation. Important properties in this respect include membrane lipids and proteins (plasma membrane ATPase, kinase, desaturase), and the concentration of membrane and cytosolic calcium. Plasma membrane ATPase appears to be an important site of cellular response to freeze-thaw stress and an alteration in the function of this enzyme is one of the earliest manifestations of stress. Our results provide evidence that these alterations could be mediated by perturbation of cellular Ca2+ and/or changes in membrane lipid composition. These results provide an insight into the mechanisms of incipient injury and recovery following injury. To understand the genetics of freezing stress resistance, we performed an interspecific hybridisation of two diploid potato species that vary in freezing tolerance and cold acclimation ability. The species were Solanum commersonii, which is freezing tolerant and able to cold-acclimate (double its freezing tolerance in 10 days at chilling temperatures) and Solanum cardiophyllum, which is freezing sensitive and unable to cold-acclimate. Analysis of the backcross progenies shows that non-acclimated freezing tolerance and acclimation ability are genetically distinct traits that segregate independently. Generation mean analysis revealed that cold-acclimation ability can be explained by a simple additive- dominance model. Our results indicate that the ability to cold-acclimate is genetically relatively simple and should be amenable to selection at the diploid level. We have recently performed lipid analysis of purified plasma membrane preparations obtained from the parents, F1 and the backcross progenies. Analysis shows that the relative increase in linoleic acid (18:2) in the plasma membrane is highly correlated to the cold acclimation ability. An increase in 18:2 co-segregated with the capacity to acclimate. Our results suggest that specific membrane lipids play a role in the genetic ability of the plant material to cold-acclimate.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Abdallah AY, Palta JP (1989) Specific changes in membrane polar lipid fatty acid composition coincide with initiation of fruit ripening and changes in freezing stress resistance of leaves of cranberry. Plant Physiology 89: 100
Amirshashi MC, Patterson FL (1956) Cold resistance of parent varieties, F2 populations and F3 lines of 20 oat crosses. Agronomy Journal 48: 184–188
Arora R, Palta JP (1986) Protoplasmic swelling as a symptom of freezing injury in onion bulb cells. Its simulation in extracellular KC1 and prevention by calcium. Plant Physiology 82: 625–629
Arora R, Palta JP (1988) In vivo perturbation of membrane-associated calcium by freeze-thaw stress in onion bulb cells. Simulation of this perturbation in extracellular KC1 and its alleviation by calcium. Plant Physiology 87: 622–628
Arora R, Palta JP (1989) Perturbation of membrane calcium as a molecular mechanism of freezing injury. In: Cherry JH (ed) Environmental stress in plants: Biochemical and biophysical mechanisms. NATO ASI Series Vol. 19. Springer-Verlag, New York, USA, pp 281–290
Arora R, Palta JP (1991) A loss in plasma membrane ATPase activity and its recovery coincides with incipient freeze-thaw injury and post-thaw recovery in onion bulb scale tissue. Plant Physiology 95: 845–852
Ashworth EN, Davis GA, Anderson JA (1985) Factors affecting ice nucleation in plant tissues. Plant Physiology 79: 1033–1037
Blum A (1988) Plant breeding for stress environments. CRC Press, Boca Raton, USA, pp 79–127
Brule-Babel AL, Fowler DB (1988) Genetic control of cold hardiness and vernalization requirement in winter wheat. Crop Science 28: 879–884
Brule-Babel AL, Fowler DB (1989) Use of controlled environments for winter cereal cold hardiness evaluation: controlled freeze tests and tissue water content as prediction tests. Canadian Journal of Plant Science 69: 355–366
Cook HW (1985) Fatty acid desaturation and chain elongation. In: Vance DE, Vance JE (eds) Biochemistry of lipids and membranes. Benjamin Cummings Publishing, California, USA, pp 181–212
Grenier G, Tremolieres A, Therrien HP, Willemot C (1972) Changements dans les lipides de la luzerne en conditions menant a l’endurcissement au froid. Canadian Journal of Botany 50: 1681–1689
Gullord M (1975) Genetics of freezing hardiness in winter wheat (Triticum aestivum L.) PhD Thesis, Michigan State University, East Lansing, USA
Gullord M, Olien CR, Everson EH (1975) Evaluation of freezing hardiness in winter wheat. Crop Science 15: 153–157
Gurr MI, Harwood JL (1991) Lipid biochemistry: An introduction. Chapman and Hall, London, UK, pp 338–344
Harbage JF, Weiss LS, Stone JM, Bamberg JB, Palta JP (1992) Increased plasma membrane linoleic acid is correlated with acclimation capacity in a Solanum population segregating for freezing tolerance and acclimation capacity. Plant Physiology 99: 10
Hellergren J, Widell S, Lundborg T (1985) ATPase in relation to freezing in purified plasma membrane vesicles from nonacclimated seedings of Pinus sylvestris. Acta Horticulturae 168: 161–166
Hepler PK, Wayne RO (1985) Calcium and plant development. Annual Review of Plant Physiology 36: 397–439
Iswari S, Palta JP (1989a) Plasma membrane ATPase as a site of functional alteration during cold acclimation and freezing injury. In: Li PH (ed) Low temperature stress physiology in crops. CRC Press, Boca Raton, USA, pp 123–137
Iswari S, Palta JP (1989b) Plasma membrane ATPase activity following reversible and irreversible freezing injury. Plant Physiology 90: 1088–1095
Iswari S, Weiss LS, Bertics PJ, Palta JP (1990) Plasma membrane bound protein kinase in cold acclimating and nonacclimating potato species. Plant Physiology 93: 84
Jian CL, Sun LH, Dong HZ, Sun DL (1982) Changes in ATPase activity during freezing injury and cold hardening. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress: Mechanisms and crop implications. Vol. 2. Academic Press, New York, USA, pp 243–259
Leshem YY (1992) Plant membranes: A biophysical approach to structure, development and senescence. Kluwer Academic Publishers, Dordrecht, The Netherlands
Leonard RT, Hepler PK (eds) (1990) Calcium in plant growth and development. American Society of Plant Physiologists, Rockville, USA
Levitt J (1980) Responses of plants to environmental stresses. Vol. 1: Chilling, freezing, and high temperature stresses. Academic Press, New York, USA
Liesenfeld DR, Auld DL, Murray GA, Swensen JB (1986) Transmittance of winter hardiness in segregating populations of peas. Crop Science 26: 49–54
Limin AE, Fowler DB (1989) The influence of cell size and chromosome dosage on cold hardiness expression in the Triticeae. Genome 32: 667–671
Lindow SE, Amy DC, Upper CD (1982) Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiology 79: 1084–1089
Lynch DV, Steponkus PL (1987) Plasma membrane lipid alterations associated with cold acclimation of winter rye seedlings (Secale cereale L. cv Puma). Plant Physiology 83: 761–767
Marshall HG (1982) Breeding for tolerance to heat and cold. In: Christiansen MN, Lewis CF (eds) Breeding plants for less favorable environments, John Wiley and Sons, New York, USA, pp 47–69
Palmgren MG, Sommarin M, Ulvskov P, Jorgensen PL (1988) Modulation of plasma membrane H+-ATPase from oat roots by lysophosphatidylcholine, free fatty acids and phospholipase A2. Physiologia Plantarum 74: 11–19
Palta, JP (1989) Plasma membrane ATPase as a key site of perturbation in response to freeze-thaw stress. In: Randall D, Blevins D, Campbell W (eds) Current topics in plant biochemistry and physiology Vol. 8. University of Missouri, Columbia, USA, pp 841–868
Palta JP, Levitt J, Stadelmann EJ (1977a) Freezing injury in onion bulb cells. I. Evaluation of the conductivity method for an analysis of ion and sugar efflux from injured cells. Plant Physiology 60: 393–397
Palta JP, Levitt J, Stadelmann EJ (1977b) Freezing injury in onion bulb cells. II. Post thawing injury or recovery. Plant Physiology 60: 398–401
Palta JP, Levitt J, Stadelmann EJ (1977c) Freezing tolerance of onion bulbs and significance of freeze-induced tissue infiltration. Cryobiology 14: 614–619
Palta JP, Levitt J, Stadelmann EJ, Burke MJ (1977d) Dehydration of onion cells: A comparison of freezing vs. desiccation and living vs. dead cells. Physiologia Plantarum 41: 273–279
Palta JP, Li PH (1978) Cell membrane properties in relation to freezing injury. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress: Mechanisms and crop implications. Academic Press, New York, USA, pp 93–115
Palta JP, Li PH (1980) Alterations in membrane transport properties by freezing injury in herbaceous plants. Evidence against the rupture theory. Physiologia Plantarum 50: 169–175
Palta JP, Meade LS (1987) Fatty acid composition changes after cold acclimation: comparison of leaf, callus and purified membranes. Plant Physiology 83: 71
Palta JP, Meade LS (1989) During cold acclimation of potato species, an increase in 18:2 and decrease in 16:0 in plasma membrane phospholipid coincides with an increase in freezing stress resistance. Plant Physiology 89: 89
Palta JP, Weiss LS (1993) Ice formation and freezing injury: An overview on the survival mechanisms and molecular aspects of injury and cold acclimation in herbaceous plants. In: Li PH, Christersson L (eds) Advances in plant cold hardiness. CRC Press, Boca Raton, USA, pp 143–176
Pearce RS (1988) Extracellular ice and cell shape in frost-stressed cereal leaves: A low temperature scanning electron microscopy study. Planta 175: 313–324
Pfahler PL (1966) Small grain improvement by breeding and selection. Report of the Florida Agriculture Experiment Station, pp 52
Pomeroy MK, Pihakaski JS, Andrews CJ (1983) Membrane properties of isolated winter wheat cells in relation to icing stress. Plant Physiology 72: 535–539
Poovaiah BW, Reddy ASN (1987) Calcium messenger systems in plants. CRC Critical Reviews in Plant Sciences 6: 47–102
de la Roche AI, Andrews CJ, Pomeroy MK, Weinberger PA, Katers M (1972) Lipid changes in winter wheat seedlings (Triticum aestivum) at temperatures inducing cold hardiness. Canadian Journal of Botany 50: 1681–1689
de la Roche AI, Pomeroy MK, Andrews CJ (1975) Changes in fatty acid composition in wheat cultivars of contrasting hardiness. Cryobiology 12: 506–512
Sakai A, Larcher W (1987) Frost survival in plants: Responses and adaptations to freezing stress. Springer-Verlag, New York, USA
Steffen KL, Arora R, Palta JP (1989) Sensitivity of photosynthesis and respiration to a freeze-thaw stress: Role of realistic freeze-thaw protocol. Plant Physiology 89: 1372–1379
Steffen KL, Palta JP (1987) Photosynthesis as a key process in plant response to low temperature: Alteration during low temperature acclimation and impairment during incipient freeze-thaw injury. In: Li PH (ed) Plant cold hardiness. Alan R. Liss, New York, USA, pp 67–99
Stone JM, Palta JM, Bamberg JB, Weiss LS, Harbage JF (1993) Inheritance of freezing resistance in tuber-bearing Solanum species: evidence for the independent control of nonacclimated freezing tolerance and cold acclimation capacity. Proceedings of the National Academy of Sciences (In press)
Stushnoff C, Fowler DB, Brule-Babel A (1984) Breeding and selection for resistance to low temperature. In: Voss PB (ed) Plant breeding - A contemporary basis. Pergamon Press, Elmsford, NY, USA, pp 115–136
Stutka J (1981) Genetic studies of frost resistance in wheat. Theoretical and Applied Genetics 59: 145–152
Stutka J (1984) A ten-parental diallele analysis of frost resistance in winter wheat. Zeitschrift fuer Pflanzenzuechtung 93: 147–157
Stutka J, Veisz O (1988) Reversal of dominance in a gene on chromosome 5A controlling frost resistance in wheat. Genome 30: 313–317
Sutinen MK, Palta JP (1989) Seasonal changes in phospholipid fatty acid composition coincide with changes in freezing stress resistance in pine needles. Plant Physiology 89: 28
Sutinen MK, Rybarczyk SJ, Palta JP (1990) Simultaneous changes in the plasma membrane polar lipids’ fatty acid composition and the vanadate-sensitive ATPase activity during cold acclimation of pine needles. Plant Physiology 93: 85
Thomashow MF (1990) Molecular genetics of cold acclimation in higher plants. In: Scandalios JG (ed) Advances in genetics, Vol. 28, Genomic responses to environmental stress. Academic Press, New York, USA, pp 99–125
Uemura M, Yoshida S (1984) Involvement of plasma membrane alterations in cold acclimation of winter rye seedlings (Secale cereale L. cv Puma). Plant Physiology 75: 818–826
Vigh L, Horvath I, van Hasselt PR, Kuiper PJC (1985) Effect of frost hardening on lipid and fatty acid composition of chloroplast thylakoid membranes in two wheat varieties of contrasting hardiness. Plant Physiology 79: 756–759
Weiss LS, Palta JP (1991a) Ice nucleation in potato leaves and the influence of thaw rates on the level of injury by freeze-thaw stress. 5th International Conference on Biological Ice Nucleation. Madison, Wisconsin, USA
Weiss LS, Palta JP (1991b) Influence of thaw rates on the level of injury by freezethaw stress in a cold hardy potato species. HortScience 26: 732
Willemot C (1975) Stimulation of phospholipid biosynthesis during frost hardening of winter wheat. Plant Physiology 55: 356–359
Yoshida S, Uemura M (1984) Protein and lipid compositions of isolated plasma membranes from orchid grass (Dactylis glomerata L.) and changes during cold acclimation. Plant Physiology 75: 31–37
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Palta, J.P., Weiss, L.S., Harbage, J.F., Bamberg, J.B., Stone, J.M. (1993). Molecular Mechanisms of Freeze-Thaw Injury and Cold Acclimation in Herbaceous Plants: Merging Physiological and Genetic Approaches. In: Jackson, M.B., Black, C.R. (eds) Interacting Stresses on Plants in a Changing Climate. NATO ASI Series, vol 16. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-78533-7_43
Download citation
DOI: https://doi.org/10.1007/978-3-642-78533-7_43
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-78535-1
Online ISBN: 978-3-642-78533-7
eBook Packages: Springer Book Archive