Abstract
The complex network of metabolic processes and reactions within the integrated system of aerobic life hinges largely on the presence of oxygen. Utilization of this highly reactive molecule in biological systems under normal metabolism and xenobiotic exposure inevitably results in the generation and accumulation of reactive oxygen species (ROS), which may lead to oxidative stress and hence damage to key molecular species. Oxidative damage has been implicated as the key factor in accelerated pathogenesis of a number of human diseases including cardiovascular, inflammatory, cancer, autoimmune, and neurodegenerative diseases. ROS also play defined functions through redox modifications of a great diversity of molecules, participating in a number of signaling pathways among other beneficial roles of its dual effect in the human metabolism. To maintain a steady balance between the toxicity of the oxidizing effects of ROS and the desired benefits, the elaborate antioxidant defense mechanism, which comprises endogenous and exogenous components, stages a constant reactive fight against excess ROS. However, the bioavailability of especially dietary antioxidants in sufficient concentrations within the human system is key to the success of this defensive war. An array of other physiological and physical variables and pharmacokinetic parameters such as absorption, distribution, and metabolism also contributes to the complex ultimate fate and effect of dietary antioxidants in humans.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction: Definitions and Overview of ROS and ANTs
The first properly recorded incidence of diseases are described in the books of paleopathology, dating back to the fifth century, with studies revealing a list of diseases that can easily form a “catalog of a pathological museum” listing cardiovascular, metabolic, inflammatory, and neurodegenerative diseases as well as cancer among other deadly diseases as the common causes of death. Could all these diseases have been a result of, or from higher levels of reactive oxygen species (ROS), which could then have eventually played a part in the progression of these diseases? One would think that these were just the harsh realities of the ancient world, but it is now evident that the ancient world experienced the same diseases that are still threatening humanity today. Could it be that the same mistakes the ancient civilizations made are repeated in this modern era? Perhaps, the answer simply lies in the balance between antioxidants (ANTs) and ROS? Diet, however, does play an important role in disease progression. Of notable interest are nutritional and phytochemical compositions of dietary foods that act as ANTs and/or ROS quenchers, purportedly delaying disease progression. Ancient and recent health cues for avoiding diseases and illnesses are well documented in the ancient manuscripts that include the cursive hieroglyphs or hieratic, paleopathology books, and even in several biblical verses, plus a pool of several hundreds of thousands of scientific articles in current journals, magazines, and books. Were the ancient Egyptians, Greeks, Romans, or the Biblical Moses far ahead of their time when they warned of diseases that were to plague humanity, yet we live in an environment full of plants with ANTs that can deliver us from the burdens? It is, however, sad that even in all the thousands of years that man has been exposed to such information, they have not taken the precaution to avoid sickness and stay well. Modern man often chooses to go against his knowledge and ignore these health “laws,” fail to live a healthy life by denying themselves recreation, rest, and healthy food, resulting in a buildup of excess ROS, leading to diseases. Perhaps, if man could follow these simple “laws” of health and assuming there are no accidents, war, or other disease vectors, we would die of “lack of breath” (old age).
ROS are chemically reactive molecules containing an unstable oxygen species with reactive chemical properties (Pelicano et al. 2004). The ROS family include free radicals such as superoxide (O2 •−) and hydroxyl radicals (HO•), containing an unpaired electron. The family also encompasses non-radical molecules such as hydrogen peroxide (H2O2). In biological systems, ROS are formed by the mitochondria during normal respiration (Tahara et al. 2009; Watson 2013) and as toxic by-products of a variety of pathways, including both enzyme-catalyzed reactions and nonenzymatic reactions. Research evidence accumulated over the recent past indicates that the most important biological sources of ROS are generated through the activity of NADPH oxidase (NOX enzymes) (Lambeth et al. 2008). ROS are also generated by exogenous sources such as ionizing radiation. Not only do ROS serve as toxic by-products of metabolism, but they are also essential messengers in cell signaling for a wide range of cellular processes including proliferation, cell cycle arrest, and cell death (Vurusaner et al. 2011), details of which will be discussed later. However, stress conditions (e.g., heavy metal presence, nutrient deficiency, pollution, pathogen attack, UV radiation, heat exposure), increase ROS levels dramatically, resulting in the propagation of a vicious cycle of chain events that lead to significant damage to cell structures and in some instances may trigger apoptosis (Pelicano et al. 2004; Watson 2013). If persistent, this eventually results in a condition known as oxidative stress. Decades of research on oxidative stress have linked it with the pathology of cancer, arteriosclerosis, cardiovascular diseases, malaria, rheumatoid arthritis, neurodegenerative diseases, and aging processes (Moure et al. 2001; Huo et al. 2009).
Living organisms possess several mechanistic systems which operate as ANTs and delay or prevent oxidative cell damage from ROS. ANTs are substances that at low concentrations delay or prevent oxidation of oxidizable cellular biomolecules such as lipids, proteins, and DNA (Vurusaner et al. 2011). Oxidation is defined as loss of electrons by an atom. A reductant or a reducing agent is a substance that donates electrons and, therefore, causes another reactant to be reduced. An oxidant or an oxidizing agent is a substance that accepts electrons and causes another reactant to be oxidized (Prior and Cao 1999). When a molecule is reduced, then the other molecule in the same reaction has to be oxidized, a phenomenon called reduction-oxidation (redox) interplay. Redox reactions underpin biological oxidation, the chain of chemical reactions in which oxygen from air is used to oxidize chemicals from the breakdown of food to provide energy for living (Prior and Cao 1999).
There are two major groups of ANTs, namely, enzymatic and nonenzymatic. The most abundant nonenzymatic ANTs are either plant-derived water-soluble substances such as vitamin C (for a minority of species including humans) and phenolic compounds or lipid-soluble vitamin E and carotenoids. Another important nonenzymatic ANT is glutathione (GSH), an essential nutrient synthesized in the body from the amino acids l-cysteine, l-glutamic acid, and glycine. Enzymatic ANTs include the primary enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Three types of SODs exist in humans, namely, cytosolic CuZn-SOD, mitochondrial Mn-SOD, and extracellular SOD (Ratnam et al. 2006). Enzymatic ANTs are endogenously produced together with some low molecular weight molecules and enzyme cofactors. Nonenzymatic ANTs are classified into various other classes, including polyphenols which form the largest class. The other classes of nonenzymatic ANTs include vitamins, carotenoids, organosulfural compounds, and minerals (Ratnam et al. 2006). The human diet has, however, changed over years, unfortunately resulting in daily intakes of ANTs far less than our ancestors. Higher levels of technology and organized agriculture which was not there some thousands of years ago have contributed in depriving modern man of antioxidant-rich diets (Benzie 2003).
Research on the mechanisms, use, and availability of ANTs such as phenolic compounds, flavonoids, vitamins, and many other macronutrients that works effectively against ROS has since generated considerable attention and resulted in thousands of papers and articles published in journals, magazines, and books (Ndhlala et al. 2010). This chapter takes a closer look at the relationship between ANTs and ROS in an attempt to understand how humans benefit from the tug of events that exists between the biomolecules.
ANTs Versus ROS: Benefiting Human Survival
Life evolved amid events that favored production of ROS which could have easily threatened the survival of organisms on the planet Earth (Ndhlala et al. 2010). Without the evolution of mechanistic adaptive techniques such as the opposing actions of ANTs and ROS, life could not have survived (Gutteridge and Halliwell 2010). In humans, increased ROS levels will result in detrimental effects including accelerated progression of diseases.
ANTs are grouped into two major classes: those preventing the initiation and those retarding the progression of oxidative chain reactions (Becker et al. 2004; Gill and Tuteja 2010). ANTs that act by preventing the initiation of oxidative processes, mainly referred to as “oxidative terminators,” achieve their mandate by physically or biologically blocking the chemical or enzymatic reduction actions which produce initiating radicals from molecular oxygen or hydroperoxides (Tubaro et al. 1998; Becker et al. 2004). The second group which slows down the rate of oxidative processes acts as “oxidative chain-breakers” by competing with the unsaturated lipids for reaction with peroxidation driving peroxy radicals, thereby resulting in a retarded reaction (Tubaro et al. 1998).
In other reactions, ANTs act in various ways, including binding metal ions, scavenging radicals, and decomposing peroxides. Often, more than one mechanism is involved, resulting in synergism (Moure et al. 2001). Synergism relates to the mechanisms of interaction of multiple elements in a system to produce an effect different from or greater than the sum of their individual effects (Becker et al. 2004). ANTs are localized in different sites, mainly around membranes while some are situated in intracellular spaces and others in the extracellular environment, as well as in aqueous and lipophilic domains. Some antioxidant molecules work in biological systems by directly interacting with other ANTs. The most reported interaction is the synergistic antioxidant action by vitamin E and vitamin C (Niki 2010).
The dynamics of antioxidant action are complex, and the only way to understand them is to follow the fate and consumption stages that occur during oxidation. When ANTs are present in a system, the order of their consumption, referred to as “pecking order,” is determined by the chemical characteristics such as redox potential and bond dissociation energy (Niki 2010). However, in heterogeneous systems, as is now well known, things do not translate from what is observed in vitro; thus, the pecking order is dependent on many other factors as well (Niki 2010). Such factors include bioavailability and possibly biotransformation. The molecules have to be absorbed, transported, distributed, and retained properly in the biological fluids, cells, and tissues. As the molecules are distributed, metabolism-derived loss-of-function could result (Shen et al. 2007).
Biological Activity of ANTs
The use of plants for food and medicine has partially been attributed to the biological efficacy of primary and secondary metabolites that possess antioxidant activities such as phenolic compounds, vitamins, and carotenoids (Ndhlala et al. 2010). Furthermore, other physiological activities of natural ANTs have been described, such as antibacterial, antiviral, and antimutagenicity (Cook and Samman 1996). Phenolic compounds are products of the shikimate and phenylpropanoid pathways and constitute a diverse and ubiquitous class of plant secondary metabolites characterized by aromatic rings and hydroxyl groups. Numerous studies aimed at investigating the beneficiary effects of phenolic compounds as natural ANTs have been carried out since the early 1870s (Shen et al. 2007). Some of the findings have led to the conclusion being drawn that human antioxidant defense systems are incomplete without dietary ANTs (Ratnam et al. 2006). Figure 176.1 represents structures of some common antioxidants.
Several studies have supported the assertion that phenolic compounds act as strong ANTs by neutralizing ROS and metal ion chelation (Chun et al. 2005). Credit is mainly given to the multiple hydroxyl groups in the chemical structures of phenolic compounds that make them ideal for redox reactions and as metal ion chelating agents. Antioxidant properties improve as the number of hydroxyl and methoxyl groups increases, hence higher antioxidant abilities of condensed and hydrolysable tannins at quenching ROS compared to monomeric phenolic acids (Hagerman et al. 1998).
Apart from phenolic compounds, a good diet will nourish humans with several other important nutritional and non-nutritional micro- and macro-molecules. These include vitamins C and E. Vitamin E (tocopherols and tocotrienols) has been reported to be the major lipid-soluble chain-breaking antioxidant in body tissues, playing an important first-line of defense on membranes, soldiering against early stages of ROS attacks (Lien et al. 1999). Vitamin C (ascorbic acid) is a water-soluble antioxidant that is involved in reduction of ROS from a variety of sources. Its role is to recycle radicals produced by oxidation of vitamin E (Baydar et al. 2007).
Besides the discussed micro- and macro-molecules, antioxidant enzymes play essential roles in protecting cells against ROS insults. Absence and/or inhibition of antioxidant enzymes will severely compromise the cellular ability to cope with ROS stress. The expression of antioxidant enzymes, such as SOD, catalase, and glutathione-S-transferase, is regulated by complex mechanisms, oxidative stress being a major factor that induces the adaptive expression of these enzymes (Pelicano et al. 2004).
In addition to antioxidant enzymes, the glutathione (GSSG/2GSH) and the thioredoxin systems are redox couple systems used by organisms in maintaining cellular redox balance by detoxifying the effect of certain ROS (Schafer and Buettner 2001). Glutathione (GSH) is a tripeptide that contains unusual peptide linkages between the amine group of cysteine and the carboxyl group of the glutamate side-chain and is endogenous. In the reduced state, the thiol group of cysteine in GSH is able to donate a reducing equivalent (H++ e−) to other unstable molecules, such as ROS. In donating an electron, GSH itself becomes reactive, but readily reacts with another reactive GSH to form a disulfide (GSSG) (Kranner and Birtić 2005). Thioredoxins are proteins that act as ANT by facilitating the reduction of other proteins by cysteine thiol-disulfide exchange. In humans, it is encoded by the TXN gene and contains two cysteine molecules which are key to thioredoxin’s ability to reduce other proteins (Koháryová and Kolárová 2008).
What Is in it for Humans?
The Benefits from ROS
ROS, being obligatory by-products emerging as a result of both normal metabolism and xenobiotic exposure, have the potential to be beneficial and harmful to cellular metabolism. The highly reactive, oxygen-containing molecular entities which are more potent and effective oxidizing agents than molecular oxygen itself have been considered important intracellular signaling molecules, which may act as mediators or second messengers in many cell functions (Ghiselli et al. 2000; Gonzalez et al. 2002). Counterintuitively as it may to expect a free-radical species, without apparent built-in selectivity, to propagate a specific signal, ROS play defined functions through redox modifications of a great diversity of molecules participating in almost every signaling pathway described to date (Covarrubias et al. 2008). In many cases, evidence points to hydrogen peroxide as the proximal signal molecule, although some studies have implicated superoxide as well. ROS have been proposed to have a role as oxygen sensors in cells that comprise essential parts of homeostatic loops directed to maintain oxygen levels in multicellular organisms under hypoxia situations (Lander 1997; Gonzalez et al. 2002). These cell systems include carotid body chemoreceptor cells, pulmonary artery smooth muscle cells, and erythropoietin-producing cells. Because of the diversity of cellular events that utilize free radicals, in some cases, their generation is required for ligand-stimulated gene expression, whereas in others, they themselves initiate a cellular response. It is now an accepted fact that ROS are signaling molecules that, as with other second messengers, transduce messages from the extracellular milieu to generate a specific cellular response. The diffusibility of some ROS through plasma membranes is a property that may contribute to determine the redox state of a community of cells or the propagation of ROS signals, mechanisms that could coordinate developmental events such as massive cell death (Covarrubias et al. 2008).
The recent uncovering on the participation of the NOX enzymes as predominant sources of ROS has brought in a unifying concept of understanding their influence in disease pathology. Body tissues involved in phagocytosis, such as neutrophils and macrophages, produce very large amounts of superoxide anion, which leads to the downstream biosynthesis of hydrogen peroxide, hypohalides, hydroxyl radical, and singlet oxygen (other ROS). These highly reactive metabolic products collectively contribute to host defense by killing invading pathogens (Lambeth 2000; Lambeth et al. 2007). Depending on cell type, source of ROS (e.g., mitochondrial versus NOX enzymes), type of ROS, and other factors, some ROS have been found to modulate apoptosis. Anti-apoptotic modulating effect is an important attribute particularly in proliferative conditions such as cancer. To this effect, an increasing number of studies point to the anti-apoptotic effects of NOX-derived ROS. In pancreatic cancer cells, NOX4-derived ROS, NOX5 in Barrett’s esophagus, NOX1 in gastric epithelium and colon, and NOX1 in endothelial cells, and were found to inhibit apoptosis (see Lambeth et al. 2008 and references therein).
Although high levels of ROS have potential toxic effects on sperm quality and function, a strong body of evidence suggests that small amounts of ROS are necessary for spermatozoa to acquire fertilizing capabilities (Aitken et al. 1989; Aitken 1997; Agarwal et al. 2003). The susceptibility of spermatozoa to oxidative stress–induced damage emanates from the fact that their plasma membranes contain large quantities of polyunsaturated fatty acids and their cytoplasm contains low concentrations of scavenging enzymes (de Lamirande and Gagnon 1995; Agarwal et al. 2003). Another important aspect of ROS is manifested by inflammatory cell–mediated defense responses against microorganisms (Zhou et al. 2007). In this context, these molecules are generated by macrophages and neutrophils and play critical roles as bactericidal, antiviral, and antitumor agents (Nathan 1992). A number of studies have shown the ability of ROS to regulate fundamental cell developmental processes such as proliferation, differentiation, death, migration, and gene transcription (Burdon and Rice-Evans 1989; Dalton et al. 1999; Allen and Tresini 2000). A function of ROS in development is likely due to the large amount of evidence, showing that ROS can regulate fundamental cellular processes.
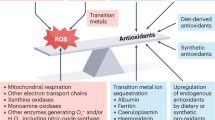
The Tilted Balance and the Benefits of ANTs
The beneficial physiological functions of ROS in the diverse tissues and cells of the human body system are dosage dependant. A balance, therefore, has to be struck between production and metabolic requirement levels. Elaborate antioxidant defense mechanism have evolved, that maintain the delicate balance between the toxicity of the oxidizing effects of ROS and the desired benefits. Under steady state conditions, this equilibrium is met by the scavenging of ROS by endogenous enzymatic and nonenzymatic ANTs. However, exposure to environmental factors (smoke, pollution, ultraviolet radiation, etc.) and pathological conditions (chronic infection, inflammation, etc.) can lead to increased ROS production, resulting in a tilted ROS/ANTs balance in favor of the oxidants, leading to oxidative stress. The consequences of oxidative stress are the key pathophysiological mechanisms in the initiation and progression of cancer, neurodegenerative disorders, cardiovascular diseases, diabetes, aging, pancreatitis, skin lesions, inflammatory diseases, sclerosis, and autoimmune disorders in humans, among others (Cosgrove et al. 1987; Jacob 1995; Ulker et al. 2003). In fact, Halliwell and Gutteridge (1990) estimate ROS to be implicated in more than 100 diseases.
Sometimes the inherent endogenous mechanisms of the human antioxidant system are sometimes incomplete without the support from the antioxidative compounds taken as foods or medicine, giving rise to a more effective complex antioxidant system in the fight against ROS-damaging effects. Through their free-radical scavenging property, ANTs delay or inhibit cellular damage. They act as radical scavengers, hydrogen donors, electron donors, peroxide decomposers, singlet oxygen quenchers, enzyme inhibitors, synergists, and metal-chelating agents (Halliwell 1997; Ratnam et al. 2006). Some ANTs induce the biosynthesis of other ANTs or defense enzymes. Components of the antioxidant system interact to provide diversity and depth to the body’s antioxidant protection system. This cooperation among the different ANTs provides greater protection against attack by ROS and thus downregulate the associated pathologic conditions. For example, ANTs have been shown to decrease oxidative stress–induced carcinogenesis by direct scavenging of ROS and by inhibiting cell proliferation secondary to protein phosphorylation (Halliwell 1997). Through in vivo experimentation, it has been evidenced that supplementing diet with ANTs can attenuate atherosclerosis in animal models (Steinberg 1986). In a nutshell, the balance between ANTs and ROS is all to the human benefit as it ensures the normal functioning of the body system and downregulates the associated damaging pathological conditions. While an enormous amount of research evidence points to this positive health benefits of ANTs in a number of diseases, several other similar studies reports on their lack of and/or negative effects.
The strong in vitro evidence that exists for the beneficial roles of ANTs in attenuating numerous disease conditions is merely mechanistic. Translating these findings into tangible benefits during human intervention trials, however, has seen very little achievements. Be that as it may, and notwithstanding the scanty and contentious in vivo evidence, it must, however, be pointed out that the role of ANTs in human health is evident and cannot be overlooked. Perhaps, the balance is tilted more in favor of the beneficial aspects than any other effect.
ANTs as a Double-Sided Sword
The greatest concern after ensuing victory in any battle is on the extent of its negative effects to the surrounding environment. Notwithstanding the purported enormous health promoting benefits from ANTs in the fight against oxidative damage in humans, it is imperative that due consideration be given to any negative effects they may have on the normal functioning of the entire body system. In this regard, ANTs have been proven, in both in vivo and in vitro models, to exhibit some dual effects.
While epidemiological evidence on vitamin E is inconclusive for their protective role in cancer, this antioxidant appears to be negatively associated with colorectal adenomas (Greenwald and McDonald 1999). Intakes of vitamin C below and above the recommended daily allowance are implicated in the increased free-radical damage to DNA (Halliwell 2000). The administration of a powerful antioxidant after the commencement of oxidative damage could result in a pro-oxidant effect and promote damage. This effect was demonstrated by Kang et al. (1998) where animals were exposed to the herbicide paraquat and administration of vitamin C as a pretreatment was protective, but when this compound was given after paraquat exposure, the damage caused by the herbicide was aggravated, presumably by interacting with released transition metals. Similar pro-oxidant effects of ANTs like vitamins C and E are reported to be involved in fatal myocardial infarctions (Sun et al. 1996; Bast and Haenen 2002). In a study by Cortés-Jofré et al. (2012), the authors concluded that there was no evidence for recommending supplements of vitamins A, C, E, selenium, either alone, or in different combinations, for the prevention of lung cancer and lung cancer mortality in healthy people. They noted that there is some evidence that the use of β-carotene supplements could be associated with a small increase in lung cancer incidence and mortality in smokers or persons exposed to asbestos. Of the ANTs shown to exhibit negative effects, vitamin E and its esters and metabolites have been implicated in a number of disorderly conditions. These include, among others, impaired blood coagulation and inhibition of cyclooxygenase enzymes, which is associated with damage to the gastrointestinal tract, resulting in ulcers (Bast and Haenen 2002). ANTs can also inhibit the oxidant-triggered signaling mechanisms that the cell uses to adapt to a free-radical insult (Halliwell 2000). Other negative effects of ANTs include suppression of the activity of other ANTs, and altering the immune system and the normal cellular protective responses to tissue damage and microbial infections (Clement and Pervaiz 1999). Reports of mutagenicity related to flavonoid-mediated oxidative damage raises major health concerns (Yamashita et al. 1999). The pro-oxidant effect exhibited by flavonoids was found to be responsible for the cytotoxic and proapoptotic effects of flavonoids isolated from various herbal medicines (Ismail and Alam 2001; Ueda et al. 2002). Findings suggest that some of the same structural attributes of flavonoids responsible for optimizing antioxidant capacity may also exacerbate oxidative stress and the associated detrimental damage to structure and functions of cellular molecules. Consequently, based on this compelling evidence, it becomes apparent that, in spite of the benefits humans derive from the antioxidant system, the body is equally exposed to the detrimental effects of some ANTs. The importance of the association between oxidative stress and disease should, therefore, not be exaggerated.
Pharmacokinetic Parameters
Understanding of the biodynamic processes that constrain dietary antioxidant release from foods, the extent of their absorption, and their ultimate fate is crucial to the comprehension of their mechanisms of action and role in disease prevention. Pharmacokinetic parameters such as absorption, distribution, metabolism, and excretion are the key important properties that determine the ultimate fate of dietary ANTs through the oral route. Once ingested, the journey of an antioxidant compound through the body system, following gut luminal events, begins with its absorption and distribution. In describing the drug discovery setting, Lipinski et al. (2001) illustrate that potency, solubility, and permeability comprise the triad required to achieve absorption through this route. Poor absorption of an antioxidant may limit its effectiveness due to its inability to obtain high systemic concentrations for extended periods of time. For example, more than 53 % of the orally administered ellagic acid, a natural phenol antioxidant found in numerous fruits and vegetables, was found remaining in the gastrointestinal tract (Teel and Martin 1988). The absorption of some antioxidant candidates is saturable, an attribute of mainly lipophilic ANTs like carotenoids and vitamin E. The high liposolubility characteristic of lycopene allows it to distribute extensively in peripheral tissues (Ratnam et al. 2006).
In order for ANTs to be absorbed, they must first be released from the food matrix and be presented to the brush-border of the small intestine in such a state that they can be absorbed into the enterocyte by passive diffusion or active transport systems (Stahl et al. 2002). Before secretion into either blood or thoracic lymph ducts in a biocompatible form, metabolic modifications and biotransformations may be required for most of the compounds. The success of this highly complex process for any given antioxidant dietary constituent, however, depends on an array of variables that include the various state of food parameters, time, as well as the body’s metabolic states. This will, in turn, affect the profile of systemic delivery of the food component and, possibly, its metabolic fate. In light of the complex nature of the pharmacokinetics of ANTs in the human system and the diversity of the variables associated with the effective absorption, distribution, and metabolism, it must therefore be pointed out that information on food nutrient content should only be used as a rough guide. The marked differences in the fecal levels of antioxidant constituents and/or metabolites between oral and intravenous routes suggest that the orally administered dietary constituents are not well absorbed.
Bioavailability
The purported biological benefits of exogenous ANTs toward human health are possibly less than that is reported as most of the research evidence is extrapolated from in vitro experimentation. This, however, presents more questions than answers with regard to the tangible benefits in the clinical context. One important question when considering the in vivo potential impacts of exogenous ANTs on disease states is their circulation levels within the body following ingestion (bioavailability). Wootton-Beard and Ryan (2011) define bioavailability as the amount and extent to which a given nutrient or therapeutic moiety is available for normal physiological functions and storage within the body. Phenolic compounds are among the most studied antioxidant compounds in this context, and the scanty data available in this regard is largely contradictory and inconclusive. The chemical structure of an antioxidant largely determines its absorption in the body system. The β-linkage of sugar moieties in polyphenols, particularly flavonoids, resists hydrolysis by pancreatic enzymes, and intestinal microflora are thought to be responsible for this hydrolysis to allow a site for conjugation. Absorption kinetics, therefore, varies considerably among dietary ANTs, owing to the heterogeneity of functional groups and their liberation from the food matrix (Ratnam et al. 2006).
Upon measuring fecal quercetin in ileostomy subjects, Hollman et al. (1997) found that human absorption of quercetin-β-glycosides from onions was 52 %, whereas absorption of quercetin without its sugar moiety, and that of quercetin-β-rutinoside was both only about 20 %. The authors also observed that peak levels were achieved less than 0.7 h after ingestion of onions, and 9 h after the rutinoside. These findings suggest that the sugar moiety of quercetin glycosides affects their absorption. Onions contain mainly glucose glycosides of quercetin and therefore, in their study, Hollman et al. (1997) concluded that conjugation with glucose enhances absorption of this flavonoid from the small gut. Later, separate related studies with pigs further indicated that the conjugation of orally administered quercetin with glucuronic and sulfuric acid appears to preferentially occur in the intestinal wall (Ader et al. 2000). In addition, the rate of elimination of quercetin metabolites seems very low, and high plasma concentrations are easily maintained with a regular supply of quercetin or rutin in the diet (Manach et al. 1997). With regard to polyphenols, a general conclusion is reached, therefore, that glycosylation influences their various physicochemical and biochemical properties on membrane permeability and subsequent bioavailability.
Bioavailability of dietary carotenoids, in general, depends on a number of factors like heat treatment, homogenization, fiber content, and presence and type of fat in the diet (Hof et al. 2000). The presence of fat in the diet has also been shown to favorably affect the absorption of lycopene (Ratnam et al. 2006). Vitamin C has relatively less bioavailability problems in comparison to other ANTs, but the elimination is reported to be very rapid, with approximately 70 % of it being removed from the body system in less than 24 h (Ratnam et al. 2006). Some ANTs are transformed in the digestive tract and marginally increase their bioavailability through their secondary metabolites. The most important dietary factor influencing the availability of carotenoids for absorption is the co-ingestion of fat. High-fat versus low-fat diets have demonstrated this requirement (Furr and Clark 1997). The current data on the bioavailability and metabolism of ANTs in humans is limited to a small number of dietary compounds, and it is thus apparent that understanding this aspect is essential for evaluating the effects of the diet on the antioxidant status in the human body system. Generally, most dietary ANTs have low bioavailability.
Conclusions
Humans live in an era with increasing concern about disease such as cancer, heart, and neurodegenerative diseases which afflict our generation. Could it be because we now live longer? Researchers have pinpointed and associated ROS with accelerated progression of these diseases, among other conditions. Nevertheless, epidemiological studies strongly suggest that antioxidants, at correct concentrations, can decrease the incidence of some disease conditions. However, one of the major challenges facing this field is the poor understanding on the functional pharmacodynamics of antioxidant agents within the human system in a clinical setting. The mechanistic nature of in vitro studies from which most of the purported antioxidant benefits are extrapolated is largely inconsistent and inconclusive and hence their limited applicability in human interventions. Bioavailability and pharmacokinetic parameters of antioxidant molecules in living systems are complicated by a plethora of physiological, chemical, and physical factors. In this regard, more questions than answers remain an intriguing and challenging situation in this very young and emerging discipline. Although preformulation studies have been conducted for some antioxidants, information on many agents remains elusive. An aggressive research elucidation using animal and/or human models is still required on the efficacy, pharmacokinetics, bioavailability, toxicology, and delivery aspects of the antioxidant agents in order to fully exploit their promising benefits in human health.
References
Ader P, Wessmann A, Wolffram S (2000) Bioavailability and metabolism of the flavonols quercetin in the pig. Free Radic Biol Med 28:1056–1067
Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–843
Aitken RJ (1997) Molecular mechanisms regulating human sperm function. Mol Hum Reprod 3:169–173
Aitken RJ, Clarkson JS, Fishel S (1989) Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod 40:183–197
Allen RG, Tresini M (2000) Oxidative stress and gene regulation. Free Radic Biol Med 28:463–499
Bast A, Haenen GRMM (2002) The toxicity of antioxidants and their metabolites. Environ Toxicol Pharmacol 11:251–258
Baydar NG, Ozkan G, Yasar S (2007) Evaluation of the antiradical and antioxidant potential of grape extracts. Food Control 18:1131–1136
Becker EM, Nissen LR, Skibsted LH (2004) Antioxidant evaluation protocols: food quality or health effects. Eur Food Res Technol 219:561–571
Benzie IFF (2003) Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integr Physiol 136:113–126
Burdon RH, Rice-Evans C (1989) Free radicals and the regulation of mammalian cell proliferation. Free Radic Res Commun 6:345–358
Chun S-S, Vattem DA, Lin Y-T, Shetty K (2005) Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem 40:809–816
Clement MV, Pervaiz S (1999) Reactive oxygen intermediates regulate cellular response to apoptotic stimuli. Free Radic Res 30:247–252
Cook NC, Samman S (1996) Flavonoids – chemistry, metabolism, cardioprotective effects, and dietary sources. Nutr Biochem 7:66–76
Cortés-Jofré M, Rueda JR, Corsini-Muñoz G, Fonseca-Cortés C, Caraballoso M, Bonfill Cosp X (2012) Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev 10:1–73
Cosgrove JP, Church DF, Pryor WA (1987) The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 22:299–304
Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, Castro-Obregón S (2008) Function of reactive oxygen species during animal development: passive or active? Dev Biol 320:1–11
Dalton TP, Shertzer HG, Puga A (1999) Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol 39:67–101
de Lamirande E, Gagnon C (1995) Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod 10:15–21
Furr HC, Clark RM (1997) Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem 8:364–377
Ghiselli A, Serafini M, Natella F, Scaccini C (2000) Total antioxidant capacity as a tool to asses redox status: critical view and experimental data. Free Radic Biol Med 29:1106–1114
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gonzalez C, Sanz-Alfayate G, Agipato MT, Gonzalez-Niño A, Rocher A, Obeso A (2002) Significance of ROS in oxygen sensing in cell systems with sensitivity to physiological hypoxia. Respir Physiol Neurobiol 132:17–41
Greenwald P, McDonald SS (1999) Antioxidants and the prevention of cancer. In: Basu TK, Temple NJ, Garg ML (eds) Antioxidants in human health and disease. CAB International, Wallingford
Gutteridge JMC, Halliwell B (2010) Antioxidants: molecules, medicines and myths. Biochem Biophys Res Commun 393:561–564
Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL (1998) High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem 46:1887–1892
Halliwell B (1997) Antioxidants in human health and disease. Annu Rev Nutr 16:33–50
Halliwell B (2000) The antioxidant paradox. Lancet 355:1179–1180
Halliwell B, Gutteridge JMC (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Hof KH, West CE, Weststrate JA, Hautvast JGAJ (2000) Dietary factors that affect the bioavailability of carotenoids. J Nutr 130:503–506
Hollman PCH, van Trijp JMP, Buysman NCP, Gaag MS, Mengelers MJB, de Vries JHM, Katan MB (1997) Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett 418:152–156
Huo Y, Qiu W-Y, Pan Q, Yao Y-F, Xing K, Lou MF (2009) Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp Eye Res 89:876–886
Ismail N, Alam M (2001) A novel cytotoxic flavonoid glycoside from Physalis angulata. Fitoterapia 72:676–679
Jacob RA (1995) The integrated antioxidant system. Nutr Res 15:755–766
Kang SA, Gang YJ, Park M (1998) In vivo dual effects of vitamin C on paraquat-induced lung damage: dependence on released metals from the damaged tissue. Free Radic Res 28:93–107
Koháryová M, Kolárová M (2008) Oxidative stress and thioredoxin system. Gen Physiol Biophys 27:71–84
Kranner I, Birtić S (2005) A modulating role for antioxidants in desiccation tolerance. Integr Comp Biol 45:734–740
Lambeth JD (2000) Regulation of the phagocyte respiratory burst oxidase by protein interactions. J Biochem Mol Biol 33:427–439
Lambeth JD, Kawahara T, Diebold B (2007) Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43:319–331
Lambeth JD, Krause K-H, Clark RA (2008) NOX enzymes as novel targets for drug development. Semin Immunopathol 30:339–363
Lander HM (1997) An essential role for free radicals and derived species in signal transduction. FASEB J 11:118–124
Lien EJ, Ren S, Bui H-H, Wang R (1999) Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic Biol Med 26:285–294
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26
Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C (1997) Bioavailability of rutin and quercetin in rats. FEBS Lett 409:12–16
Moure A, Cruz JM, Franco D, Domíngueza JM, Sineiro J, Domíngueza H, Nùñez MJ, Parajo JC (2001) Natural antioxidants from residual sources. Food Chem 72:145–171
Nathan C (1992) Nitric oxide as a secretory product of mammalian cells. FASEB J 6:3051–3064
Ndhlala AR, Moyo M, Van Staden J (2010) Natural antioxidants: fascinating or mythical biomolecules? Molecules 15:6905–6930
Niki E (2010) Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med 49:503–515
Pelicano H, Carney D, Huanga P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Prior RL, Cao G (1999) In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med 27:1173–1181
Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MNV (2006) Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release 113:189–207
Schafer FQ, Buettner GR (2001) Redox state of the cell as viewed through the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212
Shen L, Hong-Fang J, Hong-Yu Z (2007) How to understand the dichotomy of antioxidants. Biochem Biophys Res Commun 362:543–545
Stahl W, van den Berg H, Authur J, Bast A, Dainty J, Faulks RM, Gärtner C, Haenen G, Hollman P, Holst B, Kelly FJ, Polidori MC, Rice-Evans C, Southon S, van Vliet T, Viña-Ribes J, Williamson G, Astley SB (2002) Bioavailability and metabolism. Mol Aspects Med 23:39–100
Steinberg D (1986) Studies on the mechanism of action of probucol. Am J Cardiol 57:16H–21H
Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bolli R (1996) Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest 97:562–576
Tahara EB, Navarete FDT, Kowaltowski AJ (2009) Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46:1283–1297
Teel RW, Martin RM (1988) Disposition of the plant phenol ellagic acid in the mouse following oral administration by gavage. Xenobiotica 18:295–883
Tubaro F, Ghiselli A, Rapuzzi P, Maiorino M, Ursini F (1998) Analysis of plasma antioxidant capacity by competition kinetics. Free Radic Biol Med 24:1228–1234
Ueda S, Nakamura H, Masutani H, Sasada T, Takabayashi A, Yamaoka Y, Yodoi J (2002) Baicalin induces apoptosis via mitochondrial pathway as prooxidant. Mol Immunol 38:781–791
Ulker S, McMaster D, McKeown PP, Bayraktutan U (2003) Impaired activities of antioxidant enzymes elicit endothelial dysfunction in spontaneous hypertensive rats despite enhanced vascular nitric oxide generation. Cardiovasc Res 59:488–500
Vurusaner B, Poli G, Basaga H (2011) Tumor suppressor genes and ROS: complex networks of interactions. Free Radic Biol Med 52(1):7–18. doi:10.1016/j.freeradbiomed.2011.09.035
Watson J (2013) Oxidants, antioxidants and current incurability of metastatic cancers. Open Biol 3:120144
Wootton-Beard PC, Ryan L (2011) Improving public health?: the role of antioxidant-rich fruit and vegetable beverages. Food Res Int. doi:10.1016/j.foodres.2011.09.015
Yamashita N, Tanemura H, Kawanishi S (1999) Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II). Mutat Res 425:107–115
Zhou X, Ji W-J, Zhu Y, He B, Li H, Huang T-G, Li Y-M (2007) Enhancement of endogenous defenses against ROS by supra-nutritional level of selenium is more safe and effective than antioxidant supplementation in reducing hypertensive target organ damage. Med Hypotheses 68:952–956
Acknowledgments
This work was made possible by a fellowship from the Claude Leon Foundation to ARN.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Ndhlala, A.R., Ncube, B., van Staden, J. (2014). Antioxidants Versus Reactive Oxygen Species – A Tug of War for Human Benefits?. In: Laher, I. (eds) Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30018-9_181
Download citation
DOI: https://doi.org/10.1007/978-3-642-30018-9_181
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30017-2
Online ISBN: 978-3-642-30018-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences