Abstract
There are many economic and negative environmental impacts that need to be solved before biofuels become a viable replacement for some of the fossil fuel demand. One environmental problem is the great amount of water required for the production of biofuels. The use of halophilic/halotolerant algae can greatly reduce the amount of water required for biodiesel production. The use of halophiles and their enzymes for degrading cellulosic biomass might also help reduce the need for high temperatures and pH neutralization of the pretreated biomass before fermentation. Significant amounts of information have been discovered about extremophilic lignocellulolytic systems. Investigations of halophilic lignocellulolytic degradation are at their initial stages. Modern recombinant screening techniques and data mining of genomes have the potential to yield many halophilic lignocellulytic enzymes. Thus, there is great potential for developing biotechnologies with halophilic/halotolerant algae for the production of biodiesel as well as halophilic lignocellulytic enzymes for treating biomass.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
18.1 Introduction
Biofuels hold great promise as a replacement for some of our demand for fossil fuel. However, a number of environmental and economical costs have to be reduced before biofuels can be a suitable replacement for fossil fuels. These costs include the use of land, water and energy. Furthermore, these costs place the production of biofuels in direct competition with food production (Pimentel et al. 2009). To overcome some of these costs, solutions such as the efficient use of non-food biomass have to be developed and conservation of water via recycling.
Non-food plant biomass is an abundant, renewable source of stored energy. This material holds great potential for providing energy in the form of alcohols, hydrogen, and methane production, as well as electricity generation from microbial fuel cells. Typical forms of biomass include switch-grass, corn stover and wood chips. Biomass exists as cellulose and hemicellulose, encased by lignin. The purpose of the cellulose, hemicellulose, and lignin matrix is to provide structural support for the plant. Thus, this material is highly resistant to biodegradation. This also makes harsh pretreatments, high temperature and extreme pH conditions, necessary to prepare biomass for subsequent fermentation reactions (Mosier et al. 2005). Alkali pretreatment, such as lime, has been used to treat wheat straw, poplar wood, switchgrass, and cornstover. Lime can be substituted by alkaline salts during alkaline pretreatment. This can result in pH and salt concentrations similar to those found in alkaline saline lakes. Thus, bacteria that thrive under these conditions can possibly be used to further breakdown biomass after alkaline pretreatment and subsequent biofuel production.
The use of halophilic microorganisms to reduce demand for freshwater usage is not new (Woolard and Irvine 1995). To elevate the water costs of biofuel/biodiesel production, water recycle will have to be utilized. However, as water is recycled, the salts that are present will accumulate and become inhibitory to freshwater biodiesel-producing algae. Halotolerant/halophilic algae present a solution to this impediment.
18.1.1 Cellulose
Cellulose generally is a major component of biomass and exists in forms that are exceptionally stable in terms of both conformational structure and chemical bonds. This makes the molecules relatively recalcitrant due to limited solubility and high heterogeneity (Schwarz 2001). Additional hindrances to biodegradation result from the sheer size of cellulose molecules. The structure and size of cellulose preclude uptake by cells and enzymes must be secreted into the medium or bound to the outside of the cell (Beguin and Aubert 1994). However, the tremendous availability of cellulosic biomass, estimated at 30 Gt per year in global terrestrial production, has resulted in the development of very diverse, multi-faceted approaches of cellulose biodegradation in the biosphere (Cox et al. 2000). Despite the awareness of the array of cellulose biodegradative mechanisms, there have been very few biotechnologies developed from cellulolytic organisms. It is estimated that of all the microorganisms within the biosphere, only 1–2% have been transferred into an industrial or commercial applications (Gomes and Steiner 2004) and even this percentage appears to be a gross over-exageration. Given that context, there are obviously vast, untapped reserves of cellulolytic biotechnology still awaiting discovery.
18.1.2 Hemicellulose
Hemicelluloses are a broad group of heterogeneous branched and linear chain polysaccharides that account for 20–40% of lignocellulose. Within the lignocellulose complex, hemicellulose is covalently bound to lignin sheaths and interacts with cellulose through hydrogen bonds (Biely et al. 1985; Joseleau et al. 1992). These interactions aid in structural integrity and protect the cellulose from degradation by cellulases (Uffen 1997). Unlike cellulose and lignin, the composition of hemicellulose varies greatly between plant species in backbone structure, branching, and modifications. Hemicelluloses can be categorized based on the carbohydrate polymer composition. These include xylan (d-xylose), xyloglucan (d-xylose and d-glucose), glucomannan (d-glucose and d-mannose), galactoglucomannan (d-galactose, d-glucose, and d-mannose), and arabinogalactan (d-galactose and l-arabinose). A more comprehensive review of hemicellulose structure and biological function can be found in Puls and Schuseil (1993).
Due to the diversity of these polysaccharides, microorganisms involved in their degradation require the use of multiple enzymes with varying specificity and function. A genome analysis of ten representative hemicellulose-degrading bacteria revealed that each bacterium has between 4 and 14 annotated genes encoding hemicellulases (Shallom and Shoham 2003). The inherent nature of hemicellulose provides additional challenges including high molecular weight, insolubility, and rigid structure. In order to cope with these challenges, multiple hemicellulases are secreted to convert large polymers into smaller oligosaccharides, disaccharides, and monosaccharides, which can then be internalized and further metabolized (Wong et al. 1988). Total conversion of hemicellulose requires glycoside hydrolases to break the sugar backbone and branched sugar residues as well as esterases to remove acetyl and ferulic acid modifying groups (Shallom and Shoham 2003; Saha 2003).
18.1.3 Lignin
Lignin, a heterogeneous polymer made up of phenylpropanoid interunits linked by covalent bonds, is the second most abundant raw material on earth. It is a major component in the cell wall structure of many plants, providing strength and rigidity to the plant structure and helps in water transport. There are several isomers of lignin most of which are structurally complex and very resistant to microbial degradation (Li et al. 2009). Enzymes capable of degrading lignin under aerobic conditions are grouped into two main categories, peroxidases and phenol oxidases. Phenol oxidases can be further divided to include two groups of enzymes, laccases and polyphenol oxidases. On the other hand, anaerobic organisms primarily use enzymes involved in the degradation of phenols such as phenylphosphate synthase and phenylphosphate carboxylase. Many studies have focused on microbial breakdown of lignin and few have considered lignin degradation under extreme conditions of pH, temperature or high salt concentrations.
18.2 Biodegradation of Cellulose
The degradation of cellulose is generally accomplished by an inducible system of cellulolytic enzymes and proteins, working synergistically, comprising a distinct cellulase system (Wood 1975). Commonly, the term cellulase is used to describe an entire system that attacks the cellulose molecule in an extremely coordinated effort to synergistically hydrolyze cellulose within the environment and not the organism. This is important because cellulolytic microorganisms are also capable of degrading hemicellulose and coproduces xylanases along with cellulolytic enzymes (Lynd et al. 2002). The specific activity of cellulases is the hydrolysis of the β-1,4-glucosidic bonds between the glucosyl residues in cellulose (Beguin and Aubert 1994).
Cellulases can function in three different ways. Exoglucanases, cellodextrinases (1,4-β-d-glucanhydrolases) and cellobiohydrolases (1,4-β-d-glucan cellobiohydrolase) hydrolyze the glycosyl bonds at either end of the cellulose polymer chain while endoglucanases, such as 1,4-β-glucan-4-glucanhydrolases cleave the cellulose chain internally. The glycosyl bond of cellobiose can be cleaved by 1,4-β-d-glucan cellobiohydrolases and β-glucoside glycohydrolases (Voget et al. 2006). Along with the catalytic domains of the general cellulase complex, a fourth, non-catalytic component is central to cellulose degradation. In general, a carbohydrate binding module must be present in the cellulase complex to attach and position the enzymes onto the cellulose and facilitate the removal of single molecules resulting from enzymatic lysis (Schwarz 2001; Lynd et al. 2002).
18.2.1 Biodegradation of Cellulose by Halophilic Bacteria
Formal cellulase research began with the United States Army where investigations into cellulose degrading organisms were undertaken in response to alarming rates of “rot” noted in the natural materials (mostly cotton) used by combat units in the South Pacific campaigns of World War II (Reese 1976). By the time of the Vietnam War, the first research results quantifying three, functionally distinct cellulase components from crude filtrates of Trichoderma viride were published (Li et al. 1965). Since then, prospecting for relevant biotechnical resources has become more focused on extremophiles since many of the cellulases with industrial relevant characteristic have already been obtained from them (Voget et al. 2006). However, little work has been done thus far with halophiles specifically for cellulase. The current survey of biotechnical and enzymatic applications involving halophiles denotes interesting applications from the development of soy and fish sauce to holographic computer storage material to enchasing crude oil recovery (Oren 2002; Sanchez-Porro et al. 2003; Pikuta et al. 2007). However, there is no mention in the current literature of any cellulase biotechnologies developed from halophiles. This isn’t surprising as limited research has been done to study the biodegradation of cellulose by halophilic bacteria even though other extremophilic organisms, specifically alkaliphiles and thermophiles, have long been employed for their cellulosic properties (Bhat and Bhat 1997; Lynd et al. 2002; Fujinami and Fujisawa 2010). However mixed cellulolytic cultures and a pure culture of Haloarcula his panica isolated from the Waste Isolation Pilot Plant were shown to attach to and produce oxalacetic and pyruvic acids from cellulose (Vreeland et al. 1998). Recently, a carboxymethyl cellulose-degrading Marinimicrobium was isolated from the Great Salt Lake, Utah (Møller et al. 2010). Furthermore, the reported incidence of enhanced enzymatic stability and activity with the addition of salt to thermophilic cellulases has been known for some time (Bronnenmeier et al. 1995). For reasons directly related to the structure and composition their proteins, halophilic enzymes systems have thus far demonstrated enhanced utility as they are not only stable in high salt environments, but the enzymes are also found to be thermotolerant and alkalphilic and thus can be considered “polyextremophilic” (Setati 2010).
18.2.2 Uniqueness of Halophilic Cellulosic Enzymes
Enzymes produced by halophilic microorganisms possess an excess of acidic amino acids providing extensive negative charges on the enzyme surfaces (Danson and Hough 1997). This unique feature of the halophilic proteome allows these proteins to remain flexible and resist aggregation that is common to non-halophilic proteins exposed to high salt concentrations (Mevarech et al. 2000). In general, halophilic enzymes actually require high salt concentrations (mainly Cl−) to stimulate transcription and translation as well as transporter activity (Averhoff and Müller 2010). Furthermore, the solubility of halophilic enzymes at high salt concentrations has allowed for unique applications whereby aqueous/organic and non-aqueous media can be employed without substantially affecting the activity or the solubility of the enzyme (van den Burg 2003; Fukushima et al. 2005; Karbalaei-Heidari et al. 2007). While there has been a relatively long history of research and development of cellulose degrading enzymes, those specifically sourced from halophilic organisms have only come to the fore relatively recently (Huang et al. 2010). The most exhaustive review of biotechnological applications of halophilic microorganisms was undertaken by Oren (2002). Within this work, no mention was given to any halophilic cellulolytic organisms thus highlighting the embryonic stage of cellulase research and development within this particular group of extremophiles. However, based on the many instances of other enzymatic types such as DNAases, lipases, amylases, gelatinases, and proteases already employed commercially from halophilic organisms, there is great anticipation that cellulase derived from halophiles will offer up disruptive technologies in the production of cellulosic based biofuels and chemicals (van den Burg 2003; Setati 2010).
Screening for unique cellulases from extremophiles in general and halophiles specifically has been hampered by the difficulty in culturing the organisms within the laboratory in sufficient quality and quantity to perform adequate analysis. An extreme example of this was documented in the 2 years it took for investigators to culture samples of sufficient quantity for analysis (Vreeland et al. 1998). Furthermore, standard chromatographic techniques are rendered ineffective as the halophile enzymes generally exhibit high solubility in high concentrations of NaCl (Eisenberg et al. 1992). Table 18.1 illustrates the specifically identified species of halophilic bacteria strains that have undergone complete experimental assays for cellulase activity.
18.2.3 Specific Halophilic and Halotolerant Cellulases
Halocella cellulolytic a is recognized as the first true halophilic cellulolytic bacterium identified within the literature (Bolobova et al. 1992). However, the advent of genomic DNA libraries of cellulases from extremophilic environments has accelerated the discovery of halophilic cellulases. Since the first metagenome-derived halotolerant cellulase was described, there have been three other halophilic cellulases defined (Rees et al. 2003). These techniques rely on recombinant expression of isolated clones to more easily produce the cellulase enzymes rather than growing up whole cultures and isolating the enzymes from them (Rees et al. 2003). Endoglucanases are often selectively screened (Voget et al. 2006). It should be noted that the use of carboxymethylcellulose (CMC) is not ideal for enzymatic studies of cellulase production as many organisms can hydrolyze CMC but cannot degrade insoluble cellulose (Lynd et al. 2002). Such false positives are thought to be an important issue since extremophiles tend to utilize of a very diverse range of carbohydrates due to the small amounts of cellulose and few competing species in their native habitat (Lynd et al. 2002). Table 18.1 presents the known halophilic endoglucanases so far presented in the literature.
Reports from surveys of hydrolytic enzymes from extreme habitats like soda lakes, salterns, and mines have revealed that there may be many more species of halophilic organisms awaiting formal review of their cellulolytic enzymes. Table 18.2 provides reports of cellulase activities discovered from surveys of hypersaline environments. Researchers using basic assays (also listed in Table 18.2) as a proxy for cellulase activity have reported impressive numbers of halophiles that express cellulase activity. It is apparent that cellulase activities occur in extreme natural environments and halophilic organisms possess cellulolytic systems. However, the reports are fairly shallow in terms of useful biotechnical information. As an example, the report of halophilic bacterial communities in a Turkish salt lake reported that there were 14 strains collected that demonstrated cellulase activity (Birbir and Sesal 2003). However, the report lacked any specific information of the biochemical methods used to deduce the cellulase activities. While such surveys could be construed to identify high incidence of halophilic cellulase, problems with over generalized assays and techniques may be inflating the reported numbers. Conversely, the identification of so many environmental isolates producing cellulase indicates that there are opportunities to discover biotechnologies that have not been demonstrated by culture collection strains (Sanchez-Porro et al. 2003).
18.3 Degradation of Hemicellulose by Halophilic Bacteria
The diversity and mechanisms of hemicellulases has been studied extensively in mesophilic bacteria and fungi as well as certain categories of extremophilic bacteria (Gilbert and Hazlewood 1993; Shallom and Shoham 2003; Collins et al. 2005). Recent interest in halophilic bacteria has resulted in the description of novel hemicellulose degrading halophilic bacteria and archaea as well as the characterization of purified halophilic and halotolerant enzymes.
18.3.1 Xylan Degradation
Xylan is the most abundant hemicellulose component in land plants (Joseleau et al. 1992). Xylan is a heterogeneous polysaccharide composed primarily of d-xylose linked by β-1,4 glycosidic bonds. These xylopyranoside units are often substituted by a variety of acetyl, arabinosyl, and glucuronosyl groups. The composition of xylan varies greatly between plant species with regard to both backbone composition and side group residues. The degradation of xylan requires endo-β-xylanases (EC 3.2.1.8) to fragment the chain, β-xylosidases (EC 3.2.1.37) to convert short xyloogliomers into monomeric units, and auxiliary enzymes to remove side group residues (Kulkarni et al. 1999).
Within the last decade, several species of halophilic and halotolerant microorganisms have been identified with the ability to degrade xylan and other polysaccharides. These include bacteria of the genera Gracilibacillus, Chromohalobacter, Bacillus, Halomonas, and Marinimicrobium, as well as the archaeon Halorhabdus (Wainø and Ingvorsen 2003; Wejse et al. 2003; Prakash et al. 2009; Giridhar and Chandra 2010; Møller et al. 2010; Wang et al. 2010a; Yang et al. 2010). Consistent characteristics of these organisms include the ability to grow in broad ranges of NaCl as well as moderate to high alkalinity.
In addition to identifying new halophilic species capable of xylan degradation, several xylanase and xylosidase enzymes have been characterized. The size of xylanases isolated from halophilic bacteria ranged from 15 kDa in the case of Chromohalobacter sp. TPSV101 to 62 kDa in the case of strain CL8 (Wejse et al. 2003; Prakash et al. 2009). Two xylanases of 45 and 67 kDa were identified in the archaeon Halorhabdus utahensis (Wainø and Ingvorsen 2003). As observed in the archaeon, several xylanases were identified in many of the bacterial isolates. The presence of several xylanases is consistent with findings in mesophilic and as well as extremophilic bacteria (Wong et al. 1988; Collins et al. 2005). These xylanases and xylosidases show activity under a wide range of physiological conditions including temperature, NaCl concentration, and pH. Activity over a wide range of NaCl concentrations in exemplified in the xylanase isolated from the moderately halophilic bacterium Gracilibacillus sp. TSCPVG, that demonstrated sustainable activity over 0–30% NaCl (Giridhar and Chandra 2010). These enzymatic properties would be essential for extracellular activity in the extreme environments from which these organisms were isolated.
In addition to xylanases, xylosidase enzymes were either characterized or their presence inferred from hydrolysate composition. β-Xylosidase was identified in both the bacterium Gracilibacillus sp. TSCPVG and archaeon Halorhabdus utahensis (Wainø and Ingvorsen 2003; Giridhar and Chandra 2010). Due to the complete degradation of xylan to d-xylose in other cultures, one can infer the presence of a xylosidase-like enzyme in other species. While none have been specifically identified, other enzymes may exist in these halophilic microorganisms that are involved in breaking down side groups and modifications. Other potential enzymes may include α-glucuronidase (EC 3.2.1.139), acetyl xylan esterase (EC 3.1.1.72), and ferulic and p-cumaric acid esterases (EC 3.1.1.73).
18.3.2 Mannan Degradation
Mannan is a polysaccharide composed of d-mannose monomers linked by β-1,4 glycosidic bonds. The mannan homopolymer is a major component of cell walls in certain species of algae and found in some seeds (Frei and Preston 1968; Whitney et al. 1998). Frequent substitutions in mannan by d-glucose results in the heteropolymer glucomannan, a component of hemicellulose, particularly in soft trees (Whitney et al. 1998). Mannan and glucomannan can be degraded by the enzymes β-1,4 mannanase (EC 3.2.1.78), β-1,4 mannosidase (EC 3.2.1.25), and β-glucosidase (EC 3.2.1.21). As with xylanases, mannanases are usually extracellular enzymes that first break the polymer into smaller ogliomers, followed by hydrolysis to monomeric sugars (Dhawan and Kaur 2007).
While not as thoroughly investigated as xylan degraders, several species of halophilic bacteria have been isolated with the ability to metabolize mannan heteropolymers. These include organisms of the genus Marinimicrobium, Pantoea, as well an organism designated as strain NN (DSM 11805) (Wainø and Ingvorsen 1999; Møller et al. 2010; Wang et al. 2010b). Pantoea agglomerans was shown to degrade both glucomannan and galactomannan substrates, while Marinimicrobium haloxylanilyticum and strain NN were shown to only metabolize glucomannan and galactomannan, respectively. As with the xylanases previously described, these enzymes were shown to function over a broad range of NaCl, pH and temperature ranges. As an insight into the physiology of these metabolisms, it was demonstrated that the majority of mannanase and especially mannosidase activity was localized to the cell wall and membrane of strain NN (Wainø and Ingvorsen 1999). As more mannan degrading enzymes are characterized, their ecological role will become more apparent.
18.4 Lignin Degradation by Halophilic and Halotolerant Microorganisms
The abundance of plants and therefore lignin in aquatic systems such as in coastal regions and salt marshes has indicated that the majority of lignin in these environments is degraded by microbial activity (Benner et al. 1986). Since very little is known about the lignin biogeochemical cycling in such ecosystems, the identification and isolation of microorganisms from high salt environments would be of ecological importance (González et al. 1997a). Fungi and bacteria are the two main groups of microorganisms involved in ligninolytic activity and both can act either individually or together to cause complete degradation of lignin. From an industrial standpoint, lignin and lignin-based derivatives are major components in the list of waste materials generated by paper and pulp industries, tanneries, molasses-based distilleries and textile mills, and can be a serious contamination problem due to their low biodegradability and high color (Raghukumar et al. 2008). Ligninolytic enzymes have been shown to be among the best decolorizing agents for treating these wastes. The waste streams in many of these industries generally have a high salinity and hence using halotolerant or halophilic microorganisms and/or enzymes would be a favorable choice for treatment. Also, in paper industries that recycle water containing high concentration of salts like NaCl such as the pulp and paper industry, it would be practical to use enzymes that are more salt tolerant so as to have effective biopulping (converting wood chips into pulp) and biobleaching (decolorizing by using enzymes) processes (Li et al. 2002).
18.4.1 Lignin Degradation by Fungi
Of the two microbial groups capable of ligninolytic activity, fungi are known to possess powerful and extensive mechanisms of lignin degradation. By far, the most commonly studied and successful lignin degradation activity has been demonstrated in many species of white rot basidiomycetous fungi under aerobic conditions. Many marine fungal species such as Digitatispora marina, Halocyphina villosa, and Nia vibrissa grow on decaying lignocellulosic substrates. A particular marine, hypersaline-tolerant isolate, Phlebia sp. strain MG-60, isolated from mangrove stands in Okinawa, Japan, has been shown to produce many enzymes for lignin degradation. One of its enzymes, manganese peroxidase, was specifically screened for its expression under varying conditions of salt and nitrogen. Its expression is regulated by the presence of Mn2+ and a considerable increase in the level of two gene transcripts was detected when more salt was added (Kamei et al. 2008). Any excess addition of Mn2+ or NH +4 inhibited the enzyme production but the addition of salt reversed the inhibition partially or completely (Li et al. 2003). Other activity included laccase and manganese peroxidase that were capable of effectively decolorizing the dye Poly R-478, an indicator of delignification by fungi. However, an increase in salt concentration decreased this decolorization property. Furthermore MG-60 was able to whiten unbleached pulp more efficiently than the commonly studied white rot fungus for lignin degradation, Phanerochaete chrysosporium, under increased hypersaline conditions (Bucher et al. 2004). The activity of laccases, in a similar study on marine fungi, seemed to be enhanced by the addition of copper or a combination of copper and guaiacol in a nitrogen rich medium.
The lignin degrading mechanism can be applied towards bioremediation efforts as shown by the halotolerant fungus, Pestalotiopsis sp. SN-3. An efficient lignin degrader, SN-3 produces laccase and possessed a significant potential for the degradation of toxic substances in marine environments. This laccase was extremely tolerant to high salt conditions and also had high enzymatic activity. Two novel thermo-halotolerant laccase isoforms were produced by Pycnoporus sanguineus. The purified laccases showed high thermal stability and hence can have a longer shelf-life (González et al. 2008). An advantage of using laccase over other enzymes is that laccase does not require components such as manganese or hydrogen peroxide and hence preferred in environmental applications. Several researchers are actively exploring fungal species that can grow under hypersaline conditions while simultaneously breaking down lignin and dye products. For example, a fungal species of mitosporic taxa, Ulomyces chlamydosporum and two from the ascomycetes taxa, Emericella nidulans and Aspergillus phoenicis, isolated from the Dead Sea, showed very high potential as decolorizing agents (Molitoris et al. 2000). Certain marine species such as Ascocratera manglicola, Astrosphaeriella striatispora, Cryptovalsa halosarceicola, Linocarpon bipolaris and Rhizophila marina, have also shown significant amounts of lignin solubilization.
18.4.2 Bacterial Lignin Degradation
Bacterial ligninolytic activity has not received much focus until recent times. In environments such as anaerobic sediments, waterlogged wood, coastal seawater and sediments and salt marshes, bacteria dominate fungi in terms of biodegrading lignin and its polymers (González et al. 1997b). A marine, aerobic species, Sagittula stellata was identified and shown to be capable of breaking down lignin (González et al. 1997a). In addition to looking for bacterial species that possess ligninolytic activity, much effort has been undertaken to purify and characterize specific enzymes as in the case of a halophilic archaeon, Haloferax volcanii (Uthandi et al. 2009). Laccase (LccA), purified from this archaea, was able to oxidize a variety of organic substrates such as bilirubin, syringaldazine, etc. and was tolerant of high salt (0.1–1.4 M NaCl), mixed organosolvents and high temperature (55°C). Laccase has also been isolated and purified from halotolerant bacterial species such as Streptomyces psammoticus (Niladevi et al. 2008).
Cloning and expression of ligninolytic genes are the next step in the further study of lignin degradation by halotolerant/halophilic microorganisms. For example, a marine Bacillus sp., was successfully cloned and expressed with genes encoding for protocatechuate 3,4-dioxygenase. The enzymes isolated from such a bacterium were very efficient in cleaving the bonds within the lignin. The search for novel microorganisms and enzymes that can degrade lignin, along with the potential applications that this naturally occurring process holds, has lead to an improved understanding of the properties and functioning of lignin metabolism and the biogeochemical flow of complex organic molecules in hypersaline ecosystems.
18.5 Biodiesel Production by Halophilic/Halotolerant Algae
As enumerated previously, many halophilic prokaryotes have developed enzymatic moieties for utilizing polysaccharides central to biofuel development. Conversely, there are several primary producers of such polymers within highly saline environments that are of equal if not more importance in potential to supply feedstocks for biomass derived chemicals and fuels. Considerable development pressures on arable land around the globe have resulted from unsustainable development for first generation biofuel crops such as palm, sugarcane, soybeans, and corn. Combined with finite arable land, the problems with unsustainable crop derived feedstocks for biofuels has led to the coining of the term “peak soil” (Schenk et al. 2008). Microalgal culture aims to reduce the arable land and freshwater requirements of biomass for fuel and chemicals and thus thwart the “peak soil” issue. As a result, more emphasis has been placed on prospecting for phototrophic and heterotrophic halophilic primary producers that not only create unique feedstocks but also to provide an avenue for more sustainable cultivation (Mutanda et al. 2011). Central to the sustainability of industrial algal production for biofuels is utilization of marginal or even polluted sources. Halophilic cyanobacteria and algae are compatible with the effluent from pickling and tannery industries (Oren 2010), saline aquifers, produced water from oil and gas extraction, and the utilization of abandoned mine pools as water sources (Alleman 2009).
18.5.1 Microalgae
As true eukaryotes, the principal microalgae present in moderately high salinities (1–3.5 M NaCl) are from the genus Dunaliella (D. salina, D. parva, and D. viridis) as well as Asteromona gracilis and they form the basis of the food chain for higher animals such as brine shrimp and brine flies (DasSarma and Arora 2002). It is also likely that these algae are one of the primary sources of polysaccharides for non-marine, hypersaline environments because they commonly contain cellulose, and to a lesser extent mannans and xylans as fibrillar constituents of their cell walls (Barsanti and Gualtieri 2006; Lee 2008). While Dunaliella have traditionally been a commercial source for β-carotene (Ben-Amotz and Avron 1983), this halophilic green alga has also been the subject of much research in relation to biofuels. Under normal growth conditions, Dunaliella has shown promise of producing a high quality hydrocarbon from the pyroloysis of the protein fraction of the biomass (Ginzburg 1993). Under stressed conditions, such as osmotic and nitrogen deficiency, Dunaliella can produce significant quantities of carbohydrates that are ideal for methane and ethanol production (Feinberg 1984).
Diatoms from the Amphora, Nitzschia and Entomoneis genera have also been widely found in environments up to 3 M NaCl, but not in the abundance as Dunaliella (Clavero et al. 2008). Furthermore, the research on the specific polysaccharides found in halophilic diatoms is limited. However, some species have been reported to produce proline and oligosaccharides under different osmotic and nutrient stress (DasSarma and Arora 2002).
18.5.2 Cyanobacteria
These are the dominant organism in most all moderate to extreme saline impoundments and most often are comprised of Aphanothece halophytica, Dactylococcopsis salina, Phormidium ambiguum and many members of the order Oscillatoriales (O. neglecta, O. limnetica, and O. salina) (DasSarma and Arora 2002). Cellulose biosynthesis has been confirmed in many cyanobacteria including members of Oscillatoriales, but not in any specific halophilic species (Nobles et al. 2001). Furthermore, with genetic modification, it has been demonstrated that the production of osmolites may be manipulated in such a way to favor production of useful feedstocks like sucrose. Engineered mutants of organisms that typically produce glucosyl glycerol have been made to produce a simple carbohydrate like sucrose (Miao et al. 2003). Further development into the enhancement of quality and quantity of yields of both carbohydrates would create a distinct advantage for current fermentative biofuel technologies like bioethanol and biobutanol.
18.6 Conclusions
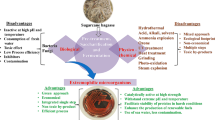
Though little work has been done to demonstrate the use of halophilic organisms for biofuel production, these organisms can be applied in this manner in two distinct ways. Halophilic algae can possibly be used to produce biodiesel and other biomass feedstocks while lowering the amount of water required for this process. Halophilic/halotolerant bacteria can possibly be used to breakdown non-food biomass that has been exposed to alkaline pretreatment for subsequent fermentation to biofuel products such as alcohols and hydrogen. Furthermore, the cellulolytic enzymes these organisms possess possibly can be used as a less harsh pretreatment of lignocellulosic biomass. Significant amounts of information have been discovered about extremophilic lignocellulolytic systems. However, investigations of halophilic lignocellulolytic degradation are nascent by comparison. For example, there have been very few studies on strict halophiles that possess cellulase complexes (Bolobova et al. 1992). Additionally, modern recombinant screening techniques and data mining of genomes has the potential to yield many halophilic lignocellulytic enzymes. This is an exciting period as there is still much to learn about these enzymes in halophilic microorganisms. Furthermore, there is great potential for developing biotechnologies with halophilic lignocellulytic enzymes in regards to treating biomass, leading to innovations in fields such as bio-fuel production.
References
Alleman D (2009) Non-traditional sources of water for coal-fired power plants. Ground Water Protection Council Annual Form, Salt Lake City, UT
Averhoff B, Müller V (2010) Exploring research frontiers in microbiology: recent advances in halophilic and thermophilic extremophiles. Res Microbiol 161:506–514
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Barsanti L, Gualtieri P (2006) Algae: anatomy, biochemistry, and biotechnology. CRC/Taylor & Francis, Boca Raton, FL
Beguin P, Aubert J-P (1994) The biological degradation of cellulose. FEMS Microbiol Rev 13:25–58
Ben-Amotz A, Avron M (1983) Accumulation of metabolytes by halotolerant algae and its industrial potential. In: Ornston LN, Balows A, Baumann P (eds) Annual review of microbiology, vol 37. Annual Review Inc, Palo Alto, CA, pp 95–119
Benner R, Moran MA, Hodson RE (1986) Biogeochemical cycling of lignocellulosic carbon in marine and freshwater ecosystems: contributions of relative procaryotes and eucaryotes. Limnol Oceanogr 31:89–100
Bhat MK, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv 15:583–620
Biely P, Puls J, Schneider H (1985) Acetyl xylan esterases in fungal cellulolytic systems. FEBS Lett 186:80–84
Birbir M, Sesal C (2003) Extremely halophilic bacterial communities in Şereflikoçhisar Salt Lake in Turkey. Turk J Biol 27:7–22
Birbir M, Ogan A, Calli B, Mertoglu B (2004) Enzyme characteristics of extremely halophilic archaeal community in Tuzkoy Salt Mine, Turkey. World J Microbiol Biotechnol 20:613–621
Bolobova AV, Simankova MC, Markovich NA (1992) Cellulase complex of a new halophilic bacterium Halocella celluloytica. Microbiology (Russia) 61:557–562
Bronnenmeier K, Kern A, Liebl W, Staudenbauer WL (1995) Purification of Thermotoga maritima enzymes for the degradation of cellulosic materials. Appl Environ Microbiol 61:1399–1407
Bucher VVC, Hyde KD, Pointing SB, Reddy CA (2004) Production of wood decay enzymes, mass loss and lignin solubilization in wood by marine ascomycetes and their anamorphs. Fungal Divers 15:1–14
Clavero E, Hernandez-Marine M, Grimalt JO, Garcia-Pichel F (2008) Salinity tolerance of diatoms from thalassic hypersaline environments. J Phycol 36:1021–1034
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Coughlan MP, Mayer F (1992) The cellulose-decomposing bacteria and their enzyme systems. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes, vol 1, 2nd edn. Springer, New York, pp 461–467
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell JJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Danson MJ, Hough DW (1997) The structural basis of protein halophilicity. Comp Biochem Physiol A Physiol 117:307–312
DasSarma S, Arora P (2002) Halophiles. In: Encyclopedia of life sciences, vol 8. Nature Publishing Group, London, pp 485–466
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27:197–216
Eisenberg H, Mevarech M, Zaccai G (1992) Biochemical, structural, and molecular genetic aspects of halophilism. Adv Protein Chem 43:1–62
Feinberg DA (1984) Fuel options from microalgae with representative chemical compositions. Solar Energy Research Institute, U.S. DOE, Golden, CO
Frei E, Preston RD (1968) Non-cellulosic structural polysaccharides in algal cell walls. III. Mannan in siphoneous green algae. Proc R Soc Lond B 169:127–145
Fujinami S, Fujisawa M (2010) Industrial applications of alkaliphiles and their enzymes – past, present and future. Environ Technol 31:845–856
Fukushima T, Mizuki T, Echigo A, Inoue A, Usami R (2005) Organic solvent tolerance of halophilic α-amylase from a haloarchaeon, Haloarcula sp. strain S-1. Extremophiles 9:85–89
Gao Z, Ruan L, Chen X, Zhang Y, Xu X (2010) A novel salt-tolerant endo-β-1,4-glucanase Cel5A in Vibrio sp. G21 isolated from mangrove soil. Appl Microbiol Biotechnol 87:1373–1382
Gilbert HJ, Hazlewood GP (1993) Bacterial cellulases and xylanases. J Gen Microbiol 139:187–194
Ginzburg B-Z (1993) Liquid fuel (oil) from halophilic algae: a renewable source of non-polluting energy. Renew Energy 3:249–252
Giridhar PV, Chandra TS (2010) Production of novel halo-alkali-thermo-stable xylanase by a newly isolated moderately halophilic and alkali-tolerant Gracilibacillus sp. TSCPVG. Process Biochem 45:1730–1737
Gomes J, Steiner W (2004) The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol 42:223–235
González JM, Mayer F, Moran MA, Hodson RE, Whitman WB (1997a) Sagittula stellata gen. nov., sp. nov., a lignin-transforming bacterium from a coastal environment. Int J Syst Bacteriol 47:773–780
González J, Mayer F, Moran MA, Hodson RE, Whitman WB (1997b) Microbulbifer hydrolyticus gen. nov., sp. nov., and Marinobacterium georgiense gen., nov., sp. nov., two marine bacteria from a lignin-rich pulp mill waste enrichment community. Int J Syst Bacteriol 47:369–376
González ED, Vallejo OD, Anaya CM, Sánchez MM, González MC (2008) Production of two novel laccase isoforms by a thermotolerant strain of Pycnoporus sanguineus isolated from an oil-polluted tropical habitat. Int Microbiol 11:163–169
Govender L, Naidoo L, Setati ME (2009) Isolation of hydrolase producing bacteria from Sua pan solar salterns and the production of endo-1,4-β-xylanase from a newly isolated haloalkaliphilic Nesterenkonia sp. Afr J Biotechnol 8:5458–5466
Huang X, Shao Z, Hong Y, Lin L, Li C, Huang F, Wang H, Liu Z (2010) Cel8H, a novel endoglucanase from the halophilic bacterium Halomonas sp. S66-4: molecular cloning, heterogonous expression, and biochemical characterization. J Microbiol 48:318–324
Joseleau J, Comtat J, Ruel K (1992) Chemical structure of xylans and their interactions in the plant cell walls. In: Kusters van Someren MA, Beldman G, Visser J, Voragen AGJ (eds) Xylans and xylanases, progress in biotechnology. Elsevier, Amsterdam, pp 1–15
Kamei I, Daikoku C, Tsutsumi Y, Kondo R (2008) Saline-dependent regulation of manganese peroxidase genes in the hypersaline-tolerant white rot fungus Phlebia sp. strain MG-60. Appl Environ Microbiol 74:2709–2716
Kanekar PP, Joshi AA, Kelkar AS, Borgave SB, Sarnaik SS (2008) Alkaline Lonar Lake, India – a treasure of alkaliphilic and halophilic bacteria. In: Sengupta M, Dalwani R (eds) Proceedings of Taal 2007: 12 World Lake Conference. The Ministry of Environment and Forests of the Government of India, Jaipur, pp 1765–1774
Karbalaei-Heidari HR, Ziaee A-A, Amoozegar AM (2007) Purification and biochemical characterization of a protease secreted by the Salinivibrio sp. strain AF-2004 and its behavior in organic solvents. Extremophiles 11:237–243
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Lee RE (2008) Phycology, 3rd edn. Cambridge University Press, New York
Li LH, Flora RM, King KW (1965) Individual roles of cellulase components derived from Trichoderma viride. Arch Biochem Biophys 111:439–447
Li X, Kondo R, Sakai K (2002) Studies on hypersaline-tolerant white-rot fungi I: screening of lignin degrading fungi in hypersaline conditions. J Wood Sci 48:147–152
Li X, Kondo R, Sakai K (2003) Studies on hypersaline-tolerant white-rot fungi IV: effects of Mn2+ and NH +4 on manganese peroxidase production and Poly R-478 decolorization by the marine isolate Phlebia sp. MG-60 under saline conditions. J Wood Sci 49:355–360
Li J, Yuan H, Yang J (2009) Bacteria and lignin degradation. Front Biol 4:29–38
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86:155–164
Miao X, Wu Q, Wu G, Zhao N (2003) Sucrose accumulation in salt-stressed cells of agp gene deletion-mutant in cyanobacterium Synechocystis sp. PP 6803. FEMS Microbiol Lett 218:71–77
Molitoris HP, Buchalo AS, Kurchenko I, Nevo E, Rawal BS, Wasser SP, Oren A (2000) Physiological diversity of the first filamentous fungi isolated from the hypersaline Dead Sea. Fungal Divers 5:55–70
Møller M, Kjeldsen K, Ingvorsen K (2010) Marinimicrobium haloxylanilyticum sp. nov., a new moderately halophilic, polysaccharide-degrading bacterium isolated from Great Salt Lake, Utah. Antonie Leeuwenhoek 98:553–565
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102:57–70
Niladevi KN, Jacob N, Prema P (2008) Evidence for a halotolerant-alkaline laccase in Streptomyces psammoticus: purification and characterization. Process Biochem 43:654–660
Nobles DR, Romanovicz DK, Brown RM Jr (2001) Cellulose in cyanobacteria. Origin of vascular plant cellulose synthase? Plant Physiol 127:529–542
Oren A (2002) Halophilic microorganisms and their environments. Kluwer Academic Publishers, Dordrecht
Oren A (2010) Industrial and environmental applications of halophilic microorganisms. Environ Technol 31:825–834
Pikuta EV, Hoover RB, Tang J (2007) Microbial extremophiles at the limits of life. Crit Rev Microbiol 33:183–209
Pimentel D, Marklein A, Toth MA, Karpoff MN, Paul GS, McCormack R, Kyriazis J, Krueger T (2009) Food versus biofuels: environmental and economic costs. Hum Ecol 37:1–12
Prakash S, Veeranagouda Y, Kyoung L, Sreeramulu K (2009) Xylanase production using inexpensive agricultural wastes and its partial characterization from a halophilic Chromohalobacter sp. TPSV 101. World J Microbiol Biotechnol 25:197–204
Prescott LM, Harley JP, Klein DA (1993) Microbial growth and metabolism and microorganisms and the environment. In: Prescott LM, Harley JP, Klein DA (eds) Microbiology, 2nd edn. Wm. C. Brown Publishers, Dubuque, pp 176–179
Puls J, Schuseil J (1993) Chemistry of hemicelluloses: relationship between hemicellulose structure and enzymes required for hydrolysis. In: Coughlan M, Hazlewood G (eds) Hemicellulose and hemicellulases. Portland Press, London, pp 1–27
Raghukumar C, Ticlo DD, Verma AK (2008) Treatment of colored effluents with lignin-degrading enzymes: an emerging role of marine-derived fungi. Crit Rev Microbiol 34:189–206
Rees H, Grant S, Jones B, Grant WD, Heaphy S (2003) Detecting cellulase and esterase enzyme activities encoded. Extremophiles 7:415–421
Reese ET (1976) History of the cellulase program at the U.S. Army Natick Development Center. Biotechnol Bioeng Symp 6:9–19
Rohban R, Amoozegar MA, Ventosa A (2009) Screening and isolation of halophilic bacteria producing extracellular hydrolyses from Howz Soltan Lake, Iran. J Ind Microbiol Biotechnol 36:333–340
Saha B (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Sanchez-Porro C, Martin S, Mellado E, Ventosa A (2003) Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol 94:295–300
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgung JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biofuel production. Bioenerg Res 1:20–43
Schwarz WH (2001) The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biotechnol 56:634–649
Setati ME (2010) Diversity and industrial potential of hydrolase-producing halophilic/halotolerant eubacteria. Afr J Biotechnol 9:1555–1560
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Uffen RL (1997) Xylan degradation: a glimpse at microbial diversity. J Ind Microbiol Biotechnol 19:1–6
Uthandi S, Saad B, Humbard MA, Maupin-Furlow JA (2009) LccA, an archaeal laccase secreted as a highly-stable glycoprotein into the extracellular medium of Haloferax volcanii. Appl Environ Microbiol 76:733–743
van den Burg B (2003) Extremophiles as a source for novel enzymes. Curr Opin Microbiol 6:213–218
Voget S, Steele HL, Streit WR (2006) Characterization of a metagenome-derived halotolerant cellulase. J Biotechnol 126:26–36
Vreeland RH, Piselli AF Jr, McDonnough S, Meyers SS (1998) Distribution and diversity of halophilic bacteria in a subsurface salt formation. Extremophiles 2:321–331
Wainø M, Ingvorsen K (1999) Production of halostable β-mannanase and β-mannosidase by strain NN, a new extremely halotolerant bacterium. Appl Microbiol Biotechnol 52:675–680
Wainø M, Ingvorsen K (2003) Production of β-xylanase and β-xylosidase by the extremely halophilic archaeon Halorhabdus utahensis. Extremophiles 7:87–93
Wang C-Y, Hsieh Y-R, Ng C-C, Chan H, Lin H-T, Tzeng W-S, Shyu Y-T (2009) Purification and characterization of a novel halostable cellulase from Salinivibrio sp. strain NTU-05. Enzyme Microb Technol 44:373–379
Wang C-Y, Chan H, Lin H-T, Shyu Y-T (2010a) Production, purification and characterisation of a novel halostable xylanase from Bacillus sp. NTU-06. Ann Appl Biol 156:187–197
Wang J, Shao Z, Hong Y, Li C, Fu X, Liu Z (2010b) A novel β-mannanase from Pantoea agglomerans A021: gene cloning, expression, purification and characterization. World J Microbiol Biotechnol 26:1777–1784
Wejse P, Ingvorsen K, Mortensen K (2003) Purification and characterisation of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles 7:423–431
Whitney SEC, Brigham JE, Darke AH, Grant Reid JS, Gidley MJ (1998) Structural aspects of the interaction of mannan-based polysaccharides with bacterial cellulose. Carbohydr Res 307:299–309
Wong KK, Tan LU, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol Mol Biol Rev 52:305–317
Wood TM (1975) Properties and mode of action of cellulases. Biotechnol Bioeng Symp 5:111–137
Woolard CR, Irvine RL (1995) Treatment of hypersaline wastewater in the sequencing batch reactor. Water Res 29:1159–1168
Yang C, Wang Z, Li Y, Niu Y, Du M, He X, Ma C, Tang H, Xu P (2010) Metabolic versatility of halotolerant and alkaliphilic strains of Halomonas isolated from alkaline black liquor. Bioresour Technol 101:6778–6784
Zhou X, Chen H, Li Z (2004) CMCase activity assay as a method for cellulase adsorption analysis. Enzyme Microb Technol 35:455–459
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Begemann, M.B., Mormile, M.R., Paul, V.G., Vidt, D.J. (2011). Potential Enhancement of Biofuel Production Through Enzymatic Biomass Degradation Activity and Biodiesel Production by Halophilic Microorganisms. In: Ventosa, A., Oren, A., Ma, Y. (eds) Halophiles and Hypersaline Environments. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-20198-1_18
Download citation
DOI: https://doi.org/10.1007/978-3-642-20198-1_18
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-20197-4
Online ISBN: 978-3-642-20198-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)