Abstract
Depending on nutritional habits, our diet may contain a substantial load of phenolics, defined as plant secondary metabolites consisting of one to several phenol groups. Their bioavailability, in other words the active fraction of ingested amounts that reaches targeted cell types or tissues where biochemical properties can act, is markedly influenced by metabolism and absorption in the gastrointestinal tract. Indeed, our intestine is the primary metabolically active site of absorption of exogenous factors in our body and harbors trillions of microbial cells with a vast metabolic potential, referred to as the intestinal microbiota. The aim of the present book chapter is to give insights into the role of phenolic compounds in human health. We will focus our attention on two families of polyphenols of importance in human nutrition, namely, the isoflavones and lignans, and will discuss in detail the role of intestinal microorganisms in regulating their metabolism and thereby health effects.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Bioavailability
- enterolignans
- equol
- health
- human nutrition
- intestinal microbiota
- isoflavones
- lignans
- microbiome
- phenolics
- phytoestrogens
1 Introduction
Nutrition, in combination with other environmental factors such as climate shifts and changes in ecosystem structure, has played a key role in evolutionary processes that made us what we are: Homo sapiens. Our brain must be constantly fueled with energy in the form of glucose, and essential nutrients such as fatty acids are required for proper brain development. Thus, selectively advantageous eating behaviors have certainly favored essential nutrient supply, efficient energy harvest from food stuff and effective mechanisms of energy storage, contributing to nutritional stability and thereby to more rapid development of cognitive functions and the emergence of our species [1]. Beyond evolutionary issues, it is nowadays acknowledged that nutrition, along with physical activity, are important factors influencing human health. In westernized countries, the long-term deleterious health effects of diets rich in calories, simple sugars, saturated fat, and red meat with respect to the development of cardiovascular diseases, colorectal cancer, and the metabolic syndrome are as much recognized as the virtue of eating enough portions of fruits and vegetables, although underlying mechanisms of actions remain to be described [2–4]. Positive effects of fruits and vegetables are usually attributed to high content of fiber, vitamins, and phenolic compounds (hereon defined as plant secondary metabolites with a backbone structure made of one or several phenol groups). Assuming that a substantial proportion of the dietary intake of common ancestor species consisted of plant materials, it is not surprising that, over millions of years of evolution, our body has inherited an efficient metabolic machinery to dispose of the large quantity of phenolic compounds that we still ingest daily as part of our omnivorous diet. Intestinal microorganisms are intrinsic parts of this metabolic machinery. Indeed, from an evolutionary perspective again, the human body can be considered as a supra-organism made of not only own eukaryotic cells, but also the hundred trillions of microorganisms that colonize various body sites such as the skin and the genital, respiratory and, most importantly with respect to nutrition, the gastrointestinal (GI) tract [5]. The intestinal microbiota is referred to as the assemblage of microbial communities and associated genomes (the metagenome) primarily colonizing the distal GI tract. Due to its highly diverse metabolic potential, the intestinal microbiota greatly alters the fate of phenolics in the human body by changing their structure and absorption rates in the gut, thereby influencing their biological effects.
In that context, the present chapter gives insights into the relevance of phenolic compounds in human nutrition. We will primarily discuss bioavailability and biological properties of isoflavones and lignans in the context of human health and disease, our main focus being the metabolic activities of intestinal bacteria.
2 Phenolics in Human Nutrition
Concoctions of plant products for the purpose of curing disease or sustaining health in human subjects have a long history of use, especially in traditional Chinese medicine. However, molecular mechanisms underlying positive effects have yet to be defined and traditional medicine therefore faces intense criticism [6]. Nevertheless, the emergence of systems biology approaches may help shedding light on host responses toward treatment with plant products that obviously contain a wealth of phenolic compounds [7–9]. Beyond these issues on the role of herbal treatment for improvement of human health, there is a plethora of epidemiological data highlighting beneficial effects associated with intake of food items rich in phenolic compounds. A well-known example of such food items is soy (or soy products) which contain elevated concentrations of the isoflavones daidzein and genistein as well as their glycosylated and methylated precursors (Fig. 78.1).
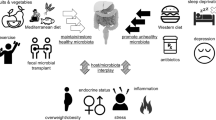
Phenolics in human nutrition: A microbiological perspective. Bioavailability of dietary compounds depends on the sum of molecular mechanisms underlying liberation of the compounds from dietary matrices, absorption, distribution into body tissues via blood circulation, metabolism (in the GI tract or target tissues), and elimination from the body. The keypad shows parameters of relevance to phenolic bioavailability. The two enlarged windows illustrate the diversity of both phenolics in food and microbial functions involved in phenolic conversion, with a focus on isoflavones and lignans. Estimates of blood concentration of daidzein, equol, and enterolignans are given in brackets (large interindividual differences are observed due to various dietary habits and ability to metabolize polyphenols). Abbreviations: ED enterodiol, EL enterolactone, GIT gastrointestinal tract, LARI lariciresinol, O-DMA O-desmethylangolensin, R residues (−H, −OH, or −CH3), SECO secoisolariciresinol, SDG secoisolariciresinol diglucoside
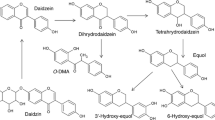
Biological properties of isoflavones were first coined in the 1940s, after infertility problems started to occur in female sheep grazing on clover pastures containing high amounts of isoflavones, and later in the 1980s in captive cheetahs fed a soy-based diet [10, 11]. These data already suggest that dietary phenolics or corresponding metabolites, such as equol, one of the two end metabolites produced by bacteria from the plant isoflavone daidzein, have the potential to interfere with highly sensitive host hormonal pathways. Since then, numerous meta-analyses and epidemiological studies including Asian populations consuming soy products on a daily basis have reported positive effects of soy intake on the development of breast cancer, bone disorders, and cardiovascular diseases [12–14].
The lignans, a family of polyphenolic compounds with a dibenzylbutane structure (Fig. 78.1), are another example of major plant phenolics relevant to human nutrition. Dietary lignans are converted to the enterolignans enterodiol (ED) and enterolactone (EL) by bacteria in the GI tract [15]. In contrast to isoflavones that occur in high concentrations almost exclusively in soy, a vast variety of food items such as flaxseeds, sesame seeds, berries (blackberry and strawberry), cereals (rye and wheat), and beverages (coffee, tea, and wine) contain detectable concentrations of lignans, which are therefore of importance in westernized diets [16]. Importantly, lignins have also been shown to be dietary precursors of enterolignans [17]. Of note, researchers originally proposed in 1980 that enterolignans were new mammalian hormones after they detected them in urinary extracts from female primates and human adults via spectrometric measurement [18, 19]. This shows that lignans share structural features with steroid hormones and are, as isoflavones, also referred to as phytoestrogens. Two years later, in 1982, the same authors reported that urinary lignans originate from food precursors [20]. Thereafter, enterolignan production has been associated with positive effects on the incidence of heart diseases as well as breast and prostate cancer [21–23].
Besides isoflavones and lignans, human food contains a wealth of phenolic compounds (Fig. 78.1). Rapid improvement in the sensitivity of analytical tools, together with the development of specific databases such as Phenol-Explorer and the USDA Flavonoid Database, have substantially contributed to the understanding of human exposure to phenolics [24]. Table 78.1 provides a non-exhaustive list of major groups of dietary phenolics and representative food sources. Depending on dietary habits, total intake of phenolics in European populations can reach up to 1 g/day or higher [26, 28, 30]. By studying dietary intake in 4,942 French adults, Scalbert et al. showed that the most dominant dietary phenolics are hydroxycinnamic acids, flavonols, and anthocyanins [30]. However, there is a direct positive association between ingested amounts of specific food products and blood concentrations of corresponding metabolites, showing that one can easily and rapidly modulate exposure to specific phenolics by modulating dietary intake. In Asian populations for instance, isoflavones intake is nearing 100 mg/day due to high intake of soy products [35]. Nevertheless, health effects of dietary phenolics do not depend solely on ingested amounts, but rather on the concentration of active compounds that reaches target tissues. In that respect, what makes lignans and isoflavones outstanding is that plant precursors are usually less biologically active so that enterolignans and equol can be seen as paradigm metabolites highlighting the relevance of bacterial activation of dietary components in the intestine. Hence, no matter which phenolics are of interest and what health effects they have, bioavailability and bacterial metabolism are matters of primary importance.
3 Bioavailability: Importance of the Intestinal Microbiota
Bioavailability refers to the proportion of absorbed doses of a molecule, and eventually metabolites thereof, which reaches sites of physiological activity. Our GI tract is of course at the front line of metabolic events regulating bioavailability due to its primary role in nutrient absorption and because oral intake is the major voluntary route of exchange with our environment (compared with passive exposure to exogenous factors via the skin and the respiratory tract). The liver and kidneys play a central role in bioavailability as well. Efficient conjugation of phenolics for the purpose of increasing water solubility, and eventually excretion, occurs in all three organs (gut, liver, kidneys) mainly via the activity of O-methyl transferases, UDP-glucuronosyltransferases, and sulfotransferases [26]. The bioavailability of dietary phenolics is thus tuned by the sum of molecular mechanisms underlying liberation from dietary matrices, absorption, metabolism (by both host and microbial cells), distribution, and excretion (Fig. 78.1).
In upper parts of the GI tract, there is a paucity of data on the fate and role of polyphenols. Their effects have been discussed in the context of oral cancer prevention [36]. Their fate in the stomach has not yet been systematically studied. Quercetin has been shown to be absorbed in the rat stomach, but only as aglycone [37]. Fast plasma appearance of anthocynins may also be explained by rapid absorption in the stomach [38]. Concerning lignans, we found that secoisolariciresinol diglucoside (SDG) (the main enterolignan precursor in flaxseed) is resistant to acid hydrolysis in vitro [39], which confirmed previous findings [40]. In the jejunum, there is good evidence that isoflavones and flavonols can be deglycosylated via lactase-phlorizin hydrolase activity and rapidly absorbed in the brush border membrane of enterocytes [41, 42]. Rat in vitro perfusion models have also been useful in demonstrating absorption of phenolic acids as well as quercetin and phloretin in the small intestine [43, 44]. However, the flavanol epigallocatechin-3-gallate can inhibit hydrolase activity in vitro, yet this inhibition is regulated by salivary proline-rich proteins [45]. This raises the question of the effect of chewing on polyphenol bioavailability via indirect or direct mechanisms such as salivary hydrolysis [46]. Plant phenolic substrates can be detected in blood and urine samples shortly after intake, which speaks in favor of rapid absorption, albeit, in low amounts. For instance, only about 2 % of the ingested dose of plant lignans was found in plasma of four individuals 1 h after intake of 50 g sesame seeds [47]. In some individuals however, plant lignans may occur in higher concentrations than enterolignans in blood samples [48]. This is also true for the isoflavone daidzein, which occurs at higher concentrations than its metabolite equol in blood samples [49], most likely because bacterial production of equol in the gut is a limiting reaction (see details in Sect. 5.1). Altogether, characterization of the metabolic network regulating phenolic bioavailability in the upper GI tract requires further investigation. In particular, very little is known about phenolic transport from gut lumen into blood stream. A recent pharmacokinetic study in human adults based on the use of equol isotopes revealed peak plasma concentrations 2–3 h after oral intake of the isotopes (350–500 ng/ml after administration of a single bolus of 20 mg) [50]. One may interpret that transport mechanisms in the gut are not region-specific, since equol is supposed to be primarily produced in distal parts of the intestine. Absorption rates of phenolics and kinetics of appearance in blood vary greatly depending on chemical structure. For instance, glucosides of quercetin (but not rhamnoglucosides) are more efficiently absorbed than aglycones [51]. However, underlying molecular mechanisms of absorption are not known. So far, only monocarboxylic acid transporters and the plasma membrane carrier bilitranslocase have been discussed for transport of phenolic acids and anthocynins, respectively [52–55]. Independently of what exactly happens in the upper GI tract, it is acknowledged that a substantial proportion of ingested polyphenols can reach the colon, where lower transit time favors bacterial conversion.
The first piece of evidence demonstrating that distal parts of the GI tract are crucial for the metabolism of phenolics is the so-called second plasma peak observed after 6–8 h postprandial when measuring phenolic metabolites in plasma samples overtime after ingestion of plant substrates [56]. Indeed, a substantial proportion of absorbed phenolics is efficiently conjugated in enterocytes and later in the liver prior to secretion back into the small intestine via the bile (enterohepatic circulation) [57]. Enterohepatic circulation thereby contributes to bacterial “re-feeding” since the bulk of glucuronidated and sulfated phenolic metabolites released in the bile can be hydrolyzed by various bacterial species [58]. Bacterial hydrolysis thus allows reabsorption of otherwise lost conjugated phenolics to be excreted in feces and thereby to delayed appearance of phenolic metabolites in the blood (second plasma peak). Another piece of evidence showing that distal gut microorganisms are crucial for phenolic metabolism is the drop in plasma and urinary concentrations of phenolics associated with alteration of intestinal microbial communities following oral antibiotic treatment [56, 59, 60]. Finally, the use of germfree mice, that is, mice that are bred in isolators under sterile conditions and are thus deprived of any living microorganisms, has provided major insights into the important role of intestinal microbial communities in shaping host physiology, including the ability to metabolize food substrates such as phenolics. To some extent, one can consider germfree mice as knockout mice, in which a multifunctional set of genes (the microbiome) has been disrupted, leading to loss of functions. Indeed, besides alteration of immune cell development [61], the absence of microorganisms in germfree animals has major impacts on energy balance [62], nutrient supply via production of short-chain fatty acids, and degradation of mucin [63] as well as phytoestrogen conversion. Enterolignans and equol, for instance, are not detectable in the intestine and body fluids of germfree rats fed phenolic-rich diets, yet gnotobiotic rats colonized with fecal suspensions from phenolic-converting human donors or with isolated active bacterial consortia regain the ability to produce active metabolites [64–67]. Taking into account that the intestinal microbial ecosystem in mammals harbors a total of up to 1014 cells belonging to more than 1,000 different species per host, each bearing approximately a few thousands of genes, it is not surprising that the absence of such diverse microbial communities is linked to disturbances in metabolic functions. In the following two sections, we will highlight specific features of intestinal microbiota that are of importance for phenolic conversion.
4 Microbial Diversity: Relevance for Phenolic Conversion
As seen above in Sect. 2, a broad array of food items contain various phenolic compounds in a wide range of concentrations (from a few micrograms up to a few hundred milligrams per 100 g), which highlights the rationale for “eating a little of everything each day” to cover supplies yet avoid adverse effects due to long-term excessive intake of a limited number of food items. With respect to chemical structure, the variety of phenolics is also quite large and the amounts ingested are driven by dietary habits, which differ markedly between individuals (Fig. 78.1). Hence, the mixture of phenolics in the intestinal lumen is determined by multiple levels of complexity and is thus highly diverse and variable.
Diversity is also a major attribute of intestinal microbial communities in mammals. Indeed, although our intestinal microbiota consists dominantly of only four of the 30 known bacterial phyla (highest taxonomic level within the superkingdom Bacteria; www.ncbi.nlm.nih.gov/Taxonomy; www.bacterio.cict.fr), namely, the Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, the diversity at low taxonomic levels (≤ genus) is very high. Most recent molecular studies refer to a few thousands different bacterial species being present in the human gut, and accordingly even more individual strains [68]. Although bacteria make up the majority of intestinal microbial populations, our intestine harbors also Archaea (two dominant methane-producing species, Methanobrevibacter smithii and Methanosphaera stadtmanae, have been described to date), eukaryotic microorganisms, such as fungi and protozoa, as well as viruses. However, the role of these microorganisms in the metabolism of phenolics is unknown (bacteriophages may, for instance, influence phenolic conversion by regulating the density of specific active bacterial populations).
As often in biology, the efficacy of one complex system (the intestinal microbiota) is greater than the sum of its biologically active parts (bacterial strains). Indeed, one key asset of the high diversity of our intestinal microbiota is that several different bacterial species can carry out one given function, such as cleaving glucose moieties of phenolics. This is referred to as “functional redundancy” (one bacterium can take over the function of another if for some reason the latter disappears). This ensures flexibility and is crucial to achieve stability and ecosystem equilibrium over time upon influence of various environmental stimuli [69]. Hence, the high diversity of our intestinal microbiota helps us cope with the high diversity of exogenous chemical compounds that we ingest. Nevertheless, in spite of this high diversity, there are a few bacterial species (50–100), and by extension a few associated bacterial functions, that make up the so-called core microbiome [70], that is, the assemblage of species/functions that are dominant (occur in high numbers) and show a high prevalence (they are found in most individuals).
Despite the notion of a core microbiome and the stability of the gut microbial ecosystem over time without major changes in dietary habits, each individual person harbors their own characteristic intestinal microbiota (in the sense of a personalized fingerprint). Indeed, there are large interindividual differences in both intestinal bacterial composition (proportion of taxa) and diversity (qualitative pattern of taxa); there is marked quantitative variation and low similarity indexes between gut samples from different individuals, even for dominant bacterial groups [68, 71, 72]. Individualized intestinal microbial patterns in adulthood are highly dependent upon a dynamic sequence of events affecting the ecosystem throughout life, especially in early life. At birth, the human body is colonized by microorganisms from the environment. Primary colonizers (aerobic or facultative anaerobic bacteria) help establish a reduced environment that is suitable for subsequent colonization by strictly anaerobic species, which largely dominate the ecosystem in adulthood. In infants below 1 to 2 years of age, the human intestinal microbiota is unstable and composition fluctuates greatly [73]. The infant gut microbiome seems not to be well equipped for efficient conversion of polyphenols. For example, equol is not detected in urine and blood samples from infants below the age of 12 months who are fed cow or breast milk [74, 75]. Delivery mode at birth (vaginal delivery vs. caesarian section) and breast versus formula milk feeding have been shown to influence microbial colonization patterns [76–79]. In early life, and very often thereafter, the intestinal ecosystem is challenged by infectious agents and antibiotic therapies. In most cases, the ecosystem shows resilience, thanks to its diversity, that is, it rapidly returns to its original state after a challenge. However, in some cases, and more likely and frequently during infancy where microbial populations are not yet fully stabilized, the ecosystem or at least specific community niches can be permanently affected [80]. Altogether, this variety of colonization and challenging events can partly explain why certain individuals harbor specific bacteria and others do not, and why the latter group therefore lacks the functions expressed by absent or subdominant bacterial species. For instance, it is well known that only about 30–50 % of human subjects produce equol from the isoflavone daidzein, meaning that one half to two thirds of human populations do not harbor equol-producing bacteria in their intestine, at least not in high enough densities [81, 82]. Likewise it has been shown that bacteria capable of catalyzing the production of enterolactone from plant lignans belong to subdominant populations, that is, they occur at densities below 108 cell/g content (compared to a total cell density of approximately 1012 cell/g) [83, 84].
In summary, our intestinal microbiome encodes numerous core functions of importance for the conversion of dietary phenolics, yet interindividual differences in the makeup of bacterial species that colonize our gut underlie interindividual differences in phenolic metabolism and thereby in possible health effects. In Sect. 5, we will give more details on active bacterial members and central metabolic reactions involved in phenolic conversion prior to focusing on health effects of isoflavones and lignans.
5 The Gut Microbiota Influences Health Effects of Phenolics
5.1 Core Bacterial Reactions and Conversion of Isoflavones and Lignans
Exceptions prove the rules: the functional diversity of intestinal microbiota implies that all plant phenolics that we ingest can be converted by microorganisms. However, there are exceptions, such as the isoflavone irilone, which seems to be resistant to bacterial conversion [85]. Gut bacteria catalyze an array of dominant core reactions that play key roles in the metabolism of a large panel of phenolic compounds, including isoflavones and lignans: (I) hydrolysis of esterified and conjugated bounds, (II) deglycosylation (removal of sugar moieties), (III) demethylation (substitution of a methyl by a hydroxyl group), (IV) dehydroxylation (reduction of hydroxyl groups), (V) dehydrogenation, (VI) reduction. Figure 78.1 gives a brief overview of the so far identified bacterial species that catalyze these reactions [86, 87]. It is noteworthy that several species occur in proximal parts of the bowel (Enterobacteriaceae, lactobacilli, lactococci, and streptococci in the stomach and small intestine), showing that bacterial metabolism of phenolics may be crucial not only in the colon, but already before, for example, for hydrolysis of conjugated metabolites secreted in the bile. Most dietary phenolics occur as biologically inert polymers or glycosides, meaning that reaction type I and II are crucial for phenolic activation and influence downstream reactions such as demethylation. As a matter of fact, the production of one given active metabolite often results from sequential reactions involving several bacterial species. For instance, production of enterolignans from SDG requires four reactions, among which demethylation and dehydroxylation are catalyzed only if the substrate has been previously deglycosylated and demethylated, respectively [15, 88]. Reaction type III (demethylation) is also crucial with respect to biological activities since most plant phenolics are methylated and are less active than hydroxylated metabolites. This is obvious, for instance, in the case of caffeic acid phenyl ester (an active phenolic constituent of honeybee propolis), for which we found that methylation of catechols markedly reduces anti-inflammatory activities [89].
The physiological advantage for bacteria to convert phenolic compounds is easily understandable in the case of deglycosylation (active species can utilize released glucose moieties as carbon and energy sources) or demethylation (acetogenic bacteria, for instance, are capable of producing energy by incorporating methyl groups into the Wood-Ljungdahl pathway of acetogenesis). In contrast, it is more difficult to identify driving forces that led to the establishment of complex phenolic-converting metabolic chains involving various distantly related bacterial species. One simplistic way to assess such a complex system is to try gaining access to individual bacterial components of the metabolic chain by means of anaerobic cultivation for subsequent in vitro characterization. Indeed, the isolation of pure bacterial cultures, in combination with the use of biochemical techniques (high performance liquid chromatography and mass spectrometry), for the purpose of metabolite identification allows description of key bacterial players in phenolic metabolism, including subdominant bacterial populations [15, 90]. Microbiologists have been culturing microorganisms for a long time, rapidly leading to major breakthroughs in biomedical research such as the identification of Mycobacterium tuberculosis by Robert Koch in 1876 or the discovery of the antibiotic penicillin by Alexander Fleming in 1928. In contrast, it is only from the 1950s onwards that the development and use of anaerobic tools by pioneers such as René Dubos, Sydney Finegold, Lillian Holdeman, Robert Hungate, Edward Moore, and Russel Schaedler gave rise to extensive culture-based work dealing with commensal bacterial communities from human intestinal samples [91–93].
In 1985, Borriello et al. were the first to study the conversion of plant lignans by fecal slurries in detail [94], yet active bacterial strains were first isolated in 2000 [88]. In the case of phenolic acids, which as mentioned above are dominant phenolics in human diet, knowledge of bacterial conversion and involved species is scant. Hydroxycinnamates (e.g., p-coumaric, ferulic, and sinapic acid) as well as benzoic acids (e.g., gallic, syringic, and vanillic acids) are rapidly degraded by intestinal bacteria and a few members of the Firmicutes are known to demethylate a variety of phenolic acids [39, 86]. Actually, this is the case of isoflavones that rapidly drew most of the attention of microbiologists working in the field of polyphenols. Reasons for this are the low proportion of equol producers among humans (30–50 %) and the fact that equol is the most potent known isoflavone metabolite. Researchers have thus embarked on a microbial “Gold Rush” attempting to isolate and identify those rare equol-producing bacteria that colonize the human gut. The first evidence for microbial equol production was published in 1995 [81], however the first equol-producing bacterium, strain Julong 732, was isolated in 2005 (and so far this isolate is still not taxonomically classified) [95]. To date, a total of 16 daidzein-converting strains have been identified (Table 78.2).
From this listing, it is obvious that proper taxonomic description is needed, as some of the isolates could belong to the same species. It is also striking that all equol-producing bacteria with a validly published name are members of the family Coriobacteriaceae. This hints at functional specialization in the gut, maybe contributing to the better survival of this bacterial group in the competitive intestinal milieu. Interestingly, some Coriobacteriaceae, such as Eggerthella spp., are dominant intestinal bacteria and can convert steroid hormones and biliary acids [58]. This shows again that core functions such as dehydroxylation are relevant to various substrates and raises the question on the influence of host hormonal status on polyphenol metabolism [115].
A major advantage of culture-based approaches is that isolated strains can be used in vivo to assess physiological roles of phenolic-converting bacteria (in e.g., germfree mice) or in vitro for isolation and characterization of active enzymes. So far, very few corresponding data have been published. Crude enzyme extracts from Asaccharobacter celatus converts daidzein to dihydrodaidzein under anaerobic conditions and a dihydrodaidzein-producing reductase from lactococci has already been cloned (UniProtKB E1CIA4 and E7FL40/1) [103, 116]. However, culturing is per definition restricted to the study of microorganisms able to be isolated and to grow in the laboratory (most recent estimation refers to a proportion of 60 % cultivable bacteria in the mouse intestine) [62]. Again, it is important to remember that one given reaction can be catalyzed by several phylogenetically distantly related bacteria, which highlights the notion of functional bacterial groups and the importance of considering intestinal microbiota as a dynamic pool of functions rather than an assemblage of taxonomic entities. A more comprehensive way to assess the bacterial conversion of phenolics at the level of the entire ecosystem (the pool of microbial functions) than culturing is to use metagenomic techniques, i.e., molecular tools dedicated to the study of the metagenome (the sum of genomes originating from the thousands of bacterial species colonizing the intestine) [68, 117]. For instance, culture- or PCR-based screening of gut metagenomic clone libraries can give direct access to bacterial genomic information involved in conversion of phenolics, metagenomic libraries being defined as collections of >10,000 Escherichia coli clones where each clone expresses functions encoded on one large DNA fragment (commonly 40,000 bp) from the gut metagenome. As an example, metagenomic clones can be cultured on agar plates containing a glucosylated phenolic substrate as sole carbon and energy source, an approach that has been already used with other kinds of substrates such as β-glucans [118]. In such an assay, only clones capable of utilizing the substrate would grow and could be further analyzed by sequencing for determination of active gene sequences. Alternatively, colorimetric reactions may also be used for detection of for instance phenolic-demethylating clones [119].
One additional key issue in the field of bacterial enzymatic conversion of polyphenols is enantiospecificity. Many polyphenols, such as isoflavones and lignans, are optically active molecules that display several asymmetric carbon atoms. So far, only S-equol has been detected as a bacterial product of daidzein conversion [95, 120]. In the case of lignans, both (+)- and (-)-enantiomers occur in plants and bacterial conversion in the gut seems to be enantiospecific and preserve absolute configuration [121]. There is strong evidence that biological activity depends upon chirality of equol [122, 123], stressing the need for stereochemical analysis of other phenolic metabolites produced by intestinal bacteria. This serves as further proof of the necessity to isolate phenolic-converting bacterial enzymes for potential biotechnological production of active metabolites [103]. Finally, the search for new bacterial metabolites (and determination of corresponding biological properties) is also of primary interest. Considering the diversity of both dietary phenolics and intestinal bacterial species, it is likely that the panel of intermediate and end metabolites produced by intestinal bacteria is much larger than hitherto observed. For instance, we have found that the lignan-dehydrogenating bacterium Lactonifactor longoviformis does not only produce enterolactone, but also the novel metabolite 2,3-bis(3,4-dihydroxybenzyl)butyrolactone, the occurrence of which in vivo along with biological activities is still to be determined [121].
In summary, the array of enzymatic reactions catalyzed by the gut microbiome alters the structure of ingested phenolics. In view of the notion of structure/activity relationship, we conclude that intestinal bacteria greatly influence the biological activities of dietary phenolics. In the case of the isoflavone daidzein the route of bacterial conversion (i.e., the production of equol or O-desmethylangolensin depending on gut bacterial composition), is key to downstream health effects (Fig. 78.1). In the following two sections, we will give detailed information on biological activities and potential health effects of isoflavones and lignans.
5.2 Health Effects of Isoflavones and the Bacterial Metabolite Equol
In recent reports, the European Food Safety Authority (EFSA) refuted claims about the role of isoflavones in body function effects (article 13.1) such as maintenance of normal blood LDL-cholesterol concentrations in the general population [124, 125]. This has two main implications (also true beyond the sole case of isoflavones): first, even when scientific rationale is sound and there is a substantial number of well-conducted studies showing an overall significant trend toward positive effects of a defined dietary compound, a major problem in nutrition research is that intake of definite food stuff may need to stretch over long life periods before one can observe significant effects, when compared, for example, with pharmacological products usually associated with instant target effects (even though long-term effects of pharmacological therapies are often also not determined, yet beneficial immediate effects indeed prevail). Thus, the preventive aspect of nutritional strategies implies to carry out studies at scales (both in terms of time and cohorts) virtually impossible to manage in order to substantiate beneficial effects. This very often hampers closing the gap between scientific evidence and clear recommendations for consumers. The second implication is that, whereas it is very difficult to corroborate findings for the “general population,” it makes sense to look at health effects of isoflavones in sensitive target groups, like infants. For these reasons, this is not our intention to provide here an exhaustive review of possible health effects of isoflavones. Instead, we will focus our attention on osteoporosis affecting menopausal women and on the effect of early exposure to isoflavones, thereby highlighting the biological properties of the bacterial metabolite equol.
Infants make up a study population of particular interest for several reasons: (1) they have not yet necessarily acquired a fully functional phenolic-metabolizing machinery (at least from a microbiological perspective), (2) the use of soy-based infant formula has become a rather common feeding alternative in westernized countries, and (3) a growing human body may be particularly sensitive to the biological properties of isoflavones. There are several published papers showing that early exposure to isoflavones has the potential to influence hormone levels and organ differentiation in the offspring of various animal species [126–129]. For instance, male marmoset twin monkeys fed soy formula milk for 30–40 days from the age of 5 days were characterized by lower mean testosterone levels in blood samples [129]. However, long-term effects must be further investigated. Furthermore, caution must be taken when interpreting results obtained using doses higher than the estimated intake of 2–10 mg isoflavones per day per kilogram body weight in infants fed soy-based formula [130, 131]. Exposure of human infants to dietary isoflavones has drawn attention of researchers since the mid-1990s. Depending on studies, isoflavone concentrations in soy-based infant formula range from 30 to 280 mg/kg [131–133]. Setchell et al. found that mean plasma concentrations of both genistein and daidzein in seven infants fed soy-based formula were 979 ng/ml (approximately 4 μmol/l) [131]. This concentration was markedly higher than in infants fed either cow-milk formula (5.3 ng/ml) or human breast milk (4.2 ng/ml), and is also higher than in adults on their usual diet. Interestingly, infants can also be exposed to isoflavones via breast milk during lactation. In seven breastfeeding mothers, ingestion of 55 mg/day isoflavone glucosides for 2–4 days increased isoflavone concentrations significantly in breast milk (from ca. 5 to 70 nmol/l) and in infant urine (from ca. 30 to 110 nmol/mg creatinine) [134]. Hence, it is clear that infants can be exposed to relatively high isoflavone concentrations and experimental work shows some significant effects of early exposure to isoflavones in animals. However, there is an obvious lack of physiological evidence in humans, as underlined in recent review papers and human infant trials [135–139].
The rationale for considering possible health effects of dietary isoflavones in infants is substantiated by in vitro and in vivo work on their biological properties. Especially, the estrogenic-like properties of isoflavones have been studied as early as in the 1950s based on the mouse uterine weight method [140], 30 years before equol was first detected in human urine [141]. Among daidzin metabolites, equol has the strongest binding affinities to estrogen receptors (ER), especially for ER-β [122, 142, 143]. Nevertheless, 17β-estradiol is 10–100 times more potent than equol. Interestingly, the R- and S-enantiomer of equol exhibit different binding affinities for ER-α (0.5 vs. 2 % of 17β-estradiol binding, respectively) or ER-β (1 vs. 20 %) [122]. Beyond binding affinities, equol can also modulate ER transcriptional activity [142, 144, 145]. Very recently, induction of estrogenic responses by equol has been demonstrated in vivo using the 3xERE-luciferase mouse model, which allows detection of estrogen activity by light production [146]. On the other hand, isoflavones have the potential to reduce estradiol bioavailability by increasing levels of circulating sex hormone–binding globulin [147, 148]. Obviously, the pro- or anti-estrogenic activities of equol depend on circulating concentrations of estradiol, which markedly vary during puberty, menstrual cycle, and menopause. Isoflavones concentrations in blood may reach up to a maximum of 10 μmol/l after ingestion of phenolic-rich food, which exceeds blood concentration of estradiol by a factor of > 10,000 [149]. Interestingly, tissue accumulation of polyphenols (including isoflavones and lignans) has been reported, which likely contributes to modulation of biological properties in target tissues [150–153].
In spite of the aforementioned properties of equol, its direct contribution to health effects is unclear. From the complex metabolite mixtures found in blood and target tissues after soy intervention, it is impossible to relate effects to only one specific molecule. Still, discoveries from the last decade may form the basis of future research to assess the exact role of equol in mediating health effects. Indeed, the fact that single equol-producing bacterial strains are now available allows the design of gnotobiological experiments using animal model of diseases. In such experiments, germfree animals colonized with an equol-producing or non-producing bacterium (a closely related inactive species or a mutant strain in which active enzymes have been knocked-out) could be compared with respect to the development of, for instance, tumors in various tissues or bone disorders in response to ingestion of daidzein-rich diets. In addition, large-scale production of pure enantiomers of equol for use in experimental or even clinical studies will surely help in deciphering direct health effects and underlying molecular mechanisms (US Patent no. 7528267 and 6716424).
To follow up on phytoestrogenic activities of isoflavones in vivo, a number of studies have looked at the effect of soy consumption on fertility parameters in adults. Again, there is evidence in animal species [154, 155], but very few data in human [156]. Alteration of semen quality by soy food or isoflavones is questionable [157, 158] and a recent meta-analysis of 15 placebo-controlled studies concluded that soy or isoflavone consumption is not associated with changes in testosterone levels in healthy men [159]. In contrast, peri- and postmenopausal women represent a target population of particular relevance. We will here focus only on the effect of isoflavones on osteoporosis, which has been intensively studied in postmenopausal women and represent a major public health problem [160]. Readers interested in the effects of isoflavones on cardiovascular risks and breast cancer may refer to already published comprehensive papers [13, 14, 161–163]. Osteoporosis is characterized by low bone mass, deterioration of bone tissue, and disruption of bone microarchitecture resulting in compromised bone strength and increased fracture risk [160]. The diagnosis of osteoporosis is primarily established by measurement of bone mineral density (BMD) [164]. Of course, genetic factors determine peak bone mass. However, studies involving twins indicate that environmental factors, including dietary habits, play a substantial role in the pathogenesis of osteoporosis [165]. Again, the EFSA refuted claims related to the use of soy isoflavones for maintenance of BMD [125]. This highlights the difficulty to reach consistency in experimental setups required for drawing conclusion on definite intake of isoflavones associated with long-term health benefits. Nevertheless, there is a growing body of valid scientific data showing overall that beneficial effects of isoflavones on bone disorders in elderly women are promising [161]. In two recent meta-analyses [166, 167], Ma et al. selected randomized controlled trials (RCT) investigating the effects of soy isoflavones on BMD and markers of bone turnover in peri- and postmenopausal women. Based on a total of 19 RCT with an intervention period of 1–24 months and isoflavone intake of 4–150 mg/day, the authors concluded that isoflavone intervention significantly attenuates bone loss of the spine in menopausal women, inhibits bone resorption, and stimulates bone formation. These results were confirmed by even more recent meta-analyses [168, 169]. However, it must be acknowledged that most studies are not appropriate for assessment of soy isoflavone consumption for more than 1 year [12]. Thus, one major remaining challenge is to characterize long-term clinically relevant effects of isoflavones prior to making statements on their use in hormone replacement therapies [170]. In a very recent double-blind RCT, Tai et al. found that treatment with 300 mg/day isoflavones for 2 years did not prevent decline of BMD in lumbar spine and proximal femur in postmenopausal Taiwanese [171].
5.3 Health Effects of Enterolignans
As for isoflavones, there is a vast number of studies investigating various biological properties and potential health effects of lignans [115]. There is good experimental evidence that lignans are beneficial with respect to the development of cardiovascular diseases and breast cancer. It is obvious however that RCT in human subjects are lacking. Interventions based on the use of flaxseeds as main lignan source have revealed promising effects with respect to reduction of prostate cancer proliferation [172, 173], tumor growth in breast cancer patients [174–176], and low-density lipoprotein (LDL) cholesterol levels [22, 177]. In addition, recent data from the EPIC study (European Prospective Investigation into Cancer and Nutrition) suggested that lignan intake decreases colon cancer risk in women [178]. However, because flaxseeds contain substantial amounts of fibers and oil, it is not possible to distinguish between direct effects of lignans and confounding or synergistic effects of fibers and oil. We will thus focus hereafter only on studies assessing health effects that can be attributed to pure lignans converted in vivo to the enterolignans ED and EL by gut bacteria. Unfortunately, viewed from that perspective, the number of human intervention trials shrinks further away. We found only two different double-blind RCT, in which authors analyzed the effect of flaxseed extracts enriched in SDG (ca. 30 % dry mass). Hallund et al. found that an intervention with 500 mg/day SDG equivalent for 6 weeks in 22 healthy postmenopausal women marginally reduced C-reactive protein concentrations and had no effect on endothelial function and plasma lipid concentrations [179–181]. In another trial involving 78 subjects with benign prostatic hyperplasia, ingestion of a flaxseed lignan extract (>300 mg/day SDG equivalent) over a 4-month period significantly improved International Prostate Symptom and Quality of Life Scores [182]. It is thus again in laboratory animals that most of the beneficial effects of pure lignans have been reported. In rodents, Lilian Thompson and colleagues found that SDG reduces or delays mammary tumor growth [183–185], affects mammary gland structure [186, 187], reduces metastasis in the lung [188] as well as colon carcinogenesis (number of aberrant crypt foci after azoxymethane treatment) [189]. In contrast, matairesinol and secoisolariciresinol did not protect against intestinal tumor formation in Min mice [190]. More recently, lariciresinol was found to attenuate mammary tumor growth in xenograft- and carcinogen-induced rat models [191]. With respect to cardiovascular risks, SDG was found to reduce the incidence of atherosclerosis in rabbits and to induce neovascularization-mediated cardioprotection in rats [192–194].
Biological properties underlying the aforementioned protective effects of lignans are not well characterized, especially in vivo. As stated in Sect. 2, plant lignans are usually less active than enterolignans, which are thus seen as paradigm metabolites for the relevance of bacterial conversion. In vitro studies showed that EL has slightly higher binding affinity for the human pregnane X receptor, which mediates induction of enzymes involved in steroid metabolism and xenobiotic detoxification, than its precursor secoisolariciresinol [195]. Moreover, EL binds to estrogen receptors, with a preference for ER-α [142, 196], and can activate estrogen responsive elements [197]. Both ED and EL modulate ER-α mRNA and protein contents and compete dose dependently with estradiol and the unsaturated fatty acid arachidonic acid for binding site on rat and human α-fetoprotein, an estradiol-binding protein [198, 199]. However, binding affinities of enterolignans appear to be 10–10,000-fold lower than those of other phytoestrogens or sex hormones. Both enterolignans and plant lignans also bind to sex hormone–binding globulin, with possible consequences on circulating levels of the sex hormones testosterone and estradiol [200]. The estrogen-dependent properties of ED and EL include as well inhibition of aromatase, 5α-reductase, and 17β-hydroxysteroid dehydrogenase, three enzymes involved in the metabolism of growth-promoting steroid hormones [201–204]. Besides, EL was found to induce the expression of the estrogen-responsive protein pS2 in human breast cancer MCF-7 cells [205]. This and other in vitro studies showed that ED and EL alter cell proliferation of various breast, colon, and prostate cell lines, as well as endothelial cells derived from bovine brain capillaries [206–210]. In vitro, both ED and EL have also higher antioxidant activities than plant precursors [211, 212]. In vivo, short-term feeding of SDG to rats only led to minor changes in the antioxidant status of hepatic tissue [213].
To conclude on the last two sections on health effects, one can say that polyphenols are generally regarded as safe and there are only a few reports on possible toxic effects (yet not in the case of isoflavones and lignans in humans) [214–217]. However, polyphenols have the potential to interact with sensitive hormonal systems. Moreover, as implied above when discussing bioavailability, efficient conjugation and excretion mechanisms as well as relatively low phenolic concentrations in blood (<200 nmol/l without intervention [51, 218, 219]), when compared with other molecules of dietary origin (sugars, amino acids, acetate, etc …), are hallmarks of efficient host metabolism dedicated to the elimination of exogenous molecules. Thus, one should not presume that biological properties of phenolics are solely synonyms of beneficial effects, for example, equol may trigger hyperplasia of rat uterine tissue [220] and lignans have been shown to affect pregnancy outcome, reproductive development, and estrous cycling in rats and women [221–223].
Isoflavones are promising with respect to improvement of osteoporosis in postmenopausal women, but long-term effects and dose/activity relationship must be further investigated. Regarding lignans, data obtained using animal models of cancer and cardiovascular disorders are promising too. However, there is a paucity of data in human subjects. In both cases (isoflavones and lignans), direct in vivo effects of bacterial metabolites is a future research area of particular interest.
6 Impact of Phenolics on Intestinal Microbiota
One fundament of intestinal ecosystems is the trialog between dietary components, intestinal microorganisms, and the host. Over the last century, medical microbiology had been a dominant field of research and the focus was mainly placed on the study of bacteria-host interactions. However, over the last 20 years, the impact of nutrition on human health and the intestinal microbiome has gained a lot more attention in westernized countries [5]. This is mainly due to: (1) research-founded breakthroughs (molecular mechanisms underlying benefits or deleterious effects of specific dietary molecules are being described); (2) shifts in public health challenges and mentalities (while many bacterial infections are no major threat anymore, chronic disorders such as allergies, obesity, and inflammatory diseases in an ever-aging population represent an increasing social and economical burden; meanwhile, many people are concerned about self-improvement of well-being via nutrition); and (3) market-driven issues (global food companies are lured by profits associated with massive consumption of functional foods and nutraceuticals).
There is nowadays strong evidence that diet greatly influences the composition of intestinal microbiota. The most studied dietary components having striking effects on microbial diversity are fat and fibers [224, 225]. In contrast, the effect of dietary microcomponents like polyphenols on intestinal microbiota is much less known, in spite of various possible mechanisms of actions. First of all, the fact that the conversion of phenolics is under the control of bacterial metabolic chains means that any substrate affecting one chain link has the potential to alter the entire system. Secondly, there is good indication that phenolic extracts and pure phenolics have antimicrobial properties and may thereby alter the growth of intestinal bacteria like clostridia, bacilli, and members of the Enterobacteriaceae [226–229]. In addition, since gene expression of enzymes catalyzing, for instance, dehydroxylation can be induced by matching substrates [230], it is possible that polyphenols directly influence core gut microbial functions. At the same time, the growth of phenolic-metabolizing bacteria may be favored if conversion provides a net energy input for the bacteria. This could lead in parallel to increased competitive advantage and thus indirect growth inhibition of other bacterial groups. Finally, certain polyphenols or metabolites thereof may interfere with quorum sensing, a molecular system that coordinates gene expression of, for instance, virulence factors according to bacterial cell density [226–229]. In spite of these mechanisms, which must still be substantiated by further investigations, there is to the best of our knowledge only 11 papers reporting effects of phenolic compounds on intestinal microbiota. In vitro experiments showed that incubation of fecal slurries with tea extracts prevented growth of clostridia [231]. Possemiers et al. showed that the hop prenylflavonoid isoxanthohumol increased the abundance of members of the Clostridium cluster XIV as well as bifidobacteria in a continuous culture system [232]. In rats, Hanske et al. found that xanthohumol does not affect the diversity of dominant fecal microbial communities, as analyzed by denaturing-gradient gel electrophoresis [233]. Smith et al. reported that a diet rich in proanthocyanidins increased the occurrence of Enterobacteriaceae and Bacteroides in rat feces [234]. The remaining papers relate to human intervention trials. Tea polyphenols increased viable counts of bifidobacteria and decreased counts of Clostridium perfringens in eight Japanese healthy adults [235], but had no major impact on fecal microbiota in six hypercholesterolemic volunteers [236]. We found in 2005 that a dietary treatment with 100 mg isoflavones per day for 1 month altered the bacterial diversity and composition in fecal samples from 39 postmenopausal women [71]. Very recently, Tzounis et al. found that a diet rich in cocoa-derived flavanols (494 mg/day) consumed for 4 weeks by 22 healthy human volunteers increased the proportion of lactic acid bacteria by a factor of two, as measured by in situ hybridization [237]. The same authors had previously reported that 150 mg/l of the flavanol monomers epicatechin and catechin stimulated growth of bifidobacteria, E. coli and members of the Firmicutes in vitro [238]. With respect to bacterial activities, Wiseman et al. found that soy consumption for 10 weeks increased beta-glucosidase activity in feces from 76 healthy young adults [239]. Finally, Hoey et al. reported two- to tenfold lower counts of bacteria in feces from ten infants (aged 4–12 months) fed a soya- versus milk-based formula [74]. However, the fecal concentration of total short chain fatty acids (ca. 45 μmol/g) as well as beta-glucosidase and glucuronidase activities (both ca. 10–25 μmol/h per g) were unchanged.
Bottom line is that the amount of data is too limited to draw firm conclusions on the impact of phenolics on intestinal microbiota. The task ahead is challenging due to the diversity of phenolics in food as well as interindividual intestinal microbial profiles. The use of next generation molecular approaches will be crucial for the identification of core responses to dietary phenolics at the level of the entire gut microbial ecosystem. High-throughput 16 S ribosomal RNA sequencing allows for instance in-depth characterization of changes in bacterial diversity. However, it will be essential to translate the meaning of such structural changes for host health development, since microbial functions are the driving force of bacteria-host interactions and changes in diversity are not necessarily linked to changes in ecosystem functions. Ecological approaches, such as metatranscriptomic or metabolomic, for gene or metabolite expression profiling could be used for identification of core microbial functional markers under the influence of dietary phenolics [240].
7 Can We Potentiate Intestinal Microbial Metabolism?
The pace of research involving phenolic compounds has rapidly increased over the last two decades (Fig. 78.2). One obvious underlying reason is the wish to prevent or cure diseases by means of natural products. Since intestinal bacteria are essential for phenolic bioavailability and associated health effects, nutritional strategies favoring production of active metabolites via the microbiome look very attractive. As seen above, the use of antibiotics is the best proof-of-concept that influencing metabolite production by targeting intestinal microbial communities is promising [56, 60, 241–244]. However, there is to date no valid data substantiating the theory of diet-driven optimization of microbial phenolic conversion.
Publication output in the field of phenolic research and intestinal microbiota. The PubMed database of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/pubmed) was searched for the number of articles per year (from 1980 until 2010) responding to the following queries: “intestinal microbiota” (black diamond) and “phenolics OR phytoestrogens OR polyphenols” (gray squares)
The link between intake of specific dietary components and phenolic metabolite production is unclear. Although increased excretion of equol has been associated with increased consumption of fat, meat, and fruits, for instance [82, 152, 245–247], and enterolignans excretion seems to correlate well with dietary intake of fibers [248–250], more work is needed to reach consensus in results. Nonetheless, it is clear that ingestion of isoflavone and lignan food substrates enhance production of equol and enterolignans [33, 251–253]. An intriguing question is however to know whether the activity or growth of phenolic-activating bacteria can be specifically induced, that is, in the case of equol, for example, whether non-equol producers on their usual diet can become producers, thanks to ingestion of appropriate plant substrates. In 12 Caucasian postmenopausal women, Védrine et al. found that isoflavone intervention (100 mg/day) increased plasma equol concentrations from 0.31 to 0.99 μmol/l in equol producers, but that the seven volunteers classified as non-equol producers did not acquire the ability to produce equol after 1 month exposure [251]. In contrast, another study in China revealed a higher proportion of equol producers among 200 healthy adults challenged with a soy-isoflavone supplement for 3 days (60 % equol producers after supplementation vs. 27 % at baseline) [254]. Here too, more work is needed to draw firm conclusions on equol phenotype changes and influence of demographic origin on phenolic bioavailability [245, 255, 256].
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit to the host [257]. Their use has drawn quite some attention for improvement of phenolic activation in the gut based on the rationale that glucosidases from probiotic lactic acid bacteria (mainly lactobacilli and bifidobacteria) may enhance phenolic bioavailability by increasing concentrations of aglycones. However, the dominance of endogenous phenolic-deglycosylating Bacteroides, Bifidobacterium, and Clostridum spp. in the intestine suggests that deglycosylation is not a limiting step in the in vivo production of active metabolites. Moreover, while there are many reports on the fermentation of soy products by probiotic bacteria, all ten intervention trials based on soy and probiotic treatment in human subjects failed to demonstrate any positive effects of probiotic bacteria [258–267]. Concerning lignans, the only one study available also failed to show any beneficial probiotic effects [268]. More interestingly, researchers in the group of Willy Verstraete at Ghent University have successfully used phenolic-converting bacteria originating from the human intestine, such as Eubacterium limosum catalyzing demethylation, to enhance the activation of isoflavones and isoxanthohumol in continuous culture systems and in rats [269, 270].
Functional food products also include prebiotics like fructooligosaccharides (FOS) and inulin, which are nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon [271]. The prebiotic concept was first coined in 1995 by Glenn Gibson and the bifidogenic effect of FOS and inulin has since then been confirmed by many studies. In contrast, there are only a few reports on the influence of prebiotics on isoflavone bioavailability. Steer and colleagues showed in vitro that 10 g/l FOS in combination with soyabean isoflavones significantly prevented genistein breakdown in continuous culture system vessels [272]. Similar results were obtained by Piaza et al. using inulin in a randomized double-blind crossover study enrolling 12 healthy postmenopausal women [273]. The authors found increased plasma concentration of daidzein and genistein after inulin treatment (approximately 7 g/day) for 21 days. Possible synergistic effects of combined isoflavone and prebiotic intervention are of particular interest with respect to health parameters such as blood lipid profiles or bone density and calcium homeostasis [274–276].
8 Conclusion
The issue of phenolics in human nutrition bears resemblance to industrialized production factories, where input of raw materials is important, yet processing strategies determine the quality of final products. That is, the amount of phenolics that we eat makes of course a difference, but metabolism within the body determines their fate and health effects. Future prospects related to phenolic bioavailability (especially bacterial metabolism) that have been evoked throughout the chapter are summarized in Table 78.3.
Intestinal microbial functions are essential for conversion of a vast majority of dietary phenolics, for example, isoflavone and lignan activation. The main future challenge for microbiologists working in the field of phenolics and human nutrition is to characterize metabolic networks at the level of the entire intestinal ecosystem, in relation to host functions. New generation molecular techniques will certainly help taking on this challenge, although computer analysis of the colossal amounts of data generated by high-throughput methods is a high hurdle for most microbiologists. It would be valuable, for instance, if large-scale human intervention trials on phenolics were designed so as to include microbiological analysis of intestinal samples via, for instance, sequencing or spectrometry analysis to characterize bacterial diversity and identify core functions of relevance to phenolics. This could lead to the discovery of phenolic-specific enterotypes, as in the sense of specific clusters of microbial species associated with functional profiles of relevance [277], thereby allowing detailed characterization of interindividual differences. The long-term objective is the ability to generate personalized meta-metabolic profiling for development of individualized nutritional strategies [278].
In view of individualized nutritional strategies, we must also say that, even though the focus of the present chapter is the metabolic potential of intestinal microorganisms, host genotype strongly determine health effects of phenolics too. Thus, a challenging task is also to assess the role of host genotype in controlling phenolic health effects, either directly via differential expression of specific key genes (coding for ER or intestinal transporters for instance) or indirectly via alteration of microbiota [279].
Finally, it is important to remember that early life periods are critical for shaping the intestinal microbiome. More effort should be put into characterizing intestinal microbiota development in infants and the implication of early dietary exposure to phenolics for health homeostasis later in life. To date, it is also not possible to provide clear recommendations with respect to dietary intake of isoflavones or lignans for treatment or prevention of diseases. Nevertheless, good evidence has been accumulating regarding improvement of bone disorders in postmenopausal women by isoflavones and cardiovascular risks as well as breast cancer by lignans. More clinical and epidemiological data are mandatory and effort should be put into performing experiments in gnotobiotic animal models of disease to draw firm conclusions on the direct role of bacterial metabolites, such as equol and enterolignans, in host health.
Abbreviations
- BMD:
-
Bone mineral density
- DNA:
-
Deoxyribonucleic acid
- E. coli :
-
Escherichia coli
- ED:
-
Enterodiol
- EFSA:
-
European Food Safety Authority
- EL:
-
Enterolactone
- ER:
-
Estrogen receptor
- FOS:
-
Fructooligosaccharides
- GI:
-
Gastrointestinal
- LDL:
-
Low-density lipoprotein
- PCR:
-
Polymerase chain reaction
- RCT:
-
Randomized controlled trials
- SDG:
-
Secoisolariciresinol diglucoside
References
Leonard WR, Snodgrass JJ, Robertson ML (2007) Annu Rev Nutr 27:311
Sluijs I, van der Schouw YT, van der AD, Spijkerman AM, Hu FB, Grobbee DE, Beulens JW (2011) Am J Clin Nutr 92:905
Gonzalez CA, Riboli E (2011) Eur J Cancer 46:2555
Crowe FL, Roddam AW, Key TJ et al (2011) Eur Heart J 32:1235
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI (2011) Nature 474:327
Qiu J (2007) Nature 448:126
Fardet A, Llorach R, Martin JF, Besson C, Lyan B, Pujos-Guillot E, Scalbert A (2008) J Proteome Res 7:2388
van Wietmarschen H, Yuan K, Lu C et al (2009) J Clin Rheumatol 15:330
Ehrman TM, Barlow DJ, Hylands PJ (2007) J Chem Inf Model 47:2316
Messina M (2010) J Nutr 140:1350S
Setchell KD, Gosselin SJ, Welsh MB, Johnston JO, Balistreri WF, Kramer LW, Dresser BL, Tarr MJ (1987) Gastroenterology 93:225
Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM (2009) Bone 44:948
Anderson JW, Bush HM (2011) J Am Coll Nutr 30:79
Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC (2009) Am J Clin Nutr 89:1145
Clavel T, Henderson G, Engst W, Dore J, Blaut M (2006) FEMS Microbiol Ecol 55:471
Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (2005) Br J Nutr 93:393
Begum AN, Nicolle C, Mila I et al (2004) J Nutr 134:120
Setchell KD, Lawson AM, Mitchell FL, Adlercreutz H, Kirk DN, Axelson M (1980) Nature 287:740
Stitch SR, Toumba JK, Groen MB, Funke CW, Leemhuis J, Vink J, Woods GF (1980) Nature 287:738
Axelson M, Sjovall J, Gustafsson BE, Setchell KD (1982) Nature 298:659
McCann MJ, Gill CI, McGlynn H, Rowland IR (2005) Nutr Cancer 52:1
Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X (2009) Am J Clin Nutr 90:288
Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J (2010) Am J Clin Nutr 92:141
Scalbert A, Andres-Lacueva C, Arita M, Kroon P, Manach C, Urpi-Sarda M, Wishart D (2011) J Agric Food Chem 59:4331
Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventos RM et al (2010) J Am Diet Assoc 110:390
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Am J Clin Nutr 79:727
Milder IE, Feskens EJ, Arts IC, Buenode Mesquita HB, Hollman PC, Kromhout D (2005) J Nutr 135:1202
Ovaskainen ML, Torronen R, Koponen JM, Sinkko H, Hellstrom J, Reinivuo H, Mattila P (2008) J Nutr 138:562
Perez-Jimenez J, Neveu V, Vos F, Scalbert A (2010) J Agric Food Chem 58:4959
Perez-Jimenez J, Fezeu L, Touvier M, Arnault N, Manach C, Hercberg S, Galan P, Scalbert A (2011) Am J Clin Nutr 93:1220
Ritchie MR, Cummings JH, Morton MS, Michael Steel C, Bolton-Smith C, Riches AC (2006) Br J Nutr 95:204
Chun OK, Chung SJ, Song WO (2007) J Nutr 137:1244
Kuijsten A, Arts IC, van’t Veer P, Hollman PC (2005) J Nutr 135:2812
Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD (2006) J Nutr 136:45
Kimira M, Arai Y, Shimoi K, Watanabe S (1998) J Epidemiol 8:168
Petti S, Scully C (2009) J Dent 37:413
Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C (2002) J Agric Food Chem 50:618
Passamonti S, Vrhovsek U, Vanzo A, Mattivi F (2003) FEBS Lett 544:210
Clavel T, Borrmann D, Braune A, Dore J, Blaut M (2006) Anaerobe 12:140
Mazur W, Fotsis T, Wahala K, Ojala S, Salakka A, Adlercreutz H (1996) Anal Biochem 233:169
Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA (2003) Eur J Nutr 42:29
Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G (2000) FEBS Lett 468:166
Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C (2001) J Nutr 131:2109
Lafay S, Morand C, Manach C, Besson C, Scalbert A (2006) Br J Nutr 96:39
Naz S, Siddiqi R, Dew TP, Williamson G (2011) J Agric Food Chem 59:2734
Walle T, Browning AM, Steed LL, Reed SG, Walle UK (2005) J Nutr 135:48
Penalvo JL, Nurmi T, Haajanen K, Al-Maharik N, Botting N, Adlercreutz H (2004) Anal Biochem 332:384
Smeds AI, Hakala K, Hurmerinta TT, Kortela L, Saarinen NM, Makela SI (2006) J Pharm Biomed Anal 41:898
Mathey J, Lamothe V, Coxam V, Potier M, Sauvant P, Bennetau-Pelissero C (2006) J Pharm Biomed Anal 41:957
Setchell KD, Zhao X, Jha P, Heubi JE, Brown NM (2009) Am J Clin Nutr 90:1029
Manach C, Williamson G, Morand C, Scalbert A, Remesy C (2005) Am J Clin Nutr 81:230S
Konishi Y, Kobayashi S, Shimizu M (2003) J Agric Food Chem 51:7296
Nicolin V, Grill V, Micali F, Narducci P, Passamonti S (2005) J Mol Histol 36:45
Vanzo A, Terdoslavich M, Brandoni A, Torres AM, Vrhovsek U, Passamonti S (2008) Mol Nutr Food Res 52:1106
Watanabe H, Yashiro T, Tohjo Y, Konishi Y (2006) Biosci Biotechnol Biochem 70:1928
Franke AA, Custer LJ, Hundahl SA (2004) Nutr Cancer 50:141
Sfakianos J, Coward L, Kirk M, Barnes S (1997) J Nutr 127:1260
Ridlon JM, Kang DJ, Hylemon PB (2006) J Lipid Res 47:241
Kilkkinen A, Pietinen P, Klaukka T, Virtamo J, Korhonen P, Adlercreutz H (2002) Am J Epidemiol 155:472
Setchell KD, Lawson AM, Borriello SP et al (1981) Lancet 2:4
Cebra JJ (1999) Am J Clin Nutr 69:1046S
Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, Gordon JI (2011) Proc Natl Acad Sci USA 108:6252
Becker N, Kunath J, Loh G, Blaut M (2011) Gut Microbes 2:25
Hanske L, Loh G, Sczesny S, Blaut M, Braune A (2009) J Nutr 139:1095
Hanske L, Loh G, Sczesny S, Blaut M, Braune A (2010) Mol Nutr Food Res 54:1405
Bowey E, Adlercreutz H, Rowland I (2003) Food Chem Toxicol 41:631
Woting A, Clavel T, Loh G, Blaut M (2010) FEMS Microbiol Ecol 72:507
Qin J, Li R, Raes J et al (2010) Nature 464:59
Mahowald MA, Rey FE, Seedorf H et al (2009) Proc Natl Acad Sci USA 106:5859
Tap J, Mondot S, Levenez F et al (2009) Environ Microbiol. doi:10.1111/j.1462-2920.2009.01982.x
Clavel T, Fallani M, Lepage P et al (2005) J Nutr 135:2786
Zoetendal EG, Akkermans AD, De Vos WM (1998) Appl Environ Microbiol 64:3854
Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE (2011) Proc Natl Acad Sci USA 108(Suppl 1):4578
Hoey L, Rowland IR, Lloyd AS, Clarke DB, Wiseman H (2004) Br J Nutr 91:607
Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ (2009) J Expo Sci Environ Epidemiol 19:223
Fallani M, Amarri S, Uusijarvi A et al (2011) Microbiology 157:1385
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R (2010) Proc Natl Acad Sci USA 107:11971
Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL (2010) Microbiology 156:3329
Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE (2005) FEMS Microbiol Lett 243:141
Willing BP, Russell SL, Finlay BB (2011) Nat Rev Microbiol 9:233
Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S (1995) J Nutr 125:2307
Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA (2000) Nutr Cancer 36:27
Possemiers S, Bolca S, Eeckhaut E, Depypere H, Verstraete W (2007) FEMS Microbiol Ecol 61:372
Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, Dore J, Blaut M (2005) Appl Environ Microbiol 71:6077
Braune A, Maul R, Schebb NH, Kulling SE, Blaut M (2010) Mol Nutr Food Res 54:929
Selma MV, Espin JC, Tomas-Barberan FA (2009) J Agric Food Chem 57:6485
Possemiers S, Bolca S, Verstraete W, Heyerick A (2011) Fitoterapia 82:53
Wang LQ, Meselhy MR, Li Y, Qin GW, Hattori M (2000) Chem Pharm Bull(Tokyo) 48:1606
Mapesa JO, Waldschmitt N, Schmoeller I, Blume C, Hofmann T, Mahungu S, Clavel T, Haller D (2011) Mol Nutr Food Res 55:1850
Clavel T, Lippman R, Gavini F, Dore J, Blaut M (2007) Syst Appl Microbiol 30:16
Bryant MP (1972) Am J Clin Nutr 25:1324
Dubos RJ, Schaedler RW (1960) J Exp Med 111:407
Moore WE, Holdeman LV (1974) Appl Microbiol 27:961
Borriello SP, Setchell KD, Axelson M, Lawson AM (1985) J Appl Bacteriol 58:37
Wang XL, Hur HG, Lee JH, Kim KT, Kim SI (2005) Appl Environ Microbiol 71:214
Maruo T, Sakamoto M, Ito C, Toda T, Benno Y (2008) Int J Syst Evol Microbiol 58:1221
Minamida K, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K (2006) J Biosci Bioeng 102:247
Minamida K, Ota K, Nishimukai M, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K (2008) Int J Syst Evol Microbiol 58:1238
Yokoyama S, Suzuki T (2008) Biosci Biotechnol Biochem 72:2660
Matthies A, Clavel T, Gutschow M, Engst W, Haller D, Blaut M, Braune A (2008) Appl Environ Microbiol 74:4847
Clavel T, Charrier C, Braune A, Wenning M, Blaut M, Haller D (2009) Int J Syst Evol Microbiol 59:1805
Schoefer L, Mohan R, Braune A, Birringer M, Blaut M (2002) FEMS Microbiol Lett 208:197
Shimada Y, Yasuda S, Takahashi M, Hayashi T, Miyazawa N, Sato I, Abiru Y, Uchiyama S, Hishigaki H (2010) Appl Environ Microbiol 76:5892
Jin JS, Nishihata T, Kakiuchi N, Hattori M (2008) Biol Pharm Bull 31:1621
Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y (2010) Int J Syst Evol Microbiol 60:1721
Matthies A, Blaut M, Braune A (2009) Appl Environ Microbiol 75:1740
Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H (2010) Arch Microbiol 192:279
Yu ZT, Yao W, Zhu WY (2008) FEMS Microbiol Lett 282:73
Hur HG, Lay JO Jr, Beger RD, Freeman JP, Rafii F (2000) Arch Microbiol 174:422
Hur HG, Beger RD, Heinze TM, Lay JO Jr, Freeman JP, Dore J, Rafii F (2002) Arch Microbiol 178:8
Wang XL, Shin KH, Hur HG, Kim SI (2005) J Biotechnol 115:261
Zhao H, Wang XL, Zhang HL, Li CD, Wang SY (2011) Appl Microbiol Biotechnol 92:803
Yokoyama S, Niwa T, Osawa T, Suzuki T (2010) Arch Microbiol 192:15
Tamura M, Tsushida T, Shinohara K (2007) Anaerobe 13:32
Adlercreutz H (2007) Crit Rev Clin Lab Sci 44:483
Thawornkuno C, Tanaka M, Sone T, Asano K (2009) Biosci Biotechnol Biochem 73:1435
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI (2007) Nature 449:804
Walter J, Mangold M, Tannock GW (2005) Appl Environ Microbiol 71:2347
Harriott OT, Frazer AC (1997) Appl Environ Microbiol 63:296
Setchell KD, Clerici C, Lephart ED et al (2005) Am J Clin Nutr 81:1072
Clavel T, Dore J, Blaut M (2006) Nutr Res Rev 19:187
Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA (2004) Bioorg Med Chem 12:1559
Brown NM, Belles CA, Lindley SL, Zimmer-Nechemias LD, Zhao X, Witte DP, Kim MO, Setchell KD (2010) Carcinogenesis 31:886
EFSA Panel on Dietetic Products (Nutrition and Allergies) (2011) EFSA J 9:2264
EFSA Panel on Dietetic Products (Nutrition and Allergies) (2009) EFSA J 7:1270
Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, He X, Supko JG (2007) Endocrinology 148:4475
Lewis RW, Brooks N, Milburn GM, Soames A, Stone S, Hall M, Ashby J (2003) Toxicol Sci 71:74
Su Y, Shankar K, Simmen RC (2009) J Nutr 139:945
Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M (2002) Hum Reprod 17:1692
Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE (1998) Am J Clin Nutr 68:1453S
Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE (1997) Lancet 350:23
Irvine CH, Fitzpatrick MG, Alexander SL (1998) Proc Soc Exp Biol Med 217:247
Franke AA, Custer LJ, Tanaka Y (1998) Am J Clin Nutr 68:1466S
Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S (2006) Am J Clin Nutr 84:406
Strom BL, Schinnar R, Ziegler EE et al (2001) JAMA 286:807
Agostoni C, Axelsson I, Goulet O et al (2006) J Pediatr Gastroenterol Nutr 42:352
Vandenplas Y, De Greef E, Devreker T, Hauser B (2011) Acta Paediatr 100:162
Chen A, Rogan WJ (2004) Annu Rev Nutr 24:33
Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ (2008) Environ Health Perspect 116:416
Cheng E, Story CD, Yoder L, Hale WH, Burroughs W (1953) Science 118:164
Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KD (1982) Biochem J 201:353
Mueller SO, Simon S, Chae K, Metzler M, Korach KS (2004) Toxicol Sci 80:14
Morito K, Hirose T, Kinjo J et al (2001) Biol Pharm Bull 24:351
Hirvonen J, Rajalin AM, Wohlfahrt G, Adlercreutz H, Wahala K, Aarnisalo P (2011) J Steroid Biochem Mol Biol 123:46
Kostelac D, Rechkemmer G, Briviba K (2003) J Agric Food Chem 51:7632
Damdimopoulou P, Nurmi T, Salminen A et al (2011) J Nutr 141:1583
Low YL, Dunning AM, Dowsett M, Luben RN, Khaw KT, Wareham NJ, Bingham SA (2006) Cancer Res 66:8980
Pino AM, Valladares LE, Palma MA, Mancilla AM, Yanez M, Albala C (2000) J Clin Endocrinol Metab 85:2797
Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P (2006) Clin Chem Lab Med 44:883
Saarinen NM, Power KA, Chen J, Thompson LU (2008) Nutr Cancer 60:245
Rickard SE, Thompson LU (1998) J Nutr 128:615
Hedlund TE, Maroni PD, Ferucci PG et al (2005) J Nutr 135:1400
Boccardo F, Lunardi GL, Petti AR, Rubagotti A (2003) Breast Cancer Res Treat 79:17
Faqi AS, Johnson WD, Morrissey RL, McCormick DL (2004) Reprod Toxicol 18:605
Guan L, Huang Y, Chen ZY (2008) Biomed Environ Sci 21:197
Cederroth CR, Auger J, Zimmermann C, Eustache F, Nef S (2010) Int J Androl 33:304
Beaton LK, McVeigh BL, Dillingham BL, Lampe JW, Duncan AM (2010) Fertil Steril 94:1717
Chavarro JE, Toth TL, Sadio SM, Hauser R (2008) Hum Reprod 23:2584
Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ (2010) Fertil Steril 94:997
Rachner TD, Khosla S, Hofbauer LC (2011) Lancet 377:1276
Cassidy A, Albertazzi P, Lise Nielsen I et al (2006) Proc Nutr Soc 65:76
Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W (2009) JAMA 302:2437
Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A (2010) Hum Reprod Update 16:745
Cummings SR, Bates D, Black DM (2002) JAMA 288:1889
Johnson ML, Lara N, Kamel MA (2009) Genome Med 1:84
Ma DF, Qin LQ, Wang PY, Katoh R (2008) Clin Nutr 27:57
Ma DF, Qin LQ, Wang PY, Katoh R (2008) Eur J Clin Nutr 62:155
Taku K, Melby MK, Takebayashi J, Mizuno S, Ishimi Y, Omori T, Watanabe S (2010) Asia Pac J Clin Nutr 19:33
Taku K, Melby MK, Kurzer MS, Mizuno S, Watanabe S, Ishimi Y (2010) Bone 47:413
Levis S, Strickman-Stein N, Doerge DR, Krischer J (2010) Contemp Clin Trials 31:293
Tai TY, Tsai KS, Tu ST et al (2011) Osteoporos Int 23:1571
Demark-Wahnefried W, Polascik TJ, George SL et al (2008) Cancer Epidemiol Biomarkers Prev 17:3577
Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT (2004) Urology 63:900
Sturgeon SR, Heersink JL, Volpe SL et al (2008) Nutr Cancer 60:612
Thompson LU, Chen JM, Li T, Strasser-Weippl K, Goss PE (2005) Clin Cancer Res 11:3828
Velentzis LS, Cantwell MM, Cardwell C, Keshtgar MR, Leathem AJ, Woodside JV (2009) Br J Cancer 100:1492
Bassett CM, Rodriguez-Leyva D, Pierce GN (2009) Appl Physiol Nutr Metab 34:965
Ward HA, Kuhnle GG, Mulligan AA, Lentjes MA, Luben RN, Khaw KT (2010) Am J Clin Nutr 91:440
Hallund J, Ravn-Haren G, Bugel S, Tholstrup T, Tetens I (2006) J Nutr 136:112
Hallund J, Tetens I, Bugel S, Tholstrup T, Bruun JM (2008) Nutr Metab Cardiovasc Dis 18:497
Hallund J, Tetens I, Bugel S, Tholstrup T, Ferrari M, Teerlink T, Kjaer A, Wiinberg N (2006) J Nutr 136:2314
Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ (2008) J Med Food 11:207
Chen J, Tan KP, Ward WE, Thompson LU (2003) Exp Biol Med (Maywood) 228:951
Rickard SE, Yuan YV, Chen J, Thompson LU (1999) Nutr Cancer 35:50
Thompson LU (1998) Baillieres Clin Endocrinol Metab 12:691
Tou JC, Thompson LU (1999) Carcinogenesis 20:1831
Ward WE, Jiang FO, Thompson LU (2000) Nutr Cancer 37:187
Li D, Yee JA, Thompson LU, Yan L (1999) Cancer Lett 142:91
Jenab M, Thompson LU (1996) Carcinogenesis 17:1343
Pajari AM, Smeds AI, Oikarinen SI, Eklund PC, Sjoholm RE, Mutanen M (2006) Cancer Lett 233:309
Saarinen NM, Warri A, Dings RP, Airio M, Smeds AI, Makela S (2008) Int J Cancer 123:1196
Penumathsa SV, Koneru S, Thirunavukkarasu M, Zhan L, Prasad K, Maulik N (2007) J Pharmacol Exp Ther 320:951
Penumathsa SV, Koneru S, Zhan L, John S, Menon VP, Prasad K, Maulik N (2008) J Mol Cell Cardiol 44:170
Prasad K (2008) Atherosclerosis 197:34
Jacobs MN, Nolan GT, Hood SR (2005) Toxicol Appl Pharmacol 209:123
Penttinen P, Jaehrling J, Damdimopoulos AE et al (2007) Endocrinology 148:4875
Pianjing P, Thiantanawat A, Rangkadilok N, Watcharasit P, Mahidol C, Satayavivad J (2011) J Agric Food Chem 59:212
Carreau C, Flouriot G, Bennetau-Pelissero C, Potier M (2008) J Steroid Biochem Mol Biol 110:176
Garreau B, Vallette G, Adlercreutz H, Wahala K, Makela T, Benassayag C, Nunez EA (1991) Biochim Biophys Acta 1094:339
Schottner M, Spiteller G, Gansser D (1998) J Nat Prod 61:119
Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Kellis JT Jr, Vickery LE (1993) J Steroid Biochem Mol Biol 44:147
Brooks JD, Thompson LU (2005) J Steroid Biochem Mol Biol 94:461
Evans BA, Griffiths K, Morton MS (1995) J Endocrinol 147:295
Wang C, Makela T, Hase T, Adlercreutz H, Kurzer MS (1994) J Steroid Biochem Mol Biol 50:205
Sathyamoorthy N, Wang TT, Phang JM (1994) Cancer Res 54:957
Cosentino M, Marino F, Ferrari M et al (2007) Pharmacol Res 56:140
Danbara N, Yuri T, Tsujita-Kyutoku M, Tsukamoto R, Uehara N, Tsubura A (2005) Anticancer Res 25:2269
Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L (1993) Proc Natl Acad Sci USA 90:2690
Lin X, Switzer BR, Demark-Wahnefried W (2001) Anticancer Res 21:3995
Mousavi Y, Adlercreutz H (1992) J Steroid Biochem Mol Biol 41:615
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU (1999) Mol Cell Biochem 202:91
Prasad K (2000) Int J Angiol 9:220
Luiten H, Yuan YV, Rickard SE, Thompson LU (1999) Nutr Res 19:1233
Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, Itoh T, Shimomura Y, Ueno T (2008) Food Chem Toxicol 46:2713
Kulling SE, Jacobs E, Pfeiffer E, Metzler M (1998) Mutat Res 416:115
Cho YM, Imai T, Ito Y, Takami S, Hasumura M, Yamazaki T, Hirose M, Nishikawa A (2009) Food Chem Toxicol 47:2150
Blaut M, Braune A, Wunderlich S, Sauer P, Schneider H, Glatt H (2006) Food Chem Toxicol 44:1940
Ozasa K, Nakao M, Watanabe Y et al (2005) J Epidemiol 15(Suppl 2):S196
Stumpf K, Pietinen P, Puska P, Adlercreutz H (2000) Cancer Epidemiol Biomarkers Prev 9:1369
Brown NM, Lindley SL, Witte DP, Setchell KD (2011) Reprod Toxicol 32:33
Orcheson LJ, Rickard SE, Seidl MM, Thompson LU (1998) Cancer Lett 125:69
Phipps WR, Martini MC, Lampe JW, Slavin JL, Kurzer MS (1993) J Clin Endocrinol Metab 77:1215
Tou JC, Chen J, Thompson LU (1998) J Nutr 128:1861
Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J (2011) Am J Clin Nutr 94:58
Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J (2010) PLoS One 5:e15046
Sklenickova O, Flesar J, Kokoska L, Vlkova E, Halamova K, Malik J (2010) Molecules 15:1270
Ozcelik B, Kartal M, Orhan I (2011) Pharm Biol 49:396
Kim MG, Lee HS (2009) J Food Sci 74:M467
Hong H, Landauer MR, Foriska MA, Ledney GD (2006) J Basic Microbiol 46:329
Doerner KC, Takamine F, LaVoie CP, Mallonee DH, Hylemon PB (1997) Appl Environ Microbiol 63:1185
Lee HC, Jenner AM, Low CS, Lee YK (2006) Res Microbiol 157:876
Possemiers S, Bolca S, Grootaert C et al (2006) J Nutr 136:1862
Hanske L, Hussong R, Frank N, Gerhauser C, Blaut M, Braune A (2005) Mol Nutr Food Res 49:868
Smith AH, Mackie RI (2004) Appl Environ Microbiol 70:1104
Okubo T, Ishihara N, Oura A, Serit M, Kim M, Yamamoto T, Mitsuoka T (1992) Biosci Biotechnol Biochem 56:588
Mai V, Katki HA, Harmsen H, Gallaher D, Schatzkin A, Baer DJ, Clevidence B (2004) J Nutr 134:473
Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP (2011) Am J Clin Nutr 93:62
Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP (2008) Br J Nutr 99:782
Wiseman H, Casey K, Bowey EA et al (2004) Am J Clin Nutr 80:692
Jacobs DM, Deltimple N, van Velzen E, van Dorsten FA, Bingham M, Vaughan EE, van Duynhoven J (2008) NMR Biomed 21:615
Atkinson C, Berman S, Humbert O, Lampe JW (2004) J Nutr 134:596
Blair RM, Appt SE, Franke AA, Clarkson TB (2003) J Nutr 133:2262
Franke AA, Halm BM, Ashburn LA (2008) Nutr Cancer 60:627
Milder IE, Kuijsten A, Arts IC, Feskens EJ, Kampman E, Hollman PC, Van’t Veer P (2007) J Nutr 137:1266
Atkinson C, Newton KM, Bowles EJ, Yong M, Lampe JW (2008) Am J Clin Nutr 87:679
Bolca S, Possemiers S, Herregat A et al (2007) J Nutr 137:2242
Gardana C, Canzi E, Simonetti P (2009) J Nutr Biochem 20:940
Adlercreutz H, Fotsis T, Heikkinen R, Dwyer JT, Woods M, Goldin BR, Gorbach SL (1982) Lancet 2:1295
Horner NK, Kristal AR, Prunty J, Skor HE, Potter JD, Lampe JW (2002) Cancer Epidemiol Biomarkers Prev 11:121
Nurmi T, Mursu J, Penalvo JL, Poulsen HE, Voutilainen S (2010) Br J Nutr 103:677
Vedrine N, Mathey J, Morand C, Brandolini M, Davicco MJ, Guy L, Remesy C, Coxam V, Manach C (2006) Eur J Clin Nutr 60:1039
Mazur WM, Uehara M, Wahala K, Adlercreutz H (2000) Br J Nutr 83:381
Luoto R, Kharazmi E, Saarinen NM, Smeds AI, Makela S, Fallah M, Raitanen J, Hilakivi-Clarke L (2010) Reprod Health 7:26
Liu B, Qin L, Liu A, Uchiyama S, Ueno T, Li X, Wang P (2010) J Epidemiol 20:377
Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G (2011) Br J Nutr 107:1201–1206
Vergne S, Sauvant P, Lamothe V, Chantre P, Asselineau J, Perez P, Durand M, Moore N, Bennetau-Pelissero C (2009) Br J Nutr 102:1642
Rijkers GT, Bengmark S, Enck P et al (2010) J Nutr 140:671S
Larkin TA, Price WE, Astheimer LB (2007) Nutrition 23:709
Larkin TA, Astheimer LB, Price WE (2009) Eur J Clin Nutr 63:238
Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS (2005) J Altern Complement Med 11:1067
Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS (2005) J Nutr 135:603
Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS (2004) J Nutr 134:1998
Tsangalis D, Wilcox G, Shah NP, Stojanovska L (2005) Br J Nutr 93:867
McMullen MH, Hamilton-Reeves JM, Bonorden MJ, Wangen KE, Phipps WR, Feirtag JM, Kurzer MS (2006) J Altern Complement Med 12:887
Bonorden MJ, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, Kurzer MS (2004) Eur J Clin Nutr 58:1635
Cohen LA, Crespin JS, Wolper C, Zang EA, Pittman B, Zhao Z, Holt PR (2007) In Vivo 21:507
Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS (2004) J Nutr 134:3277
Kekkonen RA, Holma R, Hatakka K, Suomalainen T, Poussa T, Adlercreutz H, Korpela R (2011) J Nutr 141:870
Decroos K, Eeckhaut E, Possemiers S, Verstraete W (2006) J Nutr 136:946
Possemiers S, Rabot S, Espin JC et al (2008) J Nutr 138:1310
Roberfroid M (2007) J Nutr 137:830S
Steer TE, Johnson IT, Gee JM, Gibson GR (2003) Br J Nutr 90:635
Piazza C, Privitera MG, Melilli B, Incognito T, Marano MR, Leggio GM, Roxas MA, Drago F (2007) Am J Clin Nutr 86:775
Cashman K (2003) Curr Issues Intest Microbiol 4:21
Mathey J, Mardon J, Fokialakis N et al (2007) Osteoporos Int 18:671
Wong JM, Kendall CW, de Souza R, Emam A, Marchie A, Vidgen E, Holmes C, Jenkins DJ (2010) Metabolism 59:1331
Arumugam M, Raes J, Pelletier E et al (2011) Nature 473:174
Van Duynhoven J, Vaughan EE, Jacobs DM et al (2011) Proc Natl Acad Sci USA 108(Suppl 1):4531
Spor A, Koren O, Ley R (2011) Nat Rev Microbiol 9:279
Acknowledgments
We are grateful to Prof. Hannelore Daniel (TU München, Molecular Nutrition Unit), Dr. Laura Hanske (German Institute of Human Nutrition Postdam-Rehbrücke, Department of Gastrointestinal Microbiology), Dr. Gemma Henderson (Grasslands Research Centre, AgResearch) and Dr. Augustin Scalbert (Clermont University, Department of Human Nutrition) for critically reading the manuscript and giving helpful recommendations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Clavel, T., Mapesa, J.O. (2013). Phenolics in Human Nutrition: Importance of the Intestinal Microbiome for Isoflavone and Lignan Bioavailability. In: Ramawat, K., Mérillon, JM. (eds) Natural Products. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22144-6_94
Download citation
DOI: https://doi.org/10.1007/978-3-642-22144-6_94
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-22143-9
Online ISBN: 978-3-642-22144-6
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics