Abstract
The endocannabinoid system consists of lipid-derived agonists that activate cannabinoid (CB) receptors. CB receptor agonists, namely, the phytocannabinoid Δ9-THC and the endocannabinoid anandamide, increase hunger sensation and food intake. These discoveries led to the clinical use of Δ9-THC derivatives for the treatment of cancer and HIV-related nausea and cachexia. Animal studies clarified the important role of CB1 receptors in the hypothalamus and in the limbic system in mediating orexigenic effects. In parallel, data on CB1-specific blockade either by drugs or by genetic ablation further demonstrated that CB1 inhibition protects against weight gain induced by high-fat feeding and reduces body weight in obese animals and humans. The mechanisms of weight reduction by CB1 blockade are complex: they comprise interactions with several orexigenic and anorexigenic neuropeptides and hormones, regulation of sympathetic activity, influences on mitochondrial function, and on lipogenesis. Although these mechanisms appear to be mainly mediated by the CNS, weight loss also occurs when drugs that do not reach CNS concentrations sufficient to inhibit CB1 signaling are used. The development of peripherally restricted CB1 inverse agonists and antagonists opened new routes in CB1 pharmacology because centrally acting CB1 inverse agonists, e.g., rimonabant and taranabant, exerted unacceptable side effects that precluded their further development and application as weight loss drugs. Tissue and circulating endocannabinoid concentrations are often increased in animal models of obesity and in obese humans, especially those with visceral fat accumulation. Thus, further research on CB1 inhibition is still promising to treat human obesity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endocannabinoids
- Anandamide
- Tetrahydrocannabinol
- Cannabinoid receptor
- Rimonabant
- Taranabant
- Obesity
- Cachexia
- Food intake
1 Introduction
The endocannabinoid system (ECS) consists of lipid-derived agonists activating cannabinoid (CB) receptors. Membrane phospholipid precursors, enzymes, cell membrane carriers, and transport proteins are involved in the signaling and bioavailability of endocannabinoids. The cannabinoid receptors (CB1 and CB2) are encoded by two different genes on human chromosomes: 6q14-q15 (CNR1) and 1p36.11 (CNR2). Both are heptahelical Gi/o protein-coupled receptors. Typical intracellular events following cannabinoid receptor activation are decreased cAMP synthesis, increased K+ efflux, and decreased Ca2+ influx. The cannabinoid receptors have a distinct expression pattern with rare overlap in a given cell type (Howlett et al. 2002; Pertwee et al. 2010).
In general, the ECS exerts damping actions in situations of stress or injury to facilitate cellular repair and regeneration (Pacher et al. 2006). These protective actions result from presynaptic CB1 activity in the brain modulating the release of neurotransmitters (e.g., GABA, glutamine, dopamine, norepinephrine, serotonin). Full CB1 activation results in a distinct behavioral pattern including ataxia, hypothermia, analgesia, and short-term memory impairment. For general insight into the physiology of the ECS, the reader is referred to reviews published in a previous issue of the Handbook (Abood 2005; Di Marzo et al. 2005; Howlett 2005; Pertwee 2005).
Part of the protective role of the ECS is facilitation of energy intake and storage, which in modern times may promote the development of obesity (Pagotto et al. 2006). Several animal models of obesity and obese humans exhibit increased availability of endocannabinoids in tissues and blood, and changes of expression of key genes coding for CB1 and the fatty acid amide hydrolase, FAAH (Blüher et al. 2006; Coté et al. 2007; Engeli et al. 2005; Matias et al. 2006). Whether ECS dysregulation is a consequence of the development of obesity, or a primary cause predisposing individuals to weight gain, is an unsolved matter of debate that has been discussed elsewhere (Engeli 2008a).
The phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) has been identified in 1964, but the endocannabinoid research history is short (see Table 1). The phenomenon that CB1 agonists such as Δ9-THC increase hunger sensation and food intake led to the first clinical use of cannabis for the treatment of nausea and cachexia (Abel 1975; Kirkham and Williams 2001). In parallel, CB1 inverse agonists, originally developed as therapeutics against substance abuse, showed weight-reducing activities.
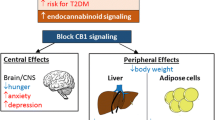
The first synthetic drug modulating the ECS and approved for weight loss was rimonabant (SR141716). Approval, however, was granted only in Europe, not in the USA. Two years later, rimonabant was withdrawn from the European market because of psychiatric side effects that prompted a negative recommendation by the EMA. This development led to a halt in research and development activities of several large pharmaceutical companies, which mostly abandoned antiobesity drugs and centrally acting CB1 inverse agonists from their agenda. This chapter will describe the molecular and physiological mechanisms by which CB1 regulates body weight and will also provide a prospect on recent developments in the field of CB1 antagonism that may generate a second wave of drugs against human obesity and associated metabolic disease.
2 Activation of CB1 Signaling Increases Hunger, Food Intake, and Body Weight
2.1 Experimental Animal Studies
A series of experiments in laboratory rodents revealed that Δ9-THC, as well as the endocannabinoids anandamide and 2-AG, increased food intake when administered orally, subcutaneously, or centrally (Kirkham and Williams 2001). A comparison between peripherally administered Δ9-THC and anandamide revealed striking similarities in the orexigenic effects. The most prominent findings were the rapid onset of feeding behavior after administration of each drug and the observation that food intake was induced in presatiated rats. Other features of feeding behavior were not different from control experiments without drugs and after fasting (Williams and Kirkham 2002). Another repeatedly reported finding was that induction of feeding behavior did not follow a strict direct dose-effect relationship. The most likely explanation is the sedative effect of higher Δ9-THC and anandamide doses which counteracts the orexigenic drive. This rather simple explanation may help to understand why earlier studies failed to conclusively show effects of cannabinoids in animals and humans (Abel 1975).
The orexigenic effects of peripherally administered anandamide in presatiated rats were prevented by prior administration of rimonabant, demonstrating for the first time the involvement of CB1 in mediating cannabinoid-stimulated food intake (Williams and Kirkham 1999). Rimonabant, however, inhibits brain and peripheral CB1; thus further proof was needed to identify the site of action. Evidence came from a study that employed direct injection of anandamide and rimonabant into the ventromedial hypothalamus in presatiated rats. Again, anandamide injection induced hyperphagia, but 30-min pretreatment with the CB1-selective inverse agonist rimonabant completely abolished this effect. Rimonabant alone had no effect on acute food intake in these experiments (Jamshidi and Taylor 2001). In animals, rimonabant also inhibited specific characteristics of cannabinoid-induced feeding, such as preferred intake of sugar and palatable food, and abolished interactions with rewarding behavior such as alcohol drinking (Arnone et al. 1997; Simiand et al. 1998). Of special importance was the finding that 2-AG injection into the nucleus accumbens shell also increased food intake in a dose- and CB1-dependent manner (Kirkham et al. 2002). The nucleus accumbens is crucial in determining the motivation to eat. By direct injection of an inhibitor of the anandamide-degrading enzyme FAAH into the nucleus accumbens, or into the parabrachial region involved in integrating gustatory signals with hypothalamic nuclei, food intake was also stimulated, and preference for palatable food increased. These effects were blocked by the specific CB1 inverse agonist AM251 (Dipatrizio and Simansky 2008; Soria-Gomez et al. 2007). Together, these studies further strengthened the important role of direct and indirect stimulation of central CB1 receptors as the site of action of cannabinoid-mediated orexigenic effects.
2.2 The Human Experience
Sporadic reports on the effects of cannabis/marijuana on increasing hunger sensation and food intake date back to ancient times and were then retrieved and repeated during the nineteenth century. Soldiers were among the first individuals participating in an observational study to prove orexigenic effects of plant cannabinoids in 1933 (Siler et al. 1933). Later on, these early findings were verified by more thoroughly controlled and standardized studies (e.g., standardized for Δ9-THC intake). Their results suggested that the orexigenic effects of marijuana in volunteers were more pronounced in the fed state than under fasting conditions. Stimulation of food intake varied with respect to time course depending on the Δ9-THC dose: under acute conditions, low-dose Δ9-THC always stimulated food intake, whereas high doses initially suppress hunger sensation, although later on, food intake was stimulated. Other common results were that quantity of food eaten increased in association with the psychoactive effects and more palatable food qualities (e.g., sweet, spicy, solid) were preferred (Gagnon and Elie 1975; Greenberg et al. 1976; Tart 1970).
Under controlled chronic conditions over 2 weeks, repeated marijuana consumption with standardized Δ9-THC doses increased body weight and food intake, although the effect on food intake was shorter and less pronounced than the gain in body weight (Foltin et al. 1988). These findings suggest that CB receptor activation not only acts in the brain to modulate hunger sensation, but may also regulate body weight on other levels. Placebo-controlled studies demonstrated that the social surroundings (e.g., being alone, having to fulfill tasks, being in social contact with others) are of crucial importance for the marijuana effects on food intake. Thus, Δ9-THC is not only able to interact with orexigenic pathways but also plays a special role for hedonistic eating behavior. This finding, if also true for endocannabinoids, might be of special importance for the development of obesity in humans because interactions between endocannabinoid signaling and the reward system may provide some explanation for the obesity epidemic in modern societies.
Ancient experience and accumulated data in healthy human subjects on the stimulating effects of Δ9-THC on eating behavior have led to the exploration of cannabis-related drugs as adjunctive treatment in disease conditions such as nausea and vomiting, unintentional weight loss, and cachexia. Nabilone is a synthetic Δ9-THC analogue with activity on CB1 and CB2 receptors, dronabinol is synthetic Δ9-THC, and Sativex® is a 1:1 mixture of plant-derived Δ9-THC and cannabidiol (Thakur et al. 2009). Nabilone and dronabinol have been evaluated for the treatment of chemotherapy-induced nausea and vomiting (Sallan et al. 1980) and are approved for this indication in some countries (Robson 2005). Cancer and HIV-related cachexia are other conditions for which the use of nabilone and dronabinol has been approved in some countries (e.g., the USA and Canada) in order to increase appetite and body weight of the patients (Beal et al. 1997; Plasse et al. 1991). Increased body weight has also been observed in patients with Alzheimer’s disease treated with dronabinol as adjunct therapy (Volicer et al. 1997). Given the psychotropic actions of these drugs, abuse and side effects clearly present matters of concern, but the safety profiles appear to be acceptable given the severity of conditions treated by these drugs, and abuse appears to be an uncommon issue (Gorter et al. 1992; Robson 2005; Ware and St Arnaud-Trempe 2010).
A recent report demonstrated that in HIV-positive marijuana smokers, the approved dronabinol dose may be too small to maintain increased appetite and food intake in the long term. Whether this finding points to tolerance development against the used dose of dronabinol, or tolerance development against the orexigenic effect of dronabinol, remains to be determined because mood effects of dronabinol were maintained at the same dose over longer periods (Bedi et al. 2010). As will be described in Sect. 3, these findings support the now accepted notion that weight changes by modulation of CB1 signaling are not only a matter of altered food intake.
3 Inhibition of CB1 Signaling Decreases Body Weight
3.1 Experimental Animal Studies
Since the description of rimonabant as a selective brain-penetrating CB1 antagonist in 1994 (Rinaldi-Carmona et al. 1994), several animal studies described the reduction of food intake and body weight by the drug. Treatment of lean Wistar rats with 10 mg/kg i.p. rimonabant significantly reduced food intake and body weight. The anorectic effect of rimonabant, however, was already diminished after 5 days, whereas the reduced body weight was maintained over 14 days of treatment (Rinaldi-Carmona et al. 1994). In contrast, lost body weight was rapidly regained if drug treatment was stopped (Carai et al. 2006). These findings were further substantiated in diet-induced obese (DIO) mice. Rimonabant 10 mg/kg p.o. significantly reduced food intake only during the first week of a 5-week treatment period. Decreased body weight was again maintained during the complete treatment period (Ravinet Trillou et al. 2003). The differential effect on food intake and body weight reduction was also demonstrated by comparing short-term rimonabant treatment with a pair-fed control group and by studying animals during a 24-h fasting period treated with rimonabant or untreated. Both experimental designs demonstrated that weight loss was more pronounced with the drug (Ravinet Trillou et al. 2003). Rimonabant was also effective in genetic models of obesity, with the fa/fa obese Zucker rat being the most widely studied model (Bensaid et al. 2003; Gary-Bobo et al. 2007; Jbilo et al. 2005). Rimonabant treatment of fa/fa rats was effective in reducing adipose tissue mass and was associated with increased adiponectin plasma concentrations, decreased inflammatory markers, decreased liver steatosis, and increased insulin sensitivity. In leptin-deficient ob/ob mice, rimonabant similarly reduced food intake and body weight. The finding that there was no difference between rimonabant treated and pair-fed animals was most likely due to the short duration of the study of only 7 days (Liu et al. 2005). However, this matter is not completely resolved because a study in DIO rats with 2 weeks’ duration also observed no differences in the reduction of body weight between rimonabant-treated animals and the pair-feeding control group (Thornton-Jones et al. 2006). Taranabant effectively reduced body weight in DIO rats over a 2-week treatment period, and somewhat different to rimonabant, the reduction in food intake appeared to be prolonged. Weight and fat mass reduction was achieved with partial CB1 receptor occupancy, and the drug’s efficiency to reduce body weight was clearly correlated to CB1 receptor occupancy in the brain (Fong et al. 2007).
Another line of evidence was developed by studying mice strains with genetic ablation of CB1 receptors. Importantly, rimonabant and taranabant had no effects on feeding behavior and body weight in CB1−/− mice (Di Marzo et al. 2001; Fong et al. 2007; Ravinet Trillou et al. 2003), and adipose tissue gene expression signatures were remarkably similar between rimonabant-treated wild-type mice and untreated CB1−/− mice (Jbilo et al. 2005). CB1−/− mice were leaner than wild-type littermates, with a specific reduction of visceral adipose tissue mass. The lean phenotype in young animals was clearly associated with a reduction of food intake, whereas in older animals, leanness and food intake were again dissociated (Cota et al. 2003). When challenged with a high-fat diet, CB1−/− mice did not develop obesity and insulin resistance and maintained a low feeding efficiency (Ravinet Trillou et al. 2004). A common finding with rimonabant treatment or CB1−/− mice studies was the reduction of plasma leptin. This finding is not surprising per se because a reduction in adipose tissue mass clearly leads to reduced circulating leptin. However, injection of leptin in wild-type mice on standard chow reduced body weight by 4.7%, whereas leptin reduced body weight by 7.5% in CB1−/− mice on the same diet. This important finding demonstrates that lack of CB1 signaling increases leptin sensitivity under these experimental conditions (Ravinet Trillou et al. 2004).
In summary, experimental animal data obtained by either pharmacological or genetic inhibition of CB1 signaling without doubt demonstrated a pronounced effect on body weight and adipose tissue mass. This weight-reducing effect is only partially accompanied by a reduction of food intake, suggesting that CB1 receptors have additional effects on energy metabolism.
3.2 Clinical Experience with CB1 Inverse Agonists
Two brain-penetrating inverse CB1 agonists, rimonabant and taranabant, have been thoroughly studied for their clinical efficacy during the last years. Weight loss was the primary endpoint in all clinical trials.
The efficacy data of rimonabant submitted to regulatory authorities were based on 6,600 overweight and obese subjects in four randomized trials that lasted for one (RIO-Lipids, RIO-Diabetes) or 2 years (RIO-Europe, RIO-North America) and tested 5 mg/day and 20 mg/day rimonabant against placebo. According to the study protocols, all subjects should have reduced caloric intake by 600 kcal/day throughout the placebo run-in and treatment periods. Primary endpoint in all trials was weight reduction. All trials followed a similar study design so that data could be combined to a large degree (Després et al. 2005; Pi-Sunyer et al. 2006; Scheen et al. 2006; van Gaal et al. 2005). The placebo-subtracted effects of 20 mg/day rimonabant were a reduction of approximately 5 kg body weight and reduction of 4 cm of waist circumference. In RIO-Diabetes, reductions of weight and waist circumference were less pronounced, but still significantly larger compared to placebo. This is a common finding in weight loss trials that recruited diabetic patients on metformin or sulfonylureas.
Weight loss with rimonabant was accompanied by favorable changes in triglycerides (−14% in addition to placebo), HDL (+8%), and fasting insulin (−4 μU/ml in nondiabetic patients). Also, a significant reduction in insulin secretion during an oral glucose load occurred. High-sensitive C-reactive protein decreased by 30%, and adiponectin increased by 40%. In RIO-Diabetes, the absolute change of hemoglobin A1c (HbA1c) compared with placebo was −0.7%, independent of concomitant oral antidiabetic drug treatment. Metabolic changes and weight loss lasted as long as rimonabant was taken. Once patients were rerandomized to placebo after 1-year treatment in RIO-North America, the treatment effects were lost, and body weight rose to the level of the ever-placebo treated group. This finding demonstrates the chronic nature of obesity and obesity-associated metabolic disease. Using the placebo data as a calibrator and analysis of covariance (ANCOVA), 50% of the favorable changes in above-mentioned metabolic variables were calculated to be due to weight loss, whereas 50% were due to rimonabant-specific effects independent of weight loss. These statistics have never been verified by controlled experiments, but point to metabolic effects of endocannabinoids and CB1 receptors (Engeli 2008b).
Before European marketing authorization of rimonabant in 2006, other phase III trials were started to broaden the knowledge on metabolic and cardiovascular effects of the drug. The randomized, double-blind, and placebo-controlled Comprehensive Rimonabant Evaluation Study of Cardiovascular Endpoints and Outcomes (CRESCENDO) included women and men ≥55 years with abdominal obesity and a history of cardiovascular disease, or at least two major cardiovascular risk factors (Topol et al. 2010). The primary endpoint was occurrence of myocardial infarction, stroke, or cardiac death. CRESCENDO enrolled 9,381 in the rimonabant (20 mg/day) group and 9,314 in the placebo group. After mean follow-up of 13.8 months, regulatory authorities requested premature discontinuation of the study due to the EMA decision to suspend marketing authorization. At this point, about half of the events required had occurred. Overall, rimonabant did not reduce occurrence of the primary endpoint. Although the event rate appeared to diverge after 1 year, the number of patients was not sufficient to assess potential beneficial actions of the drug. Gastrointestinal and neuropsychiatric side effects were of particular concern and occurred more common with rimonabant, including four completed suicides in the rimonabant and one in the placebo group. No data on body weight, waist circumference, blood pressure, glucose metabolism, or any other cardiovascular risk factors were reported (Jordan et al. 2011; Topol et al. 2010).
Reduced food intake in human subjects treated with rimonabant has only been reported in the form of abstracts, but a crossover study with taranabant was published. A high single dose of taranabant (12 mg) reduced cumulative 24-h food intake by 22% in comparison to placebo, whereas sibutramine reduced food intake by 12% compared to placebo. Reduced food intake with the high taranabant dose was present during all meals over the day, and no specific changes in macronutrient choice were observed (Addy et al. 2008).
Efficacy data of taranabant in approximately 5,850 overweight and obese patients enrolled in randomized, placebo-controlled, double-blind studies were published several months after the decision of the company to suspend further development of the drug for weight loss. In a low-dose study over 52 weeks, taranabant 2 mg/day decreased body weight by 5 kg more than placebo treatment. The proportion of patients losing 5% or 10% of initial body weight was significantly higher with taranabant 2 mg/day than with placebo and lower doses. Fat mass reduction and a reduction of waist circumference was observed accordingly (Proietto et al. 2010). In a high-dose study over 104 weeks, both higher-dose arms (4 mg/day and 6 mg/day) were prematurely stopped during the first or second year due to safety concerns, and patients of these arms were switched to 2 mg or placebo until the end of year 2. Weight reduction at week 104 was 5 kg (2 mg/day) or 6.2 kg (4 mg/day) more than with placebo (Aronne et al. 2010). Weight loss in patients with type 2 diabetes over 52 weeks with taranabant 2 mg/d was significantly greater than with placebo, but smaller than in overweight and obese patients without type 2 diabetes (Kipnes et al. 2010). In a very interesting approach different from other weight loss trials, taranabant was also tested against placebo in a randomized double-blind study after initial successful weight loss with low-calorie diet for 6 weeks (−9.6 kg). During the following year, patients on placebo gained 1.7 kg again, whereas patients on 2 mg/day taranabant lost an additional 1.2 kg (Wadden et al. 2010). These data point to the strong effects of CB1 inverse agonists on body weight regulation. On the other hand, these data also demonstrate the efficacy of well-conducted and controlled lifestyle interventions.
The rimonabant and taranabant trials clearly proved that pharmacological inhibition of CB1 signaling is efficacious to reduce and maintain lower body weight in overweight and obese patients. However, the use of brain-penetrating CB1 inverse agonists was associated with significant unwanted effects. First, gastrointestinal symptoms occurred in many patients (e.g., nausea, vomiting, diarrhea). These symptoms may result from blockade of not only central but also gastrointestinal CB1 receptors. Typically, however, most patients recovered quickly, and the symptoms disappeared, maybe because tolerance developed. Thus, weight loss by these drugs could not be explained by gastrointestinal side effects. More serious were CNS-related side effects, typically, anxiety, depressed mood, clinical relevant depression, and suicidal tendencies. As seen in the taranabant trials, these CNS side effects were dose dependent. The overall impression was that rigorous patient selection might have reduced the number of adverse CNS events and might have improved patient safety. Nevertheless, lessons learned from other new drugs clearly suggest that once a drug is marketed, rigorous patient selection rapidly vanishes in routine daily practice. Furthermore, the CNS side effects of centrally acting CB1 inverse agonists have a well-known physiological basis (Moreira et al. 2009). Both points have ultimately determined the failed introduction of this drug class into clinical practice.
4 Mechanisms of CB1-Mediated Body Weight Regulation
4.1 Food Intake and Endocannabinoid Bioavailability
Anandamide and 2-AG were measured in rat hypothalamus, in the limbic forebrain, and in the cerebellum in response to fasting and feeding. Fasting significantly increased AEA and 2-AG concentrations in the limbic forebrain and 2-AG concentrations in the hypothalamus. In response to feeding, hypothalamic 2-AG rapidly declined. No changes of endocannabinoid tissue concentrations were observed in the cerebellum, a brain region not involved in the regulation of food intake (Kirkham et al. 2002). The rapid response may reflect a decrease of CB1 signaling that, together with other signals, determines meal duration. The question then is which signals regulate postprandial endocannabinoid concentrations. A role of leptin has been discussed because leptin administration decreased elevated hypothalamic endocannabinoid concentrations in obese animal models (Di Marzo et al. 2001). However, leptin is not an acute satiety signal but rather a long-term regulator of caloric intake in relationship to fat and body mass. Also, the investigated animal models mostly had genetic defects in leptin signaling (ob/ob mice, db/db mice, fa/fa rats). Increased tissue endocannabinoid concentrations have also been described in peripheral tissues of these particular models (Maccarrone et al. 2005). Thus, the described reduction of endocannabinoids by leptin merely reflects the correction of the hormonal deficiency in these models, rather than providing a mechanistic explanation for endocannabinoid regulation by food intake. The same authors, however, also demonstrated that leptin administration is able to reduce hypothalamic anandamide and 2-AG in a normal-weight Sprague–Dawley rats. Dependency of this effect on feeding condition or daytime was not reported (Di Marzo et al. 2001).
A rapid decrease of blood endocannabinoids in response to a test meal has also been described in a small human study (Matias et al. 2006). We have reproduced this finding in a larger study with lean and obese subjects (Fig. 1, data not published). The reduction of anandamide, but not 2-AG, was observed as early as 30 min after starting food intake and was still observed 2 h after meal intake. The peak reduction of anandamide was associated with the peaks of blood glucose and insulin. Consequently, a role of insulin as a negative regulator of anandamide was recently reported (Di Marzo et al. 2009a). In the light of increased endocannabinoid availability in obese subjects and their changes with long-term weight reduction, a detailed further exploration of the role of insulin and insulin resistance on endocannabinoid availability clearly is warranted (Blüher et al. 2006; Coté et al. 2007; Di Marzo et al. 2009b; Engeli et al. 2005; Matias et al. 2006). In the hypothalamus of lean Wistar rats, however, insulin failed to acutely alter endocannabinoid concentrations (Matias et al. 2008). Thus, tissue- and species-specific mechanisms may play a role for postprandial endocannabinoid regulation. Other possible candidates for postprandial endocannabinoid changes are polyunsaturated fatty acids (Watanabe et al. 2003). In summary, disturbed postprandial regulation of CB1 endogenous agonists may represent one possible mechanism leading to increased CB1 signaling and the development of obesity.
4.2 Influence on Central Neuropeptides Regulating Food Intake
CB1 is the G-protein-coupled receptor with the largest abundance in the mammalian brain (Pertwee et al. 2010). Imaging studies identified several brain regions with high CB1 density in humans (Burns et al. 2007), and ultrastructural analyses demonstrated presynaptic CB1 expression on hypothalamic neurons in the mouse brain (Wittmann et al. 2007). Also, coexpression of CB1 and important neuropeptides (cocaine- and amphetamine-related transcript, CART; corticotrophin-releasing hormone, CRH; and, to a much lesser extent, melanocortin-concentrating hormone, MCH) in hypothalamic neurons was reported in the mouse brain (Cota et al. 2003). Thus, endocannabinoids may act as modulators of orexigenic and anorexigenic neurotransmitters and neuropeptides by presynaptic regulation of their release, as described in other brain regions and other neural functions (Wilson and Nicoll 2001). Specifically, the presynaptic reduction of norepinephrine and serotonin release by CB1 activation may have a profound effect on hunger and satiety (Piomelli 2003). As an example, sibutramine, one of the weight loss drugs of the last decade, is both a norepinephrine and serotonin reuptake inhibitor. Sibutramine’s efficacy clearly suggested a profound role of these neurotransmitters in the suppression of hunger and the rapid initiation of satiety after a meal (Stock 1997).
Autoradiography studies revealed that DIO rats had decreased CB1 receptor density in several extrahypothalamic regions, e.g., hippocampus, cortical layers I and VII, entopeduncular nucleus, and nucleus accumbens. Lower CB1 density was correlated with the cumulative energy intake from the palatable, fat-enriched diet. Consistent with earlier reports, hypothalamic CB1 density was low compared to other brain regions, but not influenced by DIO (Harrold et al. 2002). Thus, orexigenic effects of CB1 activation in the hypothalamus might be preserved with the development of obesity. The authors suggested that CB1 downregulation through high-fat feeding is the result of increased endocannabinoid availability in extrahypothalamic regions that are at least partly involved in the preference for palatable foods. But endocannabinoid concentrations were not measured in this study; thus the mechanisms of CB1 downregulation with DIO remain uncertain.
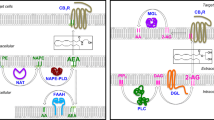
In CB1−/− mice, hypothalamic expression of the anorexigenic peptide CRH was increased, whereas expression of the orexigenic peptide CART was decreased (Cota et al. 2003). Other studies in mice also demonstrated that CB1 activation increased CART expression in hypothalamic nuclei and the nucleus accumbens, again suggesting that CART is an important downstream mediator of orexigenic endocannabinoid effects (Osei-Hyiaman et al. 2005a). For a long time, disturbances of the hypothalamic–pituitary–adrenal (HPA) axis have been discussed as a possible cause of human obesity (Wallerius et al. 2003). Interactions between CB1 signaling and the HPA axis that go beyond changes of hypothalamic CRH expression were found in CB−/− mice, but if and how these interactions may contribute to CB1-mediated weight regulation was not studied (Fig. 2).
Direct electrophysiological recordings in neurons from the lateral hypothalamus demonstrated an interaction between endocannabinoid and leptin signaling. These neurons are important because they express the orexigenic MCH and orexin neuropeptides. Stimulation of these neurons leads to CB1-mediated suppression of inhibition of local hypothalamic circuits. These effects were inhibited by leptin. Suppression of inhibition via CB1 was strongly enhanced in leptin-deficient mice (Jo et al. 2005).
4.3 Energy Metabolism
Studies in metabolic chambers did not reveal differences in body temperature, locomotor activity, or energy expenditure between CB−/− and wild-type control mice (Cota et al. 2003). This finding was unexpected because at the age of the mice during the experiment, weight differences between CB1−/− and wild-type mice could not be explained by differences in food intake. In other experiments, a single dose of rimonabant resulted in an acute increase of oxygen consumption that was not associated with increased locomotor activity in normal-weight Sprague–Dawley rats. No changes in respiratory quotient were observed. The effect was not replicated in CB1−/− mice, but the more important finding was the rapid development of tolerance, because the second dose of rimonabant already failed to increase energy expenditure (Kunz et al. 2008). These findings are in contrast to experiments with ob/ob mice. Here, 7 days of treatment with rimonabant rather robustly increased basal oxygen consumption of the animals (Liu et al. 2005), although pair feeding was associated with similar weight reduction (see Sect. 3.1). Unfortunately, energy expenditure was not measured in the pair-fed animals in this study. Rimonabant treatment enhanced skeletal muscle glucose uptake in ob/ob mice, which may enhance insulin sensitivity. In other studies, AMP-kinase did not mediate the insulin-desensitizing effect of cannabinoids in rat skeletal muscle (Kola et al. 2005), but blockade of CB1 receptors in human skeletal muscle myotubes increased AMP-kinase mRNA expression (Cavuoto et al. 2007).
One indirect calorimetry study was conducted in humans to determine effects of a single dose of taranabant on energy expenditure. This placebo-controlled, double-blind, crossover study in overweight and moderately obese men revealed that a 12-mg single dose taranabant increased resting energy expenditure significantly versus placebo, whereas a 30-mg single dose sibutramine did not. Furthermore, respiratory quotient decreased with taranabant, suggesting a preference for fat oxidation under these experimental conditions (Addy et al. 2008). Although the differences were small, the data point to an influence of CB1 inverse agonists on energy metabolism. Whether the effect was transmitted by central or peripheral effects of the drug could not have been solved by the study design.
Investigators in a recently published study knocked out CB1 receptors in forebrain and sympathetic neurons. The conditional knockout resulted in a lean phenotype, although global CB1−/− were still leaner, and protection against high-fat feeding induced obesity which was even stronger than in CB1−/− global knockout animals. Of great importance was the observation that rimonabant effects on food intake, body weight, and respiratory quotient were not anymore evident in the conditional knockout model, although these animals still expressed a reasonable number of CB1 receptors in several brain regions and peripheral organs (Quarta et al. 2010). Metabolic changes typically seen in DIO mice did not occur in the conditional knockout, suggesting that several metabolic disturbances of obesity are subject to central control. Overall, this model suggests that presynaptic CB1 receptors inhibit sympathetic activity. The conditional knockout of CB1 receptors in sympathetic neurons then led to sympathetic activation, which protected animals against high-fat feeding. The effect was partly mediated by increased brown adipose tissue thermogenesis. These data clearly point to the great importance of central regulation of energy metabolism, both in general and in mediating CB1 effects on body weight.
Nevertheless, direct effects of CB1 signaling have also been described in adipocyte mitochondria. These studies reported that CB1 activation decreased mitochondrial respiration, whereas CB1 blockade increased mitochondriogenesis and oxygen consumption in murine adipocytes (Tedesco et al. 2008, Tedesco et al. 2010). We obtained similar data in human adipocytes and observed a decrease of the oxygen consumption rate with the CB1 agonist HU210 (Fig. 3, unpublished data). Gene expression data in a murine brown adipocyte model suggested that CB1 activation led to decreased expression of the key gene uncoupling protein 1 (UCP1) (Perwitz et al. 2006). This finding points to an energy-saving effect of CB1 receptors that is not mediated by the CNS.
Presynaptic CB1 receptors regulate the release of orexigenic and anorexigenic neuropeptides. Endocannabinoids (EC) are produced by postsynaptic orexigenic and anorexigenic neurons. Whereas the effects of changes in CB1 signaling on the depicted neuropeptides were described in several studies using CB1−/− mice models or CB1 antagonists, the proposed mechanism by retrograde inhibition via CB1 receptors has as yet only been proved for MCH in specific prefornical lateral hypothalamus neurons (Jo et al. 2005). Thus, no further details are given concerning specific hypothalamic nuclei and specific neuron populations. Leptin interacts with retrograde endocannabinoid signaling by inhibition of EC synthesis (Di Marzo et al. 2001)
Energy metabolism of differentiated human SGBS adipocytes under control conditions or after 72-h treatment with the CB1 agonist HU210. HU210 significantly decreased oxygen consumption rate (OCR) under resting conditions (baseline) and after uncoupling with FCCP. Data are mean ± SEM, n = 4 independent experiments with 60 wells for each column. Group comparison by 2-way ANOVA. Significant differences are shown for the control vs. HU210 comparison (*p < 0.05)
4.4 Gastrointestinal Mechanisms
In the gastrointestinal tract, CB1 receptors are primarily expressed in the enteric nervous system. Activation of CB1 receptors decreases gastric secretion, decreases acetylcholine release, and delays gastric emptying (Storr and Sharkey 2007). In rat small intestine, anandamide concentrations increased with fasting and decreased with feeding (Gomez et al. 2002). In the same study, induction of feeding by peripherally administered CB1 agonists was inhibited through vagal ablation with capsaicin. The influence of endocannabinoids on feeding behavior can to some extent also be explained by peripheral interactions with gastrointestinal hormones. CB1 receptors and receptors for gastrointestinal hormones such as cholecystokinin (CCK), ghrelin, and orexins are colocalized on vagal nerve terminals projecting from the gastrointestinal tract to the nucleus of the solitary tract (Burdyga et al. 2004, 2006, 2010). Vagal CB1 receptors are upregulated by fasting and downregulated by feeding, and this reaction was blocked by CCK antagonists. Thus, the anorexigenic action of CCK may be in part mediated by downregulation of orexigenic CB1 receptors. Activation of ghrelin receptors also prevented the downregulation of vagal CB1 receptors.
Other interactions between the ECS and ghrelin, one of the main orexigenic gastrointestinal hormones, were also described. CB1 activation enhanced ghrelin release from the stomach. In addition, ghrelin increased hypothalamic endocannabinoid concentrations. Consequently, rimonabant reduced ghrelin’s orexigenic activity when injected into the hypothalamus and reduced circulating ghrelin in fasted and fed rats. In contrast, no interactions were observed with glucagon-like peptide, a hormone that may lead to weight reduction (Cani et al. 2004; Tucci et al. 2004). The orexigenic effects of ghrelin and CB1 signals appear to be mediated by stimulation of AMP-activated protein kinase (AMPK) in the hypothalamus (Kola et al. 2005). Whether the influence of the ECS on vagal input to the nucleus of the solitary tract contributes to CB1-mediated regulation of body weight remains to be determined.
In DIO mice, anandamide concentrations of the stomach decreased with the development of obesity, and CB1 gene expression also decreased, but gastric emptying decreased as well (Di Marzo et al. 2008). In contrast, anandamide concentrations decreased, and motility increased in the intestine of DIO mice. In this second set of experiments, the sensitivity of intestinal motility against rimonabant was also diminished with high-fat feeding (Izzo et al. 2009). Thus, the ECS has a differential effect on gastrointestinal endocannabinoid concentrations and gastrointestinal motility, depending on the site of action and the nutritional status. In a small placebo-controlled study with lean subjects, rimonabant decreased gastric accommodation in response to a test meal. Gastric sensitivity to distension and gastric motility were not altered by the drug (Ameloot et al. 2010). Whether the small effect on gastric accommodation contributes to decreased food intake and weight loss in obese patients is not known.
Another line of evidence was investigated in a rat model of the metabolic syndrome and associated cardiovascular disease, the JCR:LA-cp rat. The obese, disease-prone phenotype is due to the cp mutation that results in a stop codon in the extracellular domain of the leptin receptor (Russell et al. 2010). Treatment with rimonabant resulted in a transient reduction in food intake, and in diminished weight gain with aging, a finding consistent with several studies that have been summarized before in this paper. The most striking finding in this study was the reduction of fasting triglycerides (as seen in clinical studies as well) and the marked reduction of postprandial lymphatic apolipoprotein B48. This finding suggests a direct influence of pharmacological CB1 blockade on intestinal fatty acid resorption or enterocyte triglyceride/chylomicron synthesis. But again, whether this process contributes to a negative energy balance remains to be determined.
5 Peripheral CB1 Inhibition: New Players, More Mechanisms?
Most of the presented data in this chapter clearly point to the predominant role of the CNS in mediating CB1 effects on body weight. A similar predominance of the CNS has also been suggested by some authors with a more focused view on the regulation of glucose and lipid metabolism by modulation of CB1 signaling (Fong and Heymsfield 2009; O’Hare et al. 2011; Quarta et al. 2010). Nevertheless, other published reports speak for additional peripheral mechanisms. In a study with DIO rats, this was demonstrated by comparing ICV and sc administration of rimonabant (Nogueiras et al. 2008). Weight loss was more pronounced with systemic than CNS administration. The observed changes in adipose tissue function and gene expression were closely related to the hypophagic effect of CNS administration of rimonabant, as controlled for by pair feeding. In clear contrast to another study (O’Hare et al. 2011), glucose metabolism and insulin sensitivity was only modified by peripheral administration of rimonabant, independent of the effects on food intake (Nogueiras et al. 2008). Other authors have shown that the development of hepatic steatosis, inflammatory responses, and insulin resistance with high-fat feeding were prevented by selective knockout of hepatic CB1 receptors (Osei-Hyiaman et al. 2005b, 2008). The hepatic CB1 knockout did not mediate protection against the development of obesity with high-fat feeding, but these data nevertheless fostered the development of new CB1 drugs with restricted action in the periphery.
URB447 is a combined CB1 neutral antagonist/CB2 agonist with a 50× larger IC50 for CB1 receptors compared to rimonabant. After systemic administration, brain concentrations were below 10 pmol/g which was the lower limit of detection of the drug (LoVerme et al. 2009). Typical CNS-mediated effects of CB1 agonists such as catalepsy or hypothermia were not prevented by URB447. Interestingly, the reduction of food intake of equimolar doses of peripherally administered rimonabant and URB447 was similar, and weight reduction occurred with URB447 that was slower in onset than with rimonabant, but reached the same level at the end of the study. Weight gain in ob/ob mice was also prevented by URB447 at the same magnitude as with rimonabant (LoVerme et al. 2009).
AM6545 is a CB1 neutral antagonist with high transport capacity for members of the multidrug resistance (MDR) family. MDR transporter activity decreased brain concentrations of AM6545 to rather low values. Consequently, CB1-mediated catalepsy and hypothermia were prevented by peripherally administered rimonabant, but not by AM6545 (Tam et al. 2010). Body weight reduction in DIO mice was stronger with rimonabant, but AM6545 significantly reduced body weight as well, and pair feeding demonstrated that the body weight change with AM6545 was independent of a change in food intake. Respiratory quotient was shifted towards fat oxidation by the drug, and this effect was not observed in CB1−/− mice. Enhanced insulin sensitivity and glucose tolerance as well as amelioration of liver steatohepatitis with AM6545 treatment were also independent of decreased food intake (Tam et al. 2010).
If we accept that peripheral CB1 receptors in liver, skeletal muscle, adipose tissue, and pancreas are involved in the reported metabolic activities of peripherally restricted CB1 antagonists (Engeli 2008b), the question remains, how weight loss is mediated by these drugs. The gastrointestinal effects of the ECS (see Sect. 4.4) of course may be involved. Another possible explanation would be that peripheral effects are mediated by presynaptic CB1 receptors on sympathetic neurons (see Sect. 4.3). Sympathetic disinhibition by blockade of these receptors might occur (Quarta et al. 2010). If this is indeed the case, the clinical safety of such a drug would be of major concern.
6 Summary
CB receptor agonists increase hunger sensation and food intake. This discovery led to the clinical use of Δ9-THC derivatives for the treatment of cancer and HIV-related nausea and cachexia. Animal studies clarified the important role of CB1 receptors in the hypothalamus and in the limbic system in mediating orexigenic effects. In parallel, CB1-specific blockade either by drugs or by genetic ablation proved to protect against weight gain induced by high-fat feeding and to reduce body weight in obese animals and humans. The weight-reducing mechanisms of CB1 blockade are more complex than simply decreasing food intake. These mechanisms involve interactions with several orexigenic and anorexigenic neuropeptides and hormones, regulation of sympathetic activity, and influence on mitochondrial function, on lipogenesis, and on gastrointestinal functions including lipid absorption. Although weight reduction is to a large part mediated by the CNS, weight loss also occurs if drugs that do not reach CNS concentrations sufficient to inhibit CB1 signaling are used. The development of peripherally restricted CB1 inverse agonists and antagonists opened new routes in CB1 pharmacology because centrally acting CB1 inverse agonists, e.g., rimonabant and taranabant, were associated with unacceptable adverse reactions precluding further development and clinical application. Tissue and circulating endocannabinoids are often increased in animal models of obesity and in obese humans, especially those with visceral fat accumulation. Thus, further research on CB1 inhibition is still promising to treat obesity. But given the recent experience with CNS penetrating CB1 antagonistic drugs, a well-designed preclinical and clinical safety program has to be performed.
References
Abel EL (1975) Cannabis: effects on hunger and thirst. Behav Biol 15:255–281
Abood ME (2005) Molecular biology of cannabinoid receptors. Handb Exp Pharmacol 168:81–115
Addy C, Wright H, van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN III, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depre M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB (2008) The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab 7:68–78
Ameloot K, Janssen P, Scarpellini E, Vos R, Boesmans W, Depoortere I, Vanden BP, Tack J (2010) Endocannabinoid control of gastric sensorimotor function in man. Aliment Pharmacol Ther 31:1123–1131
Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G (1997) Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132:104–106
Aronne LJ, Tonstad S, Moreno M, Gantz I, Erondu N, Suryawanshi S, Molony C, Sieberts S, Nayee J, Meehan AG, Shapiro D, Heymsfield SB, Kaufman KD, Amatruda JM (2010) A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study. Int J Obes (Lond) 34:919–935
Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, Murphy R, Powderly W, Plasse TF, Mosdell KW, Shepard KV (1997) Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage 14:7–14
Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M (2010) Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl) 212:675–686
Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P (2003) The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63:908–914
Blüher M, Engeli S, Klöting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schön MR, Jordan J, Stumvoll M (2006) Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55:3053–3060
Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ (2004) Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 24:2708–2715
Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ (2006) Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 290:G1289–G1297
Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ (2010) Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol 299:G63–G69
Burns HD, van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, Lin LS, Liu P, Goulet MT, Gottesdiener K, Wagner JA, de Hoon J, Mortelmans L, Fong TM, Hargreaves RJ (2007) [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A 104:9800–9805
Cani PD, Montoya ML, Neyrinck AM, Delzenne NM, Lambert DM (2004) Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br J Nutr 92:757–761
Carai MA, Colombo G, Maccioni P, Gessa GL (2006) Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: preclinical and clinical data. CNS Drug Rev 12:91–99
Cavuoto P, McAinch AJ, Hatzinikolas G, Cameron-Smith D, Wittert GA (2007) Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol Cell Endocrinol 267:63–69
Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL (1998) Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 63:L113–L117
Cota D, Marsicano G, Tschöp M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U (2003) The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431
Coté M, Matias I, Lemieux I, Petrosino S, Almeras N, Després JP, Di Marzo V (2007) Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 31:692–699
Després JP, Golay A, Sjöström L (2005) Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134
Devane WA, Dysarz FA III, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G (2001) Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410:822–825
Di Marzo V, De Petrocellis L, Bisogno T (2005) The biosynthesis, fate and pharmacological properties of endocannabinoids. Handb Exp Pharmacol 168:147–185
Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, Romano B, Orlando P, Capasso F, Izzo AA (2008) The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol 153:1272–1280
Di Marzo V, Verrijken A, Hakkarainen A, Petrosino S, Mertens I, Lundbom N, Piscitelli F, Westerbacka J, Soro-Paavonen A, Matias I, Van GL, Taskinen MR (2009a) Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur J Endocrinol 161:715–722
Di Marzo V, Coté M, Matias I, Lemieux I, Arsenault BJ, Cartier A, Piscitelli F, Petrosino S, Almeras N, Després JP (2009b) Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 52:213–217
Dipatrizio NV, Simansky KJ (2008) Inhibiting parabrachial fatty acid amide hydrolase activity selectively increases the intake of palatable food via cannabinoid CB1 receptors. Am J Physiol Regul Integr Comp Physiol 295:R1409–R1414
Engeli S (2008a) Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol 20(Suppl 1):110–115
Engeli S (2008b) Peripheral metabolic effects of endocannabinoids and cannabinoid receptor blockade. Obes Facts 1:8–15
Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J (2005) Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54:2838–2843
Foltin RW, Fischman MW, Byrne MF (1988) Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 11:1–14
Fong TM, Heymsfield SB (2009) Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions. Int J Obes (Lond) 33:947–955
Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, Van der Ploeg LH, Goulet MT, Hagmann WK, Lin LS, Lanza TJ Jr, Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Francis B, Strack AM, MacIntyre DE, Shearman LP (2007) Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(t rifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther 321:1013–1022
Gagnon MA, Elie R (1975) Effects of marijuana and D-amphetamine on the appetite, food consumption and various cardio-respiratory variables in man. Union Med Can 104:914–921
Gaoni Y, Mechoulam R (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646
Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrie P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM, Bensaid M (2007) Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 46:122–129
Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez dF (2002) A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22:9612–9617
Gorter R, Seefried M, Volberding P (1992) Dronabinol effects on weight in patients with HIV infection. AIDS 6:127
Greenberg I, Kuehnle J, Mendelson JH, Bernstein JG (1976) Effects of marihuana use on body weight and caloric intake in humans. Psychopharmacology (Berl) 49:79–84
Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G (2002) Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Res 952:232–238
Hollister LE (1971) Hunger and appetite after single doses of marihuana, alcohol, and dextroamphetamine. Clin Pharmacol Ther 12:44–49
Howlett AC (2005) Cannabinoid receptor signaling. Handb Exp Pharmacol 168:53–79
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202
Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V (2009) Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol 158:451–461
Jamshidi N, Taylor DA (2001) Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 134:1151–1154
Jbilo O, Ravinet Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P (2005) The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19:1567–1569
Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW (2005) Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron 48:1055–1066
Jones D (2008) End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov 7:961–962
Jordan J, Schlaich M, Redon J, Narkiewicz K, Luft FC, Grassi G, Dixon J, Lambert G, Engeli S (2011) European Society of Hypertension Working Group on Obesity: obesity drugs and cardiovascular outcomes. J Hypertens 29:189–193
Kipnes MS, Hollander P, Fujioka K, Gantz I, Seck T, Erondu N, Shentu Y, Lu K, Suryawanshi S, Chou M, Johnson-Levonas AO, Heymsfield SB, Shapiro D, Kaufman KD, Amatruda JM (2010) A one-year study to assess the safety and efficacy of the CB1R inverse agonist taranabant in overweight and obese patients with type 2 diabetes. Diabetes Obes Metab 12:517–531
Kirkham TC, Williams CM (2001) Endogenous cannabinoids and appetite. Nutr Res Rev 14:65–86
Kirkham TC, Williams CM, Fezza F, Di Marzo V (2002) Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol 136:550–557
Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M (2005) Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 280:25196–25201
Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W (2008) Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond) 32:863–870
Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401–404
Lin LS, Lanza TJ Jr, Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Fong TM, Shen CP, Lao J, Xiao JC, Shearman LP, Stribling DS, Rosko K, Strack A, Marsh DJ, Feng Y, Kumar S, Samuel K, Yin W, Van der Ploeg LH, Goulet MT, Hagmann WK (2006) Discovery of N-[(1S,2S)-3-(4-Chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-(trifluoromethyl)pyridin-2-yl]oxy}propanamide (MK-0364), a novel, acyclic cannabinoid-1 receptor inverse agonist for the treatment of obesity. J Med Chem 49:7584–7587
Liu YL, Connoley IP, Wilson CA, Stock MJ (2005) Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 29:183–187
LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, Stella N, Xu C, Tarzia G, Piomelli D (2009) Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett 19:639–643
Maccarrone M, Fride E, Bisogno T, Bari M, Cascio MG, Battista N, Finazzi AA, Suris R, Mechoulam R, Di Marzo V (2005) Up-regulation of the endocannabinoid system in the uterus of leptin knockout (ob/ob) mice and implications for fertility. Mol Hum Reprod 11:21–28
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91:3171–3180
Matias I, Vergoni AV, Petrosino S, Ottani A, Pocai A, Bertolini A, Di Marzo V (2008) Regulation of hypothalamic endocannabinoid levels by neuropeptides and hormones involved in food intake and metabolism: insulin and melanocortins. Neuropharmacology 54:206–212
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Moreira FA, Grieb M, Lutz B (2009) Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab 23:133–144
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65
Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschöp J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschöp MH (2008) Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57:2977–2991
O’Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C (2011) Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 60:1055–1062
Osei-Hyiaman D, Depetrillo M, Harvey-White J, Bannon AW, Cravatt BF, Kuhar MJ, Mackie K, Palkovits M, Kunos G (2005a) Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology 81:273–282
Osei-Hyiaman D, Depetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G (2005b) Endocannabinoid activation at hepatic CB(1) receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115:1298–1305
Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, Kunos G (2008) Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118:3160–3169
Pacher P, Batkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R (2006) The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27:73–100
Pertwee RG (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol 168:1–51
Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631
Perwitz N, Fasshauer M, Klein J (2006) Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm Metab Res 38:356–358
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884
Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J (2006) Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295:761–775
Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG (1991) Recent clinical experience with dronabinol. Pharmacol Biochem Behav 40:695–700
Proietto J, Rissanen A, Harp JB, Erondu N, Yu Q, Suryawanshi S, Jones ME, Johnson-Levonas AO, Heymsfield SB, Kaufman KD, Amatruda JM (2010) A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. Int J Obes (Lond) 34:1243–1254
Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, Fekete C, Latorre R, Nanni C, Bucci M, Clemens LE, Heldmaier G, Watanabe M, Leste-Lassere T, Maitre M, Tedesco L, Fanelli F, Reuss S, Klaus S, Srivastava RK, Monory K, Valerio A, Grandis A, De Giorgio R, Pasquali R, Nisoli E, Cota D, Lutz B, Marsicano G, Pagotto U (2010) CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 11:273–285
Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P (2003) Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284:R345–R353
Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648
Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Robson P (2005) Human studies of cannabinoids and medicinal cannabis. Handb Exp Pharmacol 168:719–756
Russell JC, Kelly SE, Diane A, Wang Y, Mangat R, Novak S, Vine DF, Proctor SD (2010) Rimonabant-mediated changes in intestinal lipid metabolism and improved renal vascular dysfunction in the JCR:LA-cp rat model of prediabetic metabolic syndrome. Am J Physiol Gastrointest Liver Physiol 299:G507–G516
Sallan SE, Cronin C, Zelen M, Zinberg NE (1980) Antiemetics in patients receiving chemotherapy for cancer: a randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N Engl J Med 302:135–138
Scheen AJ, Finer N, Hollander P, Jensen MD, van Gaal LF (2006) Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368:1660–1672
Siler JF, Sheep WL, Bates LB, Clark GF, Cook GW, Smith WH (1933) Marihuana smoking in Panama. Mil Surg 73:269–280
Simiand J, Keane M, Keane PE, Soubrie P (1998) SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol 9:179–181
Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O (2007) Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol 151:1109–1116
Stock MJ (1997) Sibutramine: a review of the pharmacology of a novel anti- obesity agent. Int J Obes Relat Metab Disord 21(Suppl 1):S25–S29
Storr MA, Sharkey KA (2007) The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol 7:575–582
Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G (2010) Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120:2953–2966
Tart CT (1970) Marijuana intoxication common experiences. Nature 226:701–704
Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, Pasquali R, Carruba MO, Marsicano G, Lutz B, Pagotto U, Nisoli E (2008) Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 57:2028–2036
Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, Pagotto U, Carruba MO, Vettor R, Nisoli E (2010) Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes 59:2826–2836
Thakur GA, Tichkule R, Bajaj S, Makriyannis A (2009) Latest advances in cannabinoid receptor agonists. Expert Opin Ther Pat 19:1647–1673
Thornton-Jones ZD, Kennett GA, Benwell KR, Revell DF, Misra A, Sellwood DM, Vickers SP, Clifton PG (2006) The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav 84:353–359
Topol EJ, Bousser MG, Fox KA, Creager MA, Després JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, Cohen E, Gaudin C, Job B, Murphy JH, Bhatt DL (2010) Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 376:517–523
Tucci SA, Rogers EK, Korbonits M, Kirkham TC (2004) The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol 143:520–523
van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Röossner S (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397
Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ (1997) Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 12:913–919
Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, Suryawanshi S, Carofano W, Johnson-Levonas AO, Shapiro DR, Kaufman KD, Heymsfield SB, Amatruda JM (2010) A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity (Silver Spring) 18:2301–2310
Wallerius S, Rosmond R, Ljung T, Holm G, Björntorp P (2003) Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest 26:616–619
Ware MA, St Arnaud-Trempe E (2010) The abuse potential of the synthetic cannabinoid nabilone. Addiction 105:494–503
Watanabe S, Doshi M, Hamazaki T (2003) n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids 69:51–59
Williams CM, Kirkham TC (1999) Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143:315–317
Williams CM, Kirkham TC (2002) Observational analysis of feeding induced by Delta9-THC and anandamide. Physiol Behav 76:241–250
Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592
Wittmann G, Deli L, Kallo I, Hrabovszky E, Watanabe M, Liposits Z, Fekete C (2007) Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J Comp Neurol 503:270–279
Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 96:5780–5785
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Engeli, S. (2012). Central and Peripheral Cannabinoid Receptors as Therapeutic Targets in the Control of Food Intake and Body Weight. In: Joost, HG. (eds) Appetite Control. Handbook of Experimental Pharmacology, vol 209. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-24716-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-642-24716-3_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-24715-6
Online ISBN: 978-3-642-24716-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)