Abstract
Bacteria survive treatments with antimicrobial agents; they achieve this in two ways. Firstly, bacteria quickly become tolerant to these agents. This tolerance is temporary, reversible, and associated with slowing of the multiplication rate. Secondly, bacteria can undergo genetic mutations leading to permanent clonal resistance to antimicrobial agents. In patients with infections, nonmultiplying bacteria, some of which may be viable but nonculturable, exist side by side with multiplying bacteria. Current antibiotics capable of killing actively multiplying bacteria have very limited or no effect against nonmultiplying bacteria. Treatment of such infections requires a regimen of multiple antimicrobial agents in order to control nonmultiplying persistent bacteria. This is especially important in tuberculosis where there is co-existence of slowly multiplying tolerant bacteria with fast growing sensitive organisms. For this reason, a prolonged length of chemotherapy, lasting 6 months, is necessary to achieve cure. This long duration of treatment is due to the slow, inadequate effect of antibiotics on nonmultiplying persistent bacteria. Similar problems with eradication of persistent bacteria are evident in the treatment of biofilms. These bacteria serve as a pool for recurrent infections. Extended courses of antibiotics increase the likelihood of genetic resistance, raise the cost of treatments, and lead to more side effects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Nonmultiplying Bacteria and Persisters
In modern medicine, antibiotics are important in the fight against infections (Ball et al. 2004) such as bacteremia (Austrian and Gold 1964) and tuberculosis (Dineeen et al. 1976) which used to have a poor survival rate. Today, bacterial resistance to antimicrobial agents is emerging at a rate which outpaces the discovery of new antibiotics (Vashishtha 2010) and multiple-drug-resistant (MDR) bacterial pathogens (Gootz 2010) are common. This resistance profile is due to genomic modifications of bacteria by point mutations and horizontal gene transfer which lead to permanent clonal resistance to antimicrobial agents. There is another mechanism of antibiotic resistance or tolerance which is primarily dependent on the bacterial physiological state. The efficacy of the majority of antibiotics depends on the target bacteria exhibiting a high level of metabolic activity. However, once growth rate of bacteria slows down, they become insensitive to antibiotic treatment. In infectious diseases, slow or nonmultiplying tolerant bacteria coexist with fast growing sensitive organisms (Seguin et al. 2003). Antibiotics are capable of killing actively multiplying bacteria, but are almost always only partially active against slowly multiplying, or are inactive against nonmultiplying persistent bacteria (Hu et al. 2010; Coates and Hu 2006; Hu and Coates 2005). Also more than 60% of all microbial infections are caused by nonmultiplying bacteria such as those present in biofilms (Lewis 2001). The persistent bacteria are responsible for recurrent infections as seen in tuberculosis. With the currently available antibiotics, most persistent infections cannot be eradicated and are therefore associated with poor clinical outcomes (Kyd et al. 2011; Wood and Douglas 2010). Antibiotic tolerance is an important problem in patients with infections because prolonged treatment with multiple doses of antimicrobial agents is required for most bacterial infections. Compared to shorter courses with a single agent, this prolonged treatment with multiple antibiotic regimens can increase the frequency of genetic resistance associated with poor patient compliance (Coates and Hu 2006; Pechere et al. 2007), increase the cost of the treatment, and cause more side effects.
1.1 Nonmultiplying Stationary-Phase Bacteria
Bacteria grow and divide exponentially in cell suspension when growth conditions are favorable. If a certain nutrient in a medium is reduced below a threshold level, it halts key metabolic processes, such as DNA replication, and growth is arrested (Navarro Llorens et al. 2010). In order to survive nutrient deprivation, the bacteria are able to make an orderly transition from exponential growth phase to stationary phase. Cells in stationary-phase culture can survive long periods of starvation (Hu and Coates 2005) in the nonmultiplying stage (Roostalu et al. 2008). The stationary-phase nonmultiplying bacteria are able to resume growth rapidly when nutrients again become available (Siegele and Kolter 1992, 1993). Kolter et al. (1993) reported that entry into stationary phase involves a process of transition which starts at a time point in the exponential phase when DNA, proteins, and total cell mass stop increasing and continues until no further increase in cell numbers is detected. Cells are then in the stationary phase. Many factors can be responsible for this phenomenon. The most clearly defined one is starvation for a single nutrient required for growth. Some bacteria form endospores and myxospores in response to starvation (Kaiser 1986; Losick et al. 1986), but most bacterial species do not generate such differentiated cells. Only nondifferentiating bacteria are discussed in this chapter.
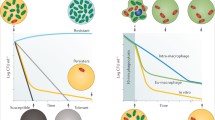
The stationary-phase response gives rise to dramatic changes in cell morphology, physiology, and gene expression (Smith 1995; Wortinger et al. 1998; Navarro Llorens et al. 2010). Lange and Hengge-Aronis examined Escherichia coli cells by light microscopy (Lange and Hengge-Aronis 1991). They found that E. coli cells became much smaller and almost spherical by reductive division when they entered into stationary phase. Some bacteria greatly reduce their size during starvation and form ultramicrocells which are less than 0.4 μm in diameter (Yourassowsky et al. 1979; Lee and Veeranagouda 2009). Changes in chromosomal topology accompanied with reduced cell division were also observed. After a few hours in stationary phase, changes in the negative superhelical density of plasmids can be detected in E. coli (Balke and Gralla 1987). The stationary-phase cultures of S. aureus produce small-colony variants (Fig. 1) which are very tolerant to antibiotics and are responsible for recurrent infections in humans (Proctor et al. 1998, 2006; Singh et al. 2009).
Colony morphology of S. aureus in stationary-phase cultures. S. aureus was grown in broth culture for 6 days. The stationary-phase culture was diluted and plated onto a nutrient agar plate. There are many small colony variants formed in the stationary-phase culture indicated by the arrow (a) compared to the colonies formed by an exponential growth phase culture (b)
The changes in cell envelope and cell membrane of starved cells mirror those which insulate and protect bacteria from stress (Martinez-Rodriguez and Mackey 2005; Siegele and Kolter 1992; Tuomanen and Tomasz 1990). For example, many stationary-phase marine bacteria coat their surfaces with hydrophobic materials that lead to cell adhesion and aggregation. Alterations in membrane composition such as fatty acids result in less fluid and less permeable membranes (Siegele and Kolter 1992; Cronan 1968).
As bacteria enter into stationary phase, their overall metabolic rate decreases but a low level of endogenous metabolism is retained (Hu et al. 2000), which enables the bacteria to take substrates into the cell during starvation and resume growth when nutrients become available (Siegele and Kolter 1992). At the same time stationary-phase bacteria synthesize and accumulate storage compounds, such as glycogen and polyphosphates and protective substances, such as trehalose (Hengge-Aronis et al. 1991).
Stationary-phase bacteria are more resistant to stress conditions such as heat shock, osmotic challenge, acidic and oxidative stress than exponential-phase bacteria (Soares et al. 2010; Hengge-Aronis 1993; Jenkins et al. 1988, 1990; Cornet et al. 2010; Davis et al. 1996; Steels et al. 1994; Arnold and Kaspar 1995; Navarro Llorens et al. 2010; Zech et al. 2009). Particularly, stationary-phase bacteria are insensitive to conventional antibiotic treatment and can only be killed by multiple doses of antibiotics at high concentration (McLeod and Spector 1996; Tuomanen and Tomasz 1990; Hu et al. 2000; Dorr et al. 2009). This is called antibiotic indifference (Jayaraman 2008).
1.2 Biofilms: Another Form of Nonmultiplying Bacteria
In nature, apart from growing in a planktonic form which is suspended or growing in a fluid environment, bacteria will also grow on surfaces to form biofilms (Trautner and Darouiche 2004). A biofilm is highly organized. It is a compact multicellular community which is on a liquid–surface interface and is embedded in a self-produced exopolysaccharide matrix. Multiplying cells occur superficially and slow or nonmultipliers live in the deeper layers. Biofilm formation is an inevitable key step in the life cycle of most microorganisms and found on many biological and nonbiological surfaces. They are associated with many infectious diseases, such as infective endocarditis, chronic skin wounds, osteomyelitis, dental plaques, infective cystic fibrosis, and infections due to indwelling medical devices such as catheters, prosthetic heart valves, and shunts (Fux et al. 2005; Mittal et al. 2009). Antibiotic therapy of infections which are associated with biofilms often lead to a poor clinical outcome, because the bacteria within the biofilms are extremely tolerant to antimicrobial agents (Lewis 2008). Successful treatment of biofilm-associated diseases requires multiple doses of high concentration antibiotic regimens or the removal of foreign body devices (Trautner and Darouiche 2004). Bacteria within biofilms are not genetically resistant strains per say. When the same cells are spread out and re-cultured in a planktonic culture, they retain sensitivity to the antibiotics (Spoering and Lewis 2001). The physiological states of the bacteria in the depth of a biofilm are very similar to those in stationary-phase planktonic culture. They are induced by nutrient starvation, high density of cell population, and accumulation of metabolic waste (Spoering and Lewis 2001; Fux et al. 2005). Biofilms are resistant to environmental stresses such as altered pH, osmolarity, and nutrient limitation. Furthermore, biofilm formation enables the bacteria to become resistant to host immune defenses (Vuong et al. 2004; Fux et al. 2005).
It has been suggested that biofilm tolerance to antibiotics and host immunity is due to the following (Lewis 2001; Fux et al. 2005): Firstly, slime matrix limits penetration of the antibiotic (Renslow et al. 2010). The exopolysaccharide matrix in which bacteria are embedded plays a key role in preventing large or small antimicrobial agents from binding to and penetrating into a biofilm. The slime matrix also protects bacteria from being engulfed during phagocytosis (Rohde et al. 2005; Vuong et al. 2004) and enhances bacterial virulence (Begun et al. 2007). Antibodies bind to the matrix and are not able to penetrate into bioflims (de Beer et al. 1997). Bacterial proteins in the biofilm matrix render them more resistant to attacks by complement (Simmons and Dybvig 2007). The exopolysaccharide matrix is likely to prevent smaller antibiotics, such as glycopeptides, crossing the diffusion barrier (Lewis 2001; Singh et al. 2010). In addition, the negatively charged exopolysaccharide binds to the positively charged antibiotics such as aminoglycosides, which effectively blocks antibiotic activity (Walters et al. 2003). Secondly, different physiological states of bacteria determine antibiotic tolerance. Biofilm formation undergoes highly regulated processes including surface attachment, cellular proliferation by cell–cell interactions, maturation by producing matrix, antibiotic tolerance development and detachment which results in bacteria regaining planktonic growth mode (O’Toole et al. 2000). In the early stage of biofilm formation, when bacteria are actively replicating, the cells are sensitive to eradication by antimicrobial agents (Gunther et al. 2009). In a mature bioflim, bacteria slow down or terminate replication and become tolerant to antibiotics. A small proportion of nonmultiplying bacteria in a biofilm cannot be eradicated by any antimicrobial agents and human immune clearance (Lewis 2008). Once the antimicrobial agents become unavailable, these persistent bacteria reform the biofilm and this leads to the relapse of infection.
1.3 Persisters
In the 1940s, Bigger (Bigger 1944) noticed that a culture of S. aureus could not be completely killed by penicillin, even at high concentration. He called these surviving bacterial cells “persisters.” Persistent cells are also present in the populations of “old” stationary-phase bacteria in batch cultures (Kaprelyants and Kell 1993; Kaprelyants et al. 1993). Old persisters can survive for a long time—it has been demonstrated that a 38-year-old stationary-phase E. coli culture contained more than 105 viable bacteria/ml in the nonmultiplying persistent stage (Eisenstark et al. 1992). Generally, persistent bacteria can be isolated by antibiotic treatment which eliminates the multiplying bacterial population (Hu et al. 2000). Persisters can be found in exponential growth culture of E. coli (Balaban et al. 2004), and this has been observed in single cells using a microfluidic device. So, persisters preexist the addition of an antibiotic to a culture and are not actually produced by such treatment. The proportion of the persistent bacteria increases upon entry into stationary phase and during biofilm formation. Persisters are nonmultiplying (Roostalu et al. 2008) and constitute 1% of the bacterial population in stationary-phase cultures and biofilms (Shah et al. 2006; Balaban et al. 2004; Spoering and Lewis 2001). These persisters are not genetically modified mutants, but are transient phenotypic variants of their parental population (Wiuff et al. 2005). They are profoundly tolerant to most marketed antibiotics (Wiuff et al. 2005; Hu et al. 2010; Keren et al. 2004a, b). If E. coli or S. aureus cells in a late stationary-phase culture are resuspended in phosphate buffered saline, the nonmultiplying persistent bacteria are very tolerant to high doses of the current antibiotics (Hu et al. 2010) as seen in Fig 2. After removal of antibiotic pressure, the small fraction of the persistent cells restore growth and become sensitive to antibiotics again (Bigger 1944).
Activities of currently marketed antibiotics against stationary-phase nonmultiplying S. aureus. The 6-day stationary-phase culture was incubated with augmentin (amoxicillin/clavulanate), azithromycin, levofloxacin, linezolid, and mupirocin at 100 μg/ml for 24 h. Viability of the bacteria was determined by colony forming unit counts. There were no antimicrobial activities observed against the stationary-phase nonmultiplying bacteria for any the tested antibiotics
1.4 Dormant Bacteria
A number of studies have indicated that there is a subgroup of dormant microbes among nonsporulating bacterial species which are unable to divide or form colonies on agar plates but are able to produce daughter cells under appropriate conditions (Kaprelyants et al. 1993). They are formed as a result of adaptation to starvation or other unfavorable conditions. The term “viable but nonculturable” (VBNC) was introduced by Colwell et al. (1985) and includes the dormant bacteria which are present in populations of some Gram-negative bacteria such as E. coli (Darcan et al. 2009), Salmonella (Turner et al. 2000), and Vibrio spp. (Chaiyanan et al. 2007; Baffone et al. 2003) starved in an aquatic environment. VBNC cells are also evident in many human pathogens such as S. enteritidis, V. cholerase, Shigella sonnei, S. flexneri (Colwell et al. 1985; Roszak et al. 1984), Listeria monocytogenes (Lindback et al. 2010; Cappelier et al. 2005, 2007), Enterococcus spp. (Lleo et al. 2001), Campylobacter jejuni (Jackson et al. 2009; Klancnik et al. 2009; Baffone et al. 2006), Helicobacter pylori (Saito et al. 2003), Staphylococcus aureus (Masmoudi et al. 2010), Mycobacterium tuberculosis (Hu et al. 2000), and Pseudomonas aeruginosa (Moore et al. 2007).
VBNC cells are usually smaller than the normal cells (Roszak and Colwell 1987). Torrellar and Morita (1981) observed very small cells of marine bacteria (ultramicrocells) which had reduced growth rates. Nilsson and colleagues (1991) also found that nonculturable V. vulnificus formed small cocci of 1.0 μm in diameter. In contrast, their rod-shaped (3.0 μm in length) vegetative cells are much larger. In general, most ultramicrobacteria of marine bacteria are unable to form colonies on agar plates (Morita 1988) but can exist as VBNCs. These cells still exhibit low metabolic activity (Barcina et al. 1989). Also, it has been found that Gram-negative and Gram-positive VBNC cells alter their cell wall components which results in robust resistance to environmental stress (Signoretto et al. 2000, 2002; Costa et al. 1999). VBNC cells can be resuscitated. Successful in vitro resuscitation and growth of S. enteritidis has been reported by Roszak et al. (1984). After addition of nutrients to microcosms (sterile river water cultures) for 25 h, the nonculturable cells produce colonies on solid agar plates.
2 Clinical Importance of Persistent Bacteria
Persistent bacteria including the VBNC forms are present in many chronic human infectious diseases (Rihl et al. 2006; Coates et al. 2008). Although persisters such as VBNC bacteria cannot cause overt disease, they form an important reservoir of latent survivors and are responsible for persistent and recurrent infections (Velazquez and Feirtag 1999). Persistent infections almost always need multiple antibiotic regimens and long periods of treatment (El Solh et al. 2008). As these persistent bacteria are nonmultiplying and cannot be cultured by traditional microbiological methods, it has proved to be difficult to detect the presence of these populations in an infection (Lleo et al. 2007; Signoretto et al. 2004). Persisters retain their pathogenicity as evidenced by maintaining their ability to adhere to human tissues and to produce toxins (Oliver 1995; Fischer-Le Saux et al. 2002; Pruzzo et al. 2002, 2003; Pommepuy et al. 1996). In chronic urinary tract infections (UTIs), a nonculturable form of E. coli with a round shape has been detected in bladder epithelial cells, and this may serve as a pool for recurrent UTIs (Anderson et al. 2003, 2004; Rivers and Steck 2001; Mulvey et al. 2001). Persistent bacteria which are present in UTIs are extremely tolerant to the highest achievable serum concentrations of all antibiotics commonly prescribed for this disease (Seguin et al. 2003). This means that eradication of persisters needs long-term antibiotic treatment, and under certain circumstances, such as catheter-associated infections, persisters cannot be eradicated by antibiotics at all, so the infected catheter has to be physically removed from the patient. This situation also applies to many other implant-associated infections. It has also been found that VBNC H. pylori is present in the gastric mucosa. These bacteria change shape from vegetative rods to an unusual round form (Kusters et al. 2006). Reactive arthritis is associated with bacterial infections which are caused by pathogens such as Chlamydia spp., Salmonella spp., Yersinia spp., Shigella spp., Campylobacter spp., and Clostridium spp. It is believed that there may be small numbers of VBNC persisters present in the joint. These bacteria cannot be cultured from synovial specimens, but their existence can be confirmed by the presence of bacterial antigens which are synthesized by metabolically active bacteria (Colmegna et al. 2004; Rihl et al. 2006). Prolonged antibiotic therapy of 12–50 weeks duration is required to cure the disease (Rihl et al. 2006) due to these persistent nonculturable bacteria.
3 Persistent M. tuberculosis
One of the most important characteristics of M. tuberculosis in the pathogenesis of the disease is its ability to persist in human tissue for the life span of the human host. During M. tuberculosis infection, the acquired immune response inhibits the replication of tubercle bacilli, but not all bacilli are destroyed. A small proportion of the bacilli remain in a dormant or persistent form (Dannebery and Rook 1994) causing latent infections. Dormant M. tuberculosis is important not only because it can survive attacks by the immune response but also because, when compared to actively growing bacteria, it is more tolerant to antibacterial agents. This means prolonged chemotherapy for 6 months is required to produce a cure (Mitchison 2004). These persistent bacteria are usually drug sensitive at relapse, so their resistance to chemotherapy is phenotypic tolerance (Hu et al. 2000) rather than genetic mutation. It is suggested that an altered physiological state of persistent M. tuberculosis accounts for its tolerance to drugs as well as the ability to survive in the host for many years. Persistence is likely to be a combined effect of both the immune system and bacterial physiology, resulting in what is generally referred to as a dormant or a latent state (Bloom and McKinney 1999).
3.1 Different Populations of M. tuberculosis in Human Lesions
It has been suggested (Mitchison 1979) that there are four different components of the bacterial population in tuberculosis lesions. As shown in Fig. 3, the majority of bacilli in the lesions of an untreated patient with tuberculosis are actively multiplying, probably at a rate similar to that in a log phase culture (population A). These bacilli may be present in the lining of open cavities with an abundant of oxygen supply, which favors the bacilli to grow rapidly. There is also a small proportion of dormant bacilli (population D) whose metabolism and growth is almost completely inhibited by an unfavorable environment, such as in closed lesions where oxygen becomes unavailable. In addition there are semidormant bacilli: Population B consists of those organisms which are inhibited by an acid environment, such as in early acute inflammatory lesions or within the phagolysosomes of macrophages. Population C is assumed to be those bacilli which contain brief or intermittent metabolic activity.
Hypothesis of special populations of tubercle bacilli within lesions killed by the bactericidal and sterilizing drugs (Mitchison 1979)
3.2 Dormant M. tuberculosis in Animal Models
The existence of dormant or persistent M. tuberculosis after chemotherapy was shown for the first time in an animal model by McCune and colleagues more than half century ago (McCune and Tompsett 1956; McCune et al. 1966b). The “Cornell model” which was named after Cornell University where the work was performed is considered to be the most useful model for demonstrating and studying mycobacterial dormancy. In this model, a large number of mice are infected intravenously with 105–106 CFU of M. tuberculosis H37Rv. Therapy with isoniazid and pyrazinamide for 12 weeks is started immediately after infection. After termination of treatment, a “sterile” state is achieved. At this stage, no tubercle bacilli are detected from homogenates of lungs or spleens of the mice by bacteriological culture and microscopy. However, the disappearance of the bacilli does not mean that the organisms have been eliminated from the tissues. About 3 months after the end of drug treatment, culturable bacilli are detected from about one-third of the mice. The reappearance of the tubercle bacilli in another two-thirds of the animals occurs within 9 months after termination of the chemotherapy. Administration of large doses of cortisone, which suppresses the immune system of the mice, accelerates the reactivation of the tubercle bacilli. The persistence of the tubercle bacilli after 12 weeks of chemotherapy is not due to the emergence of drug-resistant mutants since the bacilli which are recovered from the mice remain essentially drug sensitive (McCune et al. 1956). Some similar studies were performed later with a combination of isoniazid and rifampicin. Isoniazid and rifampicin in combination were given to M. tuberculosis infected mice for 12 months. A high relapse rate of 60% was observed by giving the mice 2 months of high dose of cortisone (Grosset 1978). The original Cornell model and the later studies show that human tuberculosis can be converted from active disease to a latent infection with chemotherapy. The dormant bacilli which survive the drug treatment can be reactivated and can cause active disease later in life. So, latent infection in animal models is characterized by the presence of the tubercle bacilli which cannot be detected by microscopy or by culture and can only be demonstrated by the emergence of active disease (McCune et al. 1966a).
3.3 Subpopulations of Nonmultiplying M. tuberculosis In Vitro
Subpopulations of nonmultiplying bacteria have been modeled in experimental cultures. M. tuberculosis is allowed to grow in a culture without agitation. The initial growth of bacilli occurs at an exponential rate with a doubling time of 16–18 h (population A) until the cell density reaches 4 × 108 CFU/ml. At this stage, dissolved oxygen becomes limiting, and the growth rate decreases while the bacilli settle to the bottom of the container (Wayne 1976, 1994). The organisms in the sediment adapt to microaerophilic conditions and enter a homogenous physiological state of dormancy. The bacilli in the deposit of the settling culture can persist for long periods after transfer to anaerobic conditions (Wayne 1977). When an old, micro-aerophilically adapted culture is treated with a high dose of rifampicin, the antibiotic kills most of the bacilli which actively replicate (A) or shows intermittent metabolic activity (population B and C), but fails to remove a small proportion of a bacterial population (D) (Hu et al. 2000) which are rifampicin tolerant persisters. These persisters rapidly lose the ability to grow on solid medium plates, but are alive and able to grow in liquid medium which contains no rifampicin (Hu et al. 2000). In such liquid subcultures, they regain the ability to grow on plates after 7 days of incubation and are then fully susceptible to rifampicin. During chemotherapy with rifampicin, tolerant bacilli that persist during treatment with rifampicin are likely to be the most difficult to kill, so that the in vitro models should reflect the likely sterilizing activity during human treatment of the drug under test, in combination with rifampicin.
4 Antibiotics Kill Nonmultiplying Bacteria
4.1 Bactericidal and Sterilizing Antibiotics
Currently, although certain antibiotics are capable of killing nonmultiplying bacteria, a very limited number of antibiotics can kill persistent or dormant bacteria. It is well established that antibiotics are ineffective against bacteria whose growth or metabolic activity has been almost completely inhibited. For example, antibiotics in the penicillin family such as penicillin, ampicillin, and amoxicillin only kill rapidly growing cells, but are ineffective against nonmultiplying cells. Although some of the antibiotics such as aminoglycosides and fluoroquinolone have activities against nongrowing bacteria such as those in biofilms, they are more effective in killing growing bacteria. In tuberculosis, antituberculous drugs have been shown to be both bactericidal and sterilizing (Fig. 3). Bactericidal activity of a drug is defined as the ability to kill rapidly replicating bacilli (population A) and is mostly effective at the beginning of treatment, such as isoniazid. The sterilizing activity of drugs such as rifampicin and pyrazinamide depends on their ability to kill semidormant bacilli (population B and C) which can persist for long periods during chemotherapy and give rise to relapses. Sterilizing activity may start early in treatment but is more evident in the later stages of treatment after bactericidal activity has declined (Mitchison and Fourie 2010). The sterilizing activity of drugs is of great clinical importance, since it determines the length of the chemotherapy. Neither bactericidal nor sterilizing drugs are currently available to kill the population D of dormant bacilli. The success of a combination of isoniazid, rifampicin, streptomycin, and pyraziamide or some other drugs in the short-course chemotherapy lies in their capacity to attack the semidormant as well as the actively replicating bacilli. In a nonmultiplying culture of M. tuberculosis, addition of fluoroquinolones such as moxifloxacin and gatifloxacin to the drug regimen of isoniazid, rifampicin, and pyraziamide reduces the numbers of persisters (Hu et al. 2003, 2006a). This indicates a need for new antibiotics which target nonmultiplying bacteria (Coates and Hu 2006, 2008) in order to shorten the length of chemotherapy.
4.2 Antimicrobials Targeting Cell Membrane and Cell Wall
There is growing evidence indicating that antimicrobial drugs against nonmultiplying bacteria are likely to target the bacterial cell membrane (Hurdle et al. 2011). The recently approved lipopeptide antibiotic, daptomycin, exhibits activity against stationary-phase nonmultiplying bacteria (Mascio et al. 2007). The mode of action of this drug is associated with modification of cell membrane potential by depolarization of the bacterial membrane (Silverman et al. 2003). Telavancin is a lipoglycopeptide derivative of vancomycin. Its mechanism of bactericidal action against nonmultiplying bacteria (Gander et al. 2005) is dependent on its ability to depolarize the bacterial cell membrane and to increase membrane permeability as well as inhibiting cell wall synthesis (Lunde et al. 2009; Nannini et al. 2010; Higgins et al. 2005). There are some antimicrobial drugs either in preclinical or clinical development targeting bacterial cell membrane or cell walls. For example, other lipoglycopeptide antibiotics such as oritavancin and dalbavancin are able to kill nonmultiplying S. aureus in stationary-phase cultures and biofilms (Belley et al. 2009; Darouiche and Mansouri 2005). The mechanism of action is to disrupt membrane potentials and to enhance the permeability of cell membranes (Belley et al. 2009). A small quinoline-derived compound HT61 was developed in an antibiotic discovery program which targets nonmultiplying bacteria from the onset of antibiotic development process (Hu et al. 2010). This is the first time that nonmultiplying cells have been targeted during the initial phases of antibiotic discovery. HT61 is very potent against nonmultiplying Gram-positive bacteria, including those that are methicillin sensitive and resistant, as well as Panton-Valentine leukocidin-carrying S. aureus. It also kills mupirocin-resistant MRSA. The action of the drug is fast, showing a complete kill within 0.5–1 h (Fig. 4). The mechanism of action of the drug is depolarization of the cell membrane and destruction of the cell wall (Hu et al. 2010). HT61 is in clinical trials with aim of decolonizing the nose of S. aureus including MRSA. Other examples of these membrane acting drugs are XF-70 and XF-73, which are porphyrin-derived compounds (Ooi et al. 2009). XF-70 and XF-73 show significant potency against nonmultiplying or slowly multiplying bacteria (Ooi et al. 2010). Ceragenins such as CSA-13 are derived from bile acid which kills bacteria by depolarization of bacterial cell membrane (Epand et al. 2010).
Potential advantages of these membrane targeting antibiotics are firstly, they are not only active against actively multiplying bacterial but most importantly they are active against nonmultiplying bacteria. Treatment using single-drug regimens may remove all populations of bacteria in different physiological states which may lead to shortened or efficient antibiotic therapy. Secondly, experimental data demonstrates that there is a very low frequency of resistance development (Van Bambeke et al. 2008; Hu et al. 2010) against this class of antibiotics. After 50 passages of S. aureus with sub-MIC concentrations of HT61, no HT61 resistant strains were selected (Hu et al. 2010). The cell membrane is essential. In order for bacteria to develop resistance to membrane active drugs, they need to modify the charges on their membrane lipids or cell membrane components. This almost always leads to death (Van Bambeke et al. 2008). Thirdly, membrane active agents normally possess rapid bactericidal activities (Hu et al. 2010) which remove the persistent bacteria prior to the drug serum level dropping below their therapeutic concentrations. In other words, these agents destroy the bacteria before resistance has a chance to occur. Mutations which are capable of conferring drug resistance cannot occur in dead bacteria.
4.3 Antipersister Formation and Waking up Dormancy
The presence of persisters in human infections is the main reason for prolonged antibiotic treatment and recurrent infection. The mechanisms in which a bacterial community forms persisters are largely unknown. Obviously, a global metabolic shut down and a program of differential gene expression play very important roles. Protein and RNA synthesis associated with stationary phase has been intensively studied in bacteria such as E. coli, S. typhimurium, and M. tuberculosis (Hu et al. 1998; Ward et al. 2010; Soares et al. 2010; Chaussee et al. 2008; Spector and Cubitt 1992; Spector et al. 1988). The stationary-phase response involves the synthesis of a characteristic set of proteins (Dong and Schellhorn 2009) which is accompanied by a gradual decrease in total protein synthesis (Groat et al. 1986; Hu et al. 1998). Most of these proteins are unique to stationary phase (De Groote et al. 2009). This essential de novo protein synthesis, accompanied by the metabolic shutdown, affords the bacteria a remarkable degree of resistance to many stress conditions. Furthermore, these processes enable the bacteria to withstand prolonged periods of stationary phase. Using transposon mutagenesis to search for persistent genes, it has been demonstrated that an intergenic region of E. coli genome is responsible for the persisters’ tolerance to high dose antibiotics (Hu and Coates 2005). In M. tuberculosis, genes which control bacterial growth have been found (Hu et al. 2006b; Parish et al. 2003). hspX gene which encodes an alpha-crystallin-like protein acts as a growth suppresser (Hu et al. 2006b; Stewart et al. 2006). Deletion of the gene results in a hyper-virulent mutant which grows faster than its parental strain (Hu et al. 2006b). This indicates that if the hspX gene product was inhibited by targeting with novel drugs, it might be possible to prevent the transition of the bacilli from log-phase growth to the nonmultiplying stage or reduce the speed of growth shifting. If persisters no longer existed or are formed at a significantly reduced speed, conventional antibiotics could be more effective.
VBNC cells can be resuscitated to normal vegetative cells when growth conditions become favorable again. Also bacterial cells are capable of producing growth promoting factors or resuscitation promoting factors such as proteins which stimulate the VBNC cells to regain growth (Mukamolova et al. 1998). If dormant bacteria in human infection can be woken up on a regular basis followed by treatments with the current antibiotic arsenal, it might be possible to effectively achieve complete sterilization and shorten the duration of chemotherapy.
5 Conclusion
Nonmultiplying persistent bacteria were discovered more than 67 years ago. It is commonly accepted that after initial exponential growth in nutrient-rich conditions, bacteria slow their metabolic activities and gradually reach a nonmultiplying stage. When bacteria grow on a solid surface, the growth mimics the profiles of planktonic culture except that biofilms are formed with a self-generated matrix. Nonmultiplying persisters, for example, VBNC cells are present in both planktonic cultures and biofilms and are also present in almost all bacterial infections. The clinical significance of these persisters is that they are profoundly tolerant to antibiotics, which leads to the need to prolong the duration of antibiotic therapy, to use high doses and to employ drug combinations. It is therefore critically important to search for antimicrobials which target nonmultiplying persisters. The most promising antibiotics discovered to date are those acting on structures such as the cell membrane or cell wall. Also, using novel agents to slow down persister formation or waking up dormant cells will be beneficial to potentiate the activities of current antibiotics. In addition, combination therapy with bactericidal and sterilizing antibiotics such as tuberculosis treatment may be able to achieve shorter and more effective antibiotic therapy with improved clinical outcomes and better patient compliance.
References
Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ (2003) Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107
Anderson M, Bollinger D, Hagler A, Hartwell H, Rivers B, Ward K, Steck TR (2004) Viable but nonculturable bacteria are present in mouse and human urine specimens. J Clin Microbiol 42:753–758
Arnold KW, Kaspar CW (1995) Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl Environ Microbiol 61:2037–2039
Austrian R, Gold J (1964) Pneumococcal bacteremia with especial reference to bacteremic Pneumococcal pneumonia. Ann Intern Med 60:759–776
Baffone W, Citterio B, Vittoria E, Casaroli A, Campana R, Falzano L, Donelli G (2003) Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int J Food Microbiol 89:31–39
Baffone W, Casaroli A, Citterio B, Pierfelici L, Campana R, Vittoria E, Guaglianone E, Donelli G (2006) Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int J Food Microbiol 107:83–91
Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S (2004) Bacterial persistence as a phenotypic switch. Science 305:1622–1625
Balke VL, Gralla JD (1987) Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol 169:4499–4506
Ball AP, Bartlett JG, Craig WA, Drusano GL, Felmingham D, Garau JA, Klugman KP, Low DE, Mandell LA, Rubinstein E, Tillotson GS (2004) Future trends in antimicrobial chemotherapy: expert opinion on the 43rd ICAAC. J Chemother 16:419–436
Barcina I, Gonzalez J, Iriberri J, Egea L (1989) Effect of visible light on progressive dormancy of Escherichia coli cells during the survival process in natural fresh water. Appl Environ Microbiol 55:6–251
Begun J, Gaiani JM, Rohde H, Mack D, Calderwood SB, Ausubel FM, Sifri CD (2007) Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses. PLoS Pathog 3:e57
Belley A, Neesham-Grenon E, McKay G, Arhin FF, Harris R, Beveridge T, Parr TR Jr, Moeck G (2009) Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother 53:918–925
Bigger JW (1944) Treatment of staphylococcal infections with penicillin. Lancet II:497–500
Bloom BR, McKinney JD (1999) The death and resurrection of tuberculosis. Nat Med 5:872–874
Cappelier JM, Besnard V, Roche S, Garrec N, Zundel E, Velge P, Federighi M (2005) Avirulence of viable but non-culturable Listeria monocytogenes cells demonstrated by in vitro and in vivo models. Vet Res 36:589–599
Cappelier JM, Besnard V, Roche SM, Velge P, Federighi M (2007) Avirulent viable but non culturable cells of Listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery. Vet Res 38:573–583
Chaiyanan S, Grim C, Maugel T, Huq A, Colwell RR (2007) Ultrastructure of coccoid viable but non-culturable Vibrio cholerae. Environ Microbiol 9:393–402
Chaussee MA, Dmitriev AV, Callegari EA, Chaussee MS (2008) Growth phase-associated changes in the transcriptome and proteome of Streptococcus pyogenes. Arch Microbiol 189:27–41
Coates AR, Hu Y (2006) New strategies for antibacterial drug design: targeting non-multiplying latent bacteria. Drugs R&D 7:133–151
Coates AR, Hu Y (2008) Targeting non-multiplying organisms as a way to develop novel antimicrobials. Trends Pharmacol Sci 29:143–150
Coates H, Thornton R, Langlands J, Filion P, Keil AD, Vijayasekaran S, Richmond P (2008) The role of chronic infection in children with otitis media with effusion: evidence for intracellular persistence of bacteria. Otolaryngol Head Neck Surg 138:778–781
Colmegna I, Cuchacovich R, Espinoza LR (2004) HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev 17:348–369
Colwell RR, Brayton BR, Grimes DJ, Roszak DB, Hug SA, Palmer LM (1985) Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implication for release of genetically engineered microorganisms. BioTechnology 3:817–820
Cornet I, Van Derlinden E, Cappuyns AM, Van Impe JF (2010) Heat stress adaptation of Escherichia coli under dynamic conditions: effect of inoculum size. Lett Appl Microbiol 51:450–455
Costa K, Bacher G, Allmaier G, Dominguez-Bello MG, Engstrand L, Falk P, de Pedro MA, Garcia-del Portillo F (1999) The morphological transition of Helicobacter pylori cells from spiral to coccoid is preceded by a substantial modification of the cell wall. J Bacteriol 181:3710–3715
Cronan JE Jr (1968) Phospholipid alterations during growth of Escherichia coli. J Bacteriol 95:2054–2061
Dannebery AM, Rook GAW (1994) Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses-dual mechanisms that control bacillary multiplication. In: Bloom BR (ed) Tuberculosis: pathogenesis, protection, and control. America Society for Microbiology press, Washington DC, pp 459–501
Darcan C, Ozkanca R, Idil O, Flint KP (2009) Viable but non-culturable state (VBNC) of Escherichia coli related to EnvZ under the effect of pH, starvation and osmotic stress in sea water. Pol J Microbiol 58:307–317
Darouiche RO, Mansouri MD (2005) Dalbavancin compared with vancomycin for prevention of Staphylococcus aureus colonization of devices in vivo. J Infect 50:206–209
Davis MJ, Coote PJ, O'Byrne CP (1996) Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology 142(10):2975–2982
de Beer D, Stoodley P, Lewandowski Z (1997) Measurement of local diffusion coefficients in biofilms by microinjection and confocal microscopy. Biotechnol Bioeng 53:151–158
De Groote VN, Verstraeten N, Fauvart M, Kint CI, Verbeeck AM, Beullens S, Cornelis P, Michiels J (2009) Novel persistence genes in Pseudomonas aeruginosa identified by high-throughput screening. FEMS Microbiol Lett 297:73–79
Dineeen P, Homan WP, Grafe WR (1976) Tuberculous peritonitis: 43 years' expereince in diagnosis and treatment. Ann Surg 184:717–722
Dong T, Schellhorn HE (2009) Global effect of RpoS on gene expression in pathogenic Escherichia coli O157:H7 strain EDL933. BMC Genomics 10:349
Dorr T, Lewis K, Vulic M (2009) SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet 5:e1000760
Eisenstark A, Miller C, Jones J, Leven S (1992) Escherichia coli genes involved in cell survival during dormancy: role of oxidative stress. Biochem Biophys Res Commun 188:1054–1059
El Solh AA, Akinnusi ME, Wiener-Kronish JP, Lynch SV, Pineda LA, Szarpa K (2008) Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am J Respir Crit Care Med 178:513–519
Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM (2010) Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob Agents Chemother 54:3708–3713
Fischer-Le Saux M, Hervio-Heath D, Loaec S, Colwell RR, Pommepuy M (2002) Detection of cytotoxin-hemolysin mRNA in nonculturable populations of environmental and clinical Vibrio vulnificus strains in artificial seawater. Appl Environ Microbiol 68:5641–5646
Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13:34–40
Gander S, Kinnaird A, Finch R (2005) Telavancin: in vitro activity against staphylococci in a biofilm model. J Antimicrob Chemother 56:337–343
Gootz TD (2010) The global problem of antibiotic resistance. Crit Rev Immunol 30:79–93
Groat RG, Schultz JE, Zychlinsky E, Bockman A, Matin A (1986) Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol 168:486–493
Grosset J (1978) The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull Int Union Tuberc 53:5–12
Gunther F, Wabnitz GH, Stroh P, Prior B, Obst U, Samstag Y, Wagner C, Hansch GM (2009) Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol Immunol 46:1805–1813
Hengge-Aronis R (1993) Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72:165–168
Hengge-Aronis R, Klein W, Lange R, Rimmele M, Boos W (1991) Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol 173:7918–7924
Higgins DL, Chang R, Debabov DV, Leung J, Wu T, Krause KM, Sandvik E, Hubbard JM, Kaniga K, Schmidt DE Jr, Gao Q, Cass RT, Karr DE, Benton BM, Humphrey PP (2005) Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 49:1127–1134
Hu Y, Coates AR (2005) Transposon mutagenesis identifies genes which control antimicrobial drug tolerance in stationary-phase Escherichia coli. FEMS Microbiol Lett 243:117–124
Hu YM, Butcher PD, Sole K, Mitchison DA, Coates AR (1998) Protein synthesis is shutdown in dormant Mycobacterium tuberculosis and is reversed by oxygen or heat shock. FEMS Microbiol Lett 158:139–145
Hu Y, Mangan JA, Dhillon J, Sole KM, Mitchison DA, Butcher PD, Coates AR (2000) Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J Bacteriol 182:6358–6365
Hu Y, Coates AR, Mitchison DA (2003) Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob Agents Chemother 47:653–657
Hu Y, Coates AR, Mitchison DA (2006a) Sterilising action of pyrazinamide in models of dormant and rifampicin-tolerant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 10:317–322
Hu Y, Movahedzadeh F, Stoker NG, Coates AR (2006b) Deletion of the Mycobacterium tuberculosis alpha-crystallin-like hspX gene causes increased bacterial growth in vivo. Infect Immun 74:861–868
Hu Y, Shamaei-Tousi A, Liu Y, Coates A (2010) A new approach for the discovery of antibiotics by targeting non-multiplying bacteria: a novel topical antibiotic for staphylococcal infections. PLoS One 5:e11818
Hurdle JG, O'Neill AJ, Chopra I, Lee RE (2011) Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75
Jackson DN, Davis B, Tirado SM, Duggal M, van Frankenhuyzen JK, Deaville D, Wijesinghe MA, Tessaro M, Trevors JT (2009) Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie Van Leeuwenhoek 96:377–394
Jayaraman R (2008) Bacterial persistence: some new insights into an old phenomenon. J Biosci 33:795–805
Jenkins DE, Schultz JE, Matin A (1988) Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol 170:3910–3914
Jenkins DE, Chaisson SA, Matin A (1990) Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol 172:2779–2781
Kaiser D (1986) Control of multicellular development: Dictyostelium and Myxococcus. Annu Rev Genet 20:539–566
Kaprelyants AS, Kell DB (1993) Dormancy in stationary-phase cultures of Micrococcus luteus: flow cytometric analysis of starvation and resuscitation. Appl Environ Microbiol 59:3187–3196
Kaprelyants AS, Gottschal JC, Kell DB (1993) Dormancy in non-sporulating bacteria. FEMS Microbiol Rev 10:271–285
Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K (2004a) Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18
Keren I, Shah D, Spoering A, Kaldalu N, Lewis K (2004b) Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol 186:8172–8180
Klancnik A, Guzej B, Jamnik P, Vuckovic D, Abram M, Mozina SS (2009) Stress response and pathogenic potential of Campylobacter jejuni cells exposed to starvation. Res Microbiol 160:345–352
Kolter R, Siegele DA, Tormo A (1993) The stationary phase of the bacterial life cycle. Annu Rev Microbiol 47:855–874
Kusters JG, van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19:449–490
Kyd JM, McGrath J, Krishnamurthy A (2011) Mechanisms of bacterial resistance to antibiotics in infections of COPD patients. Curr Drug Targets 12(4):521–530
Lange R, Hengge-Aronis R (1991) Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol 5:49–59
Lee K, Veeranagouda Y (2009) Ultramicrocells form by reductive division in macroscopic Pseudomonas aerial structures. Environ Microbiol 11:1117–1125
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007
Lewis K (2008) Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131
Lindback T, Rottenberg ME, Roche SM, Rorvik LM (2010) The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet Res 41:8
Lleo MM, Bonato B, Tafi MC, Signoretto C, Boaretti M, Canepari P (2001) Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol 91:1095–1102
Lleo MM, Benedetti D, Tafi MC, Signoretto C, Canepari P (2007) Inhibition of the resuscitation from the viable but non-culturable state in Enterococcus faecalis. Environ Microbiol 9:2313–2320
Losick R, Youngman P, Piggot PJ (1986) Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet 20:625–669
Lunde CS, Hartouni SR, Janc JW, Mammen M, Humphrey PP, Benton BM (2009) Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob Agents Chemother 53:3375–3383
Martinez-Rodriguez A, Mackey BM (2005) Physiological changes in Campylobacter jejuni on entry into stationary phase. Int J Food Microbiol 101:1–8
Mascio CT, Alder JD, Silverman JA (2007) Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob Agents Chemother 51:4255–4260
Masmoudi S, Denis M, Maalej S (2010) Inactivation of the gene katA or sodA affects the transient entry into the viable but non-culturable response of Staphylococcus aureus in natural seawater at low temperature. Mar Pollut Bull 60:2209–2214
McCune RM Jr, Tompsett R (1956) Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med 104:737–762
McCune RM Jr, McDermott W, Tompsett R (1956) The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med 104:763–802
McCune RM, Feldmann FM, Lambert HP, McDermott W (1966a) Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med 123:445–468
McCune RM, Feldmann FM, McDermott W (1966b) Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med 123:469–486
McLeod GI, Spector MP (1996) Starvation- and Stationary-phase-induced resistance to the antimicrobial peptide polymyxin B in Salmonella typhimurium is RpoS (sigma(S)) independent and occurs through both phoP-dependent and -independent pathways. J Bacteriol 178:3683–3688
Mitchison DA (1979) Basic mechanisms of chemotherapy. Chest 76:771–781
Mitchison DA (2004) Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin Respir Crit Care Med 25:307–315
Mitchison DA, Fourie PB (2010) The near future: improving the activity of rifamycins and pyrazinamide. Tuberculosis (Edinb) 90:177–181
Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K (2009) Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health 2:101–111
Moore JE, Nagano Y, Millar BC, McCalmont M, Elborn JS, Rendall J, Pattison S, Dooley JS, Goldsmith CE (2007) Environmental persistence of Pseudomonas aeruginosa and Burkholderia multivorans in sea water: preliminary evidence of a viable but non-culturable state. Br J Biomed Sci 64:129–131
Morita RY (1988) Bioavailability of energy and its relationship to growth and starvation survival in nature. Can J Microbiol 34:436–441
Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB (1998) A bacterial cytokine. Proc Natl Acad Sci USA 95:8916–8921
Mulvey MA, Schilling JD, Hultgren SJ (2001) Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579
Nannini EC, Stryjewski ME, Corey GR (2010) Telavancin's interactions with the bacterial cell membrane. Future Microbiol 5:355–358
Navarro Llorens JM, Tormo A, Martinez-Garcia E (2010) Stationary phase in gram-negative bacteria. FEMS Microbiol Rev 34:476–495
Nilsson L, Oliver JD, Kjelleberg S (1991) Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol 173:5054–5059
Oliver JD (1995) The viable but non-culturable state in the human pathogen Vibrio vulnificus. FEMS Microbiol Lett 133:203–208
Ooi N, Miller K, Hobbs J, Rhys-Williams W, Love W, Chopra I (2009) XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J Antimicrob Chemother 64:735–740
Ooi N, Miller K, Randall C, Rhys-Williams W, Love W, Chopra I (2010) XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J Antimicrob Chemother 65:72–78
O'Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Parish T, Smith DA, Kendall S, Casali N, Bancroft GJ, Stoker NG (2003) Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect Immun 71:1134–1140
Pechere JC, Hughes D, Kardas P, Cornaglia G (2007) Non-compliance with antibiotic therapy for acute community infections: a global survey. Int J Antimicrob Agents 29:245–253
Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell RR, Cormier M (1996) Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol 62:4621–4626
Proctor RA, Kahl B, von Eiff C, Vaudaux PE, Lew DP, Peters G (1998) Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin Infect Dis 27(Suppl 1):S68–S74
Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G (2006) Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305
Pruzzo C, Tarsi R, Lleo MM, Signoretto C, Zampini M, Colwell RR, Canepari P (2002) In vitro adhesion to human cells by viable but nonculturable Enterococcus faecalis. Curr Microbiol 45:105–110
Pruzzo C, Tarsi R, Lleo MM, Signoretto C, Zampini M, Pane L, Colwell RR, Canepari P (2003) Persistence of adhesive properties in Vibrio cholerae after long-term exposure to sea water. Environ Microbiol 5:850–858
Renslow RS, Majors PD, McLean JS, Fredrickson JK, Ahmed B, Beyenal H (2010) In situ effective diffusion coefficient profiles in live biofilms using pulsed-field gradient nuclear magnetic resonance. Biotechnol Bioeng 106:928–937
Rihl M, Klos A, Kohler L, Kuipers JG (2006) Infection and musculoskeletal conditions: reactive arthritis. Best Pract Res Clin Rheumatol 20:1119–1137
Rivers B, Steck TR (2001) Viable but nonculturable uropathogenic bacteria are present in the mouse urinary tract following urinary tract infection and antibiotic therapy. Urol Res 29:60–66
Rohde H, Burdelski C, Bartscht K, Hussain M, Buck F, Horstkotte MA, Knobloch JK, Heilmann C, Herrmann M, Mack D (2005) Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol Microbiol 55:1883–1895
Roostalu J, Joers A, Luidalepp H, Kaldalu N, Tenson T (2008) Cell division in Escherichia coli cultures monitored at single cell resolution. BMC Microbiol 8:68
Roszak DB, Colwell RR (1987) Metabolic activity of bacterial cells enumerated by direct viable count. Appl Environ Microbiol 53:2889–2983
Roszak DB, Grimes DJ, Colwell RR (1984) Viable but nonculturable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol 30:334–338
Saito N, Konishi K, Sato F, Kato M, Takeda H, Sugiyama T, Asaka M (2003) Plural transformation-processes from spiral to coccoid Helicobacter pylori and its viability. J Infect 46:49–55
Seguin MA, Vaden SL, Altier C, Stone E, Levine JF (2003) Persistent urinary tract infections and reinfections in 100 dogs (1989–1999). J Vet Intern Med 17:622–631
Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K (2006) Persisters: a distinct physiological state of E. coli. BMC Microbiol 6:53
Siegele DA, Kolter R (1992) Life after log. J Bacteriol 174:345–348
Siegele DA, Kolter R (1993) Isolation and characterization of an Escherichia coli mutant defective in resuming growth after starvation. Genes Dev 7:2629–2640
Signoretto C, Lleo MM, Tafi MC, Canepari P (2000) Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl Environ Microbiol 66:1953–1959
Signoretto C, Lleo MM, Canepari P (2002) Modification of the peptidoglycan of Escherichia coli in the viable but nonculturable state. Curr Microbiol 44:125–131
Signoretto C, Burlacchini G, Lleo MM, Pruzzo C, Zampini M, Pane L, Franzini G, Canepari P (2004) Adhesion of Enterococcus faecalis in the nonculturable state to plankton is the main mechanism responsible for persistence of this bacterium in both lake and seawater. Appl Environ Microbiol 70:6892–6896
Silverman JA, Perlmutter NG, Shapiro HM (2003) Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 47:2538–2544
Simmons WL, Dybvig K (2007) Biofilms protect Mycoplasma pulmonis cells from lytic effects of complement and gramicidin. Infect Immun 75:3696–3699
Singh R, Ray P, Das A, Sharma M (2009) Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol 58:1067–1073
Singh R, Ray P, Das A, Sharma M (2010) Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65:1955–1958
Smith AW (1995) Stationary phase induction in Escherichia coli – new targets for antimicrobial therapy? J Antimicrob Chemother 35:359–361
Soares NC, Cabral MP, Gayoso C, Mallo S, Rodriguez-Velo P, Fernandez-Moreira E, Bou G (2010) Associating growth-phase-related changes in the proteome of Acinetobacter baumannii with increased resistance to oxidative stress. J Proteome Res 9:1951–1964
Spector MP, Cubitt CL (1992) Starvation-inducible loci of Salmonella typhimurium: regulation and roles in starvation-survival. Mol Microbiol 6:1467–1476
Spector MP, Park YK, Tirgari S, Gonzalez T, Foster JW (1988) Identification and characterization of starvation-regulated genetic loci in Salmonella typhimurium by using Mu d-directed lacZ operon fusions. J Bacteriol 170:345–351
Spoering AL, Lewis K (2001) Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 183:6746–6751
Steels EL, Learmonth RP, Watson K (1994) Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 140(3):569–576
Stewart JN, Rivera HN, Karls R, Quinn FD, Roman J, Rivera-Marrero CA (2006) Increased pathology in lungs of mice after infection with an alpha-crystallin mutant of Mycobacterium tuberculosis: changes in cathepsin proteases and certain cytokines. Microbiology 152:233–244
Torrella F, Morita RY (1981) Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol 41:518–527
Trautner BW, Darouiche RO (2004) Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control 32:177–183
Tuomanen E, Tomasz A (1990) Mechanism of phenotypic tolerance of nongrowing pneumococci to beta-lactam antibiotics. Scand J Infect Dis Suppl 74:102–112
Turner K, Porter J, Pickup R, Edwards C (2000) Changes in viability and macromolecular content of long-term batch cultures of Salmonella typhimurium measured by flow cytometry. J Appl Microbiol 89:90–99
Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, Tulkens PM (2008) The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci 29:124–134
Vashishtha VM (2010) Growing antibiotics resistance and the need for new antibiotics. Indian Pediatr 47:505–506
Velazquez M, Feirtag JM (1999) Helicobacter pylori: characteristics, pathogenicity, detection methods and mode of transmission implicating foods and water. Int J Food Microbiol 53:95–104
Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M (2004) Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275
Walters MC 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS (2003) Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323
Ward WO, Swartz CD, Hanley NM, DeMarini DM (2010) Transcriptional characterization of Salmonella TA100 in log and stationary phase: influence of growth phase on mutagenicity of MX. Mutat Res 692:19–25
Wayne LG (1976) Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis 114:807–811
Wayne LG (1977) Synchronized replication of Mycobacterium tuberculosis. Infect Immun 17:528–530
Wayne LG (1994) Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis 13:908–914
Wiuff C, Zappala RM, Regoes RR, Garner KN, Baquero F, Levin BR (2005) Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations. Antimicrob Agents Chemother 49:1483–1494
Wood AJ, Douglas RG (2010) Pathogenesis and treatment of chronic rhinosinusitis. Postgrad Med J 86:359–364
Wortinger MA, Quardokus EM, Brun YV (1998) Morphological adaptation and inhibition of cell division during stationary phase in Caulobacter crescentus. Mol Microbiol 29:963–973
Yourassowsky E, Van Der Linden MP, Lismont MJ (1979) Growth curves, microscopic morphology, and subcultures of beta-lactamase-positive and -negative Haemophilus influenzae under the influence of ampicillin and cefamandole. Antimicrob Agents Chemother 15:325–331
Zech H, Thole S, Schreiber K, Kalhofer D, Voget S, Brinkhoff T, Simon M, Schomburg D, Rabus R (2009) Growth phase-dependent global protein and metabolite profiles of Phaeobacter gallaeciensis strain DSM 17395, a member of the marine Roseobacter-clade. Proteomics 9:3677–3697
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hu, Y., Coates, A. (2012). Nonmultiplying Bacteria are Profoundly Tolerant to Antibiotics. In: Coates, A. (eds) Antibiotic Resistance. Handbook of Experimental Pharmacology, vol 211. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28951-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-28951-4_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-28950-7
Online ISBN: 978-3-642-28951-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)