Abstract
Ashwagandha is an important traditional medical plant and has been used for more than 3,000 years in Ayurveda and traditional medicine. Ashwagandha is used extensively for pharmacological and medical purposes. Therefore, the plant has attracted scientific attention worldwide. The medicinal properties of Ashwagandha are attributed to specific secondary metabolites such as alkaloids and withsteroids–withanolides. Withanolides are C28 steroidal structures built on an ergostane framework with oxidation at C22 and C26 to form a lactone ring. Withanolides are biosynthesised through the triterpenoid source pathway, and during recent years, tremendous progress in understanding withanolide biosynthesis and genomics has been achieved. This chapter provides a glimpse on major secondary metabolites from Ashwagandha, their distribution, occurrence, biosynthesis, genomics, and biotechnology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Ayurveda is an Indian system of medicine that has evolved for over more than 3,000 years by advancing knowledge on properties of different plants to cure various diseases. A large number of drug development programmes around the world are based on plants described for their pharmacological properties in traditional system of medicine such as Ayurveda, Siddha, Unani, and traditional Chinese medicine. These traditional medical systems serve as key resources of knowledge to derive phytomolecule-based modern pharmacological data and lead structures for drug development. The Solanaceae not only harbour important food plants but also are rich in several members used as medicinal plants, as described in ancient Ayurvedic literature and other traditional pharmaceutical traditions. In addition to the written record, the importance of this family is also supported by local healing practices that rely on oral tradition. The genus Withania is a member of this family and comprises of about 20 species. Among them, W. somnifera and Withania coagulans are those most important for medical application (Tuli and Sangwan 2010). Recently, the potential of a third species, W. ashwagandha, has been identified from Indian germplasm collections using multidisciplinary approaches (Kumar et al. 2011). Particularly, different parts of W. somnifera (Ashwagandha or winter cherry) constitute essential ingredients of hundreds of Ayurvedic formulations. The pharmacological activities of W. somnifera include promotion of physiological and metabolic recovery, antiarthritic and anti-ageing effects, and improvement of cognitive functions in a geriatric context as well as the recovery from neurodegenerative disorders (Lal et al. 2006; Misra et al. 2005; Sangwan et al. 2004a). Since the nature of these pharmacological effects closely resembles those of ginseng (Panax species), Withania is often designated as ‘Indian ginseng’. In comparison to W. somnifera, W. coagulans has been far less explored for its phytochemical activity, despite its traditional use as earliest vegetable rennet suitable to coagulate milk. Recent pharmacological investigations demonstrate variable biological activities that differ from those of W. somnifera. These include antimicrobial, anti-inflammatory, antitumor, hepatoprotective, antihyperglycemic, cardiovascular, immunosuppressive, free radical scavenging, and antidepressant activities (Maurya and Akanksha 2010).
Phytochemically, several tropane alkaloids were the molecular candidates identified in Ashwagandha reported to mediate some biological activity. However, subsequent phytochemical analyses discovered a new group of abundant and highly diversified compounds subsequently termed withanolides (after the genus, where they had been discovered). Pharmacological investigations with extracts enriched in some of these withanolides matched significantly with the properties described for the plant or its part as described in Ayurveda. Thus, the curative properties of this plant could be attributed to the large and structurally diversified withanolides. Several studies could even link the therapeutic activity to individual specific withanolide moieties isolated from the herb (Kinghorn et al. 2004; Bargagna et al. 2006; Ichikawa et al. 2006; Kaileh et al. 2007). Although tropane alkaloids specific for the Solanaceae like tropine or pseudotropine had been reported also for W. somnifera long before the isolation of withanolides (Khanna et al. 1961), a link of these alkaloids with the therapeutic efficacy of Ashwagandha had not been followed or reported ever since. Nevertheless, the herb is still traded under the claim of its ‘alkaloid content’.
Withanolides constitute a novel group of compounds descending from the triterpenoids. They are diversely functionalised molecules based on an ergostane skeleton. Biosynthetically, these specialised metabolites may diverge from the ubiquitous sterol pathway at the level of 24-methylene cholesterol (Sangwan et al. 2008). Withanolides are far from widespread in the plant kingdom but are synthesised abundantly only in a few genera of the Solanaceae family, with W. somnifera yielding the most prominent amounts and diversified forms. Leaves and roots of the plant are most preferred for their therapeutic properties in traditional systems of medicine, and these are the plant parts sequestering the most significant amount of these compounds. The highly prolific sets of pharmacological activities have been linked with specific withanolides from W. somnifera and ranging from anti-inflammatory, antitumour, and antioxidative activities. Moreover, these withanolides inhibit cyclooxygenases and lipid peroxidation, modulate immunity, and restore neural functions by activating nitric oxide synthase (Rasool et al. 2000; Luvone et al. 2003).
Despite the growing body of evidence for pharmacological activities of the secondary metabolites (withanolides, withanamides, and tropane alkaloids) of Ashwagandha, information on the underlying biosynthetic pathways and the genes encoding for the enzymes of the pathways is still limited. Most of this information emerged from the New Millennium Indian Technology Leadership Initiative (NMITLI) programme on Ashwagandha funded by the Council of Scientific and Industrial Research (CSIR) in India since 2001. In addition to its own focussed research outcomes in terms of knowledge, products, and technology, the contributions and success of this cooperative research programme have tremendously catalysed the interests of the researchers worldwide for Ashwagandha.
Parallel to the understanding of the withanolide biosynthetic pathway (Sangwan et al. 2007, 2008), chemical synthesis of active withanolides A has also been pursued (Jana et al. 2011). However, given the complexities of the structures of these molecules, the chemical synthesis may remain of academic interest. At least in the near future, the plant will continue to be the sole economic source of these compounds. Therefore, programmes of plant improvement through conventional breeding and biotechnological manipulation based on knowledge on the pathway represent the most promising strategies.
2 Pharmacological Activities of Ashwagandha Extracts and Its Active Compounds
Ashwagandha has been regarded as an excellent source for anti-stress and rejuvenating activity comparable to ginseng (Panax ginseng), although the two herbs belong to different plant families (Solanaceae versus Araliaceae) that are also quite far apart phylogenetically. Traditionally, Ashwagandha has been used as a liver tonic, antiarthritic, and rejuvenating herb particularly in geriatric treatments (Jain 1991). As cellular mechanisms, suppression of free radical generation, anti-inflammatory activity; suppression of cancer cell proliferation accompanied by apoptosis through inhibition of NFκB; significant induction of axons, dendrites, presynapses, and postsynapses in the brains of mice; and amelioration of neuronal dysfunction in mice suffering from Alzheimer’s disease have been reported (Ghosal et al. 1989; Luvone et al. 2003). Various activities were found to be associated with mainly root extracts and individual pure withanolides, such as withaferin A, withanolide A, and withanolide D (Table 1; Bhattacharya et al. 2002; Oh et al. 2008; Yang et al. 2007; Bargagna et al. 2006; Malik et al. 2007; Stan et al. 2008; Schliebs et al. 1997; Mandal et al. 2008; Kushwaha et al. 2012). In addition, immunostimulatory properties have been attributed to extracts from the root of W. somnifera (Kushwaha et al. 2012). This study has revealed that selected Indian chemotypes of Ashwagandha (NMITLI-101, NMITLI-118, NMITLI-128), as well as pure withanolide–withaferin A, possessed immunomodulatory activity (Kushwaha et al. 2012). Oral administration of aqueous ethanolic extract of chemotype 101R (10 mg/kg) as well as withaferin A (0.3 mg/kg) 7 days before and after challenge with the human filarial parasite Brugia malayi offered protection in Mastomys coucha. This protection was correlated with impaired development of B. malayi larvae by pretreatment with withaferin A leading up to almost two thirds reduction in the incidence of adult worms. Moreover, among the female worms that had managed to develop, a large percentage (more than 60 %) also showed defective embryogenesis. Withanone, a close structural analogue of withaferin A and predominating abundance, has not attracted much medical interest until recently when withanone was proposed to be effective as health adjuvant. Priyandoko et al. (2011) have shown that withanone protects human cells from methoxyacetic acid (MAA)-induced toxicity (Table 1). Withanone protected normal human cells from MAA toxicity by suppressing ROS levels, DNA and mitochondrial damage, and induction of cell defence signalling pathway (Priyandoko et al. 2011). These findings warrant further basic and clinical studies that may promote the use of withanone as a health adjuvant in a variety of consumer products, where toxicity has been a concern because of the use of ester phthalates (Priyandoko et al. 2011).
Recently, withaferin A has been shown to cause the redistribution of vimentin intermediate filaments in fibroblasts from their normal arrays extending throughout the cytoplasm into perinuclear aggregates (Grin et al. 2012). Microtubules become wavier and sparser, and the number of stress fibres has been shown to increase. Very recently, withaferin A was shown to act as a strong inhibitor of the inflammatory response in islets protecting against cytokine-induced cell damage while improving survival of transplanted islets (Sorelle et al. 2013). These studies suggest that withaferin A could be incorporated as an adjunctive treatment to improve the performance of islets after transplantation (Sorelle et al. 2013). So far, withaferin A has been apparently the most studied molecule out of all withanolides and has been associated with various pharmacological activities till date (Table 1). Recently withaferin A has also been found to harbour antiviral properties, and a mechanism of action has been proposed (Grover et al. 2011) based on docking and molecular dynamics simulation studies. Withaferin A might bind with the DNA polymerase of the Herpes simplex virus. Binding of withaferin A is also shown for the aberrant tumour proteasome beta5 subunit leading to an inhibition of the tumour-related chymotrypsin-like activity of this aberrant proteasome suggested to be responsible for the antitumor effect of withaferin A (Yang et al. 2007). For a further important but relatively far less abundant withasteroid, withanolide D, a distinct set of pharmacological activities has been elucidated (Mondal et al. 2010, 2012). Withanolide D (C4β-C5β,C6β-epoxy-1-oxo-, 20b, dihydroxy-20S, 22 R-witha-2, 24-dienolide) has been shown to effectively induce apoptosis in leukaemia cell lines (MOLT-4 and K562), as well as in primary cells from patients irrespective of their lineages. This withanolide D-induced apoptosis correlated with an early accumulation of ceramide by the activation of neutral sphingomyelinase (Mondal et al. 2012). Further studies reported the withanolide D-induced cellular apoptosis in which mitochondria and p53 were intricately involved both in p53 wild-type and null cells (Mondal et al. 2012). Thus, these recent findings highlight new possibilities of recruiting withanolide D as alternative anticancer agent along with the existing chemotherapeutic agents potentially targeted towards mitochondria-mediated apoptosis (Mondal et al. 2012).
3 Diversity of Secondary Metabolites in Ashwagandha
The therapeutic potential of Ashwagandha is owing to the presence of wide variety of phytochemicals which are present in small amounts in all plant parts (Table 2). The earliest phytochemical investigations of Ashwagandha focussed on alkaloids. In fact, the therapeutic activities have been ascribed to alkaloids for a long time. Only later, withanolides were identified as the most prolific and predominant group of compounds isolated from Ashwagandha. More recently, additional new types of secondary metabolic compounds of minor abundance, such as withanamides and calystegines, have been shown to harbour pharmacological activity. This immense potential of compounds renders Ashwagandha a promising model for medicinal plants in general.
3.1 Alkaloids
Due to novel drugs derived from natural plant alkaloids (Cordell et al. 2001; Newman et al. 2003; Ortholand and Ganesan 2004) and in particular based on the presence of a tropane ring (Gross 2004), the interest for tropane alkaloids has enlarged in recent times. Tropane alkaloids are widely produced in the Solanaceae, and their potential for medical treatment has been recognised by modern medicine long back and is the base for the use of these plants in traditional systems of medicine. These drugs can be applied in different forms like pure molecules and tinctures. This group of alkaloids comprises N-methylpyrrolinium-derived nicotine alkaloids, tropine-derived true tropane alkaloids, and pseudotropine-derived nortropane alkaloids, also called calystegines (De Luca and St Pierre 2000; Drager et al. 1994; Drager 2004). The tropane alkaloids hyoscyamine (its racemic form being atropine) and scopolamine are used as anticholinergic agents acting on the parasympathetic nervous system used for the treatment of spasms as sedative agents and for dilation of the pupil (mydriasis) by ophthalmologists (Zayed and Wink 2004). The relatively newly discovered group of (pseudo) tropane alkaloids, the calystegines (Fig. 1), resemble monosaccharides in structure and have been shown to be strong glycosidase inhibitors (Asano et al. 2000), suggesting that the calystegines have potential as antidiabetic compounds. W. somnifera roots contain alkaloids in varying levels up to 0.3 %, and the leaves are reported to contain some unidentified alkaloids as well. Though alkaloids were the earliest secondary metabolites reported from Ashwagandha, no information on their biosynthetic pathway, genes, and enzymes has been available until recently.
One of the key steps of the pathway catalysed by tropinone reductases (TRs) has been analysed very recently (Kushwaha et al. 2013). Two discrete tropinone reductases (TRs) bifurcate the tropane alkaloid pathway at the intermediary stage of tropinone into tropine and pseudotropine streams similar to the situation known from some other genera of Solanaceae (Leete 1990). The catalytic reaction products of TR-1 and TR-2, respectively, lead to the generation of hyoscyamine/scopolamine and calystegines. TR-I (EC 1.1.1.206) catalyses the NADPH-dependent reduction of the 3-keto group of tropinone to the 3α-hydroxy group, whereas TR-II (EC 1.1.1.236) converts the same keto group to the 3β-hydroxy form. Sequence analysis of TRs indicates that these are the members of the short-chain dehydrogenase/reductase family. When both tropane and calystegines are produced by a given species, the relative expression levels of the two TRs in the tissue (Hashimoto et al. 1992) will determine the partitioning of metabolite flux towards each form as there seems to be no interconversion between tropine and ψ-tropine in vivo (Yamada et al. 1990). Genes of one or both TRs have been isolated from several Solanaceae species, such as Hyoscyamus niger, Datura stramonium, Solanum tuberosum, and Anisodus acutangulus, which are known to accumulate high levels of hyoscyamine, scopolamine, and calystegines (Nakajima et al. 1993; Richter et al. 2006; Kai et al. 2009). Overexpression of TR-I has been reported to considerably enhance the production of tropane alkaloids in root cultures of Atropa belladonna and A. acutangulus (Richter et al. 2005; Kai et al. 2011). Presence of tropinone reductase homologues in plant species that are not known to produce any of the tropane alkaloids (e.g. Arabidopsis thaliana, Cochlearia officinalis) suggests that these enzymes might play additional roles in other metabolic pathways (Keiner et al. 2002; Brock et al. 2006).
Until recently, biosynthesis of tropane alkaloids has been considered to occur exclusively in roots and is subsequently transported to the aerial organs. Accordingly, the expressions of the relevant genes of the metabolic pathway have been shown to be active in root tissue. However, in a recent report, a tropine-forming tropinone reductase (TR-I) cDNA was isolated from the leaf tissues of W. coagulans. The ORF was deduced to encode a polypeptide of 29.34 kDa. The recombinant His-tagged protein was functionally active implying parallel operation of the de novo biosynthetic pathway in aerial tissues. This not only represents the first report on a gene and enzyme of secondary metabolism for this commercially and medicinally important vegetable rennet species (Kushwaha et al. 2013a; 2013b) but also shows for the first time that there exists an independent tropane alkaloid synthesis in aerial tissues at least in Ashwagandha (Tables 2 and 3).
3.1.1 Multi-tissue Biosynthesis of Tropane Alkaloids
Among the secondary compounds of plants, alkaloids have been the group best studied with respect to their sites of synthesis, transport, and storage. The observations recorded in these studies suggest that there is no general site(s) of synthesis. For instance, nicotine and tropane/nortropane alkaloids of the Solanaceae plants are synthesised in the roots and transported to the aerial parts for storage, whereas the monoterpene-indole alkaloids of Rauwolfia and Catharanthus are synthesised in both the root and the leaf. The benzylisoquinoline alkaloids of Papaver are synthesised in the metaphloem (of both root and shoot) and stored in the laticifers and capsules, but the quinolizidine alkaloids in Lupinus are synthesised in the leaves and transported to roots (De Luca and St Pierre 2000; Drager 2004). In addition to tropane and its relative nicotine and calystegines for which the Solanaceae are renowned, a few genera of the family also accumulate a novel class of secondary metabolites called withanolides, the ergostane skeleton-based phytosteroids named after W. somnifera.
3.2 Withanamides and Other Secondary Metabolites
A new group of novel amido compounds has been isolated from berries of Ashwagandha (Jayaprakasam et al. 2004). The withanamides are amido-conjugated compounds of serotonin diglucoside and long-chain hydroxyl fatty acids such as withanamides A to E (Fig. 2; Table 2). Chemical characterisation using established chemical and spectral methods showed that withanamides consist of long-chain hydroxyl fatty acid moieties, glucose and serotonin. Withanamides have also been shown to be pharmacologically active as potential inhibitors of lipid peroxidation. Calystegines, as third novel group of compounds, are highly diversified in Ashwagandha. Calystegines are nonesterified polyhydroxylated alkaloids that had been discovered in roots of Calystegia sepium (Convolvulaceae) and then in A. belladonna (Solanaceae). So far, 25 calystegines that are structurally distinct have been reported for Ashwagandha (Drager et al. 1994; Drager 2004). In addition to these two novel and less abundant group of compounds, several commonly occurring flavonoids, phenolics, triterpenoids, and sterols (Table 2) have also been found in Ashwagandha (Nur-e-Alam et al. 2003; Mishra et al. 2005, 2008; Tuli and Sangwan 2010).
3.3 Withanolides from Ashwagandha
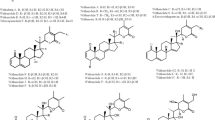
A growing body of evidence suggests that most of the therapeutic properties of Ashwagandha can be attributed to the withanolides. Withanolides are structurally similar to steroids that are ubiquitous in all organisms from microbes to man. Therefore, the withanolides are also known as withasteroids. In plants, major steroids, which are ubiquitously encountered, are sterols such as sitosterol, stigmasterol, or campestanol and brassinosteroids. Withanolides are structurally different from other steroids and not found outside the plant kingdom and are restricted mostly to the Solanaceae. The term withanolide originated from Withania, where these compounds had been discovered. Withanolides are most prolifically encountered in Ashwagandha among all Solanaceae and are characterised by a C28 basic steroidal skeleton with a nine-carbon side chain in which C22 and C26 are appropriately oxidised to form a six-membered δ-lactone ring (Fig. 3).
A 1-keto or hydroxy function in ring A is also a general feature of the withanolides. From the biogenetic point of view, the withanolides can be considered to harbour a cholestane-type structure with an extra methyl group at C-24 and various oxygenated groups or double bonds placed at different sites of the skeleton (Glotter 1991; Budhiraja et al. 2000). The withanolides were isolated during standard procedures of extraction, isolation, and structure elucidation of major as well as minor phytochemicals in the search for their potential pharmacological activities. In contrast to targeted isolation, such unbiased screens sometimes yield unexpected biologically active phytochemicals and provide a better chance to encounter novel results. Withanolides can be isolated from fresh as well as from dry material of Ashwagandha, which allows to understand metabolic intermediates accumulated under total arrest of metabolism (Sangwan et. al. 2004a, b). Conventional extraction of withanolides in pure methanol is suboptimal, but extraction of the fresh herb in 25 % methanol results efficiently extracts withanolides from the tissue (Sangwan et al. 2005; Chaurasiya et al. 2008; Lal et al. 2006). Almost all parts of Ashwagandha contain withanolides, but in specific composition. Although the withanolide profile is characteristic for each organ, some withanolide species occur in two or more plant parts. Leaves, stems, and berries (including seeds) are the most productive source of withanolides (Chaurasiya et al. 2008). Although the roots are the major object of prescriptions in Ayurvedic and folklore systems of medicines, only a few steroids and some alkaloids have been identified from root tissue (Davies and Kuttan 2002; Ray and Gupta 1994). However, recently several withanolide-type compounds have been characterised in roots (Misra et al. 2008, 2012). Among withanolide-type compounds, several had been known earlier, such as 2, 3-dihydro-3-hydroxywithaferin A, viscosalactone B, withaferin A, withanolide D, withanolide B, withanolide A, 27-hydroxy withanolide B, and 27-hydroxy withanolide A (Misra et al. 2008). The structures of these compounds were elucidated by spectroscopic methods including VIS, UV, IR, 1H NMR, 13C NMR, 2D NMR, and mass spectroscopy (Misra et al. 2008). Also, a peculiar withanolide dimer sulphide has been isolated from the roots of Ashwagandha and found to inhibit the growth of cancer cell lines (Subbaraju et al. 2006).
Phytochemical studies on the shoot of Ashwagandha yielded five new withanolides from the stem bark, namely, withasomnilide, withasomniferanolide, somniferanolide, somniferawithanolide, and somniwithanolide (Table 2).
Ashwagandha leaves contain many important withanolides, as two major compounds, withanone with a total yield of 0.222 % dry weight and withaferin A with a yield of 0.166 % dry weight, had been identified (Glotter et al. 1977; Rahman et al. 1993). Ashwagandha is peculiar, because it simultaneously produces both lead structures: the 5α-hydroxy-67α-epoxide and the 4β-hydroxy-56β-epoxide. Among the minor withanolides isolated and detected in leaves, 27-deoxy-17-hydroxywithaferin A is the most abundant along with withaferin A (0.825 % dry weight) accompanied by withanolide D with 0.047 % (Chaurasiya et al. 2007). Furthermore, other minor withanolides were 27-deoxywithaferin A, 2, 3-dihydrowithaferin A, withanolide P, withanolide F, dihydro-27-deoxywithaferin A, and dihydro-withanolide D (Table 2; Fig. 3). In addition to several known withanolides, a new dienone withanolide was reported for leaves (Rahman et al. 1993). The other new withanolide, 3α-methoxy-2,3-dihydro-27-deoxywithaferin A, was isolated from leaves of W. somnifera along with withaferin A, 27-deoxywithaferin A, 2,3-dihydrowithaferin A, and withaferin 3β-methoxy-2,3-dihydrowithaferin A (Anjuneyulu et al. 1997). The finding of withaferin A with immunosuppressive activity from shoot tips (Furmanowa et al. 2001) and the identification of very interesting withanolides of unusual chemical features from Ashwagandha leaves (Misra et al. 2005) show that the potential of leaf tissue has not yet been fully exploited. Also the Ashwagandha fruits have been shown to contain major withanolides in addition to substantial amount (10 %) of fatty acid oil rich in linoleic acid (60 % of the oil fraction). Specifically, the seeds also contain withanolides (Table 2). A specific withanolide, 7α, 17α-dihydroxy-1-oxo-5β, 6β-epoxy-22R-witha-2,24-dienolide, has been characterised through chemical identification and spectral data (Kundu et al. 1976). Two withanolides along with some minor amounts of coumarins and triterpenoids were isolated from Ashwagandha fruits (Ahmad and Douh 2002). A chloroform extract from fresh Ashwagandha berries yielded stigmasterol, its glucoside, withanone, 27-hydroxy withanolide A, along with two new withanolides, namely, iso-withanone and 6α,7α-epoxy-1α,3β,5α-trihydroxy-witha-24-enolide (Lal et al. 2006).
3.4 Chemotypic and Metabolic Diversity of Withanolides
W. somnifera occurs in several chemotypes different with respect to their withanolide composition. A withaferin A-rich chemotype has been identified from germplasm of Indian origin, though earlier reports had reported withaferin A only for two non-Indian chemotypes (one from South Africa, the other from Israel). However, Ashwagandha chemotypes from both wild and cultivated Indian populations with withaferin A in all plant parts have been identified (Kaul et al. 2009). The quantitative dynamics of withaferin A production in Indian populations, the inter-chemotypic hybrids, five previously reported chemotypes from Israel, South Africa, and India, and the inheritance of traits were analysed in a detailed chemogenetic study of this complex species. Also withanolide chemotypes have been studied extensively (Chaurasiya et al. 2009). These studies suggest two major types. In type 1, withaferin A and withanolide D with lactonisation at 5, 6 positions dominate (Fig. 3), whereas in type II, the major withanolides are withanone and withanolides with lactonisation at carbon positions 6, 7 mainly occurring in roots (Fig. 3). A wide collection of genotypes categorised as elite, wild, and cultivated accessions with respect to metabolic diversity and protein patterns were assessed by RAPD to link phytochemical with genetic diversity. This study revealed correlation of genetic clustering with chemotype with respect to the predominant presence or absence of specific withanolides. A clade rich in withaferin A as well as in withanone could be separated from a clade rich only in withaferin A, a clade rich only in withanone, and clusters rich in either withanolide D or withanolide A. This study demonstrated for the first time existence of the discrete chemotypes in the Indian population of the plant (Chaurasiya et al. 2009), and except one similar work (Dhar et al. 2006) represents the only systematic study on withanolidal diversity in relation to genetic diversity. Such information is the key to selection, genetic improvement, conservation, and management of useful accessions in gene banks as well as the development and preservation of novel chemotypes or chemotype hybrids and recombinant inbred lines for effective utilisation. Previous studies suffered from the limitation that mainly secondary sources (from botanical gardens) rather than primary samples from wild habitats had been used (Fig. 3). A countrywide collection of wild Ashwagandha accessions by a research group at the Central Institute of Medicinal and Aromatic Plants (Council of Scientific and Industrial Research) under the New Millennium Indian Technology Leadership Initiative (NMITLI) of the Indian Government has revealed that India possesses the widest chemotypic diversity of W. somnifera in the world. The huge phytochemical variability of commercially available herbal preparations with respect to even a single withanolide possibly reflects this chemotypic diversity of plants harvested from wild habitats by the drug manufacturers (Sangwan et al. 2004a).
The significance of wild collections cannot be overestimated, particularly in view of the high degree of self-pollination in this species. As a part of systematic studies on qualitative as well as quantitative phytochemical variability, several distinct lines of W. somnifera have been developed as an Indian core collection of diversity and characterised molecularly and phylogenetically (Sangwan et al. 2005, 2007; Sabir et al. 2007; Chaurasiya et al. 2009). The chemical diversity has been corroborated by high-throughput NMR metabolomics of the original chemotypes like NMITLI-101, NMITLI-108, and NMITLI-118 (Fig. 5a) developed at the Central Institute of Medicinal and Aromatic Plants (Council of Scientific and Industrial Research).
4 Tissue Specificity for the Biosynthesis of Secondary Metabolites
The secondary metabolites of W. somnifera are produced and stored in a tissue-specific manner. Even for a specific chemical subset, differential tissue distribution of different members is significant. The qualitative and quantitative profile of withanolides, as most prodigally produced metabolites in Ashwagandha, are substantially tissue specific (Chaurasiya et al. 2009). As medicinal properties of the herb are largely attributed to specific withanolide moieties, extracts prepared from different parts of the plant differ in the nature and/or efficacy of their pharmacological activities. Thus, traditional utilisation of specific tissues for specific therapeutic purposes appears to bear a scientific basis. In terms of comprehensive metabolomics, a study by Chatterjee et al. (2010) has revealed tissue-specific presence and concentration of primary and secondary metabolites in leaf versus root tissues of W. somnifera. A total of 62 major and minor primary and secondary metabolites from leaves and 48 from roots could be profiled unambiguously. These included 11 bioactive sterol–lactone molecules. The study also revealed substantial qualitative and quantitative differences between the leaf and root tissues, particularly with respect to the secondary metabolites (Chatterjee et al. 2010). Biosynthetic or metabolism-related research on withanolides has been launched only a few years ago, but there are already evidences of withanogenesis inherent to both leaf and root, contrary to the traditional view that withanolides are synthesised in the leaves and transported to roots (Sangwan et al. 2008).
As per traditional practice, both roots and leaves are prescribed for medicinal purposes in an ailment-specific manner (Kaileh et al. 2007; Sangwan et al. 2005). This practice matches with the overall withanolide richness of the two organs as well as discrete predominance of some specific withanolide moieties in each organ. Extensive phytochemical investigations of the two organs have also revealed a considerable qualitative overlap of several minor or pharmacologically non-active withanolides (Sangwan et al. 2004a, 2008). The biological function of withanolide tissue specificity is not known at this time. In fact, the functional aspects of withanolides for the plants are currently unknown beyond the general speculation that withanolides similar to other specialised metabolites might act in defence (Madina et al. 2007a, b; Sharma et al. 2007; Sangwan et al. 2008). Our preliminary observations that some W. somnifera accessions, which lack a specific major withanolide, show clearly altered flowering and fruiting behaviour and growth patterns (including a dwarf phenotype) indicate that withanolides might act also as growth regulators per se or may cross talk with brassinosteroids by competing for shared metabolic precursors. However, this hypothesis still warrants experimental verification. More insights into these questions are expected from our ongoing work to identify the enzymes and genes involved in the putative position-specific hydroxylation (by cytochrome P450) based on a metabolic model drawn from Ashwagandha root chemoinformatics, wherein withanolide B occupies an anaplerotic position for diverse metabolic transformations into withanolide A, withanolide R, withanone, and 27- hydroxy withanolide B.
Withanolide A is one of the most promising withanolides of W. somnifera in view of strong molecular evidences of its ability to induce nerve development and nerve growth promotion effect on synaptic reconstruction. Because of the root-specific production of withanolide A, root cultures particularly hairy roots that can grow rapidly in simple media and can be easily upscaled in a bioreactor form the first choice for such productions. However, despite several reports of Agrobacterium rhizogenes-transformed hairy roots of W. somnifera, the presence of withanolide A has not been detected. However, multiple shoot cultures of Ashwagandha raised under different combinations of benzyl adenine and kinetin influenced not only their morphogenetic response but also the level of withanolide A in the in vitro shoots. Interestingly, withanolide A, which is usually hardly detectable in the aerial parts of Ashwagandha, was detected in substantial amounts in multiple shoots (Sabir et al. 2008). The productivity of withanolide A in such cultures varied considerably (ca. tenfold, from 0.014 to 0.14 mg per gram fresh weight) with the change in the hormone composition as well as genotype of the explants. The enhanced de novo biogenesis of withanolide A in shoot cultures has been corroborated with radiolabeled incorporation-based biosynthetic studies using [2-(14) C] acetate as a precursor (Chaurasuya et al.2007). Production of withaferin A, the usual predominant withanolide of native shoots, has also been detected in the in vitro cultures (Sangwan et al. 2007). Although withanolides have been isolated from several tissues of the plant including roots, stem, leaf, and berries, whether these tissues are biosynthetically competent to synthesise withanolides wholly de novo or partially has remained a source of speculation. Further biogenetic analysis of withanolide A in native roots and in vitro-cultured normal roots has revealed that in planta biosynthesis of withanolide A by the roots takes place ab initio from isoprenogenic primary metabolites such as acetate and glucose.
5 Secondary Metabolites Withanolides
5.1 Biosynthesis of Withanolides
Very limited information has been available until recently on the biosynthetic aspects of withanolides. However, there is a very strong international surge in interests of researchers on biochemical, molecular, biological, and biotechnological aspects of withanolide biosynthetic pathways. A focussed and systematic series of investigations at the interface of chemistry, biochemistry, biology, and genetic and chemotypical resources in the frame of the Indian NMITLI programme has attracted global attention. Now, fundamental information about the genes and proteins involved in withanolide pathway has become available, in addition to the biochemical pathways and processes related to withanogenesis. Recent literature exhibits an increasing number of genes encoding the enzymes of the pathways involved in or related to the biosynthesis of withanolides and other secondary metabolites including functional and biochemical characterisation (Gupta et al. 2012; Akhtar et al. 2013; Sabir et al. 2013; Kushwaha et al. 2013a). Also several attempts have been made to analyse pathways from the perspectives of integration of early processes, ontogenetic regulation, and terminal transformation steps (Chaurasiya et al. 2007; Sangwan et al. 2008).
Biosynthesis of withanolides emanates from the central triterpenoid–sterol pathway with metabolic divergence even as early as the generation of 24-methylene cholesterol (Sangwan et al. 2008). The pathway comprises five segments: (a) isoprenogenic routes, (b) stem route of triterpenic isoprenoid hydrocarbons, (c) early events of cyclisation and oxidative modification common with phytosterols and brassinoids, (d) synthesis of withanolide progenitors and derived diversified withanolides, and (e) terminal conjugative transformations.
5.1.1 Isoprenogenic Routes
Unlike animal systems, plants possess two independent pathways for the synthesis of isopentenyl diphosphate (the monomeric building block for the homologous series of isoprenoids) – a mevalonate (MVA) pathway and a mevalonate-independent pathway (also called DOXP pathway). There is a huge variability in the specificity as well as in the relative participation of the two pathways that allow to accommodate the biosynthetic needs of different terpenoid subclasses (hemi-, mono-, sesqui-, tri-, tetra-terpenoids), even between the individual members within a subclass. Also other factors such as plant species, plant organ, or tissue type contribute to this variability. Recent studies, where the fate of precursors detectable by NMR through the stable 13C isotope was followed in W. somnifera (so-called retrobiosynthetic strategies), have revealed that withanolide biosynthesis recruits both MVA (mevalonate) and DOXP (deoxy xylulose pathway) pathways of isopentenyl pyrophosphate (IPP) synthesis (Chaurasiya et al. 2012). A pictorial representation of the biosynthetic systems is presented in Fig. 4. The DOXP pathway serves as a plastid-derived alternative route for isoprenoid biosynthesis. The first couple of committed and regulatory steps for terpenoid biosynthesis through the DOXP pathway is represented by the reactions catalysed by DXS and DXR, whereas the mevalonate pathway begins from acetyl coenzyme A and is regulated at the level of a step catalysed by the hydroxymethyl glutaryl coenzyme A reductase (HMGR).
Enzymes and genes of the withanolide biosynthetic pathway. ACT acetyl-CoA thiolase, HMGS 3-hydroxy3-methylglutarylco-A synthase, HMGR 3-hydroxy3-methylglutaryl CoA reductase, MVAK mevalonate kinase, MVAPK mevalonate phosphate kinase, MVAPPD mevalonate pyrophosphate decarboxylase, GPPS geranyl pyrophosphate synthase, FPP farnesyl pyrophosphate synthase, SS squalene synthase, SE squalene epoxidase, CAS cycloartenol synthase, BAS β-amyrin synthase, SMT-1 sterol methyl transferase 1, ODM obtusifoliol demethylase, dashed lines indicate multiple steps
5.1.2 Stem Route of Triterpenic Isoprenoid Hydrocarbons
The trunk route of isoprenoid biosynthesis comprises a sequence of condensation steps of different degrees of polymerisation of the monomer units to generate the series of C5, C10, C15, C20, C30, and higher prenyl pyrophosphates that provide the progenitors of mono-, sesqui-, di-, tri-, and higher terpenes. Squalene pyrophosphate serves as prenylated precursor and dephosphorylates through a carbocation mechanism under the catalysis of squalene synthase to generate squalene. Thus, squalene is a spin-off metabolite of the isoprenoid trunk route that is metabolised into diverse triterpenoids (C30) as well as their descendant specialised metabolites (C30±n) including sterols (typically C28–29), withanolides (C28), and other phytosteroids. The five-carbon IPP monomer and its isomer dimethylallyl diphosphate (DMAPP) combine in a head-to-tail manner to form geranyl diphosphate (GPP, C10 prenyl pyrophosphate) under the catalysis of GPP synthase. GPP is then released and processed by monoterpene synthases to diverse spin-off parental monoterpenes such as geraniol, linalool, myrcene, or limonene, whilst GPP produced by farnesyl diphosphate synthase (FPP synthase) remains enzyme bound for a second sub-reaction, where additional IPP in trans-configuration is coupled to yield farnesyl diphosphate (FPP, C15 prenyl pyrophosphate). FPP may again be spinned off to generate sesquiterpenes under the catalysis of sesquiterpene synthases or processed further in the trunk pathway to serve as substrate to produce higher prenyl pyrophosphates and their spin-off higher terpenoids. Thus, for triterpenoids, two molecules of FPP condense in a head-to-head manner to produce squalene (C30) under the catalytic action of squalene synthase (Fig. 4).
5.1.3 Early Events of Cyclisation and Oxidative Modification
Epoxidation of squalene to 2,3-squalene oxide involves atmospheric oxygen and the catalytic action of squalene epoxidase, a cytochrome P450 enzyme. Squalene-2, 3 epoxide occupies a highly anaplerotic position in steroid and triterpenoid biosynthesis and serves to deliver diverse progenitor molecules that are further diversified by progressive functionalisation. A battery of triterpene synthases, like cycloartenol synthase, lanosterol synthase, thalianol synthase, amyrin synthases, and lupeol synthase, catalyse these progenitors. Withanolide biosynthesis appears to follow the same early steps of squalene 2, 3-epoxide cyclisation and subsequent metabolic transformation steps as common for membrane phytosterols (like stigmasterol and sitosterol) and brassinolides. Based on a chemoinformatic analysis, it has been hypothesised that the pathway conveying withanolide biosynthesis could be a prolongation of the brassinolide pathway (Sangwan et al. 2008). Accordingly, it has been hypothesised that withanogenesis may metabolically originate from 24-methylene lophenol and/or 24-methylene cholesterol and/or campesterol (Sangwan et al. 2008). Whilst radiotracer studies carried out with 24-methylene cholesterol as precursor indicate that an intermediate from the sterol pathway is linked to withanogenesis (Lockley et al. 1976; Glotter 1991), alternative plausible metabolites have still to be tested as yet in this regard. Since the functionalisation reactions of secondary metabolism are quite versatile and can use multiple substrates, it is not very odd to examine the possibility that withanolide biosynthesis might be committed from multiple points of the sterol–brassinolide pathway (Sangwan et al. 2008). Physiologically, this hypothesis is consistent with our present knowledge on the functional aspects of sterols and brassinolides. Sterols are membrane constituents and their levels determine several critical cellular functions and processes, while brassinolides are hormones. Intracellular levels of membrane sterols and brassinolides are very low and have to be tightly controlled to maintain a balance of cellular activities, plant growth, and development. In contrast, the levels of withanolides are several orders of magnitude higher (Sangwan et al. 2008). Therefore, withanolide biosynthesis in Ashwagandha may have role in regulating sterol and brassinolide homeostasis by serving as major carbon shunt from the shared pathway segment (Sangwan et al. 2008). Although lanosterol synthesis from squalene 2,3 oxide under the enzymatic action of lanosterol synthase has been considered to be restricted to fungi, some recent studies have revealed the presence of lanosterol in a few higher plants. As function, it has been proposed to act as metabolically redundant route to secondary phytosterols/triterpenols. Although such a sterol synthase has not been found so far in W. somnifera, some derivatives of lanosterol have been recently reported to occur in Ashwagandha (Mishra et al. 2012). Considering that withanolides are based on an ergostane skeleton, their origin through such a redundant pathway should be investigated (Fig. 4).
5.1.4 Synthesis of Withanolide Progenitors and Diversified Withanolides
The nature of parental progenitor metabolites synthesised from the predicted intermediates (Sangwan et al. 2008) like 24-methylene lophenol, 24-methylene cholesterol, and campesterol is still not known. However, it seems quite certain that it involves some critical steps to generate the ergostane skeleton and additional functional groups; these include (i) C1 oxidation to a hydroxylation followed by, for most (>90 %) of the withanolides, reduction of the hydroxyl group into a keto (>C = O) function. Thereby, it implicates participation of a cytochrome P450 hydroxylase and an oxidoreductase (dehydrogenase). The structural features of the molecules suggest that oxidative transformations may involve hydroxylation at C22 as well as C26 followed by conversion of a C26 alcohol to an aldehyde and, in most cases, conversion of the aldehyde (−CHO) group into an acid (−COOH) function. These catalytic reactions can be predicted to recruit appropriate hydroxylases and other oxidoreductases. Another step is formation of a lactone (mostly) or hemiacetal (few cases) ring by closure between a C22 hydroxy group and a C26 acid group, respectively. These biochemical reactions are yet to be identified and characterised to understand the events of metabolic commitment that generate the parental metabolite for withanolide.
A myriad of withanolides that number in hundreds are derived by further functionalisation in a position- and region-specific manner. These functionalisations include epoxidation, hydroxylations, and dehydrogenations. In fact, all positions of the C28 molecule except C8, C9, and C10 can be hydroxylated individually as well as in combinations reflecting the possibility that many more withanolides can be generated than those existing in nature. Nevertheless, a substantial number of withanolides can be grouped into clusters defined by common conjugation. Accordingly, we could define two major classes of withanolides based on a conjugated hydroxy and epoxy function around C5 to C7. These are class 1 withanolides containing a 4β-hydroxy 5,6 β-epoxy group with representative major withanolides like withaferin A and withanolide D and class 2 withanolides containing a 5α,6,7,α epoxy group with representative major withanolides like withanone and withanolide A (Fig. 3). Biosynthetically, these classes may record the existence of specific cytochrome P450 hydroxylases to generate these hydroxyl and epoxy functionalities of respective orientation due to their catalytic specificity for the carbon position in functionalisation and the stereospecificity of the functional groups. From these observations and analyses, a large number of enzymes and genes can be predicted able to confer catalytic novelties with potential commercial/industrial applications. The forthcoming availability of Ashwagandha root and leaf transcriptomes under the New Millennium Indian Technology Leadership Initiative in India would provide a working platform for advanced genomics and systems biology support to accelerate the deciphering of the metabolic pathway and withanolide metabolomics.
5.1.5 Terminal Conjugative Transformations
Acylations (particularly acetylation) and glycosylations (mainly single or multiple glucosylations) are the major conjugative metabolic transformations of withanolides observed so far. Major derivatives acetoxy withanolides, withanosides, and sitoindosides are produced by the reactions that are catalysed by acyltransferases and glycosyltransferases, respectively. Several glycosyltransferases have been isolated and characterised from W. somnifera leaf and root, including cloning of the respective genes and elucidation of gene functions. An enzyme catalysing the formation of 4-acetoxy and 27-acetoxy withaferin A from withaferin A has been isolated and characterised from the leaves of W. somnifera (Chaurasiya 2007). The enzyme contains an HXXD motif as proteomic characteristic and thus belongs to the so-called BAHD family of plant acyltransferases. The family is named according to the first letter of the first four biochemically characterised enzymes of this family, namely, benzyl alcohol O-acetyltransferase (BEAT) from Clarkia breweri, anthocyanin O-hydroxycinnamoyltransferase (AHCT) from Gentiana triflora, anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT) from Dianthus caryophyllus, and deacetylvindoline 4-O-acetyltransferase (DAT) from Catharanthus roseus (D’Auria 2006).
5.2 Cellular Metabolic Cross Talk for Secondary Metabolite Production
Chemoinformatic and biogenetic comparison of triterpenes and sterols with withanolides implies that withanolides may originate through a complex and yet unknown pathway, from either 24-methylene lophenol or 24-methylene cholesterol or campesterol, or the upstream intermediates of the triterpene pathway, or analogous metabolites of the lanosterol route (Sangwan et al. 2008). However, since the stem pathway at least up to squalene is shared, the synthesis of isopentenyl diphosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) might control the withanolide pathway through governing the flux of the isoprene units. In plants, IPP and DMAPP occur by two independent routes: the classical mevalonate (MVA) pathway in the cytosol and a non-mevalonate pathway (also called deoxy xylulose pathway (DOXP) or methyl erythritol pathway (MEP)) proceeding in plastids (Lichtenthaler 1999; Rohmer 2003; Sato et al. 2003). It is considered that the synthesis of monoterpenoids and diterpenoids involves the DOXP pathway while that of triterpenes (sterols) and sesquiterpenes recruits the MVA pathway (Lichtenthaler 1999; Rohmer 2003; Bouvier et al. 2005; Cordoba et al. 2009). This generalisation is based on early studies on the relative contributions of the MVA and DOXP pathways for various classes of isoprenoids. However, the validity of this strict categorisation is challenged by more recent evidences. Normal levels of sterols (sitosterol, stigmasterol) can be synthesised through the MVA pathway (Rohmer 2003; Bouvier et al. 2005). But the sterol hyper-producing green callus of Croton stellatopilosus has been shown to recruit DOXP pathway as well in the biosynthesis of isoprenoids (De-Eknamkul and Potduang 2003). However, many sesquiterpenoid compounds, such as artemisinin, are of mixed (DOXP and MVA) or exclusively of DOXP origin (Towler and Weathers 2007). Some compounds are of mixed origin such as carrot β-caryophyllene (Hampel et al. 2005), chamomile and snapdragon flower sesquiterpenes (Adam and Zapp 1998; Dudareva et al. 2005), strawberry fruit sesquiterpenes (Hampel et al. 2005), and the Anthemis cotula allergenic anthecotuloide (Van Klink et al. 2003) are derived from both pathways. The relative carbon contribution from the MVA and DOXP pathways to withanolide biosynthesis was determined through quantitative NMR by measuring position-specific enrichment of 13C labels on individual carbons of withaferin A. 13C1-glucose fed to in vitro microshoots of W. somnifera has revealed significant contribution of the DOXP pathway to withanogenesis. Thus, withanolide biosynthesis utilises both the DOXP and the MVA pathways adding to the growing list (Chaurasiya et al. 2012), where the generalised dichotomy of isoprenoid class-specific recruitment of the two pathways is blurred. Possibly, it might be the physiological context of metabolism rather than isoprenoid class that determines the relative contribution of the two isoprenogenic routes (MVA and DOXP).
5.3 Developmental Dynamics of Withanolides
Two major withanolides, withaferin A and withanone, are accumulated in prodigal amounts in Ashwagandha leaves. However, no information is available about the developmental physiology of withanolide biogenesis. The studies on ontogenic dependence of withanolide biogenesis are highly relevant: (1) to understand the chemo-ecophysiological function of these secondary molecules, (2) to optimise harvest of bioactive phytochemical(s) from this crop, (3) to define the parameters for quality management of these herbal nutraceutical and therapeutic products, and (4) to define physiological states in studies dealing with the comparative genomics of withanogenesis. The accumulation of withaferin A and withanone was followed by TLC profiling and HPLC quantification through five stages of leaf ontogeny from very young, young, premature, mature, till senescent (Chaurasiya et al. 2007). The temporal patterns of accumulation were compared with parallel quantifications of biosynthesis using radiolabeled acetate as precursor. The levels of radioactive incorporation reported de novo biosynthesis that was found to be induced from initiating leaf development and increasing in the young leaf, such that maximal levels had accumulated by the time of leaf maturity (full expansion). The terminal degenerative and senescent phases of ontogeny seem to involve catabolic decay of withanolides, perhaps for the relocation/mobilisation of withanolide carbon not longer required for defence in tissues that are going to die anyway (Tuli and Sangwan 2010). Based on the assumption of a putative role in defence, a number of hypotheses link the production of secondary metabolites with leaf growth. For example, the optimal defence theory balances the risk for attack or damage against the costs of biosynthesis. In contrast, various resource-based theories assume that this biosynthesis is constrained by the external availability of resources and an internal trade-off in allocations between growth and defence (Chaurasiya et al. 2007).
Developmental patterns can also affect the profile of withanolide accumulation (Sidhu et al. 2011). By using NMR technology, the concentration of withanolides was found to be highest during initial stages of fruit development, whereas withanamides increased substantially during maturation of the fruits. This age-dependent shift from withanolides towards withanamides is relevant for application purposes. For instance, the mature fruit would be highly suited as antioxidant (Jayaprakasam et al. 2004), which would be beneficial in the treatment of tumours and inflammation (Jayaprakasam et al. 2003). This example illustrates that the type and developmental stages of source tissues are relevant to optimise their usage for drug and nutraceutical purposes.
6 Withanolide Pathway Genomics
Genes for the withanolide biosynthetic pathway related to upstream segment and regulatory steps of IPP synthesis (isoprenogenesis) through the MVA and DOXP pathways, the shared stem route of isoprenoid pathway, and conjugative steps of withanolide glycosylation have been cloned and characterised from W. somnifera (Table 3). The detailed biochemical and genomic significance is discussed below.
6.1 Deoxy Xylulose-5-Phosphate Synthase (DXS)
DXS constitutes the first step for the DOXP pathway of isoprenogenesis. Ashwagandha DXS gene (WsDXS) encoding a 717 amino acid polypeptide has been cloned and found to possess a similar exon–intron structure as found in the tomato homologue (Gupta et al. 2013). The gene was differentially regulated in different tissues with highest level of expression in flowers and young leaves. Moreover, the pattern differed between chemotypes. The abundance of the WsDXS transcripts parallels the content of the major withanolides withaferin A, withanone, and withanolide A. This reflects the significant contribution of DXS to deliver the IPPs required for withanolide biogeneration via the triterpenoid pathway. The expression of this gene was also induced by mechanical injury and salicylic acid and methyl jasmonate (Gupta et al. 2013).
6.2 Deoxy Xylulose Phosphate Reductase (DXR)
The reaction catalysed by DXR constitutes the next step in the DOXP pathway of isoprenogenesis. The gene has been cloned from Ashwagandha as a reading frame coding for a 475 amino acid polypeptide and, similar to DXS, harbours a putative plastid-targeting signal (Gupta et al. 2013). The comparison of WsDXS and WsDXR expression in three different chemotypes containing varying contents of withanolides, in various tissues and leaf ontogenic stages, as well as in response to chemical stimuli (SA and MeJA) and mechanical injury, indicates that WsDXS and WsDXR control the flux of carbon required for withanolide biosynthesis, for instance, during leaf development (Chaurasiya et al. 2007).
6.3 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase (HMGR)
3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGR; EC 1.1.1.34) catalyses an irreversible conversion of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) into mevalonic acid constituting the key regulatory step for the synthesis of IPP and its isomer, DMAPP, as progenitors of homologous series of isoprenoids (Chappell 1995). HMGR is the rate-limiting enzyme of the MVA pathway and thus also for withanolides. Therefore, regulatory and functional characteristics of this gene are relevant for withanolide biosynthesis. From sequence homology of WsHMGR with other plant HMGR, conserved motifs probably corresponding to the active centre can be identified. Our observations on WsHMGR suggest that its expression is responsive to wounding and exposure to methyl jasmonate and salicylic acid. Interestingly, the rapid and steady accumulation of WsHMGR transcripts in response to mechanical injury was paralleled by an identical response of WsFPPS, the gene involved in the downstream stem pathway of isoprenoid progenitor prenyl phosphate generation. WsHMGR is the first gene of Ashwagandha isoprenoid/secondary metabolite biosynthesis that has been functionally validated by pathway complementation in E. coli. It has been demonstrated that E. coli expressing WsHMGR and pAC-BETA accumulated significantly higher saffron yellow coloured β-carotene (more than twofold) as compared to the bacteria transformed with the empty vectors. This suggests that WsHMGR promoted the accumulation of β-carotene, corroborating its significance for withanolide biosynthesis (Akhtar et al. 2013).
6.4 Farnesyl Diphosphate Synthase
Similar to the initial two genes of DOXP pathway, DXR and DXS, farnesyl diphosphate synthase (WsFPPS) is much more abundantly expressed in young leaves as compared to mature leaves (Gupta et al. 2011). This gene is involved in sesquiterpene prenylphosphorylation. The abundant expression of WsFPPs in the leaf indicates that root may be less relevant for DOXP-dependent isoprenoid biosynthesis. Generally, roots and leaves can synthesise withanolides independently, but leaves are more active (Sangwan et al. 2008; Sabir et al. 2012).
6.5 Squalene Synthase
Squalene synthase (WsSQS) is a key enzyme involved in the biosynthesis of isoprenoids and catalyses a branch point diverging carbon flux from the main isoprenoid pathway towards sterol and triterpene biosynthesis. Squalene synthase causes a dimerisation of two farnesyl diphosphate (FPP) molecules into squalene. Since this gene is important for biosynthesis and regulation of withanolides, this enzymatic step has been subject of investigation in W. somnifera. A genomic fragment of 1,765 kb has been cloned and characterised recently (Bhatt et al. 2012) comprising a 1.236 bp ORF for squalene synthase. Expression of this gene was maximal in the leaf. The promotor harbours several putative regulatory cis-acting elements. Similar to DXS and DXR, squalene synthase has been shown to be upregulated by different signalling components including methyl jasmonate, salicylic acid, and 2, 4-D (Bhat et al. 2012). Meanwhile, the catalytic activity of a recombinantly expressed C-terminally truncated WsSQS could be demonstrated (Gupta et al. 2012). WsSQS transcripts are found in two open reading frames of 1,236 and 1,242 bp, respectively, corresponding to a length difference of only 2 amino acid residues, and encode 412 and 414 amino acid polypeptides, respectively (Gupta et al. 2012). A correlative investigation on WsSQS expression in a wide range of tissues demonstrates a linear correlation between transcript abundance and withanolide levels (Sabir et al. 2013). Specifically, expression of the SQS is elevated with the onset of tissue differentiation from callus to shoots matching an increase of withanolide content. In a recent study, squalene synthase has been overexpressed in W. somnifera using Agrobacterium tumefaciens-mediated transformation (Grover et al. 2013). The transgenic suspension cultures displayed a four-fold increase of SQS catalytic activity and a concomitant 2.5-fold enhancement in withanolide A content. Additionally, these transformed cell suspension cultures produced withaferin A, contrary to the non-transformed control cultures (Grover et al. 2013). These studies show clearly a direct function of SQS for withanolide biosynthesis (Gupta et al. 2012; Sabir et al. 2013; Grover et al. 2013).
6.6 Squalene Epoxidase
Squalene epoxidase (EC. 1.14.99.7) catalyses the production of 2, 3 oxidosqualene from squalene. The oxidosqualene possesses anaplerotic significance by serving as the common precursor for several triterpene hydrocarbons, triterpene alcohols, sterols, withanolides, and brassinosteroids. The gene has been cloned from W. somnifera (Razdan et al. 2013) as a 1.965 kb cDNA containing a 1.596 kb ORF (531 amino acids). A flanking fragment of 513 bp of the promotor region could be obtained through genome walking and contains several cis-elements putatively mediating responses to various biotic and abiotic stresses. Similar to the transcripts for other upstream genes of the pathway, expression of the WsSQE gene is higher in leaves as compared to shoot and root tissues (Razdan et al. 2013).
6.7 Phytosteroid Glycosyltransferases (SGT): Family Members and Functions
Sterol glycosyltransferases (SGTs) catalyse the transfer of a sugar moiety from activated (uridine diphosphate-conjugated sugars like UDP-glucose) donors to diverse acceptor molecules (aglycones) of steroidal nature. This family of genes is important for Ashwagandha, since several withanolides are found as glycosidic conjugates like withanosides and sitoindosides. Sterols and their modified counterparts are not only medically important but probably participate in stress adaptation of plants (Tuli and Sangwan 2010). The withanosides mainly comprise withanolides with one or more glucose units attached to the C-3 or C-27 positions. In a sequence of studies on W. somnifera sterol glycosyltransferases, it has been shown that the plant harbours a battery of SGTs that differ not only in their characteristic catalytic features but also in their size and intracellular localisation (Madina et al. 2007a, b; Sharma et al. 2007). Briefly, a sterol glucosyltransferase specific for the 3β-hydroxy position purified from the leaves of W. somnifera has a catalytic specificity to glycosylate both phytosterols and steroidal sapogenins (Madina et al. 2007a). A different sterol glucosyltransferase capable of transferring glucose from UDPG to the C-3 position (Sharma et al. 2007) possessed a transmembrane domain (Sharma et al. 2007). Originally, no enzyme for the glycosylation of sterols/withanolides at positions other than C-3 had been identified, though several 27-O-glucosylated pharmacologically important metabolites, like sitoindosides IX and X, are present in W. somnifera. In fact, a novel 27β-hydroxy glucosyltransferase activity has been identified and characterised that can glucosylate the C-27 hydroxy position in withanolide as well as some other sterols with a hydroxyl function at higher position. This C-27 hydroxy group-specific SGT has been suggested to function in defence responses of the plant (Madina et al. 2007b). The purified enzyme showed activity with UDP-glucose but not with UDP-galactose as sugar donor and exhibited broad sterol specificity by glucosylating a variety of sterols/withanolides with β-OH groups at C-17, C-21, and C-27 positions. An enzyme with comparable catalytic activity has not been reported earlier from plants. Both the C-3 and C-27 hydroxy group-specific glucosyltransferases follow an ordered sequential bi-substrate reaction mechanism, in which UDP-glucose binds first followed by binding of the sterol (Madina et al. 2007a, b). The catalytic activities with withanolides as substrates suggest a role for this enzyme in secondary metabolism. Results on peptide mass fingerprinting of the purified enzyme revealed their resemblance with glycuronosyltransferase-like proteins. The catalytic levels of these enzymes in the leaves of W. somnifera were enhanced, but in different levels, by application of salicylic acid and heat stress (Madina et al. 2007b). Recently, three more members of the SGT gene family have been identified in Ashwagandha (Chaturvedi et al. 2012) with amino acid sequence homology in the range of 45–67 % compared to other known plant SGTs. The transcript expression can be induced up to tenfold depending on the organ or triggering external stimulus. Recently, in addition to SGTs, a new flavonoid-specific GT was found in W. somnifera (Jadhav et al. 2012). Based on the crystal structure of plant UGTs, a structural model for this flavonoid-specific glycosyltransferases (WsFGT) could be constructed. The model predicts a GT-B-type fold (Jadhav et al. 2012) and interaction of amino acids in a conserved plant secondary product glycosyltransferase (PSPG) box with the sugar donor, while His18, Asp110, Trp352, and Asn353 are probably important for catalytic function. This structural information on the docking will be useful to understand the glycosylation mechanism of flavonoid glucosides (Jadhav et al. 2012).
7 Towards Withanolide Production In Vitro
The demand for withanolides is considerably increasing not only due to academic interest but also for the production of valuable medicinal products. Commercial cultivation of W. somnifera, however, can yield only limited amounts of withanolides for a series of reasons. These include (1) relatively long period between planting and harvesting, (2) chemotypic and developmental variations, (3) seasonal and somatic variations, (4) infections by microorganisms and herbivore attacks, and (5) environmental pollution. The comparison of W. somnifera from different geographic locations has revealed a variety of chemical profiles that are different in quantity and even quality and that are correlated with genetically distinct clusters (Sangwan et al. 2004a, b; Chaurasiya et al. 2009). Medicinal preparations from such nonuniform and non-characterised plant materials may compromise the efficacy of phytotherapy (Kushwaha et al. 2012; Mondal et al. 2010). Therefore, the establishment of efficient in vitro regeneration systems and the development of cellular technology are prerequisites to produce plant material of standardised high quality (see also chapter by Opatrný in this volume). The use of tissue culture could be an alternative method for shortening the time to obtain true-to-type plantlet regeneration and obtaining a stable production of withanolides. Reports on in vitro cultures using different Ashwagandha explants are available in the literature (Rani et al. 2003; Sabir et al. 2007; Nayak et al. 2013) and include the regeneration of plants from callus. Tissue culture of W. somnifera for the in vitro production of withanolides has been achieved elsewhere (Yu et al. 1974; Heble 1985) and also in our laboratory. Various in vitro-based approaches for the development of callus, cell suspensions, and shoot and root cultures have been developed for Ashwagandha (Table 4; Fig. 5c–i).
Tissue culture and genetic transformation of Ashwagandha. (a) Superior Ashwagandha variety growing in the CSIR-CIMAP experimental farm; (b) young Ashwagandha plant; (c) profuse callus proliferation in optimised MS medium; (d) synchronised suspension culture; (e) Agrobacterium rhizogenes-mediated hairy roots of Ashwagandha; (f) multiple shoot cultures; (g) root induction in shoot cultures of Ashwagandha; (h) shoot bud viewed under microscope; (i, j) direct rhizogenesis; (k, l) localisation of histochemical GUS expression; (m) thin layer chromatography of Ashwagandha extract isolated from control plant and transformed plant; WA withanolide A, WF withaferin A
7.1 Induction and Proliferation of Multiple Shoot Cultures
Shoot induction and proliferation of W. somnifera and the second important Ashwagandha species, W. coagulans, have been achieved (Singh et al. 2005; Sabir et al. 2007, 2008, 2013; Sharada et al. 2007). A protocol utilising a hormone combination for direct induction and regeneration of shoots was developed which was successful on several W. somnifera chemotypes (Sabir et al. 2007) and was validated in several studies involving the use of shoot cultures (Sabir et al. 2008, 2012; Mishra et al. 2012; Chaurasiya et al. 2012; Sabir et al. 2012).
Phytochemically, these multiple shoot cultures exhibited the characteristic withanolides such as withaferin A and withanolide A (Sabir et al. 2008). The accumulation of both withaferin A and withanolide D could be enhanced when 4 % sucrose was added to the medium (Table 4). Shoot cultures accumulated the rare withanolides I, G, and D, while these could not have been observed in unorganised callus (Roja et al. 1991). Shoot cultures of W. somnifera from Italy accumulated withanolide J after 20 days of in vitro growth, whereas hairy root systems of the same plant failed to produce detectable withanolides (Vitali et al. 1996). Glycoderivatives of withanolides, e.g. withanoside IV (WSG-3), withanoside VI (WSG-3A), physagulin D (WSG-P), and withastraronolide (WSC-O), were isolated from in vitro multiple shoot cultures of W. somnifera (Ahuja et al. 2009).
7.2 Callus and Cell Suspension Cultures
A fast and efficient protocol for callus induction and proliferation suitable for almost all chemotypes of W. somnifera has been established using stem, leaf, buds, and roots as explants (Fig. 5c; Sabir et al. 2008, 2009, 2012). Callus derived from axillary shoots has been demonstrated to have the best capacity for regenerating shoots (Rani et al. 2003). Only cytokinins (2.0 mg L−1 BAP alone or in association (at 1 mg L−1) with 2.0 mg L−1 Kin) have been found suitable to induce the formation of nodular green calluses (see also chapter by Šmehilova and Spíchal and Opatrný in this volume) with nodal segments as explants (Siddique et al. 2004). Field-cultivated leaves as well as seeds have demonstrated to serve as the best starting material for the induction of calluses appropriate to achieve high frequency (81 %) of shoot regeneration (Supe et al. 2006; Rout et al. 2011).
Indirect regeneration from leaf explants and a comparative analysis of withaferin A, 12-deoxywithastramonolide, and withanolide A productions in in vitro systems or greenhouse were also investigated (Dewir et al. 2010). High yields of transformed W. somnifera calluses were achieved from the infection of hypocotyls with A. rhizogenes strain MTCC-2250 (Ray and Jha 1999). We also established a protocol for regenerating tissues from root calluses (Sabir et al. 2012). Moreover, we developed a protocol for genetic transformation of W. coagulans to assess for understanding the functions of genes utilising this regeneration system with higher frequency of transformation (Mishra et al. 2012; Pandey et al. 2010).
Cell suspension cultures of W. somnifera were established, being proved to be effective in the production of withaferin A (Fig. 5d). The established cultures produced up to 25 ± 2.9 mg withaferin A L−1 when cells were elicited with 750 μM salicin compared to non-elicited cells which produced only 0.47 ± 0.03 mg withaferin A L−1 (Ciddi 2006). Leaf-derived friable cell suspension cultures were grown in highly synchronised conditions, and generated suspension cultures (see also chapter by Opatrný et al. in this issue) were also shown to possess the capacity of accumulating withanolides (Sabir et al. 2008) and also possess biotransformation potential (Sabir et al. 2011). Various abiotic elicitors (arachidonic acid, methyl jasmonate, calcium chloride, and copper sulphate) and biotic elicitors (cell extracts and culture filtrates of Alternaria alternata, Fusarium solani, and Verticillium dahliae) were tested for the ability to stimulate the accumulation of withaferin A in suspension culture of transformed cells (Baldi et al. 2008).
7.3 Root Cultures
The development of fast growing root culture system would offer a unique opportunity for producing root phytochemicals in laboratory without depending on field cultivation. Several reports are available for direct root regeneration from leaves of W. somnifera (Fig. 5i, j). Direct rhizogenesis of Ashwagandha has been reported (Sabir et al. 2013; Wasnik et al. 2009; Wadegaonkar et al. 2005). Indirect regeneration of rhizogenic roots from leaf explants and the production of withanolide were studied (Wadegaonkar et al. 2005; Wasnik et al. 2009; Sabir et al. 2013). Production of withanolide A from adventitious root cultures of W. somnifera has been reported (Nagella and Murthy 2010). Elicitation by methyl jasmonate and salicylic acid enhanced withanolide production in adventitious root cultures of W. somnifera (Sivanadhan et al. 2012a). Chitosan-mediated enhancement of withanolide (withanolide A, withanolide B, withaferin A, withanoside IV, and withanoside V) production in adventitious root cultures of W. somnifera (L.) has also been observed (Sivanadhan et al. 2012b).
7.4 Hairy Root Cultures
The establishment of hairy root cultures (Fig. 5e) of Ashwagandha and the investigation of withanolide accumulation have been reported (Kumar et al. 2005; Bandopadhyay et al. 2007; Murthy et al. 2008; Praveen and Murthy 2013). Among the different strains of A. rhizogenes, strain A4 had the highest efficiency to induce hairy roots, while strain LBA 9402 produced different morphological responses such as callus and rooty callus (Bandopadhyay et al. 2007). Accumulation of major withanolides, withaferin A, withanolide D, and withanolide A has also been found to occur in transformed root lines of W. somnifera (Ray and Jha 1999; Kumar et al. 2005; Murthy et al. 2008). The effect of growth media on root biomass accumulation (Ahuja et al. 2009; Murthy et al. 2008) and the effect of carbon sources and pH on the synthesis of withanolide A in hairy root cultures have been investigated (Nagella and Murthy 2012). Methyl jasmonate and salicylic acid were found to elicit the production of withaferin A in the hairy root cultures of W. somnifera, indicating that the accumulation and biogenesis of secondary metabolites – withanolides – in Ashwagandha are under tight regulation.
8 Agrobacterium tumefaciens-Mediated Genetic Transformation
Plant transformation mediated by A. tumefaciens has become the main method for the introduction of foreign genes into plant tissues and the subsequent regeneration of transgenic plants (see also chapter by Opatrný in this volume) and has been successfully employed to transform several medicinal plants (Gomez et al. 2007). A. tumefaciens-mediated transformation of W. somnifera was firstly attempted by Ray and Jha (1999) using a wild-type strain of A. tumefaciens, but only yielding shoot-like teratomas. Successful genetic transformation up to plant level of W. somnifera through A. tumefaciens has been reported for the first time by Pandey et al. (2010) using leaf explants (both from tissue culture and greenhouse) as starting material. A. tumefaciens strains LBA4404 and EHA101, containing the binary vector pIG121Hm, possessing nptII gene under the control of Pnos (nopaline synthase promoter), and hygromycin resistance (hptII) as selective marker, as well as gusA genes under the control of the cauliflower mosaic virus (CaMV) 35S promotor (Ohta et al. 1990), have been used for the development of transformation protocol (Fig. 5k, l). Leaf sections were co-inoculated in A. tumefaciens suspension for 10–30 min with gentle shaking, blotted dry, and placed on cocultivation medium and kept in the dark for 5 days. Several parameters such as position of leaf, age of seedling, infection time, and cocultivation duration were adjusted to improve transformation efficiency. Several distinct chemotypes of W. somnifera differing in their withanolide profile were tested and found to yield maximal frequencies of up to 77.3 % for transient and up to 1.7 % for stable transformation (Pandey et al. 2010). Although this protocol enabled for the first time genetic engineering of Ashwagandha to understand and improve the withanolide pathway, its efficiency was limited to some chemotypes. A protocol efficient for all chemotypes is still urgently required for applications in phytopharming and functional genomics. Recently, a highly efficient system for the A. tumefaciens-mediated transformation of the closely related withanolide-yielding W. coagulans has been developed to cater these requirements (Mishra et al. 2012).
8.1 Development of Efficient Protocols for Transformation of Ashwagandha
The successful and efficient transformation of plants essentially requires optimisation of several prerequisite including the competence of target cells or tissues for transformation and regeneration, the efficiency of DNA delivery, the stringency of selection, and the ability to recover fertile transgenic plants. During the past two decades, these prerequisites have been optimised leading to protocol for the improved A. tumefaciens-mediated genetic transformation of aromatic and medicinal plants (Ray and Jha 1999). Many of these factors have been evaluated in our study to generate stable transgenic plants of Ashwagandha species. The leaf from in vitro multiple shoot cultures was identified as a suitable explant for the A. tumefaciens-mediated transformation and regeneration of stable transformants of Ashwagandha species (Mishra et al. 2012). Also leaf explants have been used effectively for the transformation of other aromatic and medicinal plants (Kumar and Gupta 2008). Therefore, margins of the leaf lamina were removed in Ashwagandha to expose cut surfaces for infection, and small pieces (2–3 mm) of leaf explants (apical, middle, and basal portion) were used for infection. A. tumefaciens strain LBA 4404 with plasmid pIG121Hm was used in this study to optimise various factors like the effect of bacterial density, duration of co-inoculation, length of cocultivation, and acetosyringone concentration for Ashwagandha species transformation (Fig. 5k, l). Histochemical assays showed GUS expression in all cocultivated explants (100 % transient transformation frequency) and in different parts of plant in stem, petiole, veins, and leaf lamina of putative transformant during different developmental stages (Fig. 5k, l). The presence of the transgenes (nptII and gusA) was verified and confirmed in putative transformants by genomic PCR amplifications with genomic DNA. Withanolide (withanolide A, withanone, and withaferin) patterns as assayed by TLC showed similar patterns for the putative W. somnifera transgenic shoots to those obtained with native non-transformed shoots of in vitro shoot cultures (Fig. 5m). Morphologically, the transformed plants were identical to their untransformed counterparts. To safeguard against false positives that may result from expression of the gene in A. tumefaciens, the gusA transgene harboured an intron. Therefore, the positive GUS assay accompanied by genomic PCR analysis using gusA and nptII primers in the putative transformants provided molecular proof for the integration of the gusA gene into the plant genomic DNA and ascertained the stable genetic transformation in planta (Mishra et al. 2012). Thus, the protocol developed can be reliably used for the transfer of genes of interest to design transgenic W. somnifera plants possessing favourable traits of agronomic advantages, phytopharmaceutical chemical specialty (production of improved levels of minor, but most potent, withanolides), and other applications for metabolic engineering applications including production of heterologous proteins. Transformation also provides a powerful tool to study the function of candidate genes related to the variety of steroidal transformations unique to this medicinal plant such as the metabolic origin of withanogenesis from the central triterpenoidal route, but also to investigate the role of these genes in plant growth and development and to utilise these genes for pathway engineering in W. somnifera.
9 Summary and Future Prospects
The medicinal and pharmaceutical importance of Ashwagandha crude extracts as well as pure withanolide compounds has been tremendously increasing during recent years as evidenced by a huge increase in patents and publications on Ashwagandha in recent years. Newly discovered pharmacological roles for withanolides are being explored for modern drug development programmes, in addition to the well-known importance of Ashwagandha in traditional systems of Indian medicine and Ayurveda. In our lab, attempts have been made in a systematic manner to provide more concise and clear information about biotechnological aspects for improving quality and yield of the plant material. Also the biotechnologically generated material is assessed for further exploitation for phytotherapeutical purposes and in drug research. Earlier information particularly related to individual withanolide constituents from Ashwagandha requires further investigation and specification in terms of specific phytomolecules being associated with particular remedy, as well as establishment and definition of distinct chemotypes for sustainable utilisation. To determine precisely the phytochemical nature of the active compounds of this herb is of utmost importance to provide the appropriate doses and desired molecules for treating or preventing negative side effects of withanolide molecules or extracts. Recent efforts including those of our research group have provided new insights on how the yields of active phytomolecule(s) from Ashwagandha can be increased in a more standardised and efficient manner. The protocols for generating in vitro tissue systems, especially creating micro-clones of the developed elite varieties and hybrids, have improved leading to a unified protocol applicable to diverse geographic material for high-throughput micropropagation to take a particular accession to the field level. Various systems from unorganised or organised cells were also generated with considerable success in terms of withanolide productivity. Although in vitro-raised shoots exhibited higher amounts of withanolide A, in vitro-cultured roots exhibited lower levels of withanolide A and withanone than those of field-grown mother plants. However, certain root morphotypes accumulated withanolide A and withanone in a manner comparable to that exhibited by roots of field-grown W. somnifera. Thus, we have developed various tissue systems of W. somnifera with the ability to biosynthesise withanolides of pharmaceutical importance. Various stress conditions were studied on shoot, callus, and suspension cultures and were shown to induce the accumulation of various withanolides to a different extent. The developed tissue cultures provide also experimental models to understand the withanolide biosynthetic pathway under a convenient and controlled manner at the organ, tissue, and cellular levels, a prerequisite for large-scale production of valuable bioactive withanolides. The major withanolides were characterised in W. somnifera by using spectral and analytical techniques such as TLC, HPLC-UV photodiode array detection (PAD), evaporative light scattering detection (ELSD), and nuclear magnetic resonance (NMR). The compounds were identified as withanone, withaferin A, withanolide A, and withanolide D. Earlier reports as well as ours have suggested that the production of withanolides is closely associated with morphological differentiation and a tissue-specific pattern of withanolide production in cultures with relative expression of some of the pathway genes associated with withanolide biosynthesis. Highly efficient Agrobacterium-mediated transformation protocols for W. somnifera from A. tumefaciens or A. rhizogenes have been developed. Both systems can be used for the validation of gene functions by using genomic approaches such as gene silencing or overexpression. These culture systems also provide deep insights for the determination of metabolic structural pathways with the binary vectiors, hairy roots (transient system for initial screening) of Ashwagandha plants can be used for the expression of foreign genes or overexpression of endogenous genes for increasing withanolide production. Hairy roots could be valuable for producing withanolides in amounts sufficient for medicinal purposes and clinical trials. Since the integration of T-DNA is random, hairy roots may provide novel compounds, which are structurally diverse. Although hairy roots are versatile for different applications, they have the drawback to require maintenance in small culture flasks. The design of bioreactors for large-scale culture of hairy root systems will allow for the commercial production of withanolides of pharmacological interest. Additionally, the use of low concentration of elicitors can improve the efficiency of withanolide production. By optimising a series of parameters, cellular biomass and withanolide concentration can be increased in different developed in vitro cultures/systems. This requires a more detailed molecular understanding of withanolide biosynthesis and therefore the characterisation of the pathway genes for deciphering complete biosynthetic pathway through high-throughput metabolic profiling and sequencing. Further studies with regard to the withanolide/isoprenoid pathway-related genes of this plant would not only be useful in identifying such conjoint genes but also directly contribute to improve the biotechnological utilisation of this medicinal plant.
References
Adam KP, Zapp J (1998) Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry 48:953–959
Ahmad M, Douh A (2002) New withanolides and other constituents from the fruits of Withania somnifera. Pharm Med Chem 6:267
Ahuja A, Kaur D, Sharada M, Kumar A, Suri A, Dutt P (2009) Glycowithanolides accumulation in in vitro shoot cultures of Indian ginseng (Withania somnifera Dunal). Nat Prod Commun 4:479–482
Akhtar N, Gupta P, Sangwan NS, Sangwan RS, Trivedi PK (2013) Cloning and functional characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Withania somnifera: an important medicinal plant. Protoplasma. doi:10.1007/s00709-012-0450-2
Anjaneyulu ASR, Rao DS, Lequesne PW (1997) Withanolides, biologically active natural steroidal lactones: a review. Stud Nat Prod Chem 20:135–261
Asano N, Nash RJ, Molyneux RJ, Fleet GW (2000) Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetr 11:1645–1680
Baldi A, Singh D, Dixit VK (2008) Dual elicitation for improved production of withaferin A by cell suspension cultures of Withania somnifera. Appl Biochem Biotechnol 151:556–564
Bandopadhyay M, Jha S, Tepfer D (2007) Changes in morphological phenotypes and withanolide composition of Ri-transformed roots of Withania somnifera. Plant Cell Rep 26:599–609
Bargagna MP, Ravindranath PP, Mohan R (2006) Small molecule anti-angiogenic probes of the ubiquitin proteasome pathway: potential applications to choroidal neovascularization. Invest Ophthalmol Vis Sci 47:4138–4145
Bhat WW, Lattoo SK, Razdan S, Dhar N, Rana S, Dhar RS, Khan S, Vishwakarma RA (2012) Molecular cloning, bacterial expression and promoter analysis of squalene synthase from Withania somnifera (L.) Dunal. Gene 499:25–36
Bhattacharya SK, Bhattacharya D, Sairam K, Ghosal S (2002) Effect of Withania somnifera glycowithanolides on a rat model of tardive dyskinesia. Phytomedicine 9:167–170
Bouvier F, Rahier A, Camara B (2005) Biogenesis, molecular regulation and function of plant isoprenoids. Prog Lipid Res 44:357–429
Brock A, Herzfeld T, Paschke T, Koch M, Draeger B (2006) Brassicaceae contain nortropane alkaloids. Phytochemistry 67:2050–2057
Budhiraja RD, Krishan P, Sudhir S (2000) Biological activity of withanolides. J Sci Ind Res 59:33–54
Chappell J (1995) Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Physiol Plant Mol Biol 46:521–547
Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, Roy R, Khetrapal CL, Tuli R (2010) Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry 71:1085–1094
Chaturvedi P, Mishra M, Akhtar N, Gupta P, Mishra P, Tuli R (2012) Sterol glycosyltransferases–identification of members of gene family and their role in stress in Withania somnifera. Mol Biol Rep 39:9755–9764
Chaurasiya ND (2007) Studies on withanolides metabolism in ashwagandha (Withania somnifera). Ph.D. thesis, Kurukshetra University, Haryana
Chaurasiya ND, Gupta VK, Sangwan RS (2007) Leaf ontogenic phase related dynamics of withaferin A and withanone biogenesis in Ashwagandha (Withania somnifera) – an important medicinal herb. J Plant Biol 50:508–513
Chaurasiya ND, Uniyal GC, Lal P, Misra L, Sangwan NS, Tuli R, Sangwan RS (2008) Analysis of withanolides in root and leaf of Withania somnifera by HPLC with photo diode array and evaporative light scattering detection. Phytochem Anal 19:148–154
Chaurasiya ND, Sangwan RS, Misra LN, Tuli R, Sangwan NS (2009) Metabolic clustering of a core collection of Indian ginseng (Withania somnifera) through DNA, isoenzymes, polypeptide and withanolide profile diversity. Fitoterapia 80:496–505
Chaurasiya ND, Sangwan NS, Sabir F, Misra LN, Sangwan RS (2012) Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal). Plant Cell Rep. doi:10.1007/s00299-012-1302-4
Ciddi V (2006) Withaferin A from cell cultures of Withania somnifera. Indian J Pharm Sci 68:490–492
Cordell GA, Mary LQB, Farnsworth NR (2001) The potential of alkaloids in drug discovery. Phytother Res 15:183–205
Cordoba E, Salmi M, León P (2009) Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J Exp Bot 60:2933–2943
D’Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9:331–340
Davis L, Kuttan G (2002) Effect of Withania somnifera on CTL activity. J Exp Clin Cancer Res 21:115–118
Deb DB (1980) Enumeration. Synonymy and distribution of the Solanaceae in India. J Econ Tax Bot 1:33–54
De-Eknamkul W, Potduang B (2003) Biosynthesis of beta-sitosterol and stigmasterol in Croton sublyratus proceeds via a mixed origin of isoprene units. Phytochemistry 62:389–398
DeLuca V, St Pierre B (2000) The developmental and cell biology of alkaloid biosynthesis. Trend Plant Sci 5:168–173
Dewir YH, Chakrabarty D, Lee SH, Hahn EJ, Paek KY (2010) Indirect regeneration of Withania somnifera and comparative analysis of withanolides in in vitro and greenhouse grown plants. Biol Plant 54:357–360
Dhar RS, Verma V, Suri KA, Sangwan RS, Satti NK, Kumar A, Tuli R, Qazi GN (2006) Phytochemical and genetic analysis in selected chemotypes of Withania somnifera. Phytochemistry 67:2269–2276
Dhuley JN (2001) Nootropic like effect of ashwagandha (Withania somnifera L) in mice. Phytother Res 15:524–528
Drager B (2004) Chemistry and biology of calystegines. Nat Prod Rep 21:211–223
Drager B, Funck C, Hohler A, Mrachatz G, Nahrstedt A (1994) Calystegines as a new group of tropane alkaloids in Solanaceae. Plant Cell Tissue Organ Cult 38:235–240
Dudareva N, Andersson S, Orlova I, Gatto N, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102:933–938
Falsey RR, Marron MT, Gunaherath GM, Shirahatti N, Mahadevan D et al (2006) Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol 2:33–38
Fatima N, Anis M (2011) Thidiazuron induced high frequency axillary shoot multiplication in Withania somnifera L. Dunal. J Med Plant Res 5:6681–6687
Furmanowa M, Gajdzis–Kuls D, Ruszkowska J et al (2001) In vitro propagation of Withania somnifera and isolation of withanolides with immunosuppressive activity. Planta Med 67:146–149
Ghimire BK, Seong ES, Kim KH, Lamsal K, Yu CY, Chung M (2010) Direct shoot organogenesis from petiole and leaf discs of Withania somnifera (L.) Dunal. Afr J Biotechnol 9:7453–7461
Ghosal S, Lal J, Srivatava R, Battacharya R, Upadhyay SN, Jaiswal AK, Chattopadhyay U (1989) Anti-stress activity of sitoindosides IX and X, new C-27 glycowithanolides from Withania somnifera. Phytother Res 3:201–209
Glotter E (1991) Withanolides and related ergostane-type sterols. Nat Prod Rep 8:415–440
Glotter E, Abraham A, Gunzberg G, Kirson I (1977) Naturally occurring steroidal lactones with a 17 α–oriented side chain. Structure of withanolide E and related compounds. J Chem Soc 1:341–346
Gomez GS, Pelacho AM, Gene A (2007) The genetic manipulation of medicinal and aromatic plants. Plant Cell Rep 26:1689–1715
Grin B, Mahammad S, Wedig T, Megan MC, Tsai L, Harald H, Goldman RD (2012) Withaferin A alters intermediate filament organization, cell shape and behavior. PLoS One 7:e39065
Gross NJ (2004) Anticholinergic bronchodilatators. In: Page CP, Barnes PJ (eds) Pharmacology and therapeutics of asthma and COPD, vol 161, Handbook of experimental pharmacology. Springer, Berlin, pp 37–52
Grover A, Samuel G, Bisaria VS, Sundar D (2011) Non–nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti–herpetic mode of action of herbal drug withaferin A. BMC Bioinformatics 12:S13–S32
Grover A, Agarwal V, Shandilya A, Bisaria VS, Sundar D (2013) Enhanced withanolide production by overexpression of squalene synthase in Withania somnifera. J Biosci Bioeng. doi:10.1016/j.jbiosc.2012.12.011
Gupta P, Akhtar N, Tewari SK, Sangwan RS, Trivedi PK (2011) Differential expression of farnesyl diphosphate synthase gene from Withania somnifera in different chemotypes and in response to elicitors. Plant Growth Regul 65:93–100
Gupta N, Sharma P, Santosh KRJ, Vishwakarma RK, Khan BM (2012) Functional characterization and differential expression studies of squalene synthase from Withania somnifera. Mol Biol Rep 39:8803–8812
Gupta P, Akhtar N, Tewari SK, Sangwan RS, Trivedi PK (2013) Cloning and characterization of 2-C methyl farnesyl-δ-erythritol-4-phosphate pathway genes for isoprenoid biosynthesis from Indian ginseng, Withania somnifera. Protoplasma 250:285–295
Hampel D, Mosandl A, Wüst M (2005) Biosynthesis of mono– and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66:305–311
Hashimoto T, Nakajima K, Ongena G, Yamada Y (1992) Two tropinone reductase with distinct stereospecificities from cultured roots of Hyoscyamus niger. Plant Physiol 100:836–845
Heble MR (1985) Multiple shoot cultures: a viable alternative in vitro system for the production of known and new biologically active plant constituents. In: Neumann KH, Barz W, Reinhard E (eds) Primary and secondary metabolism of plant cell cultures. Springer, Berlin, pp 281–289
Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB (2006) Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB-regulated gene expression. Mol Cancer Ther 5:1434–1445
Ilayperuma I, Ratnasooriya WD, Weerasooriya TR (2002) Effect of Withania somnifera root extract on sexual behaviour of male rats. Asian J Androl 4:295–298
Iuvone T, Esposito G, Capasso F, Izzo A (2003) Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sci 72:1617–1625
Jadhav SK, Patel KA, Dholakia BB, Khan BM (2012) Structural characterization of a flavonoid glycosyltransferase from Withania somnifera. Bioinformation 8:943–949
Jain SK (1991) Dictionary of Indian folk medicine and ethnobotany: a reference manual of man–plant relationships, ethnic groups and ethnobotanists in India. Deep Publications, New Delhi, p 189
Jana CK, Hoecker J, Woods TM, Jessen HJ, Neuburger M, Gademann K (2011) Synthesis of withanolide A, biological evaluation of its neuritogenic properties, and studies on secretase inhibition. Angew Chem 50:8407–8411
Jayaprakasam B, Zhang Y, Seeram NP, Nair MG (2003) Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci 74:125–132
Jayaprakasam B, Strasburg GA, Nair MG (2004) Potent lipid peroxidation inhibitors from Withania somnifera fruits. Tetrahedron 60:3109–3121
Kai GY, Li L, Jiang YX, Yan XM, Zhang Y, Lu X, Liao P, Chen JB (2009) Molecular cloning, characterization of two tropinone reductases in Anisodus acutangulus and enhancement of tropane alkaloids production in AaTRI–transformed hairy roots. Biotechnol Appl Biochem 54:177–186
Kai GY, Yang S, Luo XQ, Zhou WT, Fu XQ, Zhang A, Zhang Y, Xiao JB (2011) Co-expression of AaPMT and AaTRI effectively enhances the yields of tropane alkaloids in Anisodus acutangulus hairy roots. BMC Biotechnol 11:43
Kaileh M, Berghe WV, Heyerick A, Horion J, Piette J, Libert C, De KD, Essawi T, Haegeman G (2007) Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem 282:4253–4264
Kaul MK, Kumar A, Ahuja A, Mir BA, Suri KA, Qazi GN (2009) Production dynamics of withaferin A in Withania somnifera Dunal complex. Nat Prod Res 23:1304–1311
Kaur R, Ahuja AK, Gupta BK (2004) Nutritional evaluation of fodder based total mixed ration. Indian J Anim Nutr 21:60–62
Keiner R, Kaiser H, Nakajima K, Hashimoto T, Dräger B (2002) Molecular cloning, expression and characterization of tropinone reductase II, an enzyme of the SDR family in Solanum tuberosum (L.). Plant Mol Biol 48:299–308
Khanna KL, Schwarting AE, Rother A, Bobbit JM (1961) Occurrence of tropine and pseudotropine in Withania somnifera. Lloydia 24:179–181
Khedgikar V, Kushwaha P, Gautam J, Verma A, Changkija B, Kumar A, Sharma S, Nagar GK, Singh D, Trivedi PK, Sangwan NS, Mishra PK, Trivedi R (2013) Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis. doi:10.1038/cddis.2013.294
Kinghorn AD, Su BN, Jang DS, Chang LC, Lee D, Gu J–Q, Carcanche BEJ, Pawlus AD, Lee SK, Park EJ, Cuendet M, Gills JJ, Bhat K, Park H–S, Mata GE, Song LL, Jang M, Pezzuto J (2004) Natural inhibitors of carcinogenesis. Plant Med 70:691–705
Kuboyama T, Tohda C, Komatsu K (2005) Neuritic regeneration and synaptic reconstruction induced by withanolide A. Brit J Pharmacol 144:961–971
Kumar J, Gupta P (2008) Molecular approaches for improvement of medicinal and aromatic plants. Plant Biotech Rep 2:93–112
Kumar V, Murthy KNC, Bhamidi S, Sudha CG, Ravishankar GA (2005) Genetically modified hairy roots of Withania somnifera Dunal: a potent source of rejuvenating principles. Rejuvenation Res 8:37–45
Kumar A, Mir BA, Sehgal D, Koul S, Dar TH, Maharaj KK, Soom NR, Qazi GN (2011) Utility of multidisciplinary approach for genome diagnostics of cultivated and wild germplasm resources of medicinal Withania somnifera, and status of new species, W. ashwagandha, in the cultivated taxon. Plant Syst Evol 291:141–151
Kundu AB, Mukherjee A, Dey AK (1976) A new withanolide from the seeds of Withania-somnifera. Ind J Chem 14:434–435
Kushwaha AK, Sangwan NS, Trivedi PK, Negi AS, Misra L, Sangwan RS (2013b) Tropine forming tropinone reductase gene from Withania somnifera (Ashwagandha): biochemical characteristics of the recombinant enzyme and novel physiological overtones of tissue-wide gene expression patterns. PLoS ONE 8(9):e74777. doi:10.1371/journal.pone.0074777
Kushwaha AK, Sangwan NS, Tripathi S, Sangwan RS (2013a) Molecular cloning and catalytic characterization of a recombinant tropine biosynthetic tropinone reductase from Withania coagulans leaf. Gene 516:238–247
Kushwaha S, Soni VK, Singh PK, Bano N, Kumar A, Sangwan RS, Misra–Bhattacharya S (2012) Withania somnifera chemotypes NMITLI 101R, NMITLI 118R, NMITLI 128R and withaferin A protect Mastomys coucha from Brugia malayi infection. Parasite Immunol 34:199–209
Lal P, Misra L, Sangwan RS, Tuli R (2006) New withanolides from fresh berries of Withania somnifera. Z Naturfors 61:1143–1147
Leete E (1990) Recent development in biosynthesis of the tropane alkaloids. Planta Med 56:339–352
Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Lockley WJS, Rees HH, Goodwin TW (1976) Biosynthesis of steroidal withanolides in Withania somnifera. Phytochemistry 15:937–939
Luvone T, Esposito G, Capasso F, Izzo A (2003) Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sci 72:1617–1625
Madina BR, Sharma LK, Chaturvedi P, Sangwan RS, Tuli R (2007a) Purification and characterization of a novel glucosyltransferase specific to 27b–hydroxy steroidal lactones from Withania somnifera and its role in stress responses. Biochim Biophys Acta 1774:1199–1207
Madina BR, Sharma LK, Chaturvedi P, Sangwan RS, Tuli R (2007b) Purification and physico–kinetic characterization of 3β-hydroxy specific sterol glucosyltransferase from Withania somnifera (L) and its stress response. Biochim Biophys Acta 1774:392–402
Malik F, Kumar A, Bhushan S, Khan S, Bhatia A et al (2007) Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL–60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 12:2115–2133
Mandal C, Dutta A, Mallick A, Chandra S, Misra L, Sangwan RS, Mandal C (2008) Withaferin A induces apoptosis by activating p38 mitogen–activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin in a transcription–dependent manner through mitochondrial death cascade. Apoptosis 13:1450–1464
Manickam VS, Mathavan RE, Antonisamy R (2000) Regeneration of Indian Ginseng plantlets from stem callus. Plant Cell Tissue Organ Cult 62:181–185
Mathur RS, Gupta SK et al (2006) Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J Ethnopharmacol 105:336–341
Mayola E, Gallerne C, Esposti DD et al (2011) Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down–regulation of Bcl-2. Apoptosis 16:1014–1027
Mishra S, Sangwan RS, Bansal S, Sangwan NS (2012) Efficient transgenic plant production of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture. Protoplasma. doi:10.1007/s00709-012-0428-0
Misra LN, Lal P, Sangwan RS, Sangwan NS, Uniyal GC, Tuli R (2005) Unusually sulfated and oxygenated steroids from Withania somnifera. Phytochemistry 66:2702–2707
Misra L, Lal P, Chaurasiya ND, Sangwan RS, Sinha S, Tuli R (2008a) Selective reactivity of 2-mercaptoethanol with 5β,6β-epoxide in steroids from Withania somnifera. Steroids 73:245–251
Misra LN, Mishra P, Pandey A, Sangwan RS, Sangwan NS, Tuli R (2008b) Withanolides from Withania somnifera roots. Phytochemistry 69:1000–1004
Misra LN, Misra P, Pandey A, Sangwan RS, Sangwan NS (2012) 1, 4 dioxane and ergosterol derivatives from Withania somnifera roots. J Asian Nat Prod Res 14:39–45
Mondal S, Mandal C, Sangwan RS, Chandra S, Mandal C (2010) Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. Mol Cancer 9:239
Mondal S, Bhattacharya K, Mallick A, Sangwan R, Mandal C (2012) Bak compensated for Bax in p53–null cells to release cytochrome c for the initiation of mitochondrial signaling during Withanolide D-induced apoptosis. PLoS One 7:e34277
Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR, Power JB, Hahn EJ, Paek KY (2008) Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J Int Plant Biol 50:975–981
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol 101:6735–6739
Nagella P, Murthy HN (2012) Synthesis of withanolide A depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind Crop Prod 35:241–243
Nakajima K, Hashimoto T, Yamada Y (1993) Plant Gene Register cDNA encoding tropinone reductase–ll from Hyoscyamus niger. Plant Physiol 103:1465–1466
Nayak SA, Kumar S, Satapathy K, Moharana A, Behera B (2013) In vitro plant regeneration from cotyledonary nodes of Withania somnifera (L.) Dunal and assessment of clonal fidelity using RAPD and ISSR markers. Acta Physiol Plant. doi:10.1007/s11738-012-1063-2
Newman DJ, Cragg GM, Snader KM (2003) Natural products as source of new drugs over the period. J Nat Prod 66:1022–1037
Nur–e–Alam M, Yousaf M, Qureshi S, Baig I, Nasim S (2003) A novel dimeric podophyllotoxin–type lignan and a new withanolide from Withania coagulans. Helv Chim Acta 86:607–614
Oh S, Park S, van Nocker S (2008) Genic and global functions for Paf1C in chromatin modification and gene expression in Arabidopsis. PLoS Genet 4:e100007
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of βglucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31:805–813
Ortholand JY, Ganesan A (2004) Natural products and combinatorial chemistry: back to the future. Curr Opin Chem Biol 8:271–280
Pandey V, Misra P, Chaturvedi P, Mishra MK, Trivedi PK, Tuli R (2010) Agrobacterium tumefaciens–mediated transformation of Withania somnifera (L.) Dunal: an important medicinal plant. Plant Cell Rep 29:133–141
Praveen N, Murthy HN (2013) Withanolide A production from Withania somnifera hairy root cultures with improved growth by altering the concentrations of macro elements and nitrogen source in the medium. Acta Physiol Plant 35:811–816
Priyandoko D, Ishii T, Kaul SC, Wadhwa R (2011) Ashwagandha leaf derived withanone protects normal human cells against the toxicity of methoxyacetic acid, a major industrial metabolite. PLoS One 6:e19552
Rahman AU, Jamal SA, Choudhary MI, Asif E (1993) New withanolides from Withania somnifera. J Nat Prod 56:1000–1006
Rana S, Dhar N, Bhat WW, Razdan S, Khan S, Dhar RS, Dutt P, Lattoo SK (2012) A 12 deoxy withastramonolide–rich somaclonal variant in Withania somnifera (L.) Dunal – molecular cytogenetic analysis and significance as a chemotypic resource. In Vitro Cell Dev Biol Plant 48:546–554
Rana S, Lattoo SK, Dhar N, Razdan S, Bhat WW (2013) NADPH-cytochrome P450 reductase: molecular cloning and functional characterization of two paralogs from Withania somnifera (L.) dunal. PLoS ONE 8(2):e57068. doi:10.1371/journal.pone.0057068
Rani G, Virk GS, Nagpal A (2003) Callus induction and plantlet regeneration in Withania somnifera (L.) Dunal. In Vitro Cell Dev Biol Plant 39:468–474
Rasool M, Marylatha L, Varalakshmi P (2000) Effect of Withania somnifera on lysosomal acid hydrolases in adjuvant-induced arthritis in rats. Pharma Pharmacol Commun 6:187–190
Ray AB, Gupta M (1994) Withasteroids, a growing group of naturally occurring steroidal lactone. Prog Chem Org Nat Prod 63:1–106
Ray S, Jha S (1999) Withanolide synthesis in cultures of Withania somnifera transformed with Agrobacterium tumefaciens. Plant Sci 146:1–7
Razdan S, Bhat WW, Rana S, Dhar N, Lattoo SK, Dhar RS, Vishwakarma RA (2013) Molecular characterization and promoter analysis of squalene epoxidase gene from Withania somnifera (L.) Dunal. Mol Biol Rep 40:905–916
Richter U, Rothe G, Fabian AK, Rahfeld B, Dräger B (2005) Overexpression of tropinone reductases alters alkaloid composition in Atropa belladonna root cultures. J Exp Bot 56:645–652
Rohmer M (2003) Mevalonate–independent methylerythritol phosphate pathway for isoprenoid biosynthesis elucidation and distribution. Pure Appl Chem 75:375–387
Roja G, Heble MR, Sipahimalini AT (1991) Tissue cultures of Withania somnifera: morphogenesis and withanolide synthesis. Phytother Res 5:185–187
Rout JR, Sahoo S, Das R (2011) An attempt to conserve Withania somnifera (l.) Dunal – a highly essential medicinal plant, through in vitro callus culture. Pak J Bot 43:1837–1842
Sabir F (2011) Metabolic and biochemical studies on in vitro raised tissue systems of medicinal plant Withania somnifera Dunal. Ph.D. thesis
Sabir F, Sangwan NS, Chaurasiya ND, Misra LN, Tuli R, Sangwan RS (2007) Rapid micropropagation of Withania somnifera L. accessions from axillary meristems. J Herb Spices Med Plants 13:123–133
Sabir F, Sangwan NS, Chaurasiya ND, Misra LN, Sangwan RS (2008) In vitro withanolide production by Withania somnifera L. Cultures. Z Naturforsch C 63:409–412
Sabir F, Kumar A, Tiwari P, Pathak N, Sangwan RS, Bhakuni RS, Sangwan NS (2010) Bioconversion of artemisinin to its non-peroxidic derivative deoxyartemisinin through suspension cultures of (Withania somnifera Dunal). Z Naturforsch C 65:607–612
Sabir F, Sangwan RS, Singh J, Misra L, Pathak N, Sangwan NS (2011) Biotransformation of withanolides by cell suspension cultures of (Withania somnifera Dunal). Plant Biotechnol Rep 5:127–134
Sabir F, Sangwan RS, Kumar R, Sangwan NS (2012) Salt stress induced responses in growth and metabolism in callus cultures and differentiating in vitro shoots of Indian Ginseng (Withania somnifera Dunal). J Plant Growth Reg 31:537–548
Sabir F, Mishra S, Sangwan RS, Jadaun JS, Sangwan NS (2013) Qualitative and quantitative variations in withanolides and expression of some pathway genes during different stages of morphogenesis in Withania somnifera Dunal. Protoplasma. doi:10.1007/s00709-012-0438-y
Sangwan NS, Farooqi AHA, Sabih F, Sangwan RS (2001) Regulation of essential oil production in plants. Plant Growth Regul 34:3–21
Sangwan RS, Chaurasiya ND, Misra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Qazi GN, Tuli R (2004a) Phytochemical variability in commercial herbal products and preparations of Withania somnifera (Ashwagandha). Curr Sci 86:461–465
Sangwan RS, Chaurasiya ND, Misra LN, Suri KA, Qazi GN, Tuli R, Lal P, Uniyal GC, Sangwan NS, Srivastava AK (2004b) Process for isolation of withaferin A from plant materials and products therefrom. US Patent 7,108,870, 2006
Sangwan RS, Chauraslya ND, Misra LN, Lal P, Uniyal GC, Sangwan NS (2005) An improved process for isolation of withaferin A from plant materials and products therefrom. US Patent 7108870
Sangwan RS, Chaurasiya ND, Lal P, Misra L, Uniyal GC, Tuli R, Sangwan NS (2007) Withanolide A biogeneration in in vitro shoot cultures of ashwagandha (Withania somnifera Dunal), a main medicinal plant in ayurveda. Chem Pharma Bull 55:1371–1375
Sangwan RS, Chaurasiya ND, Lal P, Misra L, Tuli R, Sangwan NS (2008) Withanolide A is inherently de novo biosynthesized in roots of the medicinal plant Ashwagandha (Withania somnifera). Physiol Plant 133:278–287
Sato Y, Ito Y, Okada S, Murakami M, Abe H (2003) Biosynthesis of the triterpenoids, botryococcenes and tetramethylsqualene in the B race of Botryococcus braunii via the non-mevalonate pathway. Tetrahed Lett 44:7035–7037
Schliebs R, Liebmann A, Bhattacharya SK, Kumar A, Ghosal S, Bigal V (1997) Systemic administration of defined extracts from Withania somnifera (Indian ginseng) and Shilajit differentially affect cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int 30:181–190
Sharada M, Ahuja A, Suri KA, Vij SP, Khajuria K, Verama V, Kumar A (2007) Withanolide production by in vitro cultures of Withania somnifera (L) Dunal and its association with differentiation. Biol Plant 51:161–164
Sharma LK, Madina BR, Chaturvedi P, Sangwan RS, Tuli R (2007) Molecular cloning and characterization of one member of 3 β–hydroxy sterol glucosyltransferase gene family in Withania somnifera. Arch Biochem Biophys 460:48–55
Siddique NA, Bari MA, Shahnewaz S, Rahman MH, Hasan MR, Khan MSI, Islam MS (2004) Plant regeneration of Withania somnifera (L.) Dunal (Ashwagandha) from nodal segments derived callus an endangered medicinal plant in Bangladesh. J Biol Sci 4:219–223
Sidhu OP, Annarao S, Chatterjee S, Tuli R, Roy R, Khetrapal CL (2011) Metabolic alterations of withania somnifera Dunal fruits at different developmental stages by NMR spectroscopy. Phytochem Anal 22:492–502
Singh AK, Varshney R, Sharma M, Agarwal SS, Bansal KC (2005) Regeneration of plants from alginate–encapsulated shoot tips of Withania somnifera (L.) Dunal, a medicinally important plant species. J Plant Physiol 163:220–223
Sivanadhan G, Arun M, Mayavan S, Rajesh M, Mariashibu TS, Manickavasagam SN, Ganapathi A (2012a) Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crop Prod 37:124–129
Sivanadhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Dev GK, Manickavasagam M, Selvaraj N, Ganapathi A (2012b) Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl Biochem Biotechnol 168:681–696
Sivanesan I (2007) Direct regeneration from apical buds explants of Withania somnifera Dunal. Ind J Biotechnol 16:125–127
Sorelle JA, Itoh T, Peng H, Kanak MA, Sugimoto K, Matsumoto S, Levy MF, Lawrence MC, Naziruddin B (2013) Withaferin A inhibits pro-inflammatory cytokine–induced damage to islets in culture and following transplantation. Diabetologia. doi:10.1007/s00125-012-2813-9
Stan SD, Zeng Y, Singh SV (2008) Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer 60(Suppl 1):51–60
Subbaraju GV, Vanisree M, Rao CV, Sivaramakrishna C, Sridhar P, Jayaprakasam B, Nair MG (2006) Ashwagandholide, a bioactive dimeric thiowithanolide isolated from the roots of Withania somnifera. J Nat Prod 69:1790–1792
Supe U, Dhote F, Roymon MG (2006) In vitro plant regeneration of Withania somnifera. Plant Tissue Cult Biotechnol 16:111–115
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep 26:2129–2136
Tuli R, Sangwan RS (2010) Ashwagandha (Withania somnifera) – a model Indian medicinal plant. Council of Scientific and Industrial Research (CSIR), Govt of India
Tursunova RN, Maslennikova VA, Abubakirov NK (1977) Withanolides in the vegetable kingdom. Chem Nat Comp 13:131–138
Van Klink J, Beckar H, Anderson S, Boland W (2003) Biosynthesis of anthecotuloide, an irregular sesquiterpene lactone from Anthemis cotula L. (Asteraceae) via a non-farnesyl diphosphate route. Org Biomol Chem 1:1503–1508
Vitali G, Conte L, Nicoletti M (1996) Withanolide composition and in vitro culture of Italian Withania somnifera. Planta Med 62:287–288
Wadegaonkar PA, Bhagwat KA, Rai MK (2005) Direct rhizogenesis and establishment of fast growing normal root organ culture of Withania somnifera Dunal. Plant Cell Tissue Organ Cult 84:223–225
Wang HC, Tsai YL, Wu YC, Chang FR, Liu MH et al (2012) Withanolides-induced breast cancer cell death is correlated with their ability to inhibit heat protein 90. PLoS One 7:e37764
Wasnik NG, Muthusamy M, Chellappan S, Vaidhyanathan V, Pulla R, Senthil K, Yang DC (2009) Establishment of in vitro root cultures and analysis of secondary metabolites in Indian ginseng – Withania somnifera. Korean J Plant Res 22:584–591
Yamada Y, Hashimoto T, Endo T, Yukimune Y, Cono J, Hamaguchi N, Drager B (1990) Biochemistry of alkaloid production in vitro. In: Charlwood RV, Rhodes MJC (eds) Secondary products from plant tissue culture. Oxford Science Publications, Oxford
Yang H, Shi G, Dou Q (2007) The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol 71:426–437
Yang ES, Choi MJ, Kim JH, Choi KS, Kwon TK (2011) Withaferin A enhances radiation–induced apoptosis in Caki cells through induction of reactive oxygen species, Bcl–2 downregulation and Akt inhibition. Chem Biol Interact 190:9–15
Yu PLC, El-Olemy MM, Stohs ST (1974) A phytochemical investigation of Withania somnifera tissue cultures. J Nat Prod 37:593–597
Zayed R, Wink M (2004) Induction of tropane alkaloid formation in transformed root cultures of Brugmansia suaveolens (Solanaceae). Z Naturfor 59:863–867
Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K (2002) Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull 50:760–765
Acknowledgements
The authors are thankful to Mr. Awadesh Srivastava and the scholars of the laboratory who have worked on Ashwagandha and contributed to various dissertations, thesis, and publications. They gratefully acknowledge New Millennium Indian Technology Leadership Initiative (NMITLI), New Delhi, and Department of Biotechnology (DBT), New Delhi (BT/PR10715/AGR/36/602/2008), for providing the financial grants to carry out various studies in author’s laboratory producing several observations cited in the article. They also gratefully acknowledge the constant encouragement and support provided by Director CSIR-CIMAP.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sangwan, N.S., Sangwan, R.S. (2014). Secondary Metabolites of Traditional Medical Plants: A Case Study of Ashwagandha (Withania somnifera). In: Nick, P., Opatrny, Z. (eds) Applied Plant Cell Biology. Plant Cell Monographs, vol 22. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-41787-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-41787-0_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-41786-3
Online ISBN: 978-3-642-41787-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)