Abstract
Phosphorus plays a significant role in several physiological and biochemical activities such as photosynthesis, transformation of sugar to starch, and other biological processes in plants. Phosphorus in soils is immobilized due to formation of insoluble complexes such as iron and aluminium hydrous oxides, crystalline and amorphous aluminium silicate and calcium carbonate. Many soil microorganisms specially Pseudomonas, Bacillus, Aspergillus and Penicillium are effective in releasing P from inorganic and organic pools of total soil P through solubilization or mineralization and are known as phosphate-solubilizing microorganisms (PSM). The major mechanisms involved in P solubilization are by the production of organic acids mainly gluconic acid (GA), 2-ketogluconic acid, citric acid and oxalic acid, which chelate the cations bound to phosphate via their hydroxyl and carboxyl groups or by the liberation of H+, thereby converting it into soluble forms. PSM help in increasing the availability of accumulated phosphates in soil for plant growth by solubilization of phosphate, which in turn increases the biological nitrogen fixation efficiency as well as crop yield.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
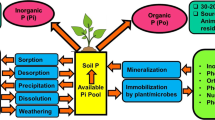
Phosphorus (P) is second major nutrient in crop productivity for its involvement in many essential processes such as cell division, development, photosynthesis, breakdown of sugar, nutrient transport within the plant, transfer of genetic characteristics from one generation to another and regulation of metabolic pathways (Tandon 1987; Armstrong 1988; Theodorou and Plaxton 1993). The maintenance of high level of soil phosphorus has been a major challenge to agricultural scientists, ecologists and farm managers because in most of the soils, phosphate is present in unavailable form due to complex formation with Ca2+, AI3+, Fe2+ or Mn2+ depending on soil pH and organic matter. The main problem of phosphorus in soil is its rapid fixation and the efficiency of P solubilization rarely exceeding 10–20%. The fixed forms of P in acidic soils are aluminium and iron phosphates while in neutral to alkaline soils as calcium phosphates. The manufacture of phosphatic fertilizers requires high-grade rock phosphate (RP) and sulphur which are getting depleted progressively and becoming costlier. The total world reserves of RP are estimated to be around 2,700 billion tons of which 80% are located in the USA, Russia, and Morocco. In India, RP deposits are estimated to be about 145 million tons but bulk of this is of poor quality and is unsuitable for manufacture of phosphatic fertilizers. Only 25% of the total P requirement is met through indigenous sources; hence, about 1.5 million tons of high grade RP is imported annually.
The concentration of total P in soil ranges from 0.01 to 0.2%, with an average approximately 0.05% (Barker 1984). But out of this only 20% of total P is available to plants. It makes about 0.2% of plant dry weight (Sahachtman et al. 1998), which indicates the importance of P availability in soil. Because of extreme reactivity and fixation of P in soil, it is unavailable for plant growth. Moreover, the applied phosphorus also gets fixed chemically with metal ions in the soil (Bagyaraj and Varma 1995; Holford 1997). Even if the total P is high, there is always a need to apply phosphatic fertilizers regularly, part of which gets fixed in soil and is unavailable to plants depending on soil pH and climatic conditions. Although various chemical transformations help in release of P immobilization, chemical fixation depletes soil of the available P. Therefore, it is important to develop technology for P solubilization for plant growth. Level of soluble P in soil can be increased either by using phosphate-solubilizing microorganisms (PSM) as bio-inoculants for solubilization of fixed soil P which can improve crop yields or application of phosphorus (P) rich compost. The use of PSM as bio-inoculants plays a vital role in maintaining soil nutrient status, structure and sustains the production base. It also reduces reliance on expensive imported phosphate; thereby a significant increase in yield was subsequently reported with inoculation of PSM in different crops.
Uptake of phosphorus by the plants is in the form of soluble orthophosphorus. In general, the availability of these ions to the plants is in the order of H2PO −14 > H2PO −24 > PO −34 . The availability also depends mainly on soil pH (Nath and Borah 1983). The soil microorganisms that are of diverse type (fungi, bacteria and actinomycetes) are responsible for solubilization of inorganic phosphates (Kapoor et al. 1989; Kucey et al. 1989; Holvorson et al. 1990; Illmer and Schinner 1992; Kapoor 1995) causing changes in pH of the soil microenvironment and by producing chelating substances that lead to the solubilization of inorganic phosphates. Iron (Fe) and aluminium (Al) at low pH and calcium (Ca) at high pH fix the available form of P into insoluble forms in soil (Dala 1973; Sanyal and De Datta 1991; Johnson and Loepper 2006; Rengel and Marschner 2005) which are not easily available.
2 Phosphate-Solubilizing Microorganisms
A large number of autotrophic and heterotrophic soil microorganisms have capacity to solubilize mineral phosphates. PSM are present in almost all the soils, although their number varies depending upon the soil and climatic conditions (Kucey et al. 1989). First time in 1903, tricalcium phosphate (TCP) solubilization was demonstrated by soil bacteria in liquid and in solid medium (Stalstrom 1903; Sackett et al. 1908). However, the extent of solubilization varies with the source of inorganic P and the microorganisms involved (Banik and Dey 1982). Phosphate-solubilizing fungi (PSF) and bacteria are known as effective organisms for phosphate solubilization (Reyes et al. 1999). Recent report suggests that PSM can be used in union with RP so that phosphorus in the RP can be made available in the soil for plant uptake (Jisha and Alagawadi 1996). Thus, PSM cause the release of nutrients into soil in naturally balanced proportion and exerts beneficial effects on plant development (Glick 1995).
The use of PSM as bio-inoculants plays a vital role in maintaining soil nutrient status and structure as it reduces reliance on expensive imported phosphatic fertilizers. Application of these bacteria along with RP resulted in increased availability of inorganic phosphate for plant utilization (Hebbara and Devi 1990; Rachewad et al. 1992; Jisha and Alagawadi 1996; Chen et al. 2006). Thus, the beneficial effect of inoculation on the availability of P to crops has led to the development of inoculum which is popularly known as phosphobacterin. The first evidence to show that inoculation of seedling with PSM increase uptake of P and crop yield was by Gerretsen (1948) on oat crop. Sundara Rao and Paul (1959) reported significant increase in the yield of barseem after inoculation with phosphobacterin. Since then, beneficial effects of inoculation with different PSM have been reported with different crops.
2.1 Major Groups of PSM
An extensive range of soil bacteria, actinomycetes, cyanobacteria and fungi belonging to the genera Pseudomonas, Enterobacter, Bacillus, Rhizobium, Agrobacterium, Microccocus, Aereobacter, Erwinia, Streptomyces, Nocardia, Aspergillus, Penicillium, Trichoderma, Anabaena, Nostoc, Calothrix and Scytonema that are able to solubilize various forms of precipitated P have been reported (Kucey et al. 1989; Roychoudhary and Kaushik 1989; Rodriguez and Fragaa 1999; Whitelaw 2000; Tran Thi Ngoc Son et al. 2006; Gulati et al. 2008; Sulbarán et al. 2009) and are generally considered to contribute a significant component of the total soil phosphatase activity (Richardson 1994).
In soil, phosphate-solubilizing bacteria (PSB) constitute 1–50% and fungi 0.5–0.1% of the total respective population (Banik and Dey 1982; Kucey 1983; Kucey et al. 1989; Chen et al. 2006). In general, fungal isolates exhibit greater P-solubilizing ability than bacteria in both liquid and solid media (Banik and Dey 1982; Gaur et al. 1973; Kucey 1983; Venkateswarlu et al. 1984). Fungi in soils are able to penetrate deep into soil more easily than bacteria, and hence may be more important to P solubilization in soils (Kucey 1983).
In addition, actinomycetes Micromonospora, Nocardia and Streptomyces have also been reported to solubilize phosphates. Yeast such as Torula sp. which is usually not present in soils has also been isolated from compost and characterized for solubilization of TCP and RP (Singh et al. 1980).
2.2 Phosphate-Solubilizing Bacteria
An extensive range of soil bacteria including actinomycetes both aerobic and anaerobic are able to solubilize various forms of insoluble inorganic phosphate compounds, such as TCP, dicalcium phosphate, hydroxyapatite and RP (Goldstein 1986; Rodriguez et al. 2000; Gulati et al. 2008; Sulbarán et al. 2009). The prominent genera involved in mineral phosphate solubilization are Pseudomonas, Bacillus, Rhizobium, Burkholderia, Achromobacter, Agrobacterium, Microccocus, Aerobacter, Flavobacterium, Erwinia, Alcaligenes, Escherichia, Serratia and Xanthomonas sp. (Li 1981; Datta et al. 1982; Venkateswarlu et al. 1984; Fernandez et al. 1984; Thomas and Shantaram 1986; Surange and Kumar 1993; Farhat et al. 2009).
The Pseudomonas sp. includes P. striata, P aeruginosa, P. putida, P vermiculosam and P. fluroscence (Sen and Paul 1957; Gaind and Gaur 1990a, b; Kumar et al. 2002; Buch et al. 2008). Population of PSB mainly depends on different soil properties such as physical, chemical properties, organic matter and P content (Kim et al. 1998).
Major Bacillus sp. known for their mineral phosphate-solubilizing abilities are B. polymyxa, B. subtilis, B. brevis, B. circulans, B. megaterium, B. mesentricus, B. mycoides, B. pulvifaciens, etc. (Sen and Paul 1958; Sundara Rao and Sinha 1963; Paul and Sundara Rao 1971; Rodriguez and Fragaa 1999; Swain and Ray 2009).
Most PSB were isolated from the rhizosphere of various plants and are known to be metabolically more active than those isolated from sources other than rhizosphere (Baya et al. 1981; Katznelson and Bose 1959; Vazquez et al. 2000). The P-solubilizing ability in bacteria is generally reduced or lost upon repeated subculturing but no such loss has been observed in the case of PSF (Kucey 1983; Sperber 1958).
P-solubilizing ability of PSB is affected by many physiological factors some of which are C and N source in medium, mineral source, pH, incubation temperature, aeration and incubation period (Gaind and Gaur 1990a, b; Vassilev and Vassileva 2003; Taiwo and Ogundiya 2008). Singh (1992) showed that PSB could solubilize 240 μg/ml and 5,300 μg/ml of TCP and mussoorie rock phosphate (MRP), respectively, after 9 weeks of incubation at 30°C under stationary conditions. Dave and Patel (1999) showed that Pseudomonas sp. release 27–163 mg of P2O5 at 37°C in 3 weeks under static conditions at cell concentrations of 6.6 × 107/ml in 100 ml of Pikovskaya’s (PVK) medium containing TCP equivalent to 225 mg P2O5 (Dave and Patel 1999). Strains of Pseudomonas sp. are capable of releasing 162 μg/ml inorganic phosphate in the medium containing TCP (Santhi 1998). Strains of Enterobacter can release inorganic phosphate ranging from 83 to 551 μg/ml in medium containing hydroxyapatite (Kim et al. 1997). Kumar and his coworker (1999) observed that strains of Acetobacter released 142–431 μg/ml of inorganic phosphate from TCP. Illmer and Schinner (1992) reported that temperature 25–30°C is optimum for P solubi1ization for Pseudomonas sp. whereas fungi solubilize more P at a slightly higher temperature, i.e. 30–35°C (Gaur 1990). B. polymyxa was found to have a higher temperature range of 35–40°C for optimum solubilization (Gaur 1990). The maximum decrease in pH was observed on third and seventh day by B. polymyxa and P. striata, respectively, which increased on further incubation (Illmer and Schinner 1992). Recent report by Farhat et al. (2009) showed that the soluble phosphorus (P) concentration in optimized medium reached 967, 500, 595 and 326 mg/l from CaHPO4, Ca3(PO4)2, hydroxyapatite and RP within 72 h of incubation after inoculion with Serratia marcescens.
Actinomycetes as PSM are of special interest since these gram-positive filamentous sporulating bacteria can flourish in extreme environments (Jiang et al. 2005; Pathom-Aree et al. 2006) and also produce various plant growth-promoting substances (Fabre et al. 1988; Manulis et al. 1994; Ikeda 2003; Jain and Jain 2007). Many workers have isolated actinomycetes strains with phosphate-solubilizing ability (Ahmad and Jha 1968; Mahmoud et al. 1973; Ibrahim and Abdel-Aziz 1977; Banik and Dey 1983; Mba 1997; Hamdali et al. 2008). Mba (1994) isolated four actinomycetes from earthworm cast of Pontoscolex corethrurus which were able to solubilize up to 7 mg soluble P/g RP within one week of incubation. Interestingly, the RP-solubilizing power of these isolates was decreased with acidification of the medium and most of the isolates were able to hydrolyse carboxymethyl cellulose (CMC). Hamdali et al. (2008) isolated 55 actinomycete cultures, which were able to solubilize RP in synthetic minimum medium. Most of these isolates were from genera Streptomyces and Micromonospora and did not produce any organic acids but found to produce siderophores rendering the P available for plants.
Many workers have used a composite culture of bacteria and fungi to enhance the P solubilization but the efficiency of such mixed inoculants depends on the compatibility among microorganisms. Kundu and Gaur (1981) found that inoculation of P. striata and Aspergillus awamori together solubilized more phosphates than dual inoculation of B. polymyxa and A. awamori.
2.3 Phosphate-Solubilizing Fungi
A large number of fungi have also been reported to solubilize insoluble forms of phosphorus. These include Aspergillus, Candida, Penicillium, Rhizopus, Cladosporium and Paecilomyces, etc. (Agnihotri 1970; Gaind and Gaur 1991; Banik and Dey 1982; Singh et al. 1984; Venkateswarlu et al. 1984; Darmwal et al. 1989; Sheshadari et al. 2000). These PSF are well known for their ability to solubilize mineral phosphates owing to their ability to produce organic acids (Mattey 1992; Reddy et al. 2002; Bojinova et al. 2008; Richa et al. 2007; Xiao et al. 2009; Khan et al. 2010), and particularly some Aspergillus and Penicillium species have been tested by inoculating directly into the soil in order to solubilize RP (Kucey 1987; Vassilev et al. 1997). Inoculation of PSF and mycorrhizal fungi also improves the physico-chemical, biochemical and biological properties of RP-amended soil (Caravaca et al. 2004).
In another study, Aspergillus tubingensis and two isolates of Aspergillus niger have also shown the highest solubilization of RP under in vitro conditions (Reddy et al. 2002). Richa et al. (2007) tested Aspergillus tubingensis and A. niger for their efficacy to solubilize RP in RP-amended soils and they observed that available P along with organic carbon was significantly increased when compared to initial soil. Also the soil pH was lowered compared to initial pH of the soil. The improvement of physico-chemical and biochemical properties of RP-amended soil with the inoculation of A. niger and mycorrhizal fungi was also reported by Caravaca et al. (2004).
Similarly, El-Azouni (2008) tested the efficacy of Aspergillus niger and Penicillium italicum to solubilize TCP in vitro and they found the release of inorganic P was 490 and 275 μg P ml−1, respectively, after one week incubation. Change in soluble P with time with respect to pH has been observed with Paeciiomyces fusisporus and other fungi (Goyal et al. 1982; Mishra et al. 1983). A nematofungus Arthrobotrys oligospora also has shown the ability to solubilize the phosphate rocks (Duponnois et al. 2006).
Arbuscular Mycorrhizae (AM) fungi are known to enhance P nutrition of plants especially in P-deficient soils by scavenging the available P due to the large surface area of their hyphae and by their high-affinity P uptake mechanisms (Hayman 1974; Moose 1980; Sanders and Tinker 1973). There are also reports of organic acid production by AM (Paul and Sundara Rao 1971) that could solubilize the insoluble mineral phosphates. As the soil phosphorus levels available to the plants increase, the amount of phosphorus also increases in the plant’s tissues and thus the concomitant carbon drain from the plant by the AM fungi making symbiosis nonbeneficial to the plant (Grant et al. 2005).
2.4 Interaction of PSB with Other Microorganisms
The PSM when used with other plant growth-promoting rhizobacteria (PGPR) act synergistically to enhance crop yields (Saxena and Tilak 1994, 1997). Co-inoculation of P. striata and AM significantly increased the soybean yield and P uptake by plants over control. Dual inoculation of Rhizobium with PSM (Perveen et al. 2002) or arbuscular mycorrhizae (AM) fungi (Zaidi et al. 2003) has been shown to improve plant growth more than with their sole inoculation in P-deficient soils. Synergistic interactions on plant growth have been observed by co-inoculation of PSB with N2 fixers such as Azotobacter (Kundu and Gaur 1984) and Azospirillum (Belimov et al. 1995) or with vesicular AM (Kim et al. 1998). Son et al. (2003, 2006) showed that co-inoculation of Bradyrhizobium japonicum and Pseudomonas sp. enhanced the number of nodules, dry weight of nodules, yield components, soil nutrient availability and uptake in soybean crop. Research workers have reported similar results with legume crops when PSBs are coinoculated with various N2 fixing bacteria (Table 4.1).
3 Mechanisms of Phosphate Solubilization
Release of organic acids by PSM has been reported as a primary mechanism of phosphate solubilization (Hilda and Fraga 2000; Khiari and Parent 2005). Besides organic acids, the production of chelating substances (2-ketogluconic acid), humic substances, mineral acids (sulphuric acids), siderophores and proton extrusion mechanisms also play an important role (Kapoor et al. 1989; Kucey et al. 1989; Illmer et al. 1995).
Growth of PSM is generally accompanied by decrease in pH of the medium and soil (Singh et al. 1980; Mishra and Banger 1985; Darmwal et al. 1989; Stevenson 2005). Reduction in pH is due to the production of organic acids, which include citric, gluconic, fumaric, malic, oxalic, lactic, 2-ketogluconic, malonic acids, etc. (Banik and Dey 1981; Venkateswarlu et al. 1984; Illmer and Schinner 1992; Vassilev et al. 1996; Hwangbo et al. 2003; Patel et al. 2008). However, quantity and quality of organic acid produced is fully dependent on type of PSM. The highest solubilization of TCP and RP was reported by citric and fumaric acid (Gaur 1990). Calcium and magnesium in RP binds to the solubilized P and carbonate thereby neutralizing the organic acids, which play a vital role in solubilization.
The release of 2-ketogluconic acid by PSM was correlated with P solubilization due to its calcium chelating ability (Firsching 1969). Moreover, these compounds form stable organometallic complexes with Fe and Al and decrease precipitation of solubilized phosphates. Chelation of calcium, especially oxalic acid helps in solubilization of insoluble phosphates (Illmer and Schinner 1992). Besides acids production and chelators, bacterial exo-polysaccharides (EPS) also play an important role in mineral phosphate solubilization. Recently, Yi et al. (2008) observed the synergistic effects of EPS and organic acid on TCP solubilization, which varied with the origin and the concentration of EPS in medium. The increase of P solubilization brought by EPS is mainly attributed to the precipitation of EPS consequently resulting in greater phosphorus released from insoluble phosphate.
Humic substances are produced in soil as a result of organic matter decomposition and contain humic and fulvic acids as their main components. Humic acids are strong chelating agents which chelate Ca2+ and release H+, and thus help in dissolution of insoluble phosphates (Gaur 1969; Pareek and Gaur 1973; Mishra et al. 1983; Banger et al. 1985; Singh and Amberger 1990). The functional group of these compounds, such as carboxylic, phenolic, hydroxylic and phenolic hydroxyl, forms stable complex with Ca, Fe and Al (Banger and Mishra 1990). They also check reprecipitation of the solubilized phosphates (Singh and Amberger 1990).
The sulphate-reducing bacteria and other heterotrophic organisms under anaerobic conditions release H2S that solubilize ferric phosphate by forming insoluble sulphides of Fe and render soluble phosphate (Gaur 1990). Rhizospheric microorganisms release CO2 forming carbonic acid (HCO3), which lowers the pH, and thus help in P solubilization (Hayman 1975). The oxidation of inorganic sulphur and pyrite to sulphuric acid by sulphur oxidizing bacteria (Thiobacillus) lowers the soil pH and hence improves the availability of phosphates (Wainwright 1984; Kapoor et al. 1991) due to formation of CaNO3 and CaSO4. Certain rhizospheric microorganisms (Rhizobium, Azotobacter, Pseudomonas) synthesize iron-chelating compounds (siderophores), which in acidic soil remove iron from ferric phosphates and render phosphate for plants (Bossier et al. 1988; Suneja and Lakshminarayana 1999; Hamdali et al. 2008).
Proton extrusion mechanism is a probable mechanism of phosphate solubilization in some microorganisms, which can solubilize phosphates without the release of organic acids in the environment (Illmer et al. 1995). Release of H+ accompanying respiration and assimilation has been observed in Penicillium aurantiogriseum and Pseudomonas sp. solubilizing hydroxyapatite (Illmer et al. 1995; Lin et al. 2006). The insoluble phosphates are solubilized at the cell surface of the microorganisms. The NH +4 /H+ exchange mechanism depends on the presence of NH +4 ions in the medium as uptake of NH +4 occurs with the concomitant release of H+ in the environment (Roos and Luchner 1984). Quality and quantity of root exudates also alter the concentration of P in the soil solution due to presence of organic ligands in the root exudates (Hinsinger 2001).
4 Estimation and Enumeration of Phosphate-Solubilizing Microorganisms
PSMs can be isolated from different sources such as soil (Khanna et al. 1979; Gupta et al. 1986; Kapoor et al. 1989; Roychoudhary and Kaushik 1989), rhizosphere (Sardina et al. 1986; Thakkar et al. 1993; Singh and Kapoor 1994), root nodules (Chhonkar and Subba Rao 1967; Surange and Kumar 1993), compost (Kapoor et al. 1989; Thakkar et al. 1993), RPs (Gaur et al. 1973) and earthworm casts (Mba 1994).
Solubilization of tricalcium phosphate (TCP) in agar medium has been used as the initial criterion for isolation and enumeration of PSM. Stalstrom (1903) first demonstrated the solubilization of TCP by soil bacteria in solid and liquid medium. Organisms growing on such media are able to solubilize P and produce clear zone around colonies due to dissolution of calcium phosphate. Mineral P solubilizers have been routinely isolated and screened using PVK medium (Pikovskaya 1948) or Sperber medium (Sperber 1957) by plate assay method, looking for halo/clear zone around colonies of potential P solubilizers (Katznelson et al. 1962; Das 1963; Bardiya and Gaur 1974; Darmwal et al. 1989). The zone of clearance is due to solubilization of inorganic phosphate mainly due to the production of organic acids in the surrounding medium (Johnston 1952; Das 1963; Sethi and Subba Rao 1968; Pareek and Gaur 1973; Kundu and Gaur 1981; Kapoor et al. 1989; Halder et al. 1990; Singal et al. 1991; Yadav and Dadarwal 1997).
Although, microorganism capable of producing zone of clearance around its colonies in plate assay method is selected as a potential P solubilizer, however, several workers have reported that many isolates which did not produce any visible zone of clearance on agar plates but could solubilize various types of insoluble inorganic phosphate in liquid medium (Louw and Webley 1959; Das 1963). Thus, the existing plate assay fails where the halo zone is inconspicuous or absent. This may be because of the varying diffusion rates of different organic acids secreted by different microorganisms (Johnston 1952). On the other hand microorganisms, which showed P solubilization in laboratory medium, do not give any solubilization in soil. For example, Whitelaw et al. (1999) showed that, even though Penicillium radicum produced GA when grown in laboratory culture, the organic acid was not the primary mechanism for solubilization of precipitated P. Hence, there is need of suitable medium for quick and reliable plate assay for screening PSM. However, the plate assay can be regarded as generally suitable for isolation and preliminary characterization of PSM.
Gupta et al. (1994) gave a modified PVK medium formulated using different concentrations of bromophenol blue (BPB) dye for screening PSM. Results revealed that as the concentration of dye increased, the clarity and visibility of the yellow coloured halo/zone improved, and most appropriate dye concentration was found to be 2.4 mg/ml. There is no correlation between halo zone formation and quantity of inorganic phosphate solubilized (Ostwal and Bhide 1972; Arora and Gaur 1979). Indicator medium containing bromothymol blue (BTB) has also been developed to enhance chances of picking up efficient PSB (Krishnaraj 1996). Advantage of using modified medium is that the incubation period required prior to selection of PSB is significantly reduced, i.e. minimum incubation period for PSB was 24 h than in original PVK medium that exceeds 7 days.
National Botanical Research Institute’s phosphate growth medium (NBRIP), which is more efficient than PVK medium was developed for screening of PSM (Nautiyal 1999). NBRIP medium was comparable to PVK agar medium; however, in broth assay, NBRIP medium consistently resulted in a 3-fold increase in P solubilization. The rate of phosphate solubilization was increased with increased concentrations of glucose (Nautiyal 1999). Also, glucose, xylose and sucrose are reported to be good carbon sources for PSF (Ahmad and Jha 1968), while for bacteria glucose, galactose and sucrose were found to be effective. However, the solubilization potential of microorganisms varies with different carbon sources depending on the type of insoluble phosphate (Ahmad and Jha 1968; Thakkar et al. 1993). In anaerobic conditions, P release is affected by the physiological state of cells and carbon sources (acetic, propionic and butyric acid) as observed under stationary conditions (Rustrain et al. 1997). The utilization of glucose directly correlates with drop in pH, which was reflected as mineral phosphate solubilization due to conversion of glucose to GA (Goldstein and Liu 1987; Liu et al. 1992). It was observed that 60 mM GA produced results in release of 0.1 mM inorganic phosphate. The amount of acid liberated by PSB roughly is more than 5 per cent of the carbohydrate consumed (Banik and Dey 1982). Phosphate solubilization ability was improved with (NH4)2SO4 at a lower concentration and was 27.1 percent more effective than KNO3 as observed by Nautiyal (1999). P. striata (PS-9) has been found to utilize urea, asparagine, ammonium sulphate, ammonium nitrate, potassium nitrate and calcium nitrate for solubilization of RP (Gaur 1990). Sodium nitrate and peptone were found to be better substrate for P solubilization by B. megaterium var. phosphaticum. P. striata solubilized the higher amount of insoluble phosphates among bacteria, and quantities of RP solubilized were much less than TCP and hydroxyapatite (Arora and Gaur 1979). However, incorporation of bacteria with RP resulted in increased availability of inorganic phosphate for plant utilization (Hebbara and Devi 1990; Rachewad et al. 1992; Jisha and Alagawadi 1996). But, in plate assay, for efficient P solubilizers, tricalcium is used instead of MRP. As TCP gets metabolized faster, it is normally used for screening studies compared to RPs (Singh and Kapoor 1994). Also, TCP solubilization is higher as compared to aluminium phosphate and ferric phosphate (Narsian et al. 1993). Microbial solubilization of RP is influenced by the physical and chemical properties of the RP and the microorganisms involved (Garg et al. 1989; Kapoor et al. 1989).
Bacteria prefer to grow in neutral to alkaline (pH 7 to 8) reaction for maximum solubilization (Wani et al. 1979) and fungi do better at slightly acidic to neutral pH (Gaur 1990). A direct correlation was obtained between decrease in pH and increase in available P of the culture media in certain cases (Sperber 1958; Agnihotri 1970; Liu et al. 1992) but others have contradictory reports that solubilization is not always proportional to the decline in pH (Mehta and Bhide 1970; Krishnaraj 1996; Asea et al. 1988; Parks et al. 1990). The form in which inorganic phosphate exists also changes according to the soil pH. Below pH 6.0, most inorganic phosphate is present as monovalent H2PO4 species. The plant uptake is also high at the pH range of 5.0–6.0, which indicates that P is primarily taken up as monovalent form (Furihata et al. 1992). It has been noted that PSB could solubilize both RP and dicalcium phosphate in unbuffered media but failed to solubilize RP in buffered media. The organic acid secreted by these bacteria was 20–50 times less than that required to solubilize P from alkaline soil (Gyaneshwar et al. 1998).
5 PSM and Yield of Crops
PSM inoculation can increase crop yields up to 70% (Verma 1993). The increase in yield is mainly attributed by the increased availability of soluble phosphorus on inoculation with PSMs, which enhance the plant growth by improving biological nitrogen fixation (Kucey et al. 1989).
Sundara Rao et al. (1963) reported increase in yields and phosphate uptake in tomato and wheat with phosphobacterin and a strain of Bacillus megaterium . Kundu and Gaur (1980) reported increased potato yield with phosphobacteria in potato. Also, increase in wheat yield with inoculation of PSM-Azotobacter chroococcum is reported. Similarly, increase in yield was observed in various crops such as chickpea, rice, etc. (Alagawadi and Gaur 1988; Kavimadan and Gaur 1971; Monod et al. 1989).
Similarly, several authors have reported increased yield of wheat (Whitelaw et al. 1997; Omar 1998), onion (Vassilev et al. 1997), alfalfa (Rodríguez et al. 1999), rice (Khan et al. 2008), maize (Richa et al. 2007) and soybean (Abd-Alla and Omar 2001) through simple inoculation of PSF. Recently, El-Azouni (2008) observed that the dual inoculation of PSF (A. niger and P. italicum) significantly increased dry matter and yield of soybean plants compared to the control TCP-amended soil in pot experiment along with significant increase in the percentage of N and P content of the plant.
6 Future Prospects
PSMs are an integral component of soil microbial community and play an important role in P cycle in soil rendering the unavailable P to plants. These PSM have enormous potential for making use of fixed P in the soil particularly in soils with low P availability in tropical and subtropical developing countries. The mechanism of phosphate solubilization by microorganisms has been studied in detail but the P solubilization is a complex phenomenon affected by many factors, such as PSM used, nutritional status of soil and environmental factors. Moreover, the stability of the PSMs after inoculation in soil is also important in P solubilization to benefit crop growth. Therefore, it needs further studies to understand the characteristics and mechanisms of phosphate solubilization by PSM. To conclude, the efforts should be made to identify, screen and characterize more PSM for their ultimate application under field conditions.
References
Abd-Alla MH, Omar SA (2001) Survival of rhizobia/bradyrhizobia and rock-phosphate-solubilizing fungus Aspergillus niger on various carriers from some agroindustrial wastes and their effects on nodulation and growth of faba bean and soybean. J Plant Nutr 24:261–272
Afzal A, Asghari B (2008) Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int J Agric Biol 10:85–88
Agnihotri VP (1970) Solubilization of insoluble phosphates by some fungi isolated from nursery seed beds. Can J Microbiol 16:877–880
Ahmad N, Jha KK (1968) Solubilization of rock phosphate by microorganisms isolated from Bihar solis. J Gen Appl Microbiol 14:89–95
Alagawadi AR, Gaur AC (1988) Associative effect of Rhizobium and phosphate solubilizing bacteria on the yield and nutrient uptake of chickpea. Plant Soil 105:241–246
Armstrong DL (1988) Role of phosphorus in plants. In: Armstrong DL (ed) Better crops with plant food. Potash and Phosphate Institute, Atlanta, USA, pp 4–5
Arora D, Gaur AC (1979) Microbial solublization of different inorganic phosphates. Indian J Expt Biol 11:1258–1261
Asea PEA, Kucey RMN, Stewart JWB (1988) Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol Biochem 20:459–464
Bagyaraj DJ, Varma A (1995) Interaction between arbuscular mycorrhizal fungi and plants: their importance in sustainable agriculture in arid and semiarid tropics. Adv Microbiol Ecol 14:119–142
Banger KC, Mishra MM (1990) Solubilization of insoluble phosphates by humic acid. Int J Trop Agric 8:209–213
Banger KC, Yadav KS, Mishra MM (1985) Transformation of rock phosphate during composting and the effect of humic acid. Plant Soil 85:259–266
Banik S, Dey BK (1981) Phosphate solubilizing microorganisms of a lateritic soil. I. Solubilization of inorganic phosphates and production of organic acids by microorganisms isolated in sucrose calcium phosphate agar plates. Zbt Bakt Abt II 136:478–486
Banik S, Dey BK (1982) Available phosphate content of an alluvial soil as influence by inoculation of some isolated phosphate solubilizing microorganisms. Plant Soil 69:353–364
Banik S, Dey BK (1983) Alluvial soil microorganisms capable of utilizing insoluble aluminium phosphate as a sole source of phosphorus. Zbt Microbiol 138:437–442
Bardiya MC, Gaur AC (1974) Isolation and screening of microorganisms dissolving low grade rock phosphate. Folia Microbiol 19:386–389
Barker SA (1984) Soil nutrient bioavailability. Wiley, New York
Baya AM, Robert SB, Ramos CA (1981) Vitamin production in relation to phosphate solubilization by soil bacteria. Soil Biol Biochem 13:527–532
Belimov AA, Kojemiakov AP, Chuvarliyeva CV (1995) Interaction between barley and mixed cultures of nitrogen fixing and phosphate-solubilizing bacteria. Plant Soil 173:29–37
Bojinova D, Velkova R, Ivanova R (2008) Solubilization of morocco phosphate by Aspergillus niger. Biores Technol 99:7348–7353
Bossier P, Hofte M, Verstraee W (1988) Ecological significance of siderophores in soil. Adv Microbiol Ecol 10:385–414
Buch A, Archana G, Naresh KG (2008) Metabolic channelling of glucose towards gluconate in phosphate solubilizing Pseudomonas aeruginosa P4 under phosphorus deWciency. Res Microbiol 159:635–642
Burdman S, Volpin H, Kigel J, Kapulnik Y, Okon Y (1996) Promotion of nod gene inducers and nodulation in common bean (Phaseolus vulgaris) root inoculated with Azospirillum brasilense. Appl Environ Microbiol 62:3030–3033
Caravaca F, Alguacil MM, Azcon R, Diaz G, Roldan A (2004) Comparing the effectiveness of mycorrhizal inoculation and amendment with sugar beet, rock phosphate and Aspergillus niger to enhance field performance of the leguminous shrub Dorycnium pentaphyllum L. Appl Soil Ecol 25:169–180
Chebotar VK, Asis CA Jr, Akao S (2001) Production of growth promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when coinoculated with Bradyrhizobium japonicum. Biol Fertil Soils 34:427–432
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Chhonkar PK, Subba Rao NS (1967) Phosphate solubilization by fungi associated with legume root nodules. Can J Microbiol 13:749–753
Dala RC (1973) Characterization of soil inorganic phosphorus. Agrochimica 17:385–396
Darmwal NS, Singh RB, Rai R (1989) Isolation of phosphate solubilizers from different sources. Curr Sci 58:570–571
Das AC (1963) Utilization of insoluble phosphates by soil fungi. J Indian Soc Soil Sci 11:203–207
Datta M, Banik S, Gupta RK (1982) Studies on the efficacy of a phytohormone producing phosphate solubilizing Bacillus firmus in augementing paddy yield in acid soils of Nagaland. Plant Soil 69:365–373
Dave A, Patel HH (1999) Inorganic phosphate solubilizing soil Pseudomonas. Indian J Microbiol 39:161–164
Derylo M, Skorupska A (1993) Enhancement of symbiotic nitrogen fixation by vitamin-secreting fluorescent Pseudomonas. Plant Soil 154:211–217
Duponnois R, Kisa M, Plenchette C (2006) Phosphate solubilizing potential of the nematofungus Arthrobotrys oligospora. J Plant Nutrit Soil Sci 169:280–282
El-Azouni IM (2008) Effect of phosphate solubilizing fungi on growth and nutrient uptake of Soybean (Glycine max L.) plants. J Appl Sci Res 4:592–598
Fabre B, Armau E, Etienne G, Legendre F, Tiraby G (1988) A simple screening method for insecticidal substances from Actinomycetes. J Antibiot 41:212–219
Farhat MB, Farhat A, Bejar W, Kammoun R, Bouchaala K, Fourati A, Antoun H, Bejar S, Chouayekh H (2009) Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch Microbiol 191:815–824
Fernandez HM, Carpena AO, Dadahiya C (1984) Solubilization of mineral phosphorus in calcareous soils by Bacillus cereus in green house experiments. Agrociencia 43:235–245
Firsching FH (1969) Chelates in analytical chemistry. Dekker, New York, pp 4–12
Furihata T, Suzuki M, Sakuri H (1992) Kinetic characterization of two phosphate uptake systems with different affinities in suspension cultures Cahtaranthus roseus protoplasts. Plant Cell Physiol 33:1151–1157
Gaind S, Gaur AC (1990a) Influence of temperature on the efficiency of phosphate solubilizing microorganisms. Indian J Microbiol 30:305–310
Gaind S, Gaur AC (1990b) Shelf life of phosphate solubilizing inoculants as influenced by type of carrier, high temperature and low moisture. Can J Microbiol 36:846–849
Gaind S, Gaur AC (1991) Thermotolerant phosphate solubilizing microorganisms and their interaction with mungbean. Plant Soil 133:141–149
Garg N, Mishra MM, Garg KL (1989) In: Mukerji KG, Singh VP, Garg KL (eds) Frontiers in applied microbiology, vol III. Rastogi, Meerut, pp 263–271
Gaur AC (1969) Studies on the availability of phosphate in soils as influenced by humic acid. Agrochimica 14:62–65
Gaur AC (1990) Phosphate solubilizing microoganisms as biofertilizers. Omega Scientific, New Delhi, India
Gaur AC, Madan M, Ostwal KP (1973) Solubilization of phosphatic compounds by native microflora of rock phosphates. Indian J Expt Biol 11:427–429
Gerretsen FC (1948) The influence of microorganisms of the phosphate intake by the plants. Plant Soil 1:51–81
Glick BR (1995) The enhancement of plant growth by free living bacteria. Can J Microbiol 32:145–148
Goldstein AH (1986) Bacterial phosphate solubilization: historical perspective and future prospects. Am J Altern Agric 1:57–65
Goldstein AH, Liu ST (1987) Molecular cloning and regulation of a mineral phosphate solubilizing gene from Erwinia herbicola. Biotechnology 5:72–74
Goyal S, Phogat VK, Mishra MM, Yadva KS (1982) Transformation of phosphorus during solubilization of rock phosphate by Aspergillus awamori. Indian J Microbiol 22:136–138
Grant C, Bitman S, Montreal M, Plenchette C, Morel C (2005) Soil and fertilizer phosphorus: effects on plant supply and mycorrhizal development. Can J Plant Sci 85:3–14
Gulati A, Rahi P, Vyas P (2008) Characterization of phosphate solubilizing fluorescent Pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr Microbiol 56:73–79
Gupta RD, Bhardwaj KKR, Marwah BC, Tripathi BR (1986) Occurrence of phosphate dissolving bacteria in some soils of North-West Himalayas under varying biosequences and climosequence. J Indian Soc Soil Sci 34:498–504
Gupta R, Singal R, Shankar A, Kuhad RC, Saxena RK (1994) Modified plate assay for screening phosphate solubilizing microorganisms. J Gen Appl Microbiol 40:255–260
Gyaneshwar P, Kumar GN, Parekh LJ (1998) Effect of buffering on the phosphate solubilization ability of microorganisms. World J Microbiol Biotechnol 14:669–673
Halder AK, Mishra AK, Bhattacharya P, Chakraborty PK (1990) Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. J Gen Appl Microbiol 36:81–92
Hamdali H, Bouizgarne B, Hafidi M, Lebrihi A, Virolle MJ, Ouhdouch Y (2008) Screening for rock phosphate solubilizing actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38:12–19
Hayman DS (1974) Plant growth responses to vesicular-arbuscular mycorrhiza VI. Effects of light and temperature. New Phytol 73:71–80
Hayman DS (1975) Phosphorus cycling by soil microorganisms and plant roots. In: Walker N (ed) Soil microbiology. Butterworths, London, pp 67–91
Hebbara M, Devi SL (1990) Effect of phosphorus solubilizing bacteria (PSB) on phosphorus availability to groundnut from rock phosphate. Curr Res 19:56–57
Hilda R, Fraga R (2000) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotech Adv 17:319–359
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root induced chemical changes: a review. Plant Soil 237:173–195
Holford ICR (1997) Soil phosphorus its measurement and its uptake by plants. Aust J Soil Res 35:227–239
Holvorson HO, Kenyan A, Kornberg HL (1990) Utilization of calcium phosphates for microbial growth at alkaline pH. Soil Biol Biochem 22:887–890
Hwangbo H, Park RD, Kim YW, Rim YS, Park KH, Kim TH, Suh JS, Kim KY (2003) 2-ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol 47:87–92
Ibrahim AN, Abdel-Aziz IM (1977) Solubilization of rock phosphate by Streptomycetes. Agrokem Alajtan 26:424–434
Ikeda T (2003) Pharmacological effects of ivermectin, an antiparasitic agent for intestinal strongyloidiasis: its mode of action and clinical efficacy. Nippon Yakurigaku Zasshi 122:527–538
Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem 24:389–395
Illmer P, Babato A, Schinner F (1995) Solubilizattion of hardly soluble AlPO4 with PSM. Soil Biol Biochem 27:265–270
Jain PK, Jain PC (2007) Isolation, characterization and antifungal activity of Streptomyces sampsonii GS 1322. Indian J Exp Biol 45:203–206
Jiang Y, Li WJ, Xu P, Tang SK, Xu LH (2005) Study on diversity of actinomycetes salt and alkaline environments. Wei Sheng Wu Xue Bae 46:191–195
Jisha MS, Alagawadi AR (1996) Nutrient uptake and yield of sorghum (Sorghum biocolor L. Moenck) inoculated with phosphate solubilzing bacteria and cellulolytic fungus in a cotton stalk amended vertisol. Microbiol Res 151:1–5
Johnson SE, Loepper RH (2006) Role of organic acids in phosphate mobilization from iron oxide. Soil Sci Soc Am J 70:222–234
Johnston HW (1952) The solubilization of phosphate: I. The action of various organic compounds on dicalcium and tricalcium phosphate. N Z J Sci Technol 33:436–444
Kapoor KK (1995) Phosphate solubilization through soil microorganisms. In: Behl KK, Khurana AL, Dogra RC (eds) Plant microbe interactions in sustainable agriculture. CCS Haryana Agricultural University, Hisar and MMB, New Delhi, pp 46–61
Kapoor KK, Mishra MM, Kukreja K (1989) Phosphate solubilization by soil microorganisms: a review. Indian J Microbiol 29:119–127
Kapoor KK, Mishra MM, Malik RS, Banger KC (1991) Solubilization of MRP by use of pyrite and Thiobacilli. Environ Ecol 9:635–641
Katznelson H, Bose B (1959) Metabolic activity and phosphate dissolving capability of bacterial isolates from wheat roots in the rhizosphere and non rhizosphere soil. Can J Microbiol 5:79–85
Katznelson H, Peterson EA, Rouat JW (1962) Phosphate dissolving microorganisms on seed and in the root zone of plants. Can J Bot 40:1181–1186
Kavimadan SK, Gaur AC (1971) Effect of seed inoculation with Pseudomonas sp. on phosphate uptake and yield of maize. Curr Sci 40:439–440
Khan SA, Hamayun M, Yoon H, Kim H, Suh S, Hwang S, Kim J, Lee I, Choo Y, Yoon U, Kong W, Lee BM, Kim J (2008) Plant growth promotion and Penicillium citrinum. BMC Microbiol 8:231
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010) Plant growth promotion by phosphate solubilizing fungi: current perspective. Arch Agron Soil Sci 56(1):73–98
Khanna SS, Chaudhary ML, Bathla RN (1979) Influence of moisture, FYM and pyrites on the solubilization of rock phosphate in calcareous soils of Haryana. Bull Indian Soc Soil Sci 12:545–549
Khiari L, Parent LE (2005) Phosphorus transformations in acid light-textured soils treated with dry swine manure. Can J Soil Sci 85:75–87
Kim KY, McDonald GA, Jordan D (1997) Solubilization of hydroxyapatite by Enerobacer agglomerans and cloned Escherichia coil in culture medium. Biol Fertil Soils 24:347–352
Kim KY, Jordan D, McDonald GA (1998) Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol Fert Soils 26:79–87
Knight TJ, Langston-Unkefer PJ (1988) Enhancement of symbiotic nitrogen fixation by a toxin releasing plant pathogen. Science 242:951–954
Krishnaraj PU (1996) Genetic characterization of mineral phosphate solubilization in Pseudomonas sp. Ph.D. Thesis, IARI, New Delhi.
Kucey RMN (1983) Phosphate solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can J Soil Sci 63:671–678
Kucey RMN (1987) Increased phosphorus uptake by wheat and field beans inoculated with a phosphate solubilizing Penicillium bilaii strain and with vesicular-arbuscular mycorrhzal fungi. Appl Environ Microbiol 55:2699–2703
Kucey RMN, Janzen HH, Leggett ME (1989) Microbially mediated increases in plant available phosphorus. Adv Agron 42:199–221
Kumar NR, Arasu VT, Gunasekaran P (2002) Genotyping of antifungal compounds producing plant growth-promoting rhizobacteria, Pseduomonas fluorescens. Curr Sci 82:1463–1466
Kundu BS, Gaur AC (1980) Effects of N2 fixing and PSM as single and composite inoculant on cotton. Indian J Microbiol 20:225–229
Kundu BS, Gaur AC (1981) Effect of single and composite cultures on rock phosphate solubilization. Haryana Agric Univ J Res 11:559–562
Kundu BS, Gaur AC (1984) Rice response to inoculation with N2-fixing and P-solubilizing microorganisms. Plant Soil 79:227–234
Li SG (1981) Studies on phosphorite decomposing microorganisms. J Soil Sci 5:33–35
Lin TF, Huang HI, Shen FT, Young CC (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Biores Technol 97:957–960
Liu ST, Lee LY, Jai CY, Hung CH, Chang YS, Wolfram JH, Rogers R, Goldstein AH (1992) Cloning of an Erwinia herbicola gene necessary for gluconic acid production and enhanced mineral phosphate solubilization in E. coli HB 101: nucleotide sequence and probable involvment in biosynthesis of the co-enzyme pyrroloquinoline quinone. J Bacteriol 174:5814–5819
Louw HA, Webley DM (1959) A study of soil bacteria dissolving certain phosphate fertilizers and related compounds. J Appl Bacteriol 22:227–233
Mahmoud SAZ, Abdel-Hafez AM, El-Sawy M, Hanafy EA (1973) Phytin-hydrolysing bacteria in soils of Egypt. Zbt Bakt Abt II 128:528–531
Monod SPI, Gupta DN, Chavan AS (1989) Enhancement of phosphate availability and phosphorus uptake in rice by phosphate solubilizing culture. J Maharashtra Agric Univ 14:178–181
Manulis S, Shafrir H, Epstein E, Lichter A, Barash I (1994) Biosynthesis of indole-3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp. Microbiology 140:1045–1050
Mattey M (1992) The production of organic acids. Rev Biotechnol 12:87–132
Mba CC (1994) Rock phosphate solubilizing and cellulolytic actinomycetes isolates of earthworm casts. Environ Manage 18:257–261
Mba CC (1997) Rock phosphate solubilizing Streptosporangium isolates from casts of tropical earthworms. Soil Biol Biochem 29:381–385
Mehta YR, Bhide VP (1970) Solubilization of tricalcium phosphate by some soil fungi. Indian J Expt Biol 8:228–229
Mishra MM, Banger KC (1985) Phosphocompost as a phosphorus source in neutral and alkaline soils. In: Mishra MM, Kapoor KK (eds) Soil biology: proceedings soil biology symposium. CCS Haryana Agricultural University, Hisar, India, pp 139–147
Mishra MM, Phogat VK, Goyal S, Yadav KS (1983) Solubilization of phosphorus from Mussoorie rock phosphate by Aspergillus awamori and humic substances. Tropic Plant Sci Res 1:221–224
Moose B (1980) Vesicular-arbuscular mycorrhiza research for tropical agriculture. Research Bulletin 194, Hawaii Institute of Tropical Agriculture and Human Resources, Honolulu, HI, USA, University of Hawaii
Narsian V, Thakkar J, Patel HH (1993) Isolation and screening of phosphate solubilizing fungi. Indian J Microbiol 34:113–118
Nath AK, Borah DK (1983) A study on the release on native and applied fixed phosphate as affected by pH and moisture regime. Indian J Agric Chem 16:247–251
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microoganisms. FEMS Microbiol Lett 170:265–270
Omar SA (1998) The role of rock-phosphate-solubilizing fungi and vesicular-arbusular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J Microbiol Biotech 14:211–218
Ostwal KP, Bhide VP (1972) Solubilization of tricalcium phosphate by soil Pseudomonas. Indian J Expt Biol 10:153–154
Pareek RP, Gaur AC (1973) Release of phosphate from tricalcium phosphate and rock phosphates by organic acids. Curr Sci 42:278–279
Parks EJ, Olson GJ, Brickman FE, Baladi F (1990) Characterization of high performance liquid chromatography (HPLC) of the solubilization of phosphorus in iron ore by a fungus. Indian J Microbiol 5:18–190
Parmar N, Dadarwal KR (1999) Stimulation of nitrogen fixation and induction of flavonoid like compounds by rhizobacteria. J Appl Microbiol 86:36–44
Patel DK, Archana G, Kumar GN (2008) Variation in the nature of organic acid secretion and mineral phosphate solubilization by Citrobacter sp. DHRSS in the presence of different sugars. Curr Microbiol 56:168–174
Pathom-Aree W, Stach JE, Ward AC, Horikoshi K, Bull AT, Goodfellow M (2006) Diversity of actinomycetes isolated from challenger deep sediment (10, 898 m) from the mariana trench. Extremophiles 10:181–189
Paul NB, Sundara Rao WVB (1971) Phosphate dissolving bacteria in rhizosphere of some cultivated legumes. Plant Soil 25:127–132
Perveen S, Khan MS, Zaidi A (2002) Effect of rhizospheric microorganisms on growth and yield of green gram (Phaseolus radiatus). Indian J Agric Sci 72:421–423
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya 7:362–370
Rachewad SN, Raut RS, Malewar GU, Ganure CK (1992) Effects of phosphate solubilizing biofertilizer on biomass production and uptake of phosphorus by sunflower. J Maharashtra Agric Univ 17:480–481
Roychoudhary P, Kaushik BD (1989) Solubilization of Mussoorie rock phosphate by cyanobacteria. Curr Sci 58:569–570
Reddy MS, Kumar S, Babita K, Reddy MS (2002) Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger. Biores Technol 84:187–189
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Reyes I, Bernier L, Simard RR, Antoun H (1999) Effect of nitrogen source on the solubilization of different inorganic phosphates by an isolate of Penicillium rugulosum and two UV induced mutants. FEMS Micobiol Ecol 28:281–290
Richa G, Khosla B, Reddy MS (2007) Improvement of maize plant growth by phosphate solubilizing fungi in rock phosphate amended soils. World J Agric Sci 3:481–484
Richardson AE (1994) Soil micro-organisms and phosphate availability. In: Pankhurst CE, Double BM, Gupts VVSR, Grace PR (eds) Soil biota management in sustainable agriculture. CSIRO, Melbourne, Australia, pp 50–62
Rodriguez H, Fragaa R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotech Adv 17:319–339
Rodriguez H, Gonzalez T, Selman G (2000) Expression of a mineral phosphate solubilizing gene from Erwinia herbicola in two rhizobacterial strains. J Biotechnol 84:155–161
Roos W, Luchner M (1984) Relationships between proton extrusion and fluxes of ammonium ions and organic acids in Penicillium cyclopium. J Gen Microbiol 130:1007–1014
Roychoudhary P, Kaushik BD (1989) Solubilization of Mussoorie rock phosphate by cynobacteria. Curr Sci 10:569–570
Rustrain E, Delgens JP, Maletta R (1997) P release by pure culture of Acinetobacter sp. Effect of growth stage with cell cultivated on various carbon sources. Lett Appl Environ Microbiol 24:144–148
Sackett WG, Palten AJ, Brown CW (1908) The solvent action of soil bacteria upon the insoluble phosphates of raw bone meal and natural rock phosphate. Zbl Bakt Abt II 20:688
Sahachtman DP, Reid RJ, Ayling SM (1998) Phosphate uptake by plants from soil to cell. Plant Physiol 116:447–453
Sanders FE, Tinker PB (1973) Phosphate flow into mycorrhizal roots. Pest Sci 4:385–395
Santhi V (1998) Mechanism of mineral phosphate solubilization and growth promotion by diverse bacteria. M.Sc. Thesis, UAS, Dharwad
Sanyal SK, De Datta SK (1991) Chemistry of phosphorus transformations in soil. Adv Soil Sci 16:1–120
Sardina MG, Boiardi JL, Erbola RJ (1986) Solubilization of phosphorus from low-grade minerals by microbial action. Biotechnol Lett 8:247–252
Saxena AK, Tilak KVBR (1994) Interaction among beneficial microorganisms. Indian J Microbiol 34:91–106
Saxena AK, Tilak KVBR (1997) Interaction of soil microorganisms with vesicular arbuscular mycorrhiza. In: Tiwari JP, Saxena G, Tiwari I, Mittal N, Chamola BP (eds) New approches in microbial ecology. Aditya Books, New Delhi, India, pp 231–250
Sen A, Paul NB (1957) Solubilization of phosphates by some common soil bacteria. Curr Sci 26:222–223
Sen A, Paul NB (1958) Occurrence of phosphobacteria in the glands of Cassia occidentalis. Indian J Agric Sci 28:21–29
Sethi RP, SubbaRao NS (1968) Solubilization of tricalcium phosphate and calcium phytate by soil fungi. J Gen Appl Microbiol 14:329–331
Sheshadari S, Kumarasamy R, Lakshminarasimham C, Ignacimuthu S (2000) Solubilization of inorganic phosphates by Azospirillium halopraeferans. Curr Sci 79:565–567
Sindhu SS, Suneja S, Goel AK, Parmar N, Dadarwal KR (2002a) Plant growth promoting effects of Pseudomonas sp. on coinoculation with Mesorhizobium sp. cicer strain under sterile and wilt sick soil conditions. Appl Soil Ecol 19:117–120
Sindhu SS, Gupta SK, Suneja S, Dadarwal KR (2002b) Enhancement of greengram nodulation and growth by Bacillus sp. Biol Plantarum 45:117–120
Singal R, Gupta R, Kuhad RC, Saxena RK (1991) Solubilization of inorganic phosphates by a Basidiomycetous fungus Cyathus. Indian J Microbiol 31:397–401
Singh S (1992) Solubilization of insoluble phosphates by bacteria. M.Sc. Thesis, CCS HAU, Hisar, India
Singh CP, Amberger A (1990) Humic substances in straw compost with rock phosphate. Biol Waste 31:165–174
Singh S, Kapoor KK (1994) Solubilization of insoluble phosphate isolated from different sources. Enviorn Ecol 12:51–55
Singh CP, Mishra MM, Yadav KS (1980) Solubilization of insoluble phosphates by thermophilic fungi. Ann Microbiol 131:289–296
Singh HP, Pareek RP, Singh TA (1984) Solubilization of rock phosphate solubilizers in broth. Curr Sci 53:1212–1213
Son TTN, Vu van Thu VU and Kobayashi H (2003) Effect of organic and bio fertilizer application on rice-soybean-rice cropping systems. In: Proceedings of the final workshop of JIRCAS Mekong Delta Project – “Development of new technologies and their practice for sustainable farming systems in the Mekong Delta”, November 25–26, 2003, pp 65–81
Son TTN, Diep CN, Giang TTM (2006) Effect of bradyrhizobia and phosphate solubilizing bacteria application on soybean in rotational system in the mekong delta. Omonrice 14:48–57
Sperber JI (1957) Solution of mineral phosphates by soil bacteria. Nature 180:994–995
Sperber JI (1958) Solution of apatite by soil microorganisms producing organic acids. Aust J Agric Res 9:778–781
Stalstrom VA (1903) Beitrag Zur Kenntrusder einwinsking sterilizer and in garung befindlieher striffe any dil loslieshkeit der phosphorus are destrical cum phosphours. Zbt Bakt Abt II 11:724–732
Stevenson FJ (2005) Cycles of soil: carbon, nitrogen, phosphorus, sulfur, micronutrients. Wiley, New York
Sulbarán M, Pérez E, Ball MM, Bahsas A, Yarzábal LA (2009) Characterization of the mineral phosphate-solubilizing activity of Pantoea agglomerans MMB051 isolated from an iron-rich soil in southeastern Venezuela (Bolívar State). Curr Microbiol 58:378–383
Sundara Rao WVB and Paul NB (1959) Bacterization of phosphobacterin, radioisotopes, fertilizers and cowdung gas plant. ICAR Proc Ser 322–326
Sundara Rao WVB, Sinha MK (1963) Phosphate dissolving microorganisms in the soil and rhizosphere. Indian J Agric Sci 33:272–278
Sundara Rao WVB, Bajpai PD, Sharma JP, Subbiah BV (1963) Solubilization of phosphates by PSM using 32P as tracer and influence of seed bacterization on the uptake by the crop. J Indian Soc Sci 11:209–219
Suneja S, Lakshminarayana K (1999) Siderophore production of Azotobacter. In: Narula N (ed) Azotobacter in sustainable agriculture. CBS, New Delhi, India, pp 64–73
Surange S, Kumar N (1993) Phosphate solubilization under varying pH by Rhizobium from tree legumes. Indian J Exptl Biol 11:427–429
Swain MR, Ray RC (2009) Biocontrol and other beneficial activities of Bacillus subtilis isolated from cowdung microflora. Microbiol Res 164:121–130
Taiwo LB, Ogundiya M (2008) Microbial solubilization of Ogun rock phosphate in the laboratory and in soil. Afr J Microbiol Res 2:308–312
Tandon HLS (1987) Phosphorus research and agricultural production in India. Fertilizer Development and Consultation Organization, New Delhi, p 172
Thakkar J, Narsian V, Patel HH (1993) I. Inorganic P solubilization by certain soil bacteria. II. Solubilization of natural rock phosphate and pure insoluble inorganic P by Aspergillus awamori. Indian J Expt Biol 31:743–747
Theodorou ME, Plaxton WC (1993) Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol 101:339–344
Thomas GV, Shantaram MV (1986) Solubilization of inorganic phosphates by bacteria from coconut plantation soils. J Plant Crops 14:42–48
Vassilev M, Vassileva N (2003) Biotechnological solubilization of rock phosphate on media containing agro-industrial wastes. Appl Microbiol Biotechnol 61:435–440
Vassilev N, Massimiliano F, Federico F (1996) Rock phosphate solubilization with gluconic acid produced by immobilized Penicillium variable P16. Biotech Tech 10:585–588
Vassilev N, Toro M, Vassileva M, Azcón R, Barea JM (1997) Rock phosphate solubilization by immobilized cells of Enterobacter sp. in fermentation and soil conditions. Biores Technol 61:29–32
Vazquez P, Holguin G, Puente EM, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Fertil Soils 30:5–6
Venkateswarlu B, Rao AY, Raina P (1984) Evaluation of phosphorus solubilization by microorganisms isolated from Aridisols. J Indian Soc Soil Sci 32:273–277
Verma LN (1993) Biofertiliser in agriculture. In: Thampan PK (ed) Organics in soil health and crop production. Peekay Tree Crops Development Foundation, Cochin, India, pp 152–183
Wainwright W (1984) Microbial sulphur oxidation in soil. Sci Prog 65:459–475
Wani PV, More BB, Patil PL (1979) Physiological studies on the activity of phosphorus solubilizing microorganisms. Indian J Microbiol 19:23–25
Wani PA, Khan MS, Zaidi A (2007) Synergistic effects of the inoculation with nitrogen fixing and phosphate-solubilizing rhizobacteria on the performance of field grown chickpea. J Plant Nutr Soil Sci 170:283–287
Whitelaw MA (2000) Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv Agron 69:99–151
Whitelaw MA, Harden TJ, Bender GL (1997) Plant growth promotion of wheat inoculated with Penicillium radicum sp. nov. Aust J Soil Res 35:291–300
Whitelaw MA, Harden TJ, Helyar KR (1999) Phosphate solubilization in solution culture by the soil fungus Penicillium radicum. Soil Biol Biochem 32:655–665
Xiao C, Chi R, He H, Qiu G, Wang D, Zhang W (2009) Isolation of phosphate-solubilizing fungi from phosphate mines and their effect on wheat seedling growth. Appl Biochem Biotechnol 159:330–342
Yadav KS, Dadarwal KR (1997) Phosphate solubilization and mobilization through soil microorganisms. In: Dadarwal KR (ed) Biotechnological approaches in soil microorganisms for sustainable crop production. Scientific Publishers, Jodhpur, India, pp 293–308
Yi Y, Huang W, Ying G (2008) Exopolysaccharide: a novel important factor in the microbial dissolution of tricalcium phosphate. World J Microbiol Biotechnol 24:1059–1065
Zaidi A, Khan MS, Amil M (2003) Interactive effect of rhizotrophic microorganisms on yield and nutrient uptake of chickpea (Cicer arietinum L.). Eur J Agron 19:15–21
Acknowledgement
The authors acknowledge the help from Mr. Harsh Kuhad, Amity University, Noida, India, during the preparation of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kuhad, R.C., Singh, S., Lata, Singh, A. (2011). Phosphate-Solubilizing Microorganisms. In: Singh, A., Parmar, N., Kuhad, R. (eds) Bioaugmentation, Biostimulation and Biocontrol. Soil Biology, vol 108. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-19769-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-19769-7_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-19768-0
Online ISBN: 978-3-642-19769-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)