Abstract
The channel kinases TRPM6 and TRPM7 are fusion proteins with an ion transport domain and an enzymatically active kinase domain. TRPM7 has been found in every mammalian tissue investigated to date. The two-in-one protein structure, the ubiquitous expression profile, and the protein’s unique biophysical characteristics that enable divalent ion transport involve TRPM7 in a plethora of (patho)physiological processes. With its prominent role in cellular and systemic magnesium homeostasis, TRPM7 emerges as a key player in embryonic development, global ischemia, cardiovascular disease, and cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Magnesium
- Calcium
- Trace metals
- Divalent cation
- Transient Receptor Potential Channel
- Adenosine triphosphate
- Channel kinase
- Magnesium homeostasis
- Embryogenesis
- Ischemia
- Breast cancer
1 Gene
The official name of the TRPM7 gene is “transient receptor potential cation channel, subfamily M, member 7.” In Homo sapiens, the TRPM7 gene is located on chromosome 15q21.2 (NCBI Gene ID 54822). Previous names for the gene include CHAK1, TRP-PLIK, and LTRPC7 (Ryazanov 2002; Runnels et al. 2001; Nadler et al. 2001). The human TRPM7 gene encodes an 1,865 amino acid protein (Schmitz et al. 2005). The molecule is unique in that it contains an ion transport domain (InterPro IPR005821) in its N-terminal section and an enzymatically active MHCK/EF2 kinase domain (InterPRo IPR004166) in the C-terminal section. Only two other mammalian genes (there are numerous channel enzymes in simple organisms) are known to code for ion channel and enzyme domain fusion proteins, namely, TRPM7’s paralog genes TRPM6 and TRPM2 (Perraud et al. 2001; Schlingmann et al. 2002; Walder et al. 2002), described elsewhere in this book. TRPM7 gene orthologs with human amino acid sequence similarity of 94.3 % to 99.89 % have been identified in chimpanzee (Pan troglodytes), mouse (Mus musculus), rat (Rattus norvegicus), cow (Bos taurus), and dog (Canis familiaris; GeneCards). In zebrafish (Danio rerio), an important model organism in biology, TRPM7 is located on chromosome 18 and shares 75 % amino acid sequence identity with the human TRPM7 gene.
Virtually nothing is known about whether the kinase and channel encoding portions of the TRPM7 gene domains can be expressed independently from each other. A splice variant in rat lacking the channel domain has been reported (Runnels et al. 2001). Human Trpm7 has 9 predicted splice variants (http://www.ensembl.org: ENST00000561267, TRPM7-001 through TRPM7-009); however, aside from the 1,865 amino acid encoding protein, the function of any of these variants remains unexplored. Finally, no genes coding for TRPM7 auxiliary subunits are known (Chubanov et al. 2004; Schmitz et al. 2005).
2 Expression
Early investigations of human tissue and cell lines by PCR with reverse transcription indicated a ubiquitous distribution pattern of TRPM7 transcripts (Nadler et al. 2001; Runnels et al. 2001). A comprehensive quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of human tissue showed that TRPM7 is widely distributed in the central nervous system as well as in the periphery, with highest expression levels in the heart, pituitary, bone, and adipose tissue (Fonfria et al. 2006).
TRPM7 is also ubiquitously expressed across mouse organs as investigated by qRT-PCR. These data show that, compared to other members of the TRP gene family, TRPM7 is the most abundantly expressed TRP channel in the majority of adult mouse organs investigated (Kunert-Keil et al. 2006). Particularly, mouse intestine, lung, kidney, and brain have strong TRPM7 expression (Kunert-Keil et al. 2006), as well as testis (Jang et al. 2012). While TRPM7 levels can vary significantly between mouse strains (Kunert-Keil et al. 2006), they seem quite constant within a particular type of strain (Vandewauw et al. 2013). Along with TRPM2, TRPM4, and TRPM8, mouse trigeminal ganglia show very high expression of TRPM7, and this gene product has a stronger representation in dorsal root ganglia along the vertebral column compared to other members of the TRPM family (Vandewauw et al. 2013). Gene expression patterns of TRPM7 during mouse development seem to occur in two waves, peaking at embryonic day 18 (E12), raising again after postnatal day 4, and maintaining stable levels into adulthood (Staaf et al. 2010). Additional studies have confirmed TRPM7 RNA expression in adult rat prostate tissue (Wang et al. 2007) and intralobar pulmonary arterial and aortic smooth muscle (Yang et al. 2006), as well as rumen epithelial cells isolated from sheep (Schweigel et al. 2008).
The assessment of TRPM7 at the protein level has been more challenging due to the paucity of highly specific antibodies. Fortunately, due to the electrogenic nature of TRPM7’s ion channel function, biophysical techniques such as whole-cell patch-clamp technique and single-channel measurements allow an estimate of the number of proteins in the plasma membrane of single live cells (Hamill et al. 1981). Endogenous TRPM7-like currents were first reported in renal cells (human HEK293), mast cells (rat RBL-2H3), and T lymphocytes (human Jurkat T) (Nadler et al. 2001). Due to the inhibition of these currents by magnesium (Mg), Mg·ATP, as well as their ability to conduct metal ions, native TRPM7-like currents were coined magnesium-nucleotide-regulated metal ion currents [MagNuM (Nadler et al. 2001; Hermosura et al. 2002)] and also magnesium-inhibited cation current [MIC (Kozak et al. 2002b)]. Subsequent investigation reported native currents with biophysical characteristics ascribed to TRPM7 in a wide variety of cell types, including the heart (Gwanyanya et al. 2004), brain (Aarts et al. 2003), and intestine (Kim et al. 2009).
Of the three cell types in which MagNuM currents were originally described, Jurkat T cells had the highest current density under the experimental conditions used (Nadler et al. 2001). Taking into account a single-channel conductance of 40 pS (Nadler et al. 2001; Li et al. 2006), this still amounts to only an estimated 30 active channels in the plasma membrane per T cell and 40 channels per HEK293 or RBL-2H3 cell. Subcellular location of TRPM7 protein in heterologous overexpression systems is to be expected (Chubanov et al. 2007), and evidence exists of native functional subcellular location in synaptic vesicles of sympathetic rat neurons (Krapivinsky et al. 2006), in tubulovesicular structures (Oancea et al. 2006), and reticular formations of vascular smooth muscle cells (Yogi et al. 2009).
3 The Channel Protein Including Structural Aspects
In analogy to other members of the TRP channel family, TRPM7 monomers are thought to form tetrameric units, modeled after voltage-gated potassium (K) channels (Mederos y Schnitzler et al. 2008; Jiang et al. 2003; Chubanov et al. 2007). The 1,865 amino acids of a human TRPM7 subunit can be subdivided into distinct domains with variable homology to TRPM7 subunits identified in other species. Four unique melastatin amino-terminal regions are linked to the 6 putative transmembrane spanning helices (Nadler et al. 2001), with the putative pore region linking segments 5 and 6. The “TRP box,” unique to all identified TRP ion channels, is a highly conserved and proline-rich 24 amino acid region C-terminal to the transmembrane domains (Venkatachalam and Montell 2007), followed by a cytoplasmic coiled-coil (CC) domain thought to underlie channel assembly and trafficking (Fujiwara and Minor 2008). The CC domain is predicted to have a four-stranded antiparallel arrangement which seems aligned with the dimer-forming atypical α-kinase domain architecture just C-terminal of the CC (Fujiwara and Minor 2008). The latter is indeed one of the unique features of TRPM7, which it shares with its paralog TRPM6: the fusion of an N-terminal functional ion channel and a C-terminally located and active serine/threonine protein kinase (Ryazanov 2002; Runnels et al. 2001; Nadler et al. 2001). Activation of caspase leads to separation of TRPM7’s kinase domain from the channel without affecting the functionality of the kinase but enhancing ion channel activity (Desai et al. 2012).
Both the CC and α-kinase domain are currently the only two TRPM7 regions where X-ray crystallography has provided structural information (Yamaguchi et al. 2001; Fujiwara and Minor 2008). The kinase domain’s 300 amino acid residues fold into a cleft-forming structure containing the active ATP-binding site. In addition, one zinc (Zn) and two Mg binding sites have been reported. Sequence analysis reveals little primary amino acid sequence similarity between catalytic domains of conventional protein kinases and TRPM7’s atypical kinase despite an overall similarity in the folding structure (Drennan and Ryazanov 2004; Yamaguchi et al. 2001). Analysis of truncation mutants using mouse TRPM7 identified two important regions in the kinase domain: residues 1553 to 1562 are essential for kinase phosphorylation activity and residues 1563 to 1670 are needed for dimer assembly (Crawley and Cote 2009).
Mass spectrometric proteomic techniques have been used on mouse and human TRPM7 to identify key phosphorylation sites. This resulted in the confirmation as well as new identification of several phosphorylation sites that are all located on the cytoplasmic C-terminus (Kim et al. 2012; Madsen et al. 2012; Matsushita et al. 2005): 3 in the CC region, 7 in a serine-threonine-rich (Ser/Thr) domain (Matsushita et al. 2005), and two P-sites of unknown function distal to the kinase domain (Kim et al. 2012). Furthermore, phosphomapping by mass spectrometry identified 47 autophosphorylation sites on TRPM7, the majority of which are located in the Ser/Thr-rich domain N-terminal of the kinase region. This part of the TRPM7 region is thought to control kinase substrate binding (Clark et al. 2008c).
4 Interacting Proteins
Information on proteins interacting with TRPM7 remains scarce, even for the kinase domain. A yeast two-hybrid screen of a rat library identified phospholipase C (PLC) as interacting partner of the TRPM7 kinase (Runnels et al. 2001). Subsequent work showed that receptor-stimulated activation of PLC causes inhibition of TRPM7 channel activity through localized phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis (Runnels et al. 2002). Furthermore, hypomagnesemic conditions increase TRPM7-kinase-regulated Ser/Thr phosphorylation in the C2 domain of PLCγ2, leading to reduced Ca signaling (Deason-Towne et al. 2012).
Involvement of TRPM7 kinase in cell motility and adhesion has been linked to its ability to phosphorylate the assembly domains of non-muscle myosin IIA, IIB, and IIC and ATP-dependent motor proteins involved in actomyosin-based cell motility (Clark et al. 2006, 2008a, b). Annexin A1, a Ca-dependent membrane-binding protein with the ability to promote membrane fusion, is also phosphorylated by the TRPM7 kinase, providing a possible link to TRPM7’s known involvement in cell growth and apoptosis (Dorovkov and Ryazanov 2004; Dorovkov et al. 2011). The TRPM7 kinase also mediates enhanced Thr phosphorylation at residue 56 of the eukaryotic elongation factor 2 (eEF2) through eEF2 kinase (Perraud et al. 2011). This specifically occurs under reduced dietary Mg and has been suggested to adjust protein translation rates to the availability of this important divalent ion.
To date, TRPM6 is possibly the best understood TRPM7-interacting protein as assessed in heterologous expression systems (Runnels 2011). Interestingly, recombinant and native TRPM6 seems to require TRPM7 for plasma membrane surface expression in mouse embryonic stem (ES), DT40, HEK293 cells, and Xenopus oocytes (Schmitz et al. 2005; Ryazanova et al. 2010; Chubanov et al. 2004, 2007), indicating that TRPM6 is inefficient in forming functional homomeric ion channels on its own. While this topic still remains somewhat controversial, supporting observations show that overexpression of TRPM6 cannot rescue cell growth arrest in chicken DT40 B cells lacking the TRPM7 protein (Schmitz et al. 2005) and, in contrast to TRPM7, cannot alter motility and proliferation of HEK293 (Chubanov et al. 2004). Furthermore, a single-point mutation at amino acid residue S141 in TRPM6 disrupts heteromeric TRPM6/TRPM7 channel formation manifesting itself as hypomagnesemia with secondary hypocalcemia (Chubanov et al. 2004). Interestingly, when cloned into the pCINeo-IRES-GFP vector, TRPM6 can be overexpressed and forms functional homomeric channels in the plasma membrane (Voets et al. 2004; Li et al. 2006). While this seems to be the only vector able to do so for unknown reasons, it presents a valuable scientific tool to study the hypothetical behavior of TRPM6 if it were expressed natively. This may provide information as to why homomeric TRPM7 channels behave differently from the heterotetramer formed by TRPM6 and TRPM7 and as to what the underlying structural features might be. It would also be interesting to elucidate whether the noncoding sequence of the TRPM6-pCINeo-IRES-GFP expression construct can influence assembly and trafficking of TRPM6.
5 A Biophysical Description of the Channel Function, Permeation, and Gating
5.1 Channel Function
Aside from representing a fusion protein, TRPM7’s most striking feature is its selectivity for divalent metal ions at hyperpolarized potentials (Monteilh-Zoller et al. 2003; Nadler et al. 2001). The strong outwardly rectifying current–voltage (I/V) signature of TRPM7 is due to voltage-dependent permeation block by extracellular divalent ions, mainly Ca and Mg (Kerschbaum et al. 2003; Nadler et al. 2001). Removal of divalent ions allows the assessment of TRPM7 single-channel characteristics at all physiological voltages, revealing a relatively large conductance of 40 pS and open times of several hundred milliseconds (Li et al. 2006). The channel itself shows no intrinsic voltage dependence, and the level of its constitutive activity is regulated by a surprising variety of intracellular and extracellular factors. Natively, most cells express only a few tens of TRPM7 proteins in the plasma membrane, and this can readily be assessed by the whole-cell patch-clamp method. However, the relative scarcity of endogenous TRPM7 in the cell’s membrane hampers the use of other, less sensitive detection methods, such as immunofluorescence or biotinylation studies, or more global protein expression evaluations by Western blot.
5.2 Kinase Function
The identification of elongation factor-2 kinase revealed a new class of protein kinases with no sequence homology to conventional eukaryotic protein kinases in regard to their catalytic domains (Ryazanov et al. 1999). There are several members of this so-called atypical or α-kinase family in mammals, and two are fused to the ion channels TRPM6 and TRPM7. The TRPM7 kinase specifically phosphorylates Ser and Thr residues in a Mg-dependent manner (Ryazanova et al. 2004). It autophosphorylates itself and phosphorylates myelin basic protein as well as histone H3. At least two of the identified autophosphorylation sites (S1511 and S1567) do not seem to influence channel behavior (Matsushita et al. 2005).
While manganese (Mn) can replace Mg to maintain kinase function, zinc (Zn) and cobalt (Co) inhibit kinase activity, while Ca plays no role (Matsushita et al. 2005; Ryazanova et al. 2004). Staurosporine, a common protein kinase inhibitor preventing ATP binding, does not interfere with TPRM7 kinase function, whereas rottlerin, a potent K+ channel activator, suppresses kinase activity at high concentrations [IC50 ~ 40 μM (Ryazanova et al. 2004)].
5.3 Channel Permeation
The first indication that TRPM7 represents a bona fide divalent ion channel at negative voltages and allows monovalent ion flux only at depolarized voltages was published in one of the original reports on TRPM7 function (Nadler et al. 2001). Detailed studies followed confirming the channel’s selectivity profile to be Zn = nickel (Ni) > barium (Ba) > Co > Mg > Mn > strontium (Sr) > cadmium (Cd) > Ca (Li et al. 2006; Monteilh-Zoller et al. 2003). Relatively large and complex structured polyamines can additionally act as permeant blockers of TRPM7 (Kerschbaum et al. 2003).
Several amino acid residues in the putative TRPM7 ion channel pore have been shown to control Ca and Mg permeability. Changing glutamic acid at residue 1047 or 1052 in the mouse channel into a neutral glutamine either strongly reduces (70 %) or even abolishes affinity to Ca or Mg, respectively (Li et al. 2007). On the other hand, changing residue E1047 into glutamine and Y1049 into proline results in linearized currents and loss of Ca permeation (Mederos y Schnitzler et al. 2008). Similar observations have been made for human TRPM7 (Numata and Okada 2008) with the corresponding key residues E1047 and E1052. In addition, aspartic acid at 1054 and 1059 also influences permeation block by divalent ions.
5.4 Channel Gating and Regulation
5.4.1 Magnesium
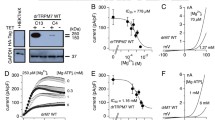
TRPM7 represents a constitutively active ion channel that is heavily regulated by a variety of physiological feedback mechanisms. One of the most important regulatory factors of channel activity is intracellular free Mg (Nadler et al. 2001), which can be mimicked by non-physiological Ba, Sr, Zn, and Mn (Kozak and Cahalan 2003). Detailed biophysical examination reveals that native TRPM7 in excised patches has two conductance states at 39 pS and 186 pS, with both reversibly inhibited by Mg (Chokshi et al. 2012c). The respective dose-response curves reveal IC50 values of 25 μM and 91 μM. Mg seems to reduce the number of active channels rather than cause an overall reduction of single-channel conductance. Mg inhibition involves two separate binding sites on the protein (Chokshi et al. 2012b), one within the kinase domain and another on the channel proper (Schmitz et al. 2003). Mg inhibitory potency measured in excised patches is about 10- to 20-fold smaller than that seen in whole-cell patch-clamp experiments where 750 μM free Mg is needed to suppress channel activity by 50 %, both in overexpression and native cell systems (Nadler et al. 2001; Demeuse et al. 2006). This suggests that additional factors in the cellular environment of TRPM7 help regulate the channel’s true physiological Mg sensitivity. Sites coordinating the Mg · ATP binding in the kinase domain are partially involved in regulating the overall affinity for Mg to the channel, since introduction of single-point mutations that abolish phosphotransferase activity (G1799D, K1648R) reduces TRPM7’s sensitivity to intracellular Mg (Schmitz et al. 2003). In contrast, autophosphorylation does not seem to play a role here, since, at least for the single-point mutants investigated (S1511/S1567), no difference can be detected compared to the wild-type (wt) channel (Matsushita et al. 2005). Interestingly, intracellular Mg seems to synergize with a variety of factors regulating TRPM7 activity, including the highly specific TRPM7 inhibitor waixenicin A, intracellular chloride, and intracellular Mg·nucleotides (Zierler et al. 2011; Yu et al. 2013; Demeuse et al. 2006).
5.4.2 Mg·Nucleotides
There is general consensus that mammalian TRPM7 is regulated by free intracellular Mg (Penner and Fleig 2007). Evidence for intracellular adenosine triphosphate (ATP) as a feedback mechanism for TPRM7 was initially controversial. Runnels et al. reported facilitation of TPM7 activity by intracellular ATP (Runnels et al. 2001), whereas in a parallel study, Nadler et al. demonstrated an inhibitory effect of ATP in its physiologically relevant form bound to Mg (Mg·ATP) (Nadler et al. 2001). This issue has been resolved and the ATP-mediated activation of TRPM7 actually is due to a decrease in free Mg caused by supplemented Na·ATP. Subsequent analyses revealed that negative feedback inhibition by Mg·ATP requires an intact nucleotide-binding site of the kinase domain involving amino acid K1648 (Schmitz et al. 2003). The binding site also helps discriminate between Mg·nucleotide (Mg·NTP) species such as Mg·GTP or Mg·TTP, since point mutations of this residue or removal of the entire kinase domain renders the channel insensitive to intracellular nucleotide regulation (Demeuse et al. 2006). Mg·adenosine diphosphate (ADP), but not adenosine monophosphate (AMP), has similar inhibitory efficacy as Mg·ATP, indicating a protection against enhanced TRPM7 activation during variations of cell energy levels. Thus, Mg chelated to nucleotides seems key to interfere with TRPM7 gating. Furthermore, inhibition by Mg·nucleotides is synergistically enhanced by intracellular free Mg. In fact, nucleotides lose any efficacy below a minimal threshold of around 200 μM free Mg (Demeuse et al. 2006). The current model therefore postulates independent binding sites for Mg and Mg·ATP, synergistically regulating channel activity. Interestingly, kinase deletion at residue 1599 renders a nonfunctional channel (Matsushita et al. 2005), while truncating the kinase domain at aa 1569 regains some channel function (Schmitz et al. 2003) and cutting the protein at residue 1510 fully recovers the ability to measure regular TRPM7 currents (Desai et al. 2012). Thus, it is tempting to speculate that the protein region between aa residues 1510 and 1599 is involved in coordinating the binding of Mg to the channel.
5.5 Receptor-Coupled TRPM7 Activity
Several studies have reported regulation of TRPM7 through PLC-dependent pathways. Co-overexpression of muscarinic receptor 1 and TRPM7 in HEK293 cells followed by charbacol stimulation leads to TRPM7 inactivation due to depletion of PIP2 in the plasma membrane (Runnels et al. 2002). Endogenous TRPM7 in CA1 hippocampal neurons is sensitive to nerve growth factor via a PLC-dependent pathway (Tian et al. 2007), and in cardiac myocytes GTP analogues lead to TRPM7 inhibition through G-protein activity and PIP2 metabolism (Macianskiene et al. 2008). In contrast, moderate overexpression of TRPM7 in neuroblastoma N1E-115 cells needs free intracellular Mg to fall below physiological levels for PLC-dependent inhibition to occur. Under normal Mg levels, TRPM7 currents are activated rather than inhibited following receptor stimulation through bradykinin, thrombin, or lysophosphatidic acid (Langeslag et al. 2007). Further evidence shows involvement of endogenous Gs/Gi-coupled receptors in TRPM7 regulation. Stimulation of acetylcholine receptors inhibits overexpressed TRPM7 currents in HEK293 cells. Isoproterenol stimulation of endogenous beta-adrenergic receptors, on the other hand, enhances TRPM7 activity and requires both a functional protein kinase A and an intact TRPM7 kinase domain (Takezawa et al. 2004).
5.6 Mechano-sensitivity and Volume
In vascular smooth muscle A7R5 cells overexpressing TRPM7, laminar flow-induced sheer stress causes channel translocation to the plasma membrane, implicating TPRM7 in cellular mechanotransduction of flow (Oancea et al. 2006). Endogenous TRPM7 in HeLa cells is directly activated by stretch or increased cell volume and does not involve exocytotic events for biomembrane incorporation (Numata et al. 2007). Exposing HEK293 cells expressing heterologous TPRM7 to varying osmotic gradients provides insight into the channel’s osmo-sensitivity mediated by molecular crowding of solutes that affect channel activity without involvement of membrane stretch (Bessac and Fleig 2007). While results are currently controversial as to the exact mechanism, it seems safe to say that changes in cell volume will affect TRPM7 channel activity.
5.7 Acidity
Acidic extracellular conditions below pH 6 greatly potentiate TRPM7 currents at negative membrane potentials (Monteilh-Zoller et al. 2003). This is due to changes in selectivity of TRPM7 that enhance monovalent ion permeation caused by direct competition of protons with divalent ions for specific binding sites in the channel pore (Jiang et al. 2005). Specifically, mutating glutamic acid residues 1047 and 1052 into nonpolar glutamine in mouse TRPM7 decreases or abolishes not only divalent ion selectivity but also pH sensitivity (Li et al. 2007). In human TRPM7, overlapping glutamic acid or aspartic acid residues have been identified in the pore region with similar results [D1054, E1052, and D1059 (Numata and Okada 2008)]. The pH sensitivity of TRPM7’s selectivity profile is an interesting biophysical feature that has to be taken into account under acidic pathological conditions.
5.8 TRPM7 Inhibitors
Several compounds have been reported to inhibit TRPM7, although most of them lack potency or specificity or both. Extracellular spermine blocks endogenous TRPM7-like currents in RBL-2H3 cells with an IC50 of 2.3 μM, whereas 20 μM of SKF-96365, a nonspecific TRP channel and voltage-gated Ca channel blocker (Singh et al. 2010), is needed for complete block (Kozak et al. 2002a). 2-Aminoethyl diphenylborinate (2-APB), a compound found to interfere with a variety of proteins involved in Ca signaling, inhibits overexpressed human TRPM7 currents with an IC50 of 174 μM (Li et al. 2006). Endogenous TRPM7-like currents can be reversibly inhibited at 50 μM 2-APB in Jurkat T lymphocytes (Prakriya and Lewis 2002). Interestingly, while 2-APB inhibits TRPM7, it activates its paralog TRPM6, making this compound a useful tool in discriminating between currents carried by these two proteins (Li et al. 2006). Furthermore, it is now known that 2-APB does not bind directly to TRPM7, but rather inhibits channel activity through an intracellular acidification mechanism (Chokshi et al. 2012a).
The first high-throughput drug-screening bioassay targeting TRPM7 was developed in 2010 using fluorescent-based Mn quench in HEK293 cells overexpressing human TRPM7 (Castillo et al. 2010). This led to the discovery of the first specific and highly potent TRPM7 inhibitor waixenicin A, a compound isolated from the soft coral Sarcothelia edmondsoni (Zierler et al. 2011). Waixenicin A blocks TRPM7 currents in a Mg-dependent manner with an IC50 of 16 nM, and TRPM7-dependent cell proliferation is inhibited with an IC50 of 3.2 μM in RBL-1 cells. Waixenicin A has no effects on other major pathways that regulate Ca influx such as TRPM2, TRPM4, and Ca release-activated Ca (CRAC) channels (Zierler et al. 2011), and the compound also does not inhibit TRPA1 at 10 μM concentrations (Zierler and Fleig unpublished data). Importantly, waixenicin A does not affect TRPM7’s sister channel TRPM6, adding another pharmacological tool for differentiating between TRPM6 and TRPM7 (Zierler et al. 2011).
Using an aequorin bioluminescence-based assay, several small conductance Ca-activated K channel inhibitors were found to act on TRPM7 (Chubanov et al. 2012), including the antimalarial plant alkaloid quinine, CyPPA, dequalinium, NS8593, SKA31, and UCL1684. Of those, the most potent compound was NS8593 with an IC50 of 1.6 μM. NS8593 is thought to be a direct channel blocker and seems to interfere with the Mg-dependent regulation of TRPM7 while also inhibiting the mobility of HEK293 (Chubanov et al. 2012). The broad-spectrum serine protease inhibitor and anticoagulant nafamostat mesylate interfere with heterologous mammalian TRPM7 with an IC50 of 27 μM (Chen et al. 2010a), and several 5-lipoxygenase inhibitors (NDGA, AA861, and MK886) inhibit TRPM7 in the higher μM range (Chen et al. 2010a). Sphingosine, the primary component of sphingolipids in the plasma membrane, and fingolimod, a structural analogue of sphingosine and FDA-approved for treatment of multiple sclerosis, are inhibitors of TRPM7 with IC50s of 600 nM and 720 nM, respectively (Qin et al. 2013). They act by reducing the open probability of the channel. Metabolites of sphingosine, such as sphingosine-1-phosphate or ceramides, have no effect. These properties are reminiscent of the sphingolipid effects reported for CRAC channels (Mathes et al. 1998). One known side effect of calcineurin inhibitors is hypomagnesemia. Cyclosporin A and FK506 (tacrolimus), important calcineurin inhibitors, affect Mg flux as assessed by MagFura measurements in the intestinal epithelial cell line CaCo (Gouadon et al. 2012). While cyclosporin A counteracts Mg accumulation, FK506 increases Mg influx without altering expression levels of TRPM6, TRPM7, or MagT1.
In conclusion, several compounds interfere with TRPM7 at various potencies or selectivity. Both 2-APB and waixenicin A could be useful tools to pharmacologically differentiate between TRPM6 and TRPM7.
6 Physiological Functions in Native Cells, Organs, and Organ systems
Early evidence pointed to TRPM7’s possible involvement in cellular Ca and Mg homeostasis as well as cell viability and proliferation (Penner and Fleig 2007). Further studies identified central roles in cell migration, exocytosis, and development (Runnels 2011), and disruption of normal TRPM7 function has been associated with the progression of cancer, severity of brain ischemia, and cardiovascular disease.
6.1 Magnesium Homeostasis
The channel’s involvement in cellular Mg homeostasis was shown through genetic knockout experiments in chicken B lymphocytes, leading to an arrest in cell proliferation and reduced intracellular Mg levels that could be rescued exclusively by high extracellular Mg supplementation (Schmitz et al. 2003). Interfering with TRPM7 channel function by a genetic knockout of the kinase domain arrests mouse embryonic stem cell proliferation that again can be rescued by high external Mg (Ryazanova et al. 2010). Such rescue of TRPM7-deficient cells is now known to be mediated by endogenous expression of alternate Mg transporters such as SLC41A1 (Kolisek et al. 2008), SLC41A2 (Sahni et al. 2007), or MagT1 (Deason-Towne et al. 2011), depending on cell type.
TRPM7 is not only important for cellular Mg homeostasis but is also involved in maintaining systemic Mg levels (Ryazanova et al. 2010). Mice heterozygotic for a TRPM7 kinase domain deletion develop hypomagnesemia compared to control mice. This seems to be caused by a deficit in Mg absorption through the colon rather than reabsorption mechanisms through the kidney. On the other hand, tissue-specific deletion of TRPM7 in T lymphocytes of mice does not alter total Mg contents of these cells (Jin et al. 2008). This is not surprising, since selective tissue-specific deletion of TRPM7 is not expected to alter overall systemic Mg homeostasis, and T cells express compensating Mg transporters such as MagT1 (Li et al. 2011).
Amidst discussions of the importance of TRPM7 in cellular and systemic Mg homeostasis, it should be remembered that the channel represents a divalent ion influx mechanism for other divalent ion species, including Ca and trace metals (Monteilh-Zoller et al. 2003). Interestingly, TRPM7’s ability to conduct Ca is currently linked to a disease-inducing role such as in neuronal ischemia or atrial fibrillation (Du et al. 2010; Aarts et al. 2003). The physiological role of Ca conductance by TRPM7 remains largely unexplored. Indeed, even TRPM7’s role in cell migration seems to be linked to its ability to conduct Mg rather than Ca (Su et al. 2011), despite a close correlation between TRPM7 plasma membrane localization and cellular Ca hot spot microdomains thought to drive cell migration (Wei et al. 2009; Clark et al. 2006). Finally, recent work implicates TRPM7-mediated Cd uptake in osteoblast cytotoxicity (Martineau et al. 2010; Levesque et al. 2008), further emphasizing TRPM7’s physiological role as a divalent ion channel mechanism.
6.2 Cell Proliferation, Cell Death, and Cell Differentiation
Genetic or pharmacological ablation of TRPM7 in proliferating tissue arrests cells at G0/G1 transition of the cell cycle (Zierler et al. 2011; Tani et al. 2007; Schmitz et al. 2003; Nadler et al. 2001; Abed and Moreau 2007; Sahni et al. 2010). When arresting RBL-2H3 cells at various stages of the cell cycle, endogenous TRPM7-like currents are significantly upregulated at the G0/G1 transition (Tani et al. 2007), further emphasizing the critical role of this channel at the transition stage from quiescence to proliferation. Differentiated mast cells, on the other hand, undergo apoptosis upon genetic suppression of TRPM7 (Ng et al. 2012), and similar observations are made in hepatic stellate cells, possibly involving the TNF-related apoptosis-inducing ligand (TRAIL) mechanism (Liu et al. 2012). Interestingly, proliferating rat embryonic hepatocytes and rat hepatoma show higher TRPM7 expression levels than adult nondividing rat hepatocytes, indicating that downregulation of endogenous TRPM7 is linked to the differentiation process (Lam et al. 2012). Thus, it appears that TRPM7 is critical for the physiology of proliferating cells to maintain cell numbers, whereas differentiated cells reduce the expression of TRPM7 to levels that sustain supplementation of cells with Ca, Mg, and trace metals.
6.3 Migration
Early observations link TRPM7 activity to maintenance of cell structure, as TRPM7 overexpression in HEK293 cells leads to rounding and detachment of cells from the substrate, which requires functional m-calpain activity and depends on Ca influx through the TRPM7 channel domain (Nadler et al. 2001; Su et al. 2006). Activation of m-calpain by TRPM7 is thought to work through stress-dependent stimulation of p38 MAP kinase and JUN kinase, as inhibitors of these proteins inhibit the cell rounding and detachment caused by overexpressing TRPM7 (Su et al. 2010). TRPM7 seems to be partially responsible for supporting activated T cell migration as well as the velocity of migration (Kuras et al. 2012), and genetic suppression of TRPM7 in migrating WI-38 fibroblasts leads to a reduced number of Ca flickers accompanied by a disruption of normal cell migratory patterns (Wei et al. 2009). Interestingly, both intracellular Mg and Ca can influence m-calpain activity (Su et al. 2010), and indeed Rac- and Cdc42-dependent polarized cell movement of fibroblasts relies on the availability of intracellular Mg and not Ca (Su et al. 2011). Further studies identify TRPM7 as controlling actomyosin contractility and cell adhesion by increasing cellular Ca levels, and this involves a phosphorylation step utilizing the channel’s kinase (Clark et al. 2006). Thus, it seems that divalent ion influx through TRPM7 is involved in cell adhesion and migration, whereas the protein’s kinase domain supports actomyosin contractility.
7 Lessons from Knockouts
The role of TRPM7 in living organisms has been investigated in several genetically tractable animal models such as mouse (M. musculus), zebrafish (D. rerio), and frog (Xenopus laevis). The roles of TRPM7-related proteins have also been explored in invertebrate species such as fruit fly (Drosophila melanogaster) and roundworm (Caenorhabditis elegans).
7.1 Mouse Trpm7
Two Trpm7 null mutant mice [Trpm7 βgeo and Trpm7 Δ17 (Table 1)] and mouse mutants lacking exons encoding the kinase domain [Trpm7 Δkinase/Δkinase (Table 1)] die at embryonic day 6.5–7.5 (e6.5–e7.5) and e7.5, respectively (Ryazanova et al. 2010; Jin et al. 2008). The reasons for this remain unclear. As briefly discussed above, mice heterozygotic for the TRPM7 kinase ablation (Trpm7 Δkinase/+) have reduced Mg levels in the blood, bone, and urine (Ryazanova et al. 2010). In contrast to wild-type control mice, a substantial fraction of heterozygotic animals die shortly after placing them on a Mg-deficient diet. In addition, Trpm7 Δkinase/+ mice exhibit behavioral alterations indicative of Mg deficiency (clasping, tremors, and seizures). Embryonic stem (ES) cells isolated from these animals show reduced TRPM7 currents due to an increased sensitivity to intracellular Mg. Thus, experiments with Trpm7 Δkinase/+ mice indicate that a key aspect of TRPM7 function is a regulation of systemic Mg homeostasis.
Conditional mutagenesis of the Trpm7fl allele (Table 1) using Cre/loxP-recombination technologies has been employed to elucidate a spatiotemporal requirement for Trpm7 during embryonic development. Here, an epiblast-restricted inactivation of Trpm7 leads to lethality indicating that TRPM7 is required within the embryo proper (Jin et al. 2008). Furthermore, global disruption of Trpm7 at different embryonic stages using a tamoxifen (TM)-inducible Cre-ER transgene uncovers embryonic lethality during e7–e9. In contrast, TM-induced mutagenesis of Trpm7 at e14.5 is compatible with prenatal development since healthy Trpm7 null pups are born with expected Mendelian inheritance. Surprisingly, the TM-induced inactivation of Trpm7 in adults causes no obvious phenotype, suggesting that Trpm7 is indispensible only before and during organogenesis (Jin et al. 2008). However, one caveat to keep in mind is the difficulty to accurately assess whether the incomplete deletion of Trpm7 observed in the tissue of this mouse model is sufficient to induce a true Trpm7 null phenotype.
Several Cre transgenic lines with tissue-specific recombination activity were used to elucidate the organ-restricted requirements of Trpm7. First, deletion of Trpm7 in the T cell lineage disrupts thymopoiesis and leads to a developmental block of thymocytes and a progressive depletion of thymic medullary cells (Jin et al. 2008). Second, disruption of Trpm7 in the embryonic ureteric bud causes ablation of the protein in collecting ducts of the postnatal kidney without obvious morphological alterations (Jin et al. 2012). In contrast, deletion of Trpm7 in the embryonic metanephric mesenchyme leads to inactivation of the gene in renal tubules of the kidney. The latter mutants show a reduction in glomeruli number, renal tubular dilation, and formation of cysts in the proximal tubules, indicating that Trpm7 is essential for nephrogenesis. Third, disruption of Trpm7 in neural crest (NC) cells at e10.5 results in loss of dorsal root ganglion sensory neurons and skin pigment cells (Jin et al. 2012). However, disruption of Trpm7 in the embryonic neural stem (NS) cells at e10.5 does not influence normal brain development. Studies with NS cells in vitro reveal that Trpm7 is not essential for their self-renewal and differentiation. In contrast, during in vitro differentiation of induced pluripotent stem cells to NS cells, Trpm7 disruption prevents the formation of the NS cell monolayer. Thus, Trpm7 seems essential for NC progenitors but dispensable once the progenitors are committed.
The role of Trpm7 in cardiogenesis has been studied by heart-restricted mutagenesis of the conditional Trpm7 allele (Sah et al. 2013). Cardiac deletion of Trpm7 at e9.0 results in congestive heart failure and death. In contrast, inactivation of Trpm7 at e13.0 produces viable mice with normal ventricular function. Deletion of Trpm7 at an intermediate time point reduces viability of the mutants to 50 %. The surviving mutant mice develop cardiomyopathy associated with heart block, impaired repolarization, and ventricular arrhythmias.
7.2 Zebrafish and Frog
Several loss-of-function mutations in zebrafish Trpm7 (zTrpm7) have been described. zTrpm7-deficient animals undergo normal early morphogenesis. However, mutant larvae exhibit multiple defects including loss of touch responsiveness, defective melanin synthesis and apoptotic death of melanophores, defective proliferation of epithelial cells in the exocrine pancreas, and lethality in late larval life (Yee et al. 2011; McNeill et al. 2007; Low et al. 2011; Elizondo et al. 2005, 2010). zTrpm7 mutant larvae have reduced total levels of Mg and Ca, and addition of supplemental Mg, but not Ca, partially rescues melanophore survival and proliferation of cells in the exocrine pancreas (Yee et al. 2011; Elizondo et al. 2010). zTrpm7 mutants develop kidney stones and express higher levels of stanniocalcin 1 (stc1) and anti-hyperphosphatemic factor, fibroblast growth factor 23 (fgf23) (Elizondo et al. 2010). Stc1 modulates total Mg and Ca levels both in mutant and wild-type larvae. The levels of Mg and Ca can be normalized in zTrpm7 mutants by a block of stc1 activity, whereas the formation of kidney stones can be prevented by knockdown of fgf23.
A role of TRPM7 in early embryonic development has also been studied by genetic manipulation of X. laevis embryos. Knockdown of Xenopus Trpm7 (xTrpm7) transcripts using morpholino oligonucleotides reveals that xTrpm7 in conjunction with noncanonical Wnt signaling regulates cell polarity and migration during gastrulation (Liu et al. 2011). The gastrulation defect can be rescued by exogenous Mg and by overexpression of the Mg transporter SLC41A2 or a dominant negative form of Rac. This suggests that TRPM7-mediated entry of Mg plays an important role in vertebrate gastrulation.
7.3 Fruit Fly and Roundworm
D. melanogaster harbors a single Trpm gene (dTrpm) encoding a channel subunit that lacks a kinase domain. dTrpm is highly expressed in the Malpighian tubules (equivalent of mammalian kidneys). dTrpm null mutants develop slowly as larvae and arrest as prepupae with morphological defects in the Malpighian tubules (Hofmann et al. 2010). dTrpm-deficient larvae show increased Mg levels in the body when raised on Mg-enriched diets indicating that dTrpm regulates removal of Mg from the hemolymph by the Malpighian tubules. dTrpm may also regulate Zn homeostasis. It was reported that dTrpm-deficient larvae exhibit low Zn levels, and this phenotype can be rescued by Zn supplementation (Georgiev et al. 2010).
C. elegans has three TRPM channels genetically related to TRPM7: GON-2, GTL-1, and GTL-2. Like dTRPM, these channels do not contain enzyme domains. GON-2 and GTL-1 are expressed in the intestine and regulate Mg uptake, while GTL-2 controls Mg excretion by the excretory cells (Teramoto et al. 2005, 2010). Moreover, gon-2/gtl-1 double mutants show reduced body Mg levels and a growth defect, which can be rescued by dietary Mg, but not Ca (Teramoto et al. 2010).
In summary, the experiments with genetically tractable animal models support the idea that TRPM7 and its genetic relatives are essential for early development, organogenesis, and regulation of Mg homeostasis.
8 Role in Hereditary and Acquired Diseases
TRPM7 is not the only ion channel whose activity is controlled by the availability of intracellular Mg · ATP. Early studies identified ATP-sensitive voltage-dependent chloride (Cl-) channels in the sarcoplasmic reticulum of rabbit skeletal muscle (Kourie 1997) and in the plasma membrane of various tissue, including mouse cortical collecting ducts (Meyer and Korbmacher 1996) and human T cells (Cahalan and Lewis 1988). CFTR ion channel gating is regulated by ATP binding and hydrolysis in synergy with intracellular Mg (Ikuma and Welsh 2000), and activity of the ATP-sensitive K+ channels in pancreatic β-cells is determined by the intracellular concentration ratio of Mg · ATP over Mg · ADP (Tarasov et al. 2004). As such, these ion channels function as sensors of cell metabolism, and any changes in availability of either glucose or oxygen will affect their channel activity with varying impact on cell (patho)physiology. For TRPM7 this was most dramatically demonstrated in a mouse model of transient global ischemia, where small interfering RNA-induced suppression of TRPM7 in the right hippocampus protected neurons from undergoing cell death compared to control (Sun et al. 2009). Ample in vitro studies corroborate the involvement of TRPM7 in oxygen-glucose deprivation-induced neuronal cell death (Aarts et al. 2003; Zhang et al. 2011), either caused by activation of channels by reactive oxygen species (Aarts et al. 2003; Coombes et al. 2011), by changes in extracellular divalent ions (Wei et al. 2007), or by TRPM7-mediated Zn2+ accumulation (Inoue et al. 2010). Epidemiologic studies inspired by these findings have performed comparative gene expression analyses in mice to link TRPM7 to brain-related diseases such as multiple sclerosis, Alzheimer’s disease, and stroke and found TRPM7 to be one of 18 common genes to be regulated in these mouse disease models (Tseveleki et al. 2010). However, a prospective, nested case-control study did not find a connection between incident risk of ischemic stroke and variations in the TRPM7 gene (Romero et al. 2009).
The TPRM7 channel kinase seems to play a role in various cardiovascular diseases. Early reports located TRPM7-like currents in pig, rat, and guinea pig ventricular myocytes (Gwanyanya et al. 2004) and most recently in human atrial myocytes (Macianskiene et al. 2012; Zhang et al. 2012a), although the channel kinase’s role in this tissue remains to be determined. More is known about TRPM7’s function in human cardiofibroblasts, where increased TRPM7-mediated Ca influx has been linked to increased myofibroblast differentiation and fibrogenesis in patients prone to atrial fibrillation (Du et al. 2010).
TRPM7 is also linked to hypertension. Vascular smooth muscle cells (VSMC) from spontaneously hypertensive rats have lower TRPM7 mRNA levels and significantly reduced intracellular Mg levels compared to VSMC from Wistar control, and this is linked to angiotensin II stimulation (Touyz et al. 2006). Interestingly, chronic angiotensin II application increases intracellular Mg in a TRPM7-dependent way leading to enhanced DNA and protein production, indicating cell growth (He et al. 2005). VSMC isolated from the ascending aorta of mouse respond to angiotensin II stimulation by upregulating TRPM7 expression, which triggers a Ca-dependent switch from contractile cell characteristics to a phenotype supporting cell proliferation (Zhang et al. 2012b). In human aortic VSMC, vascular calcification can be prevented on the cellular level by exposing cells to increasing external Mg concentrations, and the use of pharmacological tools implicates TRPM7 to be involved in this process (Louvet et al. 2013). Renal TRPM7 (and TRPM6) is downregulated in a mouse model of hereditary hypomagnesemiar (Yogi et al. 2011), which is further exacerbated by aldosterone administration to induce hypertension (Sontia et al. 2008). Mg supplementation can alleviate the effects induced by aldosterone, including hypertension, inflammation, and fibrosis.
The central function of TRPM7 in processes driving cell growth, proliferation, differentiation, and migration identifies the protein as a possible target in cancer (Sahni et al. 2010). Indeed, reducing TRPM7 expression inhibits proliferation in human head and neck carcinoma (Jiang et al. 2007) and human gastric adenocarcinoma cells (Kim et al. 2008). In other cancer cell lines, this experimental manipulation affects cell migration and invasiveness, such as in A549 lung cancer (Gao et al. 2011), human nasopharyngeal carcinoma (Chen et al. 2010b), BXPC-3 human pancreas adenocarcinoma (Rybarczyk et al. 2012), or MDA-MB-435 breast cancer cells (Meng et al. 2013). When comparing tumor tissue with normal tissue, TRPM7 is generally upregulated as assessed in human pancreatic adenocarcinoma (Rybarczyk et al. 2012; Yee et al. 2011), human breast cancer (Middelbeek et al. 2012), and rat hepatoma (Lam et al. 2012). This has led to the identification of TRPM7 as an independent predictor of poor outcome in breast cancer patients due to increased metastasis formation (Meng et al. 2013; Middelbeek et al. 2012). Patient survival is inversely related to TRPM7 expression levels in human pancreatic ductal adenocarcinoma, where TRPM7 levels increase at higher tumor staging (Rybarczyk et al. 2012).
Based on TRPM7’s unique permeation profile for both Ca and Mg, epidemiologic studies have started to look at the ratio of Ca:Mg intake and cancer risk. The T1482I polymorphism in the TRPM7 gene, thought to contribute to familial amyotrophic lateral sclerosis and Parkinsonism dementia in Guam (Hermosura et al. 2005) but not in Kii, Japan (Hara et al. 2010), is associated with elevated risk of adenomatous and hyperplastic polyps, both risk indicators of colorectal adenoma. This association is particularly strong when the Ca:Mg intake ratio is high (Dai et al. 2007) and gave reason to initiate a randomized placebo-controlled intervention clinical trial investigating whether a reduction of dietary Ca:Mg ratio lowers the risk of adenoma and hyperplastic polyps in patients who do or do not carry the T1482I allele (clinicaltrials.gov: NCT01105169). A retrospective analysis in age-matched prostate cancer patients shows a parallel increase in the serum Ca:Mg ratio and TRPM7 expression levels (Sun et al. 2013). For postmenopausal breast cancer, the medical hypothesis was brought forth that a higher ratio of Ca:Mg serum levels might parallel increased risk (Sahmoun and Singh 2010). Thus, TRPM7 as a Ca- and Mg-conducting ion channel may represent a novel target to be considered in cancer prevention and control.
References
Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115(7):863–877
Abed E, Moreau R (2007) Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif 40(6):849–865. doi:10.1111/j.1365-2184.2007.00476.x
Bessac BF, Fleig A (2007) TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J Physiol 582(Pt 3):1073–1086. doi:10.1113/jphysiol.2007.130534
Cahalan MD, Lewis RS (1988) Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43:281–301
Castillo B, Porzgen P, Penner R, Horgen FD, Fleig A (2010) Development and optimization of a high-throughput bioassay for TRPM7 ion channel inhibitors. J Biomol Screen 15(5):498–507. doi:10.1177/1087057110368294
Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW (2010a) Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One 5(6):e11161. doi:10.1371/journal.pone.0011161
Chen JP, Luan Y, You CX, Chen XH, Luo RC, Li R (2010b) TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca(2+) influx. Cell Calcium 47(5):425–432. doi:10.1016/j.ceca.2010.03.003
Chokshi R, Fruasaha P, Kozak JA (2012a) 2-aminoethyl diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels (Austin) 6(5):362–369. doi:10.4161/chan.21628
Chokshi R, Matsushita M, Kozak JA (2012b) Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am J Physiol Cell Physiol 302(7):C1004–1011. doi:10.1152/ajpcell.00422.2011
Chokshi R, Matsushita M, Kozak JA (2012c) Sensitivity of TRPM7 channels to Mg2+ characterized in cell-free patches of Jurkat T lymphocytes. Am J Physiol Cell Physiol 302(11):C1642–1651. doi:10.1152/ajpcell.00037.2012
Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A 101(9):2894–2899. doi:10.1073/pnas.0305252101
Chubanov V, Schlingmann KP, Waring J, Heinzinger J, Kaske S, Waldegger S, Mederos y Schnitzler M, Gudermann T (2007) Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J Biol Chem 282(10):7656–7667. doi:10.1074/jbc.M611117200
Chubanov V, Mederos y Schnitzler M, Meissner M, Schafer S, Abstiens K, Hofmann T, Gudermann T (2012) Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol 166(4):1357–1376. doi:10.1111/j.1476-5381.2012.01855.x
Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN (2006) TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J 25(2):290–301. doi:10.1038/sj.emboj.7600931
Clark K, Middelbeek J, Dorovkov MV, Figdor CG, Ryazanov AG, Lasonder E, van Leeuwen FN (2008a) The alpha-kinases TRPM6 and TRPM7, but not eEF-2 kinase, phosphorylate the assembly domain of myosin IIA, IIB and IIC. FEBS Lett 582(20):2993–2997. doi:10.1016/j.febslet.2008.07.043
Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN (2008b) TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J Mol Biol 378(4):790–803. doi:10.1016/j.jmb.2008.02.057
Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E, van Leeuwen FN (2008c) Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One 3(3):e1876. doi:10.1371/journal.pone.0001876
Coombes E, Jiang J, Chu XP, Inoue K, Seeds J, Branigan D, Simon RP, Xiong ZG (2011) Pathophysiologically relevant levels of hydrogen peroxide induce glutamate-independent neurodegeneration that involves activation of transient receptor potential melastatin 7 channels. Antioxid Redox Signal 14(10):1815–1827. doi:10.1089/ars.2010.3549
Crawley SW, Cote GP (2009) Identification of dimer interactions required for the catalytic activity of the TRPM7 alpha-kinase domain. Biochem J 420(1):115–122. doi:10.1042/BJ20081405
Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, Li M, Shyr Y, Zheng W (2007) The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr 86(3):743–751
Deason-Towne F, Perraud AL, Schmitz C (2011) The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett 585(14):2275–2278. doi:10.1016/j.febslet.2011.05.052
Deason-Towne F, Perraud AL, Schmitz C (2012) Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C gamma2 (PLCgamma2) using TRPM7-kinase. Cell Signal 24(11):2070–2075. doi:10.1016/j.cellsig.2012.06.015
Demeuse P, Penner R, Fleig A (2006) TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol 127(4):421–434. doi:10.1085/jgp.200509410
Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y, Clapham DE (2012) Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev Cell 22(6):1149–1162. doi:10.1016/j.devcel.2012.04.006
Dorovkov MV, Ryazanov AG (2004) Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 279(49):50643–50646. doi:10.1074/jbc.C400441200
Dorovkov MV, Kostyukova AS, Ryazanov AG (2011) Phosphorylation of annexin A1 by TRPM7 kinase: a switch regulating the induction of an alpha-helix. Biochemistry 50(12):2187–2193. doi:10.1021/bi101963h
Drennan D, Ryazanov AG (2004) Alpha-kinases: analysis of the family and comparison with conventional protein kinases. Prog Biophys Mol Biol 85(1):1–32. doi:10.1016/S0079-6107(03)00060-9
Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L (2010) TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106(5):992–1003. doi:10.1161/CIRCRESAHA.109.206771
Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM (2005) Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol 15(7):667–671. doi:10.1016/j.cub.2005.02.050
Elizondo MR, Budi EH, Parichy DM (2010) trpm7 regulation of in vivo cation homeostasis and kidney function involves stanniocalcin 1 and fgf23. Endocrinology 151(12):5700–5709. doi:10.1210/en.2010-0853
Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26(3):159–178. doi: 10.1080/1079989060063750
Fujiwara Y, Minor DL Jr (2008) X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol 383(4):854–870. doi:10.1016/j.jmb.2008.08.059
Gao H, Chen X, Du X, Guan B, Liu Y, Zhang H (2011) EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 50(6):559–568. doi:10.1016/j.ceca.2011.09.003
Georgiev P, Okkenhaug H, Drews A, Wright D, Lambert S, Flick M, Carta V, Martel C, Oberwinkler J, Raghu P (2010) TRPM channels mediate zinc homeostasis and cellular growth during Drosophila larval development. Cell Metab 12(4):386–397. doi:10.1016/j.cmet.2010.08.012
Gouadon E, Lecerf F, German-Fattal M (2012) Differential effects of cyclosporin A and tacrolimus on magnesium influx in Caco2 cells. J Pharm Pharm Sci 15(3):389–398
Gwanyanya A, Amuzescu B, Zakharov SI, Macianskiene R, Sipido KR, Bolotina VM, Vereecke J, Mubagwa K (2004) Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation. J Physiol 559(Pt 3):761–776. doi:10.1113/jphysiol.2004.067637
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100
Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, Sasaki R, Goto J, Nishizawa M, Kuzuhara S, Tsuji S (2010) TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet B Neuropsychiatr Genet 153B(1):310–313. doi:10.1002/ajmg.b.30966
He Y, Yao G, Savoia C, Touyz RM (2005) Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res 96(2):207–215. doi:10.1161/01.RES.0000152967.88472.3e
Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A (2002) Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol 539(Pt 2):445–458
Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM (2005) A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A 102(32):11510–11515. doi:10.1073/pnas.0505149102
Hofmann T, Chubanov V, Chen X, Dietz AS, Gudermann T, Montell C (2010) Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PLoS One 5(5):e10519. doi:10.1371/journal.pone.0010519
Ikuma M, Welsh MJ (2000) Regulation of CFTR Cl- channel gating by ATP binding and hydrolysis. Proc Natl Acad Sci U S A 97(15):8675–8680. doi:10.1073/pnas.140220597
Inoue K, Branigan D, Xiong ZG (2010) Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem 285(10):7430–7439. doi:10.1074/jbc.M109.040485
Jang Y, Lee Y, Kim SM, Yang YD, Jung J, Oh U (2012) Quantitative analysis of TRP channel genes in mouse organs. Arch Pharm Res 35(10):1823–1830. doi:10.1007/s12272-012-1016-8
Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R (2003) X-ray structure of a voltage-dependent K + channel. Nature 423(6935):33–41. doi:10.1038/nature01580
Jiang J, Li M, Yue L (2005) Potentiation of TRPM7 inward currents by protons. J Gen Physiol 126(2):137–150. doi:10.1085/jgp.200409185
Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG (2007) Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res 67(22):10929–10938. doi:10.1158/0008-5472.CAN-07-1121
Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE (2008) Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322(5902):756–760. doi:10.1126/science.1163493
Jin J, Wu LJ, Jun J, Cheng X, Xu H, Andrews NC, Clapham DE (2012) The channel kinase, TRPM7, is required for early embryonic development. Proc Natl Acad Sci U S A 109(5):E225–233. doi:10.1073/pnas.1120033109
Kerschbaum HH, Kozak JA, Cahalan MD (2003) Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J 84(4):2293–2305. doi:10.1016/S0006-3495(03)75035-8
Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ, So I (2008) Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci 99(12):2502–2509. doi:10.1111/j.1349-7006.2008.00982.x
Kim BJ, Park KJ, Kim HW, Choi S, Jun JY, Chang IY, Jeon JH, So I, Kim SJ (2009) Identification of TRPM7 channels in human intestinal interstitial cells of Cajal. World J Gastroenterol 15(46):5799–5804
Kim TY, Shin SK, Song MY, Lee JE, Park KS (2012) Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem Biophys Res Commun 417(3):1030–1034. doi:10.1016/j.bbrc.2011.12.085
Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A, Schweigel M (2008) SLC41A1 is a novel mammalian Mg2+ carrier. J Biol Chem 283(23):16235–16247. doi:10.1074/jbc.M707276200
Kourie JI (1997) ATP-sensitive voltage- and calcium-dependent chloride channels in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. J Membr Biol 157(1):39–51
Kozak JA, Cahalan MD (2003) MIC channels are inhibited by internal divalent cations but not ATP. Biophys J 84(2 Pt 1):922–927. doi:10.1016/S0006-3495(03)74909-1
Kozak JA, Kerschbaum HH, Cahalan MD (2002) Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol 120(2):221–235
Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE (2006) The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 52(3):485–496. doi:10.1016/j.neuron.2006.09.033
Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H (2006) Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7:159. doi:10.1186/1471-2164-7-159
Kuras Z, Yun YH, Chimote AA, Neumeier L, Conforti L (2012) KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells. PLoS One 7(8):e43859
Lam DH, Grant CE, Hill CE (2012) Differential expression of TRPM7 in rat hepatoma and embryonic and adult hepatocytes. Can J Physiol Pharmacol 90(4):435–444. doi:10.1139/Y11-136
Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K (2007) Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem 282(1):232–239. doi:10.1074/jbc.M605300200
Levesque M, Martineau C, Jumarie C, Moreau R (2008) Characterization of cadmium uptake and cytotoxicity in human osteoblast-like MG-63 cells. Toxicol Appl Pharmacol 231(3):308–317. doi:10.1016/j.taap.2008.04.016
Li M, Jiang J, Yue L (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 127(5):525–537. doi:10.1085/jgp.200609502
Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L (2007) Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem 282(35):25817–25830. doi:10.1074/jbc.M608972200
Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ (2011) Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 475(7357):471–476. doi:10.1038/nature10246
Liu W, Su LT, Khadka DK, Mezzacappa C, Komiya Y, Sato A, Habas R, Runnels LW (2011) TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev Biol 350(2):348–357. doi:10.1016/j.ydbio.2010.11.034
Liu H, Li J, Huang Y, Huang C (2012) Inhibition of transient receptor potential melastatin 7 channel increases HSCs apoptosis induced by TRAIL. Life Sci 90(15–16):612–618. doi:10.1016/j.lfs.2012.02.012
Louvet L, Buchel J, Steppan S, Passlick-Deetjen J, Massy ZA (2013) Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant 28(4):869–878. doi:10.1093/ndt/gfs520
Low SE, Amburgey K, Horstick E, Linsley J, Sprague SM, Cui WW, Zhou W, Hirata H, Saint-Amant L, Hume RI, Kuwada JY (2011) TRPM7 is required within zebrafish sensory neurons for the activation of touch-evoked escape behaviors. J Neurosci 31(32):11633–11644. doi:10.1523/JNEUROSCI.4950-10.2011
Macianskiene R, Gwanyanya A, Vereecke J, Mubagwa K (2008) Inhibition of the magnesium-sensitive TRPM7-like channel in cardiac myocytes by nonhydrolysable GTP analogs: involvement of phosphoinositide metabolism. Cell Physiol Biochem 22(1–4):109–118. doi:10.1159/000149788
Macianskiene R, Martisiene I, Zablockaite D, Gendviliene V (2012) Characterization of Mg(2)(+)-regulated TRPM7-like current in human atrial myocytes. J Biomed Sci 19:75. doi:10.1186/1423-0127-19-75
Madsen JA, Cheng RR, Kaoud TS, Dalby KN, Makarov DE, Brodbelt JS (2012) Charge-site-dependent dissociation of hydrogen-rich radical peptide cations upon vacuum UV photoexcitation. Chemistry 18(17):5374–5383. doi:10.1002/chem.201103534
Martineau C, Abed E, Medina G, Jomphe LA, Mantha M, Jumarie C, Moreau R (2010) Involvement of transient receptor potential melastatin-related 7 (TRPM7) channels in cadmium uptake and cytotoxicity in MC3T3-E1 osteoblasts. Toxicol Lett 199(3):357–363. doi:10.1016/j.toxlet.2010.09.019
Mathes C, Fleig A, Penner R (1998) Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem 273(39):25020–25030
Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, Nairn AC (2005) Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J Biol Chem 280(21):20793–20803. doi:10.1074/jbc.M413671200
McNeill MS, Paulsen J, Bonde G, Burnight E, Hsu MY, Cornell RA (2007) Cell death of melanophores in zebrafish trpm7 mutant embryos depends on melanin synthesis. J Invest Dermatol 127(8):2020–2030. doi:10.1038/sj.jid.5700710
Mederos y Schnitzler M, Waring J, Gudermann T, Chubanov V (2008) Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J 22(5):1540–1551. doi:10.1096/fj.07-9694com
Meng X, Cai C, Wu J, Cai S, Ye C, Chen H, Yang Z, Zeng H, Shen Q, Zou F (2013) TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett 333(1):96–102. doi:10.1016/j.canlet.2013.01.031
Meyer K, Korbmacher C (1996) Cell swelling activates ATP-dependent voltage-gated chloride channels in M-1 mouse cortical collecting duct cells. J Gen Physiol 108(3):177–193
Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, Wieringa B, Canisius SV, Zwart W, Wessels LF, Sweep FC, Bult P, Span PN, van Leeuwen FN, Jalink K (2012) TRPM7 is required for breast tumor cell metastasis. Cancer Res 72(16):4250–4261. doi:10.1158/0008-5472.CAN-11-3863
Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 121(1):49–60
Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A (2001) LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 411(6837):590–595. doi:10.1038.350790923507.0.2 [pii]
Ng NM, Jiang SP, Lv ZQ (2012) Retrovirus-mediated siRNA targeting TRPM7 gene induces apoptosis in RBL-2H3 cells. Eur Rev Med Pharmacol Sci 16(9):1172–1178
Numata T, Okada Y (2008) Molecular determinants of sensitivity and conductivity of human TRPM7 to Mg2+ and Ca2+. Channels (Austin) 2(4):283–286
Numata T, Shimizu T, Okada Y (2007) TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292(1):C460–467. doi:10.1152/ajpcell.00367.2006
Oancea E, Wolfe JT, Clapham DE (2006) Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98(2):245–253. doi:10.1161/01.RES.0000200179.29375.cc
Penner R, Fleig A (2007) The Mg2+ and Mg(2+)-nucleotide-regulated channel-kinase TRPM7. Handb Exp Pharmacol 179:313–328. doi:10.1007/978-3-540-34891-7_19
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411(6837):595–599. doi: 10.1038/35079100
Perraud AL, Zhao X, Ryazanov AG, Schmitz C (2011) The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k. Cell Signal 23(3):586–593. doi:10.1016/j.cellsig.2010.11.011
Prakriya M, Lewis RS (2002) Separation and characterization of currents through store-operated CRAC channels and Mg2 + -inhibited cation (MIC) channels. J Gen Physiol 119(5):487–507
Qin X, Yue Z, Sun B, Yang W, Xie J, Ni E, Feng Y, Mahmood R, Zhang Y, Yue L (2013) Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol 168(6):1294–1312. doi:10.1111/bph.12012
Romero JR, Ridker PM, Zee RY (2009) Gene variation of the transient receptor potential cation channel, subfamily M, member 7 (TRPM7), and risk of incident ischemic stroke: prospective, nested, case-control study. Stroke 40(9):2965–2968. doi:10.1161/STROKEAHA.109.558346
Runnels LW (2011) TRPM6 and TRPM7: A Mul-TRP-PLIK-cation of channel functions. Curr Pharm Biotechnol 12(1):42–53
Runnels LW, Yue L, Clapham DE (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291(5506):1043–1047. doi:10.1126/science.1058519
Runnels LW, Yue L, Clapham DE (2002) The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 4(5):329–336. doi:10.1038/ncb781
Ryazanov AG (2002) Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett 514(1):26–29
Ryazanov AG, Pavur KS, Dorovkov MV (1999) Alpha-kinases: a new class of protein kinases with a novel catalytic domain. Curr Biol 9(2):R43–45
Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG (2004) Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem 279(5):3708–3716. doi:10.1074/jbc.M308820200
Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG (2010) TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun 1:109. doi:10.1038/ncomms1108
Rybarczyk P, Gautier M, Hague F, Dhennin-Duthille I, Chatelain D, Kerr-Conte J, Pattou F, Regimbeau JM, Sevestre H, Ouadid-Ahidouch H (2012) Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int J Cancer 131(6):E851–861. doi:10.1002/ijc.27487
Sah R, Mesirca P, Mason X, Gibson W, Bates-Withers C, Van den Boogert M, Chaudhuri D, Pu W, Mangoni ME, Clapham DE (2013) The timing of myocardial Trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction and repolarization. Circulation 128(2):101–114. doi:10.1161/CIRCULATIONAHA.112.000768
Sahmoun AE, Singh BB (2010) Does a higher ratio of serum calcium to magnesium increase the risk for postmenopausal breast cancer? Med Hypotheses 75(3):315–318. doi:10.1016/j.mehy.2010.02.037
Sahni J, Nelson B, Scharenberg AM (2007) SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J 401(2):505–513. doi:10.1042/BJ20060673
Sahni J, Tamura R, Sweet IR, Scharenberg AM (2010) TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes. Cell Cycle 9(17):3565–3574. doi:10.4161/cc.9.17.12798
Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31(2):166–170. doi:10.1038/ng889
Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114(2):191–200
Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL (2005) The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem 280(45):37763–37771. doi:10.1074/jbc.M509175200
Schweigel M, Kolisek M, Nikolic Z, Kuzinski J (2008) Expression and functional activity of the Na/Mg exchanger, TRPM7 and MagT1 are changed to regulate Mg homeostasis and transport in rumen epithelial cells. Magnes Res 21(2):118–123
Singh A, Hildebrand ME, Garcia E, Snutch TP (2010) The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol 160(6):1464–1475. doi:10.1111/j.1476-5381.2010.00786.x
Sontia B, Montezano AC, Paravicini T, Tabet F, Touyz RM (2008) Downregulation of renal TRPM7 and increased inflammation and fibrosis in aldosterone-infused mice: effects of magnesium. Hypertension 51(4):915–921. doi:10.1161/HYPERTENSIONAHA.107.100339
Staaf S, Franck MC, Marmigere F, Mattsson JP, Ernfors P (2010) Dynamic expression of the TRPM subgroup of ion channels in developing mouse sensory neurons. Gene Expr Patterns 10(1):65–74. doi:10.1016/j.gep.2009.10.003
Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW (2006) TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem 281(16):11260–11270. doi:10.1074/jbc.M512885200
Su LT, Chen HC, Gonzalez-Pagan O, Overton JD, Xie J, Yue L, Runnels LW (2010) TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J Mol Biol 396(4):858–869. doi:10.1016/j.jmb.2010.01.014
Su LT, Liu W, Chen HC, Gonzalez-Pagan O, Habas R, Runnels LW (2011) TRPM7 regulates polarized cell movements. Biochem J 434(3):513–521. doi:10.1042/BJ20101678
Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, Golde TE, Orser BA, Macdonald JF, Tymianski M (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12(10):1300–1307. doi:10.1038/nn.2395
Sun Y, Selvaraj S, Varma A, Derry S, Sahmoun AE, Singh BB (2013) Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J Biol Chem 288(1):255–263. doi:10.1074/jbc.M112.393918
Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A (2004) Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A 101(16):6009–6014. doi:10.1073/pnas.0307565101
Tani D, Monteilh-Zoller MK, Fleig A, Penner R (2007) Cell cycle-dependent regulation of store-operated I(CRAC) and Mg2 + -nucleotide-regulated MagNuM (TRPM7) currents. Cell Calcium 41(3):249–260. doi:10.1016/j.ceca.2006.07.004
Tarasov A, Dusonchet J, Ashcroft F (2004) Metabolic regulation of the pancreatic beta-cell ATP-sensitive K + channel: a pas de deux. Diabetes 53(Suppl 3):S113–122
Teramoto T, Lambie EJ, Iwasaki K (2005) Differential regulation of TRPM channels governs electrolyte homeostasis in the C. elegans intestine. Cell Metab 1(5):343–354. doi:10.1016/j.cmet.2005.04.007
Teramoto T, Sternick LA, Kage-Nakadai E, Sajjadi S, Siembida J, Mitani S, Iwasaki K, Lambie EJ (2010) Magnesium excretion in C. elegans requires the activity of the GTL-2 TRPM channel. PLoS One 5(3):e9589
Tian SL, Jiang H, Zeng Y, Li LL, Shi J (2007) NGF-induced reduction of an outward-rectifying TRPM7-like current in rat CA1 hippocampal neurons. Neurosci Lett 419(2):93–98. doi:10.1016/j.neulet.2007.04.020
Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, Callera GE (2006) Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290(1):R73–78. doi:10.1152/ajpregu.00515.2005
Tseveleki V, Rubio R, Vamvakas SS, White J, Taoufik E, Petit E, Quackenbush J, Probert L (2010) Comparative gene expression analysis in mouse models for multiple sclerosis, Alzheimer's disease and stroke for identifying commonly regulated and disease-specific gene changes. Genomics 96(2):82–91. doi:10.1016/j.ygeno.2010.04.004
Vandewauw I, Owsianik G, Voets T (2013) Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci 14:21. doi:10.1186/1471-2202-14-21
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417. doi:10.1146/annurev.biochem.75.103004.142819
Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279(1):19–25. doi:10.1074/jbc.M311201200
Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31(2):171–174. doi:10.1038/ng901
Wang HP, Pu XY, Wang XH (2007) Distribution profiles of transient receptor potential melastatin-related and vanilloid-related channels in prostatic tissue in rat. Asian J Androl 9(5):634–640. doi:10.1111/j.1745-7262.2007.00291.x
Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, Mori Y, Orser BA, Xiong ZG, Jackson MF, Tymianski M, MacDonald JF (2007) TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci U S A 104(41):16323–16328. doi:10.1073/pnas.0701149104
Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H (2009) Calcium flickers steer cell migration. Nature 457(7231):901–905. doi:10.1038/nature07577
Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J (2001) Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 7(5):1047–1057
Yang XR, Lin MJ, McIntosh LS, Sham JS (2006) Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290(6):L1267–1276. doi:10.1152/ajplung.00515.2005
Yee NS, Zhou W, Liang IC (2011) Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2 + -sensitive Socs3a signaling in development and cancer. Dis Model Mech 4(2):240–254. doi:10.1242/dmm.004564
Yogi A, Callera GE, Tostes R, Touyz RM (2009) Bradykinin regulates calpain and proinflammatory signaling through TRPM7-sensitive pathways in vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol 296(2):R201–207. doi:10.1152/ajpregu.90602.2008
Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM (2011) Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J 75(2):237–245
Yu H, Zhang Z, Lis A, Penner R, Fleig A (2013) TRPM7 is regulated by halides through its kinase domain. Cell Mol Life Sci. doi:10.1007/s00018-013-1284-6
Zhang J, Zhao F, Zhao Y, Wang J, Pei L, Sun N, Shi J (2011) Hypoxia induces an increase in intracellular magnesium via transient receptor potential melastatin 7 (TRPM7) channels in rat hippocampal neurons in vitro. J Biol Chem 286(23):20194–20207. doi:10.1074/jbc.M110.148494
Zhang YH, Sun HY, Chen KH, Du XL, Liu B, Cheng LC, Li X, Jin MW, Li GR (2012a) Evidence for functional expression of TRPM7 channels in human atrial myocytes. Basic Res Cardiol 107(5):282. doi:10.1007/s00395-012-0282-4
Zhang Z, Wang M, Fan XH, Chen JH, Guan YY, Tang YB (2012b) Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ Res 111(9):1137–1146. doi:10.1161/CIRCRESAHA.112.273755
Zierler S, Yao G, Zhang Z, Kuo WC, Porzgen P, Penner R, Horgen FD, Fleig A (2011) Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem 286(45):39328–39335. doi:10.1074/jbc.M111.264341
Acknowledgments
This work was supported by NIH grant P01 GM078195 to A. F.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Fleig, A., Chubanov, V. (2014). TRPM7. In: Nilius, B., Flockerzi, V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, vol 222. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54215-2_21
Download citation
DOI: https://doi.org/10.1007/978-3-642-54215-2_21
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54214-5
Online ISBN: 978-3-642-54215-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)