Abstract
Echinoderms have an extensive endoskeleton composed of magnesian calcite, a form of calcium carbonate that contains small amounts of magnesium carbonate and occluded matrix proteins. Adult sea urchins have several calcified structures, including test, teeth, and spines, composed of numerous ossicles which form a three-dimensional meshwork of mineral trabeculae, the stereom. The biomineral development begins in 24-hour-old embryos within the primary mesenchyme cells (PMCs), the only cells producing a set of necessary matrix proteins. The deposition of the biomineral occurs in a privileged extracellular space produced by the fused filopodial processes of the PMCs. We showed for the first time that signals from ectoderm cells overlying PMCs play an important role in the regulation of biomineralization-related genes. It is believed that growth factors are produced by ectoderm cells and released into the blastocoel where they interact with cognate receptor tyrosine kinases restricted to PMCs, which activate signaling cascades regulating the expression of biomineralization-related genes. We demonstrated the implication of a TGF-beta family factor by a perturbation model in which skeleton elongation was indirectly blocked by monoclonal antibodies to an extracellular matrix (ECM) protein located on the apical surface of ectoderm. Thus, it was inferred that interfering with the binding of the ECM ligand, a member of the discoidin family, to its cell surface receptor, a βC integrin, disrupts the ectodermal cell signaling cascade, resulting in reduced or aberrant skeletons. During the last few years, we analyzed the expression of biomineralization-related genes in other examples of experimentally induced skeleton malformations, produced by the exposure to toxic metals, such as Cd and Mn or ionizing radiations, such as UV-B and X-rays. Besides the obvious toxicological implication, since the mis-expression of spicule matrix genes paralleled skeleton defects, we believe that by means of these studies we can dissect the molecular steps taking place and possibly understand the physiological events regulating embryonic biomineralization.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Basis of Biomineral Formation
Biomineralization refers to the biological processes employed by living organisms to form minerals as a result of regulated processes. A biomineral represents a complex material which incorporates both mineral and organic components exhibiting advantageous properties compared to its inorganically formed counterpart. Compared to abiotic minerals, biominerals possess additional physical and chemical characteristics which offer increased flexibility and duration. They vary in morphology, shape, and size as well as in element composition. The structure of a biomineral involves a mosaic of crystalline domains separated by occluded proteinaceous material forming a framework (Wilt 1999). The structure exhibits single crystal diffraction properties as shown by X-ray diffraction studies (Simkiss 1986).
Classically, according to the degree of biological control over the precipitated mineral, biomineralization processes can be categorized into two groups: the “biologically induced” (Lowenstam 1981) and the “biologically controlled” mineralization (Mann 1983).
In biologically induced mineralization, cell surfaces may act as causative nucleation agents which lead to crystal growth. Mineral growth is indirectly affected, but not controlled, by the biological system. The adopted mineral form is favored by metabolic processes which define the chemical conditions of the microenvironment (i.e., pH, pCO2, concentration of products resulting from secretion) (Frankel and Bazylinski 2003). As environmental conditions play a potential role in the formation of the biologically induced minerals, these biominerals exhibit heterogeneity in elemental composition, in water content, and in particle size, resulting in various external morphologies.
In biologically controlled mineralization, cellular activities direct all the stages for the precipitation of the mineral: from the determination of the initial deposition site, to nucleation, growth, and formation of the final crystalline morphology. Resulting biominerals acquire reproducible, species-specific, genetically determined structures and properties. Controlled biomineralization requires an isolated environment which serves as the mineralization site. This environment can be extracellular, intercellular or intracellular, with respect to the cells which control the process. In general, biologically induced mineralization is found in bacteria and lichens, whereas biologically controlled mineralization is found in foraminifera, cephalopod statoliths, mollusks shells, bryozoan exoskeletons, scleractinian corals, echinoderms, human bones, and teeth.
In nature, almost 50% of biominerals are calcium-bearing minerals (Lowenstam and Weiner 1989). Calcium content in living organisms is highly regulated, as it has key roles in metabolic processes at concentrations varying from 0.01 μM to 10 μM. The most abundant calcium-bearing biominerals precipitate acquiring one of the eight known calcium carbonate polymorph forms. These include seven crystalline forms: calcite, Mg-calcite, aragonite, vaterite, monohydrocalcite, protodolomite, hydrocerussite, and one amorphous calcium carbonate (ACC) form (Addadi et al. 2003). Most models of biomineralization invoke the involvement of membrane ion transporters (channels and pumps) in the delivery of Ca2+ and other ions to the calcification site (Simkiss and Wilbur 1989).
An important parameter for the formation of a biomineral is the regulation of the “isotopic composition” (Weber and Raup 1966) which establishes a physicochemical equilibrium with the microenvironment. The medium from which the mineral forms is a saturated solution, which is required at the site of mineralization only. Supersaturation can be achieved by the presence of additives which prevent the deposition of crystalline phases, such as magnesium in concentrations similar to those of the seawater (Raz et al. 2000). Another inhibitory factor of crystallization is the presence of proteins which, serving as substrates, influence the solubility of the mineral phase and stabilize different polymorphs, either ACC (Aizenberg et al. 1996) or crystalline calcium carbonate forms. In conclusion, crystal shape depends on inorganic and organic factors: pI, temperature, microenvironment growth, solubility of the mineral phase, concentration of occluded macromolecules and ions. As a result, calcium carbonate growth undergoes a series of phases and morphologies to form the final calcite structure. On the contrary, the effect of whole extracts of proteinaceous matrixes on the in vitro precipitation of calcium carbonate was shown to selectively induce the precipitation of particular polymorphs. Some authors have pointed out that the soluble or insoluble matrix, extracted from nacre, controls calcium carbonate crystal polymorphs toward aragonite or calcite formation (Falini et al. 1996). Others used chitin as a substrate and Mg2+ as an additive to induce aragonite double-layered composite film formation (Kato 2000). Studies such as those mentioned above indicate that template molecules can act as nucleators for the precipitation of the inorganic material. The surface chemistry of each template molecule and the arrangement of the amino acid groups guide the oriented nucleation of the cognate crystal face.
2 Biomineral Contents and Shapes
Examples of calcium-bearing biominerals are found in the echinoderms, a phylum of marine invertebrates. Biomineral formation has an important role in the phylum, which includes Echinoids (sea urchins and sand dollars), Asteroids (sea stars), Ophiuroids (brittle stars), Crinoids (feather stars) and Holothuroids (sea cucumbers). It supports the construction of the skeleton of the living organisms, offering support and protection. Echinoderms employ a genetically regulated process to form their calcareous skeleton by the precipitation of magnesian calcite, a form of calcium carbonate that contains small amounts of magnesium carbonate in a ratio given by the formula: (MgxCa1-x)CO3. In a high Mg/Ca ratio, amorphous calcium carbonate or aragonitic nucleation is favored over calcite (Raz et al. 2000; Mann 2001).
The magnesium carbonate content can vary from 2.5% to 39% depending on the classes and species. In general, the hardness of the biomineral is directly proportional to the concentration of magnesium. Low magnesium biominerals are found in the softer Ophiuroids and high magnesium biominerals are found in the harder Asteroids (Dubois and Chen 1989).
The skeletons of all echinoderms are made of microscopic bony plates, also called ossicles, deposited as a three-dimensional meshwork called a stereom, made of magnesian calcite and a network of interconnected holes filled with living tissue. Different types of stereom are indicative of the type of living tissue that penetrates the plates (e.g., cells, tube feet, others). The skeleton is covered by an epidermis and contains a network of internal water-filled canals or encloses a coelomic cavity bathed in coelomic fluid. The structure of the biomineral reflects complexity. Skeletal plates may remain simple or may fuse to form composite plates. They can also form tubercles, granules, fixed, or movable spines. Examples of the diversity of stereoms within the one species, Paracentrotus lividus (P. lividus), can be observed in Fig. 8.1. Among the classes, the most diverse skeletal organizations are evident in Echinoids and Holothuroids. In the former, organisms are completely surrounded by a calcified test embedded in the mesodermal stroma tissue, with only a thin layer of muscular tissue. On the contrary, Holothuroids possess an endoskeleton reduced to microscopic ossicles dispersed throughout the dermis, with a highly muscularized body wall (Smith et al. 2010). In the last 40 years, advanced high-resolution imaging techniques, such as SEM and TEM, have helped researchers to provide information about the fine structure and composition of the stereom, as well as the cellular basis of echinoderm calcification.
Paracentrotus lividus stereoms. Scanning electron micrographs of different parts of tests and spines. (a) Low magnification of all samples observed in B-I. (b) cross section of a spine fragment, (c) longitudinal surface of the spine, (d) high and (e) low magnification of external test portions,( f) fractured spine tip, (g) indented base which fits like a ball-and-socket joint over a tubercle of the test, and (h, i) integument plate. This plate shows rectilinear (box-like) stereom characteristic of the ectoderm
Various studies have been aiming to identify the proteins involved in the mineralization of the adult sea urchin test, teeth, and spines (Berman et al. 1993). The proteome of the mineralized parts of the adult sea urchin was recently described using mass spectrometry-based methods. In the Strongylocentotus purpuratus (S. purpuratus) sea urchin, 138 (Mann et al. 2008a) and 110 (Mann et al. 2008b) proteins were identified in the tooth and test/spines organic matrix respectively.
In sea urchin embryos, 231 proteins were identified in spicule matrix extracts (Mann et al. 2010). Among the most abundant proteins found are SM30 and SM50, which were originally purified biochemically and belong to the C-type lectin family. Various other lectins include: SM29, SM32, SM37, PM27 and Sp-Clect_13, as well as metalloproteases and carbonic anhydrase. Some of these proteins are expressed both in embryonic spicules and adult mineralized parts, e.g., the phosphoproteins P16 and P19 (Alvares et al. 2009) and isoforms of the C-type lectin SM30 (Killian et al. 2010). The great number of proteins identified in the above-mentioned studies has been possible thanks to the genome-wide analysis of biomineralization-related proteins (Livingston et al. 2006). Most of the proteins were found to be sea urchin specific, meaning they have no apparent homologues in other invertebrate deuterostomes or vertebrates. They include several families: the spicule-matrix proteins, the msp-130 family, cyclophilins, collagens, carbonic anhydrase, P-16, P-19, secreted Ca-binding phosphoproteins, transcription factors, ECM molecules, and proteins involved in cell-ECM interaction, such as secreted proteases. In conclusion, although for some of those proteins the biological functions have been proved, the future challenge will be to characterize and demonstrate the specific role of each protein in the growth and patterning of the skeleton.
3 Cells Involved in Adult Echinoderms Biomineralization
Teeth, spines, and the test of adult echinoderms are all formed by a terminally differentiated type of cells of mesodermal origin, called sclerocytes, specializing in the deposition of skeletal parts. Sclerocytes are the only cell population to contact the stereom and occupy the mesodermal stroma tissue of an intact mineral structure. They produce thin cell processes that closely surround the trabeculae, forming the so-called skeletal cytoplasmic sheath (Heatfield and Travis 1975). The thin processes show no organelles, while the cell body includes a well-developed and dilated Golgi complex, with associated vesicles and other organelles (Stricker 1985; Märkel et al. 1986; Dubois and Ameye 2001). Calcifying vacuoles are formed within the cytoplasmic layers, which are separated from the calcite by a vascular space. All developing ossicles appear to be surrounded by a syncytial network of sclerocytes, though it is reported that the ossicle rudiment appears either in a single cell (Crinoids, Echinoids, and Ophiuroids) or in a syncytium (Holothuroids) (Dubois and Chen 1989). The mineral skeleton is produced intracellularly or intrasyncytially (Märkel and Röser 1985). During the calcification process, the vacuoles increase in size and ramify, producing the characteristic projection of the primary plates, and new sclerocytes are incorporated in the pseudopodial syncytium (Kniprath 1974; Märkel et al. 1986). This syncytium may either envelop preexisting calcite surface on which the biomineral grows, or form independently from the preexisting stereom (Heatfield and Travis 1975).
3.1 Biomineral Formation and Regenerative Events
Echinoderms show a remarkable self-repairing ability as adults and a latent potential for regeneration of large parts of their bodies. This potential is common to all classes of the phylum as an adaptive mechanism for survival and dispersion. Lines of cells preserve the ability to differentiate into somatic and germ tissues, but little is known concerning the nature of cells and the molecular pathways involved in the different phases of echinoderm regeneration such as wound healing, growth, morphogenesis, and cell differentiation. Regeneration is in fact a characteristic type of developmental process that can involve cell turnover and tissue repair, reconstruction of external and internal organs, and regrowth of new complete adults from detached body fragments (Candia Carnevali et al. 2009). It has been reported that the dermis of regenerating spines is characterized by active sclerocytes, the principal skeleton-forming cells also known as calcoblasts or odontoblast (Märkel et al. 1986; Dubois and Ameye 2001).
Signals, activated in response to initial inflammatory events, trigger a series of intracellular processes associated with survival, cell proliferation, migration, attachment, differentiation, and eventually matrix deposition. All these steps are required to accelerate tissue repair and reconstruction, including neural, muscular, and skeletogenic tissues. Any regenerative process implies the existence of stem cells present in the circulating fluids or in the tissues in the form of resident cells, ready to be recruited after trauma (Pinsino et al. 2007). Scarce information is available on the biomineralization process following regeneration. Pioneering studies at the molecular level demonstrated the overexpression of three mRNA specific of the primary mesenchyme cells (PMCs) in regenerating spines. By in situ hybridization, it was shown that gene products were localized primarily in calcoblasts that accumulated at the regeneration sites (Drager et al. 1989). More studies are awaited in tissue calcification events, which are of fundamental importance, as they ensure that biomineralization proceeds normally in the proper sites, while ectopic mineralization is prevented elsewhere.
4 Cellular Signaling and Biomineral Formation in the Sea Urchin Embryo
Among echinoderms, the sea urchin embryo has been known for its versatility and suitability since the end of the nineteenth century, when classical embryologists performed the earliest studies on the basic mechanisms of embryo development, facilitated by the optical transparency of the embryo, along with its simplicity in shape and organization (Hörstadius 1939). Since then, the sea urchin has been utilized to a great extent for studies in several scientific fields, ranging from basic developmental biology to ecotoxicology and applied research. In addition, the ability to form a larval endoskeleton encourages the use of the sea urchin embryo for detailed studies on the mechanisms underlying biomineralization, an extremely interesting topic for nano(bio)technology. It is becoming increasingly clear that the biomineralization process is genetically controlled and a great number of steps in such biological control could exist.
Biomineralization in the sea urchin initiates from the early embryonic developmental stages and is mediated by the PMCs which express genes under a complex signaling control of transcription and growth factors (for a review see Ettensohn 2009). Schematic drawings of the initial phases of embryonic skeleton deposition and PMCs arrangement are presented in Fig. 8.2. Accumulating evidence demonstrates that the PMCs express genes which control the formation and remodeling of carbonate crystals. In sea urchin embryos, ACC is a transient phase leading to the formation of calcitic spicules (Beniash et al. 1997; Weiss et al. 2002). The formation of ACC results from a supersaturated solution produced by ions and proteins (Raz et al. 2003).
Schematic drawing the location and arrangements of PMCs. (a) Gastrula frontal and vegetal views showing the PMCs aggregates (clusters) at opposite sides of the archenteron and one of the clusters enclosing the spicule triradiate rudiment; (b) formation of PMCs aggregates and initial pseudopodial fusion; (c) alignment of PMCs and enlargement of the fused cytoplamic space, note the first sign (white dot) of biomineral deposition; and (d) development of spicule rudiment inside the cell cluster (white area), assuming the shape of a triradiate crystal (modified from Dubois and Chen 1989)
The formation of the sea urchin calcareous embryonic skeleton occurs in specialized vesicles/vacuoles within the PMCs. Cells tightly control the concentration of the ions precursors and nucleation is initiated by deposition on an organic template. The pH, pCO2, and the concentration of trace elements are regulated by membrane trafficking. After the initial precipitation occurring inside the cells, crystals are secreted to the extracellular blastocoelic environment, by fusion of vesicles with the plasma membrane, where they interact with matrix proteins to acquire the final crystal form (Wilt et al. 2008; Yang et al. 2011). Thus, the mineral is believed to be enrobed by the syncytial membrane and the cytoplasm.
The larval skeleton displays considerable morphological diversity among sea urchin species, including variations in the number, structure/shape, and size of rods. Thus, when describing the single parts of the skeleton, one should refer to the species taken into consideration. Here, we want to briefly describe for the first time the development of the skeleton of P. lividussea urchin embryo, identifying the chains of PMCs which will give rise to the different rods. In particular, as shown in Fig. 8.3, the ventral chain will give rise to the ventral transverse rod, the longitudinal chain will form the anterolateral rod, and the dorsal chain will give rise to the body and postoral rods. The larval skeleton morphology and its differences among species is interesting for evolutionary studies, both from a developmental viewpoint, to understand how these differences are produced, and from an ecological viewpoint, to understand why such differences have been generated (Zito and Matranga 2009; Ettensohn 2009).
Development of Paracentrotus lividus skeleton. Schematic drawings of skeleton development observed at (a) late gastrula; (b) prism; (c) early pluteus; (d) pluteus, ventral view; (e) pluteus, lateral view. Ventral chain, longitudinal chain, and dorsal chain indicate set of PMCs which will give raise to the ventral transverse rod, anterolateral rod and body and postoral rod, respectively. Tools to study skeleton formation in late gastrula (f, h, j) and pluteus (g, i, k) stage embryos. (f, g) differential interference contrast; (h, i) immunofluorescence with antibody to msp130; (j, k) in situ hybridization with msp130 probe
As already mentioned, the only cells competent to produce a skeleton in sea urchin embryos are the PMCs. Their morphological features and behavior, as well as the main cellular events leading to the formation of the embryonic skeleton, have been extensively described (see also this book, Chapter by Goldberg and Wilt). Considerable progress has been made in elucidating the molecular basis of PMC specification by the analysis of a gene regulatory network that operates in the large micromere descendants (Ettensohn 2009). Upstream components include several maternal and zygotic transcription factors; downstream are gene products that directly control the PMCs morphogenetic behaviors, such as ingression, migration, fusion, and deposition of the biomineralized endoskeleton. In the following, we will focus on selected aspects of skeletogenesis concerning the external cues stimulating PMCs to produce the right patterned skeleton. In particular, we will discuss on the involvement of extracellular matrix (ECM) molecules and growth factors in the process of skeletogenesis.
4.1 Extracellular Matrix
A great number of in vitro and in vivo studies highlight the great importance of the ECM during morphogenesis. In fact, in addition to its function as an extracellular space-filling scaffold, it plays a fundamental role in cell–substrate interactions, providing both spatial and temporal information to adherent cells, thus influencing cellular proliferation and viability, differentiation, and gene expression. Accordingly, interest has increased in recent years toward the identification, purification, and functional studies on ECM components, along with their ligands, and other molecules involved in cell–ECM adhesion.
The ECM of the sea urchin embryo is a very complex structure, consisting of a number of layers with many different elements. The organization of the ECM occurs in a highly regulated fashion during different developmental steps (for a review see McClay et al. 1990). In addition, it must be pointed out that it is possible to distinguish between two main ECM compartments: the apical ECM, surrounding the embryo from fertilization of the egg, and the basal lamina, localized inside the blastocoelic cavity, which forms during the late cleavage stage (see Fig. 8.4). So far, no ECM molecule with an active direct role in biomineralization has been documented in the sea urchin embryo. Nevertheless, there are a number of evidences of indirect roles played by ECM molecules during the formation of the skeleton. Among the ECM components present in the blastocoel, only ECM molecules that function in supporting or directing PMCs movements have been described until now. For example, Pamlin, a protein isolated from the basal lamina of the Hemicentrotus pulcherrimus sea urchin embryo, has been shown to promote PMC binding and migration in vitro (Katow 1995). In Lytechinus variegatus, PMCs have been observed by light microscopy to directly interact with a class of ECM fibers, localized on the basal surface of the ectoderm via their filopodia (Hodor et al. 2000). A component of such fibers is ECM3, a high-molecular-weight protein accumulated selectively in the basal lamina adjacent to the ectoderm in all regions except for the animal pole (Wessel and Berg 1995). ECM3 has been suggested as a strong candidate as a PMCs substrate molecule, with a role in providing guidance information to migrating PMCs. Interestingly, ECM3 contains an N-terminal domain similar to the mammalian chondroitin sulfate proteoglycan core protein NG2, required for the responsiveness of some cell types to PDGF, supporting the idea that it may play a role in growth factor presentation (Hodor et al. 2000). The central region of ECM3 contains five tandem repeats of a motif similar to the domain contained within the regulatory Ca2+-binding loop of the Na+-Ca2+ exchange protein, which is similar in several respects to MAFp4, a component of the aggregation factor molecular complex, mediating the species-specific sorting of sponge cells (Hodor et al. 2000).
Other ECM proteins located on the basal lamina have been described to have a role in spicule formation. For example, by microinjection of specific antibodies into the blastocoelic cavity, it has been shown that collagen in S. purpuratus (Wessel et al. 1991) and Pl-200 K protein in P. lividus (Tesoro et al. 1998) are essential for skeleton elongation and patterning.
Sea urchin metalloproteinases can modify collagen and other ECM components, as well as glycoproteins that associate with collagens and carbohydrates in the ECM. It has been shown that several inhibitors of metalloproteinases inhibit the continuation of skeletogenesis in both S. purpuratus and L. pictus embryos (Ingersoll and Wilt 1998).
The functional role played by ECM molecules in vivo has often been investigated by means of monoclonal antibodies (McAb) which interfere with their functions. Among the ECM proteins already known, Pl-nectin, isolated from P. lividus (Matranga et al. 1992) as a collagen-binding molecule, is the first described as an “indirect actor” in the ecto-mesoderm signaling (Zito et al. 1998). The protein, a discoidin family member whose complete sequence and domain architecture has been recently characterized (Fig. 8.5a) (Costa et al. 2010), is localized on the apical surface of ectoderm and endoderm cells from the blastula and gastrula stage onwards (Fig. 8.5b). By in vitro assays, it has been shown to mediate cell–substrate adhesion in a dose-dependent fashion (Fig. 8.5c), suggesting a functional role during development (Matranga et al. 1992). A homologous protein has been purified from Temnopleurus hardwickii, and named Th-nectin (Yokota et al. 1994). In the outer ECM, two other proteins called fibropellins are present after fertilization and throughout early development. At the blastula stage, fibropellins become organized into distinct fibers which form a mesh-like network over the surface of the embryo (Bisgrove et al. 1991).
Pl-nectin structure, localization and function. (a) Structure: on the left SDS-PAGE of the purified molecule showing a 210 kDa homodimer under non-reducing conditions (NR), consisting of two 105 kDa monomers under reducing conditions (R), which are held together by S-S bridges (modified from Matranga et al. 1992). On the right, three-dimensional structure of the 105 kDa C-shaped monomer, based on in silico homology modeling (modified from Costa et al. 2010). (b) Localization: indirect immunofluorescence on section with antibody to Pl-nectin. In the unfertilized egg, the protein is found within granules uniformly distributed throughout the cytoplasm. After fertilization, the protein is polarized to the apical surface of ectoderm and endoderm cells during all the developmental stages examined, until the prism stage (modified from Matranga et al. 1992). (c) Function: on the left cell adhesion assays. The protein promotes the adhesion of blastula cells to the substrate in a dose-dependent manner (histogram) (modified from Matranga et al. 1992). A monoclonal antibody (mAb) to Pl-nectin inhibits the adhesion of blastula cells to Pl-nectin-coated substrates in “in vitro” functional assays (histogram). On the right, in vivo perturbation experiments with mAb to Pl-nectin show embryos with major defects in skeleton elongation and patterning (compare control embryo with treated embryo). Primary mesenchyme cells are stained with an antibody to Msp130 showing that PMCs enter the blastocoel and position correctly (modified from Zito et al. 1998)
The function of Pl-nectin in vivo has been studied by morphogenetic assays in which embryos were cultured in the presence of a McAb to Pl-nectin (Fig. 8.5c). A high number of embryos with serious skeleton defects, but with normally developed ectoderm and endoderm structures, were found (Zito et al. 1998, 2003). It was demonstrated that skeleton deficiency was not due to a reduction in the number of PMCs ingressing the blastocoel, which were found as normal in number (usually 32 cells in P. lividus) and regularly arranged in a subequatorial ring (Zito et al. 2003). On the other hand, skeleton-deficient embryos showed a decrease in the expression levels of SM30 (Zito et al. 2003), while SM50 and MSP130 expression were not affected by the treatment with a McAb to Pl-nectin (Zito, personal communication). Concurrently, a strong reduction in the expression levels of univin, a TGF-β family growth factor, was found, while its mis-expression obtained by the injection of its mRNA was sufficient to rescue defects in skeleton elongation and SM30 expression (Zito et al. 2003). Based on these results, we proposed for the first time, a working model in which the secretion of univin, or other growth factor(s), into the blastocoel by ectodermal cells drives PMCs to synthesize SM30 and other spicule matrix proteins required for spicule growth. The competence of these ectodermal cells to produce the signal depends on their binding to Pl-nectin (see Fig. 8.6).
The interaction of ectoderm cells with Pl-nectin conditions ecto-mesoderm induction and skeleton growth. In the upper panels: low and high magnification of late gastrula embryos in which PMCs are expressing skeletogenic genes, as shown by in situ hybridization with msp130 probe. In the lower panel: schematic representation of ectoderm cells, properly interacting with the Pl-nectin contained in the ECM (hyaline layer), secrete into the blastocoel growth factors, i.e., univin, VEGF, and/or FGF, which signal PMCs to synthesize the spicule. The interaction of ectoderm cells with Pl-nectin is mediated by an integrin receptor and activates a yet unknown signaling pathway
Some parts of the proposed model have been later confirmed by our laboratory and a few others. Recently, it has been shown by in vitro immunoprecipitation and affinity chromatography experiments that Pl-nectin binds to a βC integrin subunit, suggesting that the interaction of Pl-nectin with ectoderm cells is mediated by a βC-containing integrin receptor (Zito et al. 2010). Interestingly, an integrin recognition motif (LDT) has been found in the Pl-nectin protein sequence (Costa et al. 2010). An LDT motif has been originally identified in the human mucosal addressin cell adhesion molecule (MAdCAM-1) as the binding site of the α4/β7 integrin receptor. Thus, the binding of Pl-nectin with ectoderm cells surface could result from the interaction of the LDT motif with the identified integrin receptor. Adhesion assays using competing synthetic peptides (LDT, and others) are in progress in our laboratory to confirm this hypothesis. In addition, the structure of Pl-nectin, consisting of six tandemly repeated discoidin domains, provides it with the ability to: (1) bind to ECM molecules bearing galactose and N-acetylglucosamine carbohydrate moieties (including collagen, or cell membrane surface glycoproteins), (2) bind to cell surface proteins, such as tyrosine kinase receptors and G protein-coupled receptors, and (3) form multimeric structures by the discoidin domains self-binding (Costa et al. 2010). The last property would explain the nectosome structures observed by TEM described in T. hardwikii (Kato et al. 2004).
4.2 Growth Factors
Besides the synthesis and secretion of spicule matrix proteins and the regulation of matrix–mineral interactions, PMCs require several types of cues from the surrounding embryo, including axial, temporal, and scalar signals, in order to synthesize a normal sized and patterned skeleton. Such cues have been shown to originate from the overlying ectoderm and the apical ECM (Guss and Ettensohn 1997; Zito et al. 2003; Kiyomoto et al. 2004; Duloquin et al. 2007; Röttinger et al. 2008). The attractive idea that skeleton formation is regulated by the ectodermal cues was first proposed more than 70 years ago (von Ubish, 1937), although the molecular cues implicated in such interactions are being identified only in recent years. So far, essential signals released by the ectoderm have been identified among growth factors and include univin, VEGF, and FGF (Zito et al. 2003; Duloquin et al. 2007; Röttinger et al. 2008). It seems that each of these growth factors is required for controlling skeleton morphogenesis, probably using independent pathways that are not functionally redundant.
Univin is the first gene encoding a member of the TGF-β superfamily to be identified in the sea urchin embryo (Stenzel et al. 1994). The univin amino acid sequence is closely related to zDVR-1 (zebrafish), BMPs-2 and 4 (human), decapentaplegic protein (dpp) (Drosophila), and Vg-1 (Xenopus) (Stenzel et al. 1994; Lapraz et al. 2006). At the blastula stage, univin is expressed in a circum-equatorial ring of ectodermal cells and during gastrulation is expressed in the archenteron. Univin transcripts are confined to bilateral regions of the ectoderm between the arms of the young pluteus larva (Stenzel et al. 1994; Range et al. 2007). We have shown that embryos with skeletal defects caused by ECM–ectoderm cell-binding inhibition (see above, Fig. 8.6 proposed model), and expressing low levels of univin and SM30, could be rescued by univin mRNA microinjection with the contemporaneous partial resumption of SM30 mRNA levels (Zito et al. 2003). This was the first evidence showing that a growth factor produced by ectoderm cells is the inductive signal responsible for skeleton growth. Later, Duloquin et al. (2007) demonstrated the guidance role of VEGF/VEGFR signaling for the positioning and differentiation of PMCs during gastrulation. At the early gastrula stage, the VEGFR is expressed only in the PMCs, while VEGF is expressed in two restricted areas of the ventrolateral ectoderm overlying the PMCs clusters. At the pluteus stage, VEGF transcripts are detected only in a few ectodermal cells, located at the tip of the arm buds, which face VEGFR-expressing PMCs. The impairment of VEGF/VEGFR expression, obtained by morpholinos injection, causes the inhibition of SM50 and SM30 and leads to skeleton-lacking embryos. MSP130 expression is not significantly affected, suggesting that it is not under the control of the VEGF/VEGFR signaling machinery. In the same way, Röttinger et al. (2008) have identified and characterized the FGFA gene, and its putative receptor genes FGFR1 and FGFR2. The co-localization of their transcripts during development, observed by in situ hybridization, as well as loss-of-function experiments, involving morpholinos injection, have shown that they regulate PMCs migration and dramatically affect skeleton elongation. Again, a block of SM30 and SM50 expression is demonstrated in the PMCs.
5 Ecotoxicological Approaches to the Study of Skeletogenesis
Toxicology is one of the oldest sciences. In the 1500s, Paracelsus found a way to overcome the difficulties in defining which substances are toxic and which are not, by his famous aphorism: “only dose makes the difference”. Modern ecotoxicology, can be defined as the integration of toxicology and ecology, and aims to quantify the toxic effects of stressors upon changes in the ecosystem composition (Truhaut 1977; Chapman 2002). The most relevant chemical/physical stressors for all organisms, including the marine ones, are: (1) physical agents such as ionizing radiation (UV-B and X-rays); (2) inorganic chemicals, such as heavy metals, nitrates, and nitrites; (3) organic chemicals, such as pesticides, oil, haloorganics, hydrocarbons, phenols, synthetic materials; and (4) emerging stressors, such as metallic and engineered nanoparticles, CO2, pH, temperature. Here, we focused on reports about the negative influences of chemical/physical stressors on biomineralization in echinoderms.
5.1 Metals Affecting Biomineralization
In general, although metallic elements have certain properties in common, each is a distinct element with its own physicochemical characteristics which determine its biological or toxicological properties (Duffus 2002). Exposure of cells/organisms to high levels of nonessential or essential metals causes toxicity which gained attention of many research groups (ATSDR 2008; Gerber et al. 2002; CICAD 2004; Lima et al. 2008). As an example, manganese (Mn), which at physiological concentrations is involved in cellular protection, replication and bone mineralization (ATSDR 2008; Santamaria 2008; Daly 2009), can cause toxicity when its concentration are in excess (Satyanarayana and Saraf 2007).
In sea urchins, toxicity tests on embryos and larvae have been proposed for the characterization of the effects causes by a variety of toxicants, including essential and heavy metals. Metals have been classically used as inducers of skeleton malformations in sea urchin and sea star embryos. These include cadmium (Cd), zinc (Zn), copper (Cu), chromium (Cr), lead (Pb), nickel (Ni), lithium (Li), manganese (Mn), gadolinium (Gd), whose exposure causes: reduced or absent skeleton, abnormal branching patterns, extranumerary origin sites were observed (Hardin et al. 1992; Radenac et al. 2001; Coteur et al. 2003; Russo et al. 2003; Roccheri et al. 2004; Kobayashi and Okamura 2004; Agca et al. 2009; Kiyomoto et al. 2010; Pinsino et al. 2010; Saitoh et al. 2010). In some cases, (Cd and Mn exposures) defects in biomineralization have been correlated to the expression of stress proteins, such as hsp60 and hsp70 (Roccheri et al. 2004; Pinsino et al. 2010), apoptosis (Filosto et al. 2008), and Metallothionein mRNA overexpression (Russo et al. 2003). The expression of biomineralization-related genes has been investigated only in lithium-treated and in zinc-treated embryos, by a high throughput analysis based on gene array screening and whole mount in situ hybridization, providing evidence of the differential expression of more than 4,000 and 250 genes, respectively (Poustka et al. 2007). Four genes expressed in PMCs, namely, Pmar, SMAD2, P19, SM50, seemed to be lightly upregulated upon treatment. Further investigation is needed in this direction.
5.2 Ionizing Radiations
An increase in UV-B radiation due to the thinning of the ozone hole can reach the Earth, including marine ecosystems. Although the penetration of UV-B into natural waters can vary, depending on the concentration and optical qualities of dissolved organic matter, phytoplankton, or other suspended particles (Dunne and Brown 1996), its consequences can have serious effects on organisms. Some planktonic embryos, larvae, and adults of many species are more prone to UV-B as they dwell in the top layers of the water column (Hader 2000). Harmful effects of water-penetrating UV-B radiation cause strong impairment of development as well as modifications in cellular protein composition, especially in the modulation of stress proteins levels, mis-regulation in gene expression and DNA damage (Batel et al. 1998; Schröder et al. 2005; Bonaventura et al. 2005, 2006; Holzinger and Lütz 2006; Tedetti and Sempéré 2007; Banaszak and Lesser 2009). In the following, we will briefly review the effects of ionizing radiation on the formation of the skeleton.
The effects of UV-B radiation on skeletons of echinoderms were studied at early and late stages of development on P. lividus. Embryos experimentally exposed to UV-B were lacking the skeleton or had severe skeleton abnormalities (Fig. 8.7). In addition, increased levels of the heat shock proteins 70 (hsp70) and the activation of p38 MapK were found (Bonaventura et al. 2005, 2006). Russo et al. (2010) demonstrating a direct dose-dependent relationship between UV-B exposure of sea urchin embryos and the mRNA levels of Pl-14-3-3ε, a gene involved in stress, survival, and apoptosis, suggesting its implication in the regulative cascade activated in response to UV-B irradiation. Recent evidence demonstrated that embryos exposed to high doses of X-rays lack spicules and their PMCs were delocalized inside the blastocoel. These results, together with a reduced expression of SM30 by RT-PCR and msp130 by ISH, indicate that X-rays strongly affect sea urchin embryo biomineralization (Matranga et al. 2010).
5.3 Impacts of Ocean Acidification on Biocalcification
Calcification is one of the primary targets for many studies on the impact of CO2-driven climate change in the oceans since the calcium carbonate shells or skeletons of many organisms make them potentially susceptible to dissolution in acidic waters (Orr et al. 2005). The pH shift consequent to CO2 dissolution in seawater changes the equilibrium between bicarbonate and carbonate, depleting the available carbonate pool (Gattuso et al. 2010). It has been demonstrated that CO2-induced seawater acidification alters skeletogenesis of different developing sea urchin species: Hemicentrotus pulcherrimus and Echinometra mathaei (Kurihara and Shirayama 2004), L. pictus (O’Donnell et al. 2010), P. lividus (Dupon et al. 2010), and Tripneusteus gratilla (Sheppard-Brennand et al. 2010). Embryos showed significant perturbation on both size and shape, which were enhanced upon simultaneous seawater warming and acidification, suggesting that although much emphasis has been placed on ocean acidification, embryos may not reach the skeletogenic stage because of warm waters (Sheppard-Brennand et al. 2010).
When looking at gene expression profiles using genome microarrays of L. pictus, O’Donnell et al. (2010) showed that, among genes involved in energy metabolism and biomineralization, only a few were downregulated, including Suclg-1succinyl-CoA synthetase, SM30-like, osteonectin, whereas only the Atp2a1-Ca2+ ATPase, involved in ion regulation and acid–base balance pathways was upregulated. All together, these results suggest that, although larvae are able to form an endoskeleton, increased CO2 levels have consequences on larval transcriptome. Contrary to expectations, no visible differences in developmental timing or obvious developmental skeleton abnormalities were associated with CO2 treatments in S. purpuratus. However, the trascriptomic approach has evidenced the downregulation of a few biomineralization genes, namely: cyclophylins, MSP130, MSP130-related, collagens (COLP3α, COLP4α, etc.), P19, P16, P16-like, osteonectin (Todgham and Hofmann 2009).
6 Concluding Remarks
The formation of the sea urchin skeleton offers a good model for the in vivo study of biocalcification both in adults and embryos, the latter where most of the studies were performed. In summary, to build the magnesian calcitic embryonic skeleton, cellular, microenvironmental conditions and a biomolecular toolkit are needed. PMCs, the only cells in the embryo controlling skeleton origin and growth, provide a set of specific spicule matrix proteins and supersaturated calcium carbonate niches. The process initiates with the formation of intermediate transient aggregating nanoparticles of ACC, which then crystallizes into macroscopic single rhombohedral crystals of calcite (Yang et al. 2011). Radial growth of the crystals and its branching is probably dictated by more than 200 PMCs-specific proteins, the most famous being SM30 and SM50, the first to be fully described (reviewed by Wilt 1999), which take part in the above-mentioned mechanism, possessing different roles. Within the gene repertoire of PMCs, among a great number of annotated biomineralization-related proteins, potential spicule matrix proteins are categorized as such when having at least one of the following characteristics: a lectin domain (or a C-type lectin domain), a signal sequence, pro/gly-rich or asp-rich regions (Livingston et al. 2006; Mann et al. 2010).
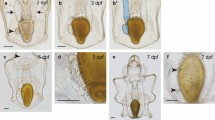
Effects of physical and chemical impacts on skeleton growth. Morphologies of P. lividus sea urchin embryos observed 48 h after fertilization. (a) control embryo at the pluteus stage, (b) UV-B embryo pulse-irradiated at the mesenchyme blastula stage (modified from Bonaventura et al. 2005), (c) X-rays embryo pulse-irradiated at the cleavage stage (modified from Matranga et al. 2010), (d) embryo continuously exposed to CdCl2 from fertilization (modified from Russo et al. 2003), and (e) embryo continuously exposed to MnCl2 from fertilization (modified from Pinsino et al. 2010)
Despite extensive information at the gene level, there is still very little information about the cellular and/or molecular mechanisms behind the biomineralization occurring in sea urchin embryos. More studies are required for a thorough description of the process which might proceed following a few main experimental directions. The first, aimed at the characterization of the role of each protein could involve in vitro crystallization tests showing the inhibition of calcium carbonate precipitation, as shown for the calprismin and caspartin, two small acidic proteins, purified from the mollusc Pinna nobilis (Marin et al. 2005). Preliminary experiments utilizing a crude extract obtained from acetic acid extraction of P. lividus tests powder (see Fig. 8.8) encourage the use of such an assay with purified recombinant spicule matrix proteins. A second experimental approach relates to studies on protein–mineral interactions where proteins might be challenged as template molecules having an effect on the patterning of the crystal.
In conclusion, despite the difference in composite “design” between vertebrates and echinoderms, the proteins related to mineralization of calcium-based skeletal tissue have remarkable similarities. Therefore, studying differences in the mineral end-products of one group will continue to illuminate processes in the other.
References
Addadi L, Raz S, Weiner S (2003) Taking advantage of disorder: amorphous calcium carbonate and its roles in biomineralization. Adv Mat 15:959–970
Agca C, Klein WH, Venutia JM (2009) Respecification of ectoderm and altered Nodal expression in sea urchin embryos after cobalt and nickel treatment. Mech Dev 126:430–442
Aizenberg J, Lambert G, Addadi L, Weiner S (1996) Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv Mat 8:222–226
Alvares K, Dixit SN, Lux E, Veis A (2009) Echinoderm Phosphorylated Matrix Proteins UTMP16 and UTMP19 Have Different Functions in Sea Urchin Tooth Mineralization. J Biol Chem 284:26149–26160
ATSDR (2008) Draft toxicological profile for manganese. Agency for toxic substances and disease registry. Division of toxicology and environmental medicine/applied toxicology branch, Atlanta, Georgia. Available via DIALOG. http://www.atsdr.cdc.gov/tox profiles/tp151-p.pdf
Banaszak AT, Lesser MP (2009) Effects of solar ultraviolet radiation on coral reef organisms. Photochem Photobiol Sci 8:1276–1294
Batel R, Fafandjel M, Blumbach B, Schröder HC, Hassanein HM, Müller IM, Müller WE (1998) Expression of the human XPB/ERCC-3 excision repair gene-homolog in the sponge Geodia cydonium after exposure to ultraviolet radiation. Mutat Res 409:123–133
Beniash E, Aizenberg J, Addadi L et al (1997) Amorphous calcium carbonate transforms into calcite durino sea urchin larval spicule growth. Proc R Soc Lond B 264:461–465
Berman A, Hanson J, Leiserowitz L, Koetzle TF, Weiner S, Addadi L (1993) Biological control of crystal texture: A widespread strategy for adapting crystal properties to function. Science 259:776–779
Bisgrove BW, Andrews ME, Raff RA (1991) Fibropellins, products of an EGF repeat-containing gene, form a unique extracellular matrix structure that surrounds the sea urchin embryo. Dev Biol 146:89–99
Bonaventura R, Poma V, Costa C, Matranga V (2005) UVB radiation prevents skeleton growth and stimulates the expression of stress markers in sea urchin embryos. Biochem Bioph Res Co 328:150–157
Bonaventura R, Poma V, Russo R, Zito F, Matranga V (2006) Effects of UV-B radiation on the development and hsp 70 expression in sea urchin cleavage embryos. Mar Biol 149:79–86
Candia Carnevali MD, Thorndyke MC, Matranga V (2009) Regenerating echinoderms: a promise to understand stem cells potential. In: Stem cells in marine organisms (eds: Rinkevich B, Matranga V) Springer, New York, pp 165–186
Chapman PM (2002) Integrating toxicology and ecology: putting the "eco" into ecotoxicology. Mar Poll Bull 44:7–15
CICAD (2004) Manganese and its compounds: environmental aspects. Concise international chemical assessment document63. WHO, Geneva, Switzerland Available via DIALOG. http://www.who.int/ipcs/publications/cicad/cicad63_rev_1.pdf
Costa C, Cavalcante C, Zito F, Yokota Y, Matranga V (2010) Phylogenetic analysis an homology modelling of Paracentrotus lividus nectin. Mol Divers 14:653–665
Coteur G, Gosselin P, Wantier P, Chambost-Manciet Y, Danis B, Pernet P, Warnau M, Dubois P (2003) Echinoderms as bioindicators, bioassays, and impact assessment tools of sediment-associated metals and PCBs in the North Sea. Arch Environ Contam Toxicol 45:190–202
Daly MJ (2009) A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol 7:237–244
Drager BJ, Harkey MA, Iwata M, Whitele AH (1989) The expression of embryonic primary mesenchyme genes of the sea urchin, Strongylocentrotus purpuratus, in the adult skeletogenic tissues of this and other species of echinoderms. Dev Biol 133:14–23
Duffus JH (2002) Effect of Cr(VI) exposure on sperm quality. Ann Occup HygMar 46:269–270
Dunne RP, Brown BE (1996) Penetration of solar UVB radiation in shallow tropical waters and its potential biological effects on coral reefs; results from the central Indian Ocean and Andaman Sea. Mar Ecol Prog Ser 144:109–118
Dupon S, Ortega-Martinez O, Thorndyke M (2010) Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19:449–462
Ettensohn CA (2009) Lessons from a gene regulatory network: echinoderm skeletogenesis provides insights into evolution, plasticity and morphogenesis. Development 136:11–21
Falini G, Albeck S, Weiner S, Addadi L (1996) Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271:67–69
Filosto S, Roccheri MC, Bonaventura R, Matranga V (2008) Environmentally relevant cadmium concentrations affect development and induce apoptosis of Paracentrotus lividus larvae cultured in vitro. Cell Biol Toxicol 24:603–610
Frankel RB, Bazylinski DA (2003) Biologically induced mineralization by bacteria. Rev Mineral Geochem 54:95–114
Gattuso JP, Gao K, Lee K, Rost B, Schulz KG (2010) Approaches and tools to manipulate the carbonate chemistry. In: Riebesell U, Fabry VJ, Hansson L, Gattuso J-P (eds) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union, Luxembourg, pp 41–52
Gerber GB, Leonard A, Hantson Ph (2002) Carcinogenicity, muta- genicity and teratogenicity of manganese compounds. Crit Rev Oncol Hematol 42:25–34
Guss KA, Ettensohn CA (1997) Skeletal morphogenesis in the sea urchin embryo: regulation of primary mesenchyme gene expression and skeletal rod growth by ectoderm-derived cues. Development 124:1899–1908
Hader DP (2000) Effects of solar UV-B radiation on aquatic ecosystems. Adv Space Res 26:2029–2040
Hardin J, Coffman JA, Black SD, McClay DR (1992) Commitment along the dorsoventral axis of the sea urchin embryo is altered in response to NiCl2. Development 116:671–685
Heatfield BM, Travis DF (1975) Ultrastructural studies of regenerating spines of the sea urchin Strongylocentrotus purpuratus. II. Cells with spherules. J Morphol 145:51–72
Hodor PG, Illies MR, Broadley S, Ettensohn CA (2000) Cell-substrate interactions during sea urchin gastrulation: migrating primary mesenchyme cells interact with and align extracellular matrix fibers that contain ECM3, a molecule with NG2-like and multiple calcium-binding domains. Dev Biol 222:181–194
Holzinger A, Lütz C (2006) Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37(190–606):207
Hörstadius S (1939) The mechanics of sea urchin development, studied by operative methods. Biol Rev 14:132–179
Ingersoll EP, Wilt FH (1998) Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev Biol 196:95–106
Kato T (2000) Polymer/calcium carbonate layered thin-film composites. Adv Mater 12:1543–1546
Kato KH, Abe T, Nakashima S, Matranga V, Zito F, Yokota Y (2004) ‘Nectosome’: a novel cytoplasmic vesicle containing nectin in the egg of the sea urchin, Temnopleurus hardwickii. Develop Growth Differ 46:239–247
Katow H (1995) Pamlin, a primary mesenchyme cell adhesion protein, in the basal lamina of the sea urchin embryo. Exp Cell Res 218:469–478
Killian CE, Croker L, Wilt FH (2010) SpSM30 gene family expression patterns in embryonic and adult biomineralized tissues of the sea urchin, Strongylocentrotus purpuratus. Gene Expr Patterns 10:135–139
Kiyomoto M, Zito F, Sciarrino S (2004) Commitment and response to inductive signals of primary mesenchyme cells of the sea urchin embryo. Dev Growth Differ 46:107–114
Kiyomoto M, Morinaga S, Ooi N (2010) Distinct embryotoxic effects of lithium appeared in a new assessment model of the sea urchin: the whole embryo assay and the blastomere culture assay. Ecotoxicology 19:563–770
Kniprath E (1974) Ultrastructure and growth of the sea urchin tooth. Calc Tiss Res 14:211–228
Kobayashi N, Okamura H (2004) Effects of heavy metals on sea urchin embryo development. Chemosphere 55:1403–1412
Kurihara H, Shirayama Y (2004) Effects of increased atmospheric CO2 on sea urchin early development. Mar Ecol Progr Series 274:161–196
Lapraz F, Röttinger E, Duboc V et al (2006) RTK and TGF-β signaling pathways genes in the sea urchin genome. Dev Biol 300:132–152
Lima PDL, Vasconcellos MC, Bahia MO, Montenegro RC, Pessoa CO, Costa-Lotufo LV, Moraes MO, Burbano RR (2008) Genotoxic and cytotoxic effects of manganese chloride in cultured human lymphocytes treated in different phases of cell cycle. Toxicol In Vitro 22:1032–1037
Livingston BT, Killian CE, Wilt F et al (2006) A genome-wide analysis of biomineralization-related proteins in the sea urchin Strongylocentrotus purpuratus. Dev Biol 300:335–348
Lowenstam HA (1981) Minerals formed by organisms. Science 211:1126–1131
Lowenstam HA, Weiner S (1989) On Biomineralization. Oxford University Press, New York
Mann S (1983) Mineralization in biological systems. Struct Bonding 54:125–174
Mann S (2001) Biomineralization: principles and concepts in bioinorganic materials chemistry. Oxford University Press, New York
Mann K, Poustka AJ, Mann M (2008a) In-depth, high-accuracy proteomics of sea urchin tooth organic matrix. Proteome Sci 6:33
Mann K, Poustka AJ, Mann M (2008b) The sea urchin (Strongylocentrotus purpuratus) test and spine proteomes. Proteome Sci 6:22
Mann K, Wilt FH, Poustka AJ (2010) Proteomic analysis of sea urchin (Strongylocentrotus purpuratus) spicule matrix. Proteome Science 8:33
Marin F, Amons R, Guichard N, Stigter M, Hecker A, Luquet G, Layrolle P, Alcaraz G, Riondet C, Westbroek P (2005) Caspartin and calprismin, two proteins of the shell calcitic prisms of the Mediterranean fan mussel Pinna nobilis. J Biol Chem 280:33895–33908
Märkel K, Röser U (1985) Comparative morphology of echinoderm calcified tissues: Histology and ultrastructure of ophiuroid scales (Echinodermata, Ophiuroida). Zoomorphology 105:197–207
Märkel K, Röser U, Mackenstedt K (1986) Ultrastructural investigations of matrix-mediated biomineralization in echinoids (Echinodermata, Echinoidea). Zoomorphology 106:232–243
Matranga V, Di Ferro D, Zito F, Cervello M, Nakano E (1992) A new extracellular matrix protein of the sea urchin embryo with properties of a substrate adhesion molecule. Roux‘s Arch Dev Biol 201:173–178
Matranga V, Zito F, Costa C, Bonaventura R, Giarrusso S, Celi F (2010) Embryonic development and skeletogenic gene expression affected by X-rays in the Mediterranean sea urchin Paracentrotus lividus. Ecotoxicology 19:530–537
McClay DR, Alliegro MC, Black SD (1990) The ontogenetic appearance of extracellular matrix during sea urchin development. In Organization and assembly of plant and animal extracellular matrix (eds: Adair WS, Mecham R). pp 1–13 Academic Press, San Diego, CA
O’Donnell MJ, Todgham AE, Sewell MA, Hammond LM, Ruggiero K, Fangue NA, Zippay ML, Hofmann GE (2010) Ocean acidification alters skeletogenesis and gene expression in larval sea urchins. Mar Ecol Progr Series 398:157–171
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner GK, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig MF, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Pinsino A, Thorndyke MC, Matranga V (2007) Coelomocytes and post-traumatic response in the common sea star Asterias rubens. Cell Stress Chap 12:332–342
Pinsino A, Matranga V, Trinchella F, Roccheri MC (2010) Sea urchin embryos as an in vivo model for the assessment of manganese toxicity: developmental and stress response effects. Ecotoxicology 19:555–562
Poustka AJ, Kühn A, Groth D, Weise V, Yaguchi S, Burke RD, Herwig R, Lehrach H, Panopoulou G (2007) A global view of gene expression in lithium and zinc treated sea urchin embryos: new components of gene regulatory networks. Genome Biol 8:R85
Radenac G, Fichet D, Miramand P (2001) Bioaccumulation and toxicity of four dissolved metals in Paracentrotus lividus sea-urchin embryo. Mar Environ Res 51:151–166
Range R, Lapraz F, Quirin M, Marro S, Besnardeau L, Lepage T (2007) Cis-regulatory analysis of nodal and maternal control of dorsal–ventral axis formation by Univin, a TGF-β related to Vg1. Development 134:3649–3664
Raz S, Weiner S, Addadi L (2000) The formation of high magnesium calcite via a transient amorphous colloid phase. Adv Mater 12:38–42
Raz S, Hamilton P, Wilt F, Weiner S, Addadi L (2003) The transient phase of amorphous calcium carbonate in sea urchin larval spicules: the involvement of proteins and magnesium ions in its formation and stabilization. Adv Funct Mater 13:480–486
Roccheri MC, Agnello M, Bonaventura R, Matranga V (2004) Cadmium induces the expression of specific stress proteins in sea urchin embryos. Biochem Biophys Res Commun 321:80–87
Röttinger E, Saudemont A, Duboc V et al (2008) FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis and regulate gastrulation during sea urchin development. Development 135:354–365
Russo R, Bonaventura R, Zito F, Schroder HC, Muller I, Muller WEG, Matranga V (2003) Stress to cadmium monitored by metallothionein gene induction in Paracentrotus lividus embryos. Cell Stress Chaperones 8:232–241
Russo R, Zito F, Costa C, Bonaventura R, Matranga V (2010) Transcriptional increase and misexpression of 14-3-3 epsilon in sea urchin embryos exposed to UV-B. Cell Stress Chaperones 15:993–1001
Saitoh M, Kuroda R, Muranaka Y, Uto N, Murai J, Kuroda H (2010) Asymmetric inhibition of spicule formation in sea urchin embryos with low concentrations of gadolinium ion. Dev Growth Differ 52:735–746
Santamaria AB (2008) Manganese exposure, essentiality & toxicity. Indian J Med Res 128:484–500
Satyanarayana YV, Saraf R (2007) Iron and manganese contamination: sources, adverse effects and control methods. J Environ Sci Eng 49:333–336
Schröder HC, Di Bella G, Janipour N, Bonaventura R, Russo R, Müller WE, Matranga V (2005) DNA damage and developmental defects after exposure to UV and heavy metals in sea urchin cells and embryos compared to other invertebrates. Prog Mol Subcell Biol 39:111–137
Sheppard-Brennand H, Soars N, Dworjanyn SA, Davis AR, Byrne M (2010) Impact of ocean warming and ocean acidification on larval development and calcification in the sea urchin tripneustes gratilla. PLoS One 5:e11372
Simkiss K (1986) The processes of biomineralization in lower plants and animals-an overview. In: Leadbeater BSC, Riding R (eds) Biomineralization in lower plants and animals, vol 30. Oxford University Press, New York, pp 19–37
Simkiss K, Wilbur K (1989) Biomineralization. Cell Biology and Mineral Deposition. Academic Press, Inc., San Diego
Smith LC, Ghosh J, Buckley MK, Clow AL, Dheilly MN, Haug T et al (2010) Echinoderm immunity. In: Soderhall K (ed) Invertebrate immunology. Landes Bioscience, Inc
Stenzel P, Angerer LM, Smith BJ, Angerer RC, Vale WW (1994) The univin gene encodes a member of the transforming growth factor-beta superfamily with restricted expression in the sea urchin embryo. Dev Biol 166:149–158
Stricker SA (1985) The ultrastructure and formation of the calcareous ossicles in the body wall of the sea cucumber Leptosynapta clarki (Echinodermata, Holothuroida). Zoomorphology 105:209–222
Tedetti M, Sempéré R (2007) Penetration of ultraviolet radiation in the marine environment. A review Photochem Photobiol 82:389–397
Tesoro V, Zito F, Yokota Y, Nakano E, Sciarrino S, Matranga V (1998) A protein of the basal lamina of the sea urchin embryo. Dev Growth Differ 40:527–535
Todgham AE, Hofmann GE (2009) Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol 212:2579–2594
Truhaut R (1977) Eco-toxicology – objectives, principles and perspectives. Ecotoxicology and Environm Safety 2:151–173
Weber JN, Raup DM (1966) Fractionation of the stable isotopes of carbon and oxygen in marine calcareous organisms—the Echinoidea. Part II. Environmental and genetic factors. Geochim Cosmochim Acta 30:705–736
Weiss IM, Tuross N, Addadi L, Weiner S (2002) Mollusk larval shell formation: amorphous calcium carbonate is a precursor for aragonite. J Exp Zool 293:478–491
Wessel G, Berg L (1995) A spatially restricted molecule of the extracellular matrix is contributed both maternally and zygotically in the sea urchin embryo. Dev Growth Diff 37:517–527
Wessel GM, Etkin M, Benson S (1991) Primary mesenchyme cells of the sea urchin embryo require an autonomously produced, nonfibrillar collagen for spiculogenesis. Dev Biol 148:261–272
Wilt F (1999) Matrix and mineral in the sea urchin larval skeleton. J Struct Biol 126:216–226
Wilt FH, Killian CE, Hamilton P, Croker L (2008) The dynamics of secretion during sea urchin embryonic skeleton formation. Exp Cell Res 314:1744–1752
Yang L, Killian CE, Kunz M, Tamura N, Gilbert PUPA (2011) Biomineral nanoparticles are space-filling. Nanoscale 3:603–609
Yokota Y, Matranga V, Zito F, Cervello M, Nakano E (1994) Nectins in sea urchin eggs and embryos. J Mar Biol Ass UK 74:27–34
Zito F, Matranga V (2009) Secondary mesenchyme cells as potential stem cells of the sea urchin embryo. In Stem cells in marine organisms (eds: Rinkevich B, Matranga V). Springer, New York, pp 187–213
Zito F, TesoroV McClay DR, Nakano E, Matranga V (1998) Ectoderm cell–ECM interaction is essential for sea urchin embryo skeletogenesis. Dev Biol 196:184–192
Zito F, Costa C, Sciarrino S, Poma V, Russo R, Angerer LM, Matranga V (2003) Expression of univin, a TGF-beta growth factor, requires ectoderm–ECM interaction and promotes skeletal growth in the sea urchin embryo. Dev Biol 264:217–227
Zito F, Burke RD, Matranga V (2010) Pl-nectin, a discoidin family member, is a ligand for betaC integrins in the sea urchin embryo. Matrix Biol 29:341–345
Acknowledgments
This work was supported by the BIOMINTEC Project (European Commission N° PITN-GA-2008-215507 grant). Authors are indebted to Prof. Frederic Marin for access to and help in the use of the SEM at the UMR 5561 CNRS BiogÕosciences, UniversitÕ de Bourgogne, 21000 Dijon, France. Authors thank Mr. Mauro Biondo for his valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Matranga, V. et al. (2011). Echinoderms as Blueprints for Biocalcification: Regulation of Skeletogenic Genes and Matrices. In: Müller, W. (eds) Molecular Biomineralization. Progress in Molecular and Subcellular Biology(), vol 52. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21230-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-21230-7_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-21229-1
Online ISBN: 978-3-642-21230-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)