Abstract
The muscarinic cholinergic system constitutes an important part of the neuronal circuitry that modulates normal cognition. Muscarinic receptor antagonists are well known to produce or exacerbate impairments in attention, learning, and memory. Conversely, both direct-acting muscarinic receptor agonists and indirect-acting muscarinic cholinergic agonists, such as acetylcholinesterase inhibitors, have shown cognition-enhancing properties, including improvements in normal cognitive function, reversal of cognitive deficits induced by muscarinic receptor antagonists, and attenuation of cognitive deficits in psychiatric and neurological disorders, such as Alzheimer’s disease and schizophrenia. However, until recently, the lack of small molecule ligands that antagonize or activate specific muscarinic acetylcholine receptor (mAChR) subtypes with high selectivity has been a major obstacle in defining the relative contributions of individual mAChRs to different aspects of cognitive function and for the development of novel therapeutic agents. These limitations may be potentially overcome by the recent discovery of novel mAChR subtype-selective compounds, notably allosteric agonists and positive allosteric modulators, which exhibit greater selectivity for individual mAChR subtypes than previous mAChR orthosteric agonists. In preclinical studies, these novel ligands have shown promising efficacy in several models for the enhancement of cognition. In this chapter, we will review the muscarinic cholinergic circuitry and pharmacology of mAChR agonists and antagonists relevant to the modulation of different aspects of cognition in animals and clinical populations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acetylcholine
- Muscarinic acetylcholine receptors
- Allosteric agonists
- Positive allosteric modulators
- Cognition
- Learning
- Memory
- Alzheimer’s disease
- Schizophrenia
- Cortex
- Hippocampus
1 Introduction
Normal cognition requires the coordination of numerous complex processes, including sensory information processing, sustained and divided attention, short- and long-term memory, and executive functions. Many neurologic and psychiatric disorders, including senile dementia, Alzheimer’s disease (AD), and schizophrenia, are associated with severe impairments in cognitive functions that are directly correlated with poor social and functional outcomes (Green 1996; Green et al. 2004; Farlow and Cummings 2007).
There is now accumulating evidence that modulation of the muscarinic cholinergic system is involved in normal cognitive processes and that imbalances in the neurotransmission of this system may account, at least in part, for the cognitive deficits associated with AD and schizophrenia. For example, nonselective muscarinic acetylcholine receptor (mAChR) antagonists produce or exacerbate impairments in cognition in animals and in healthy control, normal aging and AD populations (Domer and Schueler 1960; Pazzagli and Pepeu 1965; Drachman and Leavitt 1974; Bartus et al. 1982; Sunderland et al. 1986; Newhouse et al. 1988; Rusted and Warburton 1988). In addition, mAChR antagonists can also induce psychotomimetic-like symptoms in healthy humans and/or aggravate existing behavioral disturbances in patients with dementia or psychosis (Osterholm and Camoriano 1982; Agnoli et al. 1983; Hamborg-Petersen et al. 1984; Strauss et al. 1990). Conversely, indirect-acting mAChR agonists, such as acetylcholinesterase inhibitors (AChEIs), and direct-acting mAChR agonists can improve aspects of normal cognitive function and/or improve cognitive impairments in AD patients, and in animals, they reverse deficits induced by mAChR antagonism or lesions of cholinergic basal forebrain circuitry (Aigner and Mishkin 1986; Robbins et al. 1989a, b; Rupniak et al. 1989, 1991; Matsuoka et al. 1991; Bodick et al. 1997a, b; Cummings 2003; Shekhar et al 2008). Nonselective mAChR agonists and AChEIs have also enhanced cognitive performance, particularly in the domains of attention and memory, in schizophrenic patients (see review in Chouinard et al. 2007; Edelstein et al. 1981; Shekhar et al. 2008). Taken together, these observations have led to the hypothesis that selective activators of mAChRs may provide an important alternative approach for the treatment of the cognitive impairments associated with neurologic and psychiatric disorders, such as AD and schizophrenia.
However, while AChEIs are clinically approved for the treatment of mild-to-moderate cognitive dementia associated with AD, the effects of these compounds on deficits in memory and other cognitive functions remain modest (Amenta et al. 2001). Unfortunately, early clinical studies using direct-acting mAChR agonists for AD and schizophrenia have ultimately failed in clinical development due to a lack of true subtype selectivity that resulted in a number of dose-limiting adverse effects from nonselective activation of peripheral mAChRs (Bruno et al. 1986; Bodick et al. 1997a, b; Shekhar et al. 2008). The high conservation of the acetylcholine (ACh) binding site across the five mAChR subtypes has presented a major impediment to the development of highly selective mAChR orthosteric-site ligands. The lack of subtype-selective mAChR ligands has also limited insights into the relative roles of the mAChR subtypes in the different aspects of cognition and the clinical efficacy observed with the AChEIs and nonselective muscarinic mAChR agonists.
Using an alternative strategy, our group and others have recently identified ligands for mAChRs that activate a specific receptor subtype through action at sites that are less highly conserved and topographically distinct relative to the orthosteric binding site of ACh, termed allosteric sites. Allosteric agonists activate the receptor subtype directly in the absence of the endogenous ligand ACh, while positive allosteric modulators (PAMs) bind to an allosteric site and potentiate the effects of ACh, but have no intrinsic activity. Because mAChR PAMs can only exert their effects in the presence of ACh at a given synapse, these ligands may maintain normal temporal and spatial components of endogenous ACh neurotransmission. This latter feature may provide an important advantage in the treatment of cognitive impairments in early stage dementia or schizophrenia, as recent findings suggest that optimal levels of ACh transmission for cognition are dynamic and task dependent (Kozak et al. 2006; Hasselmo and Sarter 2011). To date, these novel allosteric activators of the different mAChR subtypes have shown efficacy in preclinical models for the enhancement of cognition, and possess suitable physiochemical properties for optimization as potential clinical candidates.
In this chapter, we will provide a brief overview of cholinergic circuitry and mAChR distribution and function in the central nervous system (CNS). We will next review the effects of different mAChR antagonists and agonists in preclinical models of cognition and in clinical populations. Finally, we will highlight recent developments with novel subtype-selective allosteric agonists and PAMs of M1 and M4 mAChRs in preclinical models for the enhancement of cognition.
2 Anatomy of the Cholinergic System
2.1 Cholinergic Cell Groups and Their Target Regions
Within the CNS, cholinergic projection neurons are organized into relatively discrete cell groups in the basal forebrain and the caudal mesencephalon. As described in the seminal work of Mesulam and colleagues (Mesulam et al. 1983), six groups of cholinergic projection neurons, termed Ch1–Ch6, can be distinguished based on their localization and projection pattern (Fig. 1). Cell groups Ch1–Ch4, located in the basal forebrain of the rat, are thought to be involved in attention, learning, and memory functions (Everitt and Robbins 1997). The cholinergic neurons of the nucleus basalis magnocellularis (Ch4), which in primates is known as the nucleus basalis of Meynert (NBM), provide wide-spread cholinergic projections throughout most of the cerebral cortex, and degeneration of these neurons is a hallmark of AD (McGeer et al. 1986). In addition, the Ch4 cells innervate the amygdaloid complex (Mesulam et al. 1983; Price and Stern 1983). Cholinergic neurons in the medial septum (Ch1) and the vertical limb of the diagonal band of Broca (Ch2) send projections to the hippocampal formation and to the medial aspects of the cortex, such as the cingulate and retrosplenial cortices (Eckenstein et al. 1988). The olfactory bulb is the recipient of cholinergic projections from the Ch3 cell group, located in the horizontal limb of the diagonal band of Broca. The cholinergic projection neurons of the caudal midbrain, which are involved in arousal, sleep, and the regulation of dopaminergic cell groups (Datta and Siwek 1997), are located in the pedunculopontine tegmental nucleus (PPTg, Ch5) and the laterodorsal tegmental nucleus (LDTg, Ch6), from where they project to the thalamus, the pontine reticular formation, and areas of the ventral midbrain (Mesulam et al. 1983; Satoh and Fibiger 1986; Clarke et al. 1987; Hallanger et al. 1987; Semba et al. 1990). The parcellation scheme developed by Mesulam and colleagues (1983) has proven to be invaluable for conceptualizing the various aspects of cholinergic function. However, the analysis of forebrain cholinergic function is complicated by the fact that non-cholinergic projection neurons are embedded in the cholinergic cell groups (Woolf et al. 1986). Therefore, results from lesion studies targeting the cholinergic basal forebrain need to be interpreted carefully (see Robbins et al. 1989a, b).
Schematic diagram illustrating the location of the cholinergic cell groups of the rat brain and their projections. (a) Sagittal view showing Ch1 (medial septum), Ch2 (vertical limb of the diagonal band of Broca [DBB]), and Ch3 (horizontal limb of the DBB) and their projections to the hippocampal formation, cerebral cortex, and olfactory bulb. (b) Sagittal view depicting Ch4 (nucleus basalis magnocellularis) and its projections throughout the cortex and amygdala, as well as Ch5 (pedunculopontine tegmental nucleus) and Ch6 (laterodorsal tegmental nucleus) innervating the thalamus, substantia nigra, and ventral tegmental area. (c) Coronal section through the striatal complex showing large cholinergic interneurons in the dorsal striatum and nucleus accumbens. Drawings are based on the work of Kimura et al. (1980), Mesulam et al. (1983), Eckenstein et al. (1988), and Gould et al. (1989). Ch1–Ch6 cholinergic cell groups; AMG amygdala; cc corpus callosum; CP caudate-putamen (striatum); HPC hippocampus; NAS nucleus accumbens; OB olfactory bulb; THAL thalamus; SN substantia nigra; VTA ventral tegmental area
2.2 Regional Distribution of Cholinergic Axons
Dense cholinergic fiber plexus originating from the basal forebrain are seen throughout neo- and allocortical areas. The laminar distribution of cholinergic fibers varies slightly across cortical areas, but layer V generally receives the most dense cholinergic fiber innervation (Eckenstein et al. 1988; Mechawar et al. 2000). The cholinergic innervation of the hippocampus is most prolific at the border between stratum oriens and pyramidal layer and in the molecular layer, while the densely packed pyramidal and granule cell layers themselves receive very little cholinergic input (Ichikawa and Hirata 1986; Schäfer et al. 1998). Cholinergic fiber density varies across the nuclei of the amydaloid complex; the most densely innervated area is the basolateral nucleus (Hellendall et al. 1986). In subcortical areas, moderate cholinergic innervations are seen in select thalamic nuclei, including the anteroventral, centromedial, and reticular nuclei (Gonzalo-Ruiz et al. 1995; Schäfer et al. 1998), and in the midbrain dopamine cell groups (Gould et al. 1989; Oakman et al. 1995; Omelchenko and Sesack 2006).
2.3 Striatal Cholinergic Interneurons
The striatal complex, including the nucleus accumbens, does not receive any extrinsic cholinergic innervation, but instead contains cholinergic interneurons as the sole source of ACh. These cholinergic interneurons are scattered throughout the striatal matrix compartment, but are largely absent from striatal patches (Gerfen and Bolam 2010). Although large cholinergic interneurons make up less than five percent of striatal neurons, their wide dendritic arbors enable them to exert control over a large striatal area (Kimura et al. 1980; Bolam et al. 1984; Phelps et al. 1985).
3 Muscarinic Receptor Distribution
For the purpose of this chapter, we will focus on the well-established distribution of the five mAChR subtypes in the rodent brain. Our description of the distribution of mAChRs will be limited to select brain regions that are thought to be involved in cognition and that either contain cholinergic neurons or receive cholinergic innervations. These areas include the cerebral cortex, hippocampus, thalamus, the basal ganglia, and basal forebrain and caudal midbrain cholinergic cell groups.
3.1 Expression of Muscarinic Receptor Message
Distribution maps of M1–M5 mAChR mRNA, obtained by in situ hybridization histochemistry, show that mAChRs are expressed throughout the rodent brain, albeit not uniformly (Fig. 2). There are pronounced differences in the overall expression levels of the five muscarinic receptors, with M1 and M5 receptors being the most and least abundant receptor subtype, respectively. Moreover, each muscarinic receptor exhibits a regional expression pattern that is strikingly different from other members of the muscarinic receptor family (Brann et al. 1988).
Distribution of M1–M5 muscarinic receptor mRNA in the mouse brain. This is a composite of images obtained from the Allen Mouse Brain Atlas (2009) developed by the Allen Institute for Brain Science (Lein et al. 2007) and available online at http://mouse.brain-map.org. CP caudate-putamen; HPC hippocampus; NAS nucleus accumbens; PFC prefrontal cortex; SN substantia nigra; VTA ventral tegmental area
The M1 receptor is not only most prominently expressed in the hippocampus, but is also abundant throughout all layers of the cortex, where the superficial layers stand out by being more intensely labeled than the remaining layers. Striatal medium spiny neurons as well as interneurons also express high levels of M1 message (Bernard et al. 1992); caudal to the striatum, subcortical M1 expression decreases along a rostro-caudal gradient from the diencephalon to the midbrain. Moderately high M2 receptor expression is found mainly in the brain regions containing cholinergic cell bodies (Vilaró et al. 1992) as well as in some thalamic nuclei including the midline, parafascicular, and reticular nuclei. In the hippocampus and cortex, M2 message is sparse; in cortical layer IV, it is completely absent. The M3 receptor is mainly expressed in the hippocampus and in the cortex, except for layers III and IV which are mostly devoid of M3 message. Very low levels of M3 mRNA are seen in the striatum and basal forebrain (Brann et al. 1988). The highest density of M4 receptors is found in the striatal complex (Vilaró et al. 1991), followed by allocortical areas, such as the hippocampus and amygdala. Expression of M4 message is relatively high in all layers of the neocortex; like M2, M4 receptor message is prominently expressed in central cholinergic neurons (Sugaya et al. 1997). The muscarinic receptor with the most restricted expression is M5. It is found in low abundance in the ventral tegmental area and the pars compacta of the substantia nigra (Vilaró et al. 1990).
3.2 Muscarinic Receptor Protein Expression
The global distribution of muscarinic receptor protein was initially assessed using a monoclonal (M35) pan-muscarinic antibody (van der Zee et al. 1989; for review, see van der Zee and Keijser 2011). With the development of subtype-selective muscarinic receptor antibodies, it became feasible to quantitate levels of receptor protein in microdissected brain regions (Li et al. 1991; Wall et al. 1991; Yasuda et al. 1993) and to determine both the cell types expressing certain mAChR subtypes and the (sub)cellular localization of mAChRs at the light and electron microscopic level (Levey et al. 1991; Hersch et al. 1994; Hersch and Levey 1995). Immunohistochemical studies demonstrated that M1–M5 protein distribution corresponds to a large degree with the mRNA expression maps indicating receptor expression at the soma and dendritic level. Furthermore, they revealed that muscarinic receptor proteins were prominently expressed presynaptically as both autoreceptors and heteroceptors (Table 1).
3.2.1 Cortex
M1, M2, and M4 are the most abundant muscarinic receptor proteins in the cortex (Levey et al. 1991). M1 protein, expressed in pyramidal cells, is enriched in layers II/III and VI, whereas M4 is localized in somata of layer II–IV cells. Terminals located in layer IV and at the border between layers V and VI exhibit strong M2 labeling, which is in agreement with the dense cholinergic innervation of these cortical layers and the role of M2 as autoreceptor (Eckenstein et al. 1988; Mechawar et al. 2000).
3.2.2 Hippocampus
The complexity of hippocampal cholinergic circuitry is illuminated by the diverse pre- and postsynaptic distribution of mAChRs, suggesting an intricate muscarinic regulation of hippocampal function. Both intrinsic neurons (pyramidal neurons, granule cells, and interneurons) and terminals originating from basal forebrain and entorhinal cortex prominently express M1–M4 receptors (see Table 1) (Levey et al. 1995b; Rouse and Levey 1996, 1997, 1998; Rouse et al. 1999, 2000).
3.2.3 Amygdala
Pyramidal neurons in the basolateral amygdala, a limbic region involved in learning and expression of fear conditioning, prominently express M1 protein (McDonald and Mascagni 2010).
3.2.4 Striatum
Approximately eighty percent and close to half of medium spiny neurons, the principal cell type in the striatum, express M1 and M4 receptor proteins, respectively (Hersch et al. 1994). Interestingly, the M4 receptor is mainly localized to the medium spiny neurons projecting to the substantia nigra reticulata (Ince et al. 1997), making M4 an interesting target to alter striatal output pathways differentially. In contrast, M2 protein is mainly expressed in striatal cholinergic interneurons, where the M2 receptor subserves the function of an autoreceptor (Hersch et al. 1994; Hersch and Levey 1995). Presynaptically located M1–M3 receptor proteins are thought to be localized to corticostriatal (M1/M3) and thalamostriatal (M2/M3) terminals (Hersch et al. 1994). Overall, the high expression of mAChRs in the striatum suggests that muscarinic ligand may be useful for modifying striatum-mediated learning processes, in particular procedural learning (Saint-Cyr et al. 1988; Cayzac et al. 2011).
3.2.5 Thalamus
Expression of mAChR proteins in the thalamus is restricted to M1 and M3 in the anterodorsal and -ventral nuclei and to M2 in the reticular nucleus (Oda et al. 2001, 2007). The thalamus as an important relay station to the cortex and striatal complex may, therefore, be subject to muscarinic regulation via M1 and/or M3 mechanisms. The presence of M2 in the reticular nucleus, whose GABAergic projections inhibit thalamic relay nuclei, suggests that M2 may play a role in global control of thalamic output (Cox et al. 1997; Pinault and Deschênes 1998).
3.2.6 Cholinergic Neurons
In the basal forebrain and other cholinergic cell groups, the principal mucarinic receptor protein is M2, which is located both in cholinergic cell bodies and in unidentified axon terminals (Levey et al. 1995a).
4 Role of Muscarinic Receptor Subtypes in Cognition
4.1 Findings with mAChR Antagonists and KO Mice
Based on an extensive literature, nonselective mAChR antagonists, such as scopolamine, disrupt multiple domains of cognitive function, from sensory information gating, attention, and memory to higher problem-solving skills in rodents, monkeys, and humans, as shown in Table 3; also see chemical structures of representative mAChR antagonists and their in vitro affinities for the different mAChR subtypes in Fig. 3 and Table 2, respectively (see Terry et al. 2006; Barak 2009; Klinkenberg and Blokland 2010 for complete reviews). For example, scopolamine, trihexyphenidyl, and benztropine produced robust dose-dependent disruptions of prepulse inhibition (PPI) of the acoustic startle reflex, a model of sensory information processing, at doses that had no effects on startle response (Jones and Shannon 2000). Scopolamine markedly decreased accuracy and/or response rates in the 5-choice serial reaction time task, a preclinical model of attentional functions used to test rats and monkeys (Jäkälä et al. 1992; Callahan et al. 1993; Jones and Higgins 1995; Higgs et al. 2000; Mirza and Stolerman 2000; Shannon and Love 2005, 2006; Shannon and Eberle 2006; Spinelli et al. 2006). In addition, scopolamine induced impairments in attention in humans, including in the attentional components of the CogState Early Phase Battery and in the digit vigilance test (Ellis et al. 2006; Fredrickson et al. 2008). With regard to learning and memory, muscarinic antagonism with scopolamine produced robust deficits in performance accuracy in numerous memory-related behavioral tasks in rodents and monkeys, including spatial memory tasks such as the Morris water maze and radial arm maze, classic Pavlovian conditioned responding, delayed non-matching to sample, and object recognition tasks (Buresová et al. 1986; Riekkinen et al. 1990; Dennes and Barnes 1993; Anagnostaras et al. 1995, 1999; Rudy 1996; Mishima et al. 2000; Feiro and Gould 2005; Betz et al. 2007; Sheffler et al. 2009; Dietrich and Jenck 2010). In humans, scopolamine decreased performance accuracy in measures of visual and verbal learning and item recognition memory tasks (Sherman et al. 2003; Green et al. 2005; Fredrickson et al. 2008; Thienel et al. 2009). Scopolamine has also been reported to produce impairments in executive functions, including attentional set-shifting in rats and Groton maze learning in humans (Chen et al. 2004; Fredrickson et al. 2008). In review of the dose-related disrupting effects of scopolamine and other nonselective mAChR antagonists, the interpretation of these effects are clearest in measures of sensory discrimination and attentional function, in which deficits are observed within a dose range that does not produce confounding effects on general motor output and/or levels of arousal as observed in models of learning and memory.
Recent findings from studies using either mAChR KO mice or antagonists are providing more defined roles for each of the mAChR subtypes in the modulation of cognition. In the case of M1 mAChRs, this particular subtype regulates a variety of physiologic effects in hippocampal and cortical brain regions, most notably enhancement of glutamatergic signaling through potentiation of N-methyl-d-aspartate (NMDA) receptor function (Marino et al. 1998). Modulation of NMDA receptor neurotransmission is key for the acquisition and consolidation of new learning and memories; and its disruption is speculated to account, at least in part, for the cognitive impairments observed in many neurological and psychiatric disorders (Marino and Conn 2002; Tsai and Coyle, 2002). Consistent with a role of M1 in learning and memory, the M1-preferring mAChR antagonist pirenzepine impaired accuracy and/or acquisition in tasks of passive avoidance, Morris water maze, and visual discrimination in rats (Fig. 3, Tables 2 and 3) (Hunter and Roberts 1988; Drinkenburg et al. 1995). Moreover, M1 mAChR KO mice have reduced long-term potentiation in response to theta burst stimulation, a physiologic endpoint thought to be procognitive in nature (Anagnostaras et al. 2003). In contrast to the effects of nonselective mAChR antagonists, M1 KO mice have shown normal performance in hippocampus-mediated tasks, including in the Morris water maze task with or without scopolamine challenge (Miyakawa et al. 2001), but distinct impairments in behavioral tasks that require medial prefrontal cortex (mPFC) function (Anagnostaras et al. 2003). For example, M1 KO mice relative to wild-type (WT) controls showed pronounced performance deficits in non-matching-to-sample tasks, including win-shift radial arm maze learning and social discrimination tests (Anagnostaras et al. 2003). Despite significant enhancement in the acquisition of contextual fear conditioning, M1 KO mice performed poorly after a time period when the task becomes independent of hippocampal function (Anagnostaras et al. 2003). In support of these findings, the highly selective M1 mAChR antagonist VU0255035 (see Fig. 3, Tables 2 and 3) had no effect on acquisition of contextual fear conditioning, a hippocampus mediated memory task (Sheffler et al. 2009). Taken together, these studies indicate a consistent role for M1 mAChR in the modulation of mPFC-mediated tasks, but future studies using the selective M1 mAChR antagonist VU0255035 are needed to further evaluate the effects of selective disruption of M1 activity in other cognitive functions.
For the role of M2 in cognition, previous studies have postulated that selective M2 mAChR antagonists may provide improvements in the cognitive deficits observed in dementia patients by increasing cholinergic signaling through antagonism of M2 mAChRs on presynaptic cholinergic terminals (Rouse et al. 2000; Zhang et al. 2002; Tzavara et al. 2003). Consistent with this hypothesis, the selective M2 mAChR antagonists, BIBN-99 and SCH57790 (see Fig. 3, Tables 2 and 3) improved performance in the passive avoidance and Morris water maze tasks in normal and aged rats, and in fixed ratio discrimination in monkeys (Table 3) (Quirion et al. 1995; Carey et al. 2001; Rowe et al. 2003). However, M2 mAChRs also function as heteroceptors localized on the axon terminals of non-cholinergic neurons that mediate presynaptic regulation of release of other neurotransmitters (Rouse et al. 2000). Not surprisingly, M2 mAChR KO mice have shown deficits in tasks of working memory and cognitive flexibility, as well as hippocampal long-term potentiation, suggesting that blockade of M2 mAChRs on both cholinergic and non-cholinergic nerve terminals may disrupt, not enhance, overall cognitive function (Tzavara et al. 2003; Seeger et al. 2004). Consistent with the M2 KO mouse cognitive phenotype AFDX116, another selective M2 mAChR antagonist decreased accuracy and increased response latencies and omissions in a rodent visual discrimination task (see Fig. 3, Tables 2 and 3) (Drinkenburg et al. 1995). Thus, more detailed studies with M2 mAChR antagonists are needed to further understand the full therapeutic potential of M2 mAChR antagonists for the treatment of clinical populations with varying levels of cholinergic tone.
To date, the relative importance of the M3 mAChR in modulating different aspects of cognitive function remains undefined. M3 mAChR KO mice have shown robust impairments in contextual fear conditioning, a classic hippocampus-mediated memory task (Poulin et al. 2010). However, there are currently no selective M3 mAChR antagonists reported in the literature, and the M3-preferring antagonist imidafenacin had no effect on performance in the Morris water maze, another hippocampus-mediated memory task (Kobayashi et al. 2007) (see Fig. 3, Tables 2 and 3). Whether selective M3 mAChR activators may have procognitive properties remains unclear as does the issue whether a viable therapeutic index could be achieved between activation of central and peripheral M3 mAChRs.
The significance of M4 mAChRs in cognitive functions remains unclear because of the pre- and postsynaptic localization of M4 mAChRs within the CNS (Levey et al. 1991; Zang and Creese 1997; Zhang et al. 2002; Tzavara et al. 2004). Previous in vivo microdialysis studies have shown significant increases in basal midbrain extracellular ACh concentrations in M4, but not M2 mAChR KO mice (Tzavara et al. 2004). Moreover, scopolamine-induced increases in midbrain extracellular ACh concentrations were dampened in the M4 mAChR KO mice (Tzavara et al. 2004). M4 mAChR KO mice also displayed increased DA efflux in response to psychotomimetics (Tzavara et al. 2004). These findings suggest that activation of M4 mAChRs may provide feedback control on basal and evoked DA release in the striatum. The tight regulation of striatal DA and ACh neurotransmission by M4 mAChRs may be critical for cognitive functions, such as procedural learning and effort-based decision making, tasks that require striatal involvement. Interestingly, the M4-preferring mAChR antagonist tropicamide disrupted PPI of the acoustic startle reflex, a task that is dependent on proper mesolimbic DA neurotransmission (Ukai et al. 2004) (Fig. 3, Tables 2 and 3). Tropicamide administration also resulted in decreased accuracy in a visuospatial delayed non-matching-to-sample task in rats (Betz et al. 2007). Studies using selective M4 mAChR agonists and antagonists need to further dissect the role of M4 mAChRs in other aspects of cognition, as will be discussed in the allosteric modulator section of this chapter.
With the expression of M5 mAChRs limited to the VTA and substantia nigra pars compacta, it is not surprising that preliminary studies with M5 mAChR KO mice have reported disruptions in the proper regulation of dopaminec-mediated behavioral tasks (Vilaró et al. 1990; Weiner et al. 1990). In particular, M5 mAChR KO mice have impaired PPI (Thomsen et al. 2007) and reduced sensitivity to the effects of different drugs of abuse (Basile et al. 2002; Fink-Jensen et al. 2003; Yamada et al. 2003; Thomsen et al. 2005; Steidl and Yeomans 2009). While there are currently no available selective M5 mAChR antagonists, the studies with M5 mAChR KO mice suggest that selective blockade of M5 mAChRs might be useful for regulating the hyperactivation of mesolimbic dopaminergic circuitry in patients with schizophrenia. Moreover, the proper function of nonneuronal M5 mAChRs expressed in the cerebrovasculature that control cerebrovasodilation and blood flow may also indirectly impact cognitive functions (Yamada et al. 2001; Araya et al. 2006). Vascular pathology has been implicated in AD, and dysfunction in cholinergic control of cerebral blood vessel dilation may contribute, in part, to the pathophysiology of this disease. Cerebrovascular deficits in M5 mAChR KO mice are associated with neuronal atrophy and deficits in performance of the novel object recognition task (Araya et al. 2006), which further support the role of M5 mAChRs in the modulation of cognitive function through nonneuronal mechanisms.
4.2 Findings with mAChR Orthosteric Agonists
Over the last 2 decades, the drive to improve cognitive impairments in patient populations with AD and other dementias has resulted in the development of two major pharmacologic approaches that modulate mACh neurotransmission, specifically indirect modulation through the enhancement of general cholinergic tone with AChEIs and direct modulation by mAChR orthosteric agonists. To date, only the AChEIs tacrine, donepezil, galantamine, and rivastigmine are clinically approved for the treatment of cognitive impairments associated with mild-to-moderate AD. While AChEIs can improve cognitive deficits in dementia patients, their therapeutic benefits are limited by a short duration of action, dose-limiting side effects, relatively modest efficacy on memory deficits, and a large population of nonresponders (Pepeu and Giovannini 2010; Birks 2006; Birks and Flicker 2006; Persson et al. 2009; Hasselmo 2006; Barten and Albright 2008).
As an alternative to the limited clinical utility of AChEIs, considerable efforts have been focused on the development of highly selective mAChR orthosteric agonists for the treatment of cognitive impairments in AD; representative chemical structures for each compound are depicted in Fig. 4 with their in vitro binding affinities at each mAChR subtype described in Table 4 and highlighted efficacy in different cognitive tasks shown in Table 5. All of the mAChR agonists presented in Table 4, including the reported M1-preferring agonist WAY-132983 and the M1/M4-preferring mAChR agonist xanomeline, exhibit relatively nonselective profiles of binding affinities across the different mAChR subtypes, underscoring the drawback of designing orthosteric site ligands that target the highly conserved ACh binding site of the five mAChR subtypes. Due to the relatively nonselective in vitro binding profiles for each of these mAChR orthosteric agonists, the role(s) of the different mAChR subtypes in the observed in vivo effects of these compounds remain unclear. However, as shown in Table 5, the majority of mAChR orthosteric agonists produced robust reversals of pharmacologic and/or lesion-induced deficits in different cognitive domains, including sensory information processing, attention, and various aspects of learning and memory. For example, oxotremorine and xanomeline reversed deficits in PPI induced by the non-selective mAChR antagonist scopolamine and the D1/D2 dopamine receptor agonist apomorphine (Jones and Shannon 2000; Stanhope et al. 2001; Jones et al. 2005) (Table 5). Cevimeline improved performance in divided or visuospatial attentional tasks in monkeys (O’Neill et al. 1999; 2003) (Table 5). In models of learning and memory, the mAChR agonists milameline, xanomeline, WAY-132983, and cevimeline enhanced performance in spatial and delayed nonmatching to sample radial arm maze tasks in scopolamine-impaired, cholinergic-lesioned, and aged rats (M’Harzi et al. 1995; Brandeis et al. 1990; Hodges et al. 1999; Bartolomeo et al. 2000) (Table 5). In addition, oxotremorine and RS-86 reversed disruptions in Morris water maze tasks induced by hemicholinium-3 (Hagan et al. 1989). Notable nonhuman primate studies include improved reversal learning in delayed non-matching-to-sample tasks after administration of mAChR agonists arecoline and RS-86 (Rupniak et al. 1989, 1992) (Table 5). Moreover, milameline also had effects on cortical EEG parameters consistent with enhanced arousal in monkeys (Schwarz et al. 1999), while sabcolemine and arecoline induced hippocampal rhythmical slow wave activity, a procognitive biomarker, in anesthetized rats (Loudon et al. 1997) (Table 5). Finally, a potential disease-modifying effect of mAChR agonists in AD has been revealed by clinical studies with sabcomeline and talsaclidine in which treated AD patients showed decreases in cerebrospinal fluid (CSF) levels of total Aβ or Aβ40 and Aβ42, indicative of a reduction in the pro-amyloidogenic processing of the amyloid precursor protein (Hock et al. 2000, 2003). These data are consistent with earlier studies using another mAChR agonist, AF102B (Fisher 2007). However, other studies have shown that decreased CSF Aβ42 may predict cognitive decline in AD (Motter et al. 1995; Galasko et al. 1998; Sunderland et al. 2003; Fagan et al. 2006) and, thus raise the question which amyloid fraction in CSF may be the most suitable biomarker for predicting, predicting pro-amyloidogenic processing of amyloid precursor protein in brain tissue (Motter et al. 1995; Galasko et al. 1998; Sunderland et al. 2003; Fagan et al. 2006). Future studies are needed to clarify these important issues in the AD literature. Taken together, there is a robust preclinical, and in some cases clinical, profile for the efficacy of mAChR agonists in the enhancement of different aspects of cognition. However, as discussed in the introduction, all of the mAChR orthosteric agonists described in Table 5 have failed to advance into further clinical development due to a lack of true subtype selectivity.
Despite the overall clinical failure of mAChR orthosteric agonists, two clinical studies with the M1/M4-preferring mAChR agonist xanomeline have provided critical proof-of-concept efficacy for the reversal of cognitive impairments and behavioral disturbances observed in AD and schizophrenia patients. In a clinical trial with mild-to-moderate AD patients, xanomeline improved aspects of cognitive performance as measured by the Alzheimer’s disease assessment scale cognitive (ADAS-cog) battery, including spoken language ability, word-finding difficulty in spontaneous speech, and constructional praxis (i.e., three-dimensional motor planning and execution) (Bodick et al. 1997a, b). Xanomeline also significantly improved a number of behavioral disturbances, including agitation, vocal outbursts, and hallucinations, observed in AD patients (Bodick et al. 1997a, b). In a separate clinical trial conducted in a small group of treatment refractory schizophrenic patients, xanomeline produced a significant enhancement in verbal learning and short-term memory functions, as well as decreased positive symptoms (Shekhar et al. 2008). The dose-limiting adverse effects observed in the xanomeline treatment groups in both clinical studies, due to the nonselective activation of peripheral mAChRs, halted further development of this compound.
4.3 Allosteric Agonists and Positive Allosteric Modulators
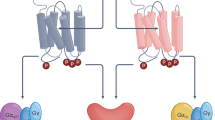
In recent years, several groups in both academia and industry have pursued a novel strategy for the discovery of mAChR ligands that stimulate a specific receptor subtype by targeting sites that are less highly conserved than the orthosteric ACh binding site, termed allosteric sites (Fig. 5a). As discussed in the following sections, allosteric activators of mAChRs exhibit high subtype selectivity and different mechanisms of action in comparison with orthosteric mAChR agonists. For example, PAMs of mAChRs exhibit no intrinsic activity at the receptor (Fig. 5b), but can bind to an allosteric site and potentiate the effects of the endogenous ligand ACh through enhancement of the affinity of ACh for the orthosteric site and/or increased coupling efficiency to the G-proteins (Fig. 5c). In contrast, allosteric mAChR agonists bind to an allosteric site on the receptor and can directly activate the receptor in the absence of ACh (Christopoulos 2002; Waelbroeck 2003; Conn et al. 2009). Discovery of these novel allosteric mAChR activators is providing exciting tools for further characterization of the roles of different mAChRs on cognition.
Schematic representation of a muscarinic acetylcholine receptor showing orthosteric and putative allosteric binding sites and effector mechanisms (a). Each of the five mAChR subtypes is a seven-transmembrane protein. Allosteric activators bind to sites other than the orthosteric Ach binding site to activate or potentiate the receptor. Muscarinic receptors are divided into two functional classes based on G-protein-coupled receptor coupling. M1, M3, and M5 mAChRs couple to Gq/G11, which results in increased intracellular calcium levels via phospholipase C activation. M2 and M4 mAChRs couple to Gi/o, resulting in the inhibition of adenylyl cyclase and ion channels. Unlike orthosteric agonists, PAMs have no intrinsic activity (b). The graph in (c) illustrates two potential modes of action of PAMs in a cell-based system: affinity modulation (PAM1) with a resulting leftward shift of the concentration–response curve and efficacy modulation (PAM2) leading to an increase in maximal response. AC adenylyl cyclase; ACh acetylcholine; cAMP cyclic AMP; IP3 inositol triphosphate; M1–M5 muscarinic cholinergic receptor subtypes 1–5; PAM positive allosteric modulator; PLC phospholipase C
4.3.1 M1 Allosteric Modulators
As shown in Fig. 6, there has been excellent progress in the identification of several M1 allosteric activators for critical proof-of-concept studies in preclinical models (see representative chemical structures for the M1 allosteric agonists and PAMs in Fig. 6 with the in vitro functional potencies at each subtype, if available, described in Table 6 and highlighted efficacy in different preclinical cognitive tasks shown in Table 7.
AC-260584 is an analog of the first-generation M1 allosteric mAChR agonist AC-42 that was shown to have activity through binding at an allosteric site on the M1 mAChR (Heinrich et al. 2009; Spalding et al. 2002; Langmead et al. 2006). AC-260584 has been reported to enhance memory functions as assessed in the novel object recognition and Morris water maze tasks in mice, as well as produce effects in preclinical models predictive of antipsychotic-like effects (Bradley et al. 2010; Vanover et al. 2008) (Table 7). Unfortunately, interpretation of the in vivo efficacy of AC-260584 is confounded by off-target effects at dopamine D2, adrenergic α1A, and serotonin 5-HT2A receptors (Heinrich et al. 2009). The M1 allosteric agonist, 77-LH-28-1, is another systemically active AC-42 analog (Langmead et al. 2008) with high selectivity for M1 and some weak M3 agonist activity (Heinrich et al. 2009) (see Fig. 6, Tables 6 and 7). Functional and site-directed mutagenesis studies have established that 77-LH-28-1not only acts as a “bi-topic” agonist that binds to a site that overlaps with the orthosteric site, but also includes an allosteric site that modulates affinity of the ACh site (Avlani et al. 2010). Several physiologic effects thought to potentiate cognition, including increased hippocampal CA1 pyramidal cell firing in vitro and in vivo and induction of synchronous network activity through increased CA3 hippocampal γ oscillations, are increased with 77-LH-28-1 treatment (Langmead et al. 2008; Buchanan et al. 2010; Jo et al. 2010; Spencer et al. 2010). Another highly selective AC-42-based compound, Lu AE51090, reversed delay-dependent memory decay in a Y-maze delayed alternation paradigm (Sams et al. 2010) (Fig. 6, Tables 6 and 7).
There are now additional second-generation, systemically active and highly selective M1 allosteric agonists and PAMs that are serving as important tools for determining the role of selective activation of M1 mAChRs in native tissue preparations and in animal models of cognition, including the M1 allosteric agonists TBPB, which is a selective and potent M1 allosteric agonist in recombinant systems (Jones et al. 2008) (Fig. 6, Tables 6 and 7). Site-directed mutagenesis studies have revealed that point mutations in the ACh binding site that reduce the activity of orthosteric mAChR agonists at M1 produce no change in the response to TBPB. A Schild analysis for the blockade of TBPB effects with the orthosteric mAChR antagonist atropine showed that TBPB interacts with the orthosteric site in a noncompetitive manner (Jones et al. 2008). Based on an allosteric ternary complex model for the actions of two molecules that interact with distinct sites on a receptor, these results collectively suggest that TBPB may act as an allosteric M1 agonist (Christopoulos and Mitchelson 1997; Jacobson et al. 2010). However, further studies are warranted as it cannot be ruled out that TBPB may act as a bi-topic agonist, similar to 77-LH-28-1 (Avlani et al. 2010). In native tissue preparations, TBPB potentiated NMDA receptor currents in CA1 hippocampal pyramindal cells, a function that is thought to contribute to the procognitive effects of mAChR agonists, as described earlier (Jones et al. 2008). In several preclinical models predictive of antipsychotic-like activity, TBPB produced efficacy at doses that do not induce the side effects associated with nonselective stimulation of peripheral mAChRs. More importantly, TBPB reversed apomorphine-induced deficits in PPI of the acoustic startle reflex and scopolamine-induced impairments in the acquisition of a hippocampal working memory task, contextual fear conditioning (Kane 2008). In addition, selective activation of M1 by TBPB increased the non-amyloidogenic processing of the amyloid precursor protein and reduced Aβ formation in vitro, as previously reported with other nonselective mAChR agonists. These data are consistent with the hypothesis that selective activation of M1 mAChRs may provide both enhancement of cognitive functions and potential disease-modifying activity for the treatment of symptoms associated with AD.
Finally, VU0357017 represents a highly potent, selective, and systemically active third-generation M1 allosteric agonist (Lebois et al. 2010) (Fig. 6, Tables 6 and 7). Unlike the other allosteric M1 agonists, VU0357017 activates the M1 mAChR at a novel allosteric site on the third extracellular loop, instead of within the seven transmembrane domain (Lebois et al. 2010). This compound potentiated NMDA receptor currents in slice electrophysiology experiments and blocked scopolamine-induced deficits in contextual fear conditioning (Lebois et al. 2010).
4.3.2 M1 Positive Allosteric Modulators
A major advance in the development of systemically active and selective M1 PAMs was the identification and characterization of benzylquinolone carboxylic acid (BQCA) (Fig. 6). In cell-based systems, BQCA is a potent PAM with a 129-fold leftward shift of the ACh concentration–response curve with high M1 selectivity that lacks agonist, potentiator, or antagonist activity at M2–M5 up to 100 μM (Ma et al. 2009) (Table 6). In addition, BQCA increases the affinity of the M1 mAChR for ACh, but does not bind at the orthosteric ACh binding site. In native tissue, BQCA increased mPFC spontaneous excitatory postsynaptic currents (sEPSCs) and potentiated carbachol-induced effects on sEPSCs frequency, and these effects were absent in M1 mAChR KO mice (Shirey et al. 2009). With in vivo electrophysiological techniques, BQCA was also shown to enhance firing rates of mPFC neurons after systemic administration (Shirey et al. 2009) (Table 7). In animal studies, BQCA reversed scopolamine-induced disruptions of the hippocampus-mediated memory task of contextual fear conditioning, increased wakefulness, decreased delta sleep, and restored deficits in mPFC-dependent discrimination reversal learning in a transgenic mouse that overexpresses a familial AD mutant form of the amyloid precursor protein (Tg2576 mice) (Ma et al. 2009; Shirey et al. 2009) (Table 7). Interestingly, BQCA also increased cortical blood flow, a process previously attributed to M5 mAChR activation based on KO studies (Yamada et al. 2001, 2003). Taken together, studies with M1 allosteric agonists and PAMs have demonstrated that selective activation of M1 produces efficacy in preclinical models of cognitive enhancement similar to the effects observed with other nonselective mAChR agonists, and indicate an important role for M1 activation in prefrontal cortex-dependent synaptic plasticity and learning.
4.3.3 M4 Positive Allosteric Modulators
There have also been recent developments in the identification of systemically active M4 PAMs, including LY2033298 and VU0152100 (Chan et al. 2008; Brady et al. 2008) (see Fig. 6 for chemical structures, and Tables 6 and 7 for in vitro properties and functional effects, respectively). LY2033298 represents a highly selective M4 PAM that robustly potentiates the response of ACh through binding at residue F186 in the third extracellular loop (o3) of the receptor (Nawaratne et al. 2010), but does not directly activate M4 mAChRs. Using rat M4 AChRs (rM4) membranes in cell-based studies, the in vitro potency of LY2033298 for potentiation of [3H]-oxotremorine-M was decreased by fivefold to sixfold in comparison with studies using human M4 AChR (hM4) membranes (hM4 EC50 = 8 nM; see Table 6). Across all in vivo models tested to date, LY2033298 had no effects when administered alone, but potentiated the effects of a subthreshold dose of the nonselective mAChR agonist oxotremorine in the inhibition of conditioned avoidance responding and reversal of apomorphine-induced disruption of the PPI (Chan et al. 2008; Leach et al. 2010; Suratman et al. 2011). The observed lower potency of LY2033298 at the rat M4 mAChR has been postulated to account for the lack of efficacy observed in animal models with the LY2033298 alone.
More recently, another highly selective, systemically active M4 mAChR PAM, VU0152100, with a 30- to 70-fold leftward shift in the ACh response was discovered (Brady et al. 2008) (Fig. 6). VU0152100 exhibits high mAChR subtype selectivity for M4 (see Table 6) relative to the other mAChRs and 15 other GPCRs that are highly expressed in the brain (Brady et al. 2008), and increases M4 mAChR receptor affinity for ACh without competing for the orthosteric ACh binding site (Brady et al. 2008). In preclinical studies, VU0152100 reversed amphetamine-induced hyperlocomotion and disruptions in the acquisition of contextual fear conditioning (Byun et al. 2011). Interestingly, these findings suggest that there is sufficient endogenous ACh tone to potentiate cholinergic responses when VU0152100 is administered alone. Although preliminary, these studies using selective M4 mAChR PAMs indicate that selective activation of M4 mAChRs produces efficacy in preclinical models predictive of antipsychosis-like activity comparable to the effects observed with xanomeline and other mAChR agonists and hint at some potential cognition enhancing effects.
5 Summary
Converging findings with subtype-selective mAChR activators and mAChR antagonists and KO mice are providing important validation for the role of the muscarinic cholinergic system in the modulation of normal cognitive functions and in the potential reversal of cognitive deficits observed in neurologic and psychiatric disorders, including AD and schizophrenia. Discovery of the novel subtype-selective mAChR ligands is also providing critical tools to better understand the relative roles of the mAChR subtypes in the different aspects of cognition and in the observed efficacy with AChEIs and orthosteric mAChR agonists. To date, selective M1 and M4 allosteric agonists and/or PAMs are providing the most promising preclinical data for the potential treatment of cognitive impairments and behavioral disturbance associated with dementia or schizophrenia.
Abbreviations
- AC:
-
Adenylyl cyclase
- ACh:
-
Acetylcholine
- AChEIs:
-
Acetylcholinesterase inhibitors
- AD:
-
Alzheimer’s disease
- ADAS-cog:
-
Alzheimer’s Disease assessment scale-cognitive
- AMG:
-
Amygdala
- BQCA:
-
Benzylquinolone carboxylic acid
- cAMP:
-
Cyclic adenosine monophosphate
- cc:
-
Corpus callosum
- CGI:
-
Clinical Global Impression scale
- CNS:
-
Central nervous system
- CP:
-
Caudate-putamen
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopamine
- DBB:
-
Diagonal band of Broca
- EC:
-
Entorhinal cortex
- EEG:
-
Electrocephalogram
- EPSC:
-
Excitatory postsynaptic current
- GABA:
-
γ-aminobutyric acid
- HPC:
-
Hippocampus
- IP3:
-
Inositol triphosphate
- KO:
-
Knockout
- LDTg:
-
Laterodorsal tegmental nucleus
- M1–M5:
-
Muscarinic receptor subtypes M1 through M5
- mAChRs:
-
Muscarinic acetylcholine receptors
- (m)PFC:
-
(Medial) prefrontal cortex
- NAM:
-
Negative allosteric modulator
- NAS:
-
Nucleus accumbens
- NBM:
-
Nucleus basalis of Meynert
- NMDA:
-
N-methyl-d-aspartate
- OB:
-
Olfactory bulb
- PAM:
-
Positive allosteric modulator
- PANSS:
-
Positive and negative syndrome scale
- PLC:
-
Phospholipase C
- PPI:
-
Prepulse inhibition
- PPTg:
-
Pedunculopontine tegmental nucleus
- SN:
-
Substantia nigra
- TBPB:
-
1-(1’-2-methylbenzyl)-1,4’-bipiperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one
- THAL:
-
Thalamus
- VTA:
-
Ventral tegmental area
- WT:
-
Wildtype
References
Agnoli A, Martucci N, Manna V, Conti L, Fioravanti M (1983) Effect of cholinergic and anticholinergic drugs on short-term memory in Alzheimer’s dementia: a neuropsychological and computerized electroencephalographic study. Clin Neuropharmacol 6:311–323
Aigner TG, Mishkin M (1986) The effects of physostigmine and scopolamine on recognition memory in monkeys. Behav Neural Biol 45:81–87
Anagnostaras SG, Maren S, Fanselow MS (1995) Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem 64:191–194
Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6:51–58
Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS (1999) Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology 21(6):731–744
Amenta F, Parnetti L, Gallai V, Wallin A (2001) Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mech Ageing Dev 122:2025–2040
Andrews JS, Grützner M, Stephens DN (1992) Effects of cholinergic and non-cholinergic drugs on visual discrimination and delayed visual discrimination performance in rats. Psychopharmacology (Berl) 106:523–530
Araya R, Noguchi T, Yuhki M, Kitamura N, Higuchi M, Saido TC, Seki K, Itohara S, Kawano M, Tanemura K, Takashima A, Yamada K, Kondoh Y, Kanno I, Wess J, Yamada M (2006) Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol Dis 24:334–344
Avlani VA, Langmead CJ, Guida E, Wood MD, Tehan BG, Herdon HJ, Watson JM, Sexton PM, Christopoulos A (2010) Orthosteric and allosteric modes of interaction of novel selective agonists of the M1 muscarinic acetylcholine receptor. Mol Pharmacol 78(1):94–104
Barak S (2009) Modeling cholinergic aspects of schizophrenia: focus on the antimuscarinic syndrome. Behav Brain Res 204:335–351
Baron SP, Wright D, Wenger GR (1998) Effects of drugs of abuse and scopolamine on memory in rats: delayed spatial alternation and matching to position. Psychopharmacology 137:7–14
Barten DM, Albright CF (2008) Therapeutic strategies for Alzheimer’s disease. Mol Neurobiol 37:171–186
Bartolomeo AC, Morris H, Buccafusco JJ, Kille N, Rosenzweig-Lipson S, Husbands MG, Sabb AL, Abou-Gharbia M, Moyer JA, Boast CA (2000) The preclinical pharmacological profile of WAY-132983, a potent M1 preferring agonist. J Pharmacol Exp Ther 292:584–596
Bartus RT, Dean RL III, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–417
Basile AS, Fedorova I, Zapata A, Liu XG, Shippenberg T, Duttaroy A, Yamada M, Wess J (2002) Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc Natl Acad Sci U S A 99:11452–11457
Bernard V, Normand E, Bloch B (1992) Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci 12:3591–3600
Besheer J, Short KR, Bevins RA (2001) Dopaminergic and cholinergic antagonism in a novel-object detection task with rats. Behav Brain Res 126:211–217
Betz AJ, McLaughlin PJ, Burgos M, Weber SM, Salamone JD (2007) The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology 194:347–359
Birks J (2006) Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 25(1):CD005593
Birks J, Flicker L (2006) Donepezil for mild cognitive impairment. Cochrane Database Syst Rev 3:CD006104
Bodick NC, Offen WW, Levey AI et al (1997a) Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol 54:465–473
Bodick NC, Offen WW, Shannon HE et al (1997b) The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord 11(Suppl 4):S16–S22
Bolam JP, Wainer BH, Smith AD (1984) Characterization of cholinergic neurons in the rat neostriatum: a combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience 12:711–718
Bolden C, Cusack B, Richelson E (1992) Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther 260:576–580
Bradley SR, Lameh J, Ohrmund L et al (2010) AC-260584, an orally bioavailable M(1) muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology 58:365–373
Brandeis R, Dachir S, Sapir M, Levy A, Fisher A (1990) Reversal of age-related cognitive impairments by an M1 cholinergic agonist, AF102B. Pharmacol Biochem Behav 36:89–95
Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW (2008) Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther 327:941–953
Brann MR, Buckley NJ, Bonner TI (1988) The striatum and cerebral cortex express different muscarinic receptor mRNAs. FEBS Lett 28:90–94
Brockel BJ, Fowler SC (1995) Effects of chronic haloperidol on reaction time and errors in a sustained attention task: partial reversal by anticholinergics and by amphetamine. J Pharmacol Exp Ther 275:1090–1098
Bruno G, Mohr E, Gillespie M, Fedio P, Chase TN (1986) Muscarinic agonist therapy of Alzheimer’s disease. A clinical trial of RS-86. Arch Neurol 43:659–661
Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR (2010) Facilitation of long-term potentiation by muscarinic M(1) receptors is mediated by inhibition of SK channels. Neuron 68:948–963
Buckley NJ, Bonner TI, Buckley CM, Brann MR (1989) Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol 35:469–476
Buresová O, Bolhuis JJ, Bures J (1986) Differential effects of cholinergic blockade on performance of rats in the water tank navigation task and in a radial water maze. Behav Neurosci 100:476–482
Bushnell PJ, Oshiro WM, Padnos BK (1997) Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology (Berl) 134:230–241
Bymaster FP, Heath I, Hendrix JC, Shannon HE (1993) Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. J Pharmacol Exp Ther 267:16–24
Byun N, Lawson K, Gore JC, Conn PJ, Jones CK (2011) Antipsychotic-like profile and reversal of cognitive impairment with the positive allosteric modulator of the M4 muscarinic acetylcholine receptor VU0152100. Abstracts of the 13th International Congress on Schizophrenia Research (ICOSR). Schizophr Bull Suppl 1:1–342
Callahan MJ, Kinsora JJ, Harbaugh RE, Reeder TM, Davis RE (1993) Continuous ICV infusion of scopolamine impairs sustained attention of rhesus monkeys. Neurobiol Aging 14:147–151
Carey GJ, Billard W, Binch H 3rd, Cohen-Williams M, Crosby G, Grzelak M, Guzik H, Kozlowski JA, Lowe DB, Pond AJ, Tedesco RP, Watkins RW, Coffin VL (2001) SCH 57790, a selective muscarinic M(2) receptor antagonist, releases acetylcholine and produces cognitive enhancement in laboratory animals. Eur J Pharmacol 431:189–200
Cayzac S, Delcasso S, Paz V, Jeantet Y, Cho YH (2011) Changes in striatal procedural memory coding correlate with learning deficits in a mouse model of Huntington disease. Proc Natl Acad Sci U S A 108:9280–9285
Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, Felder CC (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A 105:10978–10983
Chaudhuri JD, Hiltunen M, Nykänen M, Ylä-Herttuala S, Soininen H, Miettinen R (2005) Localization of M2 muscarinic receptor protein in parvalbumin and calretinin containing cells of the adult rat entorhinal cortex using two complementary methods. Neuroscience 131:557–566
Chen KC, Baxter MG, Rodefer JS (2004) Central blockade of muscarinic cholinergic receptors disrupts affective and attentional set-shifting. Eur J Neurosci 20:1081–1088
Chouinard S, Sepehry AA, Stip E (2007) Oral cholinesterase inhibitor add-on therapy for cognitive enhancement in schizophrenia: a quantitative systematic re-view, part I. Clin Neuropharmacol 30:169–182
Christopoulos A (2002) Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov 1:198–210
Christopoulos A, Mitchelson F (1997) Application of an allosteric ternary complex model to the technique of pharmacological resultant analysis. J Pharm Pharmacol 49:781–786
Clarke PB, Hommer DW, Pert A, Skirboll LR (1987) Innervation of substantia nigra neurons by cholinergic afferents from pedunculopontine nucleus in the rat: neuroanatomical and electrophysiological evidence. Neuroscience 23:1011–1019
Conn PJ, Jones CK, Lindsley CW (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci 30:148–155
Cox CL, Huguenard JR, Prince DA (1997) Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc Natl Acad Sci U S A 94:8854–8859
Cummings JL (2003) Use of cholinesterase inhibitors in clinical practice: evidence-based recommendations. Am J Geriatr Psychiatry 11:131–145
Datta S, Siwek DF (1997) Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol 77(6):2975–2988
Dawson GR, Bayley P, Channell S, Iversen SD (1994) A comparison of the effects of the novel muscarinic receptor agonists L-689,660 and AF102B in tests of reference and working memory. Psychopharmacology 113(3–4):361–368
Dennes RP, Barnes JC (1993) Attenuation of scopolamine-induced spatial memory deficits in the rat by cholinomimetic and non-cholinomimetic drugs using a novel task in the 12-arm radial maze. Psychopharmacology (Berl) 111:435–441
Dietrich H, Jenck F (2010) Intact learning and memory in rats following treat-ment with the dual orexin receptor antagonist almorexant. Psychopharmacology 212:145–154
Dillon GM, Shelton D, McKinney AP, Caniga M, Marcus JN, Ferguson MT, Kornecook TJ, Dodart JC (2009) Prefrontal cortex lesions and scopolamine impair attention performance of C57BL/6 mice in a novel 2-choice visual discrimination task. Behav Brain Res 204:67–76
Dodart JC, Mathis C, Ungerer A (1997) Scopolamine-induced deficits in a two-trial object recognition task in mice. Neuroreport 24:1173–1178
Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR (1991) Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther 256:727–733
Domer FR, Schueler FW (1960) Investigations of the amnesic properties of scopolamine and related compounds. Arch Int Pharmacodyn Ther 127:449–458
Doods H, Entzeroth M, Ziegler H, Schiavi G, Engel W, Mihm G, Rudolf K, Eberlein W (1993) Characterization of BIBN 99: a lipophilic and selective muscarinic M2 receptor antagonist. Eur J Pharmacol 242:23–30
Drinkenburg WH, Sondag HN, Coenders CJ, Andrews JS, Vossen JM (1995) Effects of selective antagonism or depletion of the cholinergic system on visual discrimination performance in rats. Behav Pharmacol 6:695–702
Drachman D, Leavitt J (1974) Human memory and the cholinergic system. A relationship to aging? Arch Neurol 30:113–121
Dudchenko P, Sarter M (1992) Behavioral microanalysis of spatial delayed alternation performance: rehearsal through overt behavior, and effects of scopolamine and chlordiazepoxide. Psychopharmacology (Berl) 107:263–270
Eckenstein FP, Baughman RW, Quinn J (1988) An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience 25:457–474
Edelstein P, Schultz JR, Hirschowitz J et al (1981) Physostigmine and lithium in the schizophrenias. Am J Psychiatry 138:1078–1081
Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ (2006) Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol 9:175–189
Ennaceur A, Meliani K (1992) Effects of physostigmine and scopolamine on rats’ performances in object-recognition and radial-maze tests. Psychopharmacology (Berl) 109:321–330
Everitt BJ, Robbins TW (1997) Central cholinergic systems and cognition. Annu Rev Psychol 48:649–684
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 59:512–519
Farlow MR, Cummings JL (2007) Effective pharmacologic management of Alzheimer’s disease. Am J Med 120:388–397
Feiro O, Gould TJ (2005) The interactive effects of nicotinic and muscarinic cholinergic receptor inhibition on fear conditioning in young and aged C57BL/6 mice. Pharmacol Biochem Behav 80:251–262
Fink-Jensen A, Fedorova I, Wörtwein G et al (2003) Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res 74:91–96
Fisher A (2007) M1 muscarinic agonists target major hallmarks of Alzheimer’s disease – an update. Curr Alzheimer Res 4:577–580
Fisher A, Brandeis R, Karton I, Pittel Z, Gurwitz D, Haring R, Sapir M, Levy A, Heldman E (1991) (+−)-cis-2-methyl-spiro(1,3-oxathiolane-5,3′)quinuclidine, an M1 selective cholinergic agonist, attenuates cognitive dysfunctions in an animal model of Alzheimer’s disease. J Pharmacol Exp Ther 257:392–403
Fornari RV, Moreira KM, Oliveira MG (2000) Effects of the selective M1 muscarinic receptor antagonist dicyclomine on emotional memory. Learn Mem 7:287–292
Fredrickson A, Snyder PJ, Cromer J, Thomas E, Lewis M, Maruff P (2008) The use of effect sizes to characterize the nature of cognitive change in psychopharmacological studies: an example with scopolamine. Hum Psychopharmacol Clin Exp 23:425–436
Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P (1998) High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol 55:937–945
Gerfen CR, Bolam JP (2010) The neuroanatomical organization of the basal ganglia. In: Steiner H, Tseng KY (eds) Handbook of basal ganglia structure and function. Academic Press, London
González I, Arévalo-Serrano J, Sanz-Anquela JM, Gonzalo-Ruiz A (2007) Effects of beta-amyloid protein on M1 and M2 subtypes of muscarinic acetylcholine receptors in the medial septum-diagonal band complex of the rat: relationship with cholinergic, GABAergic, and calcium-binding protein perikarya. Acta Neuropathol 113:637–651
Gonzalo-Ruiz A, Sanz-Anquela MJ, Lieberman AR (1995) Cholinergic projections to the anterior thalamic nuclei in the rat: a combined retrograde tracing and choline acetyl transferase immunohistochemical study. Anat Embryol (Berl) 192(4):335–49
Gould E, Woolf NJ, Butcher LL (1989) Cholinergic projections to the substantia nigra from the pedunculopontine and laterodorsal tegmental nuclei. Neuroscience 28:611–623
Green MF (1996) What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153:321–330
Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, Nathan PJ (2005) Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav 81:575–584
Green MF, Kern RS, Heaton RK (2004) Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 72:41–51
Hagan JJ, Jansen JH, Broekkamp CL (1989) Hemicholinium-3 impairs spatial learning and the deficit is reversed by cholinomimetics. Psychopharmacology 98:347–356
Hájos N, Papp EC, Acsády L, Levey AI, Freund TF (1998) Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience 82:355–376
Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH (1987) The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol 262:105–124
Hamborg-Petersen B, Nielsen MM, Thordal C (1984) Toxic effect of scopolamine eye drops in children. Acta Ophthalmol 62:485–488
Harries MH, Samson NA, Cilia J, Hunter AJ (1998) The profile of sabcomeline (SB-202026), a functionally selective M1 receptor partial agonist, in the marmoset. Br J Pharmacol 124:409–415
Hasselmo ME (2006) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16(6):710–715
Hasselmo ME, Sarter M (2011) Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36(1):52–73
Hatcher JP, Loudon JM, Hagan JJ, Clark MS (1998) Sabcomeline (SB-202026), a functionally selective M1 receptor partial agonist, reverses delay-induced deficits in the T-maze. Psychopharmacology 138:275–282
Heinrich JN, Butera JA, Carrick T, Kramer A, Kowal D, Lock T, Marquis KL, Pausch MH, Popiolek M, Sun SC, Tseng E, Uveges AJ, Mayer SC (2009) Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists. Eur J Pharmacol 605:53–56
Hellendall RP, Godfrey DA, Ross CD, Armstrong DM, Price JL (1986) The distribution of choline acetyltransferase in the rat amygdaloid complex and adjacent cortical areas, as determined by quantitative micro-assay and immunohistochemistry. J Comp Neurol 249:486–498
Hersch SM, Levey AI (1995) Diverse pre- and post-synaptic expression of m1-m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci 56:931–938
Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI (1994) Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci 14:3351–3363
Higgs S, Deacon RMJ, Rawlins JNP (2000) Effects of scopolamine on a novel choice serial reaction time task. Eur J Neurosci 12:1781–1788
Hock C, Maddalena A, Heuser I, Naber D, Oertel W, von der Kammer H, Wienrich M, Raschig A, Deng M, Growdon JH, Nitsch RM (2000) Treatment with the selective muscarinic agonist talsaclidine decreases cerebrospinal fluid levels of total amyloid beta-peptide in patients with Alzheimer’s disease. Ann N Y Acad Sci 920:285–291
Hock C, Maddalena A, Raschig A, Müller-Spahn F, Eschweiler G, Hager K, Heuser I, Hampel H, Müller-Thomsen T, Oertel W, Wienrich M, Signorell A, Gonzalez-Agosti C, Nitsch RM (2003) Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer’s disease. Amyloid 10:1–6
Hodges H, Peters S, Gray JA, Hunter AJ (1999) Counteractive effects of a partial (sabcomeline) and a full (RS86) muscarinic receptor agonist on deficits in radial maze performance induced by S-AMPA lesions of the basal forebrain and medial septal area. Behav Brain Res 99:81–92
Hunter AJ, Roberts FF (1988) The effect of pirenzepine on spatial learning in the Morris Water Maze. Pharmacol Biochem Behav 30:519–523
Ichikawa T, Hirata Y (1986) Organization of choline acetyltransferase-containing structures in the forebrain of the rat. J Neurosci 6:281–292
Ince E, Ciliax BJ, Levey AI (1997) Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse 27:357–366
Jacobson MA, Kreatsoulas C, Pascarella DM, O’Brien JA, Sur C (2010) The M1 muscarinic receptor allosteric agonists AC-42 and 1-[1′-(2-methylbenzyl)-1,4′-bipiperidin-4-yl]-1,3-dihydro-2H-benzimidazol-2-one bind to a unique site distinct from the acetylcholine orthosteric site. Mol Pharmacol 78:648–657
Jäkälä P, Sirviö J, Jolkkonen J, Riekkinen P Jr, Acsady L, Riekkinen P (1992) The effects of p-chlorophenylalanine-induced serotonin synthesis inhibition and muscarinic blockade on the performance of rats in a 5-choice serial reaction time task. Behav Brain Res 51:29–40
Jo J, Son GH, Winters BL, Kim MJ, Whitcomb DJ, Dickinson BA, Lee YB, Futai K, Amici M, Sheng M, Collingridge GL, Cho K (2010) Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci 13(10):1216–24
Jones CK, Shannon HE (2000) Muscarinic cholinergic modulation of prepulse inhibition of the acoustic startle reflex. J Pharmacol Exp Ther 294:1017–1023
Jones CK, Eberle EL, Shaw DB, McKinzie DL, Shannon HE (2005) Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. J Pharmacol Exp Ther 312:1055–1063
Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, Peterson TE, Ansari MS, Baldwin RM, Kessler RM, Deutch AY, Lah JJ, Levey AI, Lindsley CW, Conn PJ (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci 28:10422–10433
Jones DN, Higgins GA (1995) Effect of scopolamine on visual attention in rats. Psychopharmacology (Berl) 120:142–149
Kane A (2008) The in vivo characterization of TBPB, a novel allosteric agonist of M1 muscarinic receptors: implications for the role of the M1 muscarinic receptor in treatment of schizophrenia. S08 NSC 296 Thesis Defense Vanderbilt University
Kim MG, Bodor ET, Wang C, Harden TK, Kohn H (2003) C(8) substituted 1-azabicyclo[3.3.1]non-3-enes and C(8) substituted 1-azabicyclo[3.3.1]nonan-4-ones: novel muscarinic receptor antagonists. J Med Chem 46:2216–2226
Kimura H, McGeer PL, Peng F, McGeer EG (1980) Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Science 208:1057–1059
Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment. Neurosci Biobehav Rev 34:1307–1350
Kobayashi F, Yageta Y, Yamazaki T, Wakabayashi E, Inoue M, Segawa M, Matsuzawa S (2007) Pharmacological effects of imidafenacin (KRP-197/ONO-8025), a new bladder selective anti-cholinergic agent, in rats. Comparison of effects on urinary bladder capacity and contraction, salivary secretion and performance in the Morris water maze task. Arzneimittelforschung 57:147–154
Kozak R, Bruno JP, Sarter M (2006) Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex 16:9–17
Lachowicz JE, Lowe D, Duffy RA, Ruperto V, Taylor LA, Guzik H, Brown J, Berger JG, Tice M, McQuade R, Kozlowski J, Clader J, Strader CD, Murgolo N (1999) SCH 57790: a novel M2 receptor selective antagonist. Life Sci 64:535–539
Langmead CJ, Austin NE, Branch CL, Brown JT, Buchanan KA, Davies CH, Forbes IT, Fry VA, Hagan JJ, Herdon HJ, Jones GA, Jeggo R, Kew JN, Mazzali A, Melarange R, Patel N, Pardoe J, Randall AD, Roberts C, Roopun A, Starr KR, Teriakidis A, Wood MD, Whittington M, Wu Z, Watson J (2008) Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br J Pharmacol 54:1104–1115
Langmead CJ, Fry VA, Forbes IT, Branch CL, Christopoulos A, Wood MD, Herdon HJ (2006) Probing the molecular mechanism of interaction between 4-n-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine (AC-42) and the muscarinic M(1) receptor: direct pharmacological evidence that AC-42 is an allosteric agonist. Mol Pharmacol 69(1):236–46.
Lazareno S, Buckley NJ, Roberts FF (1990) Characterization of muscarinic M4 binding sites in rabbit lung, chicken heart, and NG108-15 cells. Mol Pharmacol 38:805–815
Leach K, Loiacono RE, Felder CC et al (2010) Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology 35:855–869
Leaton RN, Kreindler M (1972) Effects of physostigmine and scopolamine on operant brightness discrimination in the rat. Physiol Behav 9:121–123
Lebois EP, Bridges TM, Lewis LM, Dawson ES, Kane AS, Xiang Z, Jadhav SB, Yin H, Kennedy JP, Meiler J, Niswender CM, Jones CK, Conn PJ, Weaver CD, Lindsley CW (2010) Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M1 receptor function in the central nervous system. ACS Chem Neurosci 1:104–121
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445(7124):168–76
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11:3218–3226
Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA (1994) Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience 63:207–221
Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ (1995a) Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol 351:339–356
Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995b) Expression of ml-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15:4077–4092
Li M, Yasuda RP, Wall SJ, Wellstein A, Wolfe BB (1991) Distribution of m2 muscarinic receptors in rat brain using antisera selective for m2 receptors. Mol Pharmacol 40:28–35
Loudon JM, Bromidge SM, Brown F, Clark MS, Hatcher JP, Hawkins J, Riley GJ, Noy G, Orlek BS (1997) SB 202026: a novel muscarinic partial agonist with functional selectivity for M1 receptors. J Pharmacol Exp Ther 283:1059–1068
Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A 106:15950–15955
Marino PJ, Conn PJ (2002) Direct and indirect modulation of the N-methyl-D-aspartate receptor: potential for the development of novel antipsychotic therapies. Curr Drug Targets CNS Neurol Disord 1:1–16
Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ (1998) Activation of the genetically defined ml muscarinicreceptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A 95:11465–11470
Matsuoka N, Maeda N, Ohkubo Y et al (1991) Differential effects of physostigmine and pilocarpine on the spatial memory deficits produced by two septo-hippocampal deafferentations in rats. Brain Res 559:233–240
McDonald AJ, Mascagni F (2010) Neuronal localization of m1 muscarinic receptor immunoreactivity in the rat basolateral amygdala. Brain Struct Funct 215:37–48
McDonough JH Jr (1982) Effects of anticholinergic drugs on DRL performance of rhesus monkeys. Pharmacol Biochem Behav 17:85–90
McGeer PL, McGeer EG, Kamo H, Wong K (1986) Positron emission tomography and the possible origins of cytopathology in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 10:501–818
Means LW, Holsten RD, Long M, High KM (1996) Scopolamine- and morphine-induced deficits in water maze alternation: failure to attenuate with glucose. Neurobiol Learn Mem 66:167–175
Mechawar N, Cozzari C, Descarries L (2000) Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J Comp Neurol 428:305–318
Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10:1185–1201
M’Harzi M, Palou AM, Oberlander C, Barzaghi F (1995) Antagonism of scopolamine-induced memory impairments in rats by the muscarinic agonist RU 35,926 (CI-979). Pharmacol Biochem Behav 51:119–124
Mirza NR, Stolerman IP (2000) The role of nicotinic and muscarinic acetylcholine receptors in attention. Psychopharmacology (Berl) 148:243–250
Mishima K, Iwasaki K, Tsukikawa H, Matsumoto Y, Egashira N, Abe K, Egawa T, Fujiwara M (2000) The scopolamine-induced impairment of spatial cognition parallels the acetylcholine release in the ventral hippocampus in rats. Jpn J Pharmacol 84:163–173
Miyachi H, Kiyota H, Uchiki H, Segawa M (1999) Synthesis and antimuscarinic activity of a series of 4-(1-Imidazolyl)-2,2-diphenylbutyramides: discovery of potent and subtype-selective antimuscarinic agents. Bioorg Med Chem 7:1151–1161
Miyakawa T, Yamada M, Duttaroy A et al (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the Ml muscarinic acetylcholine receptor. J Neurosci 21:5239–5250
Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R et al (1995) Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol 38:643–648
Myhrer T, Enger S, Aas P (2004) Cognitive side effects in rats caused by pharmacological agents used to prevent soman-induced lethality. Eur J Pharmacol 483:271–279
Nakahara N, Iga Y, Saito Y, Mizobe F, Kawanishi G (1989) Beneficial effects of FKS-508 (AF102B), a selective M1 agonist, on the impaired working memory in AF64A-treated rats. Jpn J Pharmacol 51:539–547
Nawaratne V, Leach K, Felder CC, Sexton PM, Christopoulos A (2010) Structural determinants of allosteric agonism and modulation at the M4 muscarinic acetylcholine receptor: identification of ligand-specific and global activation mechanisms. J Biol Chem 285:19012–19021
Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thomason K, Mellow AM et al (1988) The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry 45:906–912
Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK (1995) Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci 15:5859–5869
Oda S, Kuroda M, Kakuta S, Kishi K (2001) Differential immunolacalization of m2 and m3 muscarinic receptors in the anteroventral and anderodorsal thalamic nuclei of the rat. Brain Res 894:109–120
Oda S, Sato F, Okada A, Akahane S, Igarashi H, Yokofujita J, Yang J, Kuroda M (2007) Immunolocalization of muscarinic receptor subtypes in the reticular thalamic nucleus of rats. Brain Res Bull 74:376–384
Omelchenko N, Sesack SR (2006) Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol 494:863–875
O’Neill J, Fitten LJ, Siembieda D, Halgren E, Kim E, Fisher A, Perryman K (1998) Effects of AF102B and tacrine on delayed match-to-sample in monkeys. Prog Neuropsychopharmacol Biol Psychiatry 22:665–678
O’Neill J, Fitten LJ, Siembieda DW, Crawford KC, Halgren E, Fisher A, Refai D (1999) Divided attention-enhancing effects of AF102B and THA in aging monkeys. Psychopharmacology (Berl) 43:123–130
O’Neill J, Siembieda DW, Crawford KC, Halgren E, Fisher A, Fitten LJ (2003) Reduction in distractibility with AF102B and THA in the macaque. Pharmacol Biochem Behav 76:301–306
Osterholm RK, Camoriano JK (1982) Transdermal scopolamine psychosis. JAMA 247:3081
Pakarinen ED, Moerschbaecher JM (1993) Comparison of the effects of scopolamine and methylscopolamine on the performance of a fixed-ratio discrimination in squirrel monkeys. Pharmacol Biochem Behav 44:815–819
Palacios JM, Bolliger G, Closse A, Enz A, Gmelin G, Malanowski J (1986) The pharmacological assessment of RS 86 (2-ethyl-8-methyl-2,8-diazaspiro-[4,5]-decan-1,3-dion hydrobromide). A potent, specific muscarinic acetylcholine receptor agonist. Eur J Pharmacol 125:45–62
Pazzagli A, Pepeu G (1965) Amnesic properties of scopolamine and brain acetylcholine in the rat. Int J Neuropharmacol 4:291–299
Pepeu G, Giovannini MG (2010) Cholinesterase inhibitors and memory. Chem Biol Interact 187:403–408
Persson CM, Wallin AK, Levander S, Minthon L (2009) Changes in cognitive domains during three years in patients with Alzheimer’s disease treated with donepezil. BMC Neurol 9:7
Phelps PE, Houser CR, Vaughn JE (1985) Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol 238:286–307
Pinault D, Deschênes M (1998) Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J Neurosci 10:3462–3469
Pitsikas N, Rigamonti AE, Cella SG, Locatelli V, Sala M, Muller EE (2001) Effects of molsidomine on scopolamine-induced amnesia and hypermotility in the rat. Eur J Pharmacol 426:193–200
Plummer KL, Manning KA, Levey AI, Rees HD, Uhlrich DJ (1999) Muscarinic receptor subtypes in the lateral geniculate nucleus: a light and electron microscopic analysis. J Comp Neurol 404:408–425
Poulin B, Butcher A, McWilliams P et al (2010) The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci U S A 107:9440–9445
Price JL, Stern R (1983) Individual cells in the nucleus basalis – diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res 269:352–356
Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H, Parent A, White N, Meaney MJ (1995) Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. J Neurosci 15:1455–1462
Riekkinen P Jr, Serviö J, Aaltonen M, Riekkinen P (1990) Effects of concurrent manipulations of nicotinic and muscarinic receptors on spatial avoidance learning. Pharmacol Biochem Behav 37:405–410
Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ (1989a) Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res 35:221–240
Robbins TW, Everitt BJ, Ryan CN, Marston HM, Jones GH, Page KJ (1989b) Comparative effects of quisqualic and ibotenic acid-induced lesions of the substantia innominata and globus pallidus on the acquisition of a conditional visual discrimination: differential effects on cholinergic mechanisms. Neuroscience 28:337–352
Rouse ST, Levey AI (1996) Expression of m1-m4 muscarinic acetylcholine receptor immunoreactivity in septohippocampal neurons and other identified hippocampal afferents. J Comp Neurol 375:406–416
Rouse ST, Levey AI (1997) Muscarinic acetylcholine receptor immunoreactivity after hippocampal commissural/associational pathway lesions: evidence for multiple presynaptic receptor subtypes. J Comp Neurol 380:382–394
Rouse ST, Levey AI (1998) Muscarinic acetylcholine receptor immunoreactivity after hippocampal commissural/associational pathway lesions: evidence for multiple presynaptic receptor subtypes. J Comp Neurol 380:382–394
Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI (1999) Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci 64:501–509
Rouse ST, Edmunds SM, Yi H, Gilmor ML, Levey AI (2000) Localization of M(2) muscarinic acetylcholine receptor protein in cholinergic and non-cholinergic terminals in rat hippocampus. Neurosci Lett 284:182–186
Rowe WB, O’Donnell JP, Pearson D, Rose GM, Meaney MJ, Quirion R (2003) Long-term effects of BIBN-99, a selective muscarinic M2 receptor antagonist, on improving spatial memory performance in aged cognitively impaired rats. Behav Brain Res 145:171–178
Rudy JW (1996) Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiol Learn Mem 65:73–81
Rupniak NM, Steventon MJ, Field MJ et al (1989) Comparison of the effects of four cholinomimetic agents on cognition in primates following disruption by scopolamine or by lists of objects. Psychopharmacology 99:189–195
Rupniak NM, Samson NA, Tye SJ, Field MJ, Iversen SD (1991) Evidence against a specific effect of cholinergic drugs on spatial memory in primates. Behav Brain Res 43:1–6
Rupniak NM, Tye SJ, Iversen SD (1992) Comparison of the effects of selective and nonselective muscarinic agonists on cognition and thermoregulation in primates. J Neurol Sci 110:222–227
Rusted JM, Warburton DM (1988) The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology 96:145–152
Saint-Cyr JA, Taylor AE, Lang AE (1988) Procedural learning and neostriatal dysfunction in man. Brain 111:941–959
Sams AG, Hentzer M, Mikkelsen GK, Larsen K, Bundgaard C, Plath N, Christoffersen CT, Bang-Andersen B (2010) Discovery of N-{1-[3-(3-oxo-2,3-dihydrobenzo[1,4]oxazin-4-yl)propyl]piperidin-4-yl}-2-phenylacetamide (Lu AE51090): an allosteric muscarinic M1 receptor agonist with unprecedented selectivity and procognitive potential. J Med Chem 53:6386–6397
Satoh K, Fibiger HC (1986) Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol 253:277–302
Savage UC, Faust WB, Lambert P, Moerschbaecher JM (1996) Effects of scopolamine on learning and memory in monkeys. Psychopharmacology (Berl) 123(1):9–14
Schäfer MK, Eiden LE, Weihe E (1998) Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system. Neuroscience 84:331–359
Schwarz RD, Callahan MJ, Coughenour LL, Dickerson MR, Kinsora JJ, Lipinski WJ, Raby CA, Spencer CJ, Tecle H (1999) Milameline (CI-979/RU35926): a muscarinic receptor agonist with cognition-activating properties: biochemical and in vivo characterization. J Pharmacol Exp Ther 291:812–822
Seeger T, Fedorova I, Zheng F et al (2004) M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24:10117–10127
Semba K, Reiner PB, Fibiger HC (1990) Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38:643–654
Shannon HE, Love PL (2005) Effects of antiepileptic drugs on attention as assessed by a five-choice serial reaction time task in rats. Epilepsy Behav 7:620–628
Shannon HE, Eberle EL (2006) Effects of biasing the location of stimulus presentation, and the muscarinic cholinergic receptor antagonist scopolamine, on performance of a 5-choice serial reaction time attention task in rats. Behav Pharmacol 17:71–85
Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, Jones CK, Niswender CM, Weaver CD, Lindsley CW, Conn PJ (2009) A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol 76:356–368
Shekhar A, Potter WZ, Lightfoot J et al (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 165:1033–1039
Sherman SJ, Atri A, Hasselmo ME, Stern CE, Howard MW (2003) Scopolamine impairs human recognition memory: data and modeling. Behav Neurosci 117:526–539
Shirey JK, Brady AE, Jones PJ et al (2009) A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 29:14271–14286
Sipos ML, Burchnell V, Galbicka G (2001) Effects of selected anticholinergics on acoustic startle response in rats. J Appl Toxicol 21(Suppl 1):S95–S101
Skjoldager P, Fowler SC (1991) Scopolamine attenuates the motor disruptions but not the attentional disturbances induced by haloperidol in a sustained attention task in the rat. Psychopharmacology (Berl) 105:93–100
Smith RD, Kistler MK, Cohen-Williams M, Coffin VL (1996) Cholinergic improvement of a naturally-occurring memory deficit in the young rat. Brain Res 707:13–21. doi:10.1016/0006-8993(95)01207-9
Spalding TA, Trotter C, Skjaerbaek N, Messier TL, Currier EA, Burstein ES, Li D, Hacksell U, Brann MR (2002) Discovery of an ectopic activation site on the M(l) muscarinic receptor. Mol Pharmacol 61:1297–1302
Spalding TA, Ma JN, Ott TR, Friberg M, Bajpai A, Bradley SR, Davis RE, Brann MR, Burstein ES (2006) Structural requirements of transmembrane domain 3 for activation by the M1 muscarinic receptor agonists AC-42, AC-260584, clozapine, and N-desmethylclozapine: evidence for three distinct modes of receptor activation. Mol Pharmacol 70:1974–1983
Spencer JP, Middleton LJ, Davies CH (2010) Investigation into the efficacy of the acetylcholinesterase inhibitor, donepezil, and novel procognitive agents to induce gamma oscillations in rat hippocampal slices. Neuropharmacology 59:437–443
Spinelli S, Ballard T, Feldon J, Higgins GA, Pryce CR (2006) Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology 51:238–250
Squire LR (1969) Effects of pretrial and posttrial administration of cholinergic and anticholinergic drugs on spontaneous alternation. J Comp Physiol Psychol 69:69–75
Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, Kennett GA, Lightowler S, Sheardown MJ, Syed R, Upton RL, Wadsworth G, Weiss SM, Wyatt A (2001) The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther 299:782–792
Steidl S, Yeomans JS (2009) M5 muscarinic receptor knockout mice show reduced morphine-induced locomotion but increased locomotion after cholinergic antagonism in the ventral tegmental area. J Pharmacol Exp Ther 328:263–275
Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM (2003) Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 289:2094–2103
Sugaya K, Clamp C, Bryan D, McKinney M (1997) mRNA for the m4 muscarinic receptor subtype is expressed in adult rat brain cholinergic neurons. Brain Res Mol Brain Res 50:305–313
Sullivan NR, Leventhal L, Harrison J, Smith VA, Cummons TA, Spangler TB, Sun SC, Lu P, Uveges AJ, Strassle BW, Piesla MJ, Ramdass R, Barry A, Schantz J, Adams W, Whiteside GT, Adedoyin A, Jones PG (2007) Pharmacological characterization of the muscarinic agonist (3R,4R)-3-(3-hexylsulfanyl-pyrazin-2-yloxy)-1-aza-bicyclo[2.2.1]heptane (WAY-132983) in in vitro and in vivo models of chronic pain. J Pharmacol Exp Ther 322:1294–1304
Sunderland T, Tariot P, Weingartner H, Murphy D, Newhouse P, Mueller E et al (1986) Pharmacologic modeling of Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 10:599–610
Suratman S, Leach K, Sexton P et al (2011) Impact of species variability and ‘probe-dependence’ on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br J Pharmacol 162:1659–1670
Strauss ME, Reynolds KS, Jayaram G, Tune LE (1990) Effects of anticholinergic medication on memory in schizophrenia. Schiz Res 3:127–129
Suzuki M, Yamaguchi T, Ozawa Y, Ohyama M, Yamamoto M (1995) Effects of (-)-S-2,8-dimethyl-3-methylene-1-oxa-8-azaspiro[4,5]decane L-tartrate monohydrate (YM796), a novel muscarinic agonist, on disturbance of passive avoidance learning behavior in drug-treated and senescence-accelerated mice. J Pharmacol Exp Ther 275(2):728–36.
Terry AV Jr, Buccafusco JJ, Borsini F, Leusch A (2002) Memory-related task performance by aged rhesus monkeys administered the muscarinic M(1)-preferring agonist, talsaclidine.Psychopharmacology (Berl) 162(3):292–300
Terry AV Jr, Parikh V, Gearhart DA, Pillai A, Hohnadel E, Warner S, Nasrallah HA, Mahadik SP (2006b) Time-dependent effects of haloperidol and ziprasidone on nerve growth factor, cholinergic neurons, and spatial learning in rats. J Pharmacol Exp Ther 318:709–724
Thienel R, Kellermann T, Schall U, Voss B, Reske M, Halfter S, Sheldrick AJ, Radenbach K, Habel U, Shah NJ, Kircher T (2009) Muscarinic antagonist effects on executive control of attention. Int J Neuropsychopharmacol 12:1307–1317
Thomsen M, Wess J, Fulton BS, Fink-Jensen A, Caine SB (2010) Modulation of prepulse inhibition through both M(1) and M (4) muscarinic receptors in mice. Psychopharmacology 208:401–416
Thomsen M, Woldbye DP, Wörtwein G, Fink-Jensen A, Wess J, Caine SB (2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci 25(36):8141–9
Thomsen M, Wortwein G, Fink-Jensen A, Woldbye DP, Wess J, Caine SB (2007) Decreased prepulse inhibition and increased sensitivity to muscarinic, but not dopaminergic drugs in M5 muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berl) 192:97–110
Tsai G, Coyle JT (2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42:165–79
Tzavara ET, Bymaster FP, Felder CC et al (2003) Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry 8:673–679
Tzavara ET, Bymaster FP, Davis RJ et al (2004) M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J 18:1410–1412
Ukai M, Okuda A, Mamiya T (2004) Effects of anticholinergic drugs selective for muscarinic receptor subtypes on prepulse inhibition in mice. Eur J Pharmacol 492:183–187
van der Zee EA, Keijser JN (2011) Localization of pre- and postsynaptic cholinergic markers in rodent forebrain: a brief history and comparison of rat and mouse. Behav Brain Res 221:356–366
van der Zee EA, Matsuyama T, Strosberg AD, Traber J, Luiten PG (1989) Demonstration of muscarinic acetylcholine receptor-like immunoreactivity in the rat forebrain and upper brainstem. Histochemistry 92:475–485
Vanover KE, Veinbergs I, Davis RE (2008) Antipsychotic-like behavioral effects and cognitive enhancement by a potent and selective muscarinic M-sub-1 receptor agonist, AC-260584. Behav Neurosci 122:570–575
Veroff AE, Bodick NC, Offen WW, Sramek JJ, Cutler NR (1998) Efficacy of xanomeline in Alzheimer disease: cognitive im-provement measured using the Computerized Neuro-psychological Test Battery (CNTB). Alzheimer Dis Assoc Disord 12:304–312
Vilaró MT, Palacios JM, Mengod G (1990) Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett 114:154–159
Vilaró MT, Wiederhold KH, Palacios JM, Mengod G (1991) Muscarinic cholinergic receptors in the rat caudate-putamen and olfactory tubercle belong predominantly to the m4 class: in situ hybridization and receptor autoradiography evidence. Neuroscience 40:159–167
Vilaró MT, Wiederhold KH, Palacios JM, Mengod G (1992) Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience 47:367–393
Waelbroeck M (2003) Allosteric drugs acting act muscarinic acetylcholine receptors. Neurochem Res 28:419–422
Wall SJ, Yasuda RP, Hory F, Flagg S, Martin BM, Ginns EI, Wolfe BB (1991) Production of antisera selective for m1 muscarinic receptors using fusion proteins: distribution oof m1 receptors in rat brain. Mol Pharmacol 39:643–649
Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW (2003) Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron 38:987–996
Watson J, Brough S, Coldwell MC, Gager T, Ho M, Hunter AJ, Jerman J, Middlemiss DN, Riley GJ, Brown AM (1998) Functional effects of the muscarinic receptor agonist, xanomeline, at 5-HT1 and 5-HT2 receptors. Br J Pharmacol 125:1413–1420
Weiner DM, Levey AI, Brann MR (1990) Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A 87:7050–7054
Wienrich M, Ceci A, Ensinger HA, Gaida W, Mendla KD, Osugi T, Raschig A, Weiser T (2002) Talsaclidine (WAL 2014 FU), a muscarinic M1 receptor agonist for the treatment of Alzheimer’s disease. Drug Dev Res 56:321–334
Woolf NJ, Hernit MC, Butcher LL (1986) Cholinergic and non-cholinergic projections from the rat basal forebrain revealed by combined choline acetyltransferase and Phaseolus vulgaris leucoagglutinin immunohistochemistry. Neurosci Lett 66:281–286
Wu MF, Jenden DJ, Fairchild MD, Siegel JM (1993) Cholinergic mechanisms in startle and prepulse inhibition: effects of the false cholinergic precursor N-aminodeanol. Behav Neurosci 107:306–316
Yamada M, Lamping KG, Duttaroy A et al (2001) Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 98:14096–14101
Yamada M, Basile AS, Fedorova I, Zhang W, Duttaroy A, Cui Y, Lamping KG, Faraci FM, Deng CX, Wess J (2003) Novel insights into M5 muscarinic acetylcholine receptor function by the use of gene targeting technology. Life Sci 74:345–353
Yamasaki M, Matsui M, Watanabe M (2010) Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci 30:4408–4418
Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB (1993) Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol 43:149–157
Zang Z, Creese I (1997) Differential regulation of expression of rat hippocampal muscarinic receptor subtypes following fimbria-fornix lesion. Biochem Pharmacol 53:1379–1382
Zhang W, Basile AS, Gomeza J et al (2002) Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci 22:1709–1717
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bubser, M., Byun, N., Wood, M.R., Jones, C.K. (2012). Muscarinic Receptor Pharmacology and Circuitry for the Modulation of Cognition. In: Fryer, A., Christopoulos, A., Nathanson, N. (eds) Muscarinic Receptors. Handbook of Experimental Pharmacology, vol 208. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-23274-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-23274-9_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23273-2
Online ISBN: 978-3-642-23274-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)