Abstract
Plants have evolved a multitude of mechanisms that adjust photosynthetic functions in the constantly fluctuating light environment. Perception of light stress in chloroplasts initiates local and systemic acclimation processes that involve complex interactions among apoplastic, chloroplastic, and mitochondrial pathways of cellular signaling. Moreover, distinct cell types seem to comprise cell-specific metabolic programs and signaling components, which elicit strictly coordinated changes in gene expression, optimization of photosynthetic machineries, and reprogramming of metabolic pathways and developmental cascades. In this chapter, we discuss the current understanding of systemic signaling in light acclimation in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Light acclimation
- Reactive oxygen species (ROS)
- Excess excitation energy
- Systemic acquired acclimation (SAA)
- Signaling
- High light
1 Introduction
Light is an important environmental factor that provides an energy source for photosynthesis, guides the activity of various metabolic programs, and modulates the growth and development during the entire life cycle of plants. On the other hand, like all living organisms, plants may also suffer detrimental effects if the incoming light energy is not handled with care. In the nature, light conditions undergo constant fluctuations due to climatic variations and seasonal alterations. Changes in cloudiness, wind, or the position of the sun may thus expose plants to light energy in amounts that exceed the need to fuel the ongoing metabolic reactions in chloroplasts. Under such conditions, the prevalence of excess light energy may imbalance the function of the photosynthetic machinery, resulting in decreased photosynthetic activity or even oxidative damage in plant cells. On the other hand, as this chapter emphasizes, perturbations in cellular metabolism elicit signals that activate various acclimation strategies in plant cells. The extent to which a plant is vulnerable to light-induced stress can vary considerably depending on the life history and pre-acclimation status of the plant. Thus, to warn shaded leaf tissues about the possibility of forthcoming scorching by sunshine, light-exposed parts of the plant elicit signals that allow shaded leaves to prepare themselves for potentially deleterious exposure to sunlight. As a whole, light acclimation is an outcome of complex interactions among developmental, environmental, and metabolic signals, which originate from different cellular compartments and converge to ensure growth and survival in changing environmental cues.
2 Local and Systemic Responses in Light Acclimation
2.1 The Concept of Light Stress
Growth and well-being of plants necessitate highly balanced light-harvesting systems that shuttle light energy for a range of light-driven redox reactions, which are tightly coupled with a multitude of pathways inside and outside the chloroplasts. Besides fueling the photo-assimilation of carbohydrates, photosynthetic redox chemistry provides NADPH and ATP for energy-demanding steps in the biosynthesis of amino acids, lipids, hormones, vitamins, and secondary metabolites, which are not only vital for basic metabolism but also have a great impact on the acclimation status of plant cells (Kirk and Leech 1972).
In linear photosynthetic electron flow, electrons are extracted from water at photosystem II (PSII) and shuttled through plastoquinone (PQ), cytochrome b6f complex (Cytb6f), plastocyanin (PC), and photosystem I (PSI) to ferredoxin–NADPH oxidoreductase (FNR) to generate NADPH. Electron transfer through Cytb6f is coupled with proton pumping to thylakoid lumen, generating a trans-thylakoid proton motive force that drives the ATP synthase. The formation of the proton gradient and the production of ATP may become enhanced through cyclic electron transfer reactions, whereby electrons become redirected from PSI to Cytb6f (Arnon 1959; Johnson 2011; Shikanai 2007). A fraction of reductive power is also targeted via PSI and the ferredoxin-thioredoxin reductase (FTR) to thioredoxins, which are small redox-active proteins that modulate the activation state of various target proteins through dithiol/disulfide exchange reactions (Keryer et al. 2004).
Absorption of excess excitation energy may cause over-reduction of the photosynthetic electron transfer chain, thus promoting light stress, which, depending on the severity of stress, may manifest itself as photoinhibition of PSII (Aro et al. 1993; Kok 1956) or enhanced accumulation of reactive oxygen species (ROS). Upon saturation of photosynthetic carbon metabolism and a consequent limitation in the availability of PSI electron acceptors, electron transfer to molecular oxygen (O2) leads to the formation of superoxide (O2 −) and hydrogen peroxide (H2O2) in chloroplast stroma (Liu et al. 2001, 2004). In PSII, imbalanced electron transfer reactions may additionally lead to the formation of singlet oxygen (1O2) (Hideg et al. 1998; Macpherson et al. 1993). Accumulation of various types of ROS is an intrinsic property of the photosynthetic electron transfer chain, and is increasingly recognized among the key mechanisms that relay stress signals in photosynthetic tissues (Foyer and Noctor 2009; Neill et al. 2002).
Different biotic and abiotic stress conditions, which slow down photosynthesis and other enzymatic activities in chloroplasts, may also promote conditions where excitation energy is in excess. Changes in redox signaling pathways and ROS metabolism represent common nominators for different stress responses, and the mechanisms of signaling and resistance against stresses induced by light, salinity, drought, cold, osmotic stress, or plant pathogens are partially overlapping (Li et al. 2009; Navrot et al. 2007). It is therefore not surprising that light acclimation and plant immunity share a level of commonalities in plants (Kangasjärvi et al. 2012; Mühlenbock et al. 2008; Trotta et al. 2011).
2.2 Biochemical and Physiological Adjustments in Local and Systemic Tissues
Plants have evolved a multitude of strategies to cope with short-term and long-term variations in light intensity. Physiological and cellular responses include leaf bending and light-induced chloroplast movements, both of which attempt to decrease the input of excess excitation energy by the photosynthetic pigment–protein complexes. In addition, light acclimation promotes distinct morphological adjustments, including more compact rosettes, thickening of the leaves, and a smaller overall leaf area than the leaves of low-light-grown plants (Kagawa et al. 2001; Park et al. 1996; Walters 2005). On biochemical level, plant acclimation to high light commonly involves enhancement of photosynthetic capacity and upregulation of antioxidant enzymes (Karpinski et al. 1999). In short term, excess excitation energy may become dissipated through the PSII subunit S (PsbS) and xanthophyll-dependent non-photochemical quenching of excitation energy (NPQ) in PSII, redox shuttling to mitochondria and photorespiration (Nunes-Nesi et al. 2011; Peterson and Havir 2001). In addition, plants have evolved tightly redox-controlled mechanisms to regulate the efficiency of the light-harvesting antenna systems to maintain redox balance in chloroplasts. A major contributor in this system is a strictly redox-regulated phosphorylation of light-harvesting (LHCII) antenna proteins by the STN7 kinase, which becomes activated upon reduction of the plastoquinone pool, and inhibited via thioredoxin activity upon reduction of chloroplast stroma (Bellafiore et al. 2005; Rintamäki et al. 2000). By these means, STN7 regulates the association of the mobile LHCII antenna complexes between PSII and PSI, a phenomenon that is a major determinant of the redox status of the thylakoid electron transfer chain (Tikkanen et al. 2006). Currently, these short-term mechanisms are understood as balancing agents that prevent the formation of unnecessary acclimation signals from chloroplasts (Tikkanen et al. 2010).
Extended periods of high light will ultimately lead to a condition where the capacity of the above-mentioned short-term regulation mechanisms become exceeded, resulting in the onset of chloroplast signals that mediate local and systemic acclimation processes in leaves (Karpinski et al. 1999). These responses involve coordinated changes in the expression of chloroplast and nuclear encoded genes, which direct optimization of photosynthetic membrane protein complexes, metabolic pathways, and reprogramming of developmental cascades to best meet the needs of the prevailing environmental cues. Thus, long-term growth under high light intensities results in optimization of photosynthetic reactions through structural modulations in the composition of the photosynthetic protein complexes and their light-harvesting antennae (Anderson et al. 1995; Walters 2005). Such adjustments necessitate tight regulation of genes related to photosynthesis and other metabolic and regulatory pathways (Sheen 1990).
During the recent years, it has become clear that perception of light stress in chloroplasts initiates acclimation signals, which are not only sensed locally in the adjacent leaf cells but also in distal tissues to initiate a systemic acquired acclimation (SAA) in nonexposed parts of the plant (Mühlenbock et al. 2008; Pogson et al. 2008; Rossel et al. 2007). Such signaling mechanisms may be highly relevant in dense populations and canopies, where sun flecks appear during the day when the position of the sun is changing. Whereas the mechanisms of SAA are starting to emerge, reciprocal signaling from shaded leaves to sun-exposed parts has not yet been recognized. Here, we discuss the current understanding of systemic signaling in light acclimation in plants.
3 Protein Components and Metabolic Interactions in Chloroplast Retrograde Signaling
During evolution, a significant proportion of genes encoding the structural and regulatory components of the photosynthetic machinery have been transferred from the chloroplast genome to the nucleus through a process denoted as horizontal gene transfer (Martin et al. 1998). Consequently, photosynthetic pigment–protein complexes are formed by chloroplast and nuclear encoded subunits, and their expression must be tightly coordinated to ensure that the photosynthetic complexes are developed and adjusted in a stoichiometric fashion under constantly changing environmental cues. Although it is clear that the chloroplast and nuclear genomes must be coordinated, the mechanisms of signaling have only started to emerge (Leister 2012). By now, a number of initiating sources for chloroplast signals as well as their consequences on the highly dynamic regulation of nuclear gene expression level have become well established, whereas the mechanisms of signal transduction are not so well understood.
Light receptors, including phytochromes and the blue light receptors CRYPTOCHROME (CRY) 1 and 2, have crucial signaling functions during the greening process, albeit CRY1 is known to mediate chloroplast signals in mature leaves as well (Kleine et al. 2007). In green leaves with fully developed chloroplasts, light-induced acclimation processes essentially involve self-regulatory feedback loops from photosynthetic components (Anderson et al. 1995; Huner et al. 1998; Walters and Horton 1995). Such self-regulation involves chloroplast retrograde signaling whereby organellar signals adjust nuclear gene expression to allow acclimation in changing environmental cues. According to Pogson et al. (2008), these signals can be divided into two classes (1) biogenic control, which ensures stoichiometric synthesis and assembly of the photosynthetic machinery, and (2) operational control, which aims at adjusting and optimizing the function of preexisting photosynthetic complexes under environmental challenges, such as light stress.
Natural environments are characterized by constant changes in temperature, water availability, and light conditions, and living organisms have to cope with these fluctuations by adjusting their metabolism accordingly. Photosynthetic organisms have evolved versatile and partially overlapping systems for sensing, signaling, and responding to a wide range of imbalances in chloroplasts. Plants monitor changes in light conditions by sensing modulations in the redox state of chloroplastic redox components, the PQ-pool PSI electron acceptors, and thiol redox components as well as formation of ROS. Depending on the eliciting signal/environmental condition, these redox components or their combinations mediate key early signaling functions to allow specific adjustment in cell functions (Tikkanen et al. 2010).
Signaling components that participate in the relay of information from chloroplasts to the nucleus have mainly been identified through genetic approaches and include the FLUORESCENT (FLU) protein, which controls the accumulation of 1O2 in chloroplasts (Baruah et al. 2009). The signaling cascade triggered by 1O2 in turn includes the chloroplastic EXECUTER (EX) proteins 1 and 2 as well as the blue light receptor CRYPTOCHROME1 (CRY1), which has also been shown to modulate the high light response in Arabidopsis (Kleine et al. 2007; Lee et al. 2007). The series of genomes uncoupled (gun) mutants revealed a set of chlorophyll biosynthesis enzymes, designated GUN2-5, which contribute to chloroplast signaling cascades through yet undefined mechanisms, albeit the role of ROS or redox-dependent signaling cascades has been implicated (Mochizuki et al. 2008; Moulin et al. 2008). GUN1 has a different function and signals through a chloroplast-localized transcription factor and connects the status of chloroplast gene expression with the regulation of photosynthesis-associated nuclear genes (Kindgren et al. 2012; Koussevitzky et al. 2007). The GUN1 pathway likely operates through the abscisic acid (ABA)-responsive transcription factor ABI4 (ABSCISIC ACID-INSENSITIVE 4), which mediates the repression of nuclear photosynthesis-associated genes, and provides a point of cross-talk with light, ABA, and sugar signaling. In fact, organellar redox signals from both chloroplasts and mitochondria are known to converge at ABI4 in the nucleus (Giraud et al. 2009; Kerchev et al. 2011).
Besides redox-based chloroplast retrograde signals, key roles for chloroplast-derived metabolites in cellular communication systems have also become evident. These include 3′-phosphoadenosine 5′-phosphate (PAP), which translocates from chloroplasts to the nucleus and modulates the expression of specific genes through inhibition of exonuclease activity (Estavillo et al. 2011). Another example of a chloroplast-derived metabolite with a role in retrograde signaling is methylerythritol cyclodiphosphate (MEcPP), an intermediate in the chloroplastic isoprenoid biosynthesis pathway (Xiao et al. 2012). Upon exposure to abiotic stresses (i.e., wounding or high light), MEcPP is accumulated and moves to the nucleus where it induces the expression of the stress marker gene HPL (Hydroperoxide lyase), whereas the expression of photosynthesis-related genes is not changed. This observation suggests that MEcPP operates on a signaling pathway that is distinct from the one elicited in the gun mutants. Altogether, these findings highlight the complexity of mechanisms that allow chloroplasts to respond to an environmental change in a highly specific manner.
4 The Role of Mitochondrial Pathways in Light Acclimation
Metabolic intermediates and reducing equivalents undergo extensive exchange between chloroplasts, cytoplasm, mitochondria, and peroxisomes (Padmasree et al. 2002). These mechanisms are particularly important under excess light, when over-reduction of electron transfer components starts to limit photosynthetic reactions and may lead to photodamage of core enzymes. Plants have evolved processes in which mitochondrial pathways have a particularly important role in the alleviation of cellular stress. In chloroplasts, the level of NADPH can be controlled through oxidation of NADPH to NADP+ by NADP-dependent malate dehydrogenase (NADP-MDH) with simultaneous reduction of oxaloacetate (OAA) to malate (Nunes-Nesi et al. 2011; Taniguchi et al. 2002). NADP-MDH in turn becomes activated upon reduction of the chloroplast Fd-thioredoxin system, and is therefore strictly regulated by light (Schepens et al. 2000). Malate is exported from chloroplasts by a process known as the malate–OAA shuttle, the translocation occurring in exchange for OAA into chloroplast stroma. Malate can be directed into the mitochondrial tricarboxylic acid cycle (TCA) (Yoshida et al. 2007). Alternatively, malate can be oxidized to OAA in the cytoplasm by a simultaneous reduction of NAD+ to NADH, and the resulting OAA and NADH can be consumed in TCA and the mitochondrial electron transport chain (mitETC), respectively. Mitochondrial pathways also participate in quenching of excess reductants through photorespiration, which involves oxidation of glycine by mitochondrial enzymes (Berry et al. 1978). This step results in the formation of NADH, which is used to reduce OAA to malate or directly incorporated into mitETC.
MitETC resides in the inner mitochondrial membrane and involves transport of electrons from NAD(P)H through NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), and to ubiqinone, localized in innermembrane space, from which it is transported to cytochrome bc1 (complex III). Cytochrome c as a free protein transports electrons from complex III to cytochrome c oxidase (complex IV), and finally to oxygen with the formation of water. During these processes, complexes I, III, and IV pump H+ from the mitochondrial matrix to the intermembrane space, creating a proton gradient that drives the ATP synthase (complex V, ATPase).
Plant mitochondria also possess an alternative pathway of mitETC through the alternative oxidase (AOX), which is not present in animal cells. AOX accepts electrons directly from ubiquinone pool, thus limiting electron flow to cytochrome c oxidase pathway. The AOX-dependent pathway does not generate proton gradient and thus does not participate in ATP synthesis through mitochondrial ATPase (Wilson 1980). In addition, plant mitochondria contain non-proton-pumping NAD(P)H dehydrogenases, which allow bypass of complex I as well (Rasmusson et al. 1998). All mitochondria possess also the uncoupling protein (UCP) which serves as yet another non-phosphorylating bypass of mitochondrial electron transfer chain by allowing relaxation of proton gradient between mitochondrial intermembrane space and matrix (Sweetlove et al. 2006).
Under long-term high light conditions, plants upregulate the level of cytochrome c oxidase rather than AOX (Yoshida et al. 2007), presumably to enhance the generation of ATP. The opposite is observed when plants are exposed to a short high light treatment when respiratory chain becomes over-reduced in part due to the high flow of reductants from chloroplasts to mitochondria and AOX becomes highly upregulated. Indeed AOX activity is critical for the maintenance of redox balance in mitochondria, and can be considered as an emergency mechanism activated upon abrupt exposure to stressful light conditions (Yoshida et al. 2011). Tight regulation of redox shuttles lowers the risk for generation of ROS (Dutilleul et al. 2003; Yoshida et al. 2007). AOX1a was found to be induced within 10–60 min after the onset of high light stress. AOX1a promoter activity and transcription abundance were shown to increase in response to treatment with ABA (Giraud et al. 2009). This regulation is likely to be mediated by the transcription factor ABI4, which was shown to interact with a promoter element of AOX1a in biochemical binding assays; moreover, aox1a promoter activity was found to be fully derepressed in abi4 mutant leaves (Giraud et al. 2009). Regulation of the expression of AOX1a via ABI4 therefore provides a direct link between chloroplastic and mitochondrial retrograde signaling (Kerchev et al. 2011; Koussevitzky et al. 2007; Pesaresi et al. 2007). Altogether, leaf mitochondria play a crucial role in light acclimation. The capacity to dissipate excess reductants through photorespiration and the malate–OAA shuttle and consequent alleviation of light stress represent a major subject in the future investigation of the topic of light tolerance in plants.
5 The Role of Bundle Sheath Cells in Systemic Signaling
The process of systemic light acclimation involves a complicated network of signals that spread through the vasculature and induce changes in gene expression and establishment of stress tolerance in the entire plant. Considering the different physiological roles of distinct leaf cell types, it is conceivable that mesophyll cells, vascular tissues, and stomatal guard cells carry out specialized roles in light acclimation. Leaf veins transport water, micronutrients, and metabolic end products between different plant organs, and have also been demonstrated to carry signaling molecules from one part to another (Robert and Friml 2009). In C3 plants, leaf veins are flanked by a layer of elongated chloroplast-containing bundle sheath cells, which due to their central position between the vein and the surrounding mesophyll tissue carry out a specific role in the relay of light-dependent acclimation signals (Leegood 2008). Vascular bundle sheath cells therefore possess far-reaching and thus far largely unidentified regulatory roles in light acclimation.
Arabidopsis mutants with reticulated leaf phenotypes have provided evidence suggesting that bundle sheath-specific metabolic processes specifically modulate leaf development and acclimation in a light-dependent manner (Barth and Conklin 2003; Kinsman and Pyke 1998; Knappe et al. 2003; Lepistö et al. 2009; Overmyer et al. 2008). These mutants typically exhibit a conditional phenotype with dark green paraveinal and light green interveinal regions caused by metabolic perturbations in at least some experimental conditions or during a specific developmental stage. Classical examples include cue1 (chlorophyll a/b binding protein underexpressed 1; Li et al. 1995), reticulata (re; González-Bayón et al. 2006), and the more recently identified ntrc deficient in a chloroplastic NADPH-dependent thioredoxin reductase (NTRC; Lepistö et al. 2009).
CUE1 encodes the phosphoenolpyruvate/phosphate translocator of the chloroplast envelope inner membrane (Knappe et al. 2003; Streatfield et al. 1999). Deficiencies in the function of CUE1 therefore deteriorate several chloroplastic metabolic routes and drastically affect the biosynthesis of aromatic amino acids (Knappe et al. 2003; Voll et al. 2003). The reticulata (re) mutant visually resembles cue1 but exhibits the reticulate phenotype conditionally when grown under long day conditions (González-Bayón et al. 2006). RE encodes a putative transmembrane protein likely to be localized in the chloroplast envelope (Zybailov et al. 2008). The same mutant was also identified as lower cell density1/radical-induced cell death2 (lcd1/rcd2) in two separate screens for ozone sensitivity (Barth and Conklin 2003; Overmyer et al. 2008). It is notable that re plants were also found to be sensitive to infection by the bacterial plant pathogen Pseudomonas syringae, suggesting that RE forms a component common for light acclimation and immunity in plants (Barth and Conklin 2003; Overmyer et al. 2008). Indeed, re plants accumulate H2O2 in the vascular leaf tissues, a phenomenon commonly observed when leaves are exposed to light stress (Karpinski et al. 1999; Overmyer et al. 2008). These findings on cue and re highlight the importance of transporters and signaling components of the chloroplast envelope membrane in plant development and acclimation.
Apart from cue1 and re, young ntrc leaves exhibit a uniform pale green phenotype, but undergo a transient phase of reticulation during greening that occurs in aging ntrc leaves (Lepistö et al. 2009). Upon aging, the veins of ntrc plants turn green, and gradually the entire leaf acquires wild-type levels of chlorophyll (Lepistö et al. 2009). NTRC is likely to regulate a multitude of thiol‐redox regulated processes in chloroplasts, such as starch metabolism (Michalska et al. 2009), chlorophyll biosynthesis, amino acid metabolism, and the biosynthesis of auxin (Lepistö et al. 2009). Moreover, NTRC has been shown to act as a reducing agent for 2-cysteine peroxiredoxin, and may thus be crucial for ROS-based signaling in chloroplasts (Pérez-Ruiz et al. 2006).
Altogether, although the molecular mechanisms underlying the reticulated pigmentation in cue1, re, and ntrc remain unresolved, these mutant characteristics have unequivocally demonstrated the existence of specified metabolic properties and unique characteristics of ROS tolerance in bundle sheath cells. Because of different physiological properties of bundle sheath cells, it can be assumed that the signaling and gene expression regulated by these signals vary as well (Fryer et al. 2003; Kangasjärvi et al. 2008, 2009).
6 Interplay Among Chloroplastic and Apoplastic Pathways in Systemic Signaling
The pioneering observation of systemic light acclimation suggested that light-induced accumulation of H2O2 in vascular leaf areas initiated systemic signaling, which induced stress resistance in high-light-illuminated Arabidopsis plants (Fryer et al. 2003; Karpinski et al. 1999). In further studies on the mechanisms of systemic signaling, ROS production in the apoplast of bundle sheath cells was associated with NADPH activity and found to become enhanced upon biosynthesis of ABA in adjacent cells of vascular parenchyma (Galvez-Valdivieso et al. 2009). In addition, Szechyńska-Hebda et al. (2010) proposed the involvement of photoelectron-physiological signaling (PEPS) in long-distance mechanisms of light acclimation.
These findings suggested the involvement of plasma membrane electrical potential, NADPH oxidases, and chloroplasts as sources of ROS in systemic signaling, and this signaling interaction most likely involves also the heterotrimeric G-proteins (Joo et al 2005). Moreover, besides H2O2, singlet oxygen also seems to accumulate predominantly along leaf vasculature (Suorsa et al. 2012), but the significance of this observation with respect to light acclimation remains obscure. NADPH oxidases are integral plasma membrane proteins composed of six transmembrane domains supporting two heme groups. The two independent hemes and FAD are required for transfer of electrons from NADPH, which serves as cytoplasmic electron donor across the membrane to oxygen, the extracellular electron acceptor, to generate the superoxide radical (Sagi and Fluhr 2006). In a secondary reaction, O2 − is dismutated to hydrogen peroxide.
Whereas the involvement of the above-mentioned components in systemic light acclimation is clear, the sequence of events remains poorly understood. Evidence has accumulated suggesting that NADPH oxidase-dependent apoplastic ROS production is followed by ROS production in the chloroplast, and that there is regulatory hierarchy in signal transfer from the apoplast to the chloroplast (Joo et al. 2005; Nomura et al. 2012). Such close signaling interaction is facilitated by the small physical distance between the chloroplast and the apoplast. In plant cells, the chloroplast envelope is often observed in a very close proximity with the plasma membrane, which should allow rapid and efficient communication between the two cellular compartments.
The finding that NADPH oxidases contribute to systemic signaling over long distances led to the formation of the concept termed the “ROS wave” (Mittler et al. 2011). Besides high light, this mechanism was shown to be induced by wounding, cold, heat, and salinity (Miller et al. 2009). Intriguingly, in all cases, formation of the ROS signal was found to depend on the specific NADPH oxidase isoform AtRbohD. Analysis of an Arabidopsis line expressing a luciferase reporter gene under the control of a ROS-responsive ZAT12 promoter revealed that application of a local stress treatment elicited luciferase activity both at the site of treatment and in systemic untreated tissues. The signal propagation was rapid, traveling at a rate of 8.4 cm/min along the stem axis, and coincided with apoplastic ROS accumulation in wild-type plants but was suppressed in atrbohD mutant plants (Miller et al. 2009). These findings led to a conclusion that AtRbohD generates a ROS signal capable of triggering and maintaining an auto-propagating ROS wave that travels from cell to cell across long distances. This also clarifies the early observations where H2O2 was shown to have a key role in systemic acclimation responses under high light stress (Fryer et al. 2003; Rossel et al. 2007). How the ROS signal is perceived and how the ROS burst becomes triggered in the neighboring cells, however, will be matters of future breakthrough.
With respect to bundle sheath-specific pathways of ROS signaling, it is intriguing that chloroplasts located along the veins in Arabidopsis leaves were found to be less sensitive to light stress than the mesophyll cell chloroplasts. This became evident upon phenotypic characterization of tapx sapx double mutants deficient in the chloroplastic stromal and thylakoid-bound ascorbate peroxidases, or tapx sapx vtc2 triple mutants deficient in both chloroplast ascorbate peroxidase and ascorbate. Both mutants showed bleaching of interveinal leaf tissues upon methyl-viologen treatment or illumination under high light (Giacomelli et al. 2007; Kangasjärvi et al 2008). Studies have also revealed that genes related to plant antioxidant network may be preferentially expressed in the paraveinal regions of leaves. These gene products include the cytoplasmic ascorbate peroxidase 2 (APX2), a microRNA (miR398) that targets the Cu/Zn superoxide dismutase (Sunkar et al. 2006) and a chloroplastic glutaredoxin (Cheng et al. 2006). The physiological significance of APX2 induction or the extent to which APX2 is required to quench the ROS in the cytoplasm of bundle sheath cells, however, is not clear. As a whole, the physiological significance of cell-type-specific expression of antioxidant components in systemic light signaling remains to be elucidated.
7 Implications for Hormonal Signaling in Systemic Light Acclimation
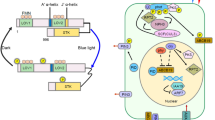
Whereas the role for ROS as a messenger in systemic signaling seems clear, it is likely that the SAA response additionally involves other thus far unknown components, which promote gene expression changes in shaded leaves. SAA might be caused by a mobile signaling molecule eliciting the response, a system analogous to the regulation of flowering through FLORIGEN, which migrates from the shoot apex to initiate flowering in shoot apical meristem of buds in response to photoperiodism (Turck et al. 2008) (Figs. 1 and 2).
Emerging mechanisms of systemic light signaling. Under excess light energy, chloroplasts initiate signals that trigger acclimation processes by adjusting gene expression in the nucleus. Over-reduction of photosynthetic electron acceptors also activates metabolic shuttles, whereby reducing equivalents are translocated to mitochondria, where the AOX pathway plays a major role in dissipating excess reducing energy under abrupt light stress. In the cytoplasm, NADPH serves as a substrate for plasmamembrane NADPH oxidases, the activity of which leads to generation of ROS in the apoplast. Through yet unknown mechanisms, successive bursts in ROS allow spreading of the stress signals to neighboring cells through apoplastic “ROS waves.” Long-distance signal transduction occurs through vascular tissues. Spreading of high light signals from exposed leaves triggers gene expression changes, classically exemplified by induction of APX2, also in the shaded leaves allowing the entire plant to prepare itself for potential future light stress
The mechanism of SAA seems to share a level of commonalities with those mediating the systemic acquired resistance (SAR), which becomes induced in uninfected parts of a pathogen-exposed plant (Mullineaux et al. 2000). Upon systemic induction of immune reactions, a mobile signaling molecule is transported to distal uninfected parts of the plant where it induces formation of ROS and the expression of pathogenesis-related genes.
In SAR, multiple phytohormones and other small signaling compounds including H2O2, jasmonic acid, salicylic acid, and azelaic acid have been suggested to function as a mobile signal from the infection site to distal leaves. However, no single substance has been found to mediate all features of SAR. Signals are transduced through the vascular tissue as well as air by volatile compound (Shah 2009). Heil and Ton (2008) proposed a model where distal defense responses are self-primed by volatile signals, while vascular signaling is needed for their full elicitation. Upon systemic signaling taking place after wounding, it has been proposed that the signal to distal leaves is transmitted through the cells of vascular parenchyma as a signal where jasmonate induces its own biosynthesis in the neighboring cells, thus moving the signal along the vascular tissue (Heil and Ton 2008). A similar kind of model might explain also the systemic acclimation to abiotic stresses.
Recent findings suggest that light acclimation involves cross-talk with components related to salicylic acid, jasmonic acid, and ethylene dependent defense signaling pathways (Mühlenbock et al. 2008). SAA, however, does not seem to depend on the perception or signaling through these stress hormones, and in this respect seems to be mediated by mechanisms different from SAR. Rossel et al. (2007) analyzed the jasmonic acid- or salicylic acid-related signaling mutants jar1-1, jin1, npr1, and NahG; all exhibited the systemic light acclimation response, albeit to a lesser extent than wild-type plants. Indeed, as yet there is no evidence that any of these hormones would directly act as a mobile signal for triggering systemic light acclimation responses in the nonexposed tissues.
A zinc finger transcription factor ZAT10 has been shown to regulate the expression of a set of same genes that are activated in distal and high light exposed leaves during SAA. ZAT10 becomes rapidly induced in leaf vasculature in both the exposed and the shaded tissues, with the exception of roots, after the onset of light stress, pointing to the role of ZAT10 in only green parts of plant. The induction of ZAT10 correlates with enhanced expression of antioxidant genes and increased tolerance against light-induced damage of PSII in the light-exposed leaves as well as tolerance to exogenous H2O2 (Rossel et al. 2007). Overexpression of ZAT10 results in a stunted mutant phenotype and promotes enhanced tolerance to various abiotic stresses including drought and high light. Thus ZAT10 may regulate the genes activated both in high light and drought (Mittler et al. 2006). The MAP kinases MAPK3 and MAPK6 have been shown to directly interact and phosphorylate ZAT10 (Nguyen et al 2012). Moreover, MAPK3 and MAPK6 mediate the onset of defense signaling in response to pathogens (Asai et al. 2002). This interaction with ZAT10 offers a route through which the responses to various stresses can be regulated and explains the elevated resistance to multiple stress conditions.
ZAT10 and another similar transcription factor ZAT12 are induced under a range of stress conditions, including high light, drought, salt, and cold (Lee et al. 2002: Sakamoto et al. 2000). Intriguingly, the induction of ZAT10 and ZAT12 was delayed upon high light illumination of the chloroplast signaling mutants gun1 and abi4 compared to wild-type plants (Koussevitzky et al. 2007), which raises the question whether the GUN1/ABI4 pathway also mediates systemic signals. A notable overlap exists in the gene expression changes taking place after shift to high light and the treatment with exogenous ABA (Kimura et al. 2003). Some of the overlap can be explained with the sudden decrease in the cell water potential after the shift to high light. What is more, functional ABA biosynthesis is required for the high light gene expression. The regulation may take place through a pathway where the protein kinase OPEN STOMATA 1 (OST1) positively regulates the accumulation of H2O2, which in turn induces the high light genes (Galvez-Valdivieso et al. 2009). One can assume that a number of yet unidentified transcription factors and other signaling components that allow plants to record and signal the severity of environmental stress are awaiting discovery.
8 APX2 as a Marker for Multiple Pathways in Light Stress Responses
Induction of the gene encoding the antioxidant enzyme APX2 is a classical marker for high light stress (Mullineaux et al. 2000). APX2 was also one of the first genes shown to be systemically upregulated in the vascular tissue of distal leaves in response to H2O2 accumulating during local high light exposure (Bechtold et al. 2008). In subsequent studies, transgenic Arabidopsis plants expressing luciferase under the promoter of APX2 have been instrumental in dissecting the components underlying systemic light acclimation in leaves. Besides high light, APX2 can be induced by heat and drought (Fryer et al. 2003). The transcriptional induction of APX2 is governed by multiple overlapping signals including light-induced reduction of the plastoquinone pool, accumulation of chloroplastic and apoplastic ROS, glutathione metabolism, ABA signaling, and the PAP-dependent retrograde signaling pathway (Ball et al. 2004; Estavillo et al. 2011; Fryer et al. 2003; Karpinski et al. 1999). Photorespiratory H2O2 production in peroxisomes, however, does not seem to have a notable role in the high-light-induced APX2 expression (Fryer et al 2003).
The high-light-inducible expression of APX2 was also found to be positively regulated by OST1 and negatively controlled by the G-protein coupled receptor. In ost1-1 mutants, APX2 was not induced under high light, whereas some other high light markers such as ELIP1 and HSP17.6 were expressed like in wild-type plants (Galvez-Valdivieso et al. 2009; Volkov et al. 2006). OST1 and the G-proteins are well-known components in the regulation of stomatal movements (Merlot et al. 2002). Thus, mechanisms governing systemic light acclimation and the stomatal aperture seem to share common components, albeit the extent to which stomatal guard cells contribute to light acclimation is currently not understood. Altogether, the amplitude of APX2 gene expression, indicative of the onset of systemic acclimation responses in bundle sheath cells, is controlled by overlapping pathways that respond to the water balance, metabolic homeostasis, and cellular ROS/redox state in Arabidopsis leaves.
9 Conclusions
Maintenance of cellular homeostasis plays a crucial role in plant “survival of the fittest.” During evolution, plants have evolved a catalogue of mechanisms to cope with harsh environmental conditions that can be expected to change in time. A key phenomenon in plant acclimation is known as systemic light signaling, whereby shaded parts of the plant receive signals from light-stressed leaf tissues and develop adjustments for potentially upcoming stress conditions. Tight regulation of redox balance and metabolite shuttling between organelles represent vital mechanisms in the maintenance of metabolic homeostasis, and allow dissipation of excess reductants that otherwise would block and damage the photosynthethic apparatus.
Systemic signaling upon perception of high light stress is of basic importance in the evolutionary calendar of plants as sessile organisms. The emerging picture on systemic light acclimation indicates that chloroplast retrograde signals cross-communicate with apoplastic pathways through yet undefined molecular interactions. Considerable research efforts are therefore still needed to identify the signaling components and receptors that mediate and determine the acclimation strategies in local and systemic leaf tissues under light stress.
References
Anderson JM, Chow WS, Park YI (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139
Arnon DI (1959) Conversion of light into chemical energy in photosynthesis. Nature 184:10–21
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977–983
Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, Karpinski S, Mullineaux PM (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16:2448–2462
Barth C, Conklin PL (2003) The lower cell density of leaf parenchyma in the Arabidopsis thaliana mutant lcd1-1 is associated with increased sensitivity to ozone and virulent Pseudomonas syringae. Plant J 35:206–218
Baruah A, Simková K, Apel K, Laloi C (2009) Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol Biol 70:547–563
Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM (2008) Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J Exp Bot 59:121–133
Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433:892–895
Berry JA, Osmond CB, Lorimer GH (1978) Fixation of 18O2 during photorespiration. Plant Physiol 62:954–967
Cheng NH, Liu JZ, Brock A, Nelson RS, Hirschi KD (2006) AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J Biol Chem 281:26280–26288
Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, de Paepe R (2003) Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 15:1212–1226
Estavillo GM, Crisp PA, Pornsiriwong W, Wirtz M, Collinge D, Carrie C, Giraud E, Whelan J, David P, Javot H, Brearley C, Hell R, Marin E, Pogson BJ (2011) Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23:3992–4012
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905
Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33:691–705
Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smirnoff N, Asami T, Davies WJ, Jones AM, Baker NR, Mullineaux PM (2009) The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21:2143–2162
Giacomelli L, Masi A, Ripoll DR, Lee MJ, van Wijk KJ (2007) Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol Biol 65:627–644
Giraud E, Van Aken O, Ho LHM, Whelan J (2009) The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol 150:1286–1296
González-Bayón R, Kinsman EA, Quesada V, Vera A, Robles P, Ponce MR, Pyke KA, Micol JL (2006) Mutations in the RETICULATA gene dramatically alter internal architecture but have little effect on overall organ shape in Arabidopsis leaves. J Exp Bot 57:3019–3031
Heil M, Ton J (2008) Long-distance signalling in plant defence. Trends Plant Sci 13:264–272
Hideg E, Kálai T, Hideg K, Vass I (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 37:11405–11411
Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 6:224–230
Johnson GN (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807:906–911
Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17:957–970
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291:2138–2141
Kangasjärvi S, Lepistö A, Hännikäinen K, Piippo M, Luomala EM, Aro EM, Rintamäki E (2008) Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem J 412:275–285
Kangasjärvi S, Nurmi M, Tikkanen M, Aro EM (2009) Cell-specific mechanisms and systemic signalling as emerging themes in light acclimation of C3 plants. Plant Cell Environ 32:1230–1240
Kangasjärvi S, Neukermans J, Li S, Aro EM, Noctor G (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J Exp Bot 63:1619–1636
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657
Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH (2011) The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23:3319–3334
Keryer E, Collin V, Lavergne D, Lemaire S, Issakidis-Bourguet E (2004) Characterization of Arabidopsis mutants for the variable subunit of ferredoxin:thioredoxin reductase. Photosynth Res 79:265–274
Kimura M, Yamamoto YY, Seki M, Sakurai T, Sato M, Abe T, Yoshida S, Manabe K, Shinozaki K, Matsui M (2003) Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem Photobiol 77:226–233
Kindgren P, Kremnev D, Blanco NE, de Dios Barajas López J, Fernández AP, Tellgren-Roth C, Kleine T, Small I, Strand A (2012) The plastid redox insensitive 2 mutant of Arabidopsis is impaired in PEP activity and high light-dependent plastid redox signalling to the nucleus. Plant J 70:279–291
Kinsman EA, Pyke KA (1998) Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development 125:1815–1822
Kirk PR, Leech RM (1972) Amino acid biosynthesis by isolated chloroplasts during photosynthesis. Plant Physiol 50:228–234
Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand A (2007) Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 144:1391–1406
Knappe S, Löttgert T, Schneider A, Voll L, Flügge UI, Fischer K (2003) Characterization of two functional phosphoenolpyruvate/phosphate translocator (PPT) genes in Arabidopsis–AtPPT1 may be involved in the provision of signals for correct mesophyll development. Plant J 36:411–420
Kok B (1956) On the inhibition of photosynthesis by intense light. Biochim Biophys Acta 21:234
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316:715–719
Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Zhu JK (2002) LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO J 21:2692–2702
Lee KP, Kim C, Landgraf F, Apel K (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA 104:10270–10275
Leegood RC (2008) Roles of the bundle sheath cells in leaves of C3 plants. J Exp Bot 59:1663–1673
Leister D (2012) Retrograde signaling in plants: from simple to complex scenarios. Front Plant Sci 3:135
Lepistö A, Kangasjärvi S, Luomala EM, Brader G, Sipari N, Keränen M, Keinänen M, Rintamäki E (2009) Chloroplast NADPH-thioredoxin reductase interacts with photoperiodic development in Arabidopsis. Plant Physiol 149:1261–1276
Li H, Culligan K, Dixon RA, Chory J (1995) CUE1: a mesophyll cell-specific positive regulator of light-controlled gene expression in Arabidopsis. Plant Cell 7:1599–1610
Li ZR, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Liu K, Sun J, Liu Y, Zhang QY, Kuang TY (2001) ESR study on superoxide radicals generated in photosystem II of higher plant. Prog Biochem Biophys 28:372–376
Liu K, Sun J, Song YG, Liu B, Xu YK, Zhang SX, Tian Q, Liu Y (2004) Superoxide, hydrogen peroxide and hydroxyl radical in D1/D2/cytochrome b-559 photosystem II reaction center complex. Photosynth Res 81:41–47
Macpherson AN, Telfer A, Truscott TG, Barber J (1993) Direct detection of singlet oxygen from isolated photosystem II reaction centres. Biochim Biophys Acta 1143:301–309
Martin W, Stoebe B, Goremykin V, Hapsmann S, Hasegawa M, Kowallik KV (1998) Gene transfer to the nucleus and the evolution of chloroplasts. Nature 393:162–165
Merlot S, Mustilli AC, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30:601–609
Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P (2009) NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA 106:9908–9913
Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2:ra45
Mittler R, Kim Y, Song L, Coutu J, Coutu A, Ciftci-Yilmaz S, Lee H, Stevenson B, Zhu JK (2006) Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett 580:6537–6542
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A (2008) The steady-state level of mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signalling in Arabidopsis. Proc Natl Acad Sci USA 105:15184–15189
Moulin M, McCormac AC, Terry MJ, Smith AG (2008) Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signalling is not due to mg-protoporphyrin IX accumulation. Proc Natl Acad Sci USA 105:15178–15183
Mühlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, Parker JE, Karpinska B, Karpinski S (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20:2339–2356
Mullineaux P, Ball L, Escobar C, Karpinska B, Creissen G, Karpinski S (2000) Are diverse signalling pathways integrated in the regulation of arabidopsis antioxidant defence gene expression in response to excess excitation energy? Philos Trans R Soc Lond B Biol Sci 355:1531–1540
Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Nguyen XC, Kim SH, Lee K, Kim KE, Liu XM, Han HJ, Hoang MH, Lee SW, Hong JC, Moon YH, Chung WS (2012) Identification of a C2H2-type zinc finger transcription factor (ZAT10) from Arabidopsis as a substrate of MAP kinase. Plant Cell Rep 31:737–745
Nomura H, Komori T, Uemura S, Kanda Y, Shimotani K, Nakai K, Furuichi T, Takebayashi K, Sugimoto T, Sano S, Suwastika IN, Fukusaki E, Yoshioka H, Nakahira Y, Shiina T (2012) Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun 3:926
Nunes-Nesi A, Araujo WL, Fernie AR (2011) Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol 155:101–107
Overmyer K, Kollist H, Tuominen H, Betz C, Langebartels C, Wingsle G, Kangasjärvi S, Brader G, Mullineaux P, Kangasjärvi J (2008) Complex phenotypic profiles leading to ozone sensitivity in Arabidopsis thaliana mutants. Plant Cell Environ 31:1237–1249
Padmasree K, Padmavathi L, Raghavendra AS (2002) Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit Rev Biochem Mol Biol 37:71–119
Park YI, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111:867–875
Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ (2006) Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18:2356–2368
Pesaresi P, Schneider A, Kleine T, Leister D (2007) Interorganellar communication. Curr Opin Plant Biol 10:600–606
Peterson RB, Havir EA (2001) Photosynthetic properties of an Arabidopsis thaliana mutant possessing a defective PsbS gene. Planta 214:142–152
Pogson BJ, Woo NS, Förster B, Small ID (2008) Plastid signalling to the nucleus and beyond. Trends Plant Sci 13:602–609
Rasmusson AG, Heiser V, Irrgang KD, Brennicke A, Grohmann L (1998) Molecular characterisation of the 76 kDa iron-sulphur protein subunit of potato mitochondrial complex I. Plant Cell Physiol 39:373–381
Rintamäki E, Martinsuo P, Pursiheimo S, Aro EM (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97:11644–11649
Robert HS, Friml J (2009) Auxin and other signals on the move in plants. Nat Chem Biol 5:325–332
Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ (2007) Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19:4091–4110
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Sakamoto H, Araki T, Meshi T, Iwabuchi M (2000) Expression of a subset of the Arabidopsis Cys(2)/His(2)-type zinc-finger protein gene family under water stress. Gene 248:23–32
Schepens I, Johansson K, Decottignies P, Gillibert M, Hirasawa M, Knaff DB, Miginiac-Maslow M (2000) Inhibition of the thioredoxin-dependent activation of the NADP-malate dehydrogenase and cofactor specificity. J Biol Chem 275:20996–21001
Shah J (2009) Plants under attack: systemic signals in defence. Curr Opin Plant Biol 12:459–464
Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2:1027–1038
Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58:199–217
Streatfield SJ, Weber A, Kinsman EA, Häusler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flugge UI, Chory J (1999) The phosphoenolopyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development and plastid-dependent nuclear gene expression. Plant Cell 11:1609–1621
Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18:2051–2065
Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro EM (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24:2934–2948
Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR (2006) Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 103:19587–19592
Szechyńska-Hebda M, Kruk J, Górecka M, Karpińska B, Karpiński S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22:2201–2218
Taniguchi M, Taniguchi Y, Kawasaki M, Takeda S, Kato T, Sato S, Tabata S, Miyake H, Sugiyama T (2002) Identifying and characterizing plastidic 2-oxoglutarate/malate and dicarboxylate transporters in Arabidopsis thaliana. Plant Cell Physiol 43:706–717
Tikkanen M, Piippo M, Suorsa M, Sirpiö S, Mulo P, Vainonen J, Vener AV, Allahverdiyeva Y, Aro EM (2006) State transitions revisited-a buffering system for dynamic low light acclimation of Arabidopsis. Plant Mol Biol 62:779–793
Tikkanen M, Grieco M, Kangasjärvi S, Aro EM (2010) Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 152:723–735
Trotta A, Wrzaczek M, Scharte J, Tikkanen M, Konert G, Rahikainen M, Holmström M, Hiltunen HM, Rips S, Sipari N, Mulo P, Weis E, von Schaewen A, Aro EM, Kangasjärvi S (2011) Regulatory subunit B′ gamma of protein phosphatase 2A prevents unnecessary defense reactions under low light in Arabidopsis. Plant Physiol 156:1464–1480
Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59:573–594
Volkov RA, Panchuk II, Mullineaux PM, Schöffl F (2006) Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61:733–746
Voll L, Häusler RE, Hecker R, Weber A, Weissenböck G, Fiene G, Waffenschmidt S, Flügge UI (2003) The phenotype of the Arabidopsis cue1 mutant is not simply caused by a general restriction of the shikimate pathway. Plant J 36:301–317
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447
Walters RG, Horton P (1995) Acclimation of Arabidopsis thaliana to light environment: regulation of chloroplast composition. Planta 197:475–481
Wilson SB (1980) Energy conservation by the plant mitochondrial cyanide-insensitive oxidase. Some additional evidence. Biochem J 190:349–360
Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K (2012) Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149:1525–1535
Yoshida K, Terashima I, Noguchi K (2007) Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant Cell Physiol 48:606–614
Yoshida K, Watanabe CK, Hachiya T, Tholen D, Shibata M, Terashima I, Noguchi K (2011) Distinct responses of the mitochondrial respiratory chain to long- and short-term high-light Environments in Arabidopsis thaliana. Plant Cell Environ 34:618–628
Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3(4):e1994
Acknowledgments
This work was supported by Academy of Finland projects 263772, 218157, and 130595 for SK and Turku University Finnish Doctoral Program in Plant Science for GK.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Konert, G., Rahikainen, M., Trotta, A., Kangasjärvi, S. (2013). Systemic Signaling in Light Acclimation of Leaves. In: Baluška, F. (eds) Long-Distance Systemic Signaling and Communication in Plants. Signaling and Communication in Plants, vol 19. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36470-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-36470-9_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36469-3
Online ISBN: 978-3-642-36470-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)