Abstract
TRPM5 is a Ca2+-activated cation channel that mediates signaling in taste and other chemosensory cells. Within taste cells, TRPM5 is the final element in a signaling cascade that starts with the activation of G protein-coupled receptors by bitter, sweet, or umami taste molecules and that requires the enzyme PLCβ2. PLCβ2 breaks down PIP2 into DAG and IP3, and the ensuing release of Ca2+ from intracellular stores activates TRPM5. Since its initial discovery in the taste system, TRPM5 has been found to be distributed in sparse chemosensory cells located throughout the digestive track, in the respiratory system, and in the olfactory system. It is also found in pancreatic islets, where it contributes to insulin secretion. This review highlights recent work on the mechanisms of the activation of the TRPM5 channel and its regulation by voltage, phosphoinositides, temperature, and pH. The distribution of the channel in the body and its functional contribution to various sensory and nonsensory processes are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Gene and Protein Structure of TRPM5
TRPM5 was first identified in an effort to find genes associated with the tumor producing condition known as Beckwith–Wiedemann syndrome (BWS), and although failing to link TRPM5 with BWS, these initial studies defined the basic structure of the gene (Prawitt et al. 2000; Enklaar et al. 2000). The human TRPM5 gene comprises 24 exons on chromosome 11, and it contains an open reading frame of 3,495 bp which predicts a protein of 1,165 amino acids (Prawitt et al. 2000). The orthologous mouse gene is located on the syntenic distal end of chromosome 7, and it contains an open reading frame that predicts a protein of 1,158 amino acids (Enklaar et al. 2000). TRPM5 shows highest homology to TRPM4 (40 % identity at the amino acid level), and it is more distantly related to other TRPM channels, such as the cold and menthol receptor TRPM8 (McKemy et al. 2002; Peier et al. 2002). Like other TRP channels, TRPM5 is thought to contain six transmembrane domains and to assemble as a tetramer (Montell et al. 2002; Clapham 2003). There is no evidence that it can co-assemble with any other TRP channel subunits, and no interacting proteins have been reported.
2 Expression Pattern
TRPM5 was first reported to be expressed in mammalian taste buds, in a subset of cells that co-express receptors for bitter, sweet, and umami tastes (Perez et al. 2002; Zhang et al. 2003). Subsequent studies showed a more wide-spread distribution of the channel (Kaske et al. 2007). Using a mouse in which the TRPM5 promoter drives expression of GFP (Clapp et al. 2006) (Fig. 1a), or direct antibody labeling, TRPM5 expression has been detected in two distinct subsets of olfactory neurons, one ciliated (Lin et al. 2007) and another microvillus (Lin et al. 2008a; Hansen and Finger 2008), in the vomeronasal organ, in the gastrointestinal tract, and in the respiratory system (Kaske et al. 2007). In most of these tissues, TRPM5 is expressed in solitary cells. Within the gastrointestinal system, TRPM5 is expressed in the stoma, small intestine, and colon in sparely distributed solitary cells. In both the respiratory system and the digestive system, the cells that express TRPM5 have been shown to be brush cells based on morphological criteria, including co-localization with villin, and CK18 (Kaske et al. 2007; Bezencon et al. 2008). TRPM5 is also expressed in pancreatic islets, where it is localized to insulin-secreting β-cells (Brixel et al. 2010; Colsoul et al. 2010). In addition, there are a few reports that TRPM5 may be expressed in the central nervous system (Dehkordi et al. 2012; Kim et al. 2012). In taste cells, the channel protein is present along the basolateral surface of the cell (Zhang et al. 2007; Kaske et al. 2007) where it may be inaccessible to apically delivered chemicals (Fig. 1b).
(a, b) Distribution of TRPM5 in taste buds of the mouse circumvallate papillae, showing (a) co-expression of TRPM5 (red) with GFP expressed under the TRPM5 promoter (green) and (b) co-localization of TRPM5 with PLCβ2 [from Zhang et al. (2007), with permission]. (c) A model for taste transduction. Binding of taste stimuli to G protein-coupled taste receptors (R) leads to dissociation of the heterotrimeric G protein. βγ subunits of the G protein activate PLCβ2, which in turn hydrolyzes PIP2 into DAG and IP3. IP3 activates IP3 receptors, which release Ca2+ from intracellular stores. Intracellular Ca2+ opens TRPM5 channels, leading to an influx of Na+ and depolarization of the cell. Note that TRPM5 is not permeable to Ca2+ and, therefore, there is no positive feedback [from Liu and Liman (2003), with permission]
3 Ion Channel Properties
3.1 Activation by Ca2+
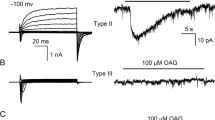
The expression pattern of TRPM5 in a subset of taste cells suggests that the channel is activated downstream of a PLC-mediated signaling cascade (Perez et al. 2002; Zhang et al. 2003). Consistent with this prediction, TRPM5 currents can be gated in heterologous cell types by stimulation of Gq-coupled receptors that activate PLC (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003; Zhang et al. 2003). PLC hydrolyzes PI(4,5)P2 into DAG and IP3, and IP3 causes release of Ca2+ from intracellular stores, and presumably one or more of these small molecules activate TRPM5. Although an initial report suggested that store depletion or elevation of IP3 directly gated TRPM5 (Perez et al. 2002; Zhang et al. 2003), subsequent studies by three laboratories independently showed that instead elevation of intracellular Ca2+ can directly gate TRPM5 channels (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003) (see Fig. 2a). That intracellular calcium is the physiological activator of TRPM5 channels is supported by several independent observations. First, activation of TRPM5 by Gq-coupled receptors is abolished when intracellular Ca2+ is strongly buffered (Liu and Liman 2003; Prawitt et al. 2003) or when IP3 receptors are inhibited with heparin (Hofmann et al. 2003). In addition, elevation of IP3 through UV-uncaging activates TRPM5 channels, and this activation is abolished if calcium is buffered (Zhang et al. 2007). Activation of native TRPM5 channels in taste cells has also been demonstrated, and the native channel display many of the same features observed for heterologously expressed channels (Zhang et al. 2007). Overall, these data support a model for the activation of TRPM5 shown in Fig. 1c. In this model, taste receptors (or other G protein-coupled receptors) signal through PLCβ2 to release Ca2+ from intracellular stores, which rapidly activates TRPM5 channels (Liu and Liman 2003). This is consistent with physiological data from taste cells and with results from targeted deletion of taste transduction molecules (Akabas et al. 1988; Hwang et al. 1990; Bernhardt et al. 1996; Wong et al. 1996; Ogura et al. 1997, 2002; Huang et al. 1999; Zhang et al. 2003).
TRPM5 is activated by intracellular calcium. (a) Activation by 40 μM Ca2+ of an inward current in a patch excised from a TRPM5-transfected CHO-K1 cell (V m = −80 mV). Neither 10 μM IP3 nor 100 μM OAG elicited a current in the same patch. (b) PIP2 partially restores TRPM5 channel activity following desensitization. Responses to 40 μM Ca2+ in the presence and absence of 20 μM PIP2 before and after desensitization. Desensitization was induced by a 30 s exposure to 40 μM Ca2+ (V m = −80 mV). (c) Activation of TRPM5 currents by 50 μM Ca2+ in patches excised from GFP+ taste cells isolated from TRPM5-GFP mice. WT and heterozygote mice show a large Ca2+-activated current that is absent in TRPM5−/− mice [from Liu and Liman (2003) and Zhang et al (2007), with permission]
Ca2+ signals can be generated from a number of different sources, and they can vary in magnitude and temporal properties (Hille 2001). For example, release of Ca2+ through ryanodine receptors generates a rapid elevation of local Ca2+ (“spark”) that can reach levels as high as 20–30 μM, a concentration that is able to activate closely opposed plasma membrane Ca2+-activated K+ channels (Wellman and Nelson 2003). On the other hand, global changes in Ca2+ concentration rarely exceed 1 μmol and can last for many seconds (Hille 2001). In understanding how TRPM5 channels are gated under physiological conditions, two questions must be answered: (1) Are the channels localized in close proximity to a Ca2+ source? (2) How sensitive to Ca2+ is the gating of the channels? As an answer to the first question, we know that TRPM5 channels are distributed across the entire plasma membrane of taste cells (Perez et al. 2002; Zhang et al. 2007; Kaske et al. 2007) (Fig. 1a). This is in striking contrast to the distribution of the pheromone-transduction channel TRPC2 which is localized to sensory microvilli of vomeronasal sensory neurons (Liman et al. 1999). The IP3 receptor and PLCβ2 show a similarly diffuse expression pattern in taste cells (Clapp et al. 2001), and therefore it is conceivable that the three molecules are localized in a signaling complex, like that which organizes signaling components of fly phototransduction (Montell et al. 2002).
Determination of the Ca2+ sensitivity of TRPM5, and of the related channel TRPM4, has been more difficult than might be expected, and there is a great deal of variation in the values for half activation of the channels by Ca2+ reported by different groups. This may be in part due to the fact that the Ca2+ sensitivity of these channels is subject to modulatory influences that are not completely understood. Perhaps the most robust and reproducible way to measure intracellular Ca2+ sensitivity is in inside-out patches (Fig. 2a). In this mode, immediately after patch excision, TRPM5 channels are activated by intracellular Ca2+ with an EC50 of 20–30 μM (Liu and Liman 2003; Ullrich et al. 2005). A similar value (8 μM) was measured for the activation of native TRPM5 channels in taste cells (Zhang et al. 2007). Ca2+ sensitivity decreases over time, possibly as a result of loss of PI(4,5)P2 from the channels, to 80 μM (Liu and Liman 2003). Under the same conditions, the structurally related channel TRPM4 is five times less sensitive to activation by intracellular Ca2+ (Zhang et al. 2005; Ullrich et al. 2005). The low sensitivity of TRPM5 channels in this recording mode argues that to be activated by physiological stimuli, the channels are most likely localized in close proximity to a Ca2+ source.
Somewhat mysteriously, the sensitivity of TRPM5 channels to activation by Ca2+ in whole cell recording mode is several orders of magnitude higher than it is in excised inside-out patches. While dose–response data is more difficult to obtain in this mode due to rundown of the current (Fig. 2c) and the need to use population data, there is nonetheless general consensus that TRPM5 can be near-maximally activated by intracellular dialysis of 1 μmol Ca2+ (Ullrich et al. 2005; Prawitt et al. 2003) [but see also Hofmann et al. (2003)]. This might reflect the loss of a factor that enhances the sensitivity of the channels to Ca2+. In addition, it is possible that perfusion of the cells with relatively low concentrations of Ca2+ elicits Ca2+ release in the vicinity of the TRPM5 channels, which further augments their activation. The high sensitivity to Ca2+ of TRPM5 channels in whole cell recoding mode could be used to argue that the channels detect global changes in Ca2+ (Prawitt et al. 2003).
3.2 Ion Selectivity
TRPM5 channels show little discrimination among the monovalent cations Na+, K+, and Cs+ and do not conduct divalent cations (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003). It is probably not coincidence that the two Ca2+-activated TRP channels are also the only TRP channels that are impermeable to Ca2+. The structural basis for the differential Ca2+ permeability of TRP channels is not known. In a detailed set of experiments, residues in the putative pore of TRPM4 were changed to the corresponding residues in a Ca2+-permeable TRP channel, TRPV6, and this conferred moderate Ca2+ permeability to the chimeric channel (Nilius et al. 2005a). However the fact that these authors were not able to identify a mutant that could confer more substantial Ca2+ permeability suggests that multiple residues or regions of the channel contribute to this process. Nonetheless, these experiments have provided experimental evidence that the region between the fifth and sixth transmembrane domain of TRPM4, and by homology TRPM5, contains the pore of the channel (Owsianik et al. 2006).
3.3 Unitary Properties
Single TRPM5 channels expressed in heterologous cells show a conductance of ~16–25 pS (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003). A similar conductance (17–21 pS) was measured for native TRPM5 channels in mouse taste cells (Zhang et al. 2007). Channel openings are short lived and flickery (Liu and Liman 2003; Zhang et al. 2007), which has precluded a detailed analysis of gating properties. This is in contrast to the long-lived openings of TRPM4 channels (bursts can last several seconds) (Launay et al. 2002; Zhang et al. 2005) and may serve as a defining feature in categorizing native channels.
3.4 Voltage-Dependent Activation
Although the activation of TRPM5 requires elevated Ca2+ levels, gating of the channel is also strongly affected by voltage (Hofmann et al. 2003; Liu and Liman 2003; Talavera et al. 2005). This is apparent in the outward rectification of TRPM5 currents in response to a voltage ramp, despite a linear I–V for the single channel conductance, and in the time-dependent relaxation of the current following a voltage step (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003; Talavera et al. 2005). Voltage-dependent activation has also been reported for TRPM8 and TRPM4 and thus may be a common feature of TRPM channels (Hofmann et al. 2003; Nilius et al. 2003, 2005b; Rohacs et al. 2005). Why should channels whose primary role is to transduce sensory signals be voltage dependent? While the answer to this question is not known, it has been hypothesized that the weak voltage dependence of these channels allows their gating to be easily modulated (Nilius et al. 2005b), a hypothesis that is supported by work on cold regulation of TRPM8, TRPV1, and TRPM5 (Voets et al. 2004; Talavera et al. 2005), decavanadate modulation of TRPM4 (Nilius et al. 2004), and PI(4,5)P2 regulation of TRPM4 and TRPM8 (Rohacs et al. 2005; Zhang et al. 2005) (see Sect. 4.2). At present the structural mechanism of voltage sensing of any of the TRP channels is not known. The fourth transmembrane of these channels contains several charged residues which might act as the voltage sensor, by analogy to voltage activation of K+ channels (Nilius et al. 2005b; Jiang et al. 2003).
3.5 Lipid Regulation
TRPM5 currents rapidly desensitize after activation (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003), a process that may play a role in the sensory adaptation of taste cells. In whole cell recording mode, rundown is observed following dialysis of intracellular Ca2+ (Fig. 2c), and similar rundown is observed in perforated patch recording following activation by bath-applied Ca2+ ionophore, arguing that rundown is not due to washout of signaling components (Liu and Liman 2003). In excised inside-out patches, rundown of TRPM5 currents is accompanied by both a change in the Ca2+ sensitivity and in the maximal magnitude of the current (Liu and Liman 2003). The phosphoinositide PI(4,5)P2 has emerged as an important cofactor in the activation of ion channels, and hydrolysis of PI(4,5)P2 has been proposed to underlie rundown of many types of PI(4,5)P2-sensitive ion channels (Suh and Hille 2005). PI(4,5)P2 is likewise a cofactor for the activation of TRPM5 (Liu and Liman 2003). Exogenous PI(4,5)P2 enhances both the Ca2+ sensitivity and magnitude of TRPM5 currents following rundown, but is ineffective prior to rundown suggesting that loss of this signaling molecule underlies desensitization (Liu and Liman 2003). Another lipid that appears to regulate TRPM5 is arachidonic acid (Oike et al. 2006). Arachidonic acid may be generated in taste cells from DAG by the actions of PLA2-IIA which is expressed in these cells, although there is presently no evidence that this pathway contributes to or is required for taste sensation.
3.6 Temperature Modulation of TRPM5
TRPM5 is structurally related to the cold-activated channel TRPM8 and more distantly related to heat-activated TRPV channels, suggesting the possibility that its activity is also thermal sensitive. Indeed warm temperatures promote activation of TRPM5, similar to the effects of heat on the TRPV channels (Talavera et al. 2005). An elegant theoretical framework has been developed to explain heat and cold activation of TRP channels which postulates that their extreme thermal sensitivity derives from their small voltage dependence (Nilius et al. 2005b). Heat acts by shifting the midpoint for voltage-dependent activation to negative voltages for TRPV1 and TRPM5 and positive voltages for TRPM8, leading to opposing thermal sensitivities of the channels (Voets et al. 2004; Talavera et al. 2005). However, unlike TRPV1, heat is not sufficient to activate TRPM5, which even at warm temperatures requires elevated Ca2+ (Talavera et al. 2005). The thermal sensitivity of TRPM5 suggests that sensation of bitter, sweet, and umami tastes might be reduced at cold temperatures. Electrophysiological recordings from mice indeed show that sweet taste is highly sensitive to temperature, although bitter and umami are unaffected (Talavera et al. 2005). Thus while these data support a role for TRPM5 in the thermal sensitivity of sweet taste, there are likely other factors that contribute to the thermal sensitivity of this process.
3.7 Pharmacology
Blockers of TRPM5 have the potential to alter taste sensation, and therefore the identification of these molecules is of great interest. One molecule that blocks TRPM5 is the bitter chemical, quinine, which is known to be a general inhibitor of ion channels. Quinine blocks TRPM5 with an EC50 of 50 μmol. The block of TRPM5 by quinine predicts that co-delivery of quinine with an agonist of the sweet receptors, which also act upstream of TRPM5, will blunt the sweet response. This is indeed what is observed, leading to the conclusion that TRPM5 is a locus for interaction between sweet and bitter tastes (Talavera et al. 2008). In a directed high-throughput screen for specific blockers of TRPM5, the chemical triphenylphosphine oxide, TPPO, was identified (Palmer et al. 2010). TPPO blocks human TRPM5 with an IC50 of 12 μmol and murine TRPM5 with an IC50 of ~30 μmol. A related compound, triphenylphosphine, was without effect, indicating that the oxygen is absolutely required for the block. TPPO appears to be specific for TRPM5, and no effect of TPPO on the activity of TRPA1, TRPV1, or TRPM4 was observed (Palmer et al. 2010).
TRPM5 is sensitive to extracellular pH level below 7.0 and is completely blocked by pH 5.9 (Liu et al. 2005). By comparison, TRPM4 is insensitive to pH levels as low as 5.4. Two residues account for most of the pH sensitivity of TRPM5—a glutamate residue in the S3–S4 linker and a His residue in the pore region (S5–S6 linker) (Liu et al. 2005). It is tempting to speculate that acid block of TRPM5 may play a functional role in taste sensation, possibly decreasing responses of taste cells to activation by bitter, sweet, or umami when consumed at acid pH.
4 Physiological Function
4.1 Taste Cells
A major advance in understanding the physiological significance of TRPM5 came with the discovery that its expression is high in the subset of taste receptor cells that mediate bitter, sweet, and umami taste (Perez et al. 2002; Zhang et al. 2003). There are five modalities of taste, of which three, bitter, sweet, and umami, are mediated by G protein-coupled receptors that bind their respective tastant (Lindemann 2001; Margolskee 2002). These receptors activate the G protein gustducin and phopholipase C (PLC) β2 (Lindemann 2001; Margolskee 2002), thereby initiating an intracellular signaling cascade that leads to membrane depolarization and release of the neurotransmitter ATP. TRPM5 is co-expressed with receptors for all three taste qualities and with gustducin and PLCβ2 indicating that it is part of the signaling pathway (Perez et al. 2002; Zhang et al. 2003) (Fig. 1a). This is supported by patch clamp recording from taste cells, which shows that a TRPM5-dependent current is activated by the elevation of intracellular IP3 or Ca2+ by UV-uncaging (Zhang et al. 2007). Moreover, membrane depolarization and ATP release by taste cells in response to tastants are dependent on a functional TRPM5 gene (Huang and Roper 2010). TRPM5 is required for normal taste, as TRPM5−/− mice are dramatically less sensitive to bitter, sweet, and umami, although they retain their ability to detect sour and salty (Zhang et al. 2003). More recently it has been shown that TRPM5 may also be an element in the transduction of fat and high-salt tastes (Liu et al. 2011; Oka et al. 2013; Ren et al. 2013).
4.2 Gut and Other Chemosensory Tissues
TRPM5 is now recognized to be expressed in the small intestine and stomach, where it may play a role in post-ingestive chemosensation (Perez et al. 2002; Kokrashvili et al. 2009). Interestingly, TRPM5 is expressed in a type of enteroendocrine cell in the duodenum that expresses the endogenous opioids, beta-endorphin and Met-enkephalin. Beta-endorphin appears to be released in response to hypertonic stimuli, and release is dependent on an intact TRPM5 gene. The mechanism by which TRPM5 is activated in this context and how signaling by these cells acts to regulate digestive function remains to be determined. TRPM5 has also been found to play a role, downstream of GPCRs, in linoleic acid-induced release of cholecystokinin by enteroendocrine cells (Shah et al. 2011).
Within the respiratory and olfactory epithelium, TRPM5 is expressed in at least two morphologically and functionally distinct populations of cells. In solitary chemoreceptors, it may function downstream of bitter receptors to detect inhaled irritants (Lin et al. 2008a, b). Goblet cells of the respiratory tract secrete Mucin 5AC, and secretion is dependent on a functional TRPM5 channel (Mitrovic et al. 2013). The subset of olfactory neurons that express TRPM5 appears to be specialized to detect pheromones and other semiochemicals (Lin et al. 2007).
4.3 Pancreatic β-Cells
TRPM5 is also expressed in pancreatic β-cells, where it plays a role in insulin secretion (Prawitt et al. 2003; Brixel et al. 2010; Colsoul et al. 2010). Insulin secretion is initiated when rising levels of glucose in the bloodstream cause its uptake into beta cells, producing a change in the ATP/ADP ratio. Elevated levels of ATP cause a block of a specific class of potassium channel (KATP) composed of KIR/SUR subunits, which depolarizes the membrane potential (Ashcroft et al. 1984; Cook and Hales 1984). Voltage-gated calcium channels open upon depolarization, leading to an elevation of intracellular Ca2+. From there, Ca2+ levels and the membrane potential oscillate, which drives pulsatile secretion of insulin (Gilon et al. 1993). In addition to the known conductances, it has been hypothesized that there must be Na+-permeable “background” current in β-cells to drive membrane depolarization upon closure of KATP channels (Ashcroft and Rorsman 1989). Ca2+-gated Na+-permeable currents have been described in pancreatic beta cells and in related cell lines where they are referred to as Ca-NS (Sturgess et al. 1987). Candidates to mediate Ca-NS and to regulate insulin secretion include TRPM4, TRPM2, and TRPM5. However, animals that carry a targeted deletion of TRPM4 do not have any defects in glucose tolerance or insulin secretion (Vennekens et al. 2007). In contrast, two groups have reported that TRPM5 channels are essential for normal insulin release. In response to glucose challenge by i.p. injection (IPGTT) or by oral administration (OGTT), TRPM5−/− animals show both a decrease in insulin secretion and a reduced glucose clearance (tolerance) as compared with WT mice (Brixel et al. 2010; Colsoul et al. 2010). Corroborating this observation, isolated islets from TRPM5−/− animals show a reduced level of insulin release to glucose challenge. The involvement of TRPM5 in insulin secretion predicts that genetic variation in the gene could contribute to the propensity to develop diabetes, a possibility that has received some support (Ketterer et al. 2011).Thus, it can be concluded that TRPM5 is part of the network of finely tuned conductances that contribute to insulin secretion and that it is a potential drug target for the control of diabetes (Colsoul et al. 2011; Liman 2010).
5 A Bittersweet Conclusion
The identification of a robust mechanism for the activation of TRPM5 in heterologous cells has facilitated the discovery of basic features of the channel and novel regulatory mechanisms that are likely to be of physiological significance. PI(4,5)P2 hydrolysis has been proposed to play an important role in desensitization of TRPM5 and may mediate sensory adaptation of taste. Thermal sensitivity of TRPM5 has been shown to contribute to the temperature dependence of sweet sensation, and acid inhibition of TRPM5 may also modulate sensory responses to taste. Finally, in the future we can look forward to structural information that will allow the design of rational chemicals to block or enhance TRPM5 function and thereby remove some of the bitterness or enhance some of the sweetness of pharmaceuticals and foods we consume.
References
Akabas MH, Dodd J, Al-Awqati Q (1988) A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science 242(4881):1047–1050
Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54(2):87–143
Ashcroft FM, Harrison DE, Ashcroft SJ (1984) Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312(5993):446–448
Bernhardt SJ, Naim M, Zehavi U, Lindemann B (1996) Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol 490(Pt 2):325–336
Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, Le Coutre J, Damak S (2008) Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 509(5):514–525. doi:10.1002/cne.21768
Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D (2010) TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch 460(1):69–76
Clapham DE (2003) TRP channels as cellular sensors. Nature 426(6966):517–524
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC (2001) Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2:6
Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC (2006) Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol 4:7
Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R (2010) Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc Natl Acad Sci USA 107(11):5208–5213
Colsoul B, Vennekens R, Nilius B (2011) Transient receptor potential cation channels in pancreatic beta cells. Rev Physiol Biochem Pharmacol 161:87–110. doi:10.1007.112.2011.2
Cook DL, Hales CN (1984) Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311(5983):271–273
Dehkordi O, Rose JE, Fatemi M, Allard JS, Balan KV, Young JK, Fatima S, Millis RM, Jayam-Trouth A (2012) Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res 1475:1–10
Enklaar T, Esswein M, Oswald M, Hilbert K, Winterpacht A, Higgins M, Zabel B, Prawitt D (2000) Mtr1, a novel biallelically expressed gene in the center of the mouse distal chromosome 7 imprinting cluster, is a member of the Trp gene family. Genomics 67(2):179–187
Gilon P, Shepherd RM, Henquin JC (1993) Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem 268(30):22265–22268
Hansen A, Finger TE (2008) Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci 9:115, 1471-2202-9-115 [pii]
Hille B (2001) Ionic channels of excitable membranes. Sinauer Associates Inc., Sunderland, MA
Hofmann T, Chubanov V, Gudermann T, Montell C (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 13(13):1153–1158
Huang YA, Roper SD (2010) Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol 588(Pt 13):2343–2350. doi:10.1113/jphysiol.2010.191106, jphysiol.2010.191106 [pii]
Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2(12):1055–1062
Hwang PM, Verma A, Bredt DS, Snyder SH (1990) Localization of phosphatidylinositol signaling components in rat taste cells: role in bitter taste transduction. Proc Natl Acad Sci USA 87(19):7395–7399
Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R (2003) The principle of gating charge movement in a voltage-dependent K + channel. Nature 423(6935):42–48
Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V (2007) TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8:49, 1471-2202-8-49 [pii]
Ketterer C, Mussig K, Heni M, Dudziak K, Randrianarisoa E, Wagner R, Machicao F, Stefan N, Holst JJ, Fritsche A, Haring HU, Staiger H (2011) Genetic variation within the TRPM5 locus associates with prediabetic phenotypes in subjects at increased risk for type 2 diabetes. Metabolism 60(9):1325–1333. doi:10.1016/j.metabol.2011.02.002, S0026-0495(11)00033-3 [pii]
Kim YS, Kang E, Makino Y, Park S, Shin JH, Song H, Launay P, Linden DJ (2012) Characterizing the conductance underlying depolarization-induced slow current in cerebellar Purkinje cells. J Neurophysiol 109(4):1174–1181, jn.01168.2011 [pii]
Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B (2009) Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 137(2):598–606, 606.e591–592
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109(3):397–407
Liman ER (2010) A TRP channel contributes to insulin secretion by pancreatic beta-cells. Islets 2(5):331–333, 12973 [pii]
Liman ER, Corey DP, Dulac C (1999) TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA 96(10):5791–5796
Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D (2007) Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA 104(7):2471–2476
Lin W, Ezekwe EA Jr, Zhao Z, Liman ER, Restrepo D (2008a) TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci 9:114
Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D (2008b) TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol 99(3):1451–1460
Lindemann B (2001) Receptors and transduction in taste. Nature 413(6852):219–225
Liu D, Liman ER (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100(25):15160–15165
Liu D, Zhang Z, Liman ER (2005) Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3-S4 and S5-S6 extracellular domains. J Biol Chem 280(21):20691–20699
Liu P, Shah BP, Croasdell S, Gilbertson TA (2011) Transient receptor potential channel type M5 is essential for fat taste. J Neurosci 31(23):8634–8642. doi:10.1523/JNEUROSCI.6273-10.2011, 31/23/8634 [pii]
Margolskee RF (2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277(1):1–4
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416(6876):52–58
Mitrovic S, Nogueira C, Cantero-Recasens G, Kiefer K, Fernandez-Fernandez JM, Popoff JF, Casano L, Bard FA, Gomez R, Valverde MA, Malhotra V (2013) TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. Elife 2:e00658. doi:10.7554/eLife.0065800658 [pii]
Montell C, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108(5):595–598
Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V (2003) Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278(33):30813–30820
Nilius B, Prenen J, Janssens A, Voets T, Droogmans G (2004) Decavanadate modulates gating of TRPM4 cation channels. J Physiol 560(Pt 3):753–765
Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T (2005a) The selectivity filter of the cation channel TRPM4. J Biol Chem 280(24):22899–22906
Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T (2005b) Gating of TRP channels: a voltage connection? J Physiol 567(Pt 1):35–44. doi:10.1113/jphysiol.2005.088377
Ogura T, Mackay-Sim A, Kinnamon SC (1997) Bitter taste transduction of denatonium in the mudpuppy Necturus maculosus. J Neurosci 17(10):3580–3587
Ogura T, Margolskee RF, Kinnamon SC (2002) Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol 87(6):3152–3155
Oike H, Wakamori M, Mori Y, Nakanishi H, Taguchi R, Misaka T, Matsumoto I, Abe K (2006) Arachidonic acid can function as a signaling modulator by activating the TRPM5 cation channel in taste receptor cells. Biochim Biophys Acta 1761(9):1078–1084
Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS (2013) High salt recruits aversive taste pathways. Nature 494(7438):472–475. doi:10.1038/nature11905, nature11905 [pii]
Owsianik G, Talavera K, Voets T, Nilius B (2006) Permeation and selectivity of TRP channels. Annu Rev Physiol 68:685–717. doi:10.1146/annurev.physiol.68.040204.101406
Palmer RK, Atwal K, Bakaj I, Carlucci-Derbyshire S, Buber MT, Cerne R, Cortes RY, Devantier HR, Jorgensen V, Pawlyk A, Lee SP, Sprous DG, Zhang Z, Bryant R (2010) Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev Technol 8(6):703–713. doi:10.1089/adt.2010.0334
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002) A TRP channel that senses cold stimuli and menthol. Cell 108(5):705–715
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5(11):1169–1176. doi:10.1038/nn952, nn952 [pii]
Prawitt D, Enklaar T, Klemm G, Gartner B, Spangenberg C, Winterpacht A, Higgins M, Pelletier J, Zabel B (2000) Identification and characterization of MTR1, a novel gene with homology to melastatin (MLSN1) and the trp gene family located in the BWS-WT2 critical region on chromosome 11p15.5 and showing allele-specific expression. Hum Mol Genet 9(2):203–216
Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA 100(25):15166–15171
Ren Z, Rhyu MR, Phan TH, Mummalaneni S, Murthy KS, Grider JR, Desimone JA, Lyall V (2013) TRPM5-dependent amiloride- and benzamil-insensitive NaCl chorda tympani taste nerve response. Am J Physiol Gastrointest Liver Physiol 305(1):G106–G117. doi:10.1152/ajpgi.00053.2013, ajpgi.00053.2013 [pii]
Rohacs T, Lopes CM, Michailidis I, Logothetis DE (2005) PI(4,5)P(2) regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8(5):626–634
Shah BP, Liu P, Yu T, Hansen DR, Gilbertson TA (2011) TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am J Physiol Cell Physiol 302(1):C210–C219. doi:10.1152/ajpcell.00209.2011, ajpcell.00209.2011 [pii]
Sturgess NC, Hales CN, Ashford ML (1987) Calcium and ATP regulate the activity of a non-selective cation channel in a rat insulinoma cell line. Pflugers Arch 409(6):607–615
Suh BC, Hille B (2005) Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol 15(3):370–378
Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438(7070):1022–1025
Talavera K, Yasumatsu K, Yoshida R, Margolskee RF, Voets T, Ninomiya Y, Nilius B (2008) The taste transduction channel TRPM5 is a locus for bitter-sweet taste interactions. FASEB J 22(5):1343–1355
Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B (2005) Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37(3):267–278
Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M (2007) Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8(3):312–320
Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430(7001):748–754
Wellman GC, Nelson MT (2003) Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34(3):211–229
Wong GT, Gannon KS, Margolskee RF (1996) Transduction of bitter and sweet taste by gustducin. Nature 381(6585):796–800
Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112(3):293–301, S0092867403000710 [pii]
Zhang Z, Okawa H, Wang Y, Liman ER (2005) Phosphatidylinositol 4,5-Bisphosphate Rescues TRPM4 Channels from Desensitization. J Biol Chem 280(47):39185–39192
Zhang Z, Zhao Z, Margolskee R, Liman E (2007) The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 27(21):5777–5786
Acknowledgments
Support was provided by the National Institutes of Health, grants R01 DC004564-12 and R21DC012747-01.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Liman, E.R. (2014). TRPM5. In: Nilius, B., Flockerzi, V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, vol 222. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54215-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-642-54215-2_19
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54214-5
Online ISBN: 978-3-642-54215-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)