Abstract
Plant immunity relies on two cell autonomous immune pathways present in each cell and on systemic signals emanating from local challenged sites, which enhance immunity in distal unchallenged cells. Activation of these different immune branches entails extensive transcriptional reprogramming of a largely common set of defense-related genes, leading to the termination or restriction of pathogen propagation at the cost of plant growth. Emerging evidence points to a role of chromatin remodeling and dynamics as a key mechanistic basis for timely and appropriate activation of immune response in plants. One such phenomenon that appears to be under epigenetic control involves defense priming that is conditioned upon immune activation or interactions with beneficial microbes. In defense priming, target defense-related genes are not actively transcribed but poised for a greater and/or faster activation upon second stimulation. Moreover, a growing list of nuclear-localized pathogen effectors also implies their possible role in the alteration of host chromatin configuration for virulence promotion. Epigenetic control of defense-related genes seems to represent an as-yet-underexplored interface during plant–pathogen interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Plants as sessile organisms cope with a wide range of microbes in an environment, including infectious pathogens that can cause disease. In addition to constitutive physical and biochemical defense barriers, plants have evolved an elaborate multilayered innate immune system to resist the majority of pathogenic microbes. Based on the feeding lifestyles, plant pathogens are largely classified into three classes: biotrophic pathogens feed on living plant cells, necrotrophic pathogens actively destroy and kill host cells to obtain nutrients, and hemi-biotrophic pathogens switch their feeding styles between the two and require living host cells during part of their life cycle (Glazebrook 2005). Plants selectively activate appropriate immune response according to the infection styles of the pathogens encountered, which is achieved at the cost of growth-related physiological processes. In addition, tradeoffs exist between different immune branches, in which the activation of one branch negatively influences another branch. This also comes at fitness costs beyond the direct energy costs required for defense execution. Critical components of plant immunity, in particular in the interactions with biotrophic and hemi-biotrophic pathogens, include two classes of immune receptors that detect nonself molecules or altered host cellular states upon pathogen challenges. Immune receptors, upon the recognition of their specific ligands, trigger a set of cellular outputs including extensive transcriptional reprogramming during immune activation. This signaling process is influenced and fine-tuned by a network of phytohormones that are also engaged in the adaptation to different abiotic stresses in the environment, thereby allowing plants to coordinate between different stress responses and growth. At present, it is thought that all living plant cells possess these immune components and, thus, the ability to detect and react to pathogens (Jones and Dangl 2006).
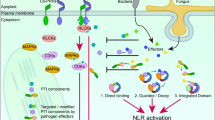
One receptor class consists of the so-called pattern recognition receptors (PRRs) that detect molecular structures typically conserved in many microbial species, designated microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs). MAMPs include bacterial flagellin, the elongation factor EF-Tu, lipopolysaccharides (LPS), peptidoglycans, and components of fungal cell walls such as chitin fragments (N-acetyl-chitooligosaccharide oligomers) (Boller and Felix 2009; Segonzac and Zipfel 2011). MAMP perception by cognate PRRs triggers immune response that restricts the invasion and/or multiplication of potential infectious microbes, termed MAMP-triggered immunity (MTI), which provides a first line of inducible basal defenses against pathogens (Boller and Felix 2009; Segonzac and Zipfel 2011). MTI activation is accompanied by a stereotypic set of defense-associated cellular outputs, such as changes of ion fluxes across the membranes, production of reactive oxygen species (ROS) into extracellular apoplastic spaces, MAPK activation, ethylene production, callose deposition, but also extensive transcriptional reprogramming and metabolic changes. Loss of single PRRs renders plants more susceptible to adapted and non-adapted pathogens, providing evidence for the significance of MTI in plant immunity (Segonzac and Zipfel 2011).
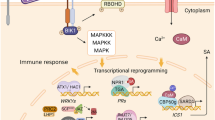
For successful infection, adapted pathogens avoid and/or suppress MTI activation with a series of effectors injected into the host, which promote virulence in the absence of their immune recognition. As a strategy to counteract them, plants evolved a second class of intracellular immune receptors, termed the disease resistance (R) proteins, that detect the structure or actions of cognate pathogen effectors encoded by avirulence (AVR) genes that are typically isolate specific. Nucleotide-binding leucine-rich repeat (NB-LRR) proteins represent the major class of R proteins. NB-LRR receptors are classified into two subclasses defined by their N-terminal domains, namely, Toll/Interleukin-1 Receptor (TIR) and coiled-coil (CC) domains. Effector recognition by R proteins triggers more dramatic immune response than MTI, designated effector-triggered immunity (ETI) (Fig. 1). ETI typically culminates in hypersensitive response (HR), a host cell death at attempted challenge sites (Chisholm et al. 2006; Jones and Dangl 2006). Like MTI, ETI also entails extensive transcriptional reprogramming of a largely overlapping set of defense-related genes. However, of note, these target genes in general undergo faster, greater, and/or more prolonged expression during ETI than during MTI (Tao et al. 2003; Caldo et al. 2004). The differences of transcriptional outputs between MTI and ETI in the amplitude and kinetics rather than in target genes per se lead to the notion that signaling events and outputs (e.g., transcriptional reprogramming) of MTI are accelerated by R protein-triggered signaling during ETI (Tao et al. 2003). However, the mechanistic basis for the differences remains almost unknown. In this respect, it should be noted that immune function of a subset of NB-LRR receptors requires their direct actions in the nucleus (Shen et al. 2007; Garcia and Parker 2009), although not all NB-LRR receptors function in the nucleus, which suggests a close functional link between their triggered ETI signaling and the nuclear machineries engaged in the control of gene expression (Shen et al. 2007; Garcia and Parker 2009). It is therefore conceivable that differential nuclear events underlie the aforementioned differences in the mode of transcriptional reprogramming between MTI and ETI.
Different immune branches in plant immunity. A first layer of inducible defenses is mounted by pattern recognition-receptors (PRR) at the membranes, upon the recognition of microbe-associated molecular patterns (MAMPs), termed MAMP-triggered immunity (MTI). Successful pathogens overcome MTI by evading PRR recognition and/or by secreting effectors into the cell that suppress MTI. Plants have evolved R proteins, of which the dominant class is represented by nucleotide-binding domain Leu-rich repeat (NB-LRR) proteins. Direct or indirect recognition of effectors leads to effector-triggered immunity (ETI). MTI and ETI activation both trigger the release of a systemic signal, which in turn leads to systemic acquired resistance (SAR)
Both MTI and ETI at local challenged sites trigger the release of a systemic signal(s), of which the identity remains elusive or controversial, to induce an enhanced state of cellular immunity at distal non-challenged sites, designated systemic acquired resistance (SAR) (Dempsey and Klessig 2012). SAR is long lasting, occasionally even for the lifetime of the plant, and effective against secondary infection by a broad range of pathogens (Durrant and Dong 2004). SAR is characterized by, e.g., accumulation of the defense-related phytohormone salicylic acid (SA) and the increased expression of a number of pathogen-related (PR) genes, encoding defense-related proteins such as antimicrobial peptides (Arabidopsis thaliana PR-1). As a master regulator for SA-based immunity and SAR, NON-EXPRESSOR OF PR GENES1 (NPR1) has been identified (Durrant and Dong 2004). Upon defense elicitation, NPR1 undergoes cellular redox state-dependent oligomer disassembly that is followed by its translocation to the nucleus, thereby interacting with members of the TGA family of basic Leu-zipper-type transcription factors (TFs) in the control of defense gene expression (Dong 2004; Durrant and Dong 2004). This represents a key mechanism that couples SA/SAR signaling with extensive transcriptional reprogramming. Moreover, as a possible basis for the long-lasting nature of SAR, subsets of defense-related genes are primed, rather than activated, in systemic unchallenged sites. The so-called defense priming holds target genes in an inactive or transiently active state but poised for faster and/or greater activation upon a subsequent pathogen attack (Conrath 2011). However, it remains elusive whether, and if so, how the aforementioned differences between MTI and ETI in transcriptional reprogramming at directly challenged sites influence the extent of SAR and/or of priming response in distal non-challenged sites.
In this chapter, we consider the potential epigenetic basis underlying transcriptional reprogramming during and after immune response, with a particular focus on the role of dynamic changes in chromatin configuration. We highlight recent studies that point to the role of chromatin-level control in the establishment and maintenance of transcription-repressive or -permissive states for defense-related genes. For the role of non-coding RNA or RNA quality control in transcriptional reprogramming, please refer to recent reviews on the topic in plants (Kanno and Habu 2011; Yaish et al. 2011; Naqvi et al. 2012).
2 Integration of Immune Receptor-Triggered Signaling with Gene Expression in the Nucleus
Protein phosphorylation cascades seem to couple signal inputs, whether upon extracellular recognition of MAMPs (MTI) or intracellular recognition of specific effectors (ETI), to gene transcription machineries in the nucleus (Tena et al. 2011). In mammals, direct outputs of MAPK signaling activated upon diverse stimuli involve histone H3 phosphorylation to condition subsequent transcriptional reprogramming (Clayton and Mahadevan 2003). In yeast, the MAPK Hog1 interacts with the Swi/Snf chromatin-remodeling complex REMODELS STRUCTURE of CHROMATIN (RSC), which then induces its recruitment to stress-responsive promoters (Mas et al. 2009). In Arabidopsis, phosphorylation activity of histone H3 and histone variant H2A.Z has been described for MPK3 and MPK6, two of major MAPKs activated in response to diverse biotic and abiotic stresses (Feilner et al. 2005). Therefore, it is plausible that MAPKs provide a direct link between immune receptor-triggered signaling and chromatin configuration changes during immune response in plants as well.
Another key basis for signal integration in the nucleus seems to be provided by nucleocytoplasmic trafficking of defense signaling components and TFs including also a subclass of NB-LRR immune receptors per se (Meier and Somers 2011). Several NB-LRR receptors that require nuclear localization for their immune function include the tobacco TIR-NB-LRR receptor N, the Arabidopsis TIR-NB-LRR receptor RPS4, and the barley CC-NB-LRR receptor MLA that confer resistance to tobacco mosaic virus, the phytopathogenic bacterium Pseudomonas syringae expressing the type III secretion (T3S) effector AvrRps4, and the powdery mildew fungus Blumeria graminis f. sp. hordei expressing cognate AvrMLA effectors, respectively (Burch-Smith and Dinesh-Kumar 2007; Shen et al. 2007; Garcia et al. 2010). Only a small portion of these NB-LRR receptor pools is localized in the nucleus, yet it plays an essential role for mounting ETI, since their enforced nuclear exclusion disables their immune function (Burch-Smith and Dinesh-Kumar 2007; Shen et al. 2007; Garcia et al. 2010). A critical nuclear action of these NB-LRR receptors involves physical interaction with DNA-binding TFs that regulate immune response, although the precise biochemical outcome of their interactions remains unclear to date (Burch-Smith and Dinesh-Kumar 2007; Shen et al. 2007).
The Arabidopsis EDS1 defines an essential non-receptor component for TIR-NB-LRR receptor-conditioned ETI. EDS1 acts as part of protein complexes with the basal defense regulators PAD4 and SAG101 (Wiermer et al. 2005), but again the precise biochemical function of the EDS1 complex(es) remains unclear. In addition, EDS1 interacts with the NB-LRR receptors RPS4, RPS6, and SNC1, but also with the phytopathogenic bacterium Pseudomonas syringae effector AvrRPS4 (Bhattacharjee et al. 2011; Heidrich et al. 2011). EDS1 shuttles between the cytoplasm and nucleus, with a small pool localized in the nucleus. Again, this small nuclear pool of EDS1, together with nuclear localization of RPS4 and AvrRPS4, is required for transcriptional reprogramming and ETI to bacterial infection that are conferred by RPS4 (Bhattacharjee et al. 2011; Heidrich et al. 2011). This reinforces the notion that critical events of ETI signaling for defense execution take place within the nucleus, and further implies that perturbations of host nuclear processes by pathogen effectors are monitored by NB-LRR receptors.
In line with this, genetic studies in Arabidopsis have revealed the genetic requirements for the components of the nuclear pore complexes in pathogen resistance, including MODIFIER OF SNC1 6 (MOS6) encoding importin α3, and MOS3 and MOS7, respectively, encoding homologs of the nucleoporin Nup96 and Nup88. MOS7 is required for proper nuclear accumulation of SNC1, EDS1, and NPR1 (Cheng et al. 2009). This further argues for the functional significance of the access of immune regulators to the nucleus and gene transcription machineries (Garcia and Parker 2009).
Of note, the aforementioned signaling from the membrane/cytoplasm to nucleus and nuclear processes is under the influence of a complex network of defense-related phytohormones. In general, salicylic acid (SA)-dependent defenses are effective against biotrophic and hemi-biotrophic pathogens, while jasmonic acid (JA) signaling together with ethylene (ET) confers effective defenses against necrotrophic pathogens and insect herbivores. These phytohormones also contribute to plant adaptation to different abiotic stress cues in a fluctuating environment. The outcome of these phytohormone interactions differs in a context-dependent manner, providing a basis for fine-tuning of immune response according to the type of pathogens encountered and the prevailing environmental conditions (Glazebrook 2005; Spoel and Dong 2008; Robert-Seilaniantz et al. 2011; Pieterse et al. 2012). Together, all these aspects of immune response predict the need for the mechanisms that can rapidly and flexibly reprogram the expression of large sets of genes at once.
3 Chromatin Remodeling and Histone Replacement in Plant Immunity
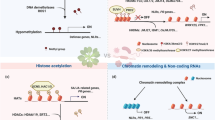
One effective way to meet such requirements in transcriptional reprogramming can be achieved through changes of chromatin configuration in eukaryotic cells. The smallest packaging unit of chromatin is termed nucleosome that consists of two copies of histone H2A, H2B, H3, and H4 wrapped by approximately 147 bp of DNA (Zhang and Reinberg 2001). The structure and function of chromatin is regulated by multiple mechanisms, including DNA methylation, ATP-dependent chromatin remodeling, replacement of histone variants, and posttranslational histone modifications such as methylation, acetylation and ubiquitination. Several of the above mechanisms have been implicated in the modulation of immune response in plants (Alvarez et al. 2010; Ma et al. 2011; Berr et al. 2012).
Replacement of histone H2A.Z with canonical histone H2A occurs through the action of a multi-subunit complex termed SWR1 in yeast and SRCAP in humans (Krogan et al. 2003; Mizuguchi et al. 2004; Cai et al. 2005). H2A.Z is typically found in the nucleosomes flanking the transcription start sites (Zilberman et al. 2008). In Arabidopsis, disruptions of a SWR1-like complex (containing PIE1) and of two of the three histone variant H2A.Z-coding genes (HTA9 and HTA11) cause in non-elicited plants transcriptional upregulation of SA-responsive SAR marker genes, spontaneous cell death, and enhanced immunity to bacterial infection (March-Diaz et al. 2008). These findings point to a role of H2A.Z deposition in the establishment and/or maintenance of transcription-repressive chromatin configuration on the target SA regulons. This might provide means by which plants avoid detrimental precocious activation of immune response in the absence of pathogens.
Genetic evidence also points to a role of several components of ATP-dependent chromatin-remodeling complexes in the repression or attenuation of these SA regulons and SA-based immunity to pathogens. These complexes contain the catalytic SUCROSE NONFERMENTING2 (SNF2) ATPase subunit. Out of the 42 SNF2 ATPase family members annotated in the Arabidopsis genome, loss of the following members results in enhanced expression of SA-responsive genes and/or enhanced basal immunity response to biotrophic or hemi-biotrophic pathogens: SPLAYED (SYD) and BRAHMA (BRM) of the SNF2 subfamily, PHOTOPERIOD-INDEPENDENT EARLY FLOWERING1 (PIE1) of the SWI/SNF-RELATED1 (SWR1) subfamily, and DECREASED DNA METHYLATION1 (DDM1) of LSH subfamily (see below).
Upon bacterial challenge of syd mutant plants as well as in non-elicited brm plants, hyper-activation of SA-responsive genes including PR1 has been observed (Bezhani et al. 2007; Walley et al. 2008). The phenotypic differences between the two mutants might reflect that SYD and BRM have a partially overlapping function, but yet a distinct set of target genes (Bezhani et al. 2007; Walley et al. 2008). It should be also noted that the upregulation of SA regulons is accompanied by reduced expression of JA/ET-inducible genes in syd plants, suggesting that the SA–JA antagonism also contributes to the observed alterations of transcriptional reprogramming in the mutant. In addition, direct SYD recruitment was selectively detected in the promoters of some of the affected JA/ET-responsive genes, i.e., VSP2 and MYC2 but not PDF1.2a. These results suggest that most of the observed mutational effects might be indirect (Walley et al. 2008). To date, the precise mechanisms by which SYD and BRM regulates defense-related genes still remain largely unknown. The presence of a bromodomain in BRM1 and ability to bind histones in vitro implies its potential interactions with acetylated histones (Farrona et al. 2007). Future studies will be needed to clarify the above suggested models.
DDM1 is required to maintain DNA methylation along the genome, although there is no proof for its direct DNA methyltransferase activity (Jeddeloh et al. 1999). Various genetic and epigenetic alterations accumulate in the progeny of hypomethylated ddm1 plants, causing the so-called bal effects that are characterized by dwarfism, curled leaves, and enhanced disease resistance that are dependent on EDS1. This is accompanied by derepression of several NB-LRR receptor-encoding genes from the RPP5 locus, of which that of SNC1 is responsible for the bal effects (Yi and Richards 2007, 2009). The RPP5 locus includes SNC1, which has been originally identified through mutagenic suppressor screens for npr1 mutant that is defective in SA-based immunity. The gain-of-function snc1 allele rescues the SA signaling defects of the npr1 mutant (Li et al. 2001; Zhang et al. 2003). In the absence of DDM1, duplication of a 55-kb region occurred between several clustered NB-LRR-encoding genes within the RPP5 locus, which increases the copy number and thus expression levels of SNC1. (Yi and Richards 2009). Comparative genome and phylogenetic studies suggest that many of NB-LRR genes were generated as a consequence of tandem gene duplication events (Baumgarten et al. 2003; Meyers et al. 2003). DDM1 might serve to prevent recombination between repeat sequences from the RPP5 cluster and thus to maintain genomic stability. This might allow plants to accommodate highly related but slightly variant repeat sequences in a cluster of homologous genes, while avoiding their mis-expression that can be detrimental to the plant. This might also serve sources for the evolution of sequence-related immune receptor-coding genes.
A separate study has revealed that MOS1, a large protein of an evolutionarily conserved BAT2 domain, can antagonize DDM1 function thus promoting Snc1 expression (Li et al. 2010). In mos1 loss-of-function mutant plants, Snc1 expression is lost and also its associated effects, i.e., constitutive autoimmunity activation, are lost. However, the expression of Snc1 is de-repressed in mos1 plants upon the disruption of DDM1. Of note, rather reduced DNA methylation levels were observed along the Snc1 promoter in mos1 plants despite the fact that Snc1 expression was repressed. This result together with the insufficiency of ddm1 mutation alone to enhance Snc1 transcript levels (Li et al. 2010), points to the complex nature of controlling the expression of the NB-LRR gene.
Recent genome-wide DNA methylation profiling of Arabidopsis plants exposed to bacterial pathogens has revealed that differentially methylated cytosines (DmCs) were enriched in gene-rich but depleted in gene-poor regions along the genome, suggesting a role of these methylation changes in transcriptional control (Dowen et al. 2012). Interestingly, although CG and CHG (where H is A, C, or T) methylations were similarly altered in response to SA and avirulent (ETI triggering), or virulent Pseudomonas syringae strains, the changes of CHH methylation levels were unique to the infection of the virulent bacterial strain among the tested stimuli, implying that differential DNA methylation patterns are associated with effective or noneffective immune response. Consistent with this, subsets of defense-related genes are mis-expressed and antibacterial immunity is enhanced in met1-3 and drm1 drm2 cmt3 mutant plants that are globally defective in maintenance of CG methylation or non-CG methylation, respectively (Dowen et al. 2012).
4 Histone Modifications During Plant Immune Response
Recent studies have uncovered an edge of dynamic changes of histone modifications during immune response and presented genetic evidence for a role taken by several histone modifiers and remodelers important for plant immunity. In general, histone modifications associated with active (transcription-permissive) chromatin include histone H3 that is mono-, di-, or tri-methylated on Lys-4 (H3K4me1, H3K4me2, or H3K4me3, respectively), H3K36me3, or acetylated H3 and H4 (H3Ac and H4Ac, respectively), and those typical of silent (transcription-repressive) chromatin include H3K9me1, H3K9me2, H3K9me3, H3K27me1, H3K27me2, or H3K27me3 (Fuchs et al. 2006; Kouzarides 2007; Pfluger and Wagner 2007; Roudier et al. 2009). In Arabidopsis, epigenome mapping studies with a focus on 11 histone modifications (H3K4me2, H3K4me3, H3K9me2, H3K9me3, H3K27me1, H3K27me2, H3K27me3, H3K36me3, H3K56ac, H4K20me1 and H2B ubiquitination) and DNA methylation have revealed that four different combinations cover ~90 % of the genome under non-stress conditions (Roudier et al. 2011). It seems likely that different combinations/patterns of histone modifications differentially influence chromatin structure and transcriptional competence of the target loci. The functional outcomes (whether permissive or repressive for gene transcription) of histone modification patterns can also vary according to the positions of these modifications with respect to the gene structure and the genomic context (Fuchs et al. 2006; Kouzarides 2007; Pfluger and Wagner 2007; Roudier et al. 2009).
All three H3K4me marks occur almost exclusively on gene coding sequences and are associated with active chromatin (Zhang et al. 2009). Increased H3K4 methylation, together with H3K9- and H3K14-acetylation, was detected at the PR1 locus in non-elicited sni1 mutant plants (Mosher et al. 2006). This seems to in part account for the recovery of PR1 expression by the sni1 mutation despite the absence of NPR1 (Li et al. 1999). An elevation of these histone H3 modifications also occurs in wild-type plants 48 h upon the application of the SA analogue benzo(1,2,3)thiadiazole-7-carbonic acid S-methyl ester (BTH). These findings indicate that the nuclear protein SNI1 antagonizes NPR1 function as a repressor of these histone modifications and thus of PR gene expression in SA-based immunity (Li et al. 1999). However, of note, another independent work fails to detect such an increase of H4K4me3 in the PR1 locus within 24 h after SA application (Alvarez-Venegas et al. 2007). This leads to a notion that active PR1 transcription is followed by the elevation of H3K4me3 and H3Ac levels, which in turn contributes to keep the PR1 chromatin in an active state. Thus, H3K4me3 and H3Ac might be associated with the establishment of a memory for the expression of defense-related genes (see below).
The major subclass of Lys-specific histone methyltransferase (HMTase) is SET (Su[var]3-9, Enhancer of Zeste, Trithorax) domain-containing enzymes, which catalyze mono- (me1), di- (me2), and/or trimethylation (me3) of different Lys residues on histone H3 and/or H4 (Hennig and Derkacheva 2009). For not all but some of Arabidopsis SET domain HMTase members tested, loss of their function results in alterations of immune response, pointing to their selective assignments to the modulation of plant immunity.
The Polycomb group (PcG) protein complex Polycomb Repressive Complex2 (PRC2) mediates H3K27me3 and thus sustains a transcription-repressive state of chromatin (Margueron and Reinberg 2011). The four core PcG subunits of PRC2 are defined by E(z), Su(z)12, Esc, and p55 in Drosophila. In Arabidopsis, homologs for these PRC2 components exist: the SET domain-containing E(z) homologs MEDEA, CURLY LEAF (CLF), and SWINGER (SWN); Su(z)12 homologs EMBRYONIC FLOWER (EMF), FERTILIZATION INDEPENDENT SEED2 (FIS2), and VERNALIZATION2 (VRN2); Esc homolog FERTILIZATION INDEPENDENT ENDOSPERM (FIE); p55 homologs MULTICOPY SUPPRESSOR OF IRA 1–5 (MSI1–MSI5). Although their catalytic activity has not been demonstrated, genetic evidence points to their role as the determinants for H3K27me3 levels in Arabidopsis (Liu et al. 2010; Jeong et al. 2011). Genetic evidence also points to pleiotropic roles of PcG proteins throughout the plant life cycle, including gametogenesis, fertilization, seed development, vegetative development, floral transition, and flower organogenesis (Kohler and Aichinger 2010; Butenko and Ohad 2011; Holec and Berger 2012). Genome-wide chromatin co-immunoprecipitation (ChIP) analysis revealed that approximately 4,400 genes (~18 %) are positive with H3K27me3 in non-stressed seedlings, suggesting the global impact of this histone mark in the control of gene expression in Arabidopsis (Zhang et al. 2007; Pontvianne et al. 2010). However, to date, the functional significance of PcG proteins has not been vigorously tested in plant immunity.
In Drosophila, as opposed to PRC2 function, Trithorax group (TrxG) proteins confer positive effects on transcription by mediating H3K4 tri-methylation. The aforementioned genome-wide ChIP analysis revealed that 12.1 % of the Arabidopsis genome carries H3K4me3 under the normal laboratory growth conditions (Zhang et al. 2009). The Arabidopsis genome encodes five TRITHORAX (trx)-like proteins (ATX1 to ATX5), which are characterized by a SET domain and a PHD domain, and seven Trx-related proteins (ATXR1 to ATXR7) (Tamada et al. 2009). ATX1 and ATX2 have been demonstrated in vitro to possess H3K4 tri- and di-methylation activity, respectively (Saleh et al. 2008; Sang et al. 2009). An Arabidopsis ortholog of Drosophila Trithorax group (trxG) H3K4 trimethylase, ATX1, acts as a positive regulator for basal defense to bacterial infection and for the expression of a high proportion of defense-related genes, including PR genes (Alvarez-Venegas et al. 2006). Transcriptional activation of WRKY70, encoding a TF that acts for balancing SA–JA signaling crosstalk, is correlated with ATX1 binding and ATX1-dependent H3K4me3 signatures at the WRKY70 promoter, suggesting that this gene defines one of ATX1 target genes in immune response (Alvarez-Venegas et al. 2007). By contrast, ATX1 binding was not detected on the PR1 locus, implying that ATX1 confers the broad effects as the sum of indirect consequences, e.g., through the upregulation of defense-related TF-coding genes. Besides H3K4 methylation activity, ATX1 also serves to recruit the TATA-binding protein and RNA polymerase II (Pol II) to the target promoters including that of WRKY70 (Ding et al. 2011). Upon the initiation of transcription, phosphorylated Pol II engaged in transcriptional elongation seems to recruit ATX1 to the transcribed gene region, where ATX1 tri-methylates histone H3. In addition to these trxG homologs, non-conserved proteins also seem to be engaged in antagonizing PRC2 function in plants (Aichinger et al. 2011). These findings suggest that less conserved, diverged mechanisms collectively mediate the equivalent function of Drosophila trxG in plants.
Suppressor screens for an Arabidopsis lesion mimic mutant, accelerated cell death11 (acd11), have revealed SDG8 (also named ASHH2), a homolog of the yeast H3K36 di-/tri-methylase SET2, that is required for basal expression of NB-LRR genes including RPM1, RPM1-conditioned ETI, and basal immunity to bacterial infection (Palma et al. 2010). In both non-elicited and benzothiadiazol (BTH)-treated sdg8 plants, H3K36me3 levels remain low on the locus encoding the NB-LRR protein LAZ5, in association with its lowered expression. Therefore, these findings suggest that SDG8-mediated H3K36me3 serves to establish and/or maintain a transcription-permissive chromatin state on subsets of NB-LRR gene loci. SDG8 also plays a crucial role in plant immunity against necrotrophic fungal pathogens through H3K36me3-mediated activation of subsets of JA/ET-inducible genes (Berr et al. 2010). However, consistent with multi-catalytic activity of SDG8 not only for H3K36me2/3 (Grini et al. 2009) but also for H3K4me3 (Cazzonelli et al. 2009), loss of SDG8 (ASHH2) also seems to influence H3K4me2 and H3K4me3 levels on the PR1 promoter upon bacterial challenge (De-La-Pena et al. 2012). The requirement of H3K9me3 for SDG8 activity has been also described in shoot branching of Arabidopsis (Dong et al. 2008). Future studies will be needed to clarify whether SDG8 directly catalyzes H3 methylation on all these Lys residues.
5 Defense Priming
In defense priming, immune response is held in an inactive or less active state but competent for more rapid and/or strong activation upon subsequent stimulation [reviewed in (Conrath 2011; Pastor et al. 2012)]. This is often accompanied by the sensitization of immune response to lower doses of defense triggers or even to stimuli of otherwise non-eliciting activity. An advantage of defense priming, compared to direct defense activation, involves sustained enhancement of host immunity at low fitness costs (van Hulten et al. 2006). Defense priming occurs upon MTI or ETI activation, colonization of nonpathogenic microbes, or wounding. Chemical compounds have also been identified to act as a trigger for defense priming upon their application on plants, such as β-aminobutyric acid (BABA). The molecular basis for defense priming remains poorly understood, but recent studies suggest a role of histone modifications, in addition to metabolic changes (accumulation of inactive precursors/derivatives for defense-promoting metabolites), modulation of defense-related hormone crosstalk, and enhanced expression of MAPKs and TFs (Conrath 2011; Pastor et al. 2012). In this chapter, we put a particular focus on defense priming that is based on changes in chromatin configuration for defense-related genes.
Histone modifications and H2A.Z replacement have been considered as a molecular basis for priming of SAR-related genes (van den Burg and Takken 2009). As mentioned above, the induction of JA/ET-inducible defense-related genes upon JA application or challenges with necrotrophic fungal pathogens is accompanied by an increase of H3K36me3 levels at the promoters of these genes in an SDG8-dependent manner (Berr et al. 2010). It is of great interest to understand whether this leads to the establishment of primed states, i.e., the acquisition of immune memories, of these genes. Moreover, using BTH as a mimic of SAR trigger, a recent study demonstrated a correlation between systemic priming of SA-inducible WRKY TF-coding genes and changes in several histone modifications. In Arabidopsis, low-dose BTH application did not activate WRKY29 and only slightly activated WRKY6 and WRKY53, in a manner reflecting their transcriptional reprogramming in systemic non-challenged leaves during pathogen-triggered SAR (Jaskiewicz et al. 2011). However, these transcript levels were greatly elevated upon water infiltration 72 h after BTH pretreatment or in systemic (distal, non-challenged) leaves 72 h after local bacterial challenges, whereas they remain low in mock controls. Primed plants exhibit an increase of H3K4me3 levels in the promoters of these WRKY genes, which occurs in an NPR1-dependent manner, suggesting that a histone-based memory underlies defense priming.
Regarding defense priming, another important question involves the heritability of the established primed states on target defense-related genes. Trans-generation inheritance of stress adaptation has been well documented for abiotic stress (Chinnusamy and Zhu 2009). However, to date, only a few recent studies support this possibility for biotic stress. Recent studies show that primed states for defense-related target genes and immune response can be transmitted to the following generations when the parent plants were exposed to pathogen challenges or exposed to priming triggering molecules. For instance, trans-generational SAR mounted upon bacterial challenges was sustained over one stress-free generation in Arabidopsis (Luna et al. 2012). This is accompanied by a shift in the balance of SA–JA signaling, i.e., enhanced SA responsiveness and reduced JA responsiveness, without significant changes in the corresponding phytohormone levels. The increase of H3K9Ac on the promoters of SA-inducible priming target genes and of H3K27me3 on a JA-inducible promoter points to a role of these histone modifications as a molecular basis for such differential primed states between SA and JA pathway genes. Moreover, trans-generational SAR occurs in non-primed drm1 drm2 cmt3 mutant plants that show reduced levels in non-CG DNA methylation, although the genomic regions and genes undergoing this DNA hypomethylation remain to be determined (Luna et al. 2012). Nevertheless, this raises the possibility that DNA hypomethylation also facilitates the trans-generational heritability. It would be of great interest to determine the sequential order and functional relationship between histone modification changes and DNA methylation changes. ETI activation and BABA application also confer defense priming that is heritable to the following generation (Slaughter et al. 2012). Not only Arabidopsis but also tomato plants exposed to JA or insect herbivory exhibit priming of JA-inducible genes and trans-generational insect resistance, in a manner requiring the JA receptor COI1 (Rasmann et al. 2012). This phenomenon also requires intact RNA-dependent DNA methylation pathway (Rasmann et al. 2012), again pointing to a role of DNA methylation changes as an underlying basis. However, carefully designed experimentation will be needed to unambiguously clarify whether the trans-generation heritability of defense priming is exclusively based on changes taking place on the chromatin-level rather than stress-induced genetic changes which may interfere with chromatin organization (Pecinka and Mittelsten Scheid 2012).
6 Target Genes of Defense Priming
Our molecular genetic work on Arabidopsis suggests that a separation of initial and sustained activation phases of MTI occurs in the presence of mal-folded PRR (Lu et al. 2009). In an ER glucosidase II β-subunit allele, designated rsw3, sustained transcriptional reprogramming, and host immunity to bacterial infection are impaired despite almost intact co-activation of other early MTI-associated outputs such as a ROS burst, MAPK activation, ET production, and initial transcriptional reprogramming. This points to the importance of sustained transcriptional reprogramming as a critical step in mounting effective immunity. Thus, it is conceivable that the target genes of this sustained transcriptional reprogramming would be closely associated with defense execution.
Genome-wide transcriptome analysis has revealed an inventory of defense-related genes, including PR1, that are mis-regulated in the mutant and thus define targets of sustained PRR signaling during MTI (Ross and Saijo et al., unpublished). In silico database analysis of these genes suggests that they are activated upon direct defense execution in diverse Arabidopsis–pathogen interactions, but remain at low expression levels in systemic tissues during SAR (Ross and Saijo et al., unpublished). Thus, these genes are also expected to include the target genes of systemic defense priming. Interestingly, these genes carry the transcription-repressive H3K27me3 and -permissive H3K4me3 histone modifications more often (56 % and 36 %, respectively) than expected (Fig. 2). The two mutually antagonistic chromatin marks are set by PcG and trxG protein complexes, respectively, and are typically associated with a gene-autonomous memory of transcription. This implies a role of these transcription memory-associated histone methylations in defense priming. This model is also consistent with the early studies on several WRKY genes (Jaskiewicz et al. 2011). Future studies will be required to reveal potential dynamics of these and other related histone modifications in the priming target loci during and after immune activation and to gain insight into the significance of the described chromatin-level changes in defense priming.
In silico analysis for H3 methylation on defense-related genes in non-elicited Arabidopsis seedlings. The Venn diagram shows the number of Arabidopsis genes carrying H3K27me3 and/or H3K4me3 out of 89 genes that are upregulated in a late MTI phase in WT plants but not in rsw3 plants (Lu et al. 2009). Further in silico comparative analysis suggests their close association with defense execution in diverse plant–pathogen interactions. The database is publicly available at the Jacobsen Lab Web site, USA (https://www.mcdb.ucla.edu/Research/Jacobsen/LabWebSite/P_EpigenomicsData.shtml)
7 Conclusions and Prospects
Prompt and robust activation of pathogen-specific immune response is crucial to effectively repel the pathogens encountered. On the other hand, stringent control of the strength and spatiotemporal spreading of defense activation are also crucial to minimize its negative influence on plant fitness. Recent progress, in particular in the reference plant Arabidopsis, has illuminated the potential importance of chromatin modification and remodeling as a means by which plants can meet these demands. However, the underlying mechanisms still remain largely unknown to date.
The engagement of histone modifications in establishing and reinforcing reversible and/or heritable patterns of gene expression has been well documented in plant development (Berr et al. 2011; Holec and Berger 2012). By contrast, the role of these regulations had not gained much interest of researchers in plant immunity until recently. However, the wealth of genetic resources and genetic tractability available in the model plant–pathogen interactions, e.g., between Arabidopsis and Pseudomonas syringae, would provide a great advantage for this emerging field as a model system for future epigenetic studies.
We propose the following stepwise regulation of histone modifications associated with transcriptional activation and attenuation of defense-related genes during immune response in plants. (1) In the absence of pathogens (or their derived elicitors), these genes are kept in a transcriptionally inactive or a basal state that is ensured by transcription-repressive or partially permissive chromatin configuration, respectively. (2) MAMP recognition, as an initial alert for the presence of potentially infectious pathogens, triggers a shift in chromatin configuration from the repressive to permissive state which either prevents the spreading of repressive histone marks and/or allows a rapid access and action of transcriptional activators. (3) Elevation of the strength of immune signaling beyond the activation threshold leads to massive activation of gene transcription, which in turn recruits defense-inducible TFs and histone modifications that would facilitate and/or reinforce the transcription of defense-related genes. (4) Following initial transcriptional changes, the persistence of active MAMP-triggered signaling or a distinct mode of signaling upon pathogen recognition (e.g., ETI signaling) leads to robust activation of gene transcription. This might be established by further spreading or acquisition of transcription-associated histone modifications and/or possibly by long-range interactions of distal genomic regions. By contrast, the absence of restimulation turns off gene transcription, which is eventually followed by the restoration of transcription-repressive (or basal, less permissive) patterns of histone modifications. (5) Upon sustained activation of gene expression (including certain posttranscriptional steps), transcription-coupled active histone modifications are firmly established and/or widely spread, which allows their persistence even after the removal of defense triggers. (6) Such long-lasting histone modification states keep the altered activation threshold, thereby providing a basis for a chromatin-level memory of immune response.
There are still many gaps in our knowledge to be filled for testing this model. To identify an inventory of target genes for systemic priming and to decipher histone modification patterns corresponding to particular chromatin states, genome-wide comparative analysis for transcriptomes (by RNA sequencing to cover possible changes in mRNA quality and non-coded RNA expression) and epigenomes (by ChIP-sequencing for different histone marks) during immune response and systemic priming will be a prerequisite. This would allow us to have a better picture of the underlying molecular events and to further generate new testable hypotheses.
This genome-wide analysis should be extended to obtain the transcriptome and epigenome profiles during MTI and ETI activation in an otherwise identical experimental platform, which is available, e.g., in Arabidopsis–P. syringae interactions. This is expected to gain insight into the mechanisms that are causative for the earlier described quantitative differences in transcriptional reprogramming between the two modes of immunity. It is possible that ETI skips or strengthens some of the stepwise regulatory processes proposed above. Of note, pathogens also seem to manipulate these host processes during infection. The transcription activator-like (TAL) effectors of the bacterial phytopathogen Xanthomonas species directly bind to specific promoter sequences in the host nucleus and activate target genes, which are otherwise repressed during immune response, for bacterial virulence promotion (Boch and Bonas 2010). This suggests the existence of host chromatin modulation activity that allows TAL effectors to access and transcribe the target genes. In addition, a growing number of effectors have been described for different pathogens that are localized in the host nuclei. It is conceivable that some of these effectors influence host gene transcription by altering chromatin configuration. Functional studies and host target identification of these effectors are expected to clarify these possibilities.
Furthermore, immune activation, whether in MTI or ETI, at directly challenged sites is linked to the activation of SAR and systemic priming in distal non-challenged sites. It will be interesting to determine whether MTI and ETI lead to significant differences in the target genes, strength, associated histone modifications, or combinations thereof of systemic priming. The overrepresentation of H3K27me3 and H3K4me3 marked by PcG and trxG proteins, respectively, at the defense-related gene loci implies a role of these modifications as a switch between non-primed and primed chromatin states of these genes in immune response. Of note, the transcripts for priming target genes accumulate barely above the background levels in systemic tissues upon defense priming, although both histone modifications typically act as a gene-autonomous memory of the preceding transcription states (Margueron and Reinberg 2011). It is of great importance to determine whether the stable acquisition of these histone modifications requires initial transcriptional reprogramming of target genes in systemic tissues as well or not.
The molecular links remain enigmatic between immune receptor-triggered signaling and chromatin modifiers/remodelers that participate in transcriptional reprogramming and priming of defense-related genes. The aforementioned genome-wide profiling of transcriptome and epigenome is expected to provide a new inventory of marker genes and histone modifications that would be valuable in further in-depth studies. In parallel, the chromatin modifiers and remodelers need to be identified that play a rate-limiting role in immune response. In this respect, the implementation of conditional gene knockout systems will be required to unambiguously assess the role of these chromatin regulators which cause dramatic pleiotropic effects during plant development and growth when they are permanently compromised.
References
Aichinger E, Villar CB, Di Mambro R, Sabatini S, Kohler C (2011) The CHD3 chromatin remodeler PICKLE and polycomb group proteins antagonistically regulate meristem activity in the Arabidopsis root. Plant Cell 23:1047–1060
Alvarez ME, Nota F, Cambiagno DA (2010) Epigenetic control of plant immunity. Mol Plant Pathol 11:563–576
Alvarez-Venegas R, Sadder M, Hlavacka A, Baluska F, Xia Y, Lu G, Firsov A, Sarath G, Moriyama H, Dubrovsky JG, Avramova Z (2006) The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes. Proc Natl Acad Sci U S A 103:6049–6054
Alvarez-Venegas R, Abdallat AA, Guo M, Alfano JR, Avramova Z (2007) Epigenetic control of a transcription factor at the cross section of two antagonistic pathways. Epigenetics 2:106–113
Baumgarten A, Cannon S, Spangler R, May G (2003) Genome-level evolution of resistance genes in Arabidopsis thaliana. Genetics 165:309–319
Berr A, McCallum EJ, Alioua A, Heintz D, Heitz T, Shen WH (2010) Arabidopsis histone methyltransferase SET DOMAIN GROUP8 mediates induction of the jasmonate/ethylene pathway genes in plant defense response to necrotrophic fungi. Plant Physiol 154:1403–1414
Berr A, Shafiq S, Shen WH (2011) Histone modifications in transcriptional activation during plant development. Biochim Biophys Acta 1809:567–576
Berr A, Menard R, Heitz T, Shen WH (2012) Chromatin modification and remodelling: a regulatory landscape for the control of Arabidopsis defence responses upon pathogen attack. Cell Microbiol 14:829–839
Bezhani S, Winter C, Hershman S, Wagner JD, Kennedy JF, Kwon CS, Pfluger J, Su Y, Wagner D (2007) Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell 19:403–416
Bhattacharjee S, Halane MK, Kim SH, Gassmann W (2011) Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 334:1405–1408
Boch J, Bonas U (2010) Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol 48:419–436
Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60:379–406
Burch-Smith TM, Dinesh-Kumar SP (2007) The functions of plant TIR domains. Sci STKE 2007:pe46
Butenko Y, Ohad N (2011) Polycomb-group mediated epigenetic mechanisms through plant evolution. Biochim Biophys Acta 1809:395–406
Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW (2005) The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem 280:13665–13670
Caldo RA, Nettleton D, Wise RP (2004) Interaction-dependent gene expression in Mla-specified response to barley powdery mildew. Plant Cell 16:2514–2528
Cazzonelli CI, Cuttriss AJ, Cossetto SB, Pye W, Crisp P, Whelan J, Finnegan EJ, Turnbull C, Pogson BJ (2009) Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell 21:39–53
Cheng YT, Germain H, Wiermer M, Bi D, Xu F, Garcia AV, Wirthmueller L, Despres C, Parker JE, Zhang Y, Li X (2009) Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. Plant Cell 21:2503–2516
Chinnusamy V, Zhu JK (2009) RNA-directed DNA methylation and demethylation in plants. Sci China C Life Sci 52:331–343
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814
Clayton AL, Mahadevan LC (2003) MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett 546:51–58
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16:524–531
De-La-Pena C, Rangel-Cano A, Alvarez-Venegas R (2012) Regulation of disease-responsive genes mediated by epigenetic factors: interaction of Arabidopsis-Pseudomonas. Mol Plant Pathol 13:388–398
Dempsey DA, Klessig DF (2012) SOS - too many signals for systemic acquired resistance? Trends Plant Sci 17:538–545
Ding Y, Avramova Z, Fromm M (2011) The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. Plant J 66:735–744
Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7:547–552
Dong G, Ma DP, Li J (2008) The histone methyltransferase SDG8 regulates shoot branching in Arabidopsis. Biochem Biophys Res Commun 373:659–664
Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR (2012) Widespread dynamic DNA methylation in response to biotic stress. Proc Natl Acad Sci U S A 109:E2183–E2191
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Farrona S, Hurtado L, Reyes JC (2007) A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMA. J Mol Biol 373:240–250
Feilner T, Hultschig C, Lee J, Meyer S, Immink RG, Koenig A, Possling A, Seitz H, Beveridge A, Scheel D, Cahill DJ, Lehrach H, Kreutzberger J, Kersten B (2005) High throughput identification of potential Arabidopsis mitogen-activated protein kinases substrates. Mol Cell Proteomics 4:1558–1568
Fuchs J, Demidov D, Houben A, Schubert I (2006) Chromosomal histone modification patterns–from conservation to diversity. Trends Plant Sci 11:199–208
Garcia AV, Parker JE (2009) Heaven’s gate: nuclear accessibility and activities of plant immune regulators. Trends Plant Sci 14:479–487
Garcia AV, Blanvillain-Baufume S, Huibers RP, Wiermer M, Li G, Gobbato E, Rietz S, Parker JE (2010) Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. PLoS Pathog 6:e1000970
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Grini PE, Thorstensen T, Alm V, Vizcay-Barrena G, Windju SS, Jorstad TS, Wilson ZA, Aalen RB (2009) The ASH1 HOMOLOG 2 (ASHH2) histone H3 methyltransferase is required for ovule and anther development in Arabidopsis. PLoS One 4:e7817
Heidrich K, Wirthmueller L, Tasset C, Pouzet C, Deslandes L, Parker JE (2011) Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334:1401–1404
Hennig L, Derkacheva M (2009) Diversity of Polycomb group complexes in plants: same rules, different players? Trends Genet 25:414–423
Holec S, Berger F (2012) Polycomb group complexes mediate developmental transitions in plants. Plant Physiol 158:35–43
Jaskiewicz M, Conrath U, Peterhansel C (2011) Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 12:50–55
Jeddeloh JA, Stokes TL, Richards EJ (1999) Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat Genet 22:94–97
Jeong CW, Roh H, Dang TV, Choi YD, Fischer RL, Lee JS, Choi Y (2011) An E3 ligase complex regulates SET-domain polycomb group protein activity in Arabidopsis thaliana. Proc Natl Acad Sci U S A 108:8036–8041
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kanno T, Habu Y (2011) siRNA-mediated chromatin maintenance and its function in Arabidopsis thaliana. Biochim Biophys Acta 1809:444–451
Kohler C, Aichinger E (2010) Antagonizing Polycomb group-mediated gene repression by chromatin remodelers. Epigenetics 5:20–23
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Krogan NJ, Keogh MC, Datta N, Sawa C, Ryan OW, Ding H, Haw RA, Pootoolal J, Tong A, Canadien V, Richards DP, Wu X, Emili A, Hughes TR, Buratowski S, Greenblatt JF (2003) A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell 12:1565–1576
Li X, Zhang Y, Clarke JD, Li Y, Dong X (1999) Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 98:329–339
Li G, Chandrasekharan MB, Wolffe AP, Hall TC (2001) Chromatin structure and phaseolin gene regulation. Plant Mol Biol 46:121–129
Li Y, Tessaro MJ, Li X, Zhang Y (2010) Regulation of the expression of plant resistance gene SNC1 by a protein with a conserved BAT2 domain. Plant Physiol 153:1425–1434
Liu C, Lu F, Cui X, Cao X (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61:395–420
Lu X, Tintor N, Mentzel T, Kombrink E, Boller T, Robatzek S, Schulze-Lefert P, Saijo Y (2009) Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc Natl Acad Sci U S A 106:22522–22527
Luna E, Bruce TJ, Roberts MR, Flors V, Ton J (2012) Next-generation systemic acquired resistance. Plant Physiol 158:844–853
Ma KW, Flores C, Ma W (2011) Chromatin configuration as a battlefield in plant-bacteria interactions. Plant Physiol 157:535–543
March-Diaz R, Garcia-Dominguez M, Lozano-Juste J, Leon J, Florencio FJ, Reyes JC (2008) Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J 53:475–487
Margueron R, Reinberg D (2011) The Polycomb complex PRC2 and its mark in life. Nature 469:343–349
Mas G, de Nadal E, Dechant R, Rodriguez de la Concepcion ML, Logie C, Jimeno-Gonzalez S, Chavez S, Ammerer G, Posas F (2009) Recruitment of a chromatin remodelling complex by the Hog1 MAP kinase to stress genes. EMBO J 28:326–336
Meier I, Somers DE (2011) Regulation of nucleocytoplasmic trafficking in plants. Curr Opin Plant Biol 14:538–546
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C (2004) ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303:343–348
Mosher RA, Durrant WE, Wang D, Song J, Dong X (2006) A comprehensive structure-function analysis of Arabidopsis SNI1 defines essential regions and transcriptional repressor activity. Plant Cell 18:1750–1765
Naqvi AR, Sarwat M, Hasan S, Roychodhury N (2012) Biogenesis, functions and fate of plant microRNAs. J Cell Physiol 227:3163–3168
Palma K, Thorgrimsen S, Malinovsky FG, Fiil BK, Nielsen HB, Brodersen P, Hofius D, Petersen M, Mundy J (2010) Autoimmunity in Arabidopsis acd11 is mediated by epigenetic regulation of an immune receptor. PLoS Pathog 6:e1001137
Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V (2012) Primed plants do not forget. Environ Exp Bot [Epub ahead of print]
Pecinka A, Mittelsten Scheid O (2012) Stress-induced chromatin changes: a critical view on their heritability. Plant Cell Physiol 53:801–808
Pfluger J, Wagner D (2007) Histone modifications and dynamic regulation of genome accessibility in plants. Curr Opin Plant Biol 10:645–652
Pieterse CM, van der Does D, Zamioudis C, Leon-Reyes A, van Wees SC (2012) Hormonal Modulation of Plant Immunity. Annu Rev Cell Dev Biol. [Epub ahead of print]
Pontvianne F, Blevins T, Pikaard CS (2010) Arabidopsis Histone Lysine Methyltransferases. Adv Bot Res 53:1–22
Rasmann S, De Vos M, Jander G (2012) Ecological role of transgenerational resistance against biotic threats. Plant Signal Behav 7:447–449
Robert-Seilaniantz A, Grant M, Jones JD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49:317–343
Roudier F, Teixeira FK, Colot V (2009) Chromatin indexing in Arabidopsis: an epigenomic tale of tails and more. Trends Genet 25:511–517
Roudier F, Ahmed I, Bérard C, Sarazin A, Mary-Huard T, Cortijo S, Bouyer D, Caillieux E, Duvernois-Berthet E, Al-Shikhley L, Giraut L, Després B, Drevensek S, Barneche F, Dèrozier S, Brunaud V, Aubourg S, Schnittger A, Bowler C, Martin-Magniette ML, Robin S, Caboche M, Colot V (2011) Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J 30:1928–1938
Saleh A, Alvarez-Venegas R, Yilmaz M, Le O, Hou G, Sadder M, Al-Abdallat A, Xia Y, Lu G, Ladunga I, Avramova Z (2008) The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell 20:568–579
Sang Y, Wu MF, Wagner D (2009) The stem cell–chromatin connection. Semin Cell Dev Biol 20:1143–1148
Segonzac C, Zipfel C (2011) Activation of plant pattern-recognition receptors by bacteria. Curr Opin Microbiol 14:54–61
Shen QH, Saijo Y, Mauch S, Biskup C, Bieri S, Keller B, Seki H, Ulker B, Somssich IE, Schulze-Lefert P (2007) Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315:1098–1103
Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B (2012) Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol 158:835–843
Spoel SH, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3:348–351
Tamada Y, Yun JY, Woo SC, Amasino RM (2009) ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell 21:3257–3269
Tao Y, Xie Z, Chen W, Glazebrook J, Chang HS, Han B, Zhu T, Zou G, Katagiri F (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15:317–330
Tena G, Boudsocq M, Sheen J (2011) Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol 14:519–529
van den Burg HA, Takken FL (2009) Does chromatin remodeling mark systemic acquired resistance? Trends Plant Sci 14:286–294
van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci U S A 103:5602–5607
Walley JW, Rowe HC, Xiao Y, Chehab EW, Kliebenstein DJ, Wagner D, Dehesh K (2008) The chromatin remodeler SPLAYED regulates specific stress signaling pathways. PLoS Pathog 4:e1000237
Wiermer M, Feys BJ, Parker JE (2005) Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8:383–389
Yaish MW, Colasanti J, Rothstein SJ (2011) The role of epigenetic processes in controlling flowering time in plants exposed to stress. J Exp Bot 62:3727–3735
Yi H, Richards EJ (2007) A cluster of disease resistance genes in Arabidopsis is coordinately regulated by transcriptional activation and RNA silencing. Plant Cell 19:2929–2939
Yi H, Richards EJ (2009) Gene duplication and hypermutation of the pathogen Resistance gene SNC1 in the Arabidopsis bal variant. Genetics 183:1227–1234
Zhang Y, Reinberg D (2001) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360
Zhang Y, Goritschnig S, Dong X, Li X (2003) A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15:2636–2646
Zhang K, Sridhar VV, Zhu J, Kapoor A, Zhu JK (2007) Distinctive core histone post-translational modification patterns in Arabidopsis thaliana. PLoS One 2:e1210
Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE (2009) Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol 10:R62
Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S (2008) Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456:125–129
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Saijo, Y., Reimer-Michalski, EM. (2013). Epigenetic Control of Plant Immunity. In: Grafi, G., Ohad, N. (eds) Epigenetic Memory and Control in Plants. Signaling and Communication in Plants, vol 18. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-35227-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-35227-0_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-35226-3
Online ISBN: 978-3-642-35227-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)