Abstract
This paper and its companion (Colmer et al., 2014) review research on the adaptation of rice (Oryza sativa L.) to the wide range of semi-aquatic environments in which it grows. The paper considers well-regulated flooding to 5–20 cm depth; the companion considers deeper flooding in rainfed conditions. Flooded environments are dominated by the very slow diffusion of gases in water and the resulting changes in soil chemical and biological conditions. Adaptations to these potentially toxic conditions hinge on an optimum ventilation network in the plant, providing O2 to the roots and rhizosphere, both being critical for favourable nutrition and tolerance of reduced-soil toxins. Rice has become a model for studying adaptation to flooded soils and flood-prone environments because of its relatively simple genome and large genetic diversity, and its extreme tolerance of flooded soils compared with other crop species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The review focuses on the mechanisms of adaptation of rice (Oryza sativa L.) to wetland environments, where the slow diffusion of gases in water results in greatly altered plant growth conditions. These mechanisms have been studied for many decades by plant physiologists, biochemists, and biophysicists, as well as by soil chemists, biologists, and physicists. Concurrently, there have been intensive agronomic and genetic studies, and rice breeding. These streams are increasingly coming together, particularly with the application of molecular genetics and bioinformatics. The great genetic diversity and relatively simple genome of rice have made it well suited to research into the mechanisms of plant adaptation to flooded environments.
This endeavour has been stimulated by the role of rice as a staple food for millions across the globe. It is the only major food crop that is cultivated in a semi-aquatic environment. Under controlled flooding and optimal management, the potential grain yield of rice is equal to that of wheat (10–11 tons ha−1 in its main agro-ecological zones—Fisher and Edmeades 2010). Rice has become one of the best explored model systems for adaptation to flooded environments. Its genome contains a multitude of adaptive mechanisms which have evolved naturally and through intense human intervention.
1.1 Rice in Wetland Ecosystems
Flooded environments are dominated by the influence of Fick’s first law of diffusion: F i = −D i dC i/dx, where F i is the flux of gas or solute i in a medium in which it has concentration gradient dC i/dx and diffusion coefficient D i. Diffusion coefficients in water are 10,000 times smaller than those in air (Armstrong 1979). So rates of diffusion of respiratory gases into and out of flooded soils are extremely slow. Thus, the flooded environment is a ‘different world’ to that of the well-aerated soils in which all other food crops are grown.
The diverse ecology of wetlands used for agriculture has been reviewed by Verhoeven and Setter (2010). Here we focus on rice and as appropriate we include other species with particular tolerance mechanisms. Rice is grown in water depths ranging from 5 to 20 cm in irrigated or rainfed paddy fields, to 0.5 to over 5 m in flood-prone areas, with floods lasting weeks to months. All these environments impose very restricted gas exchange on the submerged parts of the plant and low to zero O2 concentrations in the surrounding soil.
Few plant species offer the opportunity to understand adaptation to low O2 environments. Rice has been a model for investigation because of its significance as a food source and the resulting extensive collection and documentation of germplasm that have taken place. Though most modern rices have a common ancestry, many thousands of landraces have been catalogued from a wide range of flooding regimes.
Rice shares its ability to thrive in inundated regions with a large number of other wetland plant species (Blom and Voesenek 1996). Other species of interest include Echinochloa (barnyard grass), whose subspecies include some of the few effective weeds of rice, possibly with superior ability to grow in anoxic soils (Kennedy et al. 1980; Ismail et al. 2012). Other wetland species that have been studied include Phragmites australis, which ventilates its rhizomes via pressure flow as well as by diffusion (Armstrong et al. 1992); some wild Oryza species are perennials, but it is not clear whether these have a similar ventilation system to Phragmites. Another intensively studied species is Rumex palustris, a dicotyledon native to European flood plains; different Rumex species flourish in locations of very different water regimes over short distances (Blom and Voesenek 1996). Others include various water weeds, the best researched of which are Potamogeton species which have turions that can survive for long periods in anoxic mud and develop shoots even under anoxia (Jackson and Ram 2003; Ishizawa et al. 1999). Responses of these species relevant to the present review are discussed in the appropriate sections. A more general review is given by Colmer and Voesenek (2009).
1.2 Early Research on Rice Physiology and Soil Chemistry
This short historical account covers both the present and its companion paper. Pioneering studies were on the ventilation essential to efficient root function, but also to oxygenation of the rhizosphere to facilitate nutrient uptake and to protect the roots from the toxic compounds in the anoxic soil. This included ingenious studies initiated during the Second World War by MH van Raalte in the Botanical Gardens of Bogor in Java (van Raalte 1940, 1944). His results stimulated subsequent intensive investigations by Armstrong and colleagues, in which experimental observations were combined with modelling of O2 diffusion from the shoots to the roots and to the rhizosphere (Armstrong 1979; Armstrong and Beckett 1987).
Tolerance to anaerobiosis was a second focus, with concentration on the ability of germinating rice seedlings to develop a coleoptile (acting as a snorkel), even during anoxia. Initially these studies were on mechanisms of extension growth (Kordan 1974) and then complemented by biochemical investigations (Bertani and Reggiani 1991; Perata and Alpi 1993; Ricard et al. 1994; Gibbs and Greenway 2003; Greenway and Gibbs 2003). Germinating rice became a productive model system to elucidate mechanism of anoxia tolerance in plants. Functions of the phytohormone ethylene were another research avenue (Kende et al. 1998; Colmer et al. 2013).
The other main strand of research was on soil conditions, with emphasis on redox reactions and their effects on nutrient availability and the concentrations of toxic compounds (reviewed by Ponnaperuma 1972; Kyuma 2003; Kirk 2004).
The advent of molecular biology and the sequencing of the genomes of both indica and japonica rice subspecies (Doi et al. 2008) has rejuvenated these earlier endeavours. A logical dovetailing of genomic research with physiology, agronomy, and plant breeding has occurred, integrating with molecular genetics techniques.
1.3 Outline of the Review
The two parts of this review concern the mechanisms of adaptation of rice to its large range of environments, with special reference to the water regimes. We discuss the intriguing features of rice adaptations to this hostile environment with emphasis on aeration, gas exchange, nutrient uptake and tolerance to toxins, and on the relevant biophysics, biochemistry and molecular biology. In the present paper, we describe the ventilation network and the chemistry and physics of flooded soils. The second paper (Colmer et al. 2014) concerns deeper floods and considers water quality, underwater gas exchange, and elongation in deep water and longevity during transient complete submergence. A third paper in preparation by some of the present authors will consider the metabolic mechanisms of anoxia tolerance, for which germinating rice, particularly the coleoptile, has become an excellent model.
2 Characteristics of Rice Ecosystems
2.1 Geography
The general features of rice flooding regimes depend on the regional and local climate, and the soils and landforms, but modified to a great extent by artificial levelling and bunding of individual fields, and irrigation (Moormann and van Breemen 1978; Richardson and Vepraskas 2001). The main landforms are inland valleys in upland areas, alluvial fans and terraces in foothills, active floodplains in river basins, and coastal floodplains. There are three distinct types of hydrology in these landforms, depending on the position in the landscape: fluxial lands, in which water arrives wholly or in part from surface flow, such as in runoff or streams; phreatic, in which water arrives from groundwater that rises to the soil surface for at least part of the year; and pluvial, in which water arrives entirely from rainfall. In fluxial lands, water flowing in from neighbouring upland and upper catchments brings with it sediment and nutrients which are only slowly lost to deepwater areas downslope. Because of the net inflow of nutrients, the abundance of water, and beneficial changes in the soil resulting from chemical reduction under anoxia (Sect. 3.2), fluxial wetlands are among the most productive ecosystems on Earth. By contrast pluvial wetlands rely on nutrients brought in by rainfall or fixed biologically from the atmosphere, and they therefore tend to be much less productive. Phreatic wetlands are intermediate.
2.2 Water Regimes
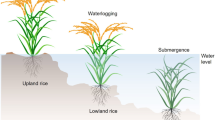
The terminology most widely used to define rice ecosystems is based on water regime (deficit, excess, or optimum), drainage (poor or good), topography (flat or undulating), and soil characteristics (with or without problems) (IRRI 1982). Four major categories are distinguished as illustrated in Fig. 1.
Characteristics of rice ecosystems (IRRI 1993). Rice ecosystems are characterised by water and land resources. Irrigated rice can occur at any point in the toposequence; the others occur as shown. See text for definitions. Reproduced by permission of IRRI
2.2.1 Irrigated
Roughly 55 % of the harvested rice area is irrigated, producing 75 % of the world’s rice (IRRI 2002). Irrigated rice is grown in levelled, bunded fields with good water control. The crop is transplanted or direct seeded in puddled soil, and shallow floodwater (5–20 cm deep) is maintained on the soil surface so that the soil is predominantly anoxic during the rice growing season. One or more crops are grown each year. In irrigated wet-season rice, water may be added as a supplement to rainfall, generally early in the season or during mid-season dry periods. In dry-season rice, crops cannot be grown without irrigation. Cloud cover is minimal and therefore solar radiation and hence yield potentials are greater than for wet-season rice.
2.2.2 Rainfed Lowland
Rainfed lowlands account for roughly 35 % of the harvested rice area globally but less than 20 % of production (IRRI 2002). The rice is grown in level to gently sloping, bunded fields that are flooded for at least part of the cropping season. Water depths may exceed 20 cm. The crop is transplanted in puddled soil or direct seeded on puddled or ploughed dry soil. The water supply is poorly controlled, with the soil alternating between aerated and anoxic conditions, and both transient submergence of the plants and drought may occur in the same season, requiring different varieties and management strategies to irrigated systems.
2.2.3 Flood-Prone
Flood-prone areas account for less than 5 % of the global harvested rice area (IRRI 2002), but where they occur they may be vital for the local economy. They are distinguished from rainfed lowlands by the depth (>50 cm) and duration (between a few days and 3 weeks) of complete submergence in flash-flood areas, to deep partial flooding in stagnant and deepwater areas, or by soil problems related to flooding (e.g. salinity, sodicity, acid sulphate, peat soil). Fields are flooded to at least 50 cm depth and often much more for periods from weeks to months. Varieties must be adapted to flash floods where the water rises rapidly but drains within 2 weeks, or to more gradual but prolonged flooding to depths exceeding 100 cm, requiring the plant to elongate to reach the surface (deepwater/’floating’ rice of over 5 m long is also represented here).
2.2.4 Upland
Upland rice accounts for only a few per cent of global production (IRRI 2002). It is grown in level to steeply sloping fields that are rarely flooded or waterlogged. No effort is made to impound water as for other rice ecosystems and the crop is direct seeded on ploughed dry soil or dibbled in wet, non-puddled soil.
2.3 Genetic Diversity of Rice
The genus Oryza originated about 130 million years ago from a common ancestor followed by introgressions of wild marsh grasses into modern rice (Khush 1997) domesticated around 9,000 years ago in Asia (Molina et al. 2011). Two distinct subspecies of O. sativa, japonica and indica, arose from independent populations more than 100,000 years ago (Sweeney and McCouch 2007), followed by a series of introgression, selection, and diversification events that occurred naturally before human intervention. Subsequent changes to the genetic composition of O. sativa have been enhanced by human activity (Vaughan et al. 2005) to produce some half a million identifiable landraces and commercial varieties of O. sativa, of which about 20 % are curated at the International Rice Research Institute (Sackville-Hamilton 2006). The contribution of continuing introgression to rice evolution has been argued (Ge and Sang 2007), but regardless of the accepted model, rice remains a dynamic species whose genetic improvement will accelerate through biotechnology (Hirochika et al. 2004; Jung et al. 2008). Within O. sativa, vast genetic potential remains to be exploited (Xu et al. 2012).
Relatively few rice genotypes have been investigated at the gene level, despite the japonica genome having been mapped more than a decade ago and in spite of intensive genomics addressing key evolutionary questions (Ge and Sang 2007). A seminal study of individual genes including a shattering gene and a pericarp colour gene has been used to establish domestication of O. sativa from various progenitors that include O. rufipogon and O. nivara (Sweeney and McCouch 2007). With this significant speciation, we speculate that many genes that confer tolerance to flooding regimes remain in modern rice cultivars. Even though at most 20 % of genetic diversity in the genus Oryza is found within the two subspecies of O. sativa (Zhu et al. 2007), enormous potential for tolerance to flooding regimes exists, as demonstrated in this review. This is particularly the case because of the diverse flooded habitats to which most rice species are habituated.
In addition to O. sativa and O. glaberrima, which have been domesticated for thousands of years in Asia and Africa, respectively, there are more than 20 wild Oryza species that remain largely unexploited. Six species share the AA genome with the two domesticated rice species and are therefore candidates for genetic improvement of cultivated rice (Sweeney and McCouch 2007; Doi et al. 2008; McNally et al. 2009). Reciprocal crosses reveal a cytoplasmic contribution to fertility that will need to be accommodated if wild germplasm is to enrich domesticated rice, with O. nivara the most compatible species with O. sativa (Naredo et al. 1997). Species such as O. rufipogon which grow as perennial weeds in wetlands are potential sources of flood tolerance genes, especially for deepwater conditions. Crosses between O. sativa and O. rufipogon demonstrate the potential for the wild relative as a donor of yield-related genes (Septiningsih et al. 2003; Sabu et al. 2009); this heritability gives hope for transfer of flood tolerance genes from the O. rufipogon gene pool.
Identification and transfer of genes conferring flood tolerance from the wild Oryza species to domesticated rice will, however, require exhaustive genomics approaches and cytogenetic techniques to facilitate introgressions of small chromosome regions containing critical genes or target QTLs (Doi et al. 2008). The search for useful alleles will accelerate as collections of wild rice relatives are screened and reproductive barriers are surmounted (Doi et al. 2008). Rapid genetic progress will specifically come from a deeper understanding of transcriptional control, as demonstrated with the Sub1A gene (Xu et al. 2006). Mazzucotelli et al. (2008) point out the importance of transcriptional regulation in developing genotypes that can tolerate thermal stresses and drought. Liu et al. (2010) report a number of anaerobically responsive motifs in O. sativa that are candidates for transcriptional control of flood tolerance genes. As critical QTLs for flood tolerance and the identities of key alleles are identified in O. sativa, tolerance genes will also be revealed in wild relatives, particularly as genome sequences become public. Specifically, improved tolerance of domesticated Oryza species to contrasting flooding regimes will require consideration of an array of gene × environment interactions. There will also be benefits for flood tolerance in more susceptible cereals because of the synteny that allows targeting of genes in related grass genomes (Xu et al. 2012).
3 Characteristics of Flooded Environments
3.1 Impaired Gas Transport
In all rice ecosystems, except upland, there is standing water on the soil surface for most of the growing season (Fig. 2). In irrigated rice, the water depths range from 5 to 20 cm. In rainfed rice much greater depths occur during flooding, and sometimes these floods last several months, in which case deepwater rices are grown. The concentrations of dissolved gases in the floodwater depend on the growth and respiration of photosynthetic algae, aquatic weeds, and non-photosynthetic organisms in the water (Roger 1996). Roger (1996) found that in irrigated rice fields with 15 cm water depth photosynthesis can lead to O2 concentrations of 0.31–0.62 mM (34–68 kPa), while at night, dissolved O2 concentration can fall to near zero. Opposite diurnal cycles occur for CO2, with associated changes in pH, which increases when CO2 decreases and vice versa. For example, Mikkelsen et al. (1978) found the floodwater pH in an irrigated rice field rose as high as 10 during the day as CO2 was removed but fell by 2 or 3 pH units at night.
Schematic profile of a flooded rice field (Kirk 2004)
In the soil beneath the floodwater, low rates of diffusion and the absence of turbulence cause O2 concentrations to drop to zero within a few hours of flooding as O2 is consumed by plant tissues and microorganisms (Ponnaperuma 1972). Anaerobic soil microbes then use alternative electron acceptors in their respiration. Dissolved CO2 formed in the respiration of these organisms escapes only slowly, so it tends to accumulate. Ponnaperuma (1972) reported CO2 pressures of 18–38 kPa in flooded soils without plants at 3 weeks after the start of flooding. There are also increases in ethylene: for example Trought and Drew (1980) found ethylene reached 0.4 Pa in a waterlogged wheat soil and Setter et al. (1988) found ethylene reached 2.5–4.5 Pa in soils in deepwater rice fields.
3.2 Changes in the Soil Following Flooding
3.2.1 Soil Physical Changes
Following flooding, air trapped in soil pores becomes compressed and further compression develops as volatile products of organic matter decomposition accumulate in the pores and as 2:1 type clays swell (Greenland 1997). As a result, large soil aggregates tend to rupture, and further rupture occurs as organic matter and oxide coatings that bind aggregates dissolve. The soil permeability declines as disaggregation proceeds and as pores become clogged with dispersed clay and other debris.
The practice of puddling the soil during land preparation for rice causes further disaggregation. The aim of puddling is to reduce losses of water through percolation, both to conserve water and to control weeds, and to facilitate transplanting. Puddling results in near complete destruction of water-stable aggregates and dispersion of fine clay particles. Some flow-through of water should be maintained so that the soil does not become entirely anoxic and toxins are washed out. Also, if the structure is completely destroyed the soil will dry only very slowly following the rice crop, and this will delay the establishment of a following dryland crop. Puddling decreases percolation rates by up to three orders of magnitude. It generally leads to an increase in total porosity because the destruction of aggregates decreases intra-aggregate pores but increases inter-aggregate and inter-domain pores. In the absence of puddling, the flooding of intra-aggregate pores results in large volumes of anaerobic conditions within aggregates, while near atmospheric O2 concentrations occur between the aggregates when the soil drains.
Repeated working of the soil for rice often results in permanent physical changes that mask the soil’s original character. Gross changes are caused by levelling, terracing, and puddling of the soil. Over time a ‘traffic’ pan of compacted soil often develops, 5–10 cm thick at 10–40 cm depth. This has a greater bulk density and is less permeable than the overlying surface soil, but has similar texture. Over time, the surface soil often becomes more coarse-textured, possibly because of weathering of clay under alternating flooding and drainage (Moormann and van Breemen 1978). Clay may also be lost from the surface during puddling by movement downslope with surface water. But equally clay may be added from upslope.
3.2.2 Soil Chemical and Biological Changes
Because O2 is excluded by submergence, soil organisms must use other soil constituents as electron acceptors (oxidising agents) in deriving energy from the oxidation of organic matter. This typically occurs in the sequence (see Kirk 2004 for a full discussion): NO3 − to N2, Mn(IV) manganese to Mn(II), Fe(III) iron to Fe(II), and then SO4 2− to S2−. Subsequently organic matter is oxidised by methanogenic bacteria to CO2 and CH4. This sequence is predicted by thermodynamics.
Concomitant with these redox reactions, there are important changes in the soil pH and alkalinity, and in the concentration of CO2 dissolved in the soil solution. Protons are consumed in the various reduction reactions and the solution pH therefore tends to increase. Initially the pH may decrease following submergence because CO2 formed in aerobic respiration escapes only very slowly, and it therefore accumulates. As CO2 continues to accumulate during anaerobic respiration and fermentation, large partial pressures develop, typically in the range 5–20 kPa (Ponnamperuma 1972). The accumulation of CO2 lowers the pH of alkaline soils and curbs the increase in pH of acid soils. As a result the pHs of all soils tend to converge following submergence in the range 6.5–7 (Ponnamperuma 1972).
As the partial pressure of CO2 increases, the concentration of HCO3 − in the soil solution increases and therefore the concentration of balancing cations in solution increases. The Mn2+ and especially Fe2+ ions formed in soil reduction displace exchangeable cations into solution. Also, the changes in pH cause changes in the charges of variable-charge clays and organic matter; thus the cation exchange capacity of acid soils tends to increase and that of alkaline soils tends to decrease.
The floodwater standing on the soil surface is usually sufficiently shallow, well mixed by wind and thermal gradients, and oxygenated by photosynthetic organisms that it is essentially aerobic (Roger 1996). However transport of O2 into the underlying soil is too slow for more than a thin layer to be aerobic (Bouldin 1968; Patrick and Delaune 1972). In this aerobic layer the concentrations of reduced species are negligible, and CO2 is the main end product of microbial respiration. In the underlying anaerobic soil, only a few millimetres away, the concentrations of Fe2+ and the various organic products of anaerobic respiration can be very large. Thus conditions change dramatically over a very short distance.
The most visible change associated with these processes is the reduction of the red and brown compounds of Fe(III) iron to blue-grey compounds of Fe(II) iron. Subsequent translocation of soluble Fe2+ to zones where O2 enters the soil—such as at the soil surface or near plant roots—produces reddish-brown mottles of insoluble Fe(III) oxides. Likewise there may be movement and re-oxidation of Mn(II) forming black Mn(IV) compounds.
Though there are exceptions—notably acid sulphate soils—a feature of flooded rice soils is that they tend not to become acidic after continuous cultivation (Kirk 2004). In upland soils, acidification occurs chiefly because of the leaching of NO3 − accompanied by base cations. But in general, little or no NO3 − is leached from flooded soils, any NO3 − entering the soil or formed in it being either absorbed by the crop or denitrified, so this process does not occur. Further, the anaerobic processes causing pH changes following flooding are reversed upon drainage and re-oxidation of the soil. Thus, unless there has been substantial movement of acid or base out of the soil during flooding, as generally there will not have been, the pH changes are reversed.
Nor do rice fields tend to become saline, except in arid or semi-arid areas (Greenland 1997). In land that is flooded for part of the year but drains naturally after the floodwater recedes, accumulated salt is removed with the draining water and there is a natural renewal of the land. Percolation and lateral drainage at the start of the following rainy season, but before the land is re-flooded, also wash out accumulated salt, as in coastal areas.
4 Root Aeration
4.1 Root Anatomy
4.1.1 Aerenchyma and Porosity
Growth of vascular plants in flooded soils requires an efficient internal aeration system to deliver O2 to submerged organs, with the additional benefit of venting CO2 produced in the roots and soil to the atmosphere. Rice and other wetland plants contain extensive longitudinally interconnected gas-filled channels—aerenchyma—which provide a low-resistance pathway for gas-phase diffusion between shoots and roots (Barber et al. 1961; Armstrong 1979). Figure 3 shows aerenchyma in different root tissues of rice.
Cross sections of primary rice roots (Butterbach-Bahl et al. 2000). (a) Radial section close to tip showing intercellular spaces (I), central cylinder (CC), and rhizodermis (RH). (b, c) Radial sections of younger (39 days) and older (72 days) basal parts showing exodermis (E), sclerenchymatous cylinder (SC), parenchyma of cortical cells (P), and aerenchyma (AE). Reproduced by permission of the authors
The aeration network is mainly based on aerenchyma, though in certain parts of the root system lacking lacunae, there may be regions with relatively high porosity due to cubic packing of cells; for example, between the end of lacunae and root apices, and in root laterals (Justin and Armstrong 1987). Maximum porosity with cubic packing if adjacent cells are perfectly spherical and only in ‘point contact’ is 21.5 %, compared to 9 % for hexagonal packing (Justin and Armstrong 1987). In practice these values are smaller in real root tissues because neighbouring cells have much more than just point contact. An example is given by Armstrong (1971) who found the porosity of the 1–2 cm zone behind the apex in rice roots grown in drained soil was 9 %, the roots not having aerenchyma and all the porosity being due to extracellular spaces between the cubically packed cells.
In a study of 41 wetland species in flooded compost, rice roots (cv. Norin 36) were eighth most porous (Justin and Armstrong 1987). At 35 % porosity rice was similar to other well-known wetland species such as Spartina anglica and Juncus effusus, but less porous than Phragmites australis (52 %) and Juncus inflexus (52 %). Porosity in the basal parts of rice roots reached approximately 45 % whereas values were approximately 10 % at 1 cm behind the apex where large aerenchyma channels are absent, but the cubical arrangement of cortical cells provides gas-filled spaces (Armstrong 1971). Moreover, rice genotypes differ in root porosity; Colmer (2003a) found a range of 29–41 % in 12 genotypes grown in stagnant deoxygenated agar. Armstrong (1971) found root porosity of rice was approximately 20 % in drained soil but 43 % in waterlogged soil. Similar findings were obtained for rice in aerated nutrient solution and in stagnant agar (Colmer 2003a). High porosity within roots in flooded anoxic soil is important because root growth depends on internal diffusion of O2 to the apex (Armstrong and Webb 1985). The capacity for this supply determines maximum rooting depth and together with a barrier in the outer cell layers of roots (Sect. 4.1.2) influences radial O2 loss and rhizosphere biogeochemical processes (Sect. 6).
Root aerenchyma are connected to lacunae at the shoot base, through which O2 enters from the atmosphere above. In a study in which porosity was measured in leaves, sheath bases, and roots of rice (cv. Calrose), Colmer and Pedersen (2008) found porosity was 26 ± 2 % (v/v) in roots, 40 ± 4 % in sheaths (basal 100 mm), and 29 ± 2 % in leaf blades including the mid-rib, with the largest lacunae in leaf blades being in the mid-rib. The importance of the shoot as the O2 source for the roots in an O2-free medium was demonstrated in experiments in which O2 was manipulated (either exogenously by gas mixtures or endogenously via light/dark periods) and dynamic tissue O2 concentrations monitored using O2 micro-electrodes and root-sleeving electrodes. A low-resistance pathway for O2 movement was present from leaf mid-rib to sheath base to roots (Colmer and Pedersen 2008). Oxygen movement along submerged shoots can also occur within surface gas films (see Colmer et al. 2013). For rice in shallow water, the very high O2 concentrations in the floodwater (0.31–0.62 mM—Sect. 3.1) may increase the O2 in the adjoining shoot tissue well above ambient, in which case the source of O2 for diffusion into the roots would be greater than ambient.
Rice genotypes also differ in the extent of shoot aerenchyma (e.g. sheaths, Parlanti et al. 2011; stems, Steffens et al. 2011) and there are differences between growth stages and development patterns. Some aerenchyma formation in shoots is ‘constitutive’, as it is in roots (Steffens et al. 2011). Ethylene signalling has been implicated in the flood-induced enhancement of aerenchyma formation in both root (Justin and Armstrong 1991; Colmer et al. 2006) and shoot tissues, with involvement also of H2O2 in the programmed cell death that forms the lacunae in shoots (Parlanti et al. 2011; Steffens et al. 2011).
That transport of O2 within aerenchyma is by diffusion was first demonstrated by van Raalte (1940), who measured concentration profiles of O2 from rice shoots to roots in semi-stagnant solution using micro-manometers. Oxygen concentrations (means of two rice cultivars) were 12.5 % near the base of the root, 6.6 % in the middle, and 4 % at the tip. Subsequently, Armstrong and co-workers have elucidated the processes of internal aeration in plant roots by use of mathematical models integrated with experimental data on axial and radial O2 profiles in maize roots and the rhizosphere, obtained with root-sleeving electrodes and micro-electrodes (Armstrong 1979; Armstrong and Beckett 1987; Gibbs et al. 1998; Darwent et al. 2003). These studies demonstrate the importance of aerenchyma in providing a low-resistance pathway for diffusion of O2 and other gases within the roots.
In addition to their large gas-filled porosity, roots of rice also contain a barrier to radial O2 loss (ROL) in the basal zones (Sect. 4.1.2). The barrier restricts O2 loss from the root, and thereby permits a greater length of root to be aerated by diffusion, resulting in a higher apical O2 concentration and an aerobic rhizosphere around the root tip (Armstrong 1979). In contrast to the data available on internal aeration and anoxia tolerance in rice, there is little information on metabolism as related to spatial and temporal differences in O2 supply. In spite of the ventilation system, the various root tissues will experience a range of O2 concentrations, as a function of their position along the diffusion path, and the external sink for O2.
4.1.2 The Barrier to O2 Loss
The barrier to radial O2 loss (ROL) from the root is located in the outer cell layers of the main axis of the roots. This has been shown for various wetland plants (Armstrong 1979; Colmer 2003a) and rice (Armstrong 1971; Colmer 2003b; Shiono et al. 2011). In the relatively few rice varieties investigated so far, the barrier starts at 1–1.5 cm behind the root tip and has a very low permeability to O2; ROL from the main axis in the basal regions was more than two orders of magnitude less than at the root tip—despite the higher expected internal concentrations in the basal zones (Armstrong 1971; Colmer 2003b). In roots of rice, the barrier to ROL is inducible, forming in stagnant or waterlogged conditions, but not (or only weakly) in aerated conditions (Colmer et al. 1998, 2006; Colmer 2003a; Shiono et al. 2011). Many wetland species have a ROL barrier in their roots; some species constitutively form such ROL barriers even in aerated conditions whereas in others it is induced by growth conditions (Colmer 2003b), but there are also notable exceptions such as Rumex palustris. The ROL barrier in R. palustris is rather weak by comparison with rice, as shown by the similar rates of ROL between root base and root tip (Visser et al. 2000). With no barrier at all, diffusion models predict a hyperbolic decrease in O2 concentration within the aerenchyma towards the root tip, and a similar profile for ROL to the medium (Armstrong 1979).
One advantage of the barrier to ROL is the potential for a greater rooting depth (Armstrong 1979). The importance of the barrier becomes less as root porosity increases, unless roots are in highly reduced substrates, which contain large concentrations of organic or inorganic toxins produced by anaerobic bacteria. Having longer roots presumably enables exploration of a greater volume of soil from which nutrients can be absorbed. However, the acclimative advantage of having a barrier to ROL may depend on soil type, with the greatest importance presumably being in highly reducing soils (Sect. 5.4). In a study with 12 genotypes of rice in stagnant agar, the barrier did not form in two of the seven upland genotypes included in the study (Colmer 2003a). So, genotypic variation for this trait within rice should allow further study of the importance of the barrier in soils with different redox potentials.
4.2 The Root O2 Budget
4.2.1 Oxygen Loss from Lateral Roots
Short, fine lateral roots account for a large proportion of the absorbing root surface in rice (Matsuo and Hoshikawa 1993) and these roots leak more O2 on a per plant basis than the adjacent primary root. Contributory factors are that these laterals have no impermeable wall layers (except under exceptional circumstances—Armstrong and Armstrong 2005) and they have a large surface area to volume ratio (Armstrong et al. 1990; Kirk 2003). Evidence for preferential loss of O2 from laterals includes measurements of Fe oxide coatings on roots placed in deoxygenated agar containing Fe(II) (Trolldenier 1988); changes in redox potential as roots grew across rows of platinum electrodes in anaerobic soil (Flessa and Fischer 1993); and the abundance of methane oxidising bacteria, which are obligate aerobes, along rice lateral roots in anaerobic soil (Gilbert et al. 1998).
Kirk (2003) developed a simple model of root aeration in rice in relation to root architecture and concluded that the basic architecture of current rice genotypes provides the best compromise between the need for internal aeration and the need for the largest possible absorbing surface per unit root mass. This architecture consists of coarse, aerenchymatous primary roots with gas-impermeable walls conducting O2 to short, fine, gas-permeable laterals.
As in rice, some other wetland species develop prolific laterals near the root base (i.e. just below the shoot–root junction) which conduct substantial amounts of O2 to the waterlogged soil. In Phragmites australis, for example, these basal laterals conduct 70–100 times more O2 to the soil than the main axes (Armstrong et al. 1990). In Phragmites, redox potentials near the root were between 200 and 600 mV, while the bulk soil was −280 mV (Armstrong et al. 1990). The O2 movement to the rhizosphere from these shallow laterals, emerging from near the root base, as opposed to deeper laterals, would mitigate drops in the O2 gradient within the aerenchyma of the main axis, in view of the short distance between the O2 source and the soil sink (Armstrong 1979). Hence substantial amounts of O2 can be released to the soil without compromising aeration of deeper root portions. Nonetheless, in an emergent aquatic plant, Eleocharis sphacelata, models assuming a 30 cm long root and 6 kPa at the root–rhizome junction indicated that ROL from laterals could lower cortical O2 concentrations at 10 cm from the rhizome–root junction from approximately 5.5 down to 1.5 kPa (Sorrell 1994).
Experiments in sterile versus non-sterile media show that the degree of oxygenation can vary substantially with the presence of bacteria: Hojberg and Sorensen (1993) found the O2 concentration at the surface of a barley root was 9 kPa in an otherwise O2-free sterile gel medium but only 2–4 kPa when non-sterilised soil extract had been added to this medium. In this latter medium, the rhizosphere respired 30–60 times faster than the bulk medium (Hojberg and Sorensen 1993).
4.2.2 Oxygen Consumption in Root Tissues
A long-standing question is the effect of different volumes of aerenchyma on the relative apportioning of O2 to the root tissues and the rhizosphere. One factor determining O2 concentration in the root tip is respiratory consumption by the root tissue along the pathway. It has been suggested that degradation of the cortex to form aerenchyma would not only decrease the physical resistance for gas-phase diffusion but also have the added benefit of reducing the amount of respiratory tissue (Williams and Barber 1961). However, using an electrical analogue and data for wetland and non-wetland plant roots, Armstrong (1979) concluded that the role in the decreased diffusional resistance for O2 provision via the aerenchyma was dominant.
Armstrong (1979) found that the length of non-wetland roots reached 6 cm and that of rice roots 10 cm under his standard conditions. The O2 concentrations at 6 cm from the root–shoot junction in rice were 15.2 kPa, decreasing to 12.0 kPa when the analogue assumed that both respiratory demand and radial O2 loss were as high as in a dryland-type root. By contrast, for a non-aerenchymatous dryland-type root, O2 at 6 cm from the root–shoot junction only increased from 2.3 to 5 kPa when the model assumed low radial O2 loss and a typical respiratory demand for a wetland plant, but with no aerenchyma.
Armstrong and Beckett (1987) extended the modelling of O2 transport and consumption within roots to include differences between tissue cylinders in the radial direction. This indicated that the various tissues across roots differ in O2 availability, and these differences become substantial in roots receiving a limited O2 supply, particularly in the most distal parts of the roots. These ‘multi-shell models’ predict energy deficits developing in two crucial tissues, which both have a very low porosity and a high metabolic activity. These tissues are the root tip, which is at the end of the longitudinal path of O2 from the shoot base, and the stele which, at least in maize, may display anaerobic catabolism even earlier than the root tip (Armstrong and Beckett 1987). These observations led to the hypothesis that, in an anoxic stele, loading of nutrients into the xylem is inhibited, leading to a substantial decrease in nutrient flow to the shoots (Gibbs et al. 1998; Colmer and Greenway 2011). For a vigorously growing crop like rice, such inhibition of nutrient transport to the shoots might be quite detrimental (but see Sect. 5.2).
4.2.3 Mechanism of Gas Movement: Diffusion or Pressure-Driven Mass Flow?
Some wetland species have an aeration potential far in excess of that provided by diffusion: bulk through-flows of “air”, described by Dacey (1980) as ‘internal winds’, are driven by real pressure differences from emergent living shoots/leaves/leaf sheaths into submerged rhizomes and the ‘exhaust’ gases escape from other emergent parts (Armstrong et al. 1991, 1992; Brix et al. 1992). Although Oryza sativa does not have rhizomes, the tillers are connected and it is conceivable that bulk gas flows could be generated between tillers having differential potentials for pressure generation. However, there is no evidence for this and in the following three truly rhizomatous Oryza species, no pressurisation or through-flow has been detected (Armstrong and Armstrong, unpublished data): O. rhizomatous (from Sri Lanka; IRGC Accession no. 105448), O. australiensis (IRGC Accession no. 103303), and O. longistaminata (from West Africa). It should be tested whether there is through-flow in any of the other wild Oryza species that possess true rhizomes.
An important controversy has been whether in rice there is a substantial contribution of mass flow to O2 provision from the atmosphere via gas films on the submerged parts of the shoots (suggested by Raskin and Kende (1983), based on short-term observations). However, mass flow along partially submerged rice shoots will not substantially enhance total O2 supply, because in the absence of a through-flow pathway, such mass flow merely replaces rather than augments flow by diffusion (Beckett et al. 1988).
4.2.4 Testing Models of Root Aeration
Experimental support for models of root aeration is equivocal. Predicted rooting depths in anoxic media as determined by gas-filled porosity influencing O2 movement to root tips are broadly in agreement with experimental data. For example, Thomson et al. (1992) found good agreement of actual root lengths with those predicted by Armstrong’s (1979) ‘root length model’, with the length of rice roots with 42 % porosity reaching 24 cm, compared to wheat roots with 22 % porosity reaching only 14 cm. These calculations did not allow for ROL, which would be much greater in wheat than in rice; however, the earlier referred to model by Armstrong (1979) showed in non-wetland roots the O2 concentration at 6 cm from the root–shoot junction would only rise from 2.3 to 5 kPa when a ROL value typical of rice was used, so its effect on maximum root length might be rather hard to pick up experimentally.
The adequacy of the internal aeration system for roots of rice can be tested by O2 measurements in root tissues such as the stele and particularly towards the tips near the end of the longest diffusion pathway. Additional data on soil oxygenation especially near lateral roots are also needed. Experience with roots of maize and P. australis has shown that, though valuable, such measurements cannot easily be used to decide whether O2 concentrations are adequate for aerobic catabolism. So, one way forward would be to dovetail these O2 measurements with checks on metabolism and root functioning in energy-dependent ion transport. One powerful approach would be the concurrent measurements of O2 concentration profiles and O2 fluxes along with ion fluxes for roots under defined conditions, using micro-electrodes, as Pang et al. (2006) have done with roots of barley. (Note: in applying such methods, interpreting O2 fluxes into roots when the shoot system is also supplying O2 can be very difficult and can lead to erroneous conclusions.) There are now also CO2 micro-electrodes (e.g. Anjos and Hahn 2008), but these have not yet been used in studies on rice or other plants.
One key question is whether the earlier mentioned O2 of 34–68 kPa (i.e. approximately two to three times higher than ambient) in the shallow floodwater of rice paddies during the day (Sect. 3.1) would substantially influence the O2 regimes of the root. Similarly, the near zero O2 concentrations at night may lower the O2 concentration at the root–shoot junction, and thus also within roots. If so, there might be diurnal fluctuations in root elongation and the thickness of the oxygenated rhizosphere. This complex situation needs to be explored by modelling dovetailed with experiments (e.g. with micro-electrodes in the field, as recently achieved with rice when completely submerged (Winkel et al. 2013)).
4.3 The Root CO2 Budget
The aeration network also plays an important role in controlling CO2 partial pressures in roots in flooded soil, despite the high CO2 levels that at times develop in the surrounding soil (Greenway et al. 2006). There are few experimental data on CO2 partial pressures within roots in flooded soils. However Greenway et al. (2006) modelled CO2 concentrations as being dependent on the removal of CO2 through root aerenchyma for given rates of CO2 production in root tissues and entry from the rhizosphere. They calculated that in 10 cm long roots with 0.6 mm diameter, 35 % porosity, and 40 kPa CO2 in the soil, the CO2 partial pressure in the root would not rise above approximately 13 kPa because there would be continuous venting to the atmosphere. The model also assumed that gas exchange between soil and root was restricted to the 10 mm tip. Greater CO2 would occur with narrower and/or longer roots (Greenway et al. 2006).
Another approach to predict CO2 partial pressures in roots in flooded soils is based on observed O2 partial pressures in the roots, by making the simplifying assumption that the only way to ventilate the CO2 produced by the plant roots is via the aerenchyma to the overlying atmosphere. It is reasonable to assume that the CO2 production at least equals the O2 consumption, i.e. that the respiratory quotient (CO2/O2) is one. Now, if the root contains 10 kPa O2, which is a rather high value for much of the roots of rice in anoxic soil, then the CO2 partial pressure would also be about 10 kPa, because equal amounts of O2 and CO2 would have to diffuse through the aerenchyma, albeit in opposite directions, and so they would require similar concentration gradients. The CO2 pressure in the root may be even greater if there is ethanol fermentation (and hence additional CO2 release). The restriction that ventilation would be only via the aerenchyma is likely for roots in many rice soils, since 10 kPa CO2 in these flooded soils is a modest value (Ponnaperuma 1972).
Even the modest CO2 partial pressure of 10 kPa may have profound consequences for the root tissues. Taking the cytoplasmic pH to be 7.5, there would be 75–90 mM HCO3 − in the cytoplasm (Greenway et al. 2006). This HCO3 − load could be coped with in three ways: (1) decarboxylation of endogenous organic acids, providing cations to balance the HCO3 −, but to a maximum of the prevailing concentration of organic anions, usually approximately 35 mM (Greenway et al. 2006); (2) increases in K+ concentration in the cytoplasm, with the risk that the K+ concentration becomes excessive; and (3) allowing a decrease in pH of the cytoplasm, which would greatly decrease the HCO3 −equilibrium concentration (a decrease of 45 % for a decrease in pH of only 7.5 down to 7.3).
The HCO3 − load may be even more acute in root tips, which can grow with internal O2 lower than 1 kPa (measurements in de-oxygenated agar solution, Armstrong and Webb 1985). So, depending on the CO2 partial pressure in the soil and still assuming a respiratory quotient of one, internal CO2 might rise to approximately 20 kPa. Thus, we suggest that at least in some rice soils, it is hard to predict whether the roots would suffer from O2 deficiency, excess CO2, or both. As one example, soils high in organic matter develop large CO2 concentrations after flooding and that would tend to increase the CO2 partial pressure within roots (Greenway et al. 2006). The opposite would be the case for soils low in CO2 because outward diffusion could mitigate the internal rise in root CO2 caused by root respiration and perhaps ethanolic fermentation. However exit of CO2 would also depend on the permeability of the outer cell layers of the roots which in rice contain a gas-diffusion barrier.
5 Root Function
5.1 Root Elongation
The limiting factor in root elongation of completely submerged rice is probably the O2 concentrations in the root apex. Evidence for this includes the very quick cessation of elongation when lights are switched off for submerged rice, when ROL from just behind the root tip rapidly dropped to zero (Waters et al. 1989). When subsequently light was again provided, both ROL and root extension resumed rapidly. Consistently, in rice, manipulation of O2 around the shoots showed that root elongation slowed when O2 at the root tip dropped to 1 kPa and only ceased altogether when O2 at the tip dropped to 0 kPa (Armstrong and Webb 1985). The simplest explanation for the cessation of elongation is that the root tips come under a severe energy crisis, so the limited energy produced is spent on maintenance, i.e. directed towards survival, rather than to growth.
Further experimentation with rice, using manipulations of O2 around the shoots, would be revealing, both for more detailed testing of the responses of extension and particularly of nutrient uptake, which as far as we know has not been done for any species.
5.2 Metabolism
Presently, there are only very few data available on metabolism in different parts of roots subjected to O2 deficiency, and as far as we know none of these is for rice. Hence we discuss first the case of maize roots, for which some data are available, and then consider under what conditions similar events may occur in rice roots.
Metabolic evidence for the effect of the O2 gradient from the root–shoot junction to the root tip of intact maize plants with roots in an anoxic medium was presented decades ago (Drew et al. 1985). The ATP/ADP ratios at 0–100 mm from the root–shoot junction were about four in both aerenchymatous and non-aerenchymatous roots, while these ratios in the root tip, at 155–200 mm from the shoot–root junction, were approximately 1.7 and 0.71 in aerenchymatous and non-aerenchymatous roots, respectively (Drew et al. 1985). Further support for O2 deficiency in the root tip, even though at least the cortex in most of the root still receives plenty of O2, is sketchy. However when whole maize roots were exposed to 0.6 mM O2 in the nutrient solution, alanine in the tip increased from 4 to 33 mM (Gibbs et al. 1998). Alanine is a reasonable indicator of O2 deficiency as it accumulates to high concentrations in anoxic rice coleoptiles and several other tissues (Gibbs and Greenway 2003).
Turning to possible O2 deficiency in the stele, in maize at locations where the cortex was at 4.3 kPa O2, O2 was below detection levels in most of the stele (Gibbs et al. 1998). Consistently, Cl− transport into the xylem was 50 % lower than in aerated roots (Gibbs et al. 1998). These observations led to the hypothesis that anoxia in the stele inhibits the transporters which load ions into the xylem vessels (Colmer and Greenway 2011). Thus, after the initial energy-dependent uptake by the cells of the epidermis and the cortex, which still receive adequate O2, the subsequent transport of ions to the xylem would become dependent on diffusion and/or bulk flow as water moves to the xylem (Colmer and Greenway 2011).
Radial gradients of O2 concentration between cortex and stele were also present for aerenchymatous maize roots (Darwent et al. 2003). Further, early stelar anoxia was suggested for the emergent aquatic species Eleocharis sphacelata, since the roots ceased elongating, though there should have been high enough O2 pressures at the root tip. Interestingly, this paper suggested that stelar anoxia was more widespread when the endodermis was lignified (Sorrell 1994). Oxygen deficiency in the stele of maize was further supported by metabolic measurements, such as concentrations of ethanol and the higher active state of the key enzyme of ethanol formation pyruvate decarboxylase in the stele than in the cortex (Thomson and Greenway 1991).
Overall these data suggest a large effect of root anaerobiosis on nutrient transport to the shoots, so it is worth exploring to what extent the events in maize occur in rice. No data on O2 concentrations in stele versus cortex are available for rice. However anoxia in the stele of rice roots is less likely than in maize roots because rice has a narrow stele, i.e. a high ratio of absorbing interface between stele and cortex to volume of respiring tissue of the stele. The higher the ratio the less likely the stele will suffer O2 deficiency. In the following, we have calculated this ratio from the areas given in publications (NB circumference × height / volume gives units mm2/mm3 = mm−1). Data of McDonald et al. (2002) for adventitious rice roots give a ratio of 19 mm−1, both under aerated and stagnant conditions, whereas data of Gibbs et al. (1998) for primary maize roots give a ratio of 6.1 mm−1. In general, wetland plants often have narrow steles: in the data of McDonald et al. (2002) for adventitious roots, seven out of ten wetland plants had ratios of 10 mm−1 or greater and all three non-wetland plants tested had ratios of 5.2–6.1 mm−1. So, impairment of ion transport to the xylem in rice is less likely than in maize. From model calculations (Armstrong and Beckett 1987), anaerobiosis in the stele of rice roots would only occur when O2 availability becomes very restricted, either when the soil is very reducing and therefore becomes a strong competitive sink for O2, or in distal root parts (e.g. stele just behind root apices and also the apices themselves).
Further exploration of these issues will not be as easy as in maize, since in that species the stele can be stripped readily from the cortex (Gibbs et al. 1998; Thomson and Greenway 1991). Apart from measuring ion fluxes to the shoot, suitable methods would be using micro-electrodes for membrane potentials, cytoplasm pH, and ion fluxes in the roots. Advantages of micro-electrodes are that the measurements can be on very specific tissues and will often respond very rapidly to a change in conditions. For example, in root hairs of Medicago sativa the imposition of anoxia depressed the pH of the cytoplasm by 0.5 within 2 min and shifted the plasma membrane potential from −160 to −125 mV (Felle 1996). Complementary measurements may be on key anaerobic metabolites and/or changes in gene expression, which are sensitive indicators of an energy deficit. Use of laser micro-dissection followed by analysis of different tissues using, for example microarrays (e.g. Rajhi et al. 2011) and other micro-assays, would also be revealing.
5.3 Nutrient Uptake
It has been speculated that the barrier to radial O2 loss may impede nutrient uptake (Armstrong 1979; Colmer 2003a). Experiments with rice roots indicate that the ROL barrier itself, however, does not substantially impede nutrient uptake. Rubinigg et al. (2002) grew rice in stagnant agar to induce the barrier and then measured uptake under aerobic conditions to exclude direct inhibitory effects of O2 deficit on uptake. They found that NO3 − net influx from 0.1 mM NO3 − in the external medium, measured using a NO3 −-specific micro-electrode, was 3–5 μmol g−1 fresh weight h−1 in roots with and without the barrier. In the former the 20–50 mm section had at most 6 % of the ROL of roots without the barrier. So, although nutrient uptake under anoxia was not tested, this experiment shows that at least if the degree of barrier formation in soil-grown roots was similar to that in the deoxygenated agar, then it would be no hindrance to uptake in flooded soil that is drained and re-aerated, as in rainfed rice.
An impermeable barrier can still have some ‘passage areas’ (‘windows’), as observed in Phragmites when O2 at the root surface was 2–2.5 kPa compared to the main adjoining areas which were close to zero O2 (Armstrong et al. 2000). Armstrong et al. (2000) postulated that the windows in the barriers might facilitate emergence of laterals or nutrient uptake, or both. The former might be important when the barrier is strong. However, the suggestion on the facilitation of transport has the problem that the fraction of these passage areas relative to the total root surface area would have to be either substantial, in which case the function of the barrier to prevent O2 loss would be compromised, or the nutrient uptake across the passage areas would have to be many fold faster than that usually encountered for root cells. However, it is possible that the cells of the passage areas have an unusually high uptake capacity, as, for example, in salt glands and in cells loading ions into the xylem.
Having excluded the possible impedance to nutrient influx by the barrier per se, the intriguing question remains whether in roots in flooded soils the epidermal cells outside the barrier to ROL can absorb nutrients even though these would be exposed to very low O2. Alternatively, diminished rates of absorption by roots with the barrier may be compensated for by greater uptake in zones without the barrier, and by lateral roots. Calculations of the extent to which N uptake by rice in flooded soil is limited by root uptake properties versus transport through the soil to root surfaces suggest that it is the fine laterals that are responsible for the bulk of the uptake (Kirk and Solivas 1997).
Colmer (2003a) found dry weights of shoots and roots in nine of 11 rice cultivars were the same or 20 % greater in waterlogged than in drained soil. Thus, either the root sections with the barrier to radial O2 movement took part in the required nutrient uptake, or other parts of the root system compensated for decreased nutrient intake in the sections with the barrier or restricted rooting depth or both. Similarly, net N uptake rates did not differ between roots of rice grown in stagnant deoxygenated agar or in aerated, nutrient solution (Rubinigg et al. 2002).
Kronzucker et al. (1998a) studied the effects of hypoxia on NH4 + fluxes in rice roots in nutrient culture using a 13 N tracer technique. They found little impairment of uptake capacity over 7 days of exposure to O2 concentrations equivalent to 15 % of air saturation. Half-lives for NH4 + exchange with sub-cellular compartments, cytoplasmic NH4 + concentrations, and efflux (as percentage of influx) were unaffected by hypoxia, but there were differences in the relative amounts of N allocated to assimilation and the vacuole versus translocation to the shoot. Measurements of influx with NH4 + concentrations in the high-affinity transporter range (2.5–500 μM) and varying O2 saturation showed that the maximum influx (V max) varied with the degree of hypoxia, but the affinity for NH4 + (K m) was unchanged.
Most experiments testing responses to anoxic media have been made at high nutrient levels, but, as shown below, the outcome at lower levels may be different. Testing response to phosphate in stagnant solution (0.1 % agar), Insalud et al. (2006) found that relative growth rates were depressed at 1.6 μM, but not at 200 μM. This growth reduction was associated with a 90 % lower P uptake at 1.6 μM Pi, but only a 50 % reduction at 200 μM Pi in stagnant compared to turbulent solution. Evidently the high-affinity P transporters that operate at lower P concentration were more affected by hypoxia. Similarly, Kotula et al. (2009a) found that rice in stagnant agar grew poorly compared to aerated solutions (photographic evidence only). This inhibitory response contrasted with the better growth and nutrient uptake in stagnant agar found by Rubinigg et al. (2002) and Colmer et al. (2006). The simplest explanation is the low nutrient concentrations used: Pi, 0.05 mM and 0.15 mM N in Kotula et al. (2009a), but 0.2 mM P and 5 mM N (7:1, NO3 −:NH4 +) in Rubinigg et al. (2002) and Colmer et al. (2006). Similarly, K and N concentrations were in the 0.15 mM range in Kotula et al. but 1 mM or higher in Colmer et al. (2006). In stagnant agar, limitations to nutrient uptake may be due to either O2 deficiency or impeded nutrient diffusion (Wiengweera et al. 1997). The relative importance of these factors can be resolved by increasing the O2 concentrations around the shoots and so elevating the O2 concentrations in the root as has been used for root elongation by Wiengweera and Greenway (2004), as well as by nutrient dose–response experiments.
Further elucidation of mechanisms of root aeration, nutrient acquisition, and growth under less optimum conditions, for example under low levels of mineral nutrition, should be a focus of future research. In addition, understanding of the importance of root growth and proliferation of laterals to access nutrients of low solubility such as phosphate (Sect. 6.2.2), and implications of root functioning in soils with fluctuating water levels (drained–flooded–drained cycles), will be important avenues for understanding of limitations for rice productivity in these conditions.
5.4 Exclusion of Toxins
The second important function of the barrier to radial O2 loss might be prevention of influx of toxic elements that are present in soil of low redox potential. Armstrong and Armstrong (2005) found that increased root cell wall suberisation, induced by high sulphide concentrations in stagnant nutrient culture, caused decreased absorption of Fe2+. Kotula et al. (2009b) found the barrier to ROL greatly decreased the diffusion of Cu2+ through the apoplast. These results do not contradict unimpaired NO3 − net uptake in the presence of the barrier (Sect. 5.3) because the uptake would be mainly by the epidermis, with subsequent flow through the symplast. Furthermore, energy-dependent NO3 − uptake would have a high ratio of flux to external concentration compared with Fe2+ uptake along a free energy gradient.
However, where this function of the barrier to block toxin entry is important (i.e. in highly reduced soil), the uptake of essential elements by the zones with the barrier may also decline markedly, because the metabolism of the epidermal cells will be inhibited even if the concentration of the soil toxin is not lethal to the epidermal cells. The lignification/suberisation of the outer cell layers can become much more intense in the presence of reduced-soil toxins, to the extent that the physical barrier prevents emergence of laterals and they grow upwards within the parent adventitious root (Armstrong and Armstrong 2005). This would result in a smaller root surface area for nutrient absorption.
5.5 Hydraulic Conductivity
Colmer and Greenway (2011) have recently reviewed the few data on this issue. The evidence is inconclusive but one positive finding was that in rice roots, the large volume of aerenchyma is unlikely to add to the hydraulic resistance since water flowed along the rim of the living cells of the strands connecting the outer root with the stele (Miyamoto et al. 2001).
Kotula et al. (2009a) have shown that the permeability of the outer cell layers in roots of rice to water was 10-fold smaller than that for O2. However, these authors emphasised that in transpiring plants this slow diffusion is irrelevant, since the hydraulic conductivity was 600–1,400 times greater than the diffusive conductivity. These data were for roots of aerated plants, so it remains important to establish the effect of the barrier to O2 loss on hydraulic conductivity.
6 The Rhizosphere
6.1 Root-Induced Changes in the Soil
As a result of O2 loss from the root, and other root-induced changes, conditions in the rhizosphere 1–2 mm from the root surface can be greatly different from those in the anoxic bulk soil. This has important consequences for the concentrations and uptake of nutrients and toxins. The main processes are as follows (Fig. 4; see Kirk 2004 for a fuller discussion).
(a) Processes in the rice rhizosphere. (b) Profiles of ferrous and ferric iron and pH in blocks of a reduced soil in contact with a planar layer of rice roots for 12 days (Kirk and Bajita 1995)
6.1.1 Oxygenation
As discussed in Sect. 4, some of the O2 diffusing down through the aerenchyma leaks out into the soil where it is consumed in biotic and abiotic processes. Mobile inorganic reductants in the soil are oxidised, particularly Fe2+ which is precipitated as Fe(OH)3 on or near the root. As a result the concentration of Fe2+ near the root falls and more Fe2+ diffuses in from the bulk soil. This is then oxidised resulting in a zone of Fe(OH)3 accumulation near the root. Hence reddish-brown deposits of ferric oxide are frequently observed on the older parts of rice roots.
The flux of O2 across a particular portion of the root depends not only on the rate of O2 transport through the root (Sect. 4.1) but also on the strength of the sink presented by the external medium. In soil, the sink strength depends on the rate of O2 diffusion into the soil, its rate of consumption by microbes and reaction with mobile reductants such as Fe2+, and the rate of Fe2+ diffusion towards the oxidation zone (Bouldin 1968). There are also differences along the root length, and between primary roots and laterals (Sect. 4.2.1). As a root grows through a portion of soil, a zone of Fe2+ depletion arises where oxidation is intense in the region of the root tip, but is rapidly filled in when the O2 supply decreases as the less permeable parts of the root grow past. Re-reduction of Fe(III) is slow compared with oxidation of Fe(II). Hence the root tips are generally white and free of ferric oxide deposits, whereas the older parts are coloured orange brown.
Measured fluxes of O2 from the roots of rice and other wetland species vary greatly, depending on the method used and experimental conditions. Sorrell and Armstrong (1994) discuss the difficulties in making these measurements. Methods include the use of polarographic electrodes to measure fluxes from sections of individual roots (Armstrong 1979); measurements of O2 release into continuously replenished, de-oxygenated solutions bathing whole root systems (Bedford et al. 1991; Kirk and Du 1997); measurements of Fe(II) oxidation in the rhizosphere near planar layers of roots in reduced soils (Begg et al. 1994; Kirk and Bajita 1995); and use of O2 micro-electrodes to measure gradients of O2 concentration near roots growing in field soils (Revsbech et al. 1999). The differences between methods reflect the various complexities listed above, and particularly the importance of differences between different parts of the root and root system, and differences over time. From the point of view of processes in the rhizosphere, the fluxes of O2 from root tips and laterals are of a similar magnitude to fluxes of nutrient ions into roots—of the order of a few nmol dm−2 root s−1—and, given the effects of radial geometry, these fluxes are sufficient to greatly alter the soil chemistry and biology close to root surfaces.
6.1.2 Acidification
The oxidation of inorganic reductants generates protons:
so the pH in the oxidation zone tends to fall. Further, because the main form of plant-available N in anaerobic soil is NH4 +, the root absorbs an excess of cations over anions. Consequently H+ is released by the root to maintain electrical neutrality, further decreasing the soil pH. Note that if N is taken up as NO3 − as a result of nitrification of NH4 + in the rhizosphere, the net acid–base change is the same because, although the root exports 2 mol less H+ for each mol of NO3 − replacing a mol of NH4 +, 2 mol of H+ are formed in the nitrification of each mol of NH4 +. Note also that Si, which is taken up in large quantities by rice plants, crosses the root as the uncharged H4SiO4 molecule (pK 1 = 9.46 at 25 °C).
The net effects of these processes will depend on the rate of H+ generation versus the rate at which H+ moves away by acid–base transfer. In flooded soils, acid–base transfer tends to be fast, both because soil diffusion coefficients increase with water content and because the high concentration of dissolved CO2 results in a high concentration of the acid–base pair H2CO3–HCO3 −. Hence protons are transferred by reaction with HCO3 − and diffusion of more HCO3 − into the acidification zone from the bulk soil. Modelling and experimental results (Begg et al. 1994; Kirk and Bajita 1995) show that over realistic ranges of conditions the rice rhizosphere will be acidified by up to 0.5 pH units as a result of these processes, and in certain circumstances by more than 1 pH unit.
6.1.3 CO2 Uptake
Because very large concentrations of dissolved CO2 develop in flooded soil (Sect. 3.2.2), in spite of root respiration the CO2 pressure outside the root may be greater than that inside it, resulting in a flow of CO2 from the soil to the atmosphere through the aerenchyma. The consequences for root physiology are discussed in Sect. 4.3. Net removal of CO2 by the root decreases the concentration of the acid H2CO3 near the root, and this may offset the acidity produced in oxidation and excess cation uptake (Begg et al. 2004).
6.2 Consequences for Nutrients and Toxins
6.2.1 Ammonium Versus Nitrate Nutrition
The principal form of plant-available N in chemically reduced, flooded soils is NH4 +, formed from the mineralisation of organic matter or from mineral fertilisers. This contrasts with well-aerated soils, where the principal form is generally NO3 −. Because NH4 + is adsorbed on soil surface, both as a freely exchangeable cation and in more tightly held forms within clay lattices, much smaller concentrations develop in the soil solution than of NO3 − in well-aerated soils. Rates of transport to roots by mass flow and diffusion are correspondingly slower and potentially limit uptake. Kirk and Solivas (1997) examined this question with a model and experiments, and concluded that in well-puddled flooded soil rates of transport will generally not limit NH4 + uptake rates for typical rice rooting densities.
Potentially more important are the consequences of NH4 + nutrition for plant physiology. Plant growth and yield are generally better when plants absorb their N as a mixture of NH4 + and NO3 − rather than as either on its own (Hawkesford et al. 2012). The reasons are not fully understood, but may involve the need to provide carbon skeletons for NH4 + assimilation in roots, compared with NO3 − assimilation in shoots, and the need to balance charges for electrical neutrality (Britto and Kronzucker 2002; Balkos et al. 2010). Some NO3 − may be formed by nitrification of NH4 + in the oxygenated rhizosphere and at the floodwater–soil interface. Kronzucker et al. (1998a, b, 1999, 2000) have found that lowland rice can be exceptionally efficient in absorbing NO3 − compared with other species, raising the possibility that rice growing in flooded soil may absorb significant amounts of NO3 − formed in the rhizosphere. This is important both because of the potential physiological benefits to the plant, and because NO3 − formed in the rhizosphere is otherwise lost through denitrification in the soil bulk.
Three lines of evidence from Kronzucker et al. (1999, 2000) suggest unusually efficient NO3 − absorption. First, steady-state influx of NO3 − and NH4 + followed Michaelis–Menten kinetics over the relevant concentration range, and V max for NO3 − was some 40 % larger than that for NH4 + (8.1 versus 5.7 μmol g−1 h−1) and K m 50 % smaller (26 ± 6 versus 51 ± 18 μM). Second, induction of the root NO3 − transporters following its re-supply to plants deprived of NO3 − for 24 h was exceptionally rapid, peaking within 2 h. For comparison, in barley—which is considered one of the most efficient NO3 − users—full induction takes up to 24 h (Kronzucker et al. 1997). Third, sub-cellular pool sizes and fluxes, estimated from the kinetics of 13N efflux out of labelled roots, indicated highly efficient NO3 − use: while similar proportions of incoming NH4 + and NO3 − were channelled into assimilation and to the vacuole, the proportion of NO3 − translocated to the shoot was larger than for NH4 + and that lost through efflux out of the roots was smaller.
The extent of NO3 − absorption by soil-grown plants will depend on its rate of formation and loss in the rhizosphere. Kirk and Kronzucker (2005) have shown with a model that substantial quantities of NO3 − can be produced and taken up through nitrification in the rice rhizosphere. Their model considers rates of O2 transport away from a root and its simultaneous consumption in biotic and abiotic processes; transport of NH4 + towards the root and its consumption in nitrification and uptake at the root surface; and transport of NO3 − formed from NH4 + towards the root and its consumption in denitrification and uptake by the root. A sensitivity analysis showed that rates of NO3 − uptake can be comparable with those of NH4 + under realistic field conditions. Also rates of denitrification and subsequent loss of N from the soil remain small even where NO3 − production and uptake are considerable.
As a result of losses by volatilisation, recoveries of N from mineral fertilisers applied to flooded rice fields are very variable, ranging from 20 to 60 %, with average recoveries between 30 and 40 % in most areas (Peng et al. 2010). Volatilisation occurs by conversion of NH4 + to NH3 in the floodwater linked to high floodwater pHs induced by algae (Sect. 3.1), and by nitrification–denitrification. Recoveries are greatly improved if fertiliser applications are split carefully to match crop demands, as is necessary to realise agronomic yield potentials (Peng et al. 2010).
6.2.2 Phosphorus
In general the changes in soil physico-chemical conditions following flooding result in P becoming more soluble, and so P availability rarely limits rice growth, at least in irrigated systems (Dobermann and Fairhurst 2000; Kirk 2004). In rainfed rice, rapid drying and re-oxidation of the soil can result in the P becoming very insoluble (Brandon and Mikkelsen 1979; Willett 1979; Huguenin-Elie et al. 2003). Re-oxidised Fe(II) compounds may be precipitated in poorly crystalline forms with large specific surface areas, on and in which P becomes immobilised. Hence upland crops grown in rotation with rice frequently suffer P deficiency even though crops on similar soils not used for rice grow healthily. The problem is in part also due to disruption of mycorrhizal networks during flooding (Ellis 1998).
The electrochemical changes and dissolution of soil solid phases in redox reactions following flooding tend to make P more soluble. An interesting question is how access of P to the root is affected by iron oxide precipitation on and near the root. Amorphous Fe(OH)3 has a large adsorbing surface and might be expected to immobilise P, impeding its access to the root. However, acidification of the rhizosphere might be expected to make P more soluble. Saleque and Kirk (1995) measured concentration profiles of P and other root-induced changes near planar layers of rice roots growing in a highly weathered P-deficient soil. They found that 90 % of the P taken up was drawn from acid-soluble pools, probably associated with Fe(II) carbonates and hydroxides. There were also narrow zones of P accumulation in an alkali-soluble pool which coincided with zones of Fe(OH)3 accumulation near the roots. The zone of P depletion coincided with a zone of acidification. Kirk and Saleque (1995) showed with a model that the acidification and the P-solubilising effect of acidity in the soil were sufficient to account for the P mobilised and absorbed by the roots. This is an extreme example, involving particularly large pH changes. But it indicates the magnitude of the effects that are possible.
Phosphorus deficiency is more prevalent in rainfed rice and aerobic soil conditions. Wissuwa and colleagues (Wissuwa and Ae 2001; Chin et al. 2011; Gamuyao et al. 2012) have explored the genetics and mechanisms of rice tolerance of P-deficient aerobic soils. They identified a major quantitative trait locus (QTL) for tolerance, Pup1. Pup1 is largely absent from irrigated rice varieties but conserved in varieties and breeding lines adapted to drought-prone environments (Chin et al. 2011). It appears to facilitate root growth under P stress by blocking the response to P stress that results in decreased root growth (Gamuyao et al. 2012).
6.2.3 Zinc
The changes in soil chemistry following flooding result in Zn being immobilised in very insoluble forms, even though total Zn contents are generally non-limiting (Kirk 2004; Impa and Johnson-Beebout 2012). This may be linked to high soil pH and excess bicarbonate, such as in the calcareous soils of the Indo-Gangetic plains of India and Bangladesh, but it also occurs in perennially wet, young non-calcareous soils, and in peats and coastal saline soils (Kirk 2004). After N, Zn deficiency is the second most important nutrient disorder in rice, affecting up to 50 % of rice soils globally (Dobermann and Fairhurst 2000). Zinc deficiency in human populations subsisting on rice is also widespread (IRRI 2010).
There is large variation in the rice germplasm in tolerance of Zn-deficient soils (Quijano-Guerta et al. 2002; Wissuwa et al. 2006). At least three mechanisms, operating together or separately, confer tolerance of seedling-stage Zn deficiency: (1) enhanced Zn uptake by secretion of Zn-chelating phytosiderophores (Arnold et al. 2010; Widodo et al. 2010; Ptashnyk et al. 2011); (2) maintenance of new root growth under low Zn and high HCO3 − concentrations (Widodo et al. 2010; Rose et al. 2011); and (3) prevention of root damage by oxidative stress linked to high HCO3 − concentrations (Frei et al. 2010; Rose et al. 2011, 2012).
The role of phytosiderophores (PS) in Fe uptake by grasses is well established, but their involvement in Zn uptake is debated (Suzuki et al. 2008). Three lines of evidence support the PS mechanism in rice. Firstly, Widodo et al. (2010) found enhanced secretion of the PS deoxymugineic acid (DMA) by a Zn-efficient rice line from a QTL mapping population. The tolerant line took up 50 % more Zn in Zn-deficient soil and this coincided with increased expression of putative ligand-efflux genes in the roots. Second, Arnold et al. (2010) found evidence supporting the PS mechanism based on differences in Zn stable isotope fractionation in the same tolerant and intolerant rice lines. They found a preference for heavy 66Zn in the tolerant rice line grown in soil, but not in the intolerant line; among alternative explanations, this could only be explained by a Zn-complexation process and uptake of complexed Zn by the roots. By contrast, rice grown in nutrient culture showed a slight Zn bias, consistent with kinetic fractionation during membrane transport of free Zn2+ ions (Weiss et al. 2005). Third, Ptashnyk et al. (2011) developed a mathematical model of PS-mediated Zn uptake, allowing for root growth, inter-root interaction, diurnal variation in PS secretion, decomposition of the PS in the soil, and the transport and interaction of the PS and Zn in the soil. It showed that (a) measured PS secretion rates were sufficient to explain measured Zn uptake rates and differences between rice genotypes and (b) there was an important interaction with rooting density, but (c) diurnally varying PS secretion had little effect on uptake.
6.2.4 Iron Toxicity
Iron toxicity is peculiar to flooded soils. It is a syndrome of disorders associated with the occurrence of Fe2+ in the soil solution. It happens over a wide range of Fe2+ concentrations, from 1,000 mg L −1 to only 10 mg L −1 in soils with poor nutrient status—especially of P or K—or with respiration inhibitors such as H2S (van Mensvoort et al. 1985; Sahrawat 2004; Becker and Asch 2005). The effects include internal damage of tissues due to excessive uptake of Fe2+; impaired nutrient uptake, especially of P, K, Ca, and Mg; and increased diseases associated with imbalanced nutrition. Three main groups of Fe toxic soils are distinguished (van Mensvoort et al. 1985): acid sulphate soils, in which extremely large concentrations of Fe2+ in the soil solution arise as a result of the soils’ peculiar mineralogy; poorly drained sandy soils in valleys receiving interflow water from adjacent higher land with highly weathered sediments; and more clayey, acid, iron-rich soils in sediments derived from highly weathered soils and which develop iron toxicity without interflow. Where Fe toxicity is associated with interflow the concentrations of dissolved Fe in the upwelling water have been found to be too small to account for the large concentrations of Fe2+ in the root zone, and most of the Fe2+ is apparently formed in situ. Therefore the interflow aggravates toxicity by some mechanism other than bringing in Fe2+, possibly involving depletion of other nutrients and upsetting the plant’s ability to exclude Fe.
In iron toxic soils, oxidation of Fe2+ in the rhizosphere is an important means of excluding it from the root. However, in acid soils with small pH buffer powers, the acidification accompanying Fe2+ oxidation may result in impaired access of nutrient cations to roots (Kirk and Bouldin 1991; Razafinjara 1999). This is because the overall concentration of the soil solution in a flooded soil depends largely on the concentration of HCO3 − buffered by dissolved CO2. Therefore, if the pH close to the root decreases below about 6.0, the concentration of anions in solution also decreases and so the concentration of cations in solution and hence their rate of diffusion to roots must decrease. Bouldin (1989) gives calculations of this effect. High rates of Fe2+ oxidation and associated H+ generation result in a low pH in the rhizosphere, especially if the soil is already acid or has a small pH buffer power. Hence the need to exclude toxic Fe2+ from the root may impair the absorption of nutrient cations by the root. Consistent with this the symptoms of iron toxicity are often alleviated by applications of K salts.
A further complication is that the lowering of the rhizosphere pH and consequent depression of HCO3 − mean that any Fe2+ entering the root will be accompanied by a proportion of Cl− or SO4 2− rather than HCO3 −. When Fe2+ enters with HCO3 −, the acidity generated in Fe2+ oxidation in the plant is neutralised by conversion of HCO3 − to CO2, which is assimilated or lost. However when Fe2+ enters with a non-volatile anion, Fe2+ oxidation will produce the equivalent amount of free H+ in the plant, with damaging effects on plant tissues (van Mensvoort et al. 1985).
7 Conclusions
The present and companion (Colmer et al. 2013) papers emphasise genotype-by-environment interactions in Oryza sativa resulting in adaptation to a huge range of flooded soil conditions. The remarkable adaptability of Oryza sativa to a large range of taxing environments is presumably related to allelic diversity in its small genome, allowing subtle variation in responses to water regimes ranging from free draining to floodwaters many metres deep. This diversity can be employed in mechanistic studies, as well as in plant breeding. The rice gene pool, so eminently adapted to flooded environments, is the key to elucidating the mechanisms of adaptation. It is far better suited to this than the relatively flooding-intolerant species Arabidopsis thaliana, which is often the default choice for molecular studies of adaptive mechanisms.
This chapter has considered the most universal feature of the various rice ecosystems: reduced anoxic soils with their large range of inorganic and organic toxins. After 70 years of research we now have a coherent picture of the essential ventilation of the plants roots and its associated rhizosphere, as well as an understanding of acquisition of essential nutrients and prevention of entry of toxic compounds. Whether the ‘avoidance’ of O2 deficiency in either tissues or rhizosphere is always effective is less clear. Even in solution culture, O2 deficiency might occur in root tips and no data are available to evaluate possible O2 deficiencies of roots in soils of very negative redox potential. To what extent nutrition is dependent on lateral roots needs further elucidation. Similarly, further work is needed on whether the barrier to O2 loss, along most of the primary root system, restricts nutrient uptake and how effectively it excludes toxins derived from the anoxic, reduced soil.
The extreme redox gradient across the rhizosphere of rice and other wetland plants means that the soil the root ‘sees’ has a much higher redox potential (i.e. less negative) than the bulk soil. This has important consequences for the transformations and uptake of nutrients and toxins. Experimental and modelling studies over the past few decades have provided a good understanding of these processes and their consequences. This understanding is beginning to be exploited in rice breeding programmes for tolerance of mineral deficiencies and toxicities. Here again the sequenced rice genome and other genetic resources in rice make it a model plant for future work.
References
Anjos TG, Hahn CEW (2008) The development of a membrane-covered microelectrode array gas sensor for oxygen and carbon dioxide measurement. Sens Actuators B Chem 135:224–229
Armstrong W (1971) Radial oxygen loss from intact rice roots as affected by distance from the root apex, respiration and waterlogging. Physiol Plant 25:192–197
Armstrong W (1979) Aeration in higher plants. Adv Bot Res 7:225–332
Armstrong J, Armstrong W (2005) Rice: sulphide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann Bot 96:625–638
Armstrong W, Beckett PM (1987) Internal aeration and the development of stelar anoxia in submerged roots. A multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol 105:221–245
Armstrong W, Webb T (1985) A critical oxygen pressure for root extension in rice. J Exp Bot 36:1573–1582
Armstrong W, Armstrong J, Beckett PM (1990) Measurement and modelling of oxygen release from roots of Phragmites australis. In: Cooper P, Finklater BC (eds) The use of constructed wetlands in water pollution control. Pergamon, Oxford, UK, pp 41–54
Armstrong W, Armstrong J, Beckett PM (1991) Convective flow in wetland plant aeration. In: Jackson MB, Davies DD, Lambers H (eds) Plant life under oxygen deprivation. SPB Publishing Company, The Hague, pp 283–302
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol 120:197–207
Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM (2000) Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot 86:687–703
Arnold T, Kirk GJD, Wissuwa W, Frei M, Zhao F-J, Mason TFD, Weiss DJ (2010) Evidence for the mechanisms of zinc uptake by rice using isotope fractionation. Plant Cell Environ 33:370–381
Balkos KD, Britto DT, Kronzucker HJ (2010) Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ 33:23–34
Barber DA, Ebert M, Evans NTS (1961) The movement of 15O through barley and rice plants. J Exp Bot 13:397–403
Becker M, Asch F (2005) Iron toxicity in rice—conditions and management concepts. J Plant Nutr Soil Sci 168:558–573
Beckett PM, Armstrong W, Justin SHFW, Armstrong J (1988) On the relative importance of convective and diffusive gas flows in plant aeration. New Phytol 110:463–468
Bedford BL, Bouldin DR, Beliveau BD (1991) Net oxygen and carbon dioxide balances in solutions bathing roots of wetland plants. J Ecol 79:943–959
Begg CBM, Kirk GJD, MacKenzie AF, Neue H-U (1994) Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytol 128:469–477
Bertani A, Reggiani R (1991) Anaerobic metabolism in rice roots. In: Jackson MB, Davies DD, Lambers H (eds) Plant life under oxygen deprivation. SPB Academic, The Hague, pp 187–199
Blom CWPM, Voesenek LACJ (1996) Flooding: the survival strategies of plants. Trends Ecol Evol 11:290–295
Bouldin DR (1968) Models for describing the diffusion of oxygen and other mobile constituents across the mud-water interface. J Ecol 56:77–87
Bouldin DR (1989) A multiple ion uptake model. J Soil Sci 40:309–319
Brandon AM, Mikkelsen DS (1979) Phosphorus transformations in alternately flooded California soils. I. Cause of plant phosphorus deficiency in rice rotation crops and correction methods. Soil Sci Soc Am J 43:989–994
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Brix H, Sorrell BK, Orr P (1992) Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnol Oceanogr 37:1420–1433
Butterbach-Bahl K, Papen H, Rennenberg H (2000) Scanning electron microscopy analysis of the aerenchyma in two rice cultivars. Phyton 40:43–55
Chin JH, Gamuyao R, Dalid C, Bustamam M, Prasetiyono J, Moeljopawiro S, Wissuwa M, Heuer S (2011) Developing rice with high yield under phosphorus deficiency: Pup1 sequence to application. Plant Physiol 156:1202–1216
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Ann Bot 91:301–309
Colmer TD (2003b) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Greenway H (2011) Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J Exp Bot 62:39–57
Colmer TD, Pedersen O (2008) Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178:326–334
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Colmer TD, Gibberd MR, Wiengweera A, Tinh TK (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J Exp Bot 49:1431–1436
Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa L.): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol 170:767–778
Colmer TD, Greenway H, Ismail AM, Kirk GJD, Atwell BJ (2014) Physiological mechanisms of flooding tolerance in rice: transient complete submergence and prolonged standing water. Prog Bot 75. doi: 10.1007/978-3-642-38797-5_8
Dacey JWH (1980) Internal winds in water lilies: an adaptation for life in anaerobic sediments. Science 210:1017–1019
Darwent MJ, Armstrong W, Armstrong J, Beckett PM (2003) Exploring the radial and longitudinal aeration of primary maize roots by means of Clark-type oxygen micro-electrodes. Russ J Plant Physiol 50:722–732
Dobermann A, Fairhurst T (2000) Rice: nutrient disorders and nutrient management. Potash & Phosphate Institute, Singapore and International Rice Research Institute, Manila
Doi K, Yasui H, Yoshimara A (2008) Genetic variation in rice. Curr Opin Plant Biol 11:144–148
Drew MC, Saglio PH, Pradet A (1985) Larger adenylate energy-charge and ATP/ADP ratios in aerenchymatous roots of Zea mays in anaerobic media as a consequence of improved oxygen transport. Planta 165:51–58
Ellis JR (1998) Post flood syndrome and vesicular-arbuscular mycorrhizal fungi. J Prod Agric 11:200–204
Felle HH (1996) Control of cytoplasmic pH under anoxic conditions and its implication for plasma membrane proton transport in Medicago sativa root hairs. J Exp Bot 47:967–973
Fisher RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Sci 50:S85–S98
Flessa H, Fischer WR (1993) Plant-induced changes in the redox potential of rice rhizospheres. Plant Soil 143:55–60
Frei M, Wang YX, Ismail AM, Wissuwa M (2010) Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Funct Plant Biol 37:74–84
Gamuyao R, Chin JH, Pariasca-Tanaka J, Pesaresi P, Catausan S, Dalid C, Slamet-Loedin I, Tecson-Mendoza EM, Wissuwa M, Heuer S (2012) The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488:535–539
Ge S, Sang T (2007) Genetics and phylogenetics of rice domestication. Curr Opin Genet Dev 17:533–538
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H (1998) Response to oxygen deficiency in primary roots of maize. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Aust J Plant Physiol 25:745–758
Gilbert B, Assmus B, Hartmann A, Frenzel P (1998) In situ localization of two methanotrophic strains in the rhizosphere of rice plants. FEMS Microbiol Ecol 25:117–128
Greenland DJ (1997) The sustainability of rice farming. CABI, Wallingford
Greenway H, Gibbs J (2003) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and for energy consuming processes. Funct Plant Biol 30:999–1030
Greenway H, Armstrong W, Colmer TD (2006) Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Ann Bot 98:9–32
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2012) Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic, London, pp 135–189
Hirochika H, Guiderdoni E, An G, Hsing YI, Eun MY, Han CD, Upadhyaya N, Ramachandran S, Zhang QF, Pereira A, Sundaresan V, Leung H (2004) Rice mutant resources for gene discovery. Plant Mol Biol 54:325–334
Hojberg O, Sorensen J (1993) Micro gradients of microbial oxygen consumption in a barley rhizosphere system. Appl Environ Microbiol 59:431–437
Huguenin-Elie O, Kirk GJD, Frossard E (2003) Phosphorus uptake by rice from soil that is flooded, drained or flooded then drained. Eur J Soil Sci 54:77–90
Impa SM, Johnson-Beebout SE (2012) Integrating soil chemistry and plant physiology research to meet the related challenges of mitigating zinc deficiency and achieving high grain Zn in rice. Plant Soil 361:3–41
Insalud N, Bell RW, Colmer TD, Rerkasem B (2006) Morphological and physiological responses of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnant solution culture. Ann Bot 98:995–1004
IRRI (1982) Terminology for rice growing environments. International Rice Research Institute, Manila
IRRI (1993) Rice research in a time of change. International Rice Research Institute, Manila
IRRI (2002) Rice almanac, 3rd edn. International Rice Research Institute, Manila
IRRI (2010) Bringing hope, improving lives. IRRI’s strategic plan 2007–2015. International Rice Research Institute, Manila
Ishizawa K, Murakami S, Kawaakami Y, Kuramochi H (1999) Growth and energy status of arrowhead tubers, pondweed turions and rice seedlings under anoxic conditions. Plant Cell Environ 22:505–514
Ismail AM, Johnson DE, Ella ES, Vergara GV, Baltazar AM (2012) Adaptation to flooding during emergence and seedling growth in rice and weeds, and implications for crop establishment. AoB Plants. doi:10.1093/aobpla/pls019
Jackson MB, Ram PC (2003) Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann Bot 91:227–241
Jung K-H, An G, Ronald PC (2008) Towards a better bowl of rice: assigning function to tens of thousands of rice genes. Nat Rev Genet 9:91–101
Justin SHFW, Armstrong W (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytol 106:465–495
Justin SHFW, Armstrong W (1991) Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol 118:49–62
Kende H, van der Knaap E, Cho HT (1998) Deep water rice: a model to study stem elongation. Plant Physiol 118:1105–1110
Kennedy RA, Barrett SCH, van der Zee D, Rumpho ME (1980) Germination and seedling growth under anaerobic conditions in Echinochloa crus-galli (barnyard grass). Plant Cell Environ 3:243–248
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35:25–34
Kirk GJD (2003) Rice root properties for internal aeration and efficient nutrient acquisition in submerged soil. New Phytol 159:185–194
Kirk GJD (2004) The biogeochemistry of submerged soils. Wiley, Chichester
Kirk GJD, Bajita JB (1995) Root-induced iron oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. New Phytol 131:129–137
Kirk GJD, Bouldin DR (1991) Speculations on the operation of the rice root system in relation to nutrient uptake. In: de Vries Penning FWT, Van Laar HH, Kropff MJ (eds) Simulation and systems analysis for rice production (SARP). Pudoc, Wageningen, pp 195–203
Kirk GJD, Du LV (1997) Changes in rice root architecture, porosity, and oxygen and proton release under phosphorus deficiency. New Phytol 135:191–200
Kirk GJD, Kronzucker HJ (2005) The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Ann Bot 96:639–646
Kirk GJD, Saleque MA (1995) Solubilization of phosphate by rice plants growing in reduced soil: prediction of the amount solubilized and the resultant increase in uptake. Eur J Soil Sci 46:247–255
Kirk GJD, Solivas JL (1997) On the extent to which root properties and transport through the soil limit nitrogen uptake by lowland rice. Eur J Soil Sci 48:613–621
Kordan HA (1974) Patterns of shoot and root growth in rice seedlings germinating in water. J Appl Ecol 11:685–690
Kotula L, Ranathunge K, Steudle E (2009a) Apoplastic barriers effectively block oxygen permeability across outer cell layers of rice roots under deoxygenated conditions: roles of apoplastic pores and of respiration. New Phytol 184:909–917
Kotula L, Ranathunge K, Schreiber L, Steudle E (2009b) Functional and chemical comparison of apoplastic barrier in roots of rice (Oryza sativa L.) grown in aerated and deoxygenated solution. J Exp Bot 60:2155–2167
Kronzucker HJ, Siddiqi MY, Glass ADM (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385:59–61
Kronzucker HJ, Kirk GJD, Siddiqi MY, Glass ADM (1998a) Effects of hypoxia on 13NH4 + fluxes in rice roots: kinetics and compartmental analysis. Plant Physiol 116:581–587
Kronzucker HJ, Schjoerring JK, Erner Y, Kirk GJD, Siddiqi MY, Glass ADM (1998b) Dynamic interactions between root NH4 + influx and long-distance N translocation in rice: insights into negative feedback processes. Plant Cell Physiol 39:1287–1293
Kronzucker HJ, Siddiqi MY, Glass ADM, Kirk GJD (1999) Nitrate-ammonium synergism in rice: a subcellular flux analysis. Plant Physiol 119:1041–1045
Kronzucker HJ, Glass ADM, Siddiqi MY, Kirk GJD (2000) Comparative kinetic analysis of ammonium and nitrate acquisition by tropical lowland rice: implications for rice cultivation and yield potential. New Phytol 145:471–476
Kyuma K (2003) Paddy soil science. Kyoto University Press, Kyoto
Liu Q, Zhang Q, Burton RA, Shirley NJ, Atwell BJ (2010) Expression of vacuolar H+-pyrophosphatase (OVP3) is under control of an anoxia-inducible promoter in rice. Plant Mol Biol 72:47–60
McDonald MP, Galway NW, Colmer TD (2002) Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dry land grass species. Plant Cell Environ 25:441–451
Matsuo T, Hoshikawa K (1993) Science of the rice plant. I. Morphology. Food and Agriculture Policy Research Center, Tokyo
Mazzucotelli A, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L (2008) Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci 174:420–431
McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, Ulat VJ, Zeller G, Clark RM, Hoen DR, Bureau TE, Stokowski R, Ballinger DG, Frazer KA, Cox DR, Padhukasahasram B, Bustamante CD, Weigel D, Mackill DJ, Bruskiewich RM, Raetsch G, Buell CR, Leung H, Leach JE (2009) Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Natl Acad Sci USA 106:12273–12278
Mikkelsen DS, De Datta SK, Obcemea WN (1978) Ammonia volatilization losses from flooded rice soils. Soil Sci Soc Am J 42:725–730
Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001) Hydraulic conductivity of rice roots. J Exp Bot 52:1835–1846
Molina J, Sikora IM, Garud IN, Flowers JM, Rubinstein S, Reynolds A, Huang P, Jackson S, Schaal BA, Bustamante CD, Boyko AR, Purugganan MD (2011) Molecular evidence for a single evolutionary origin of domesticated rice. Proc Natl Acad Sci USA 108:8351–8356
Moormann FR, van Breemen N (1978) Rice: soil, water, land. International Rice Research Institute, Manila
Naredo MEB, Juliano AB, Lu B, Jackson MT (1997) Hybridization of AA genome rice species from Asia and Australia I. Crosses and development of hybrids. Genet Resour Crop Evol 44:17–23
Pang JY, Newman I, Mendham N, Zhou M, Shabala S (2006) Microelectrode ion and O2 fluxes reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ 29:1107–1121
Parlanti S, Kudahettige NP, Lombardi L, Mensuali-Sodi A, Alpi A, Perata P, Pucciariello C (2011) Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann Bot 107:1335–1343
Patrick WH Jr, Delaune RD (1972) Characterization of the oxidized and reduced zones in flooded soils. Soil Sci Soc Am Proc 36:573–576
Peng S, Buresh RJ, Huang J et al (2010) Improving nitrogen fertilization in rice by site specific N management. A review. Agron Sustain Dev 30:649–656
Perata P, Alpi A (1993) Plant-responses to anaerobiosis. Plant Sci 93:1–17
Ponnaperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Ptashnyk M, Roose T, Jones DL, Kirk GJD (2011) Enhanced zinc uptake by rice through phytosiderophore secretion: a modelling study. Plant Cell Environ 34:2038–2046
Quijano-Guerta C, Kirk GJD, Portugal AM, Bartolome VI, McLaren GC (2002) Tolerance of rice germplasm to zinc deficiency. Field Crops Res 76:123–130
Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190:351–368
Raskin I, Kende H (1983) How does deep-water rice solve its aeration problem? Plant Physiol 72:447–454
Razafinjara AL (1999) Cation-anion balances and chemical changes in the rhizosphere of rice in an iron toxic soil. PhD thesis, University of the Philippines at Los Baños
Revsbech NP, Pederson O, Reichardt W, Briones A (1999) Microsensor analysis of oxygen and pH in the rice rhizosphere under field and laboratory conditions. Biol Fertil Soils 29:379–385
Ricard B, Couee I, Raymond P, Saglio PH, Saint-Ges V, Pradet A (1994) Plant metabolism under hypoxia and anoxia. Plant Physiol Biochem 32:1–10
Richardson JL, Vepraskas MJ (eds) (2001) Wetland soils: genesis, hydrology, landscapes, and classification. Lewis Publishers, Boca Raton, FL
Roger PA (1996) Biology and management of the floodwater ecosystem in ricefields. International Rice Research Institute, Manila
Rose MT, Rose TJ, Pariasca-Tanaka J, Widodo WM (2011) Revisiting the role of organic acids in the bicarbonate tolerance of zinc-efficient rice genotypes. Funct Plant Biol 38:493–504
Rose MT, Rose TJ, Pariasca-Tanaka J, Yoshihashi T, Neuweger H, Goesmann A, Frei M, Wissuwa M (2012) Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta 236:959–973
Rubinigg M, Stulen I, Elzenga JTM, Colmer TD (2002) Spatial patterns of radial oxygen loss and nitrate net flux along adventitious roots of rice raised in aerated or stagnant solution. Funct Plant Biol 29:1475–1481
Sabu KK, Abdullah MZ, Lim LS, Wickneswari R (2009) Analysis of heritability and genetic variability of agronomically important traits in Oryza sativa × O. rufipogon cross. Agron Res 7:97–102
Sackville-Hamilton NR (2006) How many rice varieties are there? Rice Today 5(4):50
Sahrawat KL (2004) Iron toxicity in wetland rice and the role of other nutrients. J Plant Nutr 27:1471–1504
Saleque MA, Kirk GJD (1995) Root-induced solubilization of phosphate in the rhizosphere of lowland rice. New Phytol 129:325–336
Septiningsih EM, Prasetiyono J, Lubis E, Tai TH, Tjubaryat T, Moeljopawiro S, McCouch SR (2003) Identification of quantitative trait loci for yield and yield components in an advanced backcross population derived from the Oryza sativa variety IR64 and the wild relative O. rufipogon. Theor Appl Genet 107:1419–1432
Setter TL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H (1988) Environmental factors in deepwater rice areas in Thailand: oxygen, carbon dioxide, and ethylene. In: Proceedings of the 1987 international deepwater rice workshop, International Rice Research Institute, Manila, pp 69–80
Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann Bot 107:89–99
Sorrell BK (1994) Airspace structure and mathematical-modelling of oxygen diffusion, aeration and anoxia in Eleocharis-sphacelata R-BR roots. Aust J Mar Freshw Res 45:1529–1541
Sorrell BK, Armstrong W (1994) On the difficulties of measuring oxygen release by root systems of wetland plants. J Ecol 82:177–183
Steffens B, Geske T, Sauter M (2011) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190:369–378
Suzuki M, Tsukamoto T, Inoue H, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2008) Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol Biol 66:609–617
Sweeney M, McCouch S (2007) The complex history of the domestication of rice. Ann Bot 100:951–957
Thomson CJ, Greenway H (1991) Metabolic evidence for stellar anoxia in maize roots exposed to low O2 concentrations. Plant Physiol 96:1294–1301
Thomson CJ, Colmer TD, Watkin ELJ, Greenway H (1992) Tolerance of wheat (Triticum aestivum cvs. Gamenya and Kite) and triticale (Triticosecale cv. Muir) to waterlogging. New Phytol 120:335–344
Trolldenier G (1988) Visualization of oxidizing power of rice roots and of possible participation of bacteria in iron deposition. Z Pflanzen Boden 151:117–121
Trought MCT, Drew MC (1980) Development of waterlogging damage in wheat seedlings (Triticum-aestivum). 1. Shoot and root growth in relation to changes in the concentrations of dissolved-gases in the soil solution. Plant Soil 54:77–94
van Mensvoort MEF, Lantin RS, Brinkman R, van Breemen N (1985) Toxicities of wetland soils. In: Wetland soils: characterization, classification, and utilization. International Rice Research Institute, Manila, pp 123–138
van Raalte MH (1940) On the oxygen supply of rice roots. Ann Jard Bot Buitenzorg 50:99–114
van Raalte MH (1944) On the oxidation of the environment by the roots of rice (Oryza sativa L.). Ann Jard Bot Buitenzorg Hors Ser 1:15–34
Vaughan DA, Kadowaki K, Kaga A, Tomooka N (2005) On the phylogeny and biogeography of the genus Oryza. Breed Sci 55:113–122
Verhoeven JTA, Setter TL (2010) Agricultural use of wet lands: opportunities and limitations. Ann Bot 105:155–163
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and di-cotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Waters I, Armstrong W, Thompson CJ, Setter TL, Adkins S, Gibbs J, Greenway H (1989) Diurnal changes in radial oxygen loss and ethanol metabolism in roots of submerged and non-submerged rice seedlings. New Phytol 113:439–451
Weiss DJ, Mason TFD, Zhao FJ, Kirk GJD, Coles BJ, Horstwood MSA (2005) Isotopic discrimination of zinc in higher plants. New Phytol 165:703–710
Widodo BMR, Rose T, Frei M, Pariasca-Tanaka J, Yoshihashi T, Thomson M, Hammond JP, Aprile A, Close TJ, Ismail AM, Wissuwa M (2010) Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytol 186:400–414
Wiengweera A, Greenway H (2004) Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. J Exp Bot 55:2121–2129
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convention in waterlogged soil. Ann Bot 80:115–123
Willett IR (1979) The effects of flooding for rice culture on soil chemical properties and subsequent maize growth. Plant Soil 52:373–383
Williams WT, Barber DA (1961) The functional significance of aerenchyma in plants. Symp Soc Exp Biol 15:132–144
Winkel A, Colmer TD, Ismail AM, Pedersen O (2013) Internal aeration of paddy field rice (Oryza sativa L.) during complete submergence – importance of light and floodwater O2. New Phytol 197(4):1193–1203. doi:10.1111/nph.12048
Wissuwa M, Ae N (2001) Further characterization of two QTLs that increase phosphorus uptake of rice (Oryza sativa L.) under phosphorus deficiency. Plant Soil 237:275–286
Wissuwa M, Ismail AM, Yanagihara S (2006) Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol 142:731–741
Xu K, Xia X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene response factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Xu X, Liu X, Ge S, Jensen JD, Hu F, Li X, Dong Y, Gutenkunst RN, Fang L, Huang L, Li J, He W, Zhang G, Zheng X, Zhang F, Li Y, Yu C, Kristiansen K, Zhang X, Wang J, Wright M, McCouch S, Nielsen R, Wang J, Wang W (2012) Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat Biotechnol 30:105–111
Zhu Q, Zheng X, Luo J, Gaut BS, Ge S (2007) Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice. Mol Biol Evol 24:875–888
Acknowledgement
We thank Bill Armstrong for discussions and comments on drafts—his incisive criticisms and constructive suggestions are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kirk, G.J.D., Greenway, H., Atwell, B.J., Ismail, A.M., Colmer, T.D. (2014). Adaptation of Rice to Flooded Soils. In: Lüttge, U., Beyschlag, W., Cushman, J. (eds) Progress in Botany. Progress in Botany, vol 75. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38797-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-38797-5_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38796-8
Online ISBN: 978-3-642-38797-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)